Abstract
In this study, we have investigated the ability of detoxified Shiga toxin (Stx)-converting bacteriophages Φ3538 (Δstx2::cat) (H. Schmidt et al., Appl. Environ. Microbiol. 65:3855-3861, 1999) and H-19B::Tn10d-bla (D. W. Acheson et al., Infect. Immun. 66:4496-4498, 1998) to lysogenize enteropathogenic Escherichia coli (EPEC) strains in vivo. We were able to transduce the porcine EPEC strain 1390 (O45) with Φ3538 (Δstx2::cat) in porcine ligated ileal loops but not the human EPEC prototype strain E2348/69 (O127). Neither strain 1390 nor strain E2348/69 was lysogenized under these in vivo conditions when E. coli K-12 containing H-19B::Tn10d-bla was used as the stx1 phage donor. The repeated success in the in vivo transduction of an Stx2-encoding phage to a porcine EPEC strain in pig loops was in contrast to failures in the in vitro trials with these and other EPEC strains. These results indicate that in vivo conditions are more effective for transduction of Stx2-encoding phages than in vitro conditions.
Shiga toxin (Stx)-producing Escherichia coli (STEC) strains have emerged as serious food-borne pathogens worldwide that caused large-scale outbreaks of intestinal diseases in developed countries (13, 19). Enterohemorrhagic E. coli (EHEC) O157:H7 is the predominant STEC serotype in the United States (12), United Kingdom (4), and Japan (46), frequently causing a broad spectrum of disease in humans ranging (12) from mild diarrhea to hemorrhagic colitis (36), the latter of which can progress to the hemolytic-uremic syndrome (HUS) (20), whereas other non-O157 serotypes (18) are predominant in continental Europe (4). Systemic disease, such as edema, may also develop as a result of STEC infections in swine (6). Most EHEC O157 strains produce one or more Stx, which are thought to be the major pathogenicity factors of these organisms. Shiga toxins comprise a family composed of Stx1, Stx2, and their variants (10, 11, 16, 23, 31, 34, 40, 48), which can be found in STEC strains isolated from either humans or animals (16, 32, 33).
The stx genes are part of the genome of temperate lambdoid phages, which are integrated in the chromosome of the bacterial host. At present, the ability to produce Stx has been assigned to more than 200 E. coli serotypes, which have been isolated from patients, healthy humans (27), animals, food (7), and water (25). Stx production was observed also in other members of the Enterobacteriaceae, including Citrobacter freundii (39, 44) Enterobacter cloacae (30), Shigella sonnei (42), and Shigella dysenteriae I (43).
In addition to Stx-converting phages, EHEC strains contain further mobile genetic elements. These include a chromosomal pathogenicity island termed locus of enterocyte effacement (LEE), which was first described in the enteropathogenic E. coli (EPEC) O127 strain E2348/69 (21) and a large virulence plasmid (15, 32). LEE encodes proteins responsible for the intimate adherence of EHEC to the intestinal mucosa and for the subsequent characteristic destruction of the microvilli, termed attaching and effacing lesions (24). Sequencing of the E. coli O157:H7 genome revealed the presence of additional pathogenicity islands, the function of which has yet to be elucidated (15, 32).
Similar to EHEC, EPEC strains also possess LEE, but in contrast to EHEC, the EPEC strains do not produce Stx and are not associated with HUS. Nevertheless, they are a major cause of infant diarrhea in nonindustrialized countries and are pathogenic to several animal species (27). Clonal analysis derived from multilocus enzyme electrophoresis suggests that E. coli O157:H7 evolved from a progenitor strain with the serotype O55:H7 (8, 47). Furthermore, the nearly identical sequences of the H7 flagellin gene (35) and eae alleles (22) demonstrate the close relationship between E. coli O157:H7 and E. coli O55:H7.
The dissemination of Stx-encoding phages is the most likely mechanism for the emergence of new STEC serotypes and the intergeneric spread of stx genes. Evidence for this has been provided by Schmidt et al. (38), who were able to lysogenize in vitro a variety of enteric E. coli, including two EPEC strains with a chloramphenicol acetyltransferase (cat) gene-labeled detoxified derivative of an Stx2-encoding phage isolated from E. coli O157. James et al. (17) were also able to lysogenize different wild-type E. coli and Shigella strains in vitro by using a kanamycin resistance (aph3) gene-labeled Stx2-encoding phage derivate originated from an E. coli O157 strain. Acheson et al. (2) were able to transduce a laboratory strain in the murine gastrointestinal tract with a derivative of phage H19B encoding Stx1. However, there have been so far no data about in vivo transduction of stx2 genes into wild-type E. coli strains.
The aim of this study was to establish an in vivo transduction model for wild-type E. coli strains and to examine the putative role of EPEC for the evolution of EHEC by acquisition of stx2 genes by utilizing a pig ligated ileal loop system.
MATERIALS AND METHODS
Bacterial strains and bacteriophages.
The E. coli strains used in this study are listed in Table 1. Three EPEC strains were chosen with different O antigens which were isolated from different sources. Porcine EPEC strain 1390 belonged to serogroup O45, rabbit E. coli strain 355 belongs to serogroup O15, and the human EPEC reference strain E2348/69 belonged to serotype O127:H6:K1 (21). None of these wild-type strains carried the stx1 or stx2 gene. Strain E2348/69 carried EPEC adherence factor (EAF) and was bfp-positive (see Table 4). E. coli strain MC4100 harboring detoxified Stx1 phage H-19B::Tn10d-bla was kindly provided by David Acheson, Boston, Mass. (2). Porcine EPEC O45 strain 1390 was kindly provided by John Fairbrother, Montreal, Canada (49), and was recognized as a colicin producer. The E. coli K-12 derivative C600 was used as a host strain for the propagation of phages. Phages Φ3538(Δstx2::cat) and H-19B::Tn10d-bla were induced by mitomycin C and purified as previously described (38). High-titer stock lysates of Φ3538(Δstx2::cat) and H-19B::Tn10d-bla phage were propagated from single plaques according to the method described by Sambrook et al. (37) for phage λ.
TABLE 1.
Description of E. coli strains used in this study
| Strain | Description | Reference |
|---|---|---|
| 3538 (Δstx2::cat) | Human E. coli O157:H7 strain harboring the detoxified phage φ3538 (stx2::cat) | 38 |
| MC4100 H19B-Ap-1 | E. coli K-12 strain harboring the detoxified phage H-19B::Tn10d-bla | 2 |
| 1390 | Porcine EPEC strain of serogroup O45 | 49 |
| E2348/69 | Human EPEC strain of serotype O127:H6:K1 | 21 |
| 355 | Rabbit EPEC strain of serogroup O15 | Present study |
| C600 | Laboratory strain | 37 |
TABLE 4.
MIC values of EPEC recipient 1390, phage donor 3538 (Δstx2::cat), and transductant E-12, DJ-04, and ME-08 E. coli strains
| Strain | MIC (μg/ml) ofa:
|
||||
|---|---|---|---|---|---|
| Nal | Cam | Tc | Amp | Sm | |
| 1390 | >100 | ≤4 | >40 | ≤6 | >60 |
| 3538 (Δstx2::cat) | ≤50 | >30 | ≤3 | ≤6 | ≤8 |
| E-12 | >100 | >30 | >40 | ≤6 | >60 |
| DJ-04 | >100 | >30 | >40 | ≤6 | >60 |
| ME-08 | >100 | >30 | >40 | ≤6 | >60 |
Abbreviations: Nal, nalidixic acid; Cam, chloramphenicol; Tc, tetracycline; Sm, streptomycin; Amp, ampicillin.
Selection of rifampin-resistant E. coli mutants.
Selection of spontaneous rifampin-resistant mutants was performed on Luria-Bertani (LB) agar plates containing 100 μg of rifampin (Pharmachim)/ml, and 100 μl of a culture of a rifampin-sensitive strain was spread onto the surface of a rifampin-containing LB agar plate. After overnight incubation at 37°C Rifr colonies were isolated and purified with a second selection on rifampin agar. One of these Rifr colonies was stored for further experiments.
In vitro transduction experiments. One hundred microliters of log-phase cultures of EPEC or E. coli K-12 recipient strains containing 107 CFU were mixed with 100 μl of a phage stock solution containing 104, 105, 106, or 107 PFU of phage. After 20 min of incubation at room temperature, 1 ml of LB broth was added to the cultures and incubated without shaking for 4 h at 37°C. Then they were transferred to 4 ml of fresh LB broth containing 30 μg of chloramphenicol/ml or 100 μg of ampicillin/ml, and selective enrichment of lysogenic bacteria was performed for 16 h at 37°C with shaking at 180 rpm. The bacteria were then harvested by centrifugation, transferred, and spread onto LB agar plates containing selective agents. To isolate Φ3538(Δstx2::cat) transductants, 30 μg of chloramphenicol/ml was mixed with either 50 μg of nalidixic acid/ml, when EPEC strain 1390 or E2348/69 was used as a recipient, or 100 μg of rifampin/ml. To isolate H-19B::Tn10d-bla transductants, 100 μg of ampicillin/ml was combined either with rifampin or nalidixic acid as described above. For selection of E. coli C600, 30 μg of streptomycin/ml was used. Colonies grown on selective agar after overnight incubation were investigated by PCR with stx2-specific primers HSB1 and HSB3 (38) and stx1-specific primers B54 and B55 (5).
Transduction in pig ligated ileal loops.
Phages Φ3538(Δstx2::cat) and H-19B::Tn10d-bla were induced with mitomycin C from the donor strains as described above and diluted to 3 × 107 CFU/ml. These diluted cultures were mixed to an equal amount of the potential recipient bacteria. To ensure phage absorption, these mixtures were incubated at room temperature for 20 min and kept on ice before injecting the culture to a 10-cm ligated ileal loop of 6-week-old weaned piglets. Loops were created by laparotomy in deep anesthesia and kept in postsurgical comfort as described before (26). Piglets were sacrificed 14 to 18 h after surgery, and the loop contents were spread onto LB agar plates containing different combinations of antibiotics. Tetracycline (40 μg/ml) and nalidixic acid (50 μg/ml) were used as selecting markers for strains 1390 and E2348/69, respectively, with combinations of 30 μg of chloramphenicol/ml (Δstx2::cat) and 100 μg of ampicillin/ml (stx1::bla). Twenty-four hours after incubation, the surviving colonies were tested for lactose fermentation and colicin production. Colonies with proper phenotypic markers were tested by PCR as described above. Altogether, 4 loops in 4 pigs were used in these experiments.
Phenotypic methods.
Colicin production was tested as described previously (1) by using an E. coli K-12 strain sensitive against a wide range of colicins.
O-antigen was confirmed with specific hyperimmune sera by slide agglutination.
Antibiotic resistance was examined by the disk diffusion method with the following antibiotics: streptomycin, chloramphenicol, tetracycline, ampicillin, kanamycin, gentamicin, nalidixic acid, and rifampin (Oxoid).
MICs were determined on LB agar plates containing serial dilutions of the following antibiotics: nalidixic acid (6.25 to 100 μg/ml), chloramphenicol (3.75 to 30 μg/ml), tetracycline (2.5 to 40 μg/ml), ampicillin (6.25 to 100 μg/ml), and streptomycin (7.5 to 60 μg/ml). Bacterial cultures (104 CFU) were spread onto the surface of the agar plates, and after overnight incubation, the concentration which caused reduced growth was considered the MIC.
Biochemical profiles of the donor and transductants were determined with the API identification (BioMerieux) program (rapid ID32E).
DNA methods. (i) PCR.
Potential phage recipient strains were characterized by using published PCR primer pairs B54-B55 for stx1, B56-B57 for stx2, and B52-B53 for eae (5), EAF1-EAF2 for EAF (9), and BFP1-BFP2 for bfp (14) with the respective protocols. Potential transductants were screened with primer pairs B54-B55 for stx1 (5) and HSB1-HSB3 (38) for stx2::cat.
Screening for the occupation of potential phage integration sites wrbA (32), yehV (32, 41), yecE (32), and sbcB (29) was performed by PCR under conditions described in Table 2. PCR was performed in a total volume of 50 μl containing 5 μl of template DNA, 5 μl of 10-fold-concentrated polymerase-synthesis buffer (Applied Biosystems Applera, Weiterstadt, Germany), 30 pmol of each primer, 200 μmol of each deoxynucleoside triphosphate, and 2.5 U of Taq DNA polymerase (Applied Biosystems).
TABLE 2.
PCR primers and conditions used in this study
| Primer | Sequence (5′-3′) | PCR conditions (temp [°C], time [s]) fora:
|
PCR product size (kb) | Reference | ||
|---|---|---|---|---|---|---|
| Denaturing | Annealing | Extension | ||||
| wrbA1 | ATG GCT AAA GTT CTG GTG | 94, 30 | 47, 60 | 72, 60 | 600 | This study |
| wrbA2 | CTC CTG TTG AAG ATT AGC | |||||
| sbcB1 | CAT GAT CTG TTG CCA CTC G | 94, 30 | 47, 60 | 72, 60 | 1,800 | 29 |
| sbcB2 | AGG TCT GTC CGT TTC CAC TC | |||||
| yehV-for | AAG TGG CGT TGC TTT GTG AT | 94, 30 | 54, 60 | 72, 60 | 340 | 41 |
| yehV-rev | AAC AGA TGT GTG GTG AGT GTC TG | |||||
| yecD-fwd | CGA AGA CGC CTG TAG TGC C | 94, 30 | 47, 60 | 72, 60 | 1,371 | This study |
| yecN-rev | CGC AGG GAG AAA ACC AAC TC | |||||
Each PCR was preceded by an initial denaturing step for 5 min at 94°C and followed by a final extension step after 30 cycles for 5 min at 72°C.
(ii) DNA sequencing.
The stx2::cat PCR-amplified fragments were purified by a PCR purification kit (Qiagen), and the nucleotide sequence of the amplification products was determined by using HSB1 and HSB3 PCR primers with the Dye Deoxy Terminator cycle sequencing kit with an ABI Prism 377 DNA automatic sequencer (Applied Biosystems Applera).
(iii) RFLP.
Restriction fragment length polymorphism (RFLP) of PCR-amplified fragments was performed with restriction endonuclease PvuII (Promega) after purification of the PCR product with a QIAquick PCR purification kit (Qiagen) as described by the producer.
(iv) Plaque hybridization.
Phage lysates prepared from the strain 3538 (Δstx2::cat) donor, recipient, and transductant strains after mitomycin C induction were mixed with E. coli C600 indicator bacteria in soft agar and poured onto LB agar. Plaques were transferred to nylon membranes and hybridized with a digoxigenin-labeled Δstx2::cat gene probe as described previously (38).
RESULTS
In vitro transduction of Stx-encoding phages to selected EPEC strains. (i) Characterization of recipient strains.
To find selection markers for transduction experiments, we have characterized potential recipient strains for Stx phage transduction. Rabbit E. coli strain 355 was sensitive to the tested antibiotics. To promote the selection in the transduction experiments with strain 355, we isolated a spontaneous Rifr mutant derivative (355-Rifr) from this strain as described in Materials and Methods. All of these phenotypic and the main genotypic characteristics are summarized in Table 3.
TABLE 3.
Phenotypic and genotypic characteristics of E. coli strain used in this study
| Strain | Presence of genotypic characteristic:
|
Presence of phenotypic characteristica:
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| stx1 | stx2 | eae | EAF | bfp | Lac | Col | lys | Resistance | |
| 3538 (Δstx2::cat) | − | + | − | − | − | + | − | + | Nal, Cam |
| MC4100 H19B-Ap1 | + | − | − | − | − | − | − | + | Sm, Amp |
| 1390 | − | − | + | − | − | + | + | + | Nal, Tc, Sm |
| E2348/69 | − | − | + | + | + | + | − | + | Nal, Sm, |
| 355 | − | − | + | − | − | + | − | + | Sensitive |
| C600 | − | − | − | − | − | + | − | − | Sm |
Abbreviations: Lac, lactose; Col, colicin producer; lys, lysogenic; Nal, nalidixic acid; Cam, chloramphenicol; Tc, tetracycline; Sm, streptomycin; Amp, ampicillin.
Four independent in vitro transduction experiments with different multiplicities of infection were carried out with the Stx mutant phages. In these experiments, 107 bacteria were infected with different amounts of phage, giving multiplicities of infection of 1, 10−1, 10−2, and 10−3. In these experiments, we observed variable amounts of surviving colonies on the selective LB agar plates ranging from none to about 500. Most of these colonies were tested by PCR with stx1-specific primers B54 and B55 and stx2::cat-specific primers HSB1 and HSB3. These investigations showed that none of the recipients became lysogenic for any of the Stx mutant phages. All surviving colonies were probably not transductants but spontaneous ampicillin-resistant mutants of the recipients.
(ii) Transduction of Stx mutant phages in the porcine ligated ileal loop system.
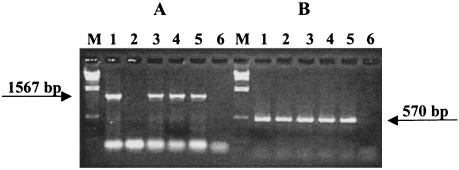
To investigate the ability of Stx2-converting bacteriophage Φ3538 (Δstx2::cat) to lysogenize EPEC strains in vivo, we conducted transduction experiments in ligated ileal loops of 6-week-old weaned piglets as described in Materials and Methods. By using porcine EPEC O45 strain 1390 (Tcr Col+) as a recipient, we isolated many Tcr and Camr lactose-fermenting (Lac+) colonies. As an additional recipient-specific marker, the colicin-production of the Tcr Camr Lac+ colonies was investigated. In two independent experiments, we tested 4 (Tcr Camr Lac+ Col+) and 25 (Tcr Camr Lac+ Col+) colonies with primers HSB1 and HSB3. Three PCR-positive transductants lysogenized with Φ3538 (Δstx2::cat) were identified. These transductant strains were derived from two different loops representing two pigs and were designated E-12, DJ-04, and ME-08. The eae-specific PCR revealed that the transductant strains all harbor the eae gene (Fig. 1). Transductants E-12, DJ-04, and ME-08 proved to be of serogroup O45. The biochemical profiles of the transductants and parental strains were identical as analyzed by the API identification program. Restriction of the HSB1-HSB3 PCR products of the transductants and wild-type strains with PvuII revealed the same pattern (Fig. 2). Nucleotide sequence analysis also confirmed the presence of stx2::cat in the transductants. Furthermore, investigation of MICs gave further evidence for the functional integration of Φ3538 (Δstx2::cat) into the host strain 1390 (Table 4).
FIG. 1.
Agarose gel electrophoresis of stx2::cat-specific (A) and eae-specific (B) PCR products. Samples: M, λ phage DNA digested with HindIII; 1, stx2::cat donor 3538, eae+; 2, EPEC recipient strain 1390, eae+; 3, transductant E-12 (stx2::cat), eae+; 4, transductant DJ-04 (stx2::cat), eae+; 5, transductant ME-08 (stx2::cat), eae+; 6, E. coli C600 (negative control).
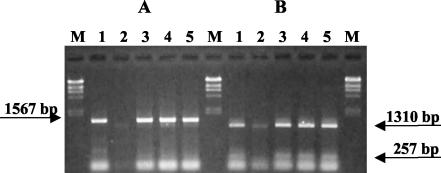
FIG. 2.
RFLP of stx2::cat-specific PCR products. The undigested (A) and PvuII-digested (B) stx2::cat amplicons were electrophoresed in a 1% agarose gel. Samples: M, λ phage DNA digested with HindIII; lane 1, strain 3538; 2, strain 1390; 3, strain E12; 4, strain DJ-04; 5, strain ME-08. The PvuII-digested stx2::cat-specific PCR uniformly yielded two fragments of 257 and 1,310 bp, respectively.
Under the same experimental conditions, we were not able to transduce the EPEC reference strain E2348/69 with phage Φ3538 (Δstx2::cat).
By repeating these loop experiments with E. coli H-19B::Tn10d-bla as the phage donor and with the same recipient strains as mentioned above, we could not isolate any transductant of strains 1390 and E2348/69.
(iii) Induction of Φ3538 (Δstx2::cat) from the lysogens.
Transductant strains E-12, DJ-04, and ME-08 and, as a control, bacterial host strain 1390 were investigated for the ability to produce infectious Φ3538 (Δstx2::cat) particles after induction with mitomycin C by the plaque method. Plaques were then hybridized with stx-specific probes. In these experiments, large amounts of phage were induced from the recipient and transductant strains, but none of the plaques hybridized with the stx2::cat-specific probe, suggesting that the plaques consist of phage other than Φ3538 (Δstx2::cat).
(iv) Chromosomal integration site of Φ3538 (Δstx2::cat).
Bacteriophage Φ3538 (Δstx2::cat) was originally isolated from E. coli O157:H7 strain 3538/96. The wrbA and sbcB genes have been described as integration sites for Stx2-encoding phages in E. coli O157:H7 (28, 31). This prompted us to screen these sites for integration of Φ3538 (Δstx2::cat) in parental strains and transductants. However, the generation of 600- and 1,800-bp PCR products in strains 1390, E-12, DJ-04, and ME-08 for wrbA and sbcB, respectively, demonstrated that these genes are intact and therefore not occupied by phage sequences. With the same approach, we investigated the yecE and yehV genes, but these genes are also not used as integration sites in any of the strains described above. Therefore, Φ3538 (Δstx2::cat) obviously uses an integration site that is yet not described for Stx phage.
DISCUSSION
STEC strains demonstrate a broad range of phenotypic and genotypic heterogeneity (27). STEC strains belong to more than 200 E. coli serotypes, and Stx production was described also for several other bacterial species (30, 39, 42, 43, 44). Bacteriophages have played an important role in the evolution of many bacterial pathogens by promoting the horizontal transfer of genes between strains of the same species as well as between distantly related bacteria. Phage can alter host bacterial properties relevant to all stages of the infectious process including bacterial adherence, colonization, invasion, exotoxin production, resistance to immune defenses and antibiotics (reviewed by Wagner and Waldor [45]). Transduction of Stx-encoding phages to nontoxigenic strains could be involved in the emergence of novel STEC serotypes (17, 38).
The genome sequences of EHEC O157 strains Sakai and EDL933 revealed the presence of 18 prophages (15, 32). Among the 18 prophages on the O157 Sakai chromosome, 13 are lambda-like phages, and in addition to Shiga toxins, they encode further virulence-related proteins, such as zinc/copper-type superoxide dismutases, Bor proteins, and Lom homologues (15). On the basis of these results, E. coli O157 bacteria might be considered phage factories, as described by Ohnishi et al. (28), releasing chimeric or mosaic phages into the environment that could lead to new pathogenic characters, first of all new STEC clones. However, the conditions, which favor lysogenic conversion in vivo, are not well established.
Recently, Strauch et al. (42) isolated and characterized an Stx-encoding temperate phage in an S. sonnei strain, which could be transduced to a nontoxigenic S. sonnei strain and to an E. coli laboratory K-12 strain. Schmidt et al. (38) lysogenized a broad range of enteric E. coli strains with a derivative of a Shiga toxin 2-encoding phage designated Φ353 8(Δstx2::cat). They were able to transduce different pathotypes, including EPEC, STEC, enterotoxigenic E. coli, enteroaggregative E. coli, enteroinvasive E. coli, and E. coli isolated from stool microflora of a healthy individual. In the EPEC group, interestingly, only 2 of 11 strains were successfully lysogenized. The lysogenized EPEC strains belonged to classical EPEC serotypes O111:H2 and O26:H, which are also known to frequently produce Stx and cause HUS (18).
We first carried out in vitro transduction experiments. In these experiments we were not able to lysogenize several selected recipient strains with the Stx mutant phage. James et al. (17) lysogenized several E. coli and Shigella strains in vitro by using an aph3 gene-labeled Stx2-encoding phage originating from an E. coli O157 strain, but neither of their 2 EPEC strains were successfully lysogenized. One reason for the failure with E. coli strains E2348/69 and 355 could be that their cryptic prophages might have mediated superimmunity (3). Another reason for the failure could be that the prophages of these EPEC strains have already occupied the potential integration sites for labeled Stx-encoding phages (38). Strain 1390 was also lysogenic, but its prophage did not prevent the integration of Φ3538 (Δstx2::cat) in the porcine loop.
In the present study, we established a suitable model for studying the in vivo spread of Stx-encoding phages. We were able to lysogenize the porcine EPEC O45 strain 1390 with phage Φ3538 (Δstx2::cat) in porcine ligated ileal loops but not the human EPEC prototype strain E2348/69. Under the same conditions, neither strain 1390 nor strain E2348/69 was lysogenized when E. coli K-12 containing H-19B::Tn10d-bla was used as the phage donor. However, two commensal non-EPEC strains of the loop flora might have been lysogenized with H-19B::Tn10d-bla, but the phage was unstable in these strains and after the first passage these phages were eliminated (data not shown). Acheson et al. (2) also lysogenized laboratory E. coli strains with phage H-19B::Tn10d-bla in the murine gastrointestinal tract in vivo. They have not detected any transduction of commensal strains, perhaps because they eliminated most of the normal intestinal flora by streptomycin application before the injection of donor and recipient E. coli K-12 strains.
The success of the in vivo transduction of Stx2 to the target EPEC strain and Stx1 to E. coli strains of the porcine intestinal flora indicates that there are important differences in the porcine intestinal environment acting as inducers of phage transduction in vivo. The exact nature of some these in vivo effectors will be determined in future studies. Among these factors, redox potential, pH value, oxygen, and perhaps a concerted action of several factors may play a role in making in vivo conditions so much different from in vitro conditions.
The bacteriophages encoding Stx2 have been shown to integrate into wrbA or sbcB in the genomes of O157:H7 strains (15, 32). Recktenwald and Schmidt (34) reported that an Stx2e-encoding bacteriophage is integrated into yecE in STEC ONT:H. Recently Shaikh and Tarr (41) analyzed the integration of Stx2- and Stx1-encoding phages into the chromosome of E. coli O157 and non-O157 STEC strains by PCR with wrbA- and yehV-specific primers, respectively. Their study revealed that stx2 bacteriophage usually occupies wrbA in stx1+ and stx2+ E. coli O157:H7 strains, but instead of wrbA, truncated bacteriophages occupy yehV in most stx1-negative and stx2-positive E. coli O157:H7 strains (41). In the present study, we have examined the integrity of wrbA, sbcB, yehV, and yecE genes by PCR in E. coli O157:H7 strain 3538 and transductant strains. Our investigations revealed that all of the previously identified stx1 and stx2 phage integration sites were intact in the donor and transductant strains, indicating that stx2 occupies a new as yet undescribed site in the chromosome of E. coli O157:H7 strain 3538 and transductants.
In conclusion, we have demonstrated that Stx-encoding phages are able to spread in vivo among wild-type enteric E. coli strains, and the failures in the in vitro trials with the same strains suggest that in vivo conditions are more effective than in vitro conditions. Results suggest that EHEC may emerge from EPEC by acquisition of stx genes, but further studies are necessary to define the bacterial features promoting the Stx-encoding phage transduction and to determine the phage integration site in the porcine EPEC strain.
Acknowledgments
This work was supported by grants from the European Union (E.U. project number QLK2-2000-0060) and by the Hungarian Basic Scientific Fund (OTKA) (no. T34970 and T37890).
We are solely responsible for the work described in this paper, and the opinions expressed are not necessarily those of the E.U.
We thank Marta Puruczki (Budapest) and Stefanie Müksch (Dresden) for skillful technical assistance.
REFERENCES
- 1.Abbott, J. D., and R. Shannon. 1958. A new method for typing Shigella sonnei using colicin production as a marker. J. Clin. Pathol. 11:71-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Acheson, D. W., J. Reidl, X. Zhang, G. T. Keusch, J. J. Mekalanos, and M. K. Waldor. 1998. In vivo transduction with Shiga toxin 1-encoding phage. Infect. Immun. 66:4496-4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campbell, A. M. 1996. Cryptic prophages, p. 2041-2046. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology. ASM Press, Washington, D.C.
- 4.Caprioli, A., and A. E. Tozzi. 1998. Epidemiology of Shiga toxin-producing Escherichia coli infections in continental Europe, p. 38-48. In J. B. Kaper and A. D. O'Brien (ed.), Escherichia coli O157:H7 and other Shiga toxin-producing E. coli strains. American Society for Microbiology, Washington, D.C.
- 5.China, B., V. Pirson, and J. Mainil. 1996. Typing of bovine attaching and effacing Escherichia coli by multiplex in vitro amplification of virulence-associated genes. Appl. Environ. Microbiol. 62:3462-3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cornick, N. A., I. Matise, J. E. Samuel, B. T. Bosworth, and H. W. Moon. 2000. Shiga toxin-producing Escherichia coli infection: temporal and quantitative relationships among colonization, toxin production, and systemic disease. J. Infect. Dis. 181:242-251. [DOI] [PubMed] [Google Scholar]
- 7.Doyle, M. P., and J. L. Schoeni. 1987. Isolation of Escherichia coli O157:H7 from retail fresh meats and poultry. Appl. Environ. Microbiol. 53:2394-2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feng, P., K. A. Lampel, H. Karch, and T. S. Whittam. 1998. Genotypic and phenotypic changes in the emergence of Escherichia coli O157:H7. J. Infect. Dis. 177:1750-1753. [DOI] [PubMed] [Google Scholar]
- 9.Franke, J., S. Franke, H. Schmidt, A. Schwarzkopf, L. H. Wieler, G. Baljer, L. Beutin, and H. Karch. 1994. Nucleotide sequence analysis of enteropathogenic Escherichia coli (EPEC) adherence factor probe and development of PCR for rapid detection of EPEC harboring virulence plasmids. J. Clin. Microbiol. 32:2460-2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friedrich, A. W., M. Bielaszewska, W. L. Zhang, M. Pulz, T. Kuczius, A. Ammon, and H. Karch. 2002. Escherichia coli harboring Shiga toxin 2 gene variants: frequency and association with clinical symptoms. J. Infect. Dis. 185:74-84. [DOI] [PubMed] [Google Scholar]
- 11.Gannon, V. P., C. Teerling, S. A. Masri, and C. L. Gyles. 1990. Molecular cloning and nucleotide sequence of another variant of the Escherichia coli Shiga-like toxin II family. J. Gen. Microbiol. 136:1125-1135. [DOI] [PubMed] [Google Scholar]
- 12.Griffin, P. M. 1995. Escherichia coli O157:H7 and other enterohemorrhagic Escherichia coli, p. 739-761. In M. J. Blaser, P. D. Smith, J. I. Ravdin, H. B. Greenberg, and R. L. Guerrant (ed.), Infections of the gastrointestinal tract. Raven Press, New York, N.Y
- 13.Griffin, P. M., and R. V. Tauxe. 1991. The epidemiology of infections caused by Escherichia coli O157:H7, other enterohemorrhagic E. coli, and the associated hemolytic uremic syndrome. Epidemiol. Rev. 13:60-98. [DOI] [PubMed] [Google Scholar]
- 14.Gunzburg, S. T., N. G. Tornieporth, and L. W. Riley. 1995. Identification of enteropathogenic Escherichia coli by PCR-based detection of the bundle-forming pilus gene. J. Clin. Microbiol. 33:1375-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayashi, T., K. Makino, M. Ohnishi, K. Kurokawa, K. Ishii, K. Yokoyama, C. Han, E. Ohtsubo, K. Nakayama, T. Murata, M. Tanaka, T. Tobe, T. Iida, H. Takami, T. Honda, C. Sasakawa, N. Ogasawara, T. Yasunaga, S. Kuhara, T. Shiba, M. Hattori, H. Shinagawa. 2001. Complete genome sequence of enterohemorrhagic Escherichia coli O157:H7 and genomic comparison with a laboratory strain K-12. DNA Res. 28:11-22. [DOI] [PubMed] [Google Scholar]
- 16.Ito, H., A. Terai, H. Kurazono, Y. Takeda, and M. Nishibuchi. 1990. Cloning and nucleotide sequencing of Vero toxin 2 variant genes from Escherichia coli O91:H21 isolated from a patient with the hemolytic uremic syndrome. Microb. Pathog. 8:47-60. [DOI] [PubMed] [Google Scholar]
- 17.James, C. E., K. N. Stanley, H. E. Allison, H. J. Flint, C. S. Stewart, R. J. Sharp, J. R. Saunders, and A. J. McCarthy. 2001. Lytic and lysogenic infection of diverse Escherichia coli and Shigella strains with a verocytotoxigenic bacteriophage. Appl. Environ. Microbiol. 67:4335-4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson, R. P., R. C. Clarke, J. B. Wilson, S. C. Read, K. Rahn, S. A. Renwick, K. A. Sandhu, D. Alves, M. A. Karmali, H. Lior, S. A. McEwen, J. S. Spika, and C. L. Gyles. 1996. Growing concerns and recent outbreaks involving non-O157:H7 serotypes of verotoxigenic Escherichia coli. J. Food. Prot. 59:1112-1122. [DOI] [PubMed] [Google Scholar]
- 19.Karch, H., M. Bielaszewska, M. Bitzan, and H. Schmidt. 1999. Epidemiology and diagnosis of Shiga toxin-producing Escherichia coli infections. Diagn. Microbiol. Infect. Dis. 34:229-243. [DOI] [PubMed] [Google Scholar]
- 20.Karmali, M. A., B. T. Steele, M. Petric, and C. Lim. 1983. Sporadic cases of haemolytic uremic syndrome associated with faecal cytotoxin and cytotoxin-producing Escherichia coli in stools. Lancet i:619-620. [DOI] [PubMed] [Google Scholar]
- 21.MacDaniel, T. K., K. G. Jarvis, M. S. Donnenberg, and J. B. Kaper. 1995. A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proc. Natl. Acad. Sci. USA 92:1664-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McGraw, E. A., J. Li, R. K. Selander, and T. S. Whittam. 1999. Molecular evolution and mosaic structure of α, β, and γ intimins of pathogenic Escherichia coli. Mol. Biol. Evol. 16:12-22. [DOI] [PubMed] [Google Scholar]
- 23.Melton-Celsa, A. R., and A. D. O'Brien. 1998. Structure, biology, and relative toxicity of Shiga toxin family members for cells and animals, p. 121-128. In J. B. Kaper and A. D. O'Brien (ed.), Escherichia coli O157:H7 and other Shiga toxin-producing E. coli strains. American Society for Microbiology, Washington, D.C.
- 24.Moon, H. W., S. C. Whipp, R. A. Argenzio, M. M. Levine, and R. A. Giannella. 1983. Attaching and effacing activities of rabbit and human enteropathogenic Escherichia coli in pig and rabbit intestines. Infect. Immun. 41:1340-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muniesa, M., and J. Jofre. 1998. Abundance in sewage of bacteriophages that infect Escherichia coli O157:H7 and that carry the Shiga toxin 2 gene. Appl. Environ. Microbiol. 64:2443-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagy, B., T. A. Casey, S. C. Whipp, and H. W. Moon. 1992. Susceptibility of porcine intestine to pilus-mediated adhesin by some isolates of piliated enterotoxigenic Escherichia coli increases with age. Infect. Immun. 60:1285-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohnishi, M., K. Kurokawa, and T. Hayashi. 2001. Diversification of Escherichia coli genomes: are bacteriophages the major contributors? Trends Microbiol. 9:481-485. [DOI] [PubMed] [Google Scholar]
- 29.Ohnishi, M., J. Terajima, K. Kurokawa, K. Nakayama, T. Murata, K. Tamura, Y. Ogura, H. Watanabe, and T. Hayashi. 2002. Genomic diversity of enterohemorrhagic Escherichia coli O157 revealed by whole genome PCR scanning. Proc. Natl. Acad. Sci. USA 99:17043-17048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paton, A. W., and J. C. Paton. 1996. Enterobacter cloacae producing a Shiga-like toxin II-related cytotoxin associated with a case of hemolytic-uremic syndrome. J. Clin. Microbiol. 34:463-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paton, A. W., J. C. Paton, M. W. Heuzenroeder, P. N. Goldwater, and P. A. Manning. 1992. Cloning and nucleotide sequence of a variant Shiga-like toxin II gene from Escherichia coli OX3:H21 isolated from a case of sudden infant death syndrome. Microb. Pathog. 13:225-236. [DOI] [PubMed] [Google Scholar]
- 32.Perna, N. T., G. Plunkett III, V. Burland, B. Mau, J. D Glasner, D. J. Rose, G. F. Mayhew, P. S. Evans, J. Gregor, H. A. Kirkpatrick, G. Posfai, J. Hackett, S. Klink, A. Boutin, Y. Shao, L. Miller, E. J. Grotbeck, N. W. Davis, A. Lim, E. T. Dimalanta, K. D. Potamousis, J. Apodaca, T. S. Anantharaman, J. Lin, G. Yen, D. C. Schwartz, R. A. Welch, and F. R. Blattner. 2001. Genome sequence of enterohaemorrhagic Escherichia coli O157:H7. Nature 409:529-533. [DOI] [PubMed] [Google Scholar]
- 33.Pierard, D., G. Muyldermans, L. Moriau, D. Stevens, and S. Lauwers. 1998. Identification of new verocytotoxin type 2 variant B-subunit genes in human and animal Escherichia coli isolates. J. Clin. Microbiol. 36:3317-3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Recktenwald, J., and H. Schmidt. 2002. The nucleotide sequence of Shiga toxin (Stx) 2e-encoding phage phiP27 is not related to other Stx phage genomes, but the modular genetic structure is conserved. Infect. Immun. 70:1896-1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reid, S. D., R. K. Selander, and T. S. Whittam. 1999. Sequence diversity of flagellin (fliC) alleles in pathogenic Escherichia coli. J. Bacteriol. 181:153-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Riley, L. W., R. S. Remis, S. D. Helgerson, H. B. McGee, J. G. Wells, B. R. Davis, R. J. Hebert, E. S. Olcott, L. M. Johnson, N. T. Hargrett, P. A. Blake, and M. L. Cohen. 1983. Hemorrhagic colitis associated with a rare Escherichia coli serotype. N. Engl. J. Med. 308:681-685. [DOI] [PubMed] [Google Scholar]
- 37.Sambrook, J., E. F. Frisch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 38.Schmidt, H., M. Bielaszewska, and H. Karch. 1999. Transduction of enteric Escherichia coli isolates with a derivative of Shiga toxin 2-encoding bacteriophage Φ3538 isolated from Escherichia coli O157:H7. Appl. Environ. Microbiol. 65:3855-3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmidt, H., M. Montag, J. Bockemühl, J. Heesemann, and H. Karch. 1993. Shiga-like toxin II-related cytotoxins in Citrobacter freundii strains from humans and beef samples. Infect. Immun. 61:534-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmidt, H., J. Scheef, S. Morabito, A. Caprioli, L. H. Wieler, and H. Karch. 2000. A new Shiga toxin 2 variant (Stx2f) from Escherichia coli isolated from pigeons. Appl. Environ. Microbiol. 66:1205-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shaikh, N., and P. I. Tarr. 2003. Escherichia coli O157:H7 Shiga toxin-encoding bacteriophages: integrations, excisions, truncations, and evolutionary implications. J. Bacteriol. 185:3596-3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Strauch, E., R. Lurz, and L. Beutin. 2001. Characterization of a Shiga toxin-encoding temperate bacteriophage of Shigella sonnei. Infect. Immun. 69:7588-7595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Strockbine, N. A., M. P. Jackson, L. M. Sung, R. K. Holmes, and A. D. O'Brien. 1988. Cloning and sequencing of the genes for Shiga toxin from Shigella dysenteriae type 1. J. Bacteriol. 170:1116-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tschäpe, H., R. Prager, W. Streckel, A. Fruth, E. Tietze, and G. Bohme. 1995. Verotoxinogenic Citrobacter freundii associated with severe gastroenteritis and cases of haemolytic uraemic syndrome in a nursery school: green butter as the infection source. Epidemiol. Infect. 114:441-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wagner, P. L., and M. K. Waldor. 2002. Bacteriophage control of bacterial virulence. Infect. Immun. 70:3985-3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Watanabe, H., A. Wada, Y. Inagaki, K. Itoh, and K. Tamura. 1996. Outbreaks of enterohaemorrhagic Escherichia coli O157:H7 infection by two different genotype strains in Japan. Lancet 348:831-832. [DOI] [PubMed] [Google Scholar]
- 47.Whittam, T. S. 1995. Genetic population structure and pathogenicity in enteric bacteria, p. 217-245. In S. Baumberg, J. P. W. Young, S. R. Saunders, and E. M. H. Wellington (ed.), Population genetics of bacteria. Cambridge University Press, Cambridge, United Kingdom.
- 48.Zhang, W., M. Bielaszewska, T. Kuczius, H. Karch. 2002. Identification, characterization, and distribution of a Shiga toxin 1 gene variant (stx(1c)) in Escherichia coli strains isolated from humans. J. Clin. Microbiol. 40:1441-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu, C., J. Harel, M. Jacques, C. Desautels, M. S. Donnenberg, M. Beaudry, and J. M. Fairbrother. 1994. Virulence properties and attaching-effacing activity of Escherichia coli O45 from swine postweaning diarrhea. Infect. Immun. 62:4153-4159. [DOI] [PMC free article] [PubMed] [Google Scholar]