Abstract
MDC1A, the second most prevalent form of congenital muscular dystrophy, results from laminin-α2 chain deficiency. This disease is characterized by extensive muscle wasting that results in extremely weak skeletal muscles. A large percentage of children with MDC1A are faced with respiratory as well as ambulatory difficulties. We investigated the effects of overexpressing insulin-like growth factor-1 (IGF-1) as a potential therapeutic target for the disease in the Lama2Dy-w mouse, a model that closely resembles human MDC1A. IGF-1 transgenic Lama2Dy-w mice showed increased survivability, body weight and muscle weight. In addition, these mice showed better ability to stand up on their hind limbs: a typical exploratory behavior seen in healthy mice. Histology and immunohistochemistry analyses revealed increased regenerative capacity and proliferation in IGF-1 transgenic Lama2Dy-w muscles. Western blot analysis showed increased phosphorylation of Akt and ERK1/2, both known to enhance myogenesis. Additionally, we saw increases in the expression of the regeneration markers MyoD, myogenin and embryonic myosin (myosin heavy chain 3, MYH3). We conclude that overexpression of IGF-1 in Lama2Dy-w mice increases lifespan and improves their overall wellbeing mainly through the restoration of impaired muscle regeneration, as fibrosis or inflammation was not impacted by IGF-1 in this disease model. Our results demonstrate that IGF-1 has a promising therapeutic potential in the treatment of MDC1A.
INTRODUCTION
MDC1A, the second most prevalent form of congenital muscular dystrophy, is caused by a defect in the laminin-α2 (LAMA2) gene. The protein encoded by this gene normally plays a crucial role in structural stability and signal transduction at the plasma membrane of muscle and Schwann cells. The absence of laminin-α2 results in poor muscle tone at birth, extremely compromised neuromuscular function and the inability to achieve independent ambulatory capacity. At the current time, there is no effective therapy for this form of congenital muscular dystrophy, and in many cases, children suffering from MDC1A succumb to premature death, either as a result of respiratory complications or failure to thrive (1,2).
Laminin-α2 belongs to a class of proteins known as laminins, which are heterotrimeric extracellular matrix (ECM) proteins comprised of α, β and γ chains. Different combinations of these three chains give rise to at least 15 distinct isoforms, which are expressed in various types of tissues (3), with laminin-α2 being predominantly expressed in muscle and peripheral nerves. It specifically interacts with α-dystroglycan, an extracellular protein belonging to the dystrophin–glycoprotein complex, and with α7β1integrin, another transmembrane protein complex. Each of these interactions has been shown to regulate PI3-kinase and stress-induced mitogen-activated protein kinase (MAPK)-mediated signaling pathways (4,5).
There are several mouse models that can be used to study the underlying mechanisms and to develop therapeutic interventions for MDC1A. The phenotype of the laminin-α2-deficient mouse model we used, Lama2Dy-w, closely resembles that of human MDC1A (6). Like humans, mice that are homozygous for the mutant allele show accelerated muscle degeneration with limited or no regenerative capacity and have an extremely short lifespan compared with their healthy littermates (7–12). Histological analysis of Lama2Dy-w muscle tissue shows significant evidence of apoptosis, increased fibrosis and severe inflammation (13–17). Several intervention strategies have been employed to alleviate muscle and nerve pathology in different mouse models of MDC1A, and have led to measurable improvements in phenotype (18–24). However, to date, no studies have examined the therapeutic effects of directly modulating regenerative capacity in laminin-α2-deficient mice.
The ability to regenerate efficiently in response to chronic muscle injury can be beneficial in the context of muscular dystrophy. In contrast to dystrophin-deficient mdx mice, a model for Duchenne muscular dystrophy (DMD), which are capable of successful regeneration for most of their lives (25,26), laminin-α2-deficient mice have limited or no regenerative capacity (8,13). Failed regeneration may be responsible in part for their poor overall growth and extremely short lifespan (27).
We hypothesized that improving the regenerative capacity of Lama2Dy-w muscles would ameliorate the pathology associated with laminin-α2 deficiency. Insulin-like growth factor-1 (IGF-1) is known to improve regeneration by enhancing processes such as proliferation, differentiation and cell survival (28,29). In addition, sustained high levels of IGF-1 can promote muscle hypertrophy (30). Muscle-specific overexpression of IGF-1 has helped to maintain regeneration efficacy in aging mice (31), and has been shown to reduce muscle pathology in dystrophic mice (32,33). To test our hypothesis, we crossed Lama2Dy-w mice with myosin light chain (MLC)/mIGF-1 transgenic mice, which overexpress a precursor of muscle-specific IGF-1 (mIGF-1) under the MLC 1/3 promoter in skeletal muscle (31). Our results demonstrate for the first time that improved regeneration mediated by overexpression of muscle-specific IGF-1 results in markedly improved longevity, overall growth and exploratory behavior of the Lama2Dy-w mice and therefore could hold therapeutic potential in laminin-α2 deficiency in humans.
RESULTS
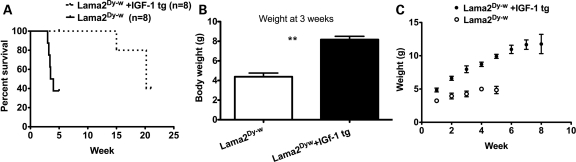
To determine the effects of IGF-1 expression on laminin-α2-deficient pathology, Lama2Dy-w mice were bred with MLC/mIGF1 transgenic mice (30). Eighty percent of Lama2Dy-w mice expressing mIGF-1 lived for at least 150 days (n= 8), well beyond the approximate 6 week lifespan commonly observed with Lama2Dy-w mice (Fig. 1A). In addition to prolonging survival, IGF-1 overexpression resulted in increased body weight of Lama2Dy-w mice. At 3 weeks of age, these mice weighed twice as much as their littermates not expressing mIGF-1 [8.171 ± 0.877 g for Lama2Dy-w+IGF-1tg (n= 7) and 4.383 ± 0.9131 g for Lama2Dy-w mice (n= 6), P< 0.001, t-test (Fig. 1B)]. Lama2Dy-w+IGF-1tg mice continued to grow until 8 weeks of age (achieving a weight of 10.91 ± 1.686 g), whereas the rare non-transgenic Lama2Dy-w mice that lived beyond 4 weeks showed a decline in their weights (Fig. 1C). It is important to note, however, that even though Lama2Dy-w+IGF-1tg mice were significantly larger than their non-transgenic littermates, they never reached the weights of their wild-type (WT) littermates (19–21 g at 4 weeks).
Figure 1.
Increased survival and body weight observed in laminin-α2-deficient mice overexpressing IGF-1. (A) Survival curves showing that most Lama2Dy-w mice died within 4 weeks of birth, whereas the lifespans of most Lama2Dy-w+IGF-1tg mice were markedly increased to at least 21 weeks (n= 8 for both groups, P< 0.009). (B) At 3 weeks, the mean body weight of Lama2Dy-w+IGF-1tg (n= 7) mice was twice that of Lama2Dy-w mice (n= 6; P< 0.001). (C) Body weight curves showing a higher growth rate in Lama2Dy-w+IGF-1tg mice than in Lama2Dy-w mice. Furthermore, a greater divergence in body weight was seen over time as Lama2Dy-w+IGF-1tg mice continued to grow, whereas Lama2Dy-w mice showed poor growth overall.
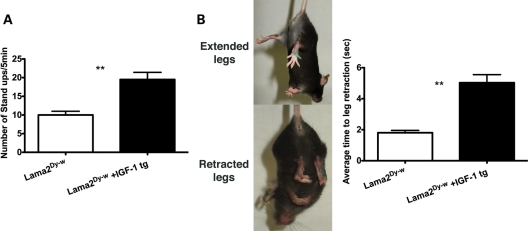
WT mice spontaneously stood up several times on their hind legs during their normal exploratory behavior, whereas Lama2Dy-w mice rarely exhibited this behavior perhaps as a result of weaker muscles. Lama2Dy-w+IGF-1tg mice showed significant improvement in this spontaneous behavior compared with their age-matched Lama2Dy-w littermates [19.50 ± 5.42 and 10.00 ± 2.83 times/5 min, respectively, P< 0.005, (Fig. 2A)]. In addition to stand up tests, we performed tail suspension tests on these mice. When suspended by their tails, WT mice spread out their limbs and digits to hold their trunks in an extended position (34). In contrast, Lama2Dy-w mice were not capable of keeping their legs extended when subjected to tail suspension. Instead, they retracted their limbs to an adducted position, indicating muscle weakness (Fig. 2B). To quantify this behavior, the time to the first episode of leg retraction was recorded in three 10 s trials per test to obtain an average time to retraction. Severity of muscle weakness was reflected by the time to first retraction, and animals were graded accordingly (severe: <3 s; moderate: 3–6 s; mild: >6 s). In this test, Lama2Dy-w+IGF-1tg mice performed significantly better compared with mice that did not express mIGF-1 [5.03 ± 1.47 and 1.801 ± 0.04 s for Lama2Dy-w+IGF-1tg and Lama2Dy-w mice, respectively, P< 0.0001 (Fig. 2B)].
Figure 2.
Overall functional activity and capacity were improved with IGF overexpression in Lama2Dy-w mice. (A) Stand up test results showed a significant increase in exploratory behavior in Lama2Dy-w+IGF-1tg mice than in Lama2Dy-w mice (P< 0.005). (B) Pictures display leg extension and leg retraction in tail suspension tests performed to assess muscle weakness, with severity graded according to the average time to leg retraction (severe:<3 s; moderate: 3–6 s; mild: >6 s). Average muscle weakness was severe in Lama2Dy-w mice and moderate in Lama2Dy-w+IGF-1tg mice.
IGF-1-overexpressing Lama2Dy-w mice also exhibited a promising delay in the progression of hind limb paralysis. Owing to a lack of basal lamina, Lama2Dy-w mice developed nerve conduction defects that subsequently resulted in hind limb paralysis by the age of 5–6 weeks. Onset of hind limb paralysis was delayed by 3–4 weeks in Lama2Dy-w+IGF-1tg mice. While these mice eventually exhibited signs of hind limb paralysis, this motor dysfunction did not appear to affect their survival.
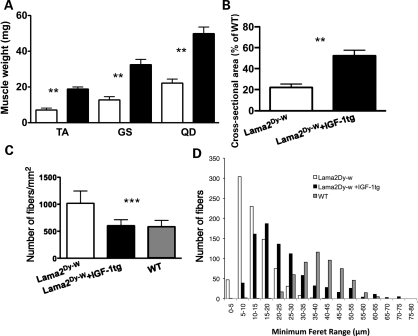
Consistent with the overall increase in body weight, the individual muscles isolated from the hind limbs of IGF-1 overexpressing Lama2Dy-w mice were also significantly larger in size and weight compared with Lama2Dy-w mice (Fig. 3A). At 6 weeks of age, the tibialis anterior (TA) muscles of Lama2Dy-w+IGF-1tg mice weighed significantly more than those isolated from Lama2Dy-w mice (18.86 ± 3.09 and 7.10 ± 2.40 mg, respectively, P< 0.0001, t-test). Similar increases were noted in the gastrocnemius/soleus muscle complex (32.48 ± 8.441 mg for Lama2Dy-w+IGF-1tg mice and 12.76 ± 4.09 mg for Lama2Dy-w mice, P= 0.0005, t-test) and quadriceps muscles (49.86 ± 9.75 mg for Lama2Dy-w+IGF-1tg mice and 22.16 ± 5.04 mg for Lama2Dy-w mice, P= 0.0002, t-test). Increased muscle weight correlated with increases in muscle cross-sectional area (Fig. 3B). For example, the mean cross-sectional area of TA muscles in Lama2Dy-w mice was ∼25% of its age- and sex-matched WT control. However, the mean cross-sectional area was increased 2-fold in the Lama2Dy-w+IGF-1tg mice (50% of its age- and sex-matched WT control). In contrast to laminin-α2-deficient muscle that had a much higher number of very small caliber fibers, TA muscles of Lama2Dy-w+IGF-1tg had larger fibers and fewer fibers per square millimeter, similar to the average WT fiber density [Lama2Dy-w= 1018 ± 227.8/mm2, Lama2Dy-w+IGF-1tg= 603.1 ± 110.7/mm2 and WT = 584.6 ± 116.6/mm2, P= 0.0042, one-way ANOVA (Fig. 3C)]. To determine fiber size distribution, we measured the minimal Feret's diameter of myofibers. This is a reliable measure of fiber cross-sectional size that is expressed as the smallest distance between two parallel tangents on opposite sides of a particle (8,35). When grouped by Feret sizes, the fibers of Lama2Dy-w+IGF-1tg muscle showed a shift towards the WT distribution compared with their non-transgenic affected littermates (Fig. 3D).
Figure 3.
Lama2Dy-w+IGF-1tg mice showed increased hind limb muscle size and myofiber hypertrophy. (A) Average muscle weights of tibialis anterior (TA), gastrocnemius/soleus complex (GS) and quadriceps muscles (QD) were significantly greater in Lama2Dy-w+IGF-1tg mice (black bars) than in its non-transgenic littermates (white bars) at 6 weeks (TA, P< 0.0001; GS, P= 0.0005; QD, P= 0.0002). (B) The mean cross-sectional area of the mid-belly portion of TA muscles in Lama2Dy-w+IGF-1tg mice was 50% (n= 6) of its age- and sex-matched WT control, a 2-fold increase compared with that of Lama2Dy-w littermates (25%, n= 4). (C) Lama2Dy-w mice showed a significant increase in the number of fibers per square millimeter, whereas Lama2Dy-w+IGF-1tg mice did not significantly vary in comparison with the WT (P= 0.0042, one-way ANOVA). (D) Minimum Feret histograms display a shift in fiber size distribution in Lama2Dy-w+IGF-1tg muscles (black bars) towards the WT (gray bars) distribution in comparison with the Lama2Dy-w (white bars) distribution. Although Lama2Dy-w mice had more fibers per square millimeter, many of those fibers were of smaller caliber.
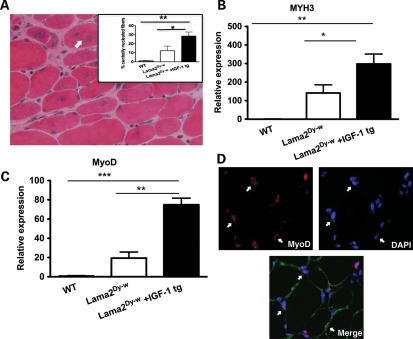
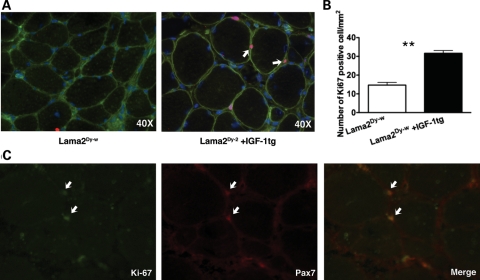
Compared with dystrophin-deficient mdx mice that are capable of efficient regeneration in response to myopathology, muscles of laminin-α2-deficient mice showed very little signs of regeneration (13,36). However, overexpression of IGF-1 significantly increased the number of centrally nucleated fibers in TA as well as soleus muscles of laminin-α2-deficient mice. More than twice the percentage of the fibers counted were centrally nucleated in Lama2Dy-w+IGF-1tg TA muscles, compared with Lama2Dy-w TA muscles (Fig. 4A). At the molecular level, we observed a 2-fold increase in the expression of embryonic myosin heavy chain (MYH3, myosin heavy chain 3) in muscle tissues of Lama2Dy-w+IGF-1tg mice compared with their non-transgenic littermates (Fig. 4B). RNA expression levels of MyoD family members (MyoD, myogenin, Myf5 and Mrf4) were measured as an index of myogenesis. Relative expression of both MyoD and myogenin was significantly higher in Lama2Dy-w+IGF-1tg muscles compared with WT as well as Lama2Dy-w littermates [relative expression for MyoD: WT = 1.146 ± 0.1385, Lama2Dy-w= 19.40 ± 10.95, Lama2Dy-w+IGF-1tg= 74.84 ± 11.83, P= 0.002, one-way ANOVA followed by Newman–Keuls multiple comparison analysis; relative expression for myogenin: WT = 1.098 ± 0.097, Lama2Dy-w= 18.35 ± 10.47, Lama2Dy-w+IGF-1tg= 71.31 ± 11.75, P= 0.0015, one-way ANOVA followed by Newman–Keuls multiple comparison analysis (Fig. 4C)]. This increase in MyoD and myogenin may contribute to improved myogenesis in IGF-1 overexpressing mice. In addition, immunohistochemistry also showed many MyoD-positive nuclei (Fig. 4D). There was no difference in the expression levels of Myf5 and Mrf4 between the three groups (data not shown). We next asked the question whether overexpression of the IGF-1 transgene increased the number of proliferating cells. Sections were stained with Ki-67, a marker for nuclei in the proliferative phase of the cell cycle. There were twice as many Ki-67-positive cells in Lama2Dy-w+IGF-1tg muscles compared with Lama2Dy-w muscles [31.67 ± 2.5 and 14.67 ± 2.517/mm2, respectively, P= 0.002, t-test (Fig. 5A and B)]. Several of these Ki-67-positive cells also stained for Pax7 (Fig. 5C).
Figure 4.
Increased muscle regeneration in Lama2Dy-w+IGF-1tg mice. (A) H&E staining of Lama2Dy-w+IGF-1tg TA muscle cross-section at 6 weeks. The image displays centrally nucleated fibers (arrow) indicating regeneration. The average percent of centrally nucleated fibers was significantly greater in Lama2Dy-w+IGF-1tg mice (30%) than in Lama2Dy-w mice (12%). (B) Transcript-level expression of embryonic myosin heavy chain (MYH3) increased 2-fold in muscle tissues of Lama2Dy-w+IGF-1tg mice compared with Lama2Dy-w mice. (C) Significant increases in relative RNA expression of MyoD (P= 0.002, one-way ANOVA) were found in Lama2Dy-w+IGF-1tg muscles compared with non-transgenic littermates. (D) Top left panel: Immunohistochemistry shows a number of MyoD (red)-positive nuclei in the interstitial space between myofibers (white arrows). Top right panel: Nuclei stained with DAPI (blue). Bottom panel: A merged image showing dystrophin (green) and DAPI (blue) with MyoD (red).
Figure 5.
Increased muscle cell proliferation observed in Lama2Dy-w mice with IGF overexpression. (A) 40× images of Lama2Dy-w (left) and Lama2Dy-w+IGF-1tg (right) TA muscles stained for Ki-67 (pink) (white arrow), dystrophin (green) and DAPI (blue). (B) The mean number of Ki67-positive cells per square millimeter was doubled in Lama2Dy-w+IGF-1tg compared with Lama2Dy-w TA muscles. (C) Immunohistochemical staining shows several Ki-67 (green)-positive nuclei stained positive for Pax7 (red), a satellite cell marker. Examples of these nuclei are indicated by white arrowheads in each panel. The last panel shows the merged image.
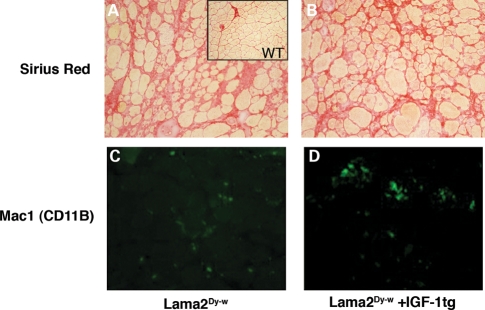
Picro-sirius red staining was used to assess the extent of fibrosis in the muscle tissues of Lama2Dy-w+IGF-1tg mice. There was no reduction in the extent of fibrosis as measured by percent fibrotic area in Lama2Dy-w+IGF-1tg muscles compared with their non-transgenic littermates (Lama2Dy-w= 27.19 ± 0.08 and Lama2Dy-w+IGF-1tg= 26.38 ± 0.04%, P= 0.8577, t-test), suggesting that IGF-1 overexpression does not reduce fibrosis in laminin-α2-deficient mice (Fig. 6A and B). Also, Mac-1 (CD11b, a marker for monocyte/macrophage) staining of muscle sections showed no difference in the amount of infiltrating inflammatory cells in Lama2Dy-w+IGF-1tg mice and littermates without the IGF-1 transgene (Fig. 6C and D). We also confirmed these results with quantitative PCR for transcript levels of CD11b, which showed no difference between the two groups (data not shown).
Figure 6.
Fibrosis and inflammation were not noticeably improved by IGF-1 overexpression in Lama2Dy-w muscles. 20× images of Picro-sirius red staining for collagen (red) of (A) Lama2Dy-w and (B) Lama2Dy-w+IGF-1tg TA muscles revealed no noticeable difference in levels of fibrosis. Immunostaining for Mac-1/CD11B (green), a marker of monocytes/macrophages, showed no change in infiltrating inflammatory cells in (C) Lama2Dy-w muscles and (D) Lama2Dy-w+IGF-1tg muscles.
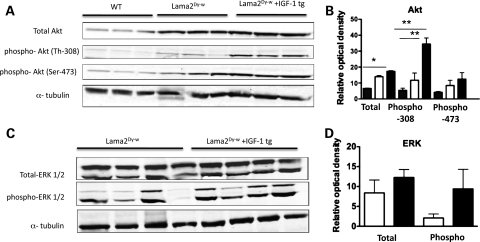
At molecular levels, IGF-1 has been shown to mediate hypertrophy and cell survival by activation of phosphatidyl-inositol 3-kinase (PI3K) and its downstream targets (32,37), as well as facilitate proliferation via RAF-1/MEK/ERK pathway activation (38). To identify the signaling pathways involved in IGF-1-mediated muscle growth and/or survival in Lama2Dy-w mice, we systematically analyzed the phosphorylation status of several downstream phospho-proteins such as those belonging to the PI3K/Akt pathway, p38 MAPK as well as ERK1/2 in muscle lysates from WT, Lama2Dy-w+IGF-1tg and Lama2Dy-w mice. Relative to WT littermates, Lama2Dy-w mice have increased levels of total Akt as well as phospho-Akt (both Ser-473 and Thr-308 forms). Although there was only a small increase in the levels of total and phospho-Ser-473 Akt in IGF-1-overexpressing Lama2Dy-w muscle, phospho-Thr-308-Akt levels were increased several folds (Fig. 7A and B). PI3K/Akt is also known to induce muscle hypertrophy by phosphorylating glycogen synthase kinase 3 (GSK3) and mammalian target of rapamycin (mTOR) (37,39). We saw a moderate increase in the phosphorylation of mTOR and its downstream target p70S6k (data not shown), whereas phosphorylation of GSK3 remained unchanged. We used qPCR to evaluate whether Akt activation inhibited transcription of MuRF1 and atrogin-1, the ubiquitin ligase E3 proteins responsible for muscle degradation (40,41) in Lama2Dy-w muscle. Although expression levels of these genes were higher (∼2-fold) in Lama2Dy-w muscles compared with WT muscles, they did not change in response to IGF-1 overexpression (data not shown). When we examined ERK activation, we found that although total ERK1/2 protein levels were similar in muscle lysates of Lama2Dy-w+IGF-1tg mice and their non-transgenic littermates, there clearly was an increase in phospho-ERK1/2 levels in Lama2Dy-w+IGF-1tg muscles (Fig. 7C and D). Lama2Dy-w+IGF-1tg and Lama2Dy-w muscles showed similar levels of both p38 and phospho-p38, suggesting that elevated MAPK pathway was required for mediating IGF-1 function (data not shown).
Figure 7.
Increased expression of downstream signaling targets of IGF-1 in Lama2Dy-w+IGF-1tg muscle lysates. (A) Western blots of total Akt, phospho-Akt (Thr-308), phospho-Akt (Ser-473) and α-tubulin in muscles isolated from 6-week-old WT, Lama2Dy-w and Lama2Dy-w+IGF-1tg mice. (B) Relative optical density analysis of phospho-Thr-308-Akt levels in Lama2Dy-w+IGF-1tg muscles (black bars) revealed a several-fold increase relative to Lama2Dy-w (white bars) and WT (gray bars), whereas total Akt and phospho-Ser-473-Akt levels increased only marginally. (C) Western blots of phospho-ERK1/2, total ERK1/2 and α-tubulin in 6-week-old muscles isolated from Lama2Dy-w and Lama2Dy-w+IGF-1tg mice. (D) Relative optical density of total ERK1/2 was similar in Lama2Dy-w and Lama2Dy-w+IGF-1tg muscle lysates. However, an increase in relative phospho-ERK1/2 levels was seen in muscles of Lama2Dy-w+IGF-1tg (black bars) compared with its non-transgenic littermates (white bars).
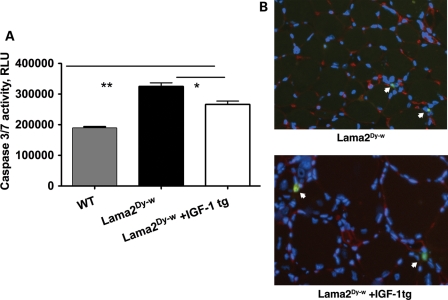
Finally, to evaluate whether IGF-1 overexpression reduced the extent of apoptosis in Lama2Dy-w muscles, we measured the enzymatic activity of caspase3/7 in muscle homogenates. One-way ANOVA followed by Newman–Keuls multiple comparison analysis showed a significant increase in caspase3/7 activity in Lama2Dy-w muscle compared with WT muscle (P< 0.0001). Although significant, there was only a 12.8% decrease in caspase activity in the muscle lysates of Lama2Dy-w+IGF-1tg mice when compared with non-transgenic littermates [n= 5, P< 0.05 (Fig. 8A)]. Using TUNEL (terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling) staining, we found only a slight decrease in the number of apoptotic nuclei in Lama2Dy-w+IGF-1tg muscle sections that did not reach statistical significance (Fig. 8B).
Figure 8.
IGF-1 overexpression leads to a decrease in Lama2Dy-w-mediated apoptosis. (A) Caspase 3/7 activity was higher in Lama2Dy-w compared with WT muscles (P< 0.0001). There was only a 12.8% decrease in caspase activity in the muscle lysates of Lama2Dy-w+IGF-1tg mice compared with its non-transgenic littermates (n= 5, P< 0.05). (B) Representative muscle sections of Lama2Dy-w (top panel) and Lama2Dy-w+IGF-1tg (bottom panel) assayed for TUNEL-positive nuclei did not show any significant difference. TUNEL-positive nuclei (green) are indicated by white arrows. These sections were also stained with PAN laminin isoform (red) and DAPI (blue).
DISCUSSION
Because of its many pathological symptoms, MDC1A is a disease that has many potential therapeutic targets. Using gene replacement therapies to correct the primary cause of MDC1A, the mutation in the LAMA2 gene would be ideal; however, this goal is presently out of the reach of our technological capabilities. Since it is not yet possible to treat the cause of the disease directly, a logical alternative is to treat its most prominent symptom: muscle wasting. A large percentage of children with MDC1A are faced with respiratory as well as ambulatory difficulties due to weakened muscles throughout their bodies. Weak esophageal muscles also present difficulties with food intake, which may result in failure to thrive (2,42). Therefore, treatment strategies that can restore muscle mass and function could greatly improve the longevity and quality of life of affected patients.
IGF-1 is one of the most potent factors in mediating bone and muscle growth (43), and has been shown to prevent muscle wasting associated with neuromuscular disease (32,44–47). IGF-1 is produced at multiple sites throughout the body: a circulating form of the hormone is produced by the liver in response to growth hormone and works in an endocrine manner, whereas other types of tissues produce IGF-1 that works in either an autocrine or a paracrine fashion. Skeletal muscle produces a particular isoform of IGF-1 known as mIGF-1 (29). Upon binding to its receptor, IGF-1 mediates muscle regeneration by promoting satellite cell proliferation/differentiation as well as hypertrophy of myofibers by altering the rate of protein synthesis and/or breakdown (30,48,49), all of which ultimately result in improved muscle mass and strength. Upregulation of the circulating form of IGF-1 is often associated with cancer (50), and therefore it is preferable to increase levels of local IGF-1 isoforms that may be sufficient to produce desired results without the added risk of carcinogenesis. Targeted expression of mIGF-1 driven by the MLC promoter in skeletal muscle ameliorates the muscle phenotype in dystrophic mdx mice (32), prevents muscle atrophy in aged mice (31) and improves regeneration in response to myoinjury (31). A short-term study in another mouse model of laminin deficiency previously demonstrated that systemic administration of IGF-1 could improve muscle size and function (46); however, they did not test the long-term benefits of IGF-1 on lifespan and growth in the context of laminin deficiency (46). In addition, recombinant human IGF-1 is an FDA-approved drug presently being considered for clinical trial in DMD, and therefore long-term therapeutic potential of IGF-1 needs to be further investigated in the context of MDC1A. Using this knowledge as a starting point, we showed in our study that overexpression of muscle-specific IGF-1 in the laminin-α2-deficient mice, Lama2Dy-w, was sufficient to significantly improve muscle weight, extend longevity and increase their overall growth and wellbeing. These observations add strength to the hypothesis that improving muscle mass by increasing regeneration could be a promising approach in treating MDC1A.
The inability to regenerate successfully in response to injury worsens the dystrophic pathology in laminin-α2-deficient mice and likely plays a significant role in disease progression in MDC1A patients. Kuang et al. (13) suggested that impaired regeneration of muscle in laminin-α2-deficient mice is due to apoptotic loss of newly formed myofibers. Although apoptosis is recognized as an important player in the disease progression, our previous studies demonstrated that inhibition of apoptosis either by genetic or by pharmacological interventions only partially ameliorated the pathology in Lama2Dy-w mice and failed to improve regeneration, as evident by the constant number of centrally nucleated fibers (7,19). We concluded that anti-apoptotic intervention improves muscle phenotype (fiber size and number), likely by facilitating postnatal survival of myofibers formed during early development, rather than by improving regeneration.
In our study here, histological analysis showed that Lama2Dy-w+IGF-1tg mice had larger muscles compared with their non-transgenic littermates. Treated mice had increased muscle cross-sectional area and a fiber distribution that was shifted towards the WT phenotype. More importantly, there was a significant increase in the number of newly regenerated (centrally nucleated) fibers, as well as proliferating satellite cells in Lama2Dy-w+IGF-1tg muscle. At the RNA level, regenerative markers such as the myogenic regulatory factors MyoD, myogenin and MYH3 (51) were expressed at significantly higher levels in IGF-1 overexpressing muscles. On the basis of these results, we conclude that the improved muscle phenotype and the overall health and survival of Lama2Dy-w mice are due to improved regeneration mediated by IGF-1.
Muscle-specific IGF-1 did not reduce fibrosis or inflammation in Lama2Dy-w muscle. Chronic inflammation and extensive accumulation of fibrotic tissue in the interstitial space are pathological signatures of laminin-α2-deficient muscles (52). Compared with DMD, where fibrosis develops later during the disease progression, extensive fibrosis is a feature of very early MDC1A pathology (52,53). Taniguchi et al. (52) showed that expression levels of several ECM components in muscle biopsies from different patients were consistently high and did not correlate with the extent of fibrosis. In addition, they showed that the major sites of induction of these ECM genes were the muscle cells themselves in MDC1A muscles, and not the cells of fibroblast origin that were present in the interstitial space. It is possible that fibrosis plays a much more etiological role in MDC1A pathology compared with that seen in classical dystrophy (52). If the underlying mechanism of the fibrotic process is different in Lama2Dy-w mice compared with mdx mice, it could explain why the IGF-1 transgene reduced fibrosis and inflammation in mdx muscles but did not affect these processes in Lama2Dy-w muscles. Alternatively, much more aggressive muscle degeneration that cannot be compensated by improved regeneration alone may occur in Lama2Dy-w mice compared with mdx mice, creating an environment for fibroblasts to infiltrate and promote fibrosis.
IGF-1 is known to promote muscle growth and cell survival by activating different protein kinase pathways including PI3K and ERK. IGF-1, in contrast to other growth factors, has been demonstrated to promote both proliferation as well as differentiation (54). Upon binding to its receptor, IGF-1 is thought to mediate proliferation by activating the MAPK pathway and to promote differentiation by activation of the PI3K/Akt pathway (55–57). However, more recent studies have shown that the PI3K/Akt pathway also plays a critical role during proliferation in response to IGF-1 (58,59). In Lama2Dy-w+IGF-1tg muscles, we saw an upregulation in both phospho-ERK1/2 (extracellular signal-regulated kinases) and phospho-Thr-308-Akt levels. Further experiments are required to delineate the role that each of these pathways plays specifically in proliferation and/or differentiation in the context of laminin-α2 deficiency. We also saw only a marginal decrease in apoptosis, measured by caspase 3/7 activities, in Lama2Dy-w+IGF-1tg muscles. Although activation of the PI3K/Akt pathway has been implicated to promote cell survival by preventing apoptosis of myotubes (4,60), the increased phosphorylation of Akt in Lama2Dy- w+IGF-1tg muscles did not correlate with the minimal reduction in the measured apoptotic index. In Lama2Dy- w muscles, activation of the PI3K/Akt pathway likely plays a role mainly in enhancing muscle regeneration. Interestingly, the anti-apoptotic effects of IGF-1 were implied but not detected in mdx mice either (32).
Laminin-α1 and agrin are binding ligands that can partially compensate for laminin-α2 deficiency, as demonstrated by transgenic overexpression in mouse models of laminin-α2 deficiency, with a likely impact on stabilizing the signaling pathways involving the dystrophin dystroglycan complex and alpha 7 beta 1 integrin. Although promising, protein replacement therapies are challenging to translate into a therapy. Similarly, pharmacological inhibition of apoptosis leads to significant improvement in disease pathology. Previously, we demonstrated that blocking apoptosis through inactivation of the Bcl2 protein family member Bax (61) results in a several-fold increase in the lifespan of Lama2Dy-w mice (19). This was accompanied by improved postnatal growth and myofiber histology. In our more recent work, we showed that pharmaceutical intervention with doxycycline (known for its antibiotic, anti-inflammatory and anti-apoptotic effects) also significantly increases survival and skeletal muscle function of Lama2Dy-w mice (7). In addition, Erb et al. (12) have shown that Omigapil, a drug that inhibits GAPDH-Siah1-mediated apoptosis, can also improve body weight and skeletal deformation of laminin-deficient mice. Although apoptosis remains an attractive target for MDC1A treatment, the above-mentioned interventions have not resulted in complete recovery. We demonstrate here that muscle-specific IGF-1 can significantly improve the lifespan and overall growth of Lama2Dy-w mice by improving regeneration capacity. Further, this enhancement in regeneration in Lama2Dy-w mice is correlated mainly with improved aspects of myogenesis and less so with inhibition of apoptosis and fibrosis/inflammation. Therefore, our study implies that targeting and improving regenerative capacity may be a useful approach, adding to the therapies currently being contemplated to treat MDC1A, with the important caveat that muscle-specific IGF-1 upregulation does not target fibrosis and underscores the potential need to develop combinatorial therapy to target the multiple disease drivers in MDC1A. Future studies to evaluate combinatorial approaches and the impact of muscle-specific IGF-1 upregulation on the satellite cell population will further our understanding of the pathophysiological mechanisms and their rescue, allowing for prioritization of translational approaches.
MATERIALS AND METHODS
Mice
Heterozygous B6.129 Lama2dy-W/+ mice carrying a targeted mutation in the Lama2 gene were kindly provided to us by Dr Jeffrey B. Miller (Boston Biomedical Institute). These transgenic mice were created at Burnham Institute, La Jolla, CA, USA, in Dr Eva Engvall's laboratory. MLC/mIGF-1 mice overexpressing muscle-specific IGF-1 (31) were kindly provided to us by Dr Elizabeth Barton at the University of Pennsylvania. To generate laminin-deficient mice carrying the IGF-1 transgene, LAMA2+/− heterozygous mice were bred with mice carrying the MLC/mIGF-1 transgene to generate the first generation. A second round of breeding was performed to generate Lama2Dy-w+IGF-1tg mice and their non-transgenic laminin-α2-deficient littermates. PCR assays on tail DNA were performed to genotype the animals. Animals were maintained at the Laboratory Animal Science Center (LASC) and Laboratory Animal Care Facility—Charles River Campus (LACF-CRC) facilities of Boston University at 12 h light and 12 h dark cycles. Animals were provided with food and water ad libitum. All animal procedures were carried out according to the protocol approved by the Institutional Animal Care and Use Committee of Boston University.
Functional capability
Two different tests were performed to evaluate muscle function: the stand up test and the tail suspension test. As a measure of functional capacity, a stand up test was performed in which the mice were placed in a new cage and exploratory behavior was quantified by measuring the number of times the animals stood up on their hind limbs during a period of 5min. This simple but practical test is most useful in evaluating how well a mouse can carryout its routine activity over the course of the study, as opposed to an invasive method that requires sacrificing the animal. A second highlight of this test is that it closely mirrors what could be accomplished in a human patient, since it is not possible to conduct invasive measurements of muscle functionality. For the tail suspension test, each animal was suspended in mid-air by the tail for a period of 10 s, and the time to the first episode of leg retraction was recorded; this procedure was repeated three times and the average time to the first episode of retraction for each mouse was calculated.
Tissue harvesting and cryosectioning
Animals were euthanized with isoflurane (Webster Veterinary, Devens, MA, USA) before quickly dissecting out the TA, gastrocnemius/soleus complex and quadriceps muscles. Muscles were weighed and snap-frozen in liquid nitrogen for RNA or protein extraction. Tissues for histology were embedded in Tissue-Tek OCT Compound (Sakura Finetek USA, Inc., Torrance, CA, USA) and frozen in isopentane (Sigma-Aldrich, St Louis, MO, USA) chilled in liquid nitrogen. Serial transverse sections (7 µm) were prepared using the Leica CM 1850 cryostat (Leica Microsystems, Inc.) and stored at −80°C.
Histology
Frozen sections for hematoxylin and eosin (H&E) staining and Picro-sirius red staining were dried at room temperature and then fixed in chilled acetone for 10 min. Mid-belly serial transverse sections (7 µm) were stained with H&E according to the manufacturer's instructions. Briefly, acetone-fixed sections were hydrated by passing through grades of alcohol and stained with hematoxylin stain 3 (Fisher Scientific, Fair Lawn, NJ, USA) for 1 min, followed by Ruben's Eosin-Phloxine Working Solution (Biocare Medical LLC) for 2 min. The sections were dehydrated by passing through grades of alcohol and mounted using Permount® (Fisher Scientific). Other sections (7 µm) were fixed and rehydrated as described above, stained with Picro-sirius Red (American MasterTech Scientific, Inc., Lodi, CA, USA) for 15 min, rinsed with 0.5% acetic acid, dehydrated with 100% ethanol, cleared with xylene for 10 min and cover-slipped with Permount® mounting medium. Images of sections were captured using a Nikon DSFi1 camera head attached to a light microscope (Nikon ECLIPSE 50i) and analyzed using NIS-Elements Basic Research 3.0 software. Genius MousePen i608 (KYE Systems Corp., Taipei Hsien, Taiwan) was used for morphometric analysis of mid-belly sections. Fiber size, percent of centrally nucleated fibers, percent fibrotic area and fiber number/area were measured in up to 24 fields (200 µm × 200 µm).
Western blot analysis
Muscle tissues (20–25 mg) were homogenized in 20 volumes of passive lysis buffer (Promega Bioscience, Inc., San Luis, CA, USA; Cat. No. E1941) containing protease and phosphatase inhibitor cocktail (Roche). Lysate was cleared by centrifugation, and protein concentration was determined using Bio-Rad Protein assay (Bio-Rad Laboratories, Hercules, CA, USA). By 10% SDS–PAGE, 30–40 µg of protein were resolved and blotted onto a nitrocellulose membrane by semi-dry transfer (Hoefer, Inc., Holliston, MA, USA). Blots were blocked with Odyssey blocking buffer diluted with TBS-Tween (TBST; 50 mm Tris–HCl, 150 mm NaCl, pH 7.6, and 0.1% Tween 20) and incubated overnight at 4°C with the following primary antibodies: anti-phospho-Akt-Thr-308 (1:800), anti-phospho-Akt-Ser-473 (1:800), total Akt (1:1000), phospho-ERK1/2 (1:800), total ERK1/(1:1000) (Cell Signaling Technology, Danvers, MA, USA) and anti-α-tubulin (1:5000) (Sigma-Aldrich). Blots were washed four times, 5 min each with TBST and incubated for 60 min with 1:2000 goat anti-rabbit IR dye 800 (LI-COR Biosciences, Lincoln, NE, USA) and 1:5000 goat anti-mouse Alexa Fluor 680 (Invitrogen, Inc., Carlsbad, CA, USA). Blots were washed as above and scanned on Odyssey Infrared Imaging System (LI-COR Biosciences). Densitometric analyses were performed using Odyssey Image Analysis software.
Gene expression
RNA from muscle tissue was extracted with TRIzol reagent (Invitrogen, Inc.) according to the manufacturer's instructions. RNA was treated with Turbo DNase I (Ambion, Austin, TX, USA) and cleaned using RNeasy Mini Kit (Qiagen, Inc., Valencia, CA, USA). Using the High Capacity cDNA Reverse Transcription Kit, 1 µg of RNA was reverse-transcribed (Applied Biosystem, Foster City, CA, USA). cDNA was amplified using TaqMan assays. Relative gene expression was determined using the ddCt method, with GAPDH as the endogenous control.
Immunostaining
Frozen tissue sections (7 µm) were fixed with 4% paraformaldehyde, blocked for 60 min with blocking buffer (5% normal serum, 0.3% Triton X-100 in PBS) containing mouse-on-mouse (MOM) blocking agent (Vector Laboratories, Inc., Burlingame, CA, USA). Sections were stained with rabbit anti-Ki67 (1:200) (Thermo Fisher Scientific, Pittsburg, PA, USA), mouse anti-dystrophin (1:200) and anti-CD11b (1:50) (BD Biosciences, San Jose, CA, USA) for 60 min, followed by washing with PBS. The sections were subsequently stained with goat anti-rabbit Alexa Fluor 568 (1:200) and goat anti-mouse Alexa Fluor 488 (1:200) (Invitrogen, Inc.) in the dark for 60 min, followed by DAPI staining for 10 min. Sections were washed as above and mounted with Vectashield (Vector Laboratories). Double immunostaining for MyoD1 and dystrophin as well as for Pax7 and Ki67 was done sequentially. Sections were stained with mouse monoclonal anti-MyoD1 clone 5.8 A (1:100) (Dako) and polyclonal rabbit anti-dystrophin (1:200) (Abcam, Cambridge, MA, USA) for 2 h each. Optimal staining for Pax7 (1:3) (Developmental Studies Hybridoma Bank) was achieved by staining overnight at 4°C, followed by staining with Ki67 (1:200) (Thermo Fisher Scientific) for 60 min at room temperature. Blocking, washing and labeling with secondary antibodies were done as described above.
Caspase assay
Apoptosis was assessed by Caspase Glow 3/7 Assay (Promega Bioscience; Cat. No. G8090) and was performed according to the manufacturer's instructions.
TUNEL assay
Frozen muscle sections were immunostained for visualization of the apoptotic nuclei by TUNEL assay, using ApopTag Plus Fluorscein In Situ Apoptosis Detection Kit (Chemicon International-Millipore, Billerica, MA, USA; Cat. No. S7111). These sections were subsequently stained for laminin, using 1:100 dilution PAN laminin antibody (Sigma-Aldrich; Cat. No. L9393), for 1 h at room temperature and detected using goat anti-rabbit Alexa Fluor 568 (Invitrogen) secondary antibody.
Statistics
Statistical analysis was performed using the unpaired two tailed t-test, one-way ANOVA, followed by Newman–Keuls multiple comparison analysis as appropriate, using GraphPad Prism 4 software. Data are presented as mean ± standard deviation.
FUNDING
This work was supported with the grants awarded to Mahasweta Girgenrath from National Institute of Health (R21 AR056628-01A1), Muscular Dystrophy Association (Development grant), Cure CMD and SAM.
ACKNOWLEDGEMENTS
We would like to thank Drs Jennifer Chen, Stefan Girgenrath and Anne Rutkowski for their valuable comments.
Conflict of Interest statement. None declared.
REFERENCES
- 1.Muntoni F., Voit T. The congenital muscular dystrophies in 2004: a century of exciting progress. Neuromuscul. Disord. 2004;14:635–649. doi: 10.1016/j.nmd.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 2.Muntoni F., Bertini E., Bonnemann C., Brockington M., Brown S., Bushby K., Fiszman M., Korner C., Mercuri E., Merlini L., et al. 98th ENMC International Workshop on Congenital Muscular Dystrophy (CMD), 7th Workshop of the International Consortium on CMD, 2nd Workshop of the MYO CLUSTER project GENRE. 26–28th October, 2001, Naarden, The Netherlands. Neuromuscul. Disord. 2002;12:889–896. doi: 10.1016/s0960-8966(02)00068-8. [DOI] [PubMed] [Google Scholar]
- 3.Colognato H., Yurchenco P.D. Form and function: the laminin family of heterotrimers. Dev. Dyn. 2000;218:213–234. doi: 10.1002/(SICI)1097-0177(200006)218:2<213::AID-DVDY1>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 4.Langenbach K.J., Rando T.A. Inhibition of dystroglycan binding to laminin disrupts the PI3K/AKT pathway and survival signaling in muscle cells. Muscle Nerve. 2002;26:644–653. doi: 10.1002/mus.10258. [DOI] [PubMed] [Google Scholar]
- 5.Laprise P., Poirier E.M., Vezina A., Rivard N., Vachon P.H. Merosin-integrin promotion of skeletal myofiber cell survival: differentiation state-distinct involvement of p60Fyn tyrosine kinase and p38alpha stress-activated MAP kinase. J. Cell Physiol. 2002;191:69–81. doi: 10.1002/jcp.10075. [DOI] [PubMed] [Google Scholar]
- 6.Miyagoe Y., Hanaoka K., Nonaka I., Hayasaka M., Nabeshima Y., Arahata K., Takeda S. Laminin alpha2 chain-null mutant mice by targeted disruption of the Lama2 gene: a new model of merosin (laminin 2)-deficient congenital muscular dystrophy. FEBS Lett. 1997;415:33–39. doi: 10.1016/s0014-5793(97)01007-7. [DOI] [PubMed] [Google Scholar]
- 7.Girgenrath M., Beermann M.L., Vishnudas V.K., Homma S., Miller J.B. Pathology is alleviated by doxycycline in a laminin-alpha2-null model of congenital muscular dystrophy. Ann. Neurol. 2009;65:47–56. doi: 10.1002/ana.21523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bentzinger C.F., Barzaghi P., Lin S., Ruegg M.A. Overexpression of mini-agrin in skeletal muscle increases muscle integrity and regenerative capacity in laminin-alpha2-deficient mice. FASEB J. 2005;19:934–942. doi: 10.1096/fj.04-3376com. [DOI] [PubMed] [Google Scholar]
- 9.Meinen S., Barzaghi P., Lin S., Lochmuller H., Ruegg M.A. Linker molecules between laminins and dystroglycan ameliorate laminin-alpha2-deficient muscular dystrophy at all disease stages. J. Cell Biol. 2007;176:979–993. doi: 10.1083/jcb.200611152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moll J., Barzaghi P., Lin S., Bezakova G., Lochmuller H., Engvall E., Muller U., Ruegg M.A. An agrin minigene rescues dystrophic symptoms in a mouse model for congenital muscular dystrophy. Nature. 2001;413:302–307. doi: 10.1038/35095054. [DOI] [PubMed] [Google Scholar]
- 11.Eusebio A., Oliveri F., Barzaghi P., Ruegg M.A. Expression of mouse agrin in normal, denervated and dystrophic muscle. Neuromuscul. Disord. 2003;13:408–415. doi: 10.1016/s0960-8966(03)00036-1. [DOI] [PubMed] [Google Scholar]
- 12.Erb M., Meinen S., Barzaghi P., Sumanovski L.T., Courdier-Fruh I., Ruegg M.A., Meier T. Omigapil ameliorates the pathology of muscle dystrophy caused by laminin-alpha2 deficiency. J. Pharmacol. Exp. Ther. 2009;331:787–795. doi: 10.1124/jpet.109.160754. [DOI] [PubMed] [Google Scholar]
- 13.Kuang W., Xu H., Vilquin J.T., Engvall E. Activation of the lama2 gene in muscle regeneration: abortive regeneration in laminin alpha2-deficiency. Lab. Invest. 1999;79:1601–1613. [PubMed] [Google Scholar]
- 14.Hayashi Y.K., Tezak Z., Momoi T., Nonaka I., Garcia C.A., Hoffman E.P., Arahata K. Massive muscle cell degeneration in the early stage of merosin-deficient congenital muscular dystrophy. Neuromuscul. Disord. 2001;11:350–359. doi: 10.1016/s0960-8966(00)00203-0. [DOI] [PubMed] [Google Scholar]
- 15.Vachon P.H., Loechel F., Xu H., Wewer U.M., Engvall E. Merosin and laminin in myogenesis; specific requirement for merosin in myotube stability and survival. J. Cell Biol. 1996;134:1483–1497. doi: 10.1083/jcb.134.6.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arahata K., Engel A.G. Monoclonal antibody analysis of mononuclear cells in myopathies. I: Quantitation of subsets according to diagnosis and sites of accumulation and demonstration and counts of muscle fibers invaded by T cells. Ann. Neurol. 1984;16:193–208. doi: 10.1002/ana.410160206. [DOI] [PubMed] [Google Scholar]
- 17.Spencer M.J., Tidball J.G. Do immune cells promote the pathology of dystrophin-deficient myopathies? Neuromuscul. Disord. 2001;11:556–564. doi: 10.1016/s0960-8966(01)00198-5. [DOI] [PubMed] [Google Scholar]
- 18.Dominov J.A., Kravetz A.J., Ardelt M., Kostek C.A., Beermann M.L., Miller J.B. Muscle-specific BCL2 expression ameliorates muscle disease in laminin {alpha}2-deficient, but not in dystrophin-deficient, mice. Hum. Mol. Genet. 2005;14:1029–1040. doi: 10.1093/hmg/ddi095. [DOI] [PubMed] [Google Scholar]
- 19.Girgenrath M., Dominov J.A., Kostek C.A., Miller J.B. Inhibition of apoptosis improves outcome in a model of congenital muscular dystrophy. J. Clin. Invest. 2004;114:1635–1639. doi: 10.1172/JCI22928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gawlik K., Miyagoe-Suzuki Y., Ekblom P., Takeda S., Durbeej M. Laminin alpha1 chain reduces muscular dystrophy in laminin alpha2 chain deficient mice. Hum. Mol. Genet. 2004;13:1775–1784. doi: 10.1093/hmg/ddh190. [DOI] [PubMed] [Google Scholar]
- 21.Gawlik K.I., Akerlund M., Carmignac V., Elamaa H., Durbeej M. Distinct roles for laminin globular domains in laminin alpha1 chain mediated rescue of murine laminin alpha2 chain deficiency. PLoS One. 2010;5:e11549. doi: 10.1371/journal.pone.0011549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gawlik K.I., Durbeej M. Transgenic overexpression of laminin alpha1 chain in laminin alpha2 chain-deficient mice rescues the disease throughout the lifespan. Muscle Nerve. 2010;42:30–37. doi: 10.1002/mus.21616. [DOI] [PubMed] [Google Scholar]
- 23.Gawlik K.I., Li J.Y., Petersen A., Durbeej M. Laminin alpha1 chain improves laminin alpha2 chain deficient peripheral neuropathy. Hum. Mol. Genet. 2006;15:2690–2700. doi: 10.1093/hmg/ddl201. [DOI] [PubMed] [Google Scholar]
- 24.Gawlik K.I., Mayer U., Blomberg K., Sonnenberg A., Ekblom P., Durbeej M. Laminin alpha1 chain mediated reduction of laminin alpha2 chain deficient muscular dystrophy involves integrin alpha7beta1 and dystroglycan. FEBS Lett. 2006;580:1759–1765. doi: 10.1016/j.febslet.2006.02.027. [DOI] [PubMed] [Google Scholar]
- 25.Itagaki Y., Saida K., Iwamura K. Regenerative capacity of mdx mouse muscles after repeated applications of myo-necrotic bupivacaine. Acta Neuropathol. 1995;89:380–384. doi: 10.1007/BF00309633. [DOI] [PubMed] [Google Scholar]
- 26.McGeachie J.K., Grounds M.D., Partridge T.A., Morgan J.E. Age-related changes in replication of myogenic cells in mdx mice: quantitative autoradiographic studies. J. Neurol. Sci. 1993;119:169–179. doi: 10.1016/0022-510x(93)90130-q. [DOI] [PubMed] [Google Scholar]
- 27.Girgenrath M., Kostek C.A., Miller J.B. Diseased muscles that lack dystrophin or laminin-alpha2 have altered compositions and proliferation of mononuclear cell populations. BMC Neurol. 2005;5:7. doi: 10.1186/1471-2377-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Engert J.C., Berglund E.B., Rosenthal N. Proliferation precedes differentiation in IGF-I-stimulated myogenesis. J. Cell Biol. 1996;135:431–440. doi: 10.1083/jcb.135.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Florini J.R., Ewton D.Z., Coolican S.A. Growth hormone and the insulin-like growth factor system in myogenesis. Endocr. Rev. 1996;17:481–517. doi: 10.1210/edrv-17-5-481. [DOI] [PubMed] [Google Scholar]
- 30.Adams G.R., McCue S.A. Localized infusion of IGF-I results in skeletal muscle hypertrophy in rats. J. Appl. Physiol. 1998;84:1716–1722. doi: 10.1152/jappl.1998.84.5.1716. [DOI] [PubMed] [Google Scholar]
- 31.Musaro A., McCullagh K., Paul A., Houghton L., Dobrowolny G., Molinaro M., Barton E.R., Sweeney H.L., Rosenthal N. Localized Igf-1 transgene expression sustains hypertrophy and regeneration in senescent skeletal muscle. Nat. Genet. 2001;27:195–200. doi: 10.1038/84839. [DOI] [PubMed] [Google Scholar]
- 32.Barton E.R., Morris L., Musaro A., Rosenthal N., Sweeney H.L. Muscle-specific expression of insulin-like growth factor I counters muscle decline in mdx mice. J. Cell Biol. 2002;157:137–148. doi: 10.1083/jcb.200108071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shavlakadze T., White J., Hoh J.F., Rosenthal N., Grounds M.D. Targeted expression of insulin-like growth factor-I reduces early myofiber necrosis in dystrophic mdx mice. Mol. Ther. 2004;10:829–843. doi: 10.1016/j.ymthe.2004.07.026. [DOI] [PubMed] [Google Scholar]
- 34.Bittner R.E., Anderson L.V., Burkhardt E., Bashir R., Vafiadaki E., Ivanova S., Raffelsberger T., Maerk I., Hoger H., Jung M., et al. Dysferlin deletion in SJL mice (SJL-Dysf) defines a natural model for limb girdle muscular dystrophy 2B. Nat. Genet. 1999;23:141–142. doi: 10.1038/13770. [DOI] [PubMed] [Google Scholar]
- 35.Briguet A., Courdier-Fruh I., Foster M., Meier T., Magyar J.P. Histological parameters for the quantitative assessment of muscular dystrophy in the mdx-mouse. Neuromuscul. Disord. 2004;14:675–682. doi: 10.1016/j.nmd.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 36.Connolly A.M., Keeling R.M., Mehta S., Pestronk A., Sanes J.R. Three mouse models of muscular dystrophy: the natural history of strength and fatigue in dystrophin-, dystrophin/utrophin-, and laminin alpha2-deficient mice. Neuromuscul. Disord. 2001;11:703–712. doi: 10.1016/s0960-8966(01)00232-2. [DOI] [PubMed] [Google Scholar]
- 37.Rommel C., Bodine S.C., Clarke B.A., Rossman R., Nunez L., Stitt T.N., Yancopoulos G.D., Glass D.J. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat. Cell Biol. 2001;3:1009–1013. doi: 10.1038/ncb1101-1009. [DOI] [PubMed] [Google Scholar]
- 38.Grey A., Chen Q., Xu X., Callon K., Cornish J. Parallel phosphatidylinositol-3 kinase and p42/44 mitogen-activated protein kinase signaling pathways subserve the mitogenic and antiapoptotic actions of insulin-like growth factor I in osteoblastic cells. Endocrinology. 2003;144:4886–4893. doi: 10.1210/en.2003-0350. [DOI] [PubMed] [Google Scholar]
- 39.Bodine S.C., Stitt T.N., Gonzalez M., Kline W.O., Stover G.L., Bauerlein R., Zlotchenko E., Scrimgeour A., Lawrence J.C., Glass D.J., et al. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat. Cell Biol. 2001;3:1014–1019. doi: 10.1038/ncb1101-1014. [DOI] [PubMed] [Google Scholar]
- 40.Sandri M., Sandri C., Gilbert A., Skurk C., Calabria E., Picard A., Walsh K., Schiaffino S., Lecker S.H., Goldberg A.L. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 2004;117:399–412. doi: 10.1016/s0092-8674(04)00400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bodine S.C., Latres E., Baumhueter S., Lai V.K., Nunez L., Clarke B.A., Poueymirou W.T., Panaro F.J., Na E., Dharmarajan K., et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001;294:1704–1708. doi: 10.1126/science.1065874. [DOI] [PubMed] [Google Scholar]
- 42.Philpot J., Bagnall A., King C., Dubowitz V., Muntoni F. Feeding problems in merosin deficient congenital muscular dystrophy. Arch. Dis. Child. 1999;80:542–547. doi: 10.1136/adc.80.6.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goldspink G. Loss of muscle strength during aging studied at the gene level. Rejuvenation Res. 2007;10:397–405. doi: 10.1089/rej.2007.0597. [DOI] [PubMed] [Google Scholar]
- 44.Dobrowolny G., Giacinti C., Pelosi L., Nicoletti C., Winn N., Barberi L., Molinaro M., Rosenthal N., Musaro A. Muscle expression of a local Igf-1 isoform protects motor neurons in an ALS mouse model. J. Cell Biol. 2005;168:193–199. doi: 10.1083/jcb.200407021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gehrig S.M., Ryall J.G., Schertzer J.D., Lynch G.S. Insulin-like growth factor-I analogue protects muscles of dystrophic mdx mice from contraction-mediated damage. Exp. Physiol. 2008;93:1190–1198. doi: 10.1113/expphysiol.2008.042838. [DOI] [PubMed] [Google Scholar]
- 46.Lynch G.S., Cuffe S.A., Plant D.R., Gregorevic P. IGF-I treatment improves the functional properties of fast- and slow-twitch skeletal muscles from dystrophic mice. Neuromuscul. Disord. 2001;11:260–268. doi: 10.1016/s0960-8966(00)00192-9. [DOI] [PubMed] [Google Scholar]
- 47.Palazzolo I., Stack C., Kong L., Musaro A., Adachi H., Katsuno M., Sobue G., Taylor J.P., Sumner C.J., Fischbeck K.H., et al. Overexpression of IGF-1 in muscle attenuates disease in a mouse model of spinal and bulbar muscular atrophy. Neuron. 2009;63:316–328. doi: 10.1016/j.neuron.2009.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bark T.H., McNurlan M.A., Lang C.H., Garlick P.J. Increased protein synthesis after acute IGF-I or insulin infusion is localized to muscle in mice. Am. J. Physiol. 1998;275:E118–E123. doi: 10.1152/ajpendo.1998.275.1.E118. [DOI] [PubMed] [Google Scholar]
- 49.Barton-Davis E.R., Shoturma D.I., Sweeney H.L. Contribution of satellite cells to IGF-I induced hypertrophy of skeletal muscle. Acta Physiol. Scand. 1999;167:301–305. doi: 10.1046/j.1365-201x.1999.00618.x. [DOI] [PubMed] [Google Scholar]
- 50.LeRoith D., Roberts C.T., Jr The insulin-like growth factor system and cancer. Cancer Lett. 2003;195:127–137. doi: 10.1016/s0304-3835(03)00159-9. [DOI] [PubMed] [Google Scholar]
- 51.Rantanen J., Hurme T., Lukka R., Heino J., Kalimo H. Satellite cell proliferation and the expression of myogenin and desmin in regenerating skeletal muscle: evidence for two different populations of satellite cells. Lab. Invest. 1995;72:341–347. [PubMed] [Google Scholar]
- 52.Taniguchi M., Kurahashi H., Noguchi S., Sese J., Okinaga T., Tsukahara T., Guicheney P., Ozono K., Nishino I., Morishita S., et al. Expression profiling of muscles from Fukuyama-type congenital muscular dystrophy and laminin-alpha 2 deficient congenital muscular dystrophy; is congenital muscular dystrophy a primary fibrotic disease? Biochem. Biophys. Res. Commun. 2006;342:489–502. doi: 10.1016/j.bbrc.2005.12.224. [DOI] [PubMed] [Google Scholar]
- 53.Noguchi S., Tsukahara T., Fujita M., Kurokawa R., Tachikawa M., Toda T., Tsujimoto A., Arahata K., Nishino I. cDNA microarray analysis of individual Duchenne muscular dystrophy patients. Hum. Mol. Genet. 2003;12:595–600. [PubMed] [Google Scholar]
- 54.Machida S., Booth F.W. Insulin-like growth factor 1 and muscle growth: implication for satellite cell proliferation. Proc. Nutr. Soc. 2004;63:337–340. doi: 10.1079/PNS2004354. [DOI] [PubMed] [Google Scholar]
- 55.Coolican S.A., Samuel D.S., Ewton D.Z., McWade F.J., Florini J.R. The mitogenic and myogenic actions of insulin-like growth factors utilize distinct signaling pathways. J. Biol. Chem. 1997;272:6653–6662. doi: 10.1074/jbc.272.10.6653. [DOI] [PubMed] [Google Scholar]
- 56.Jones N.C., Fedorov Y.V., Rosenthal R.S., Olwin B.B. ERK1/2 is required for myoblast proliferation but is dispensable for muscle gene expression and cell fusion. J. Cell Physiol. 2001;186:104–115. doi: 10.1002/1097-4652(200101)186:1<104::AID-JCP1015>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 57.Tamir Y., Bengal E. Phosphoinositide 3-kinase induces the transcriptional activity of MEF2 proteins during muscle differentiation. J. Biol. Chem. 2000;275:34424–34432. doi: 10.1074/jbc.M005815200. [DOI] [PubMed] [Google Scholar]
- 58.Chakravarthy M.V., Abraha T.W., Schwartz R.J., Fiorotto M.L., Booth F.W. Insulin-like growth factor-I extends in vitro replicative life span of skeletal muscle satellite cells by enhancing G1/S cell cycle progression via the activation of phosphatidylinositol 3'-kinase/Akt signaling pathway. J. Biol. Chem. 2000;275:35942–35952. doi: 10.1074/jbc.M005832200. [DOI] [PubMed] [Google Scholar]
- 59.Machida S., Spangenburg E.E., Booth F.W. Forkhead transcription factor FoxO1 transduces insulin-like growth factor's signal to p27Kip1 in primary skeletal muscle satellite cells. J. Cell Physiol. 2003;196:523–531. doi: 10.1002/jcp.10339. [DOI] [PubMed] [Google Scholar]
- 60.Xiong Y., Zhou Y., Jarrett H.W. Dystrophin glycoprotein complex-associated Gbetagamma subunits activate phosphatidylinositol-3-kinase/Akt signaling in skeletal muscle in a laminin-dependent manner. J. Cell Physiol. 2009;219:402–414. doi: 10.1002/jcp.21684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Borner C. The Bcl-2 protein family: sensors and checkpoints for life-or-death decisions. Mol. Immunol. 2003;39:615–647. doi: 10.1016/s0161-5890(02)00252-3. [DOI] [PubMed] [Google Scholar]