Abstract
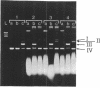
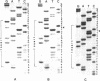
The mutagenic activity of a series of longer chain O6-n-alkylguanine residues (O6-n-propyl, O6-n-butyl, O6-n-octyl) has been analyzed using a plasmid molecule (pUC 9) in which single O6-alkylguanines were positioned in the unique Pstl recognition site by shot gun ligation (Nucleic Acids Res. 13, 3305-3316 (1985)) of overlapping synthetic oligonucleotides. After transfection of these vectors into E. coli cells having normal DNA repair systems, progeny plasmids were produced, of which 2.6%, 2.8% and 4.3% were mutated in their Pstl site when containing O6-n-propylguanine, O6-n-butylguanine, O6-n-octylguanine, respectively. DNA sequence analysis of mutant plasmid genomes revealed that O6-n-propylguanine and O6-n-butylguanine induced exclusively G-->A transitions located specifically at the preselected site. O6-n-octylguanine induced apart from G-->A transitions (70%) also targeted G-->T transversions (30%). These results indicate that the mutation frequency of longer chain O6-alkylguanines can be substantial in cells with normal repair systems and that the mutation pattern depends on the nature of the alkyl group.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balmain A., Brown K. Oncogene activation in chemical carcinogenesis. Adv Cancer Res. 1988;51:147–182. doi: 10.1016/s0065-230x(08)60222-5. [DOI] [PubMed] [Google Scholar]
- Basu A. K., Essigmann J. M. Site-specifically alkylated oligodeoxynucleotides: probes for mutagenesis, DNA repair and the structural effects of DNA damage. Mutat Res. 1990 Nov-Dec;233(1-2):189–201. doi: 10.1016/0027-5107(90)90162-w. [DOI] [PubMed] [Google Scholar]
- Basu A. K., Essigmann J. M. Site-specifically modified oligodeoxynucleotides as probes for the structural and biological effects of DNA-damaging agents. Chem Res Toxicol. 1988 Jan-Feb;1(1):1–18. doi: 10.1021/tx00001a001. [DOI] [PubMed] [Google Scholar]
- Borowy-Borowski H., Chambers R. W. A study of side reactions occurring during synthesis of oligodeoxynucleotides containing O6-alkyldeoxyguanosine residues at preselected sites. Biochemistry. 1987 May 5;26(9):2465–2471. doi: 10.1021/bi00383a010. [DOI] [PubMed] [Google Scholar]
- Chambers R. W. Site-specific mutagenesis in cells with normal DNA repair systems: transitions produced from DNA carrying a single O6-alkylguanine. Nucleic Acids Res. 1991 May 11;19(9):2485–2488. doi: 10.1093/nar/19.9.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers R. W., Sledziewska-Gojska E., Hirani-Hojatti S., Borowy-Borowski H. uvrA and recA mutations inhibit a site-specific transition produced by a single O6-methylguanine in gene G of bacteriophage phi X174. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7173–7177. doi: 10.1073/pnas.82.21.7173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers R. W., Sledziewska-Gojska E., Hirani-Hojatti S. In vivo effect of DNA repair on the transition frequency produced from a single O6-methyl- or O6-n-butyl-guanine in a T:G base pair. Mol Gen Genet. 1988 Aug;213(2-3):325–331. doi: 10.1007/BF00339598. [DOI] [PubMed] [Google Scholar]
- Coulondre C., Miller J. H. Genetic studies of the lac repressor. IV. Mutagenic specificity in the lacI gene of Escherichia coli. J Mol Biol. 1977 Dec 15;117(3):577–606. doi: 10.1016/0022-2836(77)90059-6. [DOI] [PubMed] [Google Scholar]
- Druckrey H., Preussmann R., Ivankovic S., Schmähl D. Organotrope carcinogene Wirkungen bei 65 verschiedenen N-Nitroso-Verbindungen an BD-Ratten. Z Krebsforsch. 1967;69(2):103–201. [PubMed] [Google Scholar]
- Ellison K. S., Dogliotti E., Connors T. D., Basu A. K., Essigmann J. M. Site-specific mutagenesis by O6-alkylguanines located in the chromosomes of mammalian cells: influence of the mammalian O6-alkylguanine-DNA alkyltransferase. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8620–8624. doi: 10.1073/pnas.86.22.8620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer P. B., Foster A. B., Jarman M., Tisdale M. J. The alkylation of 2'-deoxyguanosine and of thymidine with diazoalkanes. Some observations on o-alkylation. Biochem J. 1973 Sep;135(1):203–213. doi: 10.1042/bj1350203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goth R., Rajewsky M. F. Persistence of O6-ethylguanine in rat-brain DNA: correlation with nervous system-specific carcinogenesis by ethylnitrosourea. Proc Natl Acad Sci U S A. 1974 Mar;71(3):639–643. doi: 10.1073/pnas.71.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green C. L., Loechler E. L., Fowler K. W., Essigmann J. M. Construction and characterization of extrachromosomal probes for mutagenesis by carcinogens: site-specific incorporation of O6-methylguanine into viral and plasmid genomes. Proc Natl Acad Sci U S A. 1984 Jan;81(1):13–17. doi: 10.1073/pnas.81.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundström T., Zenke W. M., Wintzerith M., Matthes H. W., Staub A., Chambon P. Oligonucleotide-directed mutagenesis by microscale 'shot-gun' gene synthesis. Nucleic Acids Res. 1985 May 10;13(9):3305–3316. doi: 10.1093/nar/13.9.3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R. C., Reddy M. V., Randerath K. 32P-postlabeling analysis of non-radioactive aromatic carcinogen--DNA adducts. Carcinogenesis. 1982;3(9):1081–1092. doi: 10.1093/carcin/3.9.1081. [DOI] [PubMed] [Google Scholar]
- Kleihues P., Margison G. P. Carcinogenicity of N-methyl-N-nitrosourea: possible role of excision repair of O6-methylguanine from DNA. J Natl Cancer Inst. 1974 Dec;53(6):1839–1841. [PubMed] [Google Scholar]
- Koehl P., Burnouf D., Fuchs R. P. Construction of plasmids containing a unique acetylaminofluorene adduct located within a mutation hot spot. A new probe for frameshift mutagenesis. J Mol Biol. 1989 May 20;207(2):355–364. doi: 10.1016/0022-2836(89)90259-3. [DOI] [PubMed] [Google Scholar]
- Loechler E. L., Green C. L., Essigmann J. M. In vivo mutagenesis by O6-methylguanine built into a unique site in a viral genome. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6271–6275. doi: 10.1073/pnas.81.20.6271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loveless A. Possible relevance of O-6 alkylation of deoxyguanosine to the mutagenicity and carcinogenicity of nitrosamines and nitrosamides. Nature. 1969 Jul 12;223(5202):206–207. doi: 10.1038/223206a0. [DOI] [PubMed] [Google Scholar]
- Mackay W., Benasutti M., Drouin E., Loechler E. L. Mutagenesis by (+)-anti-B[a]P-N2-Gua, the major adduct of activated benzo[a]pyrene, when studied in an Escherichia coli plasmid using site-directed methods. Carcinogenesis. 1992 Aug;13(8):1415–1425. doi: 10.1093/carcin/13.8.1415. [DOI] [PubMed] [Google Scholar]
- Miller E. C. Some current perspectives on chemical carcinogenesis in humans and experimental animals: Presidential Address. Cancer Res. 1978 Jun;38(6):1479–1496. [PubMed] [Google Scholar]
- Mitra G., Pauly G. T., Kumar R., Pei G. K., Hughes S. H., Moschel R. C., Barbacid M. Molecular analysis of O6-substituted guanine-induced mutagenesis of ras oncogenes. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8650–8654. doi: 10.1073/pnas.86.22.8650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto K., Tanaka A., Yamaha T. Reaction of 1-n-propyl-1-nitrosourea with DNA in vitro. Carcinogenesis. 1983 Nov;4(11):1455–1458. doi: 10.1093/carcin/4.11.1455. [DOI] [PubMed] [Google Scholar]
- Pauly G. T., Powers M., Pei G. K., Moschel R. C. Synthesis and properties of H-ras DNA sequences containing O6-substituted 2'-deoxyguanosine residues at the first, second, or both positions of codon 12. Chem Res Toxicol. 1988 Nov-Dec;1(6):391–397. doi: 10.1021/tx00006a011. [DOI] [PubMed] [Google Scholar]
- Reid T. M., Lee M. S., King C. M. Mutagenesis by site-specific arylamine adducts in plasmid DNA: enhancing replication of the adducted strand alters mutation frequency. Biochemistry. 1990 Jul 3;29(26):6153–6161. doi: 10.1021/bi00478a007. [DOI] [PubMed] [Google Scholar]
- Richardson K. K., Richardson F. C., Crosby R. M., Swenberg J. A., Skopek T. R. DNA base changes and alkylation following in vivo exposure of Escherichia coli to N-methyl-N-nitrosourea or N-ethyl-N-nitrosourea. Proc Natl Acad Sci U S A. 1987 Jan;84(2):344–348. doi: 10.1073/pnas.84.2.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saffhill R., Margison G. P., O'Connor P. J. Mechanisms of carcinogenesis induced by alkylating agents. Biochim Biophys Acta. 1985 Dec 17;823(2):111–145. doi: 10.1016/0304-419x(85)90009-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmeiser H., Dipple A., Schurdak M. E., Randerath E., Randerath K. Comparison of 32P-postlabeling and high pressure liquid chromatographic analyses for 7,12-dimethylbenz[a]anthracene--DNA adducts. Carcinogenesis. 1988 Apr;9(4):633–638. doi: 10.1093/carcin/9.4.633. [DOI] [PubMed] [Google Scholar]
- Singer B., Essigmann J. M. Site-specific mutagenesis: retrospective and prospective. Carcinogenesis. 1991 Jun;12(6):949–955. doi: 10.1093/carcin/12.6.949. [DOI] [PubMed] [Google Scholar]
- Singer B., Kuśmierek J. T. Chemical mutagenesis. Annu Rev Biochem. 1982;51:655–693. doi: 10.1146/annurev.bi.51.070182.003255. [DOI] [PubMed] [Google Scholar]
- Singer B. O-alkyl pyrimidines in mutagenesis and carcinogenesis: occurrence and significance. Cancer Res. 1986 Oct;46(10):4879–4885. [PubMed] [Google Scholar]
- Spiegelhalder B., Preussmann R. Occupational nitrosamine exposure. 1. Rubber and tyre industry. Carcinogenesis. 1983 Sep;4(9):1147–1152. doi: 10.1093/carcin/4.9.1147. [DOI] [PubMed] [Google Scholar]
- Strauss B. S. Translesion DNA synthesis: polymerase response to altered nucleotides. Cancer Surv. 1985;4(3):493–516. [PubMed] [Google Scholar]
- Sukumar S., Notario V., Martin-Zanca D., Barbacid M. Induction of mammary carcinomas in rats by nitroso-methylurea involves malignant activation of H-ras-1 locus by single point mutations. Nature. 1983 Dec 15;306(5944):658–661. doi: 10.1038/306658a0. [DOI] [PubMed] [Google Scholar]
- Swann P. F. Why do O6-alkylguanine and O4-alkylthymine miscode? The relationship between the structure of DNA containing O6-alkylguanine and O4-alkylthymine and the mutagenic properties of these bases. Mutat Res. 1990 Nov-Dec;233(1-2):81–94. doi: 10.1016/0027-5107(90)90153-u. [DOI] [PubMed] [Google Scholar]
- Ullmann A., Jacob F., Monod J. Characterization by in vitro complementation of a peptide corresponding to an operator-proximal segment of the beta-galactosidase structural gene of Escherichia coli. J Mol Biol. 1967 Mar 14;24(2):339–343. doi: 10.1016/0022-2836(67)90341-5. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Zarbl H., Sukumar S., Arthur A. V., Martin-Zanca D., Barbacid M. Direct mutagenesis of Ha-ras-1 oncogenes by N-nitroso-N-methylurea during initiation of mammary carcinogenesis in rats. 1985 May 30-Jun 5Nature. 315(6018):382–385. doi: 10.1038/315382a0. [DOI] [PubMed] [Google Scholar]