Abstract
Thiopurine methyltransferase (TPM T) activity exhibits monogenic co-dominant inheritance, with ethnic differences in the frequency of occurrence of variant alleles. With conventional thiopurine doses, homozygous TPMT-deficient patients (~1 in 178 to 1 in 3,736 individuals with two nonfunctional TPMT alleles) experience severe myelosuppression, 30–60% of individuals who are heterozygotes (~3–14% of the population) show moderate toxicity, and homozygous wildtype individuals (~86–97% of the population) show lower active thioguanine nucleolides and less myelosuppression. We provide dosing recommendations (updates at http://www.pharmgkb.org) for azathioprine, mercaptopurine (MP), and thioguanine based on TPMT genotype.
The purpose of this guideline is to provide information with which to interpret clinical thiopurine methyltransferase (TPMT) genotype tests so that the results can be used successfully to guide the dosing of thiopurines. Although most of the dosing recommendations have been generated from clinical studies in only a few diseases, we have extrapolated recommended doses to all conditions, given the pharmacokinetic characteristics of the genotype/phenotype associations. This is the first guideline developed by the Clinical Pharmacogenetics Implementation Consortium, which is part of the National Institutes of Health’s Pharmacogenomics Research Network.1 The consortium is a community-driven organization that is developing peer-reviewed, freely available gene/drug guidelines that are published in full at PharmGKB (http://www.pharmgkb.org). Guidelines for the use of phenotypic tests (i.e., TPMT activity and thiopurine metabolite levels) and analyses of cost effectiveness are beyond the scope of this article.
FOCUSED REVIEW OF THE LITERATURE
The review of the literature focused on TPMT genotype and thiopurine use (Supplementary Data online), with reviews2–5 being used as summaries of earlier literature.
Gene: TPMT
Background
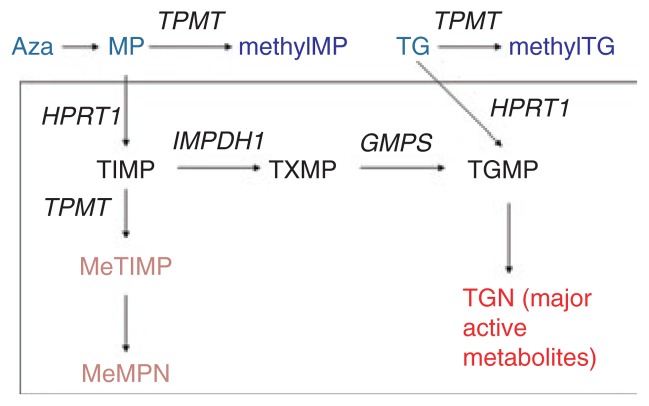
TPMT activity is inherited as a monogenic co-dominant trait (Supplementary Figure S1 online). It methylates mercaptopurine (MP) and thioguanine (Figure 1), causing an inverse relationship between TPMT activity and concentrations of active thioguanine nucleotide (TGN) metabolites. With conventional doses of thiopurines, individuals (~1 in 178 to 1 in 3,736) who inherit two inactive TPMT alleles (homozygous deficient) universally experience severe myelosuppression; a high proportion of those who are heterozygous show moderate to severe myelosuppression, and those who are homozygous for wild-type TPMT alleles have lower levels of TGN metabolites and consequently a lower risk of myelosuppression. 6–9 There are substantial ethnic differences in the frequencies of low-activity variant alleles (Supplementary Tables S3 and S4 online).
Figure 1.
Azathioprine (Aza), mercaptopurine (MP), and thioguanine (TG) are all prodrugs that are inactivated by thiopurine methyltransferase (TPMT). All three agents give rise to the same active thioguanine nucleotide (TGN) metabolites. Methylthioinosine monophosphate (MeTIMP) is a form of methylmercaptopurine nucleotide (MeMPN) which also has some activity (see text) and is formed from the secondary metabolite thioinosine monophosphate (TIMP). GMPS, guanosine monophosphate synthetase; HPRT1, hypoxanthine phosphoribosyltransferase; IMPDH1, inosine monophospate dehydrogenase; TGMP, thioguanosine monophosphate; TXMP, thioxanthosine monophosphate.
Three TPMT single-nucleotide polymorphisms account for >90% of inactivating alleles, and therefore genotyping tests have a high likelihood of being informative.10,11 Complementary phenotype laboratory tests can be helpful adjuncts to genotyping tests (Supplementary Data online, Other Considerations).
Interpretation of genetic tests
Genetic testing analyzes the DNA sequence at each of the important single-nucleotide polymorphism locations in the TPMT gene (Supplementary Data online). Each named ★ allele is defined by the genotype at one or more specific single-nucleotide polymorphisms (Supplementary Table S1 online) and is associated with a distinctive level of enzyme activity (Supplementary Table S2 online). Table 1 summarizes the assignment of the likely TPMT phenotype on the basis of the most common ★ allele diplotypes, and these assignments are used to link genotypes with thiopurine dosing. Although inactivating TPMT alleles have been extensively studied in several populations (Supplementary Tables S3 and S4 online), one of the limitations inherent in a commercial genotype-only test is that rare or previously undiscovered variants will generally not be detected.
Table 1.
Assignment of likely thiopurine methyltransferase phenotypes based on genotypes
| Likely phenotype | Genotypes | Examples of diplotypes |
|---|---|---|
| Homozygous wild-type or normal, high activity (constitutes ~86–97%a of patients) | An individual carrying two or more functional (*1) alleles | *1/*1 |
| Heterozygote or intermediate activity (~3–14%a of patients) | An individual carrying one functional allele (*1) plus one nonfunctional allele (*2, *3A, *3B, *3C, or *4) | *1/*2, *1/*3A, *1/*3B, *1/*3C, *1/*4 |
| Homozygous variant, mutant, low, or deficient activity (~1 in 178 to 1 in 3,736 patientsa) | An individual carrying two nonfunctional alleles (*2, *3A, *3B, *3C, or *4) | *3A/*3A, *2/*3A, *3C/*3A, *3C/*4, *3C/*2, *3A/*4 |
See Supplementary Data online for estimates of phenotype frequencies among different ethnic/geographic groups.
Available genetic test options
See Supplementary Data online.
Incidental findings
No diseases have been linked to variations in TPMT in the absence of drug treatment.3
Other considerations
Other genes, such as ITPA, have been linked to variations in thiopurine pharmacokinetics and dynamics, but their effect is weaker than that of TPMT, and these other genes are not currently used clinically.
Drugs
Background
Three thiopurines are used clinically: azathioprine (a prodrug for MP), MP, and thioguanine. Although all three medications share many of the same pharmacologic effects, MP and azathioprine are used for nonmalignant immunologic disorders, MP for lymphoid malignancies, and thioguanine for myeloid leukemias.
Because azathioprine is a prodrug for MP, the two drugs can be considered to have identical interactions with TPMT; that is, TPMT catabolizes MP to inactive methylMP, leaving less parent drug available for eventual anabolism to active TGNs (Figure 1). The secondary metabolite of MP, TIMP, is also a substrate for TPMT, and methylTIMP (and further phosphorylated metabolites, methylMP nucleotides or MeMPN) have some activity (mostly immunosuppressive and hepatotoxic); they inhibit de novo purine synthesis and may contribute to some of the adverse effects of thiopurines.3,7,12 Individuals who inherit two nonfunctional TPMT alleles are at 100% risk for life-threatening myelosuppression, due to high TGNs, if they receive chronic therapy with conventional doses of MP (or azathioprine). Despite having higher TGNs than wild-type homozygotes, only ~30–60% of patients who are heterozygous for TPMT are unable to tolerate full doses of MP or azathioprine.7,13,14 Some heterozygotes may have good thiopurine tolerance because they have lower concentrations (and thus fewer toxic effects) of the methylMP nucleotides (MeMPN) than do homozygous wild-type carriers, thereby allowing tolerance of higher TGNs. There is therefore more debate over the dosing of azathioprine and MP in patients who are heterozygous for TPMT as compared with those who are homozygous deficient, although heterozygotes are at significantly higher risk for toxicity than wild-type patients.15
Although there is lower affinity between thioguanine and TPMT than between MP and TPMT, TPMT has a significant impact on the pharmacokinetics of thioguanine and thereby on its therapeutic effects. Thioguanine is directly inactivated by TPMT to its inactive methylthioguanine base, leaving less of the drug available for anabolism to active TGN metabolites. There is no analogous secondary metabolite of thioguanine that can undergo activation through TPMT (i.e., there are no methylTIMP or methylMP nucleotides); as a result, patients receiving thioguanine are able to tolerate substantially higher TGN concentrations than are those receiving MP or azathioprine.16
Although there are fewer clinical data for thioguanine than for MP, the inverse relationship between TPMT activity and risk of toxicity should be more straightforward for thioguanine than for MP.17 Therefore, within each TPMT phenotypic group, the decreases in initial recommended dosages are similar for thioguanine, MP, and azathioprine (Table 2).
Table 2.
Recommended dosing of thiopurines by thiopurine methyltransferase phenotype
| Phenotype | MP |
Azathioprine
|
TG |
|||||
|---|---|---|---|---|---|---|---|---|
| Implications for MP and azathioprine pharmacologic measures |
Dosing recommendations for MP |
Classification of recommen- dationsa |
Dosing recommendations for azathioprine |
Classification of recommen- dationsa |
Implications
for pharmacologic measures after TG |
Dosing recommendations for TG |
Classification of recommen- dationsa |
|
| Homozygous wild-type or normal, high activity | Lower concentrations of TGN metabolites, higher methylTIMP, this is the “normal” pattern | Start with normal starting dose (e.g., 75 mg/m2/d or 1.5 mg/kg/d) and adjust doses of MP (and of any other myelosuppressive therapy) without any special emphasis on MP compared to other agents. Allow 2 weeks to reach steady state after each dose adjustment.4,25,29 | Strong | Start with normal starting dose (e.g., 2–3 mg/kg/d) and adjust doses of azathioprine based on disease-specific guidelines. Allow 2 weeks to reach steady state after each dose adjustment.4,27,29 | Strong | Lower concentrations of TGN metabolites, but note that TGN after TG are 5–10× higher than TGN after MP or azathioprine | Start with normal starting dose. Adjust doses of TG and of other myelosuppressive therapy without any special emphasis on TG. Allow 2 weeks to reach steady state after each dose adjustment.4,16 | Strong |
|
| ||||||||
| Heterozygote or intermediate activity | Moderate to high concentrations of TGN metabolites; low concentrations of methylTIMP | Start with reduced doses (start at 30–70% of full dose: e.g., at 50 mg/m2/d or 0.75 mg/kg/d) and adjust doses of MP based on degree of myelosuppression and disease-specific guidelines. Allow 2–4 weeks to reach steady state after each dose adjustment. In those who require a dosage reduction based on myelosuppression, the median dose may be ~40% lower (44 mg/m2) than that tolerated in wild-type patients (75 mg/m2).6,12 In setting of myelosuppression, and depending on other therapy, emphasis should be on reducing MP over other agents.4,13,15,21,23,25,29,31,32 | Strong | If disease treatment normally starts at the “full dose”, consider starting at 30–70% of target dose (e.g., 1–1.5 mg/kg/d), and titrate based on tolerance. Allow 2–4 weeks to reach steady state after each dose adjustment.4,27,29,31 | Strong | Moderate to high concentrations of TGN metabolites; but note that TGN after TG are 5–10× higher than TGN after MP or azathioprine | Start with reduced doses (reduce by 30–50%) and adjust doses of TG based on degree of myelosuppression and disease-specific guidelines. Allow 2–4 weeks to reach steady state after each dose adjustment. In setting of myelosuppression, and depending on other therapy, emphasis should be on reducing TG over other agents.4,16 | Moderate |
|
| ||||||||
| Homozygous variant, mutant, low, or deficient activity | Extremely high concentrations of TGN metabolites; fatal toxicity possible without dose decrease; no methylTIMP metabolites | For malignancy, start with drastically reduced doses (reduce daily dose by 10-fold and reduce frequency to thrice weekly instead of daily, e.g., 10 mg/m2/d given just 3 days/week) and adjust doses of MP based on degree of myelosuppression and disease-specific guidelines. Allow 4–6 weeks to reach steady state after each dose adjustment. In setting of myelosuppression, emphasis should be on reducing MP over other agents. For nonmalignant conditions, consider alternative nonthiopurine immunosuppressant therapy.4,24,29,31 | Strong | Consider alternative agents. If using azathioprine start with drastically reduced doses (reduce daily dose by 10-fold and dose thrice weekly instead of daily) and adjust doses of azathioprine based on degree of myelosuppression and disease-specific guidelines. Allow 4–6 weeks to reach steady state after each dose adjustment. Azathioprine is the likely cause of myelosuppression.27,29–31,33 | Strong | Extremely high concentrations of TGN metabolites; fatal toxicity possible without dose decrease | Start with drastically reduced doses16 (reduce daily dose by 10-fold and dose thrice weekly instead of daily) and adjust doses of TG based on degree of myelosuppression and disease-specific guidelines. Allow 4–6 weeks to reach steady state after each dose adjustment. In setting of myelosuppression, emphasis should be on reducing TG over other agents. For nonmalignant conditions, consider alternative nonthiopurine immunosuppressant therapy.4 | Strong |
MP, mercaptopurine; TG, thioguanine; TGN, thioguanine nucleotide; TIMP, secondary metabolite of MP.
Rating scheme is described in Supplementary Data online.
Linking genetic variability to variability in drug-related phenotypes
There is substantial evidence linking TPMT genotype to phenotypic variability (see Supplementary Table S5 online). Dose adjustments based on TPMT genotype have reduced thiopurine-induced adverse effects without compromising desired antitumor and immunosuppressive therapeutic effects in several clinical settings (Supplementary Table S5 online). This body of evidence, rather than randomized clinical trials, provides the basis for most of the dosing recommendations in Table 2.
Dosage recommendations
Thiopurines are most commonly used to treat nonmalignant conditions but are also critical anticancer agents. The approach to dosing adjustments based on TPMT status may differ depending on the clinical indication and the propensity to initiate therapy at higher vs. lower starting doses. We and others18–23 advocate testing for TPMT status prior to initiating thiopurine therapy, so that starting dosages can be adjusted accordingly.
Thiopurines are used as immunosuppressants in inflammatory bowel disease, rheumatoid arthritis, and other immune conditions. In most of these diseases, the selection of medications is carried out stepwise, with multiple nonthiopurine (and nonmyelosuppressive) agents being available as alternatives. Several consensus guidelines for treatment of nonmalignant diseases5,24 explicitly recommend preemptive TPMT testing coupled with customized starting doses of thiopurines. A survey calling for responses from pediatric gastroenterologists revealed that 61% of child patients were tested for TPMT before starting thiopurine therapy,25 and the average rates of preemptive testing reported by non–cancer specialists in the United Kingdom were 47–94%.5
In nonmalignant conditions, if one starts with low doses in all patients in order to avoid severe toxicity in the minority with a TPMT defect, one risks disease progression during the period of upward dosage titration.26 In nonmalignant conditions, full starting doses are recommended for homozygous wild-type carriers, reduced doses (30–70% of target dose) in those who are heterozygous for TPMT,27 and substantially reduced doses (or use of an alternative agent) in the rare homozygous deficient patients (Table 2).5,26
Thiopurines have a unique role in the treatment of several malignancies. Conventional starting doses of thiopurines are generally “high” because these doses have been derived from trials heavily weighted by the ~86–97% of the population who are wild-type for TPMT and receive maximal tolerable doses by the standards of anticancer treatment (hence, full doses should be given to those who are homozygous wild-type for TPMT; Table 2). Given that starting doses have tended to be high (e.g., 75 mg/m2 of MP) in cancer (e.g., in acute lymphoblastic leukemia), lower-than-normal starting doses should be used in heterozygous deficient patients14,16,22,28 and markedly reduced doses (at least 10-fold reduction) in homozygous deficient patients29 (Table 2). This approach has decreased the risk of acute toxicity without compromising relapse rates in acute lymphoblastic leukemia.30 Even at these markedly reduced dosages, erythrocyte TGN concentrations in homozygous deficient patients remain well above those tolerated and achieved by the majority of patients (who are wild-type for TPMT).5,29
There are varying practices about when—and even whether—to test for TPMT status in oncology patients who receive thiopurines. Because of the rarity of malignancies and defective TPMT genotypes, no randomized clinical trials have proven the benefit of customizing starting doses of thiopurine based on TPMT status in cancer settings. Nevertheless, many cancer clinicians preemptively test TPMT status to customize starting doses of thiopurines, basing their decision on the strong mechanistic data and retrospective analyses of clinical trials supporting a lower dose in those with a TPMT defect (Supplementary Table S5 online). Thiopurines are almost always used as part of combination chemotherapy that contains multiple myelosuppressive medications. Therefore, a trial-and-error approach (i.e., starting thiopurine therapy without ascertaining the TPMT status) has some disadvantages. The duration of myelosuppression varies substantially—an extremely long period of myelosuppression can result if conventional thiopurine doses are given to a patient with low TPMT activity, thereby delaying ongoing chemotherapy. Also, it is impossible to determine, through clinical monitoring alone, which of several myelosuppressive agents is the most likely cause of myelosuppression. Another reason to test every patient preemptively is that even a short full-dose course of thiopurines can result in death or severe myelosuppression in the rare homozygous deficient individual.3,31 Such an eventuality could be avoided by preemptive testing and starting with dramatically decreased doses (more than 10-fold lower than normal doses) of thiopurine or choosing an alternative therapy for the potentially at-risk patients.
Some of the clinical data on which dosing recommendations are based (Table 2) rely on measures of TPMT phenotype rather than genotype; however, because TPMT genotype is so strongly linked to TPMT phenotype, these recommendations should apply regardless of the method used to assess TPMT status.
Recommendations for incidental findings
Not applicable.
Other considerations
Complementary clinical laboratory tests are available to measure thiopurine metabolites in erythrocytes: TGNs (for MP, azathioprine, and thioguanine) and MeMPN nucleotides (or methylTIMP) for those on MP or azathioprine (see Supplementary Data online for details).
Potential benefits and risks for the patient
One of the benefits of preemptive TPMT testing is that doses that are customized on the basis of TPMT status reduce the likelihood of acute myelosuppression without compromising disease control.5,7,22,28 The risks would be that a proportion of heterozygotes may spend a period of time at lower thiopurine doses than they can eventually tolerate, because only ~30–60% of heterozygous patients receiving conventional thiopurine doses experience severe myelosuppression.5,7,14 However, because steady state is reached in 2–4 weeks, any period of “underdosing” should be short, and in studies using this approach—at least in acute lymphoblastic leukemia and inflammatory bowel disease— outcomes were not compromised.5,7,22,27,28
A possible risk to the patient is an error in genotyping.5 Because genotypes are lifelong test results, any such error could stay in the medical record for the life of the patient.
Caveats: appropriate use and/or potential misuse of genetic tests
Usually, thiopurines are administered orally every day for a period of at least several months. Genotype-based starting doses are just that—starting doses—and in most diseases, titration to an acceptable degree of myelosuppression is required. Clinicians should continue to evaluate markers of disease progression and/or myelosuppression to adjust thiopurine doses upward or downward from the genotype-directed starting doses. One caveat is that some serious long-term adverse effects (secondary tumors) have been associated with the use of thiopurine therapy in patients with defective TPMT activity, even in the absence of severe acute myelosuppression; it is not known whether capping doses of thiopurines in those with a TPMT defect will ameliorate the risk of these late-developing adverse effects (secondary cancer). Some adverse reactions to thiopurines, such as pancreatitis and hepatotoxicity, are not related to low TPMT activity.
Supplementary Material
Acknowledgments
We acknowledge the critical input of Drs L. Ramsey, J. Kawedia, and C. Fernandez, and members of the Clinical Pharmacogenetics Implementation Consortium of the Pharmacogenomics Research Network, funded by the National Institutes of Health. This work is supported by NIH UO1 GM 92666, CA 21765, PharmGKB (R24-GM61374), U19 HL065962-10, 2U19GM061390-11, and ALSAC.
Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines reflect expert consensus based on clinical evidence and peer-reviewed literature available at the time they are written and are intended only to assist clinicians in decision making and to identify questions for further research. New evidence may have emerged since the time a guideline was submitted for publication. Guidelines are limited in scope and are not applicable to interventions or diseases that are not specifically identified. Guidelines do not account for individual variations among patients, and cannot be considered inclusive of all proper methods of care or exclusive of other treatments. It remains the responsibility of the health-care provider to determine the best course of treatment for a patient. Adherence to any guideline is voluntary, with the ultimate determination regarding its application to be made solely by the clinician and the patient. CPIC assumes no responsibility for any injury or damage to persons or property arising out of or related to any use of CPIC’s guidelines, or for any errors or omissions.
Footnotes
SUPPLEMENTARY MATERIAL is linked to the online version of the paper at http://www.nature.com/cpt
CONFLICT OF INTEREST
W.E.E. and M.V.R. have received patent royalties from TPMT genotyping tests. The other authors declared no conflict of interest.
References
- 1.Relling MV, Klein TE. CPIC: Clinical Pharmacogenetics Implementation Consortium of the Pharmacogenomics Research Network. Clin Pharmacol Ther. 89:474–477. doi: 10.1038/clpt.2010.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sandborn WJ. Pharmacogenomics and IBD: TPMT and thiopurines. Inflamm Bowel Dis. 2004;10(suppl 1):S35–S37. doi: 10.1097/00054725-200402001-00008. [DOI] [PubMed] [Google Scholar]
- 3.Evans WE. Pharmacogenetics of thiopurine S-methyltransferase and thiopurine therapy. Ther Drug Monit. 2004;26:186–191. doi: 10.1097/00007691-200404000-00018. [DOI] [PubMed] [Google Scholar]
- 4.Weinshilboum R. Inheritance and drug response. N Engl J Med. 2003;348:529–537. doi: 10.1056/NEJMra020021. [DOI] [PubMed] [Google Scholar]
- 5.Ford LT, Berg JD. Thiopurine S-methyltransferase (TPMT) assessment prior to starting thiopurine drug treatment; a pharmacogenomic test whose time has come. J Clin Pathol. 2010;63:288–295. doi: 10.1136/jcp.2009.069252. [DOI] [PubMed] [Google Scholar]
- 6.Black AJ, et al. Thiopurine methyltransferase genotype predicts therapylimiting severe toxicity from azathioprine. Ann Intern Med. 1998;129:716–718. doi: 10.7326/0003-4819-129-9-199811010-00007. [DOI] [PubMed] [Google Scholar]
- 7.Relling MV, et al. Mercaptopurine therapy intolerance and heterozygosity at the thiopurine S-methyltransferase gene locus. J Natl Cancer Inst. 1999;91:2001–2008. doi: 10.1093/jnci/91.23.2001. [DOI] [PubMed] [Google Scholar]
- 8.Lennard L, Van Loon JA, Weinshilboum RM. Pharmacogenetics of acute azathioprine toxicity: relationship to thiopurine methyltransferase genetic polymorphism. Clin Pharmacol Ther. 1989;46:149–154. doi: 10.1038/clpt.1989.119. [DOI] [PubMed] [Google Scholar]
- 9.Lennard L, Lilleyman JS, Van Loon J, Weinshilboum RM. Genetic variation in response to 6-mercaptopurine for childhood acute lymphoblastic leukaemia. Lancet. 1990;336:225–229. doi: 10.1016/0140-6736(90)91745-v. [DOI] [PubMed] [Google Scholar]
- 10.Schaeffeler E, et al. Comprehensive analysis of thiopurine S-methyltransferase phenotype-genotype correlation in a large population of German-Caucasians and identification of novel TPMT variants. Pharmacogenetics. 2004;14:407–417. doi: 10.1097/01.fpc.0000114745.08559.db. [DOI] [PubMed] [Google Scholar]
- 11.Yates CR, et al. Molecular diagnosis of thiopurine S-methyltransferase deficiency: genetic basis for azathioprine and mercaptopurine intolerance. Ann Intern Med. 1997;126:608–614. doi: 10.7326/0003-4819-126-8-199704150-00003. [DOI] [PubMed] [Google Scholar]
- 12.Nygaard U, Toft N, Schmiegelow K. Methylated metabolites of 6-mercaptopurine are associated with hepatotoxicity. Clin Pharmacol Ther. 2004;75:274–281. doi: 10.1016/j.clpt.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 13.Evans WE, et al. Preponderance of thiopurine S-methyltransferase deficiency and heterozygosity among patients intolerant to mercaptopurine or azathioprine. J Clin Oncol. 2001;19:2293–2301. doi: 10.1200/JCO.2001.19.8.2293. [DOI] [PubMed] [Google Scholar]
- 14.Stocco G, et al. Genetic polymorphism of inosine triphosphate pyrophosphatase is a determinant of mercaptopurine metabolism and toxicity during treatment for acute lymphoblastic leukemia. Clin Pharmacol Ther. 2009;85:164–172. doi: 10.1038/clpt.2008.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higgs JE, Payne K, Roberts C, Newman WG. Are patients with intermediate TPMT activity at increased risk of myelosuppression when taking thiopurine medications? Pharmacogenomics. 2010;11:177–188. doi: 10.2217/pgs.09.155. [DOI] [PubMed] [Google Scholar]
- 16.Lennard L, Lilleyman JS. Individualizing therapy with 6-mercaptopurine and 6-thioguanine related to the thiopurine methyltransferase genetic polymorphism. Ther Drug Monit. 1996;18:328–334. doi: 10.1097/00007691-199608000-00003. [DOI] [PubMed] [Google Scholar]
- 17.McBride KL, Gilchrist GS, Smithson WA, Weinshilboum RM, Szumlanski CL. Severe 6-thioguanine-induced marrow aplasia in a child with acute lymphoblastic leukemia and inhibited thiopurine methyltransferase deficiency. J Pediatr Hematol Oncol. 2000;22:441–445. doi: 10.1097/00043426-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Schwab M, et al. Azathioprine therapy and adverse drug reactions in patients with inflammatory bowel disease: impact of thiopurine S-methyltransferase polymorphism. Pharmacogenetics. 2002;12:429–436. doi: 10.1097/00008571-200208000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Gurwitz D, et al. Improving pharmacovigilance in Europe: TPMT genotyping and phenotyping in the UK and Spain. Eur J Hum Genet. 2009;17:991–998. doi: 10.1038/ejhg.2009.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fargher EA, et al. Current use of pharmacogenetic testing: a national survey of thiopurine methyltransferase testing prior to azathioprine prescription. J Clin Pharm Ther. 2007;32:187–195. doi: 10.1111/j.1365-2710.2007.00805.x. [DOI] [PubMed] [Google Scholar]
- 21.Aricó M, et al. The seventh international childhood acute lymphoblastic leukemia workshop report: Palermo, Italy, January 29–30, 2005. Leukemia. 2005;19:1145–1152. doi: 10.1038/sj.leu.2403783. [DOI] [PubMed] [Google Scholar]
- 22.Schmiegelow K, et al. Thiopurine methyltransferase activity is related to the risk of relapse of childhood acute lymphoblastic leukemia: results from the NOPHO ALL-92 study. Leukemia. 2009;23:557–564. doi: 10.1038/leu.2008.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Relling MV, Altman RB, Goetz MP, Evans WE. Clinical implementation of pharmacogenomics: overcoming genetic exceptionalism. Lancet Oncol. 2010;11:507–509. doi: 10.1016/S1470-2045(10)70097-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anstey AV, Wakelin S, Reynolds NJ. British Association of Dermatologists Therapy, Guidelines and Audit Subcommittee. Guidelines for prescribing azathioprine in dermatology. Br J Dermatol. 2004;151:1123–1132. doi: 10.1111/j.1365-2133.2004.06323.x. [DOI] [PubMed] [Google Scholar]
- 25.Colletti RB, et al. Variation in care in pediatric Crohn disease. J Pediatr Gastroenterol Nutr. 2009;49:297–303. doi: 10.1097/MPG.0b013e3181919695. [DOI] [PubMed] [Google Scholar]
- 26.Sandborn WJ. Rational dosing of azathioprine and 6-mercaptopurine. Gut. 2001;48:591–592. doi: 10.1136/gut.48.5.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meggitt SJ, Gray JC, Reynolds NJ. Azathioprine dosed by thiopurine methyltransferase activity for moderate-to-severe atopic eczema: a double-blind, randomised controlled trial. Lancet. 2006;367:839–846. doi: 10.1016/S0140-6736(06)68340-2. [DOI] [PubMed] [Google Scholar]
- 28.Schmiegelow K, et al. Long-term results of NOPHO ALL-92 and ALL-2000 studies of childhood acute lymphoblastic leukemia. Leukemia. 2010;24:345–354. doi: 10.1038/leu.2009.251. [DOI] [PubMed] [Google Scholar]
- 29.Evans WE, Horner M, Chu YQ, Kalwinsky D, Roberts WM. Altered mercaptopurine metabolism, toxic effects, and dosage requirement in a thiopurine methyltransferase-deficient child with acute lymphocytic leukemia. J Pediatr. 1991;119:985–989. doi: 10.1016/s0022-3476(05)83063-x. [DOI] [PubMed] [Google Scholar]
- 30.Relling MV, Pui CH, Cheng C, Evans WE. Thiopurine methyltransferase in acute lymphoblastic leukemia. Blood. 2006;107:843–844. doi: 10.1182/blood-2005-08-3379. [DOI] [PubMed] [Google Scholar]
- 31.Lennard L. Clinical implications of thiopurine methyltransferase–optimization of drug dosage and potential drug interactions. Ther Drug Monit. 1998;20:527–531. doi: 10.1097/00007691-199810000-00014. [DOI] [PubMed] [Google Scholar]
- 32.Krynetski EY, Evans WE. Pharmacogenetics of cancer therapy: getting personal. Am J Hum Genet. 1998;63:11–16. doi: 10.1086/301941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaskas BA, et al. Safe treatment of thiopurine S-methyltransferase deficient Crohn’s disease patients with azathioprine. Gut. 2003;52:140–142. doi: 10.1136/gut.52.1.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.