1. INTRODUCTION
The detection and measurement of neuronal discharge activity of the brain provides unique insight into the neurophysiological mechanisms of brain function. Despite limitations on the interpretation of neuronal discharge activity in sedated or anesthetized animals, most neurophysiological experiments in rodents continue to be performed using sedation or general anesthesia (Wilson et al., 1996, Goto et al., 2001, Kasanetz et al., 2006). In these studies, neuronal recordings are generally completed under sedation while restraining the animal’s head in a stereotaxic frame using ear bars and mouth piece. Although this technique permits stabilized neuronal recordings from specific locations and is relatively easy to perform, the spontaneous neuronal activity is altered by the anesthetic agents (Nicoll et al., 1982, Schonewille et al., 2006, Alkire et al., 2008). While a number of investigators have correlated neuronal discharge activity with various behaviors and movements in anesthetized animals, the abolishment of such activities by the anesthesia compromises the interpretation of such studies.
A number of investigators have circumvented the need for general anesthetics by performing neuronal recordings in freely moving animals using implanted electrode arrays (Pager et al., 1984, Korshunov et al., 1995, Anderson et al., 2003, Jackson et al., 2007, Eliades et al., 2008). This technique has an advantage of allowing stabilized recordings from individual nuclei for long periods of time and correlates discharge activity with behavior. Moreover, this technique permits recording of brain activity with minimal physical and behavioral restrictions for the animal. However, implementation of this technique is costly and technically difficult.
Various head-restraining techniques have previously been instituted to permit single-unit extracellular recordings along multiple tracks in unanaesthetized rodents (Parry et al., 1993, Soulière et al., 2000, Heck et al., 2002, Fa et al., 2003). Because of perceived limitations posed by currently available devices, we presently undertook to develop and test a novel stereotaxic apparatus. The advantages of our setup include: 1) simplified and strong stabilization of a head fixture with an eight point skull fixation, 2) a light weight design, which permits quick and reliable securing of the head fixture without the added time and weight of traditional epoxy-based techniques, 3) adaptability of the head fixture to readily fit various skull sizes, 4) a novel and accurate skull alignment technique which circumvents the initial requirement of ear bars and 5) excellent access to the animal during experimental conditions.
2. MATERIALS AND METHODS
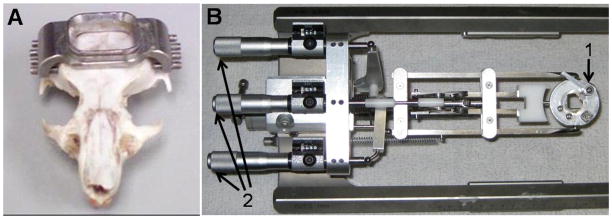
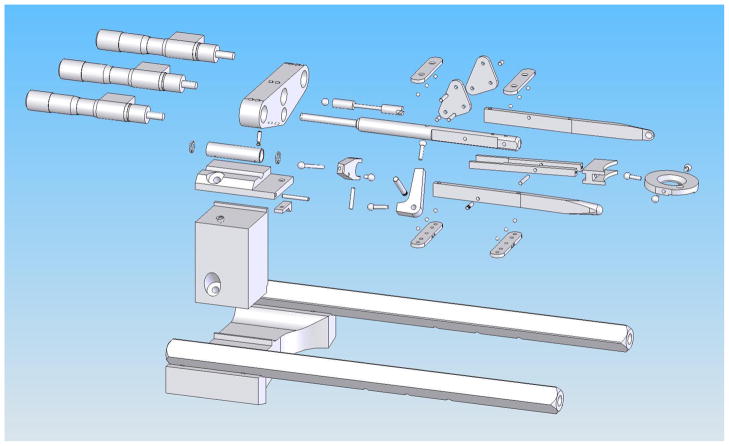
The stereotaxic apparatus is composed of two principal components: 1) a stainless steel head fixture, which is mounted to the skull (Fig. 1A) and 2) a stereotaxic positioner used to accurately achieve the flat skull position for stereotaxic alignment (Figs. 1B, 2, 3). The apparatus was designed in collaboration with Custom Design & Fabrication, a mechanical and electronics support facility under the Department of Radiology at Virginia Commonwealth University, Richmond, VA.
Fig. 1.
Head fixture and stereotaxic positioner. A. Stainless steel head fixture. B. Positioning System. 1. Cam-lock for securing the head fixture, 2. Micrometer adjustments for yaw, pitch, and roll.
Fig. 2.
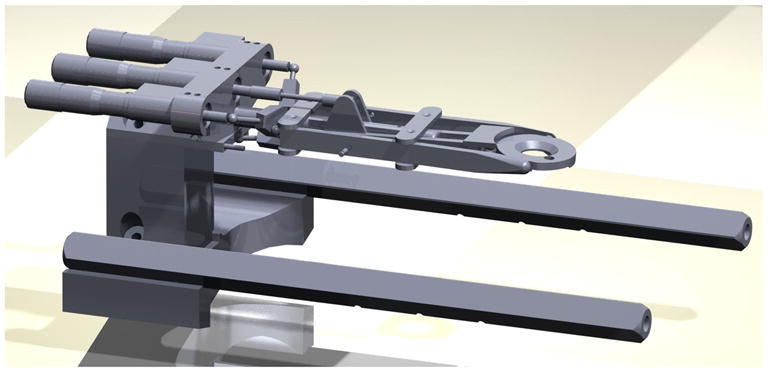
Computer-aided design (CAD) drawing of the custom-fabricated stereotaxic positioner. The positioner was designed to fit via an elevated base onto a standard large animal sterotaxic frame.
Fig. 3.
Computer-aided design (CAD) explosion drawing illustrating the individual parts of the stereotaxic positioner.
2.1. Apparatus
2.1.1. Head fixture
The head fixture is constructed from stainless steel and weighs 2.8 grams. The skull mounted fixture is designed to remain secure for longer periods than adhesives can provide, to minimize the size and weight of the head fixture so that it could be easily supported by the animal, and to facilitate rapid and precise repositioning for repeat recording sessions. The fixture has remained secure in studies lasting up to two weeks in rats weighing 45–220 gms. Also, for repeat recording sessions the animal’s head position can be precisely reestablished within minutes.
The fixture is secured to the skull using eight miniature 0–80 stainless steel set screws. The faces of these screws are machined flat with the exception of a 0.3 mm long center spike. The flat surface distributes the clamping pressure over the 1.2 mm diameter face of the screw and the spike prevents the screw from sliding on the bone. The eight point mounting allows the fixture to conform to the skull contour and distribute the clamping forces over a fairly large area. This mounting provides excellent stability of the skull for neuronal recordings.
A 1 × 1 cm (adaptable sized) opening in the center of the fixture (Fig. 1A and Fig. 3) provides ample access for passing microelectrodes over a large surface area. This protruding access port serves as the mounting point for securing the fixture to the positioner. A cam forces the fixture into a V shaped corner wedge locking the chamber onto the stereotaxic positioner (Fig 1B, #1). A tapered groove on the perimeter of the access port mates with the cam and wedge to ensure precise fixture alignment. Between recording sessions, the access port is sealed with a plastic cap (not shown) which slides into the tapered groove.
2.1.2. Stereotaxic positioner
A positioning system (Fig. 1B, 2, 3) was designed to be mounted onto a standard sized large animal stereotaxic frame. Three micrometer heads permit head alignment in three directions (yaw, pitch, and roll) to achieve the flat skull position required for accurate regional brain targeting using standard stereotaxic atlases. Once proper alignment is achieved, the values from the digital readouts on the micrometer heads are recorded allowing the position to be quickly and accurately restored later after the skull reference points have been removed.
2.2. Implantation of head fixture
All animals and procedures used for the study were approved by the Institutional Animal Care and Use Committee (IACUC) at Virginia Commonwealth University. A total of 18 juvenile Gunn rats (45–220 gms in weight), a strain of Wistar rats, were used for this study. Animals of different weights were selected to test the stability of the head fixture on skulls of different sizes. Extracellular neuronal recordings were performed for 2–3 hrs a day for up to two weeks on 13 of 18 animals used in this study. The five remaining animals were used for post-mortem verification of accuracy of targeting. For these animals, the neuronal recordings were restricted to a single session to ascertain accurate histological reconstruction of the location of recording tracks.
During surgery, the rats were placed on a flat Plexiglas platform with their mouth and nose inserted into a cone device to administer isofluorane anesthesia (1–2%). The body temperature was maintained at 37.0 ± 0.5°C with a regulated heating pad. Using sterile techniques, an incision was made along the sagittal plane of the head. The skin was retracted to expose the skull bone to just below the lateral ridges of the skull. Using a dental scraper, the upper edges of the temporalis muscles were retracted from the temporal bones and all tissues covering the exposed bone were cleared. The head fixture was then visually centered on the skull. While griping the head fixture in place with one hand, the fixture was firmly attached using the miniature screws positioned just below the ridges of the parietal bone of the skull. Once the head fixture is mounted, the skin abuts the edges of the fixture. Electromyography (EMG) fine wire electrodes (A-M Systems, Carlsborg, WA) were implanted into hip muscles and soldered to a micro-circuit board. A slot made in the plastic cap holds the micro-circuit board when the cap is secured on the chamber. Dental acrylic was applied along the inside of the chamber opening to enhance binding of the fixture and prevent leakage of saline placed to prevent drying of the tissues. After the surgery, the animals were returned to their cage and allowed 24 hours for recovery. No overt signs of stress, pain or change in behavior were evident after implantation of the head fixture. The head fixtures have been well tolerated afterwards and the skin has consistently healed well along the edge of the fixtures.
2.3. Skull alignment
On the day of the first recording session, in order to precisely target brain regions using a standard stereotaxic atlas, it is necessary to accurately achieve a flat skull position. We developed a unique tool to achieve this critical skull alignment and circumvent the need for ear bars and a mouth piece. We designed a system to adjust for the movement of the head in space, which follows a three axis coordinate system: yaw, pitch and roll. Yaw refers to the rotation about the Z or vertical axis, pitch about the Y or transverse axis and roll about the X or longitudinal axis. Our custom alignment tool (Fig. 2) consists of a clear Plexiglas stand that slides along the outer support rail of the stereotaxic frame. The platform is also fitted with a sensitive dial indicator and retractable probe. The retractable probe is connected to a pair of retractable arms that when level on the skull is reflected by a zero reading on the indicator dial.
On the first day of recordings, the rat’s heads were immobilized by clamping the head fixture into the stereotaxic positioner. Next, principal landmarks (bregma, lambda and the inter-aural line) were visually identified and the skull aligned as follows:
Yaw alignment (Fig. 3A)
The midline, connecting the bregma to lambda, was identified on the skull. The yaw alignment was achieved by adjusting the corresponding micrometer on the positioner such that the bregma-lambda line was visually centered within a reticule scribed on the platform.
Pitch alignment (Fig. 3B)
The dial test assembly was mounted such that the arms of the test indicator gently touch the midline. The corresponding micrometer on the positioner was adjusted until the dial test indicator was zeroed. Several measures were obtained at different points along the bregma-lambda line and their average was used for alignment.
Roll alignment (Fig. 3C)
The alignment procedure was carried out following a similar process as for the pitch alignment, except the probes of the dial indicator are oriented perpendicular to the midline and a third micrometer is adjusted accordingly.
The skull alignment is painless, non-invasive, and is performed without the use of anesthesia. The skull alignment needs to be done only once and with experience, was quickly performed in about 5 minutes. The values from the digital readouts on micrometers are recorded so that the appropriate skull position can be realigned at the start of each recording session. Thus, the skull can be rapidly realigned without the use of ear and mouth piece, minimizing setup related distress for the animal.
2.4. Craniotomy
After aligning the skull, the animals were anesthetized with isofluorane (1–2%) with the head restrained within the positioning mechanism. A 3.5 mm burr hole centered at 2 mm caudally and 1.5 mm laterally to the bregma reference point targeting the entopeduncular nucleus (EP) or globus pallidus (GP) was drilled into the bone exposing the underlying dura mater. The craniotomy was completed on the side contralateral to EMG electrodes using a drill supported by an adjustable stereotaxic arm. At the start of subsequent recording sessions, scar tissue that accumulated over the exposed dura mater was carefully removed and the chamber opening was thoroughly cleaned.
2.5. Microelectrode recordings
After drilling the burr hole, the anesthesia was discontinued and the animals promptly awoke. The animals consistently adapted quickly to the head-restraint and no separate training or habituation period was required. Within about 30 min. of the initial period of head restraining, neuronal recording sessions were initiated. In subsequent daily recording sessions, the head fixture could be easily and quickly restored, was mostly well tolerated, and recordings could be initiated immediately without an adaptation period. During recording sessions, the animal stood on the Plexiglass platform whose height was adjusted to position the animal to stand comfortably and freely move its limbs. Also, soft gauze was placed on either side of the animal, room noise was minimized and contact with the animals was limited to comfort the animal. In-between recording tracks, the animals, were regularly given formula to keep them hydrated.
A mini-XYZ-manipulator (Thomas Recording, Giessen, Germany) was mounted onto a stereotaxic arm and a concentric micro-drive head with 300 μm intra-electrode spacing was used to simultaneously introduce up to 3 glass coated tungsten microelectrodes (80 μm diameter) into the posterolateral (motor) territory of EP or GP. The following coordinates were used for targeting of the microelectrodes: 1) EP, 1.0–1.5 mm posterior from bregma and 2.9–3.4 mm lateral from midline, 2) GP, 0–1.0 mm anterior from bregma and 2.9–4.6 mm lateral from the midline. Extracellular recordings were collected by independently advancing the microelectrodes (impedance: 1 – 2 MΩ) to the targeted areas. Each nucleus was readily identified by its characteristic neuronal firing pattern. The optic tract, just below the EP, was readily identified by its distinct response to light flashes. During the recordings, this provided confirmation of the location of the microelectrodes. The location and firing patterns of cells and the borders of encountered nuclei along each microelectrode track were plotted and were superimposed on transparencies of parasagittal sections from the Paxinos and Watson atlas to estimate the precise location in the brain. During the recording sessions, the state of vigilance of the animals was routinely monitored.
The recorded neuronal activity was displayed over two oscilloscope screens (HAMEG Instruments, Mainhausen, Germany) and connected to an audioamplifier for aural monitoring of the signal. EMG activity was monitored continuously on a desktop computer using Sort Client (Plexon Inc., Dallas, TX). Both, neuronal and EMG activity were collected for a minimum of 120 secs at a sampling rate of 40 kHz and were passed into a Plexon pre-amplifier (gain = 50, bandwidth 0.07–8 kHz).
2.6 Histology
In five animals, at the end of the recording session, the rats were deeply anesthetized with sodium pentobarbital and perfused transcardially with formalin. The brains were removed and each hemisphere was paraffin embedded in the sagittal plane. The brains were sliced at 20 μm throughout the hemisphere and representative sections were stained with cresyl violet. 3.
RESULTS
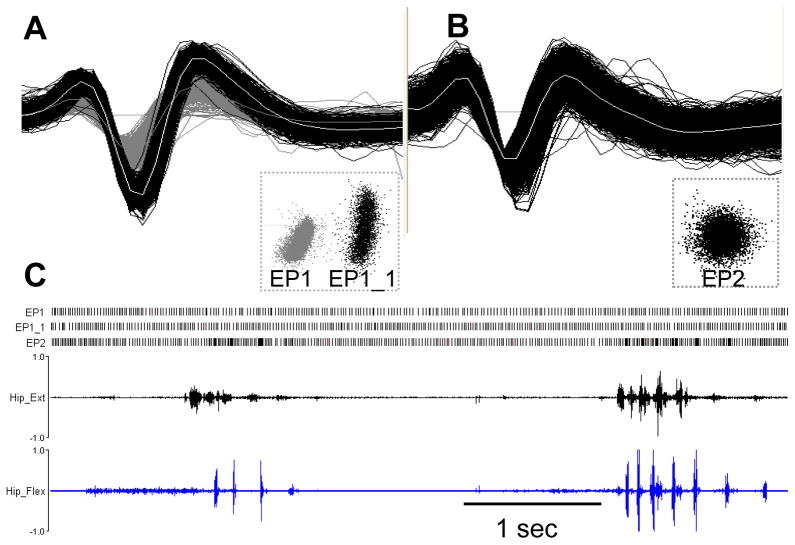
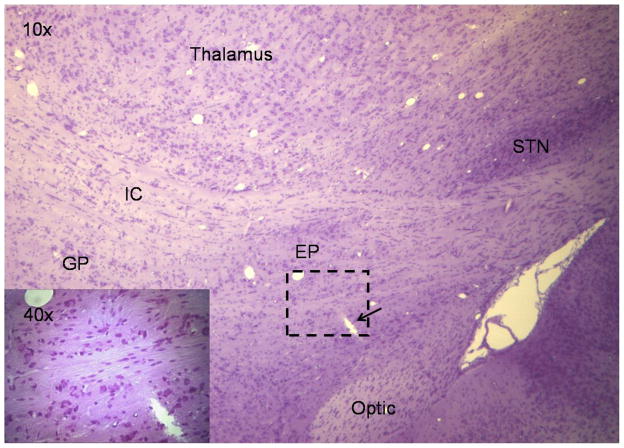
The assembly provided excellent stabilization of the skull during neuronal recordings, while the large opening at the center of the fixture permitted stereotaxic access at any desired angle. Based on physiological mapping to a standard atlas (Paxinos and Watson et al., 1998) in 18 animals, the initial targeting was within 0.5 ± 0.3 mm, which is comparable to standard ear bar guidance methods, 0.4 ± 0.3 mm for juvenile Wistar rats (Paxinos and Watson, 1998). Fig. 4 depicts an example of multi-unit recordings collected from the motor portion of EP during active movements in a head-restrained rat. Fig. 4A shows two EP units, EP1 and EP1_1, collected on a single microelectrode while Fig. 4B shows another EP unit, EP2, simultaneously collected on an adjacent microelectrode 300 μm apart. These units were readily discriminated into separate clusters using Principal Component Analysis (PCA) based on the size and shape of action potentials. Fig. 4C shows a raster display of these three units during periods of rest and active movements. Alternating agonist-antagonist bursts in hip muscles during the voluntary movements were associated with recurrent discharge bursts in the EP2 neuron. On the other hand, simultaneously recorded activity in EP1 and EP1_1 neurons showed regular, stereotypical tonic discharge firing throughout the recording period. These examples highlight that our head-restraint system permits stabilized recordings during active movements. Reconstruction of microelectrode recording tracts from histological sections confirmed the accuracy of the targeting system (Fig. 5).
Fig. 4.
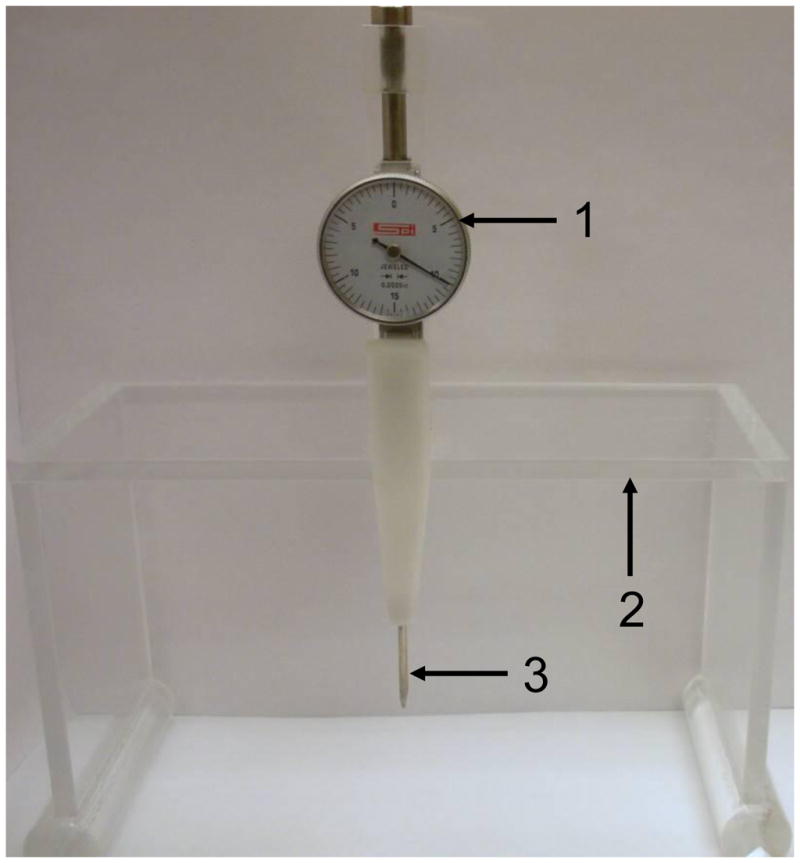
Customized tool for measuring skull alignment. 1. Dial test indicator 2. Plexiglas platform that slides along the stereotaxic frame 3. Retractable arms for measuring the leveling of the skull.
Fig. 5.
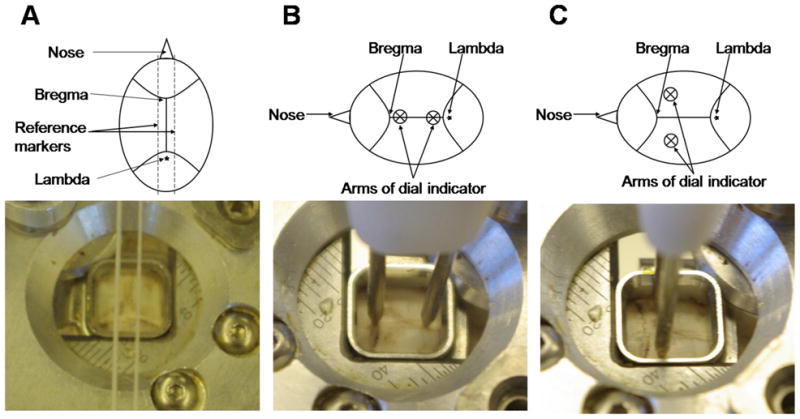
Adjustments for establishing a flat skull position. A. Yaw measurement using reference markers B. Pitch measurement using arms of the dial indicator C. Roll measurement using arms of the dial indicator.
4. DISCUSSION
In this study, we described a novel technique to perform neuronal recordings in awake, head-restrained young and juvenile rats. The head fixture and positioning system allow excellent stabilization of the head and accurate stereotaxic localization over multiple sessions of recording. We demonstrated the ability to maintain uninterrupted multi-unit recordings in deep brain nuclei in awake head-restrained actively moving animals. Based on superimposing detailed physiological reconstructions of the recording tracts onto the Paxinos and Watson rat atlas, the accuracy of our positioning system compared favorably with reported accuracy estimates using standard techniques (Paxinos and Watson et al., 1998). Our technique allows faster setup time during surgery and during multi-day recording sessions and appreciably reduces the weight of the implant thereby easing the setup process and reducing the stress to the animals.
A number of techniques have previously been introduced to record single unit activity in awake head-restrained rodents. One of the most utilized techniques involves immobilizing the head using a metallic headpost while recording through a separate recording chamber (Parry et al., 1993, Heck et al., 2002, Schonewille et al., 2006, Bryant et al., 2009). This method generally requires placing multiple screws into the skull and building up an epoxy base that secures in combination the supporting screws, headpost, and chamber. An alternative approach involves cementing a U-shaped headpiece to the skull which in turn is secured to a stereotaxic restraining frame (Soulière et al., 2000, Mahon et al., 2006). This technique allows high-quality single-unit recordings and provides wide stereotaxic access. Another group developed a head-restraining system using a restraining bridge and dummy ear bars (Fa et. al., 2002) which permits maintaining the head of the animal in the same spatial position as when using the conventional ear bars and mouth piece.
Our recording system offers a number of additional advantages over current recording systems. The durability of the head fixture was successfully established in animals of different sizes ranging from 45 to 220 gms in weight. Thus, based on our experience from recording in very young and juvenile rats, our system can be expected to be reliable for investigating young and adult rats. Our system is less cumbersome than existing systems, allowing greater access to the animal during experimental conditions. The positioning system permits relatively easy alignment of the head with the animal fully awake, while circumventing the need for using invasive ear bars. The positioning mechanism in turn allows rapid and accurate targeting of desired brain regions over multiple days by simply adjusting 3 dials (yaw, pitch and roll) at the start of each session. Although we chose to record from the basal ganglia and to simultaneously record EMG activity, the chamber could, without major design modification, be readily enlarged and adapted to access more lateral portions of the brain or to record, for example, EEG activity. Additionally, our system would readily permit micro-dialysis experiments, brain micro-stimulation, lesions and pharmacology studies.
The present study has some shortcomings. Although the animals mostly tolerated the 2–3 hour recording sessions very well and habituated quickly to the recording conditions, intense grooming uncommonly caused changes in amplitude or complete loss of neuronal activity. At such times, the recording was stopped and care was taken to make the animal comfortable usually by giving a food reward. Rarely the behavior persisted and the recording sessions were terminated prematurely. During early experiments the head fixture dislodged from the skull (in 4 animals) within 24–48 hrs of mounting because of improper mounting as a result of lack of experience on our part. None of the head fixtures dislodged in the last 18 animals used for this study. For the purpose of the current study, we have recorded from these animals for up to two weeks after implanting the head-restraint. Although, we expect the fixture to remain stable for several weeks after implantation further studies need to be performed to verify this. Over long periods of time, the head fixture would likely get dislodged because of increased bone porosity and growth of animal’s skull. Additionally, although we are confident that our physiological reconstructions of microelectrode recording tracts strongly support the accuracy of our positioning system, we also recognize the shortcoming posed by the limited anatomical support in our study.
In summary, in large part due to limitations of current restraining techniques, many investigators continue to investigate neuronal activity in sedated or anesthetized animals despite major limitations in the interpretation of such data. The use of our novel technique allows high quality stabilized neuronal recordings in awake head-restrained animals during periods of rest and active movements. The system can be very quickly attached to the skull and accurately aligned, is relatively lightweight, and can be quickly resecured for multiple recording sessions. By allowing daily repeat microelectrode studies in rodents without sedation or anesthesia, the use of our novel positioning system could more readily lead to greater advances in the understanding, and ultimately, the treatment of movement disorders and other neurological conditions.
Fig. 6.
Single unit extracellular discharge activity in the entopeduncular nucleus (EP) in a head-restrained rat during movement. A. Two neurons collected on a single microelectrode B. A third neuron collected simultaneously on an adjacent microelectrode 300 μm apart. The distinct clusters and shape of each unit illustrates the excellent quality of recordings obtained using our head-restraint system. C. Raster displays for these three neurons during periods of rest and active movements. The persistent tonic discharge activity in the EP1 and EP1_1 neurons during movement and the movement-related discharge bursts on a stable tonic background in the EP2 neuron together highlight the excellent stability of the head restraining system.
Fig. 7.
Anatomical support for accuracy of the positioning system. A. Nissl stained 10x sagittal section demonstrating the tip of a microelectrode (arrow) just below the lateral motor portion of EP. B. The dashed area in figure A. is shown magnified at 40x. STN- subthalamic nucleus, EP- entopeduncular nucleus, GP- globus pallidus, IC-internal capsule, Optic- optic tract.
Acknowledgments
This research was supported by NIH NINDS grant R01NS47151 and NIDCD grant R01DC00369 to SMS and Thomas F. and Kate Miller Jeffress Memorial Trust award to MSB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson M, O’Mara S. Analysis of recordings of single-unit firing and population activity in the dorsal subiculum of unrestrained, freely moving rats. J Neurophysiol. 2003;90:655–65. doi: 10.1152/jn.00723.2002. [DOI] [PubMed] [Google Scholar]
- Alkire M, Hudetz A, Tononi G. Consciousness and anesthesia. Science. 2008;322:876–80. doi: 10.1126/science.1149213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilkey DK, Muir GM. A low cost, high precision subminiature microdrive for extracellular unit recording in behaving animals. J Neurosci Methods. 1999;92:87–90. doi: 10.1016/s0165-0270(99)00102-8. [DOI] [PubMed] [Google Scholar]
- Bryant J, Roy S, Heck D. A technique for stereotaxic recordings of neuronal activity in awake, head-restrained mice. J Neurosci Methods. 2009;178:75–9. doi: 10.1016/j.jneumeth.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliades S, Wang X. Chronic multi-electrode neural recording in free-roaming monkeys. J Neurosci Methods. 2008;172:201–14. doi: 10.1016/j.jneumeth.2008.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fà M, Mereu G, Ghiglieri V, Meloni A, Salis P, Gessa G. Electrophysiological and pharmacological characteristics of nigral dopaminergic neurons in the conscious, head-restrained rat. Synapse. 2003;48:1–9. doi: 10.1002/syn.10177. [DOI] [PubMed] [Google Scholar]
- Goto Y, O’Donnell P. Network synchrony in the nucleus accumbens in vivo. 2001;21:4498–504. doi: 10.1523/JNEUROSCI.21-12-04498.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck D, Kmmell F, Thach W, Aertsena A. Dynamic correlation of neuronal activity in rat cerebellar cortex modulated by behavior. Ann NY Acad Sci. 2002;978:156–63. doi: 10.1111/j.1749-6632.2002.tb07563.x. [DOI] [PubMed] [Google Scholar]
- Jackson A, Fetz E. Compact movable microwire array for long-term chronic unit recording in cerebral cortex of primates. J Neurophysiol. 2007;98:3109–18. doi: 10.1152/jn.00569.2007. [DOI] [PubMed] [Google Scholar]
- Kasanetz F, Riquelme L, O’Donnell P, Murer MG. Turning off cortical ensembles stops striatal Up states and elicits phase perturbations in cortical and striatal slow oscillations in rat in vivo. J Physiol (Lond) 2006;577:97–113. doi: 10.1113/jphysiol.2006.113050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korshunov VA. Miniature microdrive for extracellular recording of neuronal activity in freely moving animals. J Neurosci Methods. 1995;57:77–80. doi: 10.1016/0165-0270(94)00130-9. [DOI] [PubMed] [Google Scholar]
- Mahon S, Vautrelle N, Pezard L, Slaght S, Deniau J, Chouvet G, Charpier S. Distinct patterns of striatal medium spiny neuron activity during the natural sleep-wake cycle. 2006;26:12587–95. doi: 10.1523/JNEUROSCI.3987-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll RA, Madison DV. General anesthetics hyperpolarize neurons in the vertebrate central nervous system. Science. 1982;217:1055–7. doi: 10.1126/science.7112112. [DOI] [PubMed] [Google Scholar]
- Pager J. A removable head-mounted microdrive for unit recording in the free-behaving rat. 1984;33:843–8. doi: 10.1016/0031-9384(84)90057-x. [DOI] [PubMed] [Google Scholar]
- Parry TJ, McElligott JG. A method for restraining awake rats using head immobilization. 1993;53:1011–5. doi: 10.1016/0031-9384(93)90283-l. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4. San Diego, CA: Academic Press; 1998. [Google Scholar]
- Schonewille M, Khosrovani S, Winkelman BHJ, Hoebeek F, De Jeu MTG, Larsen I, Van der Burg J, Schmolesky M, Frens M, De Zeeuw C. Purkinje cells in awake behaving animals operate at the upstate membrane potential. Nat Neurosci. 2006;9:459–61. doi: 10.1038/nn0406-459. [DOI] [PubMed] [Google Scholar]
- Soulire F, Urbain N, Gervasoni D, Schmitt P, Guillemort C, Fort P, Renaud B, Luppi PH, Chouvet G. Single-unit and polygraphic recordings associated with systemic or local pharmacology: a multi-purpose stereotaxic approach for the awake, anaesthetic-free, and head-restrained rat. J Neurosci Res. 2000;61:88–100. doi: 10.1002/1097-4547(20000701)61:1<88::AID-JNR11>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Wilson CJ, Kawaguchi Y. The origins of two-state spontaneous membrane potential fluctuations of neostriatal spiny neurons. 1996;16:2397–410. doi: 10.1523/JNEUROSCI.16-07-02397.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]