Abstract
Mammalian DNA replication initiates at multiple sites along chromosomes at different times, following a temporal replication program. Homologous alleles typically replicate synchronously; however, mono-allelically expressed genes such as imprinted genes, allelically excluded genes and genes on the female X chromosome replicate asynchronously. We have used a chromosome engineering strategy to identify a human autosomal locus that controls this replication timing program in cis. We show that Cre/loxP-mediated rearrangements at a discrete locus at 6q16.1 result in delayed replication of the entire chromosome. This locus displays asynchronous replication timing that is coordinated with other mono-allelically expressed genes on chromosome 6. Characterization of this locus revealed mono-allelic expression of a large intergenic non-coding RNA, which we have named asynchronous replication and autosomal RNA on chromosome 6, ASAR6. Finally, disruption of this locus results in the activation of the previously silent alleles of linked mono-allelically expressed genes. We previously found that chromosome rearrangements involving eight different autosomes display delayed replication timing, and that cells containing chromosomes with delayed replication timing have a 30–80-fold increase in the rate at which new gross chromosomal rearrangements occurred. Taken together, these observations indicate that human autosomes contain discrete cis-acting loci that control chromosome-wide replication timing, mono-allelic expression and the stability of entire chromosomes.
INTRODUCTION
Morphological differences between chromosomes within the same cell were first observed in mammalian cells over 40 years ago (1,2). These alterations, referred to as ‘incomplete condensation' or ‘pulverization' of chromosomes, could occur on one or a few chromosomes during mitosis. In addition, these abnormally condensed chromosomes synthesized DNA after the normally condensed chromosomes had ceased replication (3,4). However, whether or not the chromosomes displaying these abnormal phenotypes contained structural abnormalities was not determined in these earlier studies. Subsequently, we found that certain tumor-derived chromosome rearrangements exhibit a significant delay in replication timing (DRT), which is characterized by a >2 h delay in both the initiation as well as the completion of DNA synthesis along the entire length of the chromosome (5). Chromosomes with DRT also display a significant delay in mitotic chromosome condensation (DMC) that is characterized by an under-condensed appearance during mitosis and a concomitant delay in the phosphorylation of serine 10 of histone H3 (5,6). Chromosomes with DRT/DMC were detected in 5 of 7 tumor-derived cell lines and 5 of 13 primary tumor samples (5). DRT/DMC was also detected on ∼5% of inter-chromosomal translocations induced by exposure to ionizing radiation (7). Finally, we found that cells containing chromosomes with DRT/DMC have a 30–80-fold increase in the rate at which new gross chromosomal rearrangements occur, indicating that DRT/DMC causes genomic instability (8). Taken together, these observations indicate that DRT/DMC is a common phenotype in cancer cells and in cells exposed to ionizing radiation.
We have developed a chromosome engineering strategy that allows for the systematic analysis of chromosomes with DRT/DMC (7,8). This strategy is based on the Cre/loxP site-specific recombinase system to generate precise chromosomal rearrangements (Supplementary Material, Fig. S1). Using this system, we previously identified four balanced translocations, each displaying DRT/DMC on only one of the derivative chromosomes (8). In this report, we show that Cre/loxP-mediated translocations or deletions at a discrete locus on human chromosome 6 result in DRT/DMC. In addition, we show that the rearrangements that cause DRT/DMC disrupt a mono-allelically expressed, asynchronously replicating, long intergenic non-coding RNA, which we have named asynchronous replication and autosomal RNA on chromosome 6, ASAR6. Finally, we show that the genetic alterations that cause DRT/DMC also result in bi-allelic expression of two linked mono-allelically expressed genes.
RESULTS
‘Alternative partner' analysis
Our models for the generation of DRT/DMC predict that either disruption of discrete chromosomal loci that promote normal replication timing of entire chromosomes or that juxtaposition of incompatible chromosome domains at certain translocation breakpoints results in delayed replication that subsequently results in delayed mitotic condensation (6,8). In either case, the information that is responsible for the phenotype of the engineered translocations with DRT/DMC must be linked in cis to one or both of the loxP cassettes. Therefore, to determine whether one or both loxP integration sites are necessary for generating the phenotype, we created ‘alternative partner’ translocations with an existing pair of loxP-tagged chromosomes, which we had previously shown to generate a Cre-dependent balanced translocation displaying DRT/DMC (8) (Supplementary Material, Figs S2 and S3). Thus, generating different translocations with these existing loxP-tagged chromosomes would allow us to determine whether DRT/DMC segregates with one or both of the integration sites, and would allow us to determine whether DRT/DMC occurs only with certain chromosomal exchanges and not with others. Figure 1A shows a schematic representation of this alternative partner analysis for chromosomes 6 and 10 in the P175 cell line (8) (see Supplementary Material for a more detailed description of this alternative partner analysis). In total, we have characterized four new balanced translocations involving chromosome 6 and three new balanced translocations involving chromosome 10 (Supplementary Material, Figs S4–S12).
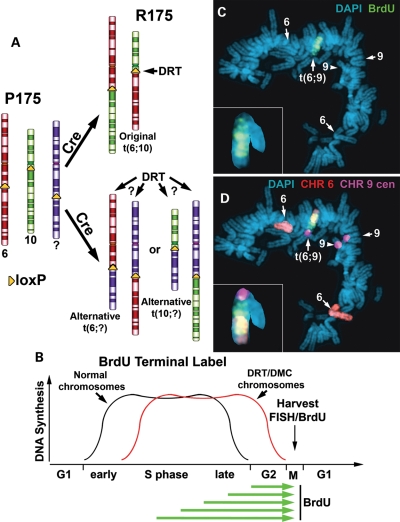
Figure 1.
Alternative partner analysis. (A) Illustration of the loxP integration sites, chromosomes 6 (red) and 10 (green) and balanced translocation, t(6;10), in P175/R175. The random integration of a new loxP cassette is expected to integrate into a third chromosome (purple) and generate a new Cre-dependent translocation. (B) BrdU terminal labeling procedure. BrdU is added to the media for increasing times (green arrows) and cultures are harvested for mitotic spreads and processed for FISH and BrdU incorporation. (C and D) Cells containing a t(6;9) were incubated with BrdU for 4.5 h and processed for BrdU incorporation (green) and for FISH using probes for chromosome 6 (CHR 6 paint, red) and chromosome 9 centromere (CHR 9 cen, pseudo-colored purple) (D). The inset shows an enlarged image of the chromosome 9 derivative. Chromosomes were stained with DAPI.
We next used a BrdU ‘terminal label’ protocol to measure the chromosome replication timing of these new translocations (Fig. 1B). This protocol allows us to visualize the latest replicating regions of chromosomes. Accordingly, the banded pattern of BrdU incorporation allows us to detect actively replicating regions of chromosomes, and differences in replication timing between chromosome pairs are seen as differences in this banding pattern. In addition, measuring the total amount of BrdU incorporation in individual chromosomes allows us to quantify any differences in the normally synchronous replication timing of homologous chromosome pairs. First, to characterize the replication timing of the chromosomes involved in the alternative partner translocations, we carried out an extensive analysis of chromosome replication timing in the parental P175 cells prior to the generation of any Cre-mediated rearrangements (Supplementary Material, Figs S13–S16). Analysis of the banding pattern of BrdU incorporation at multiple time points indicated that the replication timing of each pair of chromosomes, 1, 4, 5, 6, 7, 8, 9, 10 and 17, was consistent with the known replication timing maps for these chromosomes (9,10). In addition, analysis of the banding pattern and quantification of the BrdU incorporation indicated that each pair of chromosomes replicated synchronously (Supplementary Material, Figs S13–S16). Therefore, the alterations in replication timing of the rearranged chromosomes, described below, are due to Cre-mediated events and not to pre-existing replication timing differences in this set of chromosomes.
Figure 1C and D shows an example of BrdU incorporation into the chromosomes of a mitotic cell containing a chromosome 6 alternative partner, t(6;9)(q15;p21) (Supplementary Material, Fig. S7). The only chromosome showing detectable BrdU incorporation in this mitotic spread was the chromosome 9 derivative of the t(6;9), indicating that it displays DRT. Comparing the banded pattern of BrdU incorporation of the t(6;9) with the non-rearranged chromosomes 6 and 9 in P175 cells (Supplementary Material, Figs S13 and S14) indicated that the chromosome 9 derivative was delayed in replication by at least 4h. Note that the chromosome 9 derivative of the t(6;9) displays DRT, but does not display DMC in this mitotic cell. Analysis of additional mitotic spreads indicated that the chromosome 9 derivative does indeed display the DMC phenotype (Supplementary Material, Fig. S7c). A second example of DRT without DMC on this t(6;9) is shown in Supplementary Material, Figure S8. Given these inconsistencies in detecting DMC, we have concentrated on the replication timing of the chromosome rearrangements described below.
DRT was also detected on two other chromosome 6 alternative partner translocations, a t(6;17)(q15;q25) and a t(6;7)(q15;q36) (Supplementary Material, Figs S5 and S6, respectively). However, analysis of a fourth translocation involving chromosome 6, t(6;8)(q15;q24.1), indicated that it did not display DRT (Supplementary Material, Fig. S9). Therefore, three of four alternative partner translocations involving this chromosome 6 loxP cassette integration site display DRT. The reason for the apparent ‘normal’ replication timing of the t(6;8) translocation is currently unknown. Regardless, we found that four of five translocations at this loxP integration site, including the original t(6;10), display DRT. Furthermore, we found that only one derivative chromosome of these balanced translocations displays DRT, and that DRT segregated with the distal portion of chromosome 6. In contrast, none of the new alternative partner translocations involving chromosome 10, a t(1:10)(p22.3;q11.2), a t(4;10)(q25;q11.2) and a t(5;10)(q35.1;q11.2), displays DRT (Supplementary Material, Figs S9, S10 and S11, respectively). These observations suggest that the chromosome 6 integration site is required for generating DRT on this set of inter-chromosomal translocations, and that the chromosome 10 integration site plays only a passive role in generating DRT/DMC on the original t(6;10).
Chromosome 6 deletions display DRT
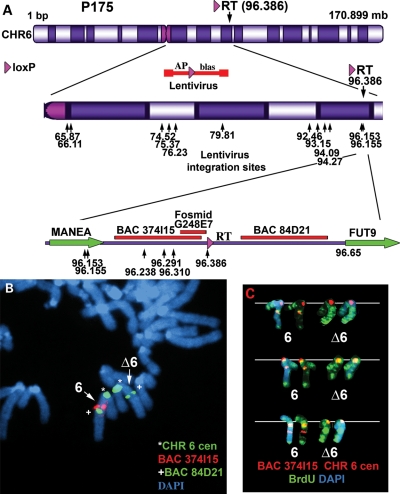
We next determined whether deletions in chromosome 6, nested at the original loxP-3′RT integration site in P175 cells, also display DRT. For this analysis, we used a lentiviral vector to generate pools of clones containing new AP-loxP cassette integrations in P175 cells. The rationale behind this set of experiments was based on previous studies showing that intra-chromosomal Cre events are markedly more efficient than inter-chromosomal Cre events (11). Thus, expression of Cre in pools of lentiviral infected cells would reconstitute the selectable marker (Aprt) at a higher frequency in cells containing lentiviral integrations near the original loxP-3′RT cassette. A schematic diagram of the lentiviral construct is shown in Figure 2A (also see Supplementary Material, Figs S1 and S17a). In addition, because this scheme can generate deletions in only one direction with respect to the original loxP-3′RT integration site (i.e. proximal), we also modified the original loxP-3′RT integration site in P175 cells so that deletions could be generated distal to the original loxP cassette (Supplementary Material, Figs S18 and S19). Using this random lentiviral integration approach, we have generated more than 50 independent deletions in chromosome 6, and these nested deletions extend either proximal or distal from the original loxP-3′RT cassette integration site. To characterize these deletions in molecular detail, we first cloned and sequenced the loxP-3′RT integration site in P175 genomic DNA [96 386 321 base pairs (NCBI Build 36/hg18); see Supplementary Material]. A schematic illustration of this integration site, showing the location of the two closest protein-coding genes MANEA and FUT9, is shown in Figure 2A. We next characterized the deletions using multiple independent assays, including: Southern blot hybridizations (Supplementary Material, Fig. S17b); junction PCR designed to span the genome-loxP-3′RT cassette (Supplementary Material, Fig. S17c and d); LAM-PCR (12) to identify the lentiviral 5′-LTR integration sites (Supplementary Material, Table S1); Affymetrix SNP micro-array and PCR-based assays for loss of heterozygosity and copy number changes (Supplementary Material, Figs S20 and S21); and fluorescence in situ hybridization (FISH) using BACs or Fosmids located within the deleted regions (Figs 2A–C, 3G; Supplementary Material, Figs S22, S23 and S25). A partial set of the lentiviral integration sites for the proximal deletions is shown in Figure 2A (Supplementary Material, Table S1). The largest deletion proximal to the loxP-3′RT integration site is ∼30 Mb and extends to near the centromere, and the smallest proximal deletion is ∼76 kb. The largest deletion distal to the original loxP cassette is ∼26 Mb, and the smallest distal deletion is only ∼18 kb (Supplementary Material, Fig S19 and Table S1).
Figure 2.
Chromosome 6 proximal deletions. (A) Diagram of chromosome 6 showing the orientation and integration site (in megabases) of the original loxP-3′RT cassette, structure of the AP-loxP lentivirus and the locations of the 5′LTR-genome junctions for 15 lentiviral integration sites (arrows), BACs RP11-374I15 (374I15) and CTD-84D21 (84D21), Fosmid G248P86031E7 (G248E7) and two protein-coding genes, MANEA and FUT9. (B) Deletion of BAC 374I15 from one copy of chromosome 6 (∼2.1 Mb deletion). Mitotic cells were processed for FISH using probes for the chromosome 6 centromere (green), BAC 374I15 (red) and a second BAC 84D21 (green). (C) Replication timing assay on an ∼16.58 Mb deletion. Cells were incubated with BrdU for 4.5 h, harvested and processed for BrdU incorporation (green) and FISH, using probes for the chromosome 6 centromere (red) and BAC 374I15 (red). Chromosomes from three independent mitotic spreads are shown. Chromosomes were stained with DAPI (blue).
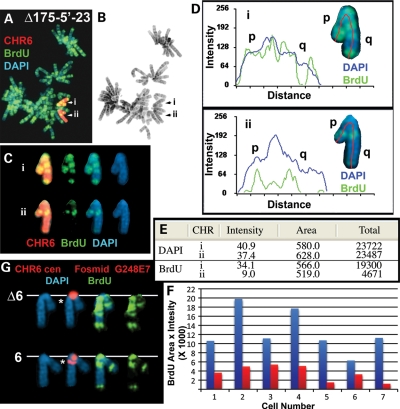
Figure 3.
Delayed replication timing on an ∼76 kb deletion. Cells were incubated with BrdU for 4.5 h, harvested and processed for FISH, using either a chromosome 6 paint (A–E; red) or a chromosome 6 centromeric probe (red) plus Fosmid G248E7 (red), and DAPI (blue) (G). (B) Reverse DAPI banding of the chromosomes shown in (A). (C) The two chromosome 6s from (A) were cut out and aligned. (D) Pixel-intensity profiles of the BrdU incorporation (green), and DAPI (blue) staining along the two chromosome 6s from (A). (E) The pixel-intensity (average intensity × area) for each chromosome showing the total amount of BrdU incorporation or DAPI staining. (F) Quantification of the BrdU incorporation in seven different pairs of chromosome 6s. The red and blue bars represent the two chromosomes identified by the chromosome 6 paint.
We next assayed the replication timing of the chromosome 6s in a subset of these deletion clones. Figure 2C shows examples of the BrdU incorporation pattern in cells containing an ∼16.6 Mb proximal deletion. Cultures were incubated with BrdU for 4.5 h, and mitotic cells were harvested, processed for BrdU incorporation and subjected to FISH, using a chromosome 6 centromeric probe plus a BAC from the deleted region (Fig. 2A). The FISH signal from the chromosome 6 centromeric probe allowed us to identify both chromosome 6s, and the presence or absence of the BAC allowed us to distinguish between the non-deleted or deleted chromosomes, respectively. Comparing the BrdU incorporation patterns between chromosome 6s in multiple cells indicated that the deleted 6s were delayed in replication timing by >2 h. Similarly, DRT was detected on all of the proximal deletions assayed, including deletions of: ∼21.8 Mb (Supplementary Material, Fig. S22), ∼2.1 Mb (not shown), ∼233 kb (not shown), ∼231 kb (Supplementary Material, Fig. S23), ∼94 kb (Supplementary Material, Fig. S24) and ∼76 kb (Fig. 3). Importantly, delayed replication was detected on both arms of the deleted chromosomes (Fig. 3A–D; Supplementary Material, Figs S22–S24), indicating that the effects of these genetic alterations cross the centromere. In contrast, the deletions distal to the original loxP-3′RT integration site, including deletions of ∼25.9 Mb (Supplementary Material, Fig. S25a–c), ∼1.4 Mb (Supplementary Material, Fig. S25d) and ∼18 kb (Supplementary Material, Fig. S25e), did not display DRT. Although the extent of DRT in any given cell can be quite variable, we have not detected any significant differences between the different deletion mutations in chromosome 6. Taken together, these observations indicate that DRT occurs with deletions as small as ∼76 kb proximal to the original loxP-3′RT integration site, and does not occur with deletions distal to this integration site, even with deletions as large as ∼26 Mb.
Mono-allelic expression and DRT
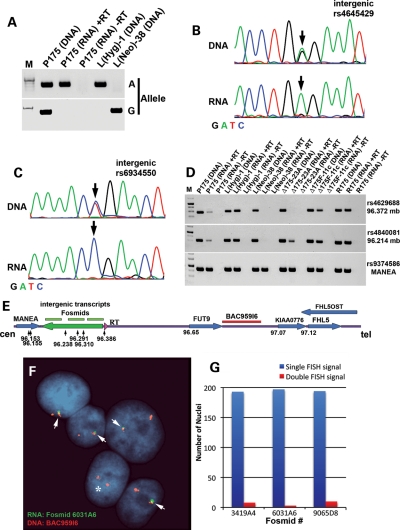
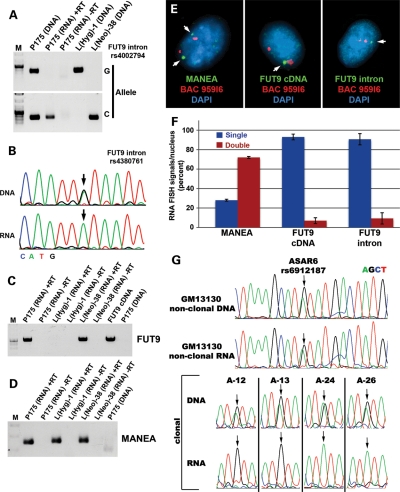
Previous reports have indicated that Cre/loxP-mediated deletion of the Xist gene from adult somatic cells causes a delay in the replication timing of the entire X chromosome (13,14). This delayed replication phenotype appears to be similar to the DRT phenotype observed on the autosomes described here and previously (5–8). Therefore, given the similarities in replication timing defects associated with Xist gene deletions and the deletions in chromosome 6, we next determined whether the region of chromosome 6 responsible for DRT is transcribed. Our first indication of transcription from the intergenic region between MANEA and FUT9 came from a report that identified RNAs expressed in human embryonic stem cells that were associated with the RNA-binding protein FOX2 (15). To confirm expression of this region in our cell culture system, we used RT-PCR to assay numerous locations within the intergenic region between MANEA and FUT9. First, we used primers designed to amplify unique sequences from RNA extracted from HTD114 (the parent cells of P175), P175 and two chromosome 6 mono-chromosomal hybrids (mouse L cells, each containing one of the two chromosome 6s from HTD114). We detected RNA expression from HTD114, P175 and one of the mono-chromosomal hybrids (Supplementary Material, Table S2; Fig. 4D). Consistent with this observation, allele-specific RT-PCR of a heterozygous single-nucleotide polymorphism (SNP) indicated that the RNA transcripts expressed in P175 cells were mono-allelically expressed (Fig. 4A). In addition, Figure 4B and C shows sequencing traces from two different SNPs that are heterozygous in genomic DNA, but homozygous in cDNA from P175 cells, again indicating that this intergenic region is mono-allelically expressed. In total, we have detected mono-allelic expression of 4 heterozygous SNPs and 29 additional non-polymorphic locations between MANEA and the loxP-3′RT integration site in P175 cells (Supplementary Material, Table S2). Additional PCR and sequencing reactions from HTD114 and the chromosome 6 mono-chromosomal hybrids indicated that the chromosome that contains the loxP-3′RT integration site in P175 contains the expressed allele for this entire region, spanning positions 96 171 771 to 96 373 551 of chromosome 6, representing >200 kb of genomic DNA (Fig. 4E; Supplementary Material, Table S2). In addition, cells containing the smallest deletion that causes DRT, ∼76 kb, lack expression from the deleted region as expected, but retain expression of the mono-allelic transcripts proximal to the deletion (Fig. 4D), indicating the existence of transcriptional start sites within the non-deleted region. Expression of this intergenic region was also detected in R175 cells (Fig. 4D; Supplementary Material, Table S2), indicating that the translocation event that generated the t(6;10) does not interfere with expression of this region. As expected, the chromosome 6 deletions that do not result in DRT, i.e. distal to the loxP-3′RT integration site, also do not interfere with expression of these mono-allelic transcripts (Fig. 4D). Furthermore, RNA–DNA FISH analysis showed mono-allelic expression of this intergenic region (Fig. 4E–G; Supplementary Material, Fig. S27). Because this intergenic region also displays asynchronous replication (see what follows), we have named these non-coding transcripts asynchronous replication and autosomal RNA on chromosome 6, ASAR6.
Figure 4.
Mono-allelic expression at chromosome 6q16.1. (A) Allele-specific RT-PCR from the intergenic region between MANEA and the loxP-3′RT integration site. PCRs, using forward primers containing single-base differences (allele G or A; see Supplementary Material, Table S3) at their 3′ ends, plus a common reverse primer, for SNP rs4645429. PCRs were carried out on genomic DNAs isolated from P175, two mono-chromosomal hybrids containing the two different chromosome 6s from HTD114 [L(Hyg)-1 contains the loxP-3′RT integrated chromosome 6, and L(Neo)-38 contains the other chromosome 6], and either with (+RT) or without (−RT) reverse transcriptase on P175 RNA. (B and C) DNA-sequencing traces from PCRs designed to detect SNPs rs4645429 and rs6934550. The top panels show the traces from P175 DNA, and the bottom panels show the traces from P175 cDNA (RNA). The arrows mark the location of the SNPs. (D) Mono-allelic expression of the intergenic region in mono-chromosomal hybrids and in the chromosome 6 deletions. RT-PCRs were carried out on RNA isolated from P175, L(Hyg)-1, L(Neo)-38, Δ175-23a (∼76 kb proximal deletion), Δ175F-11c (∼25.9 Mb distal deletion) and R175 [t(6;10)]. Genomic DNAs from each cell line served as positive controls. PCRs were carried out using primers designed to detect SNP rs4629688 (located within the smallest deletion), rs4840081 (located proximal to the smallest deletion) and rs9374586 (located within MANEA). Sequencing of the PCR products confirmed that all primers amplified the correct chromosome position (not shown). (E) Schematic diagram of the region of chromosome 6 between MANEA and FHL5 showing the location of the smallest proximal deletions (arrows), the three Fosmids used as probes in the RNA FISH (green) and BAC959I6 used in the DNA FISH (red) in (F) and (G). (F) RNA–DNA FISH using Fosmid G248P86031A6 (6031A6). (G) The number of single (blue) and double (red) sites of RNA expression. Two hundred cells were scored for RNA FISH signals that were associated with the DNA FISH signal for three Fosmid probes [see (E) and Supplementary Material, Fig. S27].
Consistent with our observations of mono-allelic expression at this locus, a previous study, using the density of LINE-1 elements to identify mono-allelically expressed genes, predicted that the FUT9 and FHL5 genes (located ∼180 and ∼730 kb distal to the loxP integration site, respectively; Fig. 4E) would be mono-allelically expressed (16). Therefore, we next determined whether FUT9 and FHL5 were also mono-allelically expressed in our cell system. For this analysis, we used allele-specific RT-PCR of heterozygous SNPs (Fig. 5A), RT-PCR followed by sequencing of heterozygous SNPs located within introns (Fig. 5B; Supplementary Material, Table S2), RT-PCR using primers spanning exons in mono-chromosomal hybrids (Fig. 5C) and RNA–DNA FISH for expression of primary transcripts (Fig. 5E and F; Supplementary Material, Figs S28 and S30). This analysis indicated that the FUT9 gene is indeed mono-allelically expressed. In addition, we detected mono-allelic expression from within and distal to the FHL5 gene (Supplementary Material, Table S2 and Fig. S26a). However, exon-specific primers, which spanned large introns, failed to generate RT-PCR products for FHL5 (Supplementary Material, Fig. S26b), indicating that properly spliced FHL5 protein-coding transcripts were not expressed. In addition, the transcripts detected from the FHL5 region were generated from the antisense orientation (Supplementary Material, Fig. S26c). In addition, both FUT9 and these FHL5 opposite strand transcripts (FHL5OST) were not expressed from the chromosome 6 that contains the loxP-3′RT cassette (Supplementary Material, Table S2). Therefore, the chromosome 6 rearrangements that cause DRT/DMC were generated on the chromosome 6 that expresses ASAR6, but is silent for both FUT9 and FHL5OST. In addition, analysis of the parental HTD114 cells indicated that FUT9 is also mono-allelic, and that HTD114 and P175 cells express the same allele, indicating that integration of the original loxP-3′RT cassette was not responsible for mono-allelic expression of this region in P175 cells (Supplementary Material, Table S2). In contrast, the MANEA and KIAA0776 genes are bi-allelically expressed in HTD114 and P175 cells (Supplementary Material, Table S2 and Fig. S32). A similar analysis of other known or predicted mono-allelically expressed genes present on chromosome 6 indicated either no or bi-allelic expression in P175 cells (Supplementary Material, Table S5).
Figure 5.
Mono-allelic expression of FUT9 and random mono-allelic expression of ASAR6. (A) Allele-specific RT-PCR of an SNP located in the first intron of FUT9. PCR using forward primers containing single-base differences (Allele G or C; see Supplementary Material, Table S3) at their 3′ ends, plus a common reverse primer, for SNP rs4002794. PCRs were carried out on genomic DNAs from P175, the two mono-chromosomal hybrids [L(Hyg)-1 and L(Neo)-38], either with (+RT) or without (−RT) reverse transcriptase on P175 RNA. (B) DNA-sequencing traces from PCRs designed to detect SNP rs4380761, located in the second intron of FUT9. The top panel shows the trace from P175 DNA, and the bottom panel shows the trace from P175 cDNA (RNA). The arrows mark the location of the SNP. (C) Exon-specific RT-PCR for expression of FUT9. PCRs were carried out, with primers designed to detect exons 2 and 3 from the spliced FUT9 cDNA, either with (+RT) or without (−RT) reverse transcriptase, on RNA from P175 and the mono-chromosomal hybrids L(Hyg)-1 and L(Neo)-38. PCR products derived from a cloned FUT9 cDNA indicated correctly spliced products from P175 and L(Neo)-38. PCR products were not detected from P175 DNA, because the intron spanned by these two primers is >90 kb. (D) Exon-specific RT-PCR for expression of MANEA. PCRs, using primers designed to amplify exons 2 and 3 from the MANEA cDNA, either with (+RT) or without (−RT) reverse transcriptase, on RNA from P175 cells and the mono-chromosomal hybrids L(Hyg)-1 and L(Neo)-38. Sequencing reactions confirmed the exon junctions for properly spliced MANEA cDNA (not shown). (E) RNA–DNA FISH for expression of MANEA and FUT9. P175 cells were subjected to RNA FISH (green), using a cDNA probe for MANEA (left panel), a cDNA probe for FUT9 (middle panel) and an intronic probe for FUT9 (right panel). Slides were subsequently fixed and processed for DNA FISH (red) using BAC 959I6, located distal to FUT9 (Fig. 4E). Arrows mark the sites of expression, and the nuclear DNA was detected with DAPI. RNA FISH signals associated with DNA FISH signals were scored. (F) Quantification of the single (blue) and double (red) sites of RNA FISH signals for MANEA and FUT9. Error bars indicate the error of the standard mean. (G) Random mono-allelic expression of ASAR6. Non-clonal and clonal lymphoblastoid (GM1310) cell lines were assayed for expression of ASAR6. DNA sequence traces from PCRs designed to detect SNP rs6912187. The top panels show the traces from non-clonal DNA and cDNA (RNA). The bottom panels show the traces from DNA and cDNA (RNA) isolated from four independent clones (A12, A13, A24 and A26) isolated from GM13130. The arrows mark the location of the SNP.
We next determined whether the mono-allelic expression from this region of chromosome 6 was imprinted or random. For this analysis, we examined non-clonal and clonal cell lines derived from lymphoblast cultures for expression of SNPs located within ASAR6. Figure 5G–I shows that both alleles of ASAR6 were detected in the RNA of the non-clonal line, and that either one or the other allele was detected in the clonal lines. This analysis indicated that ASAR6 is subject to random mono-allelic expression in these lymphoblastoid cells.
Asynchronous replication on chromosome 6
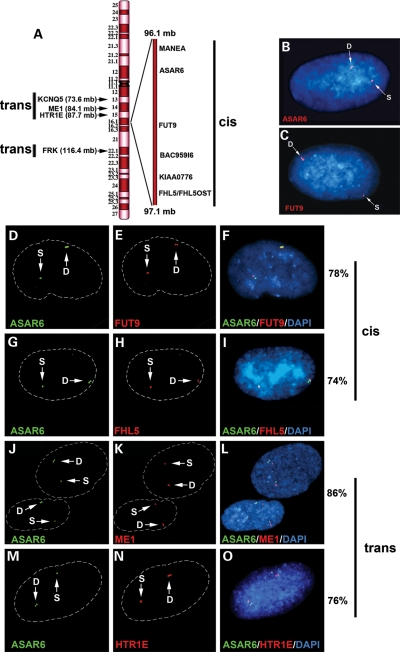
Because mono-allelically expressed genes display asynchronous replication (reviewed in 17), we next tested whether the region of chromosome 6 between MANEA and FHL5 also displays asynchronous replication. Loci can be identified as replicating either synchronously or asynchronously using a FISH-based assay (18). FISH analysis of interphase nuclei pulse-labeled with BrdU allows selective examination of cells in S-phase. This assay also utilizes a methanol/acetic acid fixation, which destroys the nuclear structure and allows for a relatively accurate analysis of replication timing (19,20). Using a probe to a particular chromosomal site, some cells display two single-hybridization dots, indicating that neither allele has replicated (an SS pattern); other cells display two double dots, indicating that both alleles have replicated (a DD pattern); and a third class of cells contains one single dot and one double dot, indicating that only one of the two alleles (an SD pattern) has replicated (Supplementary Material, Fig. S36). Asynchronously replicating loci show the SD pattern in 30–50% of S-phase cells, whereas synchronously replicating genes typically present the SD pattern in only 10–20% of S-phase cells (20–22). We used primary human skin fibroblasts and this FISH assay to quantify the number of hybridization signals per locus present in S-phase nuclei. We found that five different probes from this region of chromosome 6, spanning ∼1 Mb of genomic DNA including MANEA, ASAR6, FUT9, BAC959I6 and FHL5, as well as four additional chromosome 6 mono-allelically expressed genes (HTR1E, KCNQ5, ME1 and FRK) located ∼10–20 Mb from ASAR6, all show asynchronous replication (Fig. 6A–C; Table 1).
Figure 6.
Asynchronous replication of random mono-allelically expressed genes on chromosome 6. (A) Schematic diagram of chromosome 6 showing the location of the genes and loci assayed for asynchronous replication. The ∼1 Mb region of chromosome 6 between MANEA and FHL5 is expanded on the right. The coordination in asynchronous replication of chromosome 6 mono-allelically expressed genes with ASAR6 was found to be either in cis or in trans as indicated. Examples of the SD pattern in single nuclei for ASAR6 (B) and FUT9 (C). The BrdU staining is not shown. (D–O) Coordination in the asynchronous replication timing along chromosome 6. Low-passage primary human skin fibroblasts were analyzed using a two-color FISH assay combining BAC probes for ASAR6 (green) with BAC or Fosmid probes for FUT9, FHL5, ME1 and HTR1E (red; see Supplementary Material, Table S4 for probes). DNA was stained with DAPI. Arrows mark the location of the FISH signals. Dashed white lines show the outline of the nuclei. Single dots (S) represent un-replicated loci and double dots (D) represent replicated loci. The percentage of cells that simultaneously displayed the SD pattern for both probes was determined in 100 cells for the cis versus trans configuration.
Table 1.
FISH analysis of human loci
| Locus | Position | %SD |
|---|---|---|
| MANEA | 6q16.1 | 36 |
| ASAR6 | 6q16.1 | 43 |
| FUT9 | 6q16.1 | 37 |
| BAC959I6 | 6q16.1 | 44 |
| FHL5 | 6q16.1 | 34 |
| HTR1E | 6q15 | 33 |
| KCNQ5 | 6q13 | 33 |
| ME1 | 6q14.1 | 39 |
| FRK | 6q22.1 | 34 |
| PTK6 | 20q13.3 | 19 |
| LARP | 5q33.2 | 24 |
| C9orf43 | 9q32 | 19 |
The percentage of the single–double (%SD) pattern was determined using FISH.
Previous studies have shown that the random mono-allelically expressed genes present on a given autosome are coordinated in their asynchronous replication so that the alleles on one homolog replicate earlier than the alleles on the other (21,22). Therefore, we next tested whether the asynchronously replicating loci described above also display coordination in their asynchronous replication. The level of coordination was examined using a two-color FISH assay and scoring cells that simultaneously displayed the SD signal for both loci (21,22). For this analysis, we used a BAC probe representing ASAR6 in combination with probes representing MANEA, FUT9, BAC959I6 and FHL5 (Fig. 6A and Table 1). We found that the asynchronous replication of ASAR6 was coordinated in cis with FUT9 (78/100 cells, P < 1 × 10−5; Fig. 6D–F), FHL5 (74/100 cells, P < 1 × 10−5; Fig. 6G–I), BAC959I6 (70/100 cells, P < 1 × 10−4; Supplementary Material, Fig. S37a–c) and MANEA (78/100 cells, P < 1 × 10−5; Supplementary Material, Fig. S37d–f).
Because asynchronous replication on human autosomes is coordinated at the whole chromosome level (21), we also assayed other mono-allelically expressed genes located either centromeric or telomeric to ASAR6 (Fig. 6A). Interestingly, we found that the asynchronous replication of ASAR6 was also coordinated with these other chromosome 6 asynchronous genes; however, the coordination was in trans. Thus, the earlier replicating allele of ASAR6 was linked to the later replicating alleles of ME1 (86/100 cells, P < 1 × 10−5; Fig. 6J–L), HTR1E (76/100 cells, P < 1 × 10−5; Fig. 6M–O), KCNQ5 (74/100 cells, P < 1 × 10−5; Supplementary Material, Fig. S37g–i) and FRK (74/100 cells, P < 1 × 10−5; not shown).
Disruption of mono-allelic expression
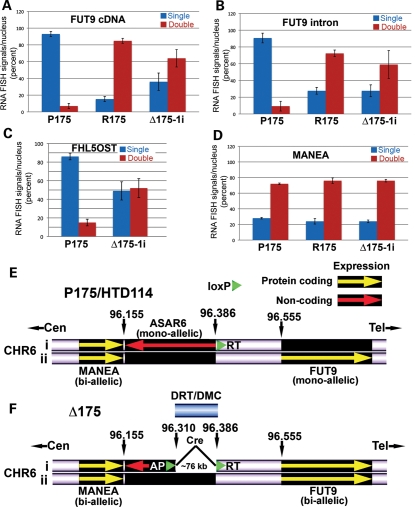
We next tested whether the genetic alterations in chromosome 6 that cause DRT also affect mono-allelic expression of FUT9 and FHL5OST. For this analysis, we used RNA–DNA FISH to assay their expression in R175 and Δ175-1i [containing the original t(6;10) or an ∼230 kb proximal deletion, respectively]. Figure 7A–D shows that these genetic alterations result in bi-allelic expression of both FUT9 and FHL5OST (also see Supplementary Material, Figs S28–S35). Taken together, our data indicate that mono-allelic transcription of ASAR6 is occurring only on the chromosome 6 that is ‘inactive’ for FUT9 and FHL5OST, and that the Cre-mediated rearrangements that result in DRT also result in expression of the previously silent FUT9 and FHL5OST alleles in cis. A schematic illustration of the location of the ASAR6 transcripts, the location of the smallest deletion that causes DRT and the activation of the previously silent FUT9 allele are shown in Figure 7E and F.
Figure 7.
Loss of mono-allelic expression at 6q16.1. (A–D) Bi-allelic expression of previously mono-allelically expressed genes in R175 and Δ175-1i. Slides were processed for RNA–DNA FISH and examined for sites of RNA hybridization using FUT9 cDNA (A), FUT9 intron (B), FHL5OST Fosmid (G248P86054G4) (C) or MANEA cDNA (D) probes. The DNA probe was BAC 959I6 (Fig. 4E). The percentage of cells (P175, R175 and Δ175-1i) from three independent hybridizations that displayed single (blue bars) or double (red bars) sites of RNA hybridization are indicated (also see Supplementary Material, Figs S28–S35). Error bars indicate the error of the standard mean. (E) Schematic diagram of the region of the long arm of chromosome 6 near the loxP-3′RT integration site in P175. Both chromosome 6s are indicated (i and ii), showing expression of protein-coding genes (yellow arrows) and non-coding regions (red arrow). (F) Schematic diagram of the same region of chromosome 6 shown in (E). The smallest Cre/loxP-mediated deletion that we have generated which induces DRT is ∼76 kb.
DISCUSSION
The observations described here are consistent with previous reports showing that, like X chromosomes, autosome pairs display coordination in their asynchronous replication timing of mono-allelically expressed genes (21–23). Interestingly, deletion of the Xist gene from adult somatic cells results in a delayed replication phenotype (13,14) that appears to be similar to the DRT phenotype described for certain autosomal rearrangements (5,7,8). In this report, we show that inter-chromosomal translocations or deletions at a specific location on human chromosome 6 result in delayed replication of the entire chromosome. These data define a cis-acting locus responsible for chromosome-wide replication timing of a human autosome. Furthermore, because the vast majority of chromosome 6 genes are not subject to mono-allelic expression and asynchronous replication, our data indicate that disruption of the ASAR6 locus delays replication timing of both asynchronously and synchronously replicating genes along the entire chromosome.
Although parallels between X inactivation and the coordinated replication asynchrony of autosomal mono-allelically expressed genes have been made (17,21–25), a previous report showed that not all random mono-allelically expressed alleles are expressed from the same homolog (26). These observations suggest that although autosomal asynchronous replication timing is coordinated in cis (21–23), mono-allelic expression is not (26,27). However, it should be pointed out that the asynchronous replication timing of these ‘non-coordinated’ mono-allelically expressed genes was not assayed. In this report, we show that ASAR6 shows asynchronous replication that is coordinated in cis with the mono-allelically expressed genes FUT9 and FHL5OST, even though ASAR6 is expressed from the opposite homolog. In addition, we found that ASAR6 shows coordination in asynchronous replication with other mono-allelically expressed genes located at a distance along chromosome 6, but this coordination was in trans. Moreover, the apparent mono-allelic expression observed with certain transcripts can arise via mechanisms that may not be associated with asynchronous replication timing (reviewed in 28). Therefore, a detailed analysis of the coordinated replication timing in combination with allele-specific expression assays is required to determine whether the ‘non-coordinated’ expression pattern is a characteristic of certain genes or is a characteristic of all random mono-allelically expressed genes located on autosomes.
The deletions that cause DRT disrupt the ASAR6 RNA and result in bi-allelic expression of the previously silent alleles of the mono-allelically expressed genes FUT9 and FHL5OST. However, in contrast to XIST RNA, ASAR6 RNA does not appear to coat the entire chromosome. Interestingly, ASAR6 transcripts can be detected in human embryonic stem cells (E.P.S. and M.J.T., unpublished data; also see reference 15), but are not expressed in all adult tissues (Supplementary Material, Fig. S38). In contrast, Xist remains expressed on the inactive X in most if not all adult tissues (reviewed in 29). However, Xist is apparently not absolutely required for the maintenance of X chromosome silencing, as Xist deletion after X-inactivation does not automatically result in global X-reactivation (30,31). Therefore, it will be interesting to determine whether disruption of the ASAR6 locus in cells that do not express ASAR6 RNA also results in delayed replication. We believe that this is a distinct possibility, as disruption of the silent Xist gene causes a delay in the replication timing of the active X chromosome (13).
Previously, we used ionizing radiation to generate chromosome rearrangements in mouse and human cells and found that ∼5% of inter-chromosomal translocations involving autosomes display DRT/DMC (7). Furthermore, our original ‘chromosome-engineering’ screen for DRT/DMC identified five balanced translocations, involving eight different autosomes (8). In more recent studies, we have generated a set of nested deletions in chromosome 15, anchored at the original loxP integration site in the P268 cell line [see Supplementary Material, Fig. S2 and reference (8)], and found that the deleted 15s also display DRT (N.D., L.S. and M.J.T., unpublished data). On the basis of these observations, we propose that all mammalian autosomes contain discrete cis-acting loci that function to regulate chromosome-wide replication timing.
MATERIALS AND METHODS
Cell culture
HTD114 cells are a human APRT-deficient cell line derived from the HT1080 fibrosarcoma (32). This cell line and all of its subclones, including P175, the alternative partner clones (R-lines) and the deletion clones (Δ-lines), were grown in DMEM (Gibco) supplemented with 10% fetal bovine serum (Hyclone). HTD114 derivatives were grown as above with the addition of 500 mg/ml Geneticin (Gibco), 200 mg/ml Hygromycin B (Calbiochem) and/or 10 µg/ml Blasticidin S HCl (Invitrogen). Lentiviral infections were carried out using a multiplicity of infection of <0.1, according to standard procedures (Invitrogen). The HTD114 R-line and Δ-line derivatives were grown in DMEM supplemented with 10% dialyzed fetal bovine serum (Hyclone), 10 mg/ml azaserine (Sigma) and 10 mg/ml adenine (Sigma) to facilitate selection for Aprt-expressing cells. APRT counter-selection was carried out in 10 µg/ml diaminopurine (Sigma). Low-passage primary human skin fibroblasts were obtained from ATCC and cultured in DMEM plus 10% fetal bovine serum (Hyclone). The lymphoblast cell line GM13130 was obtained from the Coriell Institute and cultured in RPMI (GIBCO) media supplemented with 15% fetal bovine serum (Hyclone). Clones of GM13130 were obtained by limiting dilution in 96-well dishes, expanded and harvested for DNA and RNA. Cells were grown in a humidified incubator at 37°C in a 5% carbon dioxide atmosphere.
DNA FISH
Trypsinized cells were centrifuged at 1000 r.p.m. for 10 min in a swinging bucket rotor. The cell pellet was re-suspended in 75 mm potassium chloride for 15–30 min at 37°C, re-centrifuged at 1000 r.p.m. for 10 min and fixed in 3:1 methanol:acetic acid. Fixed cells were added drop-wise to microscope slides to generate mitotic chromosome spreads using standard methods (33). Slides with mitotic spreads were baked at 85°C for 20 min and then treated with 0.1 mg/ml RNAase for 1 h at 37°C. After RNAase treatment, the slides were washed in 2× SSC (1× SSC is 150 mm NaCl and 15 mm sodium citrate) with three changes for 3 min each and dehydrated in 70, 90, and 100% ethanol for 3 min each. The slides were denatured in 70% formamide in 2× SSC at 70°C for 3 min, and whole chromosome paints were used according to the manufacturer's recommendations and hybridization solutions (American Laboratory Technologies and Vysis). Detection of digoxigenin-dUTP probes utilized a three-step incubation of slides with sheep FITC-conjugated anti-digoxigenin antibodies (Roche), followed by rabbit FITC-conjugated anti-sheep antibodies (Roche), followed by goat FITC-conjugated anti-rabbit antibodies (Jackson Laboratories). Slides were stained with DAPI (12.5 mg/ml) or propidium iodide (0.3 mg/ml), cover slipped and viewed under UV fluorescence with appropriate filters (Olympus).
Centromeric, BAC and Fosmid probes
Mitotic chromosome spreads were prepared as described above. Slides were treated with RNase at 100 µg/ml for 1 h at 37°C and washed in 2× SSC and dehydrated in 70, 90 and 100% ethanol. Chromosomal DNA was denatured at 75°C for 3 min in 70% formamaide/2× SSC, followed by dehydration in ice-cold 70, 90 and 100% ethanol. BAC and Fosmid DNAs were nick-translated using standard protocols to incorporate biotin-11-dUTP or digoxigenin-dUTP (Invitrogen). BAC and Fosmid DNAs were directly labeled with Cy3-dUTP, FITC-dUTP, Spectrum Orange-dUTP or Spectrum Green-dUTP (Vysis, Abbott Laboratories) using nick-translation or random priming using standard protocols. Final probe concentrations varied from 40–60 ng/µl. Centromeric probe cocktails (Vysis) plus BAC or Fosmid DNAs were denatured at 75°C for 10 min and prehybridized at 37°C for 30 min. Probes were applied to slides and incubated overnight at 37°C. Post-hybridization washes consisted of three 3 min rinses in 50% formamide/2× SSC, three 3 min rinses in 2× SSC and finally three 3 min rinses in PN buffer (0.1 m Na2HPO4+0.0 m NaH2PO4, pH 8.0, +2.5% Nonidet NP-40), all at 45°C. Signal detection was carried out as described (34). Amplification of biotinylated probe signal utilized alternating incubations of slides with anti-avidin (Vector) and FITC-ExtrAvidin (Sigma). Slides were then counterstained with either propidium iodide (2.5 µg/ml) or DAPI (15 µg/ml) and viewed under UV fluorescence (Olympus).
RNA–DNA FISH
Cells were plated on microscope slides at ∼45% confluence and incubated overnight in complete media in a 37°C humidified CO2 incubator. Slides were rinsed one time with sterile RNase-free PBS. Slides were incubated for 30 s in CSK buffer (100 mm NaCl, 300 mm sucrose, 3 mm MgCl2,10 mm PIPES, pH 6.8), 5 min in CSK buffer plus 0.1% Triton X-100, and then for an additional 30 s in CSK buffer at room temperature. Cells were fixed in 4% paraformaldehyde in PBS for 10 min at room temperature. Slides were rinsed in 70% ETOH and stored in 70% ETOH at 4°C until use. Just prior to use, slides were dehydrated through an ETOH series (70, 90 and 100%) and allowed to air-dry. Denatured probes were prehybridized with Cot-1 DNA at 37°C for 30 min. Slides were hybridized at 37°C for 14–16 h. Slides were washed as follows: three times in 50% formamide/2× SSC at 42°C for 5 min, three times in 2× SSC at 42°C for 5 min, three times in 4× SSC/0.1% Tween 20 at room temperature for 3 min. Slides were then fixed in 4% paraformaldehyde in PBS for 5 min at room temperature, and briefly rinsed in 2× SSC at room temperature. The slides were then dehydrated in 70, 90 and 100% ETOH and then processed for DNA FISH, including the RNAase treatment step, as described above. Slides were then counterstained with either propidium iodide (2.5 µg/ml) or DAPI (15 µg/ml) and viewed under UV fluorescence (Olympus). Z-stack images were generated using the Cytovision workstation.
Replication timing assay
The BrdU replication timing assay was performed on exponentially dividing cultures as follows: asynchronously growing cells were exposed to 20 µg/ml of BrdU (Sigma) for 4.5, 5, 6, 7, 8 or 9 h. Mitotic cells were harvested in the absence of colcemid, treated with 75 mm KCl for 15–30 min at 37°C, fixed in 3:1 methanol:acetic acid and dropped on wet ice-cold slides. The chromosomes were denatured in 70% formamide in 2× SSC at 70°C for 3 min and processed for DNA FISH, as described above. The incorporated BrdU was then detected using an FITC-labeled anti-BrdU antibody (Becton Dickinson). Slides were stained with propidium iodide (0.3 mg/ml), cover slipped and viewed under UV fluorescence.
All images were captured with an Olympus BX fluorescent microscope using a 100× objective, automatic filter-wheel and Cytovision workstation. Individual chromosomes were identified with either chromosome-specific paints or centromeric probes in combination with BACs from the deleted regions. Utilizing the Cytovision workstation, each chromosome was isolated from the metaphase spread and a line drawn along the middle of the entire length of the chromosome. The Cytovision software was used to calculate the pixel area and intensity along each chromosome for each fluorochrome occupied by the DAPI and BrdU (FITC) signals. The total amount of fluorescent signal was calculated by multiplying the average pixel intensity by the area occupied by those pixels.
PCR and expression analysis
Genomic DNA and total RNA were isolated from HTD114 cells and its sub-clones using trizol reagent (Invitrogen). Total human RNA samples were from the Ambion FirstChoice Human Total RNA Survey Panel. cDNA was prepared using the SuperScriptTM III First-Strand Synthesis System (Invitrogen). Reverse transcriptase reactions were performed in the presence or absence of reverse transcriptase on 5 µg of total RNA. PCR (genomic and RT-PCR) was performed in a 25–50 µl volume using 50–100 ng of genomic DNA or 1–2 µl of cDNA (50–100 ng of input RNA equivalent), 1× Standard Taq Buffer (New England Biolabs, Inc.), 200 µm of each deoxynucleotide triphosphates, 0.2 µm of each primer and 3U of Taq DNA Polymerase (New England Biolabs, Inc.) under the following reaction conditions: 95°C for 2 min, followed by 35–45 cycles of 95°C for 30 s, 55–62°C for 45 s and 72°C for 1 min, with a final extension time of 10 min at 72°C. PCR products were separated on 1% agarose gels, stained with ethidium bromide and photographed under ultraviolet light illumination. Sequencing of PCR products was carried out at the Vollum Institute DNA Sequencing Core Facility.
SUPPLEMENTARY MATERIAL
FUNDING
E.P.S. was supported by a National Science Foundation Graduate Research Fellowship, an ARCS Foundation Scholarship, a Tartar Trust Fellowship and a Vertex Fellowship. N.D. was supported by the Pre-Doctoral Training Grant in Molecular Hematology, T32 HL00781. This work was supported by grants from the National Cancer Institute, CA104693 and CA131967, to M.J.T.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr Michael Harkey, at the Fred Hutchinson Cancer Research Center's Clonal Analysis Core, for the LAM-PCR analysis and Dr Phil Soriano for the Flp expression construct. We are grateful to Dr Mark Groudine, Dr David Kabat and Dr Sarah Smolik for critical comments on the manuscript. We are also grateful to Dr Andrew Chess for providing the genotypes of the GM13130 lymphoblast cell line. We are grateful to DNASTAR, Inc., for the gift of the Lasergene DNA sequence analysis software.
Conflict of Interest statement. None declared.
REFERENCES
- 1.Nichols W.W., Levan A., Aula P., Norrby E. Extreme chromosome breakage induced by measles virus in different in vitro systems. Hereditas. 1964;51:380. doi:10.1111/j.1601-5223.1964.tb01943.x. [Google Scholar]
- 2.Nichols W.W., Levan A., Aula P., Norrby E. Chromosome damage associated with measles virus in vitro. Hereditas. 1965;54:101. doi: 10.1111/j.1601-5223.1965.tb02008.x. doi:10.1111/j.1601-5223.1965.tb02008.x. [DOI] [PubMed] [Google Scholar]
- 3.Kato H., Sandberg A.A. Chromosome pulverization in human cells with micronuclei. J. Natl Cancer Inst. 1968;40:165–179. [PubMed] [Google Scholar]
- 4.Miles C.P., O'Neill F. 3H labeling patterns of permanent cell line chromosomes showing pulverization or accentuated secondary constrictions. J. Cell Biol. 1969;40:553–561. doi: 10.1083/jcb.40.2.553. doi:10.1083/jcb.40.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith L., Plug A., Thayer M. Delayed replication timing leads to delayed mitotic chromosome condensation and chromosomal instability of chromosome translocations. Proc. Natl Acad. Sci. USA. 2001;98:13300–13305. doi: 10.1073/pnas.241355098. doi:10.1073/pnas.241355098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang B.H., Smith L., Huang J., Thayer M. Chromosomes with delayed replication timing lead to checkpoint activation, delayed recruitment of aurora B and chromosome instability. Oncogene. 2007;26:1852–1861. doi: 10.1038/sj.onc.1209995. doi:10.1038/sj.onc.1209995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Breger K.S., Smith L., Turker M.S., Thayer M.J. Ionizing radiation induces frequent translocations with delayed replication and condensation. Cancer Res. 2004;64:8231–8238. doi: 10.1158/0008-5472.CAN-04-0879. doi:10.1158/0008-5472.CAN-04-0879. [DOI] [PubMed] [Google Scholar]
- 8.Breger K.S., Smith L., Thayer M.J. Engineering translocations with delayed replication: evidence for cis control of chromosome replication timing. Hum. Mol. Genet. 2005;14:2813–2827. doi: 10.1093/hmg/ddi314. doi:10.1093/hmg/ddi314. [DOI] [PubMed] [Google Scholar]
- 9.Camargo M., Cervenka J. Patterns of DNA replication of human chromosomes. II. Replication map and replication model. Am. J. Hum. Genet. 1982;34:757–780. [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen S.M., Cobb E.R., Cordeiro-Stone M., Kaufman D.G. Identification of chromosomal bands replicating early in the S phase of normal human fibroblasts. Exp. Cell Res. 1998;245:321–329. doi: 10.1006/excr.1998.4258. doi:10.1006/excr.1998.4258. [DOI] [PubMed] [Google Scholar]
- 11.Mills A.A., Bradley A. From mouse to man: generating megabase chromosome rearrangements. Trends Genet. 2001;17:331–339. doi: 10.1016/s0168-9525(01)02321-6. doi:10.1016/S0168-9525(01)02321-6. [DOI] [PubMed] [Google Scholar]
- 12.Harkey M.A., Kaul R., Jacobs M.A., Kurre P., Bovee D., Levy R., Blau C.A. Multiarm high-throughput integration site detection: limitations of LAM-PCR technology and optimization for clonal analysis. Stem Cells Dev. 2007;16:381–392. doi: 10.1089/scd.2007.0015. doi:10.1089/scd.2007.0015. [DOI] [PubMed] [Google Scholar]
- 13.Diaz-Perez S., Ouyang Y., Perez V., Cisneros R., Regelson M., Marahrens Y. The element(s) at the nontranscribed Xist locus of the active X chromosome controls chromosomal replication timing in the mouse. Genetics. 2005;171:663–672. doi: 10.1534/genetics.105.043026. doi:10.1534/genetics.105.043026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diaz-Perez S.V., Ferguson D.O., Wang C., Csankovszki G., Tsai S.C., Dutta D., Perez V., Kim S., Eller C.D., Salstrom J., et al. A deletion at the mouse Xist gene exposes trans-effects that alter the heterochromatin of the inactive X chromosome and the replication time and DNA stability of both X chromosomes. Genetics. 2006;174:1115–1133. doi: 10.1534/genetics.105.051375. doi:10.1534/genetics.105.051375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yeo G.W., Coufal N.G., Liang T.Y., Peng G.E., Fu X.D., Gage F.H. An RNA code for the FOX2 splicing regulator revealed by mapping RNA-protein interactions in stem cells. Nat. Struct. Mol. Biol. 2009;16:130–137. doi: 10.1038/nsmb.1545. doi:10.1038/nsmb.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allen E., Horvath S., Tong F., Kraft P., Spiteri E., Riggs A.D., Marahrens Y. High concentrations of long interspersed nuclear element sequence distinguish monoallelically expressed genes. Proc. Natl Acad. Sci. USA. 2003;100:9940–9945. doi: 10.1073/pnas.1737401100. doi:10.1073/pnas.1737401100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldmit M., Bergman Y. Monoallelic gene expression: a repertoire of recurrent themes. Immunol. Rev. 2004;200:197–214. doi: 10.1111/j.0105-2896.2004.00158.x. doi:10.1111/j.0105-2896.2004.00158.x. [DOI] [PubMed] [Google Scholar]
- 18.Selig S., Okumura K., Ward D.C., Cedar H. Delineation of DNA replication time zones by fluorescence in situ hybridization. EMBO J. 1992;11:1217–1225. doi: 10.1002/j.1460-2075.1992.tb05162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Azuara V., Brown K.E., Williams R.R., Webb N., Dillon N., Festenstein R., Buckle V., Merkenschlager M., Fisher A.G. Heritable gene silencing in lymphocytes delays chromatid resolution without affecting the timing of DNA replication. Nat. Cell Biol. 2003;5:668–674. doi: 10.1038/ncb1006. doi:10.1038/ncb1006. [DOI] [PubMed] [Google Scholar]
- 20.Mostoslavsky R., Singh N., Tenzen T., Goldmit M., Gabay C., Elizur S., Qi P., Reubinoff B.E., Chess A., Cedar H., et al. Asynchronous replication and allelic exclusion in the immune system. Nature. 2001;414:221–225. doi: 10.1038/35102606. doi:10.1038/35102606. [DOI] [PubMed] [Google Scholar]
- 21.Ensminger A.W., Chess A. Coordinated replication timing of monoallelically expressed genes along human autosomes. Hum. Mol. Genet. 2004;13:651–658. doi: 10.1093/hmg/ddh062. doi:10.1093/hmg/ddh062. [DOI] [PubMed] [Google Scholar]
- 22.Singh N., Ebrahimi F.A., Gimelbrant A.A., Ensminger A.W., Tackett M.R., Qi P., Gribnau J., Chess A. Coordination of the random asynchronous replication of autosomal loci. Nat. Genet. 2003;33:339–341. doi: 10.1038/ng1102. doi:10.1038/ng1102. [DOI] [PubMed] [Google Scholar]
- 23.Schlesinger S., Selig S., Bergman Y., Cedar H. Allelic inactivation of rDNA loci. Genes Dev. 2009;23:2437–2447. doi: 10.1101/gad.544509. doi:10.1101/gad.544509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krueger C., Morison I.M. Random monoallelic expression: making a choice. Trends Genet. 2008;24:257–259. doi: 10.1016/j.tig.2008.03.005. doi:10.1016/j.tig.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 25.Zakharova I.S., Shevchenko A.I., Zakian S.M. Monoallelic gene expression in mammals. Chromosoma. 2009;118:279–290. doi: 10.1007/s00412-009-0206-8. doi:10.1007/s00412-009-0206-8. [DOI] [PubMed] [Google Scholar]
- 26.Gimelbrant A., Hutchinson J.N., Thompson B.R., Chess A. Widespread monoallelic expression on human autosomes. Science. 2007;318:1136–1140. doi: 10.1126/science.1148910. doi:10.1126/science.1148910. [DOI] [PubMed] [Google Scholar]
- 27.Ohlsson R. Genetics. Widespread monoallelic expression. Science. 2007;318:1077–1078. doi: 10.1126/science.1150705. doi:10.1126/science.1150705. [DOI] [PubMed] [Google Scholar]
- 28.Tycko B. Allele-specific DNA methylation: beyond imprinting. Hum. Mol. Genet. 2010;19:R210–220. doi: 10.1093/hmg/ddq376. doi:10.1093/hmg/ddq376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Payer B., Lee J.T. X chromosome dosage compensation: how mammals keep the balance. Annu. Rev. Genet. 2008;42:733–772. doi: 10.1146/annurev.genet.42.110807.091711. doi:10.1146/annurev.genet.42.110807.091711. [DOI] [PubMed] [Google Scholar]
- 30.Brown C.J., Willard H.F. The human X-inactivation centre is not required for maintenance of X-chromosome inactivation. Nature. 1994;368:154–156. doi: 10.1038/368154a0. doi:10.1038/368154a0. [DOI] [PubMed] [Google Scholar]
- 31.Csankovszki G., Panning B., Bates B., Pehrson J.R., Jaenisch R. Conditional deletion of Xist disrupts histone macroH2A localization but not maintenance of X inactivation. Nat. Genet. 1999;22:323–324. doi: 10.1038/11887. doi:10.1038/11887. [DOI] [PubMed] [Google Scholar]
- 32.Zhu Y., Bye S., Stambrook P.J., Tischfield J.A. Single-base deletion induced by benzo[a]pyrene diol epoxide at the adenine phosphoribosyltransferase locus in human fibrosarcoma cell lines. Mutat. Res. 1994;321:73–79. doi: 10.1016/0165-1218(94)90122-8. doi:10.1016/0165-1218(94)90122-8. [DOI] [PubMed] [Google Scholar]
- 33.Helm S. Cancer Cytogenetics. New York: Wiley-Liss; 1995. [Google Scholar]
- 34.Trask B., Pinkel D. Flow Cytometry. New York: Academic Press; 1990. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.