Abstract
The RNA-binding protein TDP-43 has been linked to amyotrophic lateral sclerosis (ALS) both as a causative locus and as a marker of pathology. With several missense mutations being identified within TDP-43, efforts have been directed towards generating animal models of ALS in mouse, zebrafish, Drosophila and worms. Previous loss of function and overexpression studies have shown that alterations in TDP-43 dosage recapitulate hallmark features of ALS pathology, including neuronal loss and locomotor dysfunction. Here we report a direct in vivo comparison between wild-type and A315T mutant TDP-43 overexpression in Drosophila neurons. We found that when expressed at comparable levels, wild-type TDP-43 exerts more severe effects on neuromuscular junction architecture, viability and motor neuron loss compared with the A315T allele. A subset of these differences can be compensated by higher levels of A315T expression, indicating a direct correlation between dosage and neurotoxic phenotypes. Interestingly, larval locomotion is the sole parameter that is more affected by the A315T allele than wild-type TDP-43. RNA interference and genetic interaction experiments indicate that TDP-43 overexpression mimics a loss-of-function phenotype and suggest a dominant-negative effect. Furthermore, we show that neuronal apoptosis does not require the cytoplasmic localization of TDP-43 and that its neurotoxicity is modulated by the proteasome, the HSP70 chaperone and the apoptosis pathway. Taken together, our findings provide novel insights into the phenotypic consequences of the A315T TDP-43 missense mutation and suggest that studies of individual mutations are critical for elucidating the molecular mechanisms of ALS and related neurodegenerative disorders.
INTRODUCTION
Amyotrophic lateral sclerosis (ALS) is an adult-onset, progressive neurodegenerative disorder characterized by motor neuron dysfunction, which leads to paralysis and respiratory failure followed by death, generally within 5 years from diagnosis. About 20% of all ALS patients also exhibit fronto-temporal lobar degeneration, which is characterized by neurodegeneration of the frontal and temporal lobes (1). Approximately 10% of all ALS cases are inherited (familial ALS, fALS) and have been linked to a number of loci, including superoxide dismutase (SOD1), alsin (a GPTase), senataxin (a DNA/RNA helicase), VAMP/synaptobrevin-associated protein B, P150 dynactin, angiogenin, TAR DNA-binding protein (TDP-43) and FUsed in Sarcoma (Fus) (2–8). The remaining 90% of ALS cases are sporadic (sALS) and remain poorly understood.
Extensive pathological studies have identified TDP-43 as a common component of cytoplasmic inclusions found in almost all non-SOD1 cases of ALS studied to date (9–11) as well as in other neurodegenerative disorders (reviewed in 1). Histological examinations of human tissue obtained at autopsy have defined distinct subtypes of TDP-43-positive cytoplasmic inclusions ranging in shape from filamentous to round aggregates that are present in neurons and sometimes in the surrounding glia (12). Recently, several articles reported the identification of TDP-43 gene mutations in both fALS and sALS patients of diverse ethnicities (3,11,13–18). Thus, TDP-43 has emerged as a common denominator for the majority of ALS cases known to date, and studying its function has the potential to provide valuable insights into the pathology of neurodegeneration.
TDP-43 protein consists of two RNA recognition motifs (RRM1 and 2) as well as a glycine-rich domain within the C terminus (19). In vitro assays have demonstrated that TDP-43 binds with high-affinity UG-rich sequences, consistent with a role in mRNA splicing (20). Except for a single mutation found in the first RNA-binding domain of TDP-43, all other mutations found in ALS patients lie in the C-terminus, including the glycine-rich domain (3,17,18). These mutations are amino acid substitutions that are thought to increase TDP-43 phosphorylation and target it for degradation by the proteasome (3). The TDP-43 protein is ubiquitously expressed and co-localizes with Survival of Motor Neuron (SMN) and gemin proteins in the nucleus. Its cellular functions are just beginning to be understood and include transcriptional repression, splicing, miRNA biogenesis, apoptosis and cell division (reviewed in 1). In cultured neurons, TDP-43 associates with RNA granules and co-purifies with beta-actin and CaMKII mRNAs. Furthermore, TDP-43 co-localizes with fragile X mental retardation protein (FMRP) and Staufen in an activity-dependent manner, suggesting that TDP-43 may regulate synaptic plasticity in vivo by controlling the transport and splicing of synaptic mRNAs (21).
Recently, an avalanche of articles demonstrated the requirement for TDP-43 function in various aspects of neuronal development and function in Drosophila neurons (22–26). Loss-of-function and overexpression studies showed that both a TDP-43 deficit and excess human TDP-43 (hTDP-43) lead to a decrease in the size of the larval neuromuscular junction (NMJ) as well as reduced motility (23,25). In contrast, overexpression of hTDP-43 in the dendritic arborization neurons leads to an overgrown dendritic arbor, a phenotype which is less pronounced when the M337V or Q331K ALS variants of TDP-43 are overexpressed (26). Recently, a comparison of cellular and functional phenotypes resulting from expression of various TDP-43 variants revealed a requirement for TDP-43′s RNA-binding activity in neurons (22). Although these and other recent reports demonstrate the presence of several features of ALS pathology in Drosophila, worms, mice or zebrafish models (27–30), more clarity is needed regarding the phenotypic consequences of overexpressing wild-type versus missense mutations of TDP-43 in neurons.
Here we report the direct comparison of a range of phenotypes produced by expressing wild-type and A315T mutant TDP-43 in the Drosophila nervous system. We also show that TDP-43 neurotoxicity is modulated by the proteasome, HSP70 chaperone and apoptotic pathways. We generated transgenic Drosophila expressing either fly or hTDP-43 variants in two neuronal models: the retina and motor neurons. We found that TDP-43 expression in photoreceptor neurons leads to the formation of cytoplasmic and axonal aggregates in developing retina. Adult eyes expressing TDP-43 variants exhibit progressive neuronal loss and neurodegeneration in a dose-dependent manner. When comparing transgenic lines that express TDP-43 at similar levels, we found that the Drosophila variants are more potent than their human counterparts. Similar differential effects between fly and hTDP-43 as well as between wild-type and the A315T allele were found in motor neurons. Notably, wild-type Drosophila TDP-43 (TBPH) expression in motor neurons led to a relocalization from the nucleus, where it is normally found, to the cytoplasm, where it formed visible aggregates. In contrast, the TBPH A315T mutant as well as the hTDP-43 variants remained restricted to the nucleus regardless of their level of expression (i.e. moderate versus high). For both Drosophila and human variants, wild-type TDP-43 expression was more detrimental than A315T to viability and motor neuron survival, as evidenced by earlier lethality and neuronal apoptosis phenotypes in the nervous system. When expressed at comparable levels with wild-type TBPH, the A315T TBPH variant was restricted to the nucleus and showed modest evidence for neuronal death. Although these results might suggest a direct correlation between the amount of mislocalized (cytoplasmic) TDP-43 and apoptosis in motor neurons, our analyses of the hTDP-43 transgenes indicate that cell death can occur in the absence of cytoplasmic aggregation and is likely due to neurotoxic effects caused by excess wild-type TDP-43 or the presence of the A315T allele. Interestingly, although both the wild-type and A315T alleles of hTDP-43 remain restricted to the nucleus, expression of wild-type hTDP-43 (hwt) results in a dramatic loss of motor neurons, but expression of the hA315T mutant leads to just a few cells expressing markers of apoptosis. Adult survival and climbing ability also appear to be more severely affected by overexpression of wild-type TDP-43 than that of the A315T variant. In contrast, larval turning behavior is significantly more affected by expression of A315T than that of wild-type TDP-43. Taken together, our results demonstrate that wild-type and A315T mutant TDP-43 exert differential toxicity. The wild-type allele has more dramatic effects on neuronal death, adult survival and climbing ability, whereas the A315T allele has a more pronounced effect on larval turning behavior, which requires complex motor neuron coordination across anterior–posterior and dorsal–ventral axes. Our work also reveals a dose-dependent effect of TDP-43 expression as evidenced by the direct correlation between TDP-43 protein levels and neurotoxic phenotypes. RNA interference (RNAi) and genetic interaction experiments show that wild-type and A315T overexpression mimic a loss-of-function phenotype and suggest a dominant-negative effect. Finally, genetic manipulations of proteasome function, the HSP70 chaperone and caspase inhibitors suggest that TDP-43 toxicity is modulated by proteasome-mediated degradation, protein folding and the apoptosis pathways.
RESULTS
Overexpression of wild-type and A315T mutant TDP-43 in the eye leads to neurodegeneration accompanied by cell loss
To directly compare the in vivo phenotypes of wild-type TDP-43 with the effects of individual missense mutations found in ALS patients (3), we generated transgenic flies expressing both wild-type and mutant TDP-43 under the control of the UAS promoter. This strategy allows for tissue-specific expression during Drosophila development using the bipartite Gal4-UAS system (31). Using the GMR Gal4 driver, we expressed wild-type and A315T mutant human and TBPH variants in the developing retina. After surveying several transgenic lines for each TDP-43 variant, we chose wild-type and A315T mutant-expressing lines (one of each) that are comparable in levels of expression (Fig. 1G and H for hTDP-43, and Supplementary Material, Fig. S1 for TBPH). In addition, we chose a higher expressing A315T line (hA315T HE, Fig. 1G and H). As shown in Figure 1A–C, expression of hTDP-43 (wild-type and A315T mutant) has no visible phenotype in the eye at 25°C. Despite the absence of surface phenotypes, retinal sections through the adult eyes exhibit evidence of cell loss and neurodegeneration (Fig. 1A′–C′), suggesting that hTDP-43 expression is toxic in the retina. These results were substantiated by our findings that when expressed at higher levels (by raising the flies at 29°C, which increases Gal4 activity), both wild-type and A315T mutant hTDP-43 expression led to visible signs of retinal neurodegeneration (Fig. 1D–F). Plastic sections through these retina showed clear evidence of cell loss (Fig. 1D′–F′). Similar results were obtained with additional hTDP-43 transgenic lines (including hA315T HE), suggesting that the milder phenotypes at 25°C are likely due to the presence of a threshold for hTDP-43 toxicity rather than a positional effect (due to the randomness of transgene insertion in the genome). A similar dose response was observed for wild-type TBPH, which leads to severe eye neurodegeneration at 25°C but is 100% lethal when overexpressed at higher levels (by raising the flies at 29°C). We also found that retinal cell loss has an aging component, as suggested by the finding that hTDP-43-expressing adults (both wild-type and A315T), despite showing little or no visible phenotypes at 25°C when 1–3 days old (DO) (Fig. 1B and C), they all exhibited a visible loss of pigmentation at 5 DO. This phenotype worsens as animals age (compare Fig. 1B with 7A and Fig. 1C with 7E).
Figure 1.
Overexpression of human wild-type and A315T mutant TDP-43 leads to neurodegeneration in the adult retina. (A–F) Expression of hwt and hA315T results in normal surface phenotypes at 25°C (B and C compared with A). Both hTDP-43 transgenes exhibit strong surface phenotypes when expressed at higher levels (29°C, see E and F) compared with controls (D). Genotypes as indicated. Anterior right, dorsal up. (A′–F′) Corresponding plastic sections indicate that regardless of the surface phenotype, the retinas undergo cell loss. Note large areas of cell loss within the retina (arrows). Adult eyes shown are from 1–2-day-old flies. (G) Western blot analyses showing hTDP-43 expression. Note similar levels of TDP-43 expression for the wild-type (hwt) and A315T (hA315T) transgenes as well as increased expression levels in an additional hA315T line (hA315T HE). Genotypes as indicated, on top. Blotting antibodies as indicated on the right. Tubulin was used as a loading control. (H) Quantification of relative protein levels from Western blot analysis. Scale bar (A′): 30 µm.
Taken together, these results indicate that when expressed at comparable levels, Drosophila and hTDP-43 overexpression have similar consequences, although the fly transgenes appear to be more potent than their human equivalents. Thus, although the TDP-43 gene, TBPH, is highly conserved in Drosophila, our data suggest that some differences may exist between the fly and hTDP-43 pathways in vivo. Indeed, flies harbor an additional, previously unreported TDP-43 homolog, namely CG7804, which is 41.6% identical to TBPH and 34.5% identical to hTDP-43 (Supplementary Material, Fig. S2). The presence of CG7804 may account for the fact that the wild-type TBPH transgene is more toxic than hwt despite being expressed at comparable levels (Supplementary Material, Fig. S1). Our results also support the concept that TDP-43 and its toxicity in vivo are dose- and age-dependent.
Wild-type and mutant TDP-43 accumulate in axonal aggregates in the developing eye
One of the hallmarks of ALS pathology is the accumulation of cytoplasmic inclusions containing TDP-43 (32). Previous reports include both the presence and absence of TDP-43 aggregates in models ranging from yeast to flies, zebrafish, cultured neurons and mice (22–24,27–29,33,34). We also asked whether overexpression of wild-type TDP-43 and the A315T mutant transgenes alters the subcellular localization of TDP-43 and leads to the formation of cytoplasmic aggregates. To this end, we compared the distribution of individual, YFP-tagged hTDP-43 transgenes (wild-type and A315T) with nuclear GFP. As seen in Figure 2, both TDP-43 variants accumulate in axonal aggregates when expressed in developing eye discs (Fig. 2A–F′, arrowheads). When examining their subcellular distribution in comparison with GFP-NLS at high magnification, we found that all TDP-43 variants used in this study exhibit some nuclear as well as some cytoplasmic presence (compare Fig. 2D″ with E″ and F″). When comparing their subcellular localization, the A315T mutant appears diffuse (asterisk in Fig. 2F″) in comparison with wild-type TDP-43, which exhibits a more particulate distribution (arrowhead in Fig. 2E″). Similar results were obtained expressing TBPH (Supplementary Material, Fig. S2). These data indicate that all transgenes examined in this study exhibit some level of redistribution from the nucleus into the axons of the developing retina.
Figure 2.
Overexpression of TDP-43 in larval eye imaginal discs leads to altered cytoplasmic localization and axonal aggregates. (A–F) Single-confocal slices (1 µm each) showing hTDP-43 localization when expressed in the developing retina with GMR-Gal4. hTDP-43 visualized via individual fluorescent tags [as indicated (B, C and E–F″), compare with GFP-NLS (A, D–D″)]. Filamentous actin labeled with phalloidin (phall), and DNA stained with Hoechst (as indicated). Note TDP-43 aggregates in axons (arrowheads). (D′–F′) High magnification views of optic stalk show TDP-43 aggregates in axons (white arrowheads, D′–F′), whereas GFP-NLS remains restricted to nuclei (D′). (D″–F″) High-magnification insets showing the localization of GFP-NLS (D″) and TDP-43 wt and A315T in relation to the nucleus (E″–F″). Stainings as indicated. Note that hwt forms more pronounced aggregates than hA315T, which appears more diffusely distributed (asterisk). Individual nuclei are circled in red. Red arrowheads indicate some amount of depletion from the nucleus hwt. Scale bar (A): 50 µm.
Wild-type TBPH accumulates in cytoplasmic aggregates when expressed in motor neurons
We next compared the subcellular distribution of TDP-43 variants in motor neurons. Using the D42 Gal4 driver (35), we expressed YFP-tagged wild-type and A315T hTDP-43 (Fig. 3B–B″ and C–C″, respectively) as well as RFP-tagged, wild-type and A315T TBPH (Fig. 3D–D″ and E–E″, respectively) and then compared their localization with that of nuclear GFP (GFP-NLS, Fig. 3A–A″) or RFP (RFP-NLS, data not shown). These experiments showed that when expressed at similar levels, the hTDP-43 variants remained restricted to the nucleus (compare white arrows in Fig. 3A–C′). Although some cells expressing hwt exhibit a ring-like distribution at the edge of the nucleus (arrowheads in Fig. 3B and B′), hA315T is clearly restricted to the nucleus (arrows in Fig. 3C and C′). Interestingly, a fraction of wild-type TBPH redistributed to the cytoplasm and formed visible aggregates within the neuropil (red arrows in Fig. 3D′), whereas the TBPH A315T allele was mostly restricted to the nucleus (arrows in Fig. 3E and E′). Thus, of all transgenes used in this study, wild-type TBPH is the sole variant to exhibit a pronounced exit from the nucleus. These data indicate that motor neurons handle expression of Drosophila and human, both wild-type and A315T mutant TDP-43 differently than photoreceptor neurons where all TDP-43 variants accumulated in axonal aggregates.
Figure 3.
Subcellular localization of TDP-43 in motor neurons. (A–E) D42-Gal4-driven expression of GFP-NLS (A, control) and TDP-43 variants (B–E) in ventral ganglia of third instar larvae. Genotypes indicated on the top, and stainings shown on the left. Images shown represent projections of 1 µm confocal slices. GFP (or YFP) and RFP indicate tags used to visualize TDP-43 variants, and DNA visualized using Hoechst. (A′–E″) High-magnification views of ventral ganglia shown in (A)–(E). Both human transgenes (wt and A315T) remained restricted to the nucleus (white arrows in B and B′, and C and C′). Note cell with peripheral nuclear localization in hTDP-43 wt (white arrowhead in B and B′). Wild-type TBPH translocates to the cytoplasm and forms axonal aggregates (red arrows in D′). The fly A315T mutant protein is mostly restricted to the nucleus (white arrows in E and E′). Hoechst staining of the samples shown in (A′)–(E′) labels DNA. Scale bars: 30 μm in (A), 15 μm in (A′).
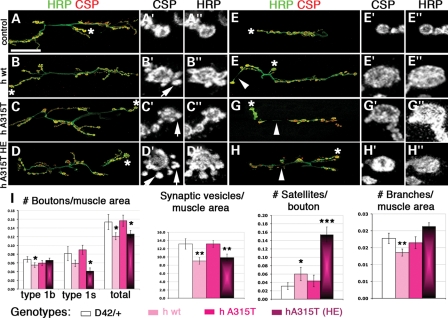
Motor neurons expressing TDP-43 variants exhibit morphological defects at the NMJ synapse
To determine whether TDP-43 overexpression has an impact on the ability of motor neurons to form synaptic connections, we examined the morphology of the larval NMJ (Fig. 4). The larval NMJ synapse at muscles 6/7 consists of structural varicosities referred to as type 1s and type 1b synaptic boutons that form when motor neuron terminals innervate the surface of post-synaptic muscles (36). We labeled the pre-synaptic motor neuron membrane at the NMJ with horseradish peroxidase (HRP) antibodies and the synaptic vesicles within boutons with cysteine string protein (CSP) antibodies (37) (Fig. 4A–D″ and E–H″). Using these markers, we measured the effect of TDP-43 variants on the overall size of the NMJ synapse by quantifying the total number of boutons (as marked by HRP), the area occupied by synaptic vesicles (as indicated by CSP) as well as the number of satellite boutons and axonal branches (using both CSP and HRP labels) (Fig. 4I). First, quantification of the number of type 1s and 1b as well as the total number of boutons (1s plus 1b) per muscle area showed that overexpression of hwt led to a significant decrease in the total number of boutons (Fig. 4I). When equal levels of hA315T protein were expressed at the NMJ, we found no significant changes in bouton numbers (Fig. 4I). However, when higher levels of A315T (hA315T HE; Fig. 1G and H) were expressed, there was a significant decrease in the number of synaptic boutons at the NMJ, similar to the effect of the lower expressing human wild-type TDP-43 line (Fig. 4I).
Figure 4.
NMJ morphology is altered by overexpression of TDP-43 variants. (A–H″) Larval NMJs at muscles 6/7, abdominal segment A3 (A–D″) and abdominal segment A6 (E–H″). Genotypes shown on the left, and stainings indicated on the top. Selected terminal type 1b boutons (marked with asterisks) are shown in (A′–D′) and (A′–D′, HRP only), and likewise for A6 in (E′–H′) and (E′–H′, HRP only). Arrowheads indicate thinning of the HRP-stained neuronal membrane. Arrows indicate satellite boutons. D42-Gal4-driven overexpression of TDP-43 (wild-type and A315T) affects various aspects of synaptic morphology [see (I) for quantitative analyses at A3, which include a high expressing hA315T transgene (A315T HE)]. Student's t-test was used to determine statistical significance. ***P< 0.001; **P< 0.01; *P< 0.05. Scale bar (A): 45 μm.
We also quantified the total area occupied by synaptic vesicles and found that hwt leads to a significant decrease compared with controls (Fig. 4I). In addition, our morphological analyses revealed the presence of supranumerary satellite boutons (arrow in Fig. 4B′) due to expression of hwt. These structures represent abnormal synaptic growths and suggest potential defects in microtubule organization and intracellular trafficking. As with the total number of boutons, comparable levels of hA315T had no effect, but when the higher hA315T-expressing line (hA315T HE) was used, the area occupied by synaptic vesicles was decreased and the number of satellite boutons was increased, similar to the effect of hwt (Fig. 4I). These results suggest that TDP-43 may control synaptic function at the NMJ (Fig. 5A).
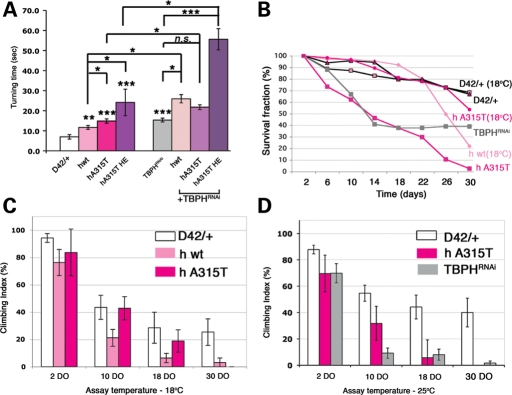
Figure 5.
Overexpression of TDP-43 variants acts as a dominant negative and affects locomotor activity and survival. (A) Larvae expressing D42-driven human wild-type, hA315T mutant TDP-43 (including hA315T HE) and TBPH RNAi transgenes (alone or in combination) take significantly longer to turn over following a ventral-up inversion. Genotypes as indicated. (B) hTDP-43 expression or TBPH RNAi in motor neurons cause a dramatic decrease in adult survival. (C and D) Adult climbing assays performed on the adult survivors show severe motor impairment at both 18 (C) and 25°C (D). Student's t-test was used to determine statistical significance. ***P< 0.001; **P< 0.01; *P< 0.05.
Additional quantifications included the number of axonal branches per muscle area (Fig. 4I). hwt but not hA315T or hA315T HE led to a significant decrease in the number of axonal branches at the NMJ (Fig. 4I). These data are consistent with a previous report indicating that hwt overexpression leads to fewer boutons and branches at the NMJ compared with controls (23).
We also investigated whether TDP-43-overexpressing larvae exhibit signs of motor neuron degeneration as reported in ALS patients. Neurodegeneration at the larval NMJ has been shown to manifest through the presence of synaptic ‘footprints’, i.e. structural remains of boutons that are positive for post-synaptic Dlg but lack presynaptic markers such as CSP and HRP (38). Because no obvious ‘footprints’ were found in the larval anterior segment A3 (data not shown), we also examined the more posterior segment A6, which is thought to be more sensitive to motor neuron degeneration, presumably due to the increased length of the motor neuron axons. Although some thinning of the neuronal membrane (as indicated by HRP staining) was observed with all TDP-43 variants (arrowheads, Fig. 4E–H; Supplementary Material, Fig. S3D–F), no terminal boutons positive for Dlg but showing reduced CSP staining were found (data not shown; Supplementary Material, Fig. S3). Experiments using fly transgenes also show that when comparable levels of wild-type and A315T mutant TBPH are expressed in motor neurons, the wild-type allele has a more pronounced effect on the size of the NMJ (as indicated by its effect on the total number of boutons per muscle area) and the number of satellite boutons (Supplementary Material, Fig. S4). Taken together, these data indicate that TDP-43 regulates NMJ morphology and that despite some differences between the effects of Drosophila and hTDP-43, the wild-type allele has more pronounced phenotypic effects at the NMJ than the A315T variant when expressed at similar levels. Furthermore, our results indicate that by expressing it at higher levels, hA315T generates toxicity comparable with that of hwt in regard to bouton numbers, synaptic vesicle area and satellite boutons (Fig. 4I).
Locomotor activity, viability and survival are impaired regardless of the presence of detectable TDP-43 cytoplasmic aggregates
The defects detected at the NMJ, in particular the decrease in the area occupied by synaptic vesicles and the increased number of satellite boutons, led us to hypothesize that overexpression of TDP-43 variants may lead to locomotor defects. To test this possibility, we assayed locomotor ability using larval turning assays (39). In brief, crawling third instar larvae were gently rolled ventral side up, and the time needed to turn back to dorsal side up was recorded. As seen in Figure 5A, both hwt and the hA315T allele led to a significant increase in the time needed to turn over and resume crawling on the ventral side. Notably, in this assay, the A315T mutant phenotype was significantly worse than that of hwt. In keeping with our previous observation that TDP-43 toxicity is dose-dependent, we found that the hA315T HE transgene leads to a higher level of impairment in larval motor coordination as measured by larval turning assays (Fig. 5A). Furthermore, upon comparison with the turning behavior of TBPH RNAi in motor neurons (D42::TBPHRNAi), we found that overexpression of both hTDP-43 variants mimics a loss-of-function phenotype (Fig. 5A). Similar results were obtained with the TBPH transgenes, except that no significant differences were noted between the effect of wild-type and A315T (Supplementary Material, Fig. S5A).
An outstanding question in the field is whether the TDP-43 variants act as loss- or gain-of function mutants. To further address this issue, we coexpressed our hTDP-43 variants together with TBPH RNAi (Fig. 5A), which results in a significant downregulation of the endogenous Drosophila TBPH expression (Supplementary Material, Fig. S5B; note that TBPHRNAi targets both Drosophila homologs, TBPH and CG7804). These experiments show that the reduction of TBPH by RNAi enhances the toxic effect of both wild-type and A315T hTDP-43 expression in motor neurons and support the notion that hTDP-43 overexpression in motor neurons may act as a dominant negative, at least in regard to larval turning behavior.
Expression of TDP-43 during development has dramatic effects on the viability of the fly. Wild-type and A315T TBPH expression in motor neurons leads to lethality at both the larval and pupal stages. Comparable levels of wild-type and A315T hTDP-43 are less toxic than those of Drosophila counterparts and exerted differential effects on viability and survival when expressed in motor neurons (Fig. 5B and Table 1). We found that expression of hwt was semilethal at 25°C but adults were viable at 18°C. When expressed at comparable levels, the hTDP-43 A315T mutant had no detectable effect on viability (adults were obtained at both 18 and 25°C). Higher levels of A315T, namely hA315T HE expression, in motor neurons resulted in 100% pupal lethality at 25°C. Although viable adults were obtained with both wild-type and A315T mutant hTDP-43 expression, we did observe an effect on survival. As seen in Figure 5B, control flies exhibited a 30% decrease in survival 30 days after eclosion (at both 18 and 25°C). In contrast, expression of hwt at 18°C led to a 78% decrease in survival, whereas the A315T-expressing adults exhibited a 47% reduction in survival at 18°C and 98% at 25°C. Because only few adults expressing hwt were obtained at 25°C, both survival and adult locomotor assays with the wild-type transgene were performed at 18°C only (Fig. 5B and C).
Table 1.
Summary of TDP-43 phenotypes
| Transgene/phenotypes | TBPH wt | TBPH A315T | hTDP-43 wt | hTDP-43 A315T |
|---|---|---|---|---|
| Adult eyes | ||||
| Surface phenotype | Depigmentation (at 25°C) | Depigmentation (at 25°C) | Depigmentation (29°C, age-dependent at 25°C) | Depigmentation (29°C, age-dependent at 25°C) |
| Neuronal loss | Yes | Yes | Yes | Yes |
| Eye discs | ||||
| Cellular localization | Nuclear/cytoplasmic | Nuclear/cytoplasmic | Nuclear/cytoplasmic | Nuclear/cytoplasmic |
| Axonal aggregates | Yes | Yes | Yes | Yes |
| Motor neurons | ||||
| Cellular localization | Nuclear/cytoplasmic | Nuclear | Nuclear | Nuclear |
| Neurite aggregates | Yes | No | No | No |
| Locomotor function | ||||
| Larval turning | Impaired | Impaired | Impaired | Impaired |
| Adult climbing | ND (lethal) | ND (lethal) | Impaired | Impaired |
| Motor neuron death | Yes | Yes, few cells | Yes | Yes, few cells |
| Viability | Larval/pupal lethal | Pupal lethal | Adult semilethal (25°C), viable (18°C) | Adult viable (18°C, 25°C) HE line, pupal lethal |
| NMJ synapse | ||||
| Number of boutons | Increased | NC | Decreased | NC (decreased for HE) |
| Synaptic area | Decreased | Decreased | Decreased | NC |
| Number of satellites | Increased | NC | Increased | NC (decreased for HE) |
| Number of branches | NC | NC | Decreased | NC |
| Survival | ND (due to larval/pupal lethality) | ND (due to pupal lethality) | Affected | Affected |
NC, no change; ND, not determined.
Next, we tested the effects of wild-type and A315T mutant hTDP-43 on adult locomotor function. Using climbing assays (40), we found that at 18°C, control flies exhibited a progressive decline in their ability to climb (94.3% for 2 DO to 25.4% for 30 DO flies) (Fig. 5C), whereas expression of hTDP-43 variants had a more significant impact. hwt-expressing adults showed a decline in climbing ability from 76.3% at 2 DO to 3.1% at 30 DO. The A315T-expressing adults were comparable with controls except for the last time point (83.7% at 2 DO to 0% at 30 DO). At 25°C, the A315T allele showed a progressive and significant decline in climbing ability compared with controls (Fig. 5D). Just like the effect on survival, the impairment in locomotor function due to TDP-43 overexpression mimicked the effect of TDP-43 loss (using TBPH RNAi; Fig. 5D).
In keeping with our previous results, these data are consistent with a graded effect whereby the Drosophila variants have more severe phenotypic consequences than the human alleles (compare Supplementary Material, Fig. S5A with Fig. 5A). Furthermore, these results indicate that when expressed at comparable levels, hwt is more detrimental to viability, survival and adult climbing than the A315T allele. As with the larval turning results, these effects mimic the phenotypes due to TDP-43 loss of function in motor neurons, suggesting that TDP-43 overexpression may also act as a dominant negative in regard to survival and motor neuron function. Interestingly, hA315T is more detrimental than hwt for larval turning but not adult climbing behavior. This finding supports the notion that certain behaviors may require more complex integration of neuromuscular function and thus may be more sensitive to the A315T mutation than other phenotypes. Taken together, our results suggest that A315T may impair neuromuscular function through a mechanism distinct from that of wild-type TDP-43.
Cytoplasmic aggregation of TDP-43 is not required for apoptotic death in motor neurons
A hallmark of ALS pathology is motor neuron dysfunction accompanied by apoptotic death (41). Although in the retina there is clear evidence for cell loss (Fig. 1), we wanted to determine whether our transgenes also affect motor neuronal survival. To test this possibility, we performed terminal dUTP nick end labeling (TUNEL) assays in the larval ventral ganglia, where motor neurons reside. As seen in Figure 6, apoptosis was detected in several cells that coincide with wild-type TBPH-expressing motor neurons [compare Fig. 6C and C′ with A and A′ (positive control) and B and B′ (negative control)]. Apoptotic death due to A315T TBPH expression in motor neurons was very modest (Fig. 6D and D′). The human transgenes expressing wild-type and A315T mutant hTDP-43 at comparable levels did not show any evidence of apoptosis in the larval ventral ganglia. The larvae expressing hA315T HE contained a few TUNEL-positive cells and died as pupae (data not shown).
Figure 6.
Motor neuron apoptosis due to TDP-43 overexpression. (A–D) TUNEL staining marks apoptotic cells. Genotypes/treatment indicated on top, and stainings shown on the left. (A and A′) RFP-NLS expressing ventral ganglia treated with HCl exhibits widespread TUNEL staining. (B and B′) RFP-NLS expressing ventral ganglia shows no indication of cell death (negative control). (C and C′) Wild-type TBPH expression results in some apoptotic cells within the ventral ganglia (arrows, compare C′ with A′ and B′). (D and D′) Few apoptotic cells can be detected in A315T TBPH expressing ventral ganglia (arrows). (E) GFP NLS expression in adult motor neurons using D42 Gal4. Thoracic segments T1, 2, 3 as shown. Arrows (E–G) indicate areas where motor neurons are located. Note: The region marked by red inset in (E) is shown at high magnification for different thoracic ganglia (assayed for apoptosis) in (E1′)–(G″). (E1′ and E1″) TUNEL staining in the T1/T2 region of a GFP-NLS adult thoracic ganglia treated with HCl (positive control). (E2′ and E2″) GFP-NLS expression does not induce apoptosis (negative control). (F′–G″) Adult thoracic ganglia expressing hwt (F′ and F″) and hA315T mutant (G′ and G″). Motor neurons visualized via fluorescent protein tag when either hwt (F) or hA315T mutant TDP-43 (G) is expressed with the D42 driver. Note the dramatic reduction in motor neurons as a result of hwt expression (F). TUNEL assays indicate the presence of apoptotic cells (arrows) when hwt (F′ and F″) or, to a lesser extent, A315T mutant hTDP-43 (G′ and G″) are expressed in adult motor neurons. Scale bar: 70 μm (A), 125 μm (E) and 70 μm (E1′).
Given that the hTDP-43 variant expression produced viable adults as opposed to the TBPH variants that were lethal at earlier stages, it is possible that cell death in the animals expressing hTDP-43 at comparable levels may occur later in development, in the adult nervous system. To this end, we performed TUNEL assays in adult thoracic ganglia expressing either wild-type or A315T mutant hTDP-43. Indeed, as shown in Figure 6F–F″, hwt expression results in a dramatic loss of motor neurons as evidenced by: (i) a visible reduction in the number of cells expressing the hTDP-43 transgene (Fig. 6F, compare with E) and the presence of TUNEL-positive cells that coincide with transgene expression (compare Fig. 6F′ and F″ with controls in Fig. 6E1′–E2″). In contrast, no obvious signs of cell loss were present in the context of hA315T expression (Fig. 6G, compare with E). TUNEL assays, however, indicate the presence of a few apoptotic cells that coincide with hA315T-expressing cells (Fig. 6G′ and G″). Taken together, our results with the fly and human transgenes support the notion that regardless of its subcellular localization (nuclear or cytoplasmic), TDP-43 overexpression leads to motor neuron death by apoptosis. These data also substantiate our previous observations that wild-type TDP-43 exerts higher toxicity than the A315T variant (Table 1).
TDP-43 toxicity is modulated by the proteasome and the HSP70 chaperone activities as well as the apoptosis pathway
Previous studies have suggested that the mechanisms underlying the toxicity of TDP-43 include hyperphosphorylation of the mutated variants, followed by excessive targeting to the proteasome, which may eventually become functionally overwhelmed (3,30). To test whether the proteasome contributes to TDP-43 neurotoxicity in vivo, we used a dominant-negative form of the β2 subunit of the 20S proteasome complex, namely prosβ (42). Overexpression of prosβ alone in the eye does not produce a visible phenotype (Fig. 7I); however, when coexpressed with wild-type and A315T mutant hTDP-43, it enhanced the depigmentation phenotype due to TDP-43 overexpression (Fig. 7A and E with B and F, respectively). Although this enhancement was observed throughout adult life, the data shown are from 15-day-old adults when the hTDP-43 phenotypes are more clearly visible at 25°C. Our results are consistent with a scenario where TDP-43 overexpression may be handled by the proteasome, which when further impaired by our dominant-negative approach contributes to enhanced toxicity.
Figure 7.
TDP-43 toxicity is modulated by the proteasome, HSP70 activities and the apoptosis pathway. (A and E) Compound eyes expressing hwt (A) or A315T mutant hTDP-43 (hA315T, E) at 25°C, in 15-day-old adults, exhibit age-dependent depigmentation. Genotypes as indicated on the left, and interacting genes on the top. All transgenes expressed with GMR-Gal4. (B and F) Coexpression of prosβ enhances the depigmentation phenotype due to TDP-43 overexpression (arrows). (C, D, G and H) Coexpression of Hsp70 (C and G) or the p35 caspase inhibitor (D and H) alleviates the eye depigmentation phenotypes due to TDP-43 overexpression. All comparisons were performed with similarly aged flies. (I–K) Overexpression of prosβ, Hsp70 or p35 alone does not lead to visible eye phenotypes.
To gain further insight into the mechanisms underlying the neurotoxic effects of TDP-43, we also tested whether overexpression of the HSP70 chaperone can rescue the eye phenotypes due to wild-type and A315T overexpression. HSP70 was chosen due to its ability to rescue the toxicity of polyglutamine, α-synuclein and RNA-mediated neurodegeneration in Drosophila models, in some instances by alleviating the presence of protein aggregates (43–45). As seen in Figure 7, overexpression of human HSP70 alleviates the depigmentation phenotype due to hTDP-43 (wild-type and A315T) expression in the eye (compare Fig. 7C and G with A and E, respectively). These data, together with the prosβ interaction, suggest that TDP-43 toxicity may be due to aggregates that pose an overwhelming stress on the proteasome but may be mitigated by excess HSP70 chaperone activity.
Although we have detected clear evidence of cell death in the eye, we wanted to determine whether inhibition of apoptosis can mitigate the eye phenotypes due to wild-type and A315T overexpression. As seen in Figure 7D and H (compare with Fig. 7A and E, respectively), overexpression of the p35 baculovirus protein, a caspase inhibitor (46), rescues, although not perfectly, the TDP-43 phenotypes in the adult eye. These results are consistent with the notion that caspase-mediated apoptotic pathways mediates in part the toxicity due to TDP-43 overexpression in the eye.
DISCUSSION
Despite the discovery of several missense mutations in the RNA-binding protein TDP-43, the mechanisms of neurodegeneration involving the individual alleles remain poorly understood. To uncover the consequences of TDP-43 mutations linked to ALS, we performed a direct in vivo comparison of the neuronal and functional phenotypes resulting from overexpression of wild-type and the A315T mutant TDP-43 in the Drosophila nervous system. Expression of either allele (wild-type or A315T) of TDP-43 in photoreceptor and motor neurons led to effects on viability, locomotor function and survival. In the developing retina, overexpression of the TDP-43 variants used in this study resulted in the formation of cytoplasmic and axonal aggregates accompanied by neuronal loss. The eye tissue showed the highest sensitivity to overexpression of TDP-43 and revealed the most dramatic alterations in the subcellular localization of TDP-43, resembling those found in postmortem ALS tissues (9,11). When TDP-43 variants were expressed in motor neurons, we also found several hallmark features of ALS, including cytoplasmic aggregates, cell death and defects in locomotor ability (Table 1). To our surprise, when expressed at comparable levels, wild-type TDP-43 was more toxic than the A315T mutant in regard to viability, survival, NMJ anatomy and adult climbing behavior but not for larval turning behavior. We also found that by simply increasing expression of the mutant allele we were able to recapitulate several, although not all, phenotypes resulting from overexpression of wild-type TDP-43. These include effects on viability, and select NMJ features such as decreased synaptic area and malformed synaptic boutons that may be more sensitive to mutations in TDP-43 than others (e.g. axon branching). This is particularly interesting because, on the one hand, it shows that TDP-43 is inherently toxic, consistent with previous in vivo and in vitro studies (22,23,26,28,29,33,47,48), whereas, on the other hand, our data show that wild-type and A315T mutant TDP-43 exert differential toxicity when evaluated by a repertoire of cellular and behavioral assays. These results emphasize the need for detailed studies of individual missense mutations in TDP-43, in particular those that mimic the mutations identified in ALS patients. Such studies are needed to identify allele-specific neuronal and synaptic defects that could provide clues to their mechanism of action and lead to the development of personalized therapeutic interventions.
We found that when expressed at comparable levels, the TBPH variants were more toxic than their human counterparts. A possible explanation for this difference is provided by the fact that the Drosophila genome encodes an additional, previously unreported homolog of TDP-43, namely CG7804. This locus encodes a second, highly homologous TDP-43 protein in Drosophila that contains the RNA-binding domains RRM1/2 but lacks the C-terminal glycine-rich region. The function of this predicted, truncated, additional TDP-43 protein, as well as the significance of its loss during evolution, remains to be investigated. Although the fly and hTDP-43 pathways may not be identical, most differences seem to be mitigated by the fact that by simply expressing higher levels of hA315T (hA315T HE) we can recapitulate several of the neuronal and functional phenotypes resulting from wild-type TBPH overexpression, the most toxic of the transgenes in this study. Thus, dosage appears to directly correlate with toxicity.
TDP-43 has been previously shown to be required for the proper morphology of the NMJ synapse (23,25) and our findings further support this. In addition, our morphological analyses revealed novel phenotypes, including the reduced area occupied by synaptic vesicles, which appears to be most sensitive to alterations in TDP-43 (both wild-type and A315T) and suggests the presence of functional defects at the synapse. Indeed, larval turning assays, which rely on complex motor coordination along the anterior–posterior and dorsal–ventral axes of the larvae, showed that both the wild-type and A315T alleles affect locomotor ability. It is intriguing that larval turning is the only assay in which the A315T mutant shows higher toxicity than the wild-type allele and it remains to be seen what are the molecular mechanisms underlying this effect. Additional evidence for TDP-43′s effect on locomotor function is provided by the adult climbing assays. Although these results suggest that TDP-43 overexpression affects synaptic function, more work is needed to determine the underlying defects at the NMJ synapse. For example, it would be interesting to determine what specific aspects of synaptic transmission may be affected by altering TDP-43 function in presynaptic motor neurons. Knowing which parameters of synaptic function are impaired due to expression of missense mutations in TDP-43 will provide important clues to the mechanisms underlying the pathology of ALS.
Our results indicate that cytoplasmic aggregates are not required for motor neuron death, consistent with previous findings using hwt (24). Upon wild-type TBPH overexpression, motor neuron death was sparsely detected in larval ventral ganglia before lethality ensued. When hwt was overexpressed, adult motor neuron numbers were significantly reduced in thoracic ganglia and we found strong evidence for apoptotic death. In contrast, overexpression of A315T TBPH led to barely detectable apoptosis, and hA315T resulted in some cells positive for TUNEL staining. Surprisingly, apoptosis detection was also marginal in the ventral ganglia of the higher expressing hA315T HE larvae. Although our findings on the extent of apoptotic death may not be in full agreement with previous reports (22,23), it is likely that differences in expression levels and type of neuron or animal model studied may account for the differences observed. We would also like to note that in the case of the Drosophila transgenes, their effect on viability may have overshadowed the motor neuron phenotypes. For example, overexpression of TBPH, which overall has more pronounced effects than the hTDP-43 variants, may have led to lethality due to the inability of the transgenic larvae to feed properly. Indeed, the wild-type TBPH expressing larvae appeared ‘skinny’ and developmentally delayed. The TBPH transgenics that died before reaching adulthood may have succumbed to starvation and/or delayed growth, thus precluding us from detecting extensive motor neuron death, which may otherwise have been observed later in development. This scenario is further substantiated by our findings with the hTDP-43 variants, which exhibited motor neuron apoptosis in the adult but not larval nervous system.
Several questions remain regarding the molecular mechanisms that mediate the dominant effect of TDP-43 overexpression. How can overexpression of wild-type TDP-43 mimic a loss-of-function phenotype? One possibility is that excess protein is sequestered in cytoplasmic or nuclear aggregates, which may affect the stoichiometry of physiological TDP-43 complexes, thus mimicking a loss-of-function phenotype (49). Overall, our data suggest that the precise amount of TDP-43 expression is critical to proper neuronal and synaptic function and alterations in TDP-43 levels and/or function are responsible for the observed phenotypes. The dominant effects of TDP-43 overexpression are not unlike those obtained with other RNA-binding proteins including FMRP and Argonaute 1, which result in visible phenotypes when overexpressed in the eye (50,51). Thus, we suggest that the phenotypes resulting from wild-type and mutant TDP-43 overexpression stem from defects in gene expression, in particular the dysregulation of RNA targets that are just beginning to be discovered (52).
The genetic interactions with the proteasome support the notion that the insoluble TDP-43 products that arise from overexpression or the presence of missense mutations (3,23,30,53) may normally be processed by the proteasome degradation machinery. By impairing the function of the proteasome, insoluble TDP-43 products may not be cleared efficiently, which could account for the enhanced toxicity we observed in the eye. Although this result is not entirely unexpected and previous studies have shown that pharmacological inhibition of the proteasome enhances the accumulation of C-terminal fragments of TDP-43 (3), our results provide an in vivo demonstration for the involvement of the proteasome in TDP-43 neurotoxicity. Future biochemical studies will focus on whether the amount of insoluble TDP-43 products is increased upon proteasome impairment or whether additional mechanisms account for the enhanced neurotoxicity we observed in the eye. Although it remains to be seen whether cytoplasmic and axonal TDP-43 aggregates are cleared by HSP70 coexpression, our discovery that excess HSP70 chaperone alleviates the phenotype of TDP-43 in the eye supports the notion that TDP-43 misfolding underlies its toxicity in the eye. Not surprisingly, given the dramatic cell loss evident in the retina, we found that inhibition of caspases through overexpression of the p35 baculovirus protein also alleviates, although does not completely rescue, the phenotype of TDP-43 overexpression in the eye. Taken together, these findings indicate that commonalities may exist between TDP-43 and other known proteinopathies and suggest that therapies based on targeting the proteasome function, HSP70 chaperone activity or caspases may be effective for ALS as well as other neurodegenerative disorders in which these pathways have been implicated.
In summary, our results support previous findings and provide further evidence that wild-type and mutant TDP-43 overexpression in Drosophila recapitulates hallmark aspects of ALS pathology. Perhaps the most surprising result is that, when expressed at comparable levels, wild-type TDP-43 exerted higher toxicity than the A315T allele, in regard to viability, survival, neuronal loss and adult locomotor function but not larval locomotor behavior. Although a mouse model of A315T overexpression exhibits ubiquitin-positive cytoplasmic aggregates and other features of ALS pathology (28), a recent article reports the presence of rather weak pathological hallmarks in an ALS patient carrying this allele (54). These findings suggest that the genetic background may influence TDP-43 phenotypes and are consistent with a recent report that ataxin 2 constitutes a risk factor for ALS (47). Taken together, our results and these published data indicate that detailed phenotypic analyses of different mutants in animal models such as Drosophila are critical for elucidating the biology of TDP-43 and the mechanisms of neurodegenerative disease. In addition, our work suggests that perhaps animal models based on individual missense mutations that mimic those found in human patients will serve as better models for genetic and drug screens that can lead to novel effective and potentially personalized therapies.
MATERIALS AND METHODS
Drosophila genetics
All Drosophila stocks and crosses were kept on standard yeast/cornmeal/molasses food at 25°C unless otherwise noted. TBPH cDNA GH09868 was obtained from the Drosophila Genome Project in the pOT2 vector. Following PCR amplification and site-directed mutagenesis (QuikChange II, Stratagene), the inserts (wild-type and A315T) were cloned into the pUAST destination vectors pTGW and pTRW (pUAST with N-terminal GFP and RFP, respectively; Drosophila Genome Resource Center), using the Gateway Technology (Invitrogen). hTDP-43, wild-type and A315T with YFP C-terminal tag (in pRS416 yeast expression vector, from A. Gitler) were cloned into the NotI and KpnI sites of the pUAST germline transformation vector. Transgenic lines were mapped and balanced using standard genetic techniques and include GFP TBPH wild-type, RFP TBPH wild-type and A315T, hTDP-43 wild-type YFP, hTDP-43 A315T YFP. Gal4 drivers used in this study include the eye-specific GMR Gal4 R13 and the motor neuron driver D42 Gal4 (35). TBPH RNAi lines were obtained from the Vienna Drosophila RNAi Center (lines w1118; P{GD6943}v38377 and w1118;P{GD6943}v38377) and used to generate a double-RNAi recombinant stock. GMR Gal4 wild-type hTDP-43 and GMR Gal4 hA315T stocks were generated using standard meiotic recombination techniques. For genetic interactions, the following stocks were used: (i) w1118;P{w[+mC]=UAS-Prosbeta2[1]}1B (dominant-negative form of the 20S proteasome core subunit); (ii) w1118;P{w[+mC]=UAS-Hsap\HSPA1L.W}53.1/CyO (human molecular chaperone Hsp70) and (iii) w[*];P{w[+mC]=UAS-p35.H}BH2 (baculovirus-derived apoptosis inhibitor).
Western blots
To determine the relative expression levels of the various TDP-43 transgenes used in this study, Drosophila heads were collected from adults expressing wild-type and A315T mutant, Drosophila or hTDP-43, using the GMR Gal4 driver. Following homogenization in 2× Laemmli buffer, the lysates were resolved on SDS–PAGE and then transferred to a PVDF membrane (Millipore). Drosophila and hTDP-43 transgenes were detected using rabbit polyclonal anti-GFP (Invitrogen) at 1/3000, rabbit polyclonal anti-TARDBP (Abcam) at 1/1250 or rabbit polyclonal anti-TBPH at 1/3000. Tubulin was used as a loading control and was detected using a mouse monoclonal anti-tubulin antibody (Millipore) at 1/1000. The secondary antibody used was goat anti-mouse- or goat anti-rabbit-conjugated HRP, as appropriate, at 1/1000 (Thermo Scientific). Proteins of interest were visualized using SuperSignal West Femto Substrate (Thermo Scientific). Protein levels were quantified using NIH Image software.
Immunohistochemistry and imaging
Adult fly eyes were imaged with a Leica MZ6 microscope equipped with an Olympus DP71 camera and controlled by Olympus DP Controller and Olympus DP Manager software. Individual images were processed using Adobe Photoshop CS2 (Adobe).
Larval NMJ preparations have been described previously (55). Briefly, wandering third instar larvae were filleted, pinned out on Sylgard dishes, fixed in 3.5% formaldehyde in PBS, pH 7.2, for 20 min and then permeabilized with 0.1% Triton X-100. Following treatment in a blocking agent consisting of 2% BSA and 5% NGS, the fillets were stained with anti-DCSP2 at 1/300 (DSHB), anti-HRP-FITC at 1/50 (Sigma) and anti-Dlg polyclonal at 1/900 (gift of Peter Bryant). Secondary antibodies at 1/1000 were from Molecular Probes. Larval muscles 6 and 7 were imaged in abdominal segments A3 and A6 on a Nikon PCM 2000 confocal microscope and were displayed as a projection of 1 µm serial sections. Type 1b, 1s, and satellite boutons as well as branches were manually counted and total CSP area was determined using Metamorph Image Analysis software (Universal Imaging). All measurements were divided by total muscle area to take into account variations in the size of the individual larvae.
Eye discs and larval ventral ganglia were dissected and prepared as above. Eye discs were stained with either rhodamine phalloidin (1/300), FITC phalloidin (1/150; Sigma) or Alexa 647 phalloidin (1/300) (Molecular Probes) as well as Hoechst 33342 (Invitrogen) at 1/10 000 and, if appropriate, anti-GFP-FITC (Rockland) at 1/200. Ventral ganglia were stained as above except that instead of phalloidin, they were stained with anti-HRP-FITC or TRITC (Sigma) at 1/50. They were imaged on a Zeiss Meta 510 confocal microscope and displayed as projections of 1 µm serial Z sections.
Eye sections
Adult eyes were embedded and sectioned as described previously (51). In brief, following the removal of the proboscis, adult heads were fixed in Trump's fixative (4% paraformaldehyde, 1% glutaraldehyde, 100 mm cacodylate buffer, pH 7.2, 2 mm sucrose, 0.5 mm EGTA) overnight. The following day, the samples were washed three times in 100 mm cacodylate buffer with 264 mm sucrose, then placed in a 0.5% OsO4 in 100 mm cacodylate buffer for 1 h and rinsed for 10 min in 100 mm cacodylate buffer with 264 mm sucrose. Following a dehydration protocol using ethanol and propylene oxide, samples were embedded in Embed 812 and baked at 65°C overnight. Using a Reichert Jung Ultracut E Ultramicrotome, 1 µm thick plastic sections were cut and stained with 1% toluidine blue in 1% sodium borate. Images were acquired using a Zeiss Axioplan microscope equipped with an Olympus DP71 camera and controlled by Olympus DP Controller and Olympus DP Manager software.
TUNEL assay
TUNEL assays were performed on larval ventral and adult thoracic ganglia, using the In Situ Cell Death Detection Kit (Roche, Indianapolis, IN, USA). Briefly, ganglia were fixed in semi-intact preparations with 3.5% formaldehyde in PBS, pH 7.2, for 20 min. After permeabilization with 0.05% Triton X-100, the ganglia were treated with 10 µg/ml proteinase K (Fermentas) for 10 min at 37° C. The ganglia were then dissected into microtiter dishes. The positive control for the TUNEL reaction consisted of ganglia treated with 2 N HCl for 30 min. The TUNEL reaction was carried out for 1.5 h at 37° C as per manufacturer's instructions using, as appropriate, a TMR-Red or an FITC label. Ganglia were then stained with Hoechst 33342 (Invitrogen) at 1/10 000 and, if appropriate, anti-GFP-FITC (Rockland) at 1/200. Preps were mounted in 4% N-propyl gallate in 95% glycerol and imaged on a Zeiss Meta 510 confocal microscope. Ganglia were displayed as projections of 1 µm serial Z sections.
Locomotor function assays
Larval turning assays
Third instar wandering larvae were placed on a grape juice plate at room temperature. After becoming acclimated, crawling larvae were gently turned onto their backs (ventral side up) and monitored until they were able to turn back (dorsal side up) and continue their forward movement. The amount of time that it took each larva to complete this task was recorded. Three to five trials of 8 to 10 larvae were performed for each genotype. Student's t-test was performed to assess statistical significance.
Climbing assays
Expression of the Drosophila transgenes using D42 Gal 4 was lethal before adulthood, primarily at the pupal stage. Therefore, climbing assays were performed with adults expressing wild-type and A315T mutant hTDP-43 in motor neurons. Owing to effects on viability from these transgenes as well, flies were raised at both 25 and 18°C. Ten (1-day-old) adult males of each genotype were collected and tested for their climbing ability starting when 2 DO and every 4 days thereafter, until they reached 30 days after eclosion. Five to ten such cohorts (50–100 males) were tested for each genotype. To assess their climbing ability, the flies were transferred to an empty vial marked at 5cm from the bottom (40). After being allowed to acclimate to the new environment (∼30s), flies were gently tapped down to the bottom of the vial and then the time it took each fly to pass the 5cm mark was recorded. All flies that climbed the 5cm up the vial in 18 s or less passed, whereas those that could not climb that high or took longer than 18 s failed. Both the number of flies that passed and the number of flies surviving were recorded each time the test was performed. The climbing index for each genotype was calculated as the number of flies that passed the climbing test, normalized to the number of survivors on the day of the test. Survivability was calculated by dividing the number of flies alive on each day by the number alive on day 2.
SUPPLEMENTARY MATERIAL
FUNDING
This work was supported by the Jim Himelic Foundation and by the Muscular Dystrophy Association (in part through support from Mr and Mrs Dunham in memory of their daughter Susan Hope Nearing Dunham; MDA173230 to D.C.Z.). Partial support was also provided by the Undergraduate Biology Research Program funded by the Howard Hughes Medical Institute (HHMI 5205889 to J.T. and B.G.).
Supplementary Material
ACKNOWLEDGEMENTS
We are indebted to the Himelic family for their generous support. We also thank Drs Bruce Coull, David Labiner and Katalin Scherer (Department of Neurology, University of Arizona) for their support and suggestions; Aaron Gitler (University of Pennsylvania) for the human TDP-43 constructs; Fabian Feiguin and Francisco Baralle (ICGEB, Trieste) for the TBPH antibody; Gabrielle Boulianne (University of Toronto) for helpful discussions and sharing unpublished results; Peter Bryant (University of California, Irvine) for the polyclonal Dlg antibody; Drosophila Genome Resource Center, Bloomington Stock Center and the Vienna Drosophila RNAi Center for reagents; Iowa Hybridoma Bank for CSP antibodies; Dan Marenda (Drexel University) for suggestions with the climbing assays; Genetics Services for generating transgenic flies; Gio Bosco (Department of Molecular and Cellular Biology, University of Arizona) for access to the Olympus DP71 camera; John Hildebrand (Department of Neuroscience, University of Arizona) for the use of the Zeiss microscope/Olympus imaging system; Sam Ward (Department of Molecular and Cellular Biology, University of Arizona) for the MetaMorph software; Carl Boswell (Department of Molecular and Cellular Biology, University of Arizona) for help with confocal imaging; Patty Jansma (Department of Neuroscience, University of Arizona) for help with adult eye sections; Annette Estevez for helpful comments on the manuscript.
Conflict of Interest statement. None declared.
REFERENCES
- 1.Banks G.T., Kuta A., Isaacs A.M., Fisher E.M. TDP-43 is a culprit in human neurodegeneration, and not just an innocent bystander. Mamm. Genome. 2008;19:299–305. doi: 10.1007/s00335-008-9117-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosen D.R., Siddique T., Patterson D., Figlewicz D.A., Sapp P., Hentati A., Donaldson D., Goto J., O'Regan J.P., Deng H.X., et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- 3.Kabashi E., Valdmanis P.N., Dion P., Spiegelman D., McConkey B.J., Vande Velde C., Bouchard J.P., Lacomblez L., Pochigaeva K., Salachas F., et al. TARDBP mutations in individuals with sporadic and familial amyotrophic lateral sclerosis. Nat. Genet. 2008;40:572–574. doi: 10.1038/ng.132. [DOI] [PubMed] [Google Scholar]
- 4.Beleza-Meireles A., Al-Chalabi A. Genetic studies of amyotrophic lateral sclerosis: controversies and perspectives. Amyotroph. Lateral Scler. 2009;10:1–14. doi: 10.1080/17482960802585469. [DOI] [PubMed] [Google Scholar]
- 5.Vance C., Rogelj B., Hortobagyi T., De Vos K.J., Nishimura A.L., Sreedharan J., Hu X., Smith B., Ruddy D., Wright P., et al. Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science. 2009;323:1208–1211. doi: 10.1126/science.1165942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valdmanis P.N., Daoud H., Dion P.A., Rouleau G.A. Recent advances in the genetics of amyotrophic lateral sclerosis. Curr. Neurol. Neurosci. Rep. 2009;9:198–205. doi: 10.1007/s11910-009-0030-9. [DOI] [PubMed] [Google Scholar]
- 7.Lagier-Tourenne C., Cleveland D.W. Rethinking ALS: the FUS about TDP-43. Cell. 2009;136:1001–1004. doi: 10.1016/j.cell.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwiatkowski T.J., Jr, Bosco D.A., Leclerc A.L., Tamrazian E., Vanderburg C.R., Russ C., Davis A., Gilchrist J., Kasarskis E.J., Munsat T., et al. Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science. 2009;323:1205–1208. doi: 10.1126/science.1166066. [DOI] [PubMed] [Google Scholar]
- 9.Maekawa S., Leigh P.N., King A., Jones E., Steele J.C., Bodi I., Shaw C.E., Hortobagyi T., Al-Sarraj S. TDP-43 is consistently co-localized with ubiquitinated inclusions in sporadic and Guam amyotrophic lateral sclerosis but not in familial amyotrophic lateral sclerosis with and without SOD1 mutations. Neuropathology. 2009;29:672–683. doi: 10.1111/j.1440-1789.2009.01029.x. [DOI] [PubMed] [Google Scholar]
- 10.Tan C.F., Eguchi H., Tagawa A., Onodera O., Iwasaki T., Tsujino A., Nishizawa M., Kakita A., Takahashi H. TDP-43 immunoreactivity in neuronal inclusions in familial amyotrophic lateral sclerosis with or without SOD1 gene mutation. Acta Neuropathol. 2007;113:535–542. doi: 10.1007/s00401-007-0206-9. [DOI] [PubMed] [Google Scholar]
- 11.Van Deerlin V.M., Leverenz J.B., Bekris L.M., Bird T.D., Yuan W., Elman L.B., Clay D., Wood E.M., Chen-Plotkin A.S., Martinez-Lage M., et al. TARDBP mutations in amyotrophic lateral sclerosis with TDP-43 neuropathology: a genetic and histopathological analysis. Lancet Neurol. 2008;7:409–416. doi: 10.1016/S1474-4422(08)70071-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neumann M., Sampathu D.M., Kwong L.K., Truax A.C., Micsenyi M.C., Chou T.T., Bruce J., Schuck T., Grossman M., Clark C.M., et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- 13.Daoud H., Valdmanis P.N., Kabashi E., Dion P., Dupre N., Camu W., Meininger V., Rouleau G.A. Contribution of TARDBP mutations to sporadic amyotrophic lateral sclerosis. J. Med. Genet. 2009;46:112–114. doi: 10.1136/jmg.2008.062463. [DOI] [PubMed] [Google Scholar]
- 14.Gitcho M.A., Baloh R.H., Chakraverty S., Mayo K., Norton J.B., Levitch D., Hatanpaa K.J., White C.L., III, Bigio E.H., Caselli R., et al. TDP-43 A315T mutation in familial motor neuron disease. Ann. Neurol. 2008;63:535–538. doi: 10.1002/ana.21344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuhnlein P., Sperfeld A.D., Vanmassenhove B., Van Deerlin V., Lee V.M., Trojanowski J.Q., Kretzschmar H.A., Ludolph A.C., Neumann M. Two German kindreds with familial amyotrophic lateral sclerosis due to TARDBP mutations. Arch. Neurol. 2008;65:1185–1189. doi: 10.1001/archneur.65.9.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pamphlett R., Luquin N., McLean C., Jew S.K., Adams L. TDP-43 neuropathology is similar in sporadic amyotrophic lateral sclerosis with or without TDP-43 mutations. Neuropathol. Appl. Neurobiol. 2009;35:222–225. doi: 10.1111/j.1365-2990.2008.00982.x. [DOI] [PubMed] [Google Scholar]
- 17.Rutherford N.J., Zhang Y.J., Baker M., Gass J.M., Finch N.A., Xu Y.F., Stewart H., Kelley B.J., Kuntz K., Crook R.J., et al. Novel mutations in TARDBP (TDP-43) in patients with familial amyotrophic lateral sclerosis. PLoS Genet. 2008;4:e1000193. doi: 10.1371/journal.pgen.1000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sreedharan J., Blair I.P., Tripathi V.B., Hu X., Vance C., Rogelj B., Ackerley S., Durnall J.C., Williams K.L., Buratti E., et al. TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science. 2008;319:1668–1672. doi: 10.1126/science.1154584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson B.S., McCaffery J.M., Lindquist S., Gitler A.D. A yeast TDP-43 proteinopathy model: exploring the molecular determinants of TDP-43 aggregation and cellular toxicity. Proc. Natl Acad. Sci. USA. 2008;105:6439–6444. doi: 10.1073/pnas.0802082105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buratti E., Baralle F.E. Characterization and functional implications of the RNA binding properties of nuclear factor TDP-43, a novel splicing regulator of CFTR exon 9. J. Biol. Chem. 2001;276:36337–36343. doi: 10.1074/jbc.M104236200. [DOI] [PubMed] [Google Scholar]
- 21.Wang I.F., Wu L.S., Chang H.Y., Shen C.K. TDP-43, the signature protein of FTLD-U, is a neuronal activity-responsive factor. J. Neurochem. 2008;105:797–806. doi: 10.1111/j.1471-4159.2007.05190.x. [DOI] [PubMed] [Google Scholar]
- 22.Voigt A., Herholz D., Fiesel F.C., Kaur K., Muller D., Karsten P., Weber S.S., Kahle P.J., Marquardt T., Schulz J.B. TDP-43-mediated neuron loss in vivo requires RNA-binding activity. PLoS One. 2010;5:e12247. doi: 10.1371/journal.pone.0012247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Y., Ray P., Rao E.J., Shi C., Guo W., Chen X., Woodruff E.A., III, Fushimi K., Wu J.Y. A Drosophila model for TDP-43 proteinopathy. Proc. Natl Acad. Sci. USA. 2010;107:3169–3174. doi: 10.1073/pnas.0913602107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanson K.A., Kim S.H., Wassarman D.A., Tibbetts R.S. Ubiquilin modifies TDP-43 toxicity in a Drosophila model of amyotrophic lateral sclerosis (ALS) J. Biol. Chem. 2010;285:11068–11072. doi: 10.1074/jbc.C109.078527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feiguin F., Godena V.K., Romano G., D'Ambrogio A., Klima R., Baralle F.E. Depletion of TDP-43 affects Drosophila motoneurons terminal synapsis and locomotive behavior. FEBS Lett. 2009;583:1586–1592. doi: 10.1016/j.febslet.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 26.Lu Y., Ferris J., Gao F.B. Frontotemporal dementia and amyotrophic lateral sclerosis-associated disease protein TDP-43 promotes dendritic branching. Mol. Brain. 2009;2:30. doi: 10.1186/1756-6606-2-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ash P.E., Zhang Y.J., Roberts C.M., Saldi T., Hutter H., Buratti E., Petrucelli L., Link C.D. Neurotoxic effects of TDP-43 overexpression in C. elegans. Hum. Mol. Genet. 2010;19:3206–3218. doi: 10.1093/hmg/ddq230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wegorzewska I., Bell S., Cairns N.J., Miller T.M., Baloh R.H. TDP-43 mutant transgenic mice develop features of ALS and frontotemporal lobar degeneration. Proc. Natl Acad. Sci. USA. 2009;106:18809–18814. doi: 10.1073/pnas.0908767106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kabashi E., Lin L., Tradewell M.L., Dion P.A., Bercier V., Bourgouin P., Rochefort D., Bel Hadj S., Durham H.D., Velde C.V., et al. Gain and loss of function of ALS-related mutations of TARDBP (TDP-43) cause motor deficits in vivo. Hum. Mol. Genet. 2009;19:671–683. doi: 10.1093/hmg/ddp534. [DOI] [PubMed] [Google Scholar]
- 30.Liachko N.F., Guthrie C.R., Kraemer B.C. Phosphorylation promotes neurotoxicity in a Caenorhabditis elegans model of TDP-43 proteinopathy. J. Neurosci. 2010;30:16208–16219. doi: 10.1523/JNEUROSCI.2911-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brand A.H., Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 32.Arai T., Hasegawa M., Akiyama H., Ikeda K., Nonaka T., Mori H., Mann D., Tsuchiya K., Yoshida M., Hashizume Y., et al. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem. Biophys. Res. Commun. 2006;351:602–611. doi: 10.1016/j.bbrc.2006.10.093. [DOI] [PubMed] [Google Scholar]
- 33.Johnson B.S., Snead D., Lee J.J., McCaffery J.M., Shorter J., Gitler A.D. TDP-43 is intrinsically aggregation-prone and ALS-linked mutations accelerate aggregation and increase toxicity. J. Biol. Chem. 2009;284:20329–20339. doi: 10.1074/jbc.M109.010264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barmada S.J., Skibinski G., Korb E., Rao E.J., Wu J.Y., Finkbeiner S. Cytoplasmic mislocalization of TDP-43 is toxic to neurons and enhanced by a mutation associated with familial amyotrophic lateral sclerosis. J. Neurosci. 2010;30:639–649. doi: 10.1523/JNEUROSCI.4988-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gustafson K., Boulianne G.L. Distinct expression patterns detected within individual tissues by the GAL4 enhancer trap technique. Genome. 1996;39:174–182. doi: 10.1139/g96-023. [DOI] [PubMed] [Google Scholar]
- 36.Koh Y.H., Gramates L.S., Budnik V. Drosophila larval neuromuscular junction: molecular components and mechanisms underlying synaptic plasticity. Microsc. Res. Tech. 2000;49:14–25. doi: 10.1002/(SICI)1097-0029(20000401)49:1<14::AID-JEMT3>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 37.Ranjan R., Bronk P., Zinsmaier K.E. Cysteine string protein is required for calcium secretion coupling of evoked neurotransmission in Drosophila but not for vesicle recycling. J. Neurosci. 1998;18:956–964. doi: 10.1523/JNEUROSCI.18-03-00956.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eaton B.A., Davis G.W. LIM Kinase1 controls synaptic stability downstream of the type II BMP receptor. Neuron. 2005;47:695–708. doi: 10.1016/j.neuron.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 39.Bodily K.D., Morrison C.M., Renden R.B., Broadie K. A novel member of the Ig superfamily, turtle, is a CNS-specific protein required for coordinated motor control. J. Neurosci. 2001;21:3113–3125. doi: 10.1523/JNEUROSCI.21-09-03113.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Le Bourg E., Lints F.A. Hypergravity and aging in Drosophila melanogaster. 4. Climbing activity. Gerontology. 1992;38:59–64. doi: 10.1159/000213307. [DOI] [PubMed] [Google Scholar]
- 41.Neumann M. Molecular neuropathology of TDP-43 proteinopathies. Int. J. Mol. Sci. 2009;10:232–246. doi: 10.3390/ijms10010232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Belote J.M., Fortier E. Targeted expression of dominant negative proteasome mutants in Drosophila melanogaster. Genesis. 2002;34:80–82. doi: 10.1002/gene.10131. [DOI] [PubMed] [Google Scholar]
- 43.Warrick J.M., Chan H.Y., Gray-Board G.L., Chai Y., Paulson H.L., Bonini N.M. Suppression of polyglutamine-mediated neurodegeneration in Drosophila by the molecular chaperone HSP70. Nat. Genet. 1999;23:425–428. doi: 10.1038/70532. [DOI] [PubMed] [Google Scholar]
- 44.Auluck P.K., Chan H.Y., Trojanowski J.Q., Lee V.M., Bonini N.M. Chaperone suppression of alpha-synuclein toxicity in a Drosophila model for Parkinson's disease. Science. 2002;295:865–868. doi: 10.1126/science.1067389. [DOI] [PubMed] [Google Scholar]
- 45.Jin P., Zarnescu D.C., Zhang F., Pearson C.E., Lucchesi J.C., Moses K., Warren S.T. RNA-mediated neurodegeneration caused by the fragile X premutation rCGG repeats in Drosophila. Neuron. 2003;39:739–747. doi: 10.1016/s0896-6273(03)00533-6. [DOI] [PubMed] [Google Scholar]
- 46.Zhou L., Schnitzler A., Agapite J., Schwartz L.M., Steller H., Nambu J.R. Cooperative functions of the reaper and head involution defective genes in the programmed cell death of Drosophila central nervous system midline cells. Proc. Natl Acad. Sci. USA. 1997;94:5131–5136. doi: 10.1073/pnas.94.10.5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Elden A.C., Kim H.J., Hart M.P., Chen-Plotkin A.S., Johnson B.S., Fang X., Armakola M., Geser F., Greene R., Lu M.M., et al. Ataxin-2 intermediate-length polyglutamine expansions are associated with increased risk for ALS. Nature. 2010;466:1069–1075. doi: 10.1038/nature09320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ritson G.P., Custer S.K., Freibaum B.D., Guinto J.B., Geffel D., Moore J., Tang W., Winton M.J., Neumann M., Trojanowski J.Q., et al. TDP-43 mediates degeneration in a novel Drosophila model of disease caused by mutations in VCP/p97. J. Neurosci. 2010;30:7729–7739. doi: 10.1523/JNEUROSCI.5894-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang C., Tan W., Whittle C., Qiu L., Cao L., Akbarian S., Xu Z. The C-terminal TDP-43 fragments have a high aggregation propensity and harm neurons by a dominant-negative mechanism. PLoS ONE. 2010;5:e15878. doi: 10.1371/journal.pone.0015878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jin P., Zarnescu D.C., Ceman S., Nakamoto M., Mowrey J., Jongens T.A., Nelson D.L., Moses K., Warren S.T. Biochemical and genetic interaction between the fragile X mental retardation protein and the microRNA pathway. Nat. Neurosci. 2004;7:113–117. doi: 10.1038/nn1174. [DOI] [PubMed] [Google Scholar]
- 51.Zarnescu D.C., Jin P., Betschinger J., Nakamoto M., Wang Y., Dockendorff T.C., Feng Y., Jongens T.A., Sisson J.C., Knoblich J.A., et al. Fragile X protein functions with lgl and the par complex in flies and mice. Dev. Cell. 2005;8:43–52. doi: 10.1016/j.devcel.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 52.Sephton C.F., Cenik C., Kucukural A., Dammer E.B., Cenik B., Han Y., Dewey C.M., Roth F.P., Herz J., Peng J., et al. Identification of neuronal RNA targets of TDP-43-containing ribonucleoprotein complexes. J. Biol. Chem. 2011;286:1204–1215. doi: 10.1074/jbc.M110.190884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou H., Huang C., Chen H., Wang D., Landel C.P., Xia P.Y., Bowser R., Liu Y.J., Xia X.G. Transgenic rat model of neurodegeneration caused by mutation in the TDP gene. PLoS Genet. 2010;6:e1000887. doi: 10.1371/journal.pgen.1000887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cairns N.J., Perrin R.J., Schmidt R.E., Gru A., Green K.G., Carter D., Taylor-Reinwald L., Morris J.C., Gitcho M.A., Baloh R.H. TDP-43 proteinopathy in familial motor neuron disease with TARDBP A315T mutation: a case report. Neuropathol. Appl. Neurobiol. 2010;36:673–679. doi: 10.1111/j.1365-2990.2010.01121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Estes P.S., Roos J., van der Bliek A., Kelly R.B., Krishnan K.S., Ramaswami M. Traffic of dynamin within individual Drosophila synaptic boutons relative to compartment-specific markers. J. Neurosci. 1996;16:5443–5456. doi: 10.1523/JNEUROSCI.16-17-05443.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.