Abstract
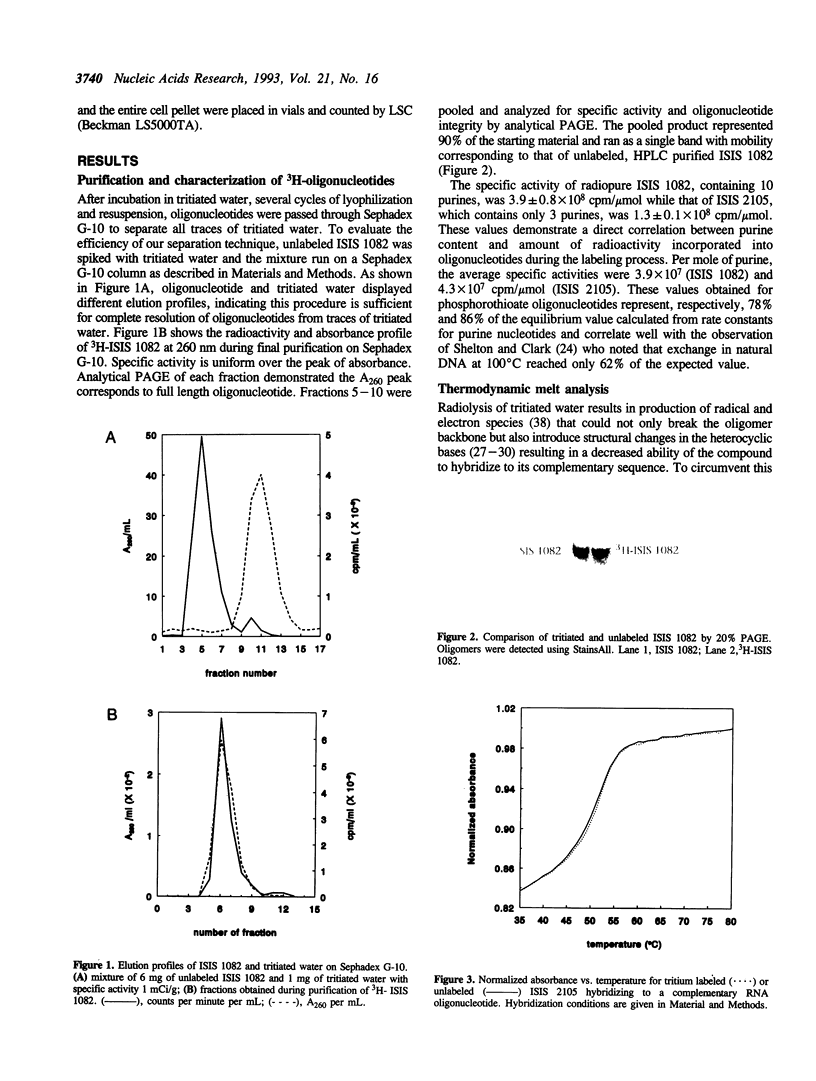
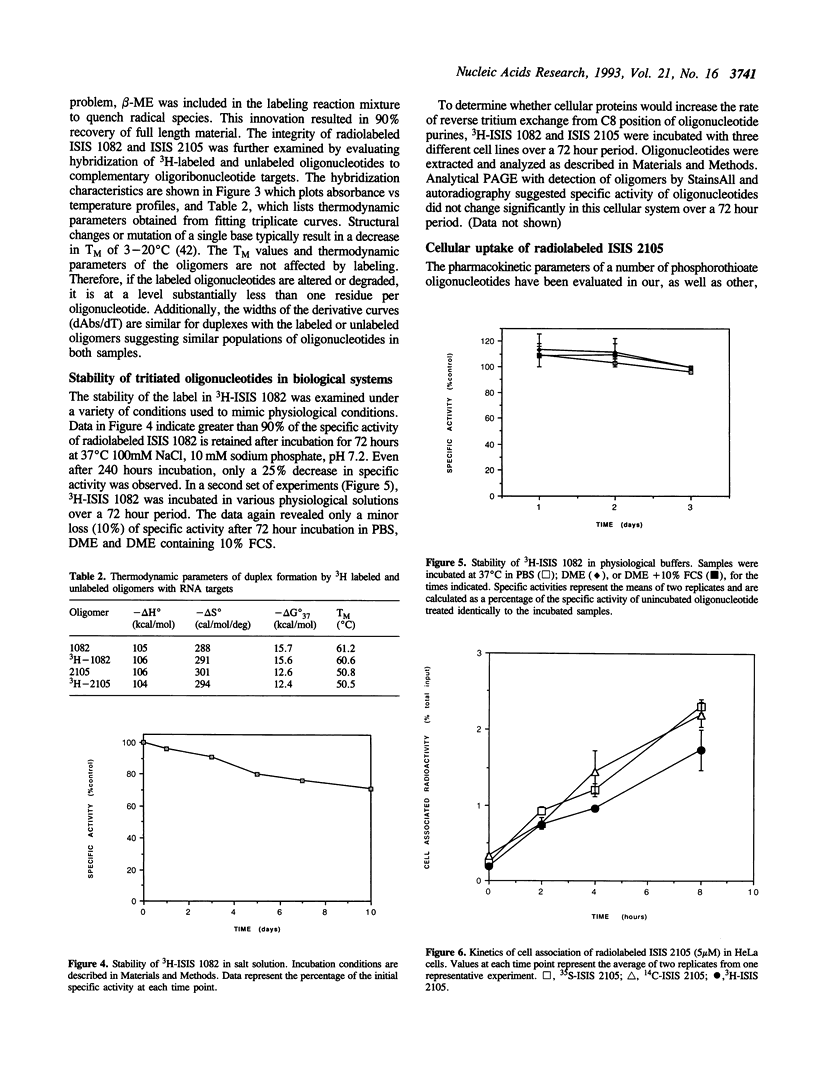
We describe a simple, efficient, procedure for labeling oligonucleotides to high specific activity (< 1 x 10(8) cpm/mumol) by hydrogen exchange with tritiated water at the C8 positions of purines in the presence of beta-mercaptoethanol, an effective radical scavenger. Approximately 90% of the starting material is recovered as intact, labeled oligonucleotide. The radiolabeled compounds are stable in biological systems; greater than 90% of the specific activity is retained after 72 hr incubation at 37 degrees C in serum-containing media. Data obtained from in vitro cellular uptake experiments using oligonucleotides labeled by this method are similar to those obtained using 35S or 14C-labeled compounds. Because this protocol is solely dependent upon the existence of purine residues, it should be useful for radiolabeling modified as well as unmodified phosphodiester oligonucleotides.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benevides J. M., Thomas G. J., Jr Dependence of purine 8C-H exchange on nucleic acid conformation and base-pairing geometry: a dynamic probe of DNA and RNA secondary structures. Biopolymers. 1985 Apr;24(4):667–682. doi: 10.1002/bip.360240407. [DOI] [PubMed] [Google Scholar]
- Chiang M. Y., Chan H., Zounes M. A., Freier S. M., Lima W. F., Bennett C. F. Antisense oligonucleotides inhibit intercellular adhesion molecule 1 expression by two distinct mechanisms. J Biol Chem. 1991 Sep 25;266(27):18162–18171. [PubMed] [Google Scholar]
- Crooke R. M., Hoke G. D., Shoemaker J. E. In vitro toxicological evaluation of ISIS 1082, a phosphorothioate oligonucleotide inhibitor of herpes simplex virus. Antimicrob Agents Chemother. 1992 Mar;36(3):527–532. doi: 10.1128/aac.36.3.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crooke R. M. In vitro toxicology and pharmacokinetics of antisense oligonucleotides. Anticancer Drug Des. 1991 Dec;6(6):609–646. [PubMed] [Google Scholar]
- Doppler-Bernardi F., Felsenfeld G. In vitro incorporation of tritium into native DNA. Biopolymers. 1969;8(6):733–741. doi: 10.1002/bip.1969.360080604. [DOI] [PubMed] [Google Scholar]
- Freier S. M., Albergo D. D., Turner D. H. Solvent effects on the dynamics of (dG-dC)3. Biopolymers. 1983 Apr;22(4):1107–1131. doi: 10.1002/bip.360220408. [DOI] [PubMed] [Google Scholar]
- Fritzsche H. Infrared studies of deoxyribonucleic acids, their constituents and analogues. 3. Evidence of slow isotopic exchange of hydrogen attached to carbon in DNA. Biochim Biophys Acta. 1967 Nov 21;149(1):173–179. doi: 10.1016/0005-2787(67)90699-5. [DOI] [PubMed] [Google Scholar]
- Lapiashvili G. N., Lesnik E. A., Maslova R. N., Varshavsky Y. M. A kinetic study of 1H leads to 3H exchange in C(8) H-groups of purinic residues in DNA. Nucleic Acids Res. 1977 Jul;4(7):2181–2189. doi: 10.1093/nar/4.7.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesnik E. A., Maslova R. N., Agranovich I. M., Varshavsky YaM Conformational peculiarities of polynucleotides with a nonrandom base sequence according to the 1H----3H exchange rate in C8H groups of purinic residues. J Biomol Struct Dyn. 1987 Dec;5(3):601–614. doi: 10.1080/07391102.1987.10506415. [DOI] [PubMed] [Google Scholar]
- Maslova R. N., Lasnik E. A., Varshavsky YaM Hydrogen exchange rate in C(8)H groups of purine residues as a tool for estimation of their pKa values in nucleic acids. FEBS Lett. 1974 Dec 15;49(2):181–187. doi: 10.1016/0014-5793(74)80507-7. [DOI] [PubMed] [Google Scholar]
- Maslova R. N., Lesnik E. A., Varshavsky Y. M. Studies on the effect of conformation on the rate of the slow isotope hydrogen exchange in polyadenylic acid. Biochem Biophys Res Commun. 1969 Feb 7;34(3):260–265. doi: 10.1016/0006-291x(69)90825-0. [DOI] [PubMed] [Google Scholar]
- Monia B. P., Johnston J. F., Ecker D. J., Zounes M. A., Lima W. F., Freier S. M. Selective inhibition of mutant Ha-ras mRNA expression by antisense oligonucleotides. J Biol Chem. 1992 Oct 5;267(28):19954–19962. [PubMed] [Google Scholar]
- Narang S. A., Brousseau R., Hsiung H. M., Michniewicz J. J. Chemical synthesis of deoxyoligonucleotides by the modified triester method. Methods Enzymol. 1980;65(1):610–620. doi: 10.1016/s0076-6879(80)65063-0. [DOI] [PubMed] [Google Scholar]
- Nielsen P. E., Egholm M., Berg R. H., Buchardt O. Sequence-selective recognition of DNA by strand displacement with a thymine-substituted polyamide. Science. 1991 Dec 6;254(5037):1497–1500. doi: 10.1126/science.1962210. [DOI] [PubMed] [Google Scholar]
- Puglisi J. D., Tinoco I., Jr Absorbance melting curves of RNA. Methods Enzymol. 1989;180:304–325. doi: 10.1016/0076-6879(89)80108-9. [DOI] [PubMed] [Google Scholar]
- Searcy D. G. Techniques for DNA hybridization in vitro using non-radioactive DNA and DNA made radioactive by neutron activation, alkylation with radioactive alkylating agents, and by exchange with 3H2O. Biochim Biophys Acta. 1968 Sep 24;166(2):360–370. doi: 10.1016/0005-2787(68)90223-2. [DOI] [PubMed] [Google Scholar]
- Shelton K. R., Clark J. M., Jr In vitro tritium labelling of DNA. Biochem Biophys Res Commun. 1968 Dec 9;33(5):850–854. doi: 10.1016/0006-291x(68)90239-8. [DOI] [PubMed] [Google Scholar]
- Stawinski J., Hozumi T., Narang S. A., Bahl C. P., Wu R. Arylsulfonyltetrazoles, new coupling reagents and further improvements in the triester method for the synthesis of deoxyribooligonucleotides. Nucleic Acids Res. 1977 Feb;4(2):353–371. doi: 10.1093/nar/4.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein A., Iversen P. L., Subasinghe C., Cohen J. S., Stec W. J., Zon G. Preparation of 35S-labeled polyphosphorothioate oligodeoxyribonucleotides by use of hydrogen phosphonate chemistry. Anal Biochem. 1990 Jul;188(1):11–16. doi: 10.1016/0003-2697(90)90521-a. [DOI] [PubMed] [Google Scholar]
- Studencki A. B., Wallace R. B. Allele-specific hybridization using oligonucleotide probes of very high specific activity: discrimination of the human beta A- and beta S-globin genes. DNA. 1984;3(1):7–15. doi: 10.1089/dna.1.1984.3.7. [DOI] [PubMed] [Google Scholar]
- Tomasz M., Olson J., Mercado C. M. Mechanism of the isotopic exchange of the C-8 hydrogen of purines in nucleosides and in deoxyribonucleic acid. Biochemistry. 1972 Mar 28;11(7):1235–1241. doi: 10.1021/bi00757a019. [DOI] [PubMed] [Google Scholar]
- Yamamoto O., Fuji I., Ogawa M. Difference in DNA strand break by gamma- and beta-irradiations: an in vitro study. Biochem Int. 1985 Aug;11(2):217–223. [PubMed] [Google Scholar]




