Abstract
Background
Horizontal gene transfer (HGT) is recognized as one of the major forces for bacterial genome evolution. Many clinically important bacteria may acquire virulence factors and antibiotic resistance through HGT. The comparative genomic analysis has become an important tool for identifying HGT in emerging pathogens. In this study, the Serine-Aspartate Repeat (Sdr) family has been compared among different sources of Staphylococcus aureus (S. aureus) to discover sequence diversities within their genomes.
Methodology/Principal Findings
Four sdr genes were analyzed for 21 different S. aureus strains and 218 mastitis-associated S. aureus isolates from Canada. Comparative genomic analyses revealed that S. aureus strains from bovine mastitis (RF122 and mastitis isolates in this study), ovine mastitis (ED133), pig (ST398), chicken (ED98), and human methicillin-resistant S. aureus (MRSA) (TCH130, MRSA252, Mu3, Mu50, N315, 04-02981, JH1 and JH9) were highly associated with one another, presumably due to HGT. In addition, several types of insertion and deletion were found in sdr genes of many isolates. A new insertion sequence was found in mastitis isolates, which was presumably responsible for the HGT of sdrC gene among different strains. Moreover, the sdr genes could be used to type S. aureus. Regional difference of sdr genes distribution was also indicated among the tested S. aureus isolates. Finally, certain associations were found between sdr genes and subclinical or clinical mastitis isolates.
Conclusions
Certain sdr gene sequences were shared in S. aureus strains and isolates from different species presumably due to HGT. Our results also suggest that the distributional assay of virulence factors should detect the full sequences or full functional regions of these factors. The traditional assay using short conserved regions may not be accurate or credible. These findings have important implications with regard to animal husbandry practices that may inadvertently enhance the contact of human and animal bacterial pathogens.
Introduction
Staphylococcus aureus (S. aureus) is a highly adaptive and versatile gram-positive bacterium that presents growing and formidable global challenges for human and animal health concerns [1]. S. aureus can cause diseases ranging from superficial skin infections to life-threatening diseases such as pneumonia meningitis osteomyelitis endocarditis toxic shock syndrome (TSS) chest pain bacteremia and sepsis in human [2]. S. aureus also colonizes a range of other mammals including companion animals such as dogs, cats and horses [3], and livestock such as cows, pigs and goats [4], [5]. It can also colonize birds such as chickens and turkeys [6], [7], [8]. Thus, understanding the pathogenesis of S. aureus in different hosts is very important.
Comparative analyses of different S. aureus genomes have revealed that many strains have independently acquired genes from members of their surrounding microflora that confer antibiotic resistance and/or encode virulence factors [9], [10], [11]. The horizontal gene transfer (HGT) of mobile genetic elements (MGEs) among bacteria is the primary mode for the spread of antibiotic resistance and virulence factors in clinically important pathogens [11], [12]. MGEs consist of viruses, plasmids and associated elements (insertion sequences, transposons and integrons) that are either self-transmissible or use mobile plasmids and viruses as vehicles for their dissemination [13], [14]. A hypothesis that specific combinations of virulence factors encoded within MGEs are exchanged among strains by a “plug and play” mechanism has been proposed to explain occurrence of clones that are particularly well-adapted for causing certain diseases or infecting specific hosts [15], [16].
In comparison with the sequenced S. aureus strains associated with human infection, allelic variation in bovine strain RF122, also known as strain ET3-1, was high among virulence and surface-associated genes involved in host colonization, toxin production, iron metabolism, antibiotic resistance and gene regulation [16]. It is interesting that the majority of the RF122-unique genes were encoded by MGEs [16]. Furthermore, genes encoding well-known virulence factors such as spa, clfA, sdrC and ebh in RF122 contained premature codons and thus are pseudogenes [16]. Recently, McCarthy and Lindsay [17] confirmed that many of the S. aureus surface protein genes were missing or truncated in 58 strains with published sequences from various types of hosts. Thus, it is plausible that surface proteins are potential targets for horizontal gene transfer of mobile genetic elements. The Serine-Aspartate Repeat (Sdr) family is one type of the cell wall-anchored proteins. However, whether these genes are inclined to mutation like other surface protein genes has not been reported.
The Sdr proteins in S. aureus are members of the Microbial Surface Components Recognizing Adhesive Matrix Molecules (MSCRAMM) family encoded by the tandemly arrayed sdrC, sdrD and sdrE genes [18]. In addition, sdrF, sdrG and sdrH have been reported in Staphylococcus epidermidis (S. epidermidis) [19]. The Sdr proteins are characterized by the presence of an R region containing various numbers of the Ser-Asp dipeptides. The Sdr proteins have a similar structural organization. A signal peptide is followed by an A region which is similar in size among the different members of the Sdr family. However, they are not closely related with only 20–30% identical amino acid residues [18]. The A region is followed by B repeats. The Sdr proteins have two, three or five additional 110- to 113-residue sequences (B repeats) that are tandemly repeated in SdrC, SdrE and SdrD, respectively. The B repeats are followed by the R region. The C termini contain LPXTG motifs and hydrophobic amino acid segments. However, SdrH in S. epidermidis has a short 60-residue A region at its N terminus followed by the R domain without B repeats [19]. In addition, SdrH in S. epidermidis has a unique 277-residue C region and a C-terminal hydrophobic segment, without the LPXTG motif [19]. A few ligands for Sdr proteins in S. aureus have been identified: bone sialoprotein as a ligand for Bbp (bone sialo-binding protein), which is an allelic variant of SdrE [20], and beta-neurexin as a ligand for SdrC [21]. Moreover, the ligands for SdrF and SdrG in S. epidermidis were type I collagen and fibrinogen, respectively [22], [23].
The function of Sdr proteins in S. aureus remains unknown. However, there have been a few studies which reported a strong correlation between sdr genes of S. aureus and certain human diseases according to the distributional assay of sdr genes. Peacock et al. [24] demonstrated a strong correlation between S. aureus invasiveness and the presence of one of the allelic variants of the sdrE gene. Moreover, Trad et al. [25] reported a significantly higher prevalence of the sdrD gene in S. aureus strains responsible for bone infections. Sabat et al. [26] also showed that the sdrD gene was significantly associated with osteomyelitis but not with blood infections. On the other hand, there were no significant correlations of sdrE with blood infections and with osteomyelitis [26]. While sdrD was significantly associated with methicillin-resistant S. aureus (MRSA) strains, the sdrE distribution did not differ between the MSSA and MRSA strains [26]. Nevertheless, there has been little systemic research on the distribution of sdr genes in S. aureus isolates from bovine mastitis.
The traditional assay for distribution of genes involves amplifying the most conserved regions of genes, usually 100–1000 bp, in order to confirm the presence or absence of the targets [26], [27]. With the rapid development of sequencing technology and much cheaper prices for sequencing, it is now feasible to sequence whole length of a target gene or a targeted function region for identifying the presence of the gene and revealing mutations in the un-conserved regions at the same time. In the current study, therefore, the whole length of both the A region and B repeats of sdrC, sdrD, sdrE genes and the whole length of sdrH gene were amplified. Our results revealed several insertion and deletion mutation sites in these sdr genes. Further bio-informatics analyses showed the potential existence of horizontal gene transfer of mobile genetic elements. In addition, the correlation between the distribution of sdr genes in these isolates and clinical or subclinical symptoms was calculated.
Results
Sequence information revealed the existence of mutations in sdr genes in bovine mastitis isolates
To identify whether there was any difference between the specific sequences of sdrC, sdrD, sdrE and sdrH from bovine mastitis and those of the same genes in sequenced strains, sequence alignment was performed with DNAMAN software (version 6.0). As shown in Table 1, the A region and B repeats for sdrC in isolates from Ontario and Western Canada and the A region and B repeats for sdrD from all four regions shared 99.15% and 99.84% DNA sequence identity with those from the MRSA strain named TCH130, respectively. Interestingly, sdrD from all four regions contained a 1623_1626delATCT deletion mutation in the C-terminus of the A region, resulting in a frameshift that terminated translation at 553Leu, and loss of 832aa at the C-terminus. The A region and B repeats for sdrE in isolates from Ontario and Western Canada shared 98.83% DNA sequence identity with those from strain JKD6159. The full sequence of sdrH in isolates from Ontario and Western Canada shared 98.65% sequence identity with strains Mu3 and Mu50. The A region and B repeats for SdrC, sdrE and the full sequence of sdrH in isolates from Quebec and Eastern Canada shared 100.00%, 99.96% and 100.00% DNA sequence identity with those from strain RF122, respectively. On the other hand, the A region and B repeats for sdrD in isolates from Quebec and Eastern Canada shared 99.84% sequence identity with those from strain TCH130.
Table 1. DNA sequence identities (%) of sdr genes between S. aureus isolates in Canada and sequenced S. aureus strains.
| Strain | Ontario & Western Canada Isolates | Quebec & Eastern Canada Isolates | ||||||
| sdrC(2001)* | sdrD(3209) | sdrE(2649) | sdrH(1233) | sdrC(1969) | sdrD(3209) | sdrE(2649) | sdrH(1299) | |
| RF122 | 90.91 | - | 87.79 | 89.17 | 100.00 | - | 99.96 | 100.00 |
| MRSA252 | 90.10 | - | 87.13 | 93.66 | 95.42 | - | 95.16 | 87.55 |
| ST398 | 90.81 | 89.70 | - | 91.20 | 95.92 | 89.70 | - | 91.91 |
| ED133 | 90.61 | 89.95 | 96.43 | 92.70 | 95.52 | 89.95 | 89.09 | 93.10 |
| JKD6159 | 88.58 | 89.31 | 98.83 | 90.63 | 91.08 | 89.31 | 87.56 | 90.35 |
| TCH130 | 99.15 | 99.84 | 96.70 | 99.36 | 90.81 | 99.84 | 86.72 | 89.32 |
| MSSA476 | 94.99 | 93.68 | 96.32 | 89.84 | 91.12 | 93.68 | 88.56 | 86.05 |
| MW2 | 94.94 | 93.62 | 96.32 | 96.80 | 91.07 | 93.62 | 88.56 | 91.18 |
| ED98 | 95.53 | 99.60 | 97.63 | 97.71 | 88.94 | 99.60 | 88.19 | 90.80 |
| N315, JH1, JH9, 04-02981 | 95.48 | 99.63 | 97.60 | 97.71 | 88.94 | 99.63 | 88.12 | 90.80 |
| Mu50, Mu3 | 95.53 | 99.63 | 97.56 | 98.65 | 88.94 | 99.63 | 88.08 | 89.99 |
| TW20 | 94.39 | 94.44 | 96.17 | 96.80 | 89.60 | 94.44 | 88.53 | 91.18 |
| NCTC8325 | 94.39 | 94.32 | - | 96.72 | 89.60 | 94.32 | - | 91.10 |
| COL | 94.44 | 94.48 | 95.56 | 97.71 | 89.55 | 94.48 | 87.97 | 90.88 |
| Newman | 94.44 | 94.51 | 95.56 | 96.80 | 89.55 | 94.51 | 87.97 | 91.18 |
| USA300_FRP3757USA300_TCH1516 | 94.39 | 94.44 | 95.63 | 97.16 | 89.50 | 94.44 | 88.04 | 90.36 |
*Nucleotide numbers of the A region and B repeats of sdrC, sdrD and sdrE and the full sequence of sdrH in bovine mastitis isolates.
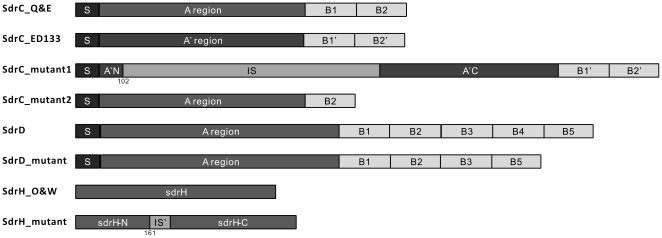
Several types of insertion and deletion in specific sdr sequences were found (Figure 1). An insertion sequence was found in the A region of sdrC from three subclinical isolates of one individual cow in Eastern Canada at codon 102 (Figure 1). Interestingly, even the sequences for flanking regions of the insertion of these three isolates were different from others from the same region. The BLAST results in NCBI revealed that the flanking sequences and the “insertion sequence” in this mutant were found in the S. aureus strain ED133 (ST133) sequence. ED133 was isolated from ovine clinical mastitis in France [28]. Moreover, a deletion mutation was found in the B repeats of sdrC in 23 isolates from 14 cows in Eastern Canada (11 clinical isolates from 7 cows and 12 subclinical isolates from 7 cows), with one B repeat lost and only one B repeat remaining in the B repeats of sdrC (Figure 1). Similarly, one B repeat was lost in the B repeats of sdrD in 58 clinical and subclinical isolates from Quebec (2 subclinical isolates from 2 cows), Eastern Canada (14 clinical isolates from 12 cows and 11 subclinical isolates from 9 cows), Western Canada (3 clinical isolates from 3 cows and 16 subclinical isolates from 12 cows) and Ontario (7 clinical isolates from 7 cows and 5 subclinical isolates from 4 cows), with 4 B repeats remaining in the B repeats of sdrD (Figure 1). In addition, an insertion mutation was detected in the R domain of sdrH from 4 subclinical isolates of 2 cows in Ontario (Figure 1). The insertion sequence was 3 “DNPKPKPDPKPDP” repeats at codon 161. It seems that these 3 repeats were the product of a duplication of the same adjacent repeats in the R domain of sdrH mutants. However, no sequence diversity was found in sdrE.
Figure 1. Insertion and deletion mutations in sdr genes of bovine mastitis-associated S. aureus.
S represents the signal sequence while A and A' regions stand for putative ligand-binding A region. B1, B2, B3, B4, B5, B1′ and B2′ designate single B repeat. A'N and A'C are flanking regions of the insertion sequence which are identical to the N-terminus and the C-terminus of SdrC_ED133 A region, respectively; IS denotes an insertion sequence in the A region of SdrC_ED133; sdrH-N and sdrH-C represent flanking regions of the 3 repeats insertion sequence which are identical with N-terminus and the C-terminus of SdrH_O&W; IS' represents 3x repeats insertion sequence in sdrH mutant. 102 and 161 are the locations for the insertion. SdrC_Q&E represent the normal SdrC proteins in isolates from Quebec and East Canada; SdrC_mutant1 represents the insertion sequence found in SdrC in Canadian isolates E48, E49 and E50. SdrC_mutant2 represents the deletion sequence found in SdrC in Canadian isolates E5-16, CE6-14, CE16 and CE18; SdrD represents the normal SdrD proteins in Canadian isolates; SdrD_mutant represents the deletion sequence found in SdrD in Canadian isolates Q14, Q17, E7, E13, E21-24, E28-30, E45, E48, CE6-10, CE13, CE16, CE18-23, CE28, O3, O4, O18-20, CO4, CO7, CO8, CO11, CO16, CO18, CO20, O3, O4, O18-20, W9, W13, W15, W16, W20-23, W25, W32, W33, W35-37, W39, W40, CW3, CW4 and CW18; SdrH_O&W represents the normal SdrH proteins in isolates from Ontario and Western Canada; SdrH_mutant represents the insertion sequence found in SdrH in Ontario and Western Canada isolates O21-24.
Alignment of sequencing classified bovine mastitis isolates into two types
The alignment results of sdrC, sdrD, sdrE and sdrH genes from bovine mastitis isolates indicated that the sdrC, sdrE and sdrH genes could be classified into two types. The A region and B repeats for sdrC and sdrE as well as sdrH gene in isolates from Quebec and Eastern Canada were identical, and were classified in the same type; while the A region and B repeats for sdrC and sdrE and the sdrH gene in isolates from Ontario and Western Canada shared the identical sequence but were different from isolates from Quebec and Eastern Canada. Thus, they belonged to another type. The type of sdrC, sdrE and sdrH in same isolate was identical. The sdrC gene was used as an example and shown in Figures 2 and S1. The sdrC genes from bovine mastitis isolates in Canada were classed into 2 types: One type for isolates from Quebec and Eastern Canada and another type for isolates from Ontario and Western Canada. There were no differences in amplified sdr PCR fragments between clinical and subclinical isolates from the same region. The A region and B repeats of sdrD in isolates from all four Canadian regions were identical.
Figure 2. Bovine mastitis-associated S. aureus isolates was classified according to sdr genes.
Alignment of a partial DNA sequence of sdrC gene of bovine mastitis isolates classifies S. aureus isolates. The same classification can be obtained by alignment of the full sequence of sdrC gene, as shown in Figure S1. sdrC_CQ, sdrC_CE, sdrC_CO and sdrC_CW represent sdrC genes from clinical isolates of Quebec, Eastern Canada, Ontario and Western Canada, respectively. sdrC_Q, sdrC_E, sdrC_O and sdrC_W represent sdrC genes from subclinical isolates of Quebec, Eastern Canada, Ontario and Western Canada, respectively. sdrCQ&E and sdrCO&W stand for two different types of sdrC gene in isolates from the Quebec and Eastern Canada region, and from the Ontario and Western Canada region, respectively.
The distribution of sdrC, sdrD, sdrE and sdrH of S. aureus was associated with clinical and subclinical isolates of bovine mastitis
The contribution of particular binding factors to S. aureus pathogenesis in bovine mastitis is poorly understood. In order to find the relationship between the bovine mastitis and the distribution of sdr genes in S. aureus, the distribution of sdrC, sdrD, sdrE and sdrH of S. aureus between clinical and subclinical isolates of bovine mastitis was investigated. As shown in Table 2, the sdrC gene was present in all investigated isolates (n = 218). However, in 2 subclinical strains from one individual cow in Ontario (of the total 134 subclinical strains), only the sdrC gene (sdrD negative, sdrE negative and sdrH negative) was found in the sdr locus. Fifteen subclinical strains from 10 different cows (5 from Western Canada, 4 from Quebec and 1 from Ontario) only contained sdrC and sdrH genes (sdrD negative, sdrE negative). Almost all of the isolates contained sdrH gene, except for 3 subclinical isolates, with 2 from Ontario and 1 from Western Canada. All clinical isolates from Ontario and Quebec contained sdrD and sdrE genes.
Table 2. Distribution of sdr genes from clinical and subclinical isolates of bovine mastitis from different Canadian regions.
| Source of isolates* | No. of isolates | sdrC[No.(%)] | sdrD[No.(%)] | sdrE[No.(%)] | sdrH[No.(%)] |
| CW | 18 | 18(100.0) | 17(94.4) | 15(83.3) | 18(100.0) |
| W | 38 | 38(100.0) | 32(84.2) | 20(52.6) | 37(97.4) |
| CO | 16 | 16(100.0) | 16(100.0) | 15(93.8) | 16(100.0) |
| O | 24 | 24(100.0) | 19(79.2) | 19(79.2) | 22(91.7) |
| CQ | 20 | 20(100.0) | 17(85.0) | 20(100.0) | 20(100.0) |
| Q | 25 | 25(100.0) | 5(20.0) | 19(76.0) | 25(100.0) |
| CE | 30 | 30(100.0) | 22(73.3) | 29(96.7) | 30(100.0) |
| E | 47 | 47(100.0) | 43(91.5) | 46(97.9) | 47(100.0) |
| All Clinical | 84 | 84(100.0) | 72(85.7) | 79(94.0) | 84(100.0) |
| All Subclinical | 134 | 134(100.0) | 99(73.9) | 104(77.6) | 131(97.8) |
*CW, CO, CQ and CE represent clinical isolates from Western Canada, Ontario, Quebec and Eastern Canada, respectively. W, O, Q and E represent subclinical isolates from Western Canada, Ontario, Quebec and Eastern Canada, respectively.
A significant association between the sdrC-positive, sdrH-positive, sdrD-negative, sdrE-negative gene profile and subclinical strains was found (15/119 versus 0/84; Fisher's exact test; P = 0.0006). Among the tested isolates, the presence of sdrD was significantly associated with clinical isolates (99/35 versus 72/12; Fisher's exact test; P = 0.0431), especially in isolates from Quebec (5/20 versus 17/3; Fisher's exact test; P<0.0001). In addition, the presence of sdrE was significantly associated with clinical isolates (104/30 versus 79/5; Fisher's exact test; P = 0.0011), especially in isolates from Quebec (19/6 versus 20/0; Fisher's exact test; P = 0.0265) and Western Canada (20/18 versus 15/3; Fisher's exact test; P = 0.0386).
Gene phylogenetic trees, based on sdrC, sdrD, sdrE and sdrH, revealed evolutional relationships among S. aureus strains and mastitis isolates
In order to better understand the relationship between the variation of sdrC, sdrD, sdrE and sdrH and the evolution of S. aureus strains, gene phylogenetic trees of sdrC, sdrD, sdrE and sdrH were constructed, using both the A region and B repeats of sdrC, sdrD, sdrE and full sequence of sdrH of 21 sequenced S. aureus strains and 218 mastitis isolates.
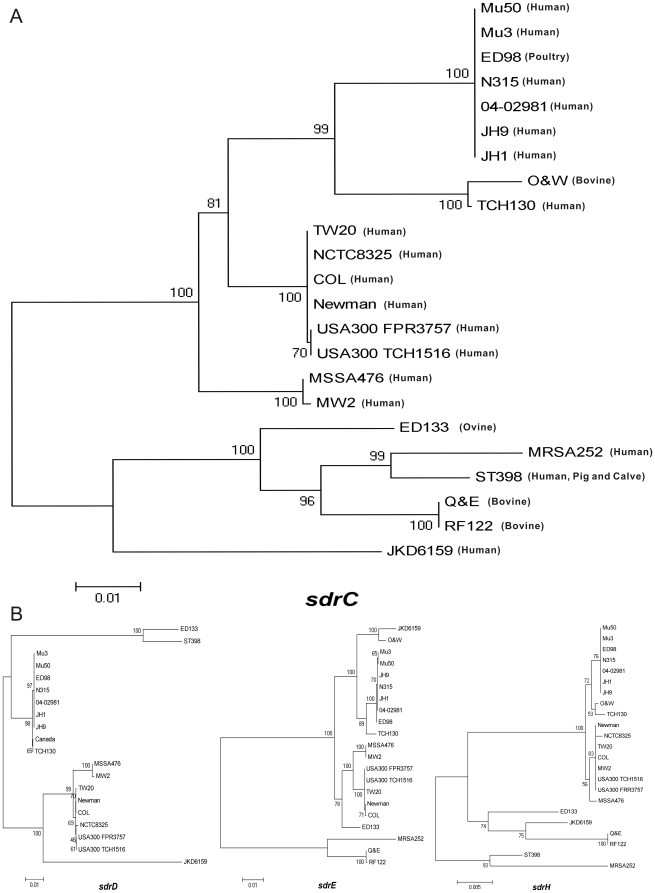
As shown in Figure 3A, the phylogenetic tree of sdrC divided strains and isolates into several clusters. One main cluster included strains Mu50, ED98, Mu3, N315, 04-02981, JH1, JH9, TCH130 and Ontario and Western Canada isolates. Another main cluster included strains Newman, COL, NCBC8325, TW20, USA300 TCH1516 and USA300 FPR3757 and this cluster was more conserved than the former cluster. Ovine-associated strain ED133 [28], bovine-associated strain RF122 [16], swine-associated strain ST398 [29], human-associated strain MRSA252 [30] and Quebec and Eastern Canada isolates were highly divergent from the above two clusters but showed phylogenetical similarity to one another. Strain JKD6159 [31] had the highest divergence from all other strains and isolates.
Figure 3. Phylogenetic trees of sdr genes revealed the evolutional relationship among S. aureus strains and isolates.
The graph was constructed by using MEGA software (version 4.0) with the NJ method and bootstrap values were provided as percents over 1000 replications. Alignment gaps were considered complete deletion. A) Phylogenetic tree of sdrC gene. Q&E represents the isolates from Quebec and Eastern Canada; O&W represents the isolates from Ontario and Western Canada. B) Phylogenetic trees of sdrD, sdrE and sdrH genes. Canada in the sdrD phylogenetic tree denotes the isolates from all four Canadian regions.
The phylogenetic tree of sdrD showed similar clustering to that of sdrC (Figure 3B), but one main cluster, which including strains Mu50, ED98, Mu3, N315, 04-02981, JH1, JH9, TCH130 and Ontario and Western Canada isolates, was more conserved than another main cluster, which including strains Newman, COL, NCBC8325, TW20, USA300 TCH1516 and USA300 FPR3757. Strains ED133 and ST398 were similar with each other and JKD6159 was still the most genetically diverse S. aureus strain. Strains RF122 and MRSA252 did not have sdrD and were not included for constructing the phylogenetic tree. The sdrE and sdrH phylogenetic trees were also similar in organization to those of sdrC and sdrD (Figure 3B), however, strain JKD6159 appeared to be closely related to Ontario and Western Canada isolates. The sdrE gene seems less variable than sdrC and sdrD genes according to the sdrE phylogenetic tree. Strains ST398 and NCTC8325 were not included for constructing the phylogenetic tree of sdrE, due to their lack of sdrE sequences.
The concatenated sequences of the seven multilocus sequence typing (MLST) of all the 21 S. aureus strains used in this study were also used for constructing the phylogenetic tree of strains, as described [30]. The results from the sdr phylogenetic trees (Figure 3) were in general agreement with the one from MLST typing (Figure S2). Figure 3 showed the relationship among strains MRSA252, ED133, ST398, RF122 and JKD6159. However, such relationship was not evident in the MLST typing.
Discussion
One of the novel findings from this study was the discovery of possible horizontal gene transfer between bovine S. aureus isolates and strains from other species. The A region and B repeats of sdrC, sdrE and the full sequence of sdrH in bovine mastitis isolates from Quebec and Eastern Canada shared 100.00%, 99.96% and 100.00% sequence identities with those in RF122, respectively (Table 1). However, the A region and B repeats of sdrD from all Canadian regions shared 99.84% sequence identities with genes from TCH130. RF122 is a bovine mastitis strain, while TCH130 is a human-associated strain (Table 3). Some bovine mastitis isolates from Quebec and Eastern Canada did not contain sdrD gene, while others did. These results suggested isolates from Quebec and Eastern Canada without sdrD might be homologous to RF122 strain, since RF122 does not contain the sdrD gene. The sdrD in S. aureus isolates from Quebec and Eastern Canada could be obtained through the horizontal transfer from TCH130 or a strain related to TCH130, since these two sdrD were almost identical. Alternatively, a genetic drift might have occurred in isolates with from Quebec and Eastern Canada and lost sdrD gene. However, only a gene with minor or no function may be lost due to a genetic drift [32]. Considering the fact that the sdrD gene is strongly associated with serious human diseases [25], [26], [27] and the clinical mastitis as shown in this study, it is unlikely that the genetic drift is responsible for our observed sequence diversity of sdrD. The A region and B repeats for sdrC and sdrE in isolates from Quebec and Eastern Canada also showed 95.42% and 95.16% of sequence identities with those of MRSA252, respectively (Table 1). RF122 and MRSA252 have been reported to share 14 different DNA sequence blocks [33]. In addition, RF122 and MRSA252 were the only 2 published strains which do not contain a sdrD gene, suggesting that these two strains were evolutionarily similar. Interestingly, sdrC from Quebec and Eastern Canada isolates contained premature stop codons and thus was a pseudogene, as was also reported for RF122 [16]. On the other hand, the A region and B repeats for sdrC in Quebec and Eastern Canada isolates also showed 95.52% and 95.92% sequence identities with those of strains ED133 (an ovine mastitis strain) and ST398 (a swine strain), respectively (Table 1 and Table 3). The sdrC gene in strains ED133 and ST398 was not truncated. Therefore, the sdrC locus in bovine strains and isolates seems not essential for inducing bovine mastitis.
Table 3. S. aureus strains evaluated in this study.
| Strain | MLST Type* | Geographic origin | Year | Comments** | Host and diseases |
| Mu50 | ST5 | Japan | 1997 | HA-MRSA/VISA | Human with Vancomycin resistance |
| Mu3 | ST5 | Japan | 1996 | MRSA/hetero-VISA | Human with Pneumonia |
| ED98 | ST5 | Northern Ireland | 1996–1997 | N/A*** | Poultry with BCO |
| N315 | ST5 | Japan | 1982 | HA-MRSA/VSSA | Human |
| 04–02981 | ST225 | Köln, Germany | 2004 | MRSA | Human |
| JH1 | ST105 | New York, USA | 2000 | MRSA/VSSA | Human, the earliest isolate of JH9 |
| JH9 | ST105 | New York, USA | 2000 | MRSA/VISA | Human with Vancomycin resistance |
| TCH130 | ST72 | Houston, USA | 2001 | MRSA | Human with pneumonia |
| TW20 | ST239 | London, UK | 2003 | MRSA | Human with Bacteremia |
| NCTC8325 | ST8 | Colindale, UK | 1940s | MSSA | Human with Sepsis |
| COL | ST250 | Colindale, UK | 1961 | MRSA | Human with Penicillinase-negative |
| Newman | ST8 | UK | 1952 | MSSA | Human with tubercular Osteomyelitis |
| USA300-FPR3757 | ST8 | San Francisco, USA | 2002–2004 | CA-MRSA | Human with HIV-positive |
| USA300-TCH1516 | ST8 | San Francisco, USA | 2002–2004 | CA-MSSA | Human with sepsis |
| MSSA476 | ST1 | Oxford, UK | 1998 | CA-MSSA | Human with Osteomyelitis and Bacteremia |
| MW2 | ST1 | North Dakota, USA | 1998 | CA-MRSA | Human with septic arthritis and septicaemia |
| ED133 | ST133 | France | 1997 | N/A | Ovine mastitis |
| MRSA252 | ST36 | Oxford, UK | 1997 | HA-MRSA | Human with Septicemia |
| ST398 | ST398 | Netherlands | 2006 | MRSA | Human with Endocarditis, Also highly infect pigs and calves. |
| RF122 | ST151 | Ireland | 1993 | MSSA | Bovine mastitis |
| JKD6159 | ST93 | Australia | 2003 | CA-MRSA | Human with Septicemia |
| CW1-18 | N/A | Canada | 2007–2008 | MSSA | Bovine mastitis |
| W1-38 | N/A | Canada | 2006–2007 | MSSA | Bovine mastitis |
| CO1-16 | N/A | Canada | 2007 | MSSA | Bovine mastitis |
| O1-24 | N/A | Canada | 2007 | MSSA | Bovine mastitis |
| CQ1-20 | N/A | Canada | 2007 | MSSA | Bovine mastitis |
| Q1-25 | N/A | Canada | 2007 | MSSA | Bovine mastitis |
| CE1-30 | N/A | Canada | 2007 | MSSA | Bovine mastitis |
| E1-47 | N/A | Canada | 2007 | MSSA | Bovine mastitis |
*MLST represents multilocus sequence typing.
**HA-MRSA means hospital-acquired methicillin-resistant S. aureus; CA-MSSA is the community-acquired methicillin-sensitive S. aureus; VISA is vancomycin-intermediate level-resistant S. aureus; VSSA represents vancomycin sensitive S. aureus.
***N/A, Not available.
Possible horizontal gene transfer between bovine S. aureus isolates and strains from other species was also evident in Ontario and Western Canada isolates. The sdrC and sdrD genes in isolates from Ontario and Western Canada shared 99.15% and 99.84% (Table 1) of sequence identities with those from S. aureus strain TCH130, which came from a 2 year old child with pneumonia (Human Microbiome Project, http://www.ncbi.nlm.nih.gov/nuccore/ACHD00000000). Some isolates from Ontario and Western Canada contained both sdrC and sdrD, while others only contained sdrC, not sdrD. The sdrE gene in isolates from Ontario and Western Canada shared 96.70% and 98.83% of sequence identities with those from TCH130 and JKD6159, respectively (Table 1). The sdrC, sdrD, sdrE and sdrH gene in isolates from Ontario and Western Canada also shared an average of 95.51%, 99.62%, 97.60% and 98.02% sequence identify with genes from the second cluster which including strains ED98, Mu3, Mu50, N315, JH1, JH9 and 04-02981, respectively. S. aureus ED98 was isolated from a chicken with bacterial chondronecrosis with ostemyelitis (BCO) in Ireland [7], [8] and the others were human-associated S. aureus strains (Table 3). The identical sdrC, sdrD, sdrE, and sdrH genes between the human-associated S. aureus strains and ED98 suggest that HGT may have occurred among them.
Insertion and deletion were detected in the A region and B repeats of sdrC and sdrD genes (Figure 1). Some of them may be due to mobile genetic elements. An insertion sequence in the sdrC A region was found to be similar to the C-terminal region of Enterococcus faecium transposase IS1216V (EMBL: L40841), sharing 46% identity. In addition, its organizational structure was similar to transposon IS1272 [34]. As shown in Figure 1, the sdrC in this mutant from an Eastern Canada cow was totally different from that of other cows in the same region. Interestingly, the flanking sequences of this insertion within sdrC and the “insertion sequence” in this mutant were found in the S. aureus strain ED133 (ST133) sequence [28]. However, the insertion sequence in ED133 genome was outside of the A region and was called “putative insertion element protein”. It was plausible that the putative insertion element helped to move the sdrC of ED133 strain into a bovine mastitis isolate. If this should be the case, our report is the first to show the horizontal gene transfer from an ovine mastitis S. aureus strain to a bovine mastitis isolate. Horizontal gene transfer had been reported before among S. aureus strains from different hosts, for example, between strains of human and poultry [7], or between strains of human and bovine [33].
Another insertion was found in the R domain of sdrH in S. aureus isolates. The insertion sequence in sdrH mutants was three conserved “DNPKPKPDPKPDP” repeats at codon 161. sdrH gene was first reported in S. epidermidis [19]. By analyzing the putative sdrH genes of genomes of 21 sequenced S. aureus strains, it was found that the structure of sdrH gene in S. aureus was similar to that of in S. epidermidis. The insertion mutation seems a product of duplication of the 3 conserved “DNPKPKPDPKPDP” repeats as a whole unit in the wild type sdrH gene.
One deletion was detected in sdrC and sdrD genes of S. aureus isolates, with one B repeat lost. Bacterial simple sequence repeats (SSRs) are prone to high rates of mutation through slipped strand mispairing that result in expansions or contractions in the number of repeat units [35], [36]. Hence, the loss of B repeat could be due to the slip-stand mispairing. One study of the B repeats of SdrF, a S. epidermidis surface protein containing four B repeats, showed that a single B repeat of S. epidermidis 9491 retained the capacity to bind to its ligand [22].
The phylogenetic trees of sdrC, sdrD, sdrE and sdrH consistently showed the relationship between our S. aureus isolates and the published S. aureus strains. As shown in Figure 3, the 21 published S. aureus strains and our isolates were divided into several clusters by sdr genotyping. In the sdrC phylogenetic tree (Figure 3A), the S. aureus isolates from Quebec and Eastern Canada were phylogenetically similar to strain TCH130, while the Ontario and Western Canada isolates were homologous with strain RF122, suggesting that the S. aureus isolates in Canada had two totally different ancestors. In addition, MRSA252 and three other strains (ST398, RF122 and ED133) seem to share a common ancestor. This notion was also supported by the results of sdrD, sdrE and sdrH phylogenetic trees. Strain JKD6159 showed the highest divergence from other strains or isolates in sdrC, sdrD and sdrE phylogenetic trees, suggesting that JKD6159 was in the farthest evolutional end among the 21 strains. This was consistent with the fact that JKD6159 was a distant strain from Australian [31] (Table 3). A previous study showed that the genome from strain 04-02981 was co-linear with N315 and JH1 [37], which were confirmed in this study. The results from the phylogenetic trees indicated that the first main cluster containing strains Mu50, ED98, Mu3, N315, 04-02981, JH1, JH9, TCH130 and Ontario and Western Canada isolates was less conservative in sdrC, but more conservative in sdrD and equally conservative in sdrE and sdrH, in comparison with another main cluster containing strains Newman, COL, NCBC8325, TW20, USA300 TCH1516 and USA300 FPR3757. Our results suggested that these two main clusters have developed different strategies for the sdr gene evolution. The function of Sdr proteins in S. aureus pathogenesis remains unknown. Thus, it is not feasible at this time to postulate how the sequence diversity of sdr genes affects biological functions of the translated proteins. In addition, an evolutionary relationship should exist among S. aureus strains from different species. However, such a relationship was less clear in the phylogenetic tree using MLST typing in comparison with sdr phylogenetic trees.
Another major finding from this study was a significant association between the presence of sdrE and clinical strains (104/30 versus 79/5; Fisher's exact test; P = 0.0011). Another significant association was also found between the sdrC-positive, sdrH-positive, sdrD-negative, sdrE-negative gene profile and subclinical isolates (15/119 versus 0/84; Fisher's exact test; P = 0.0006), suggesting that these isolates had a substantially decreased potential to establish clinical bovine mastitis. Our result was supported by Sabat et al. [26]. They showed that the sdrC-positive, sdrD-negative, sdrE-negative gene profile was not found in the strains collected from bone infections [26]. A third significant association was found between the sdrD positive gene profile and clinical isolates, suggesting that sdrD was important for the pathogenesis of bacteria but not essential for bacterial survival, since some isolates did not have sdrD. A previous study [26] showed that there was a strong association between the presence of the sdrD gene and MRSA responsible for bone infections. In addition, Trad et al. [25] showed a significantly higher prevalence of sdrD in bone infection isolates than in nasal isolates. South African methicillin-susceptible S. aureus (MSSA) isolates were more likely than MRSA isolates to carry virulence gene sdrD [27]. The same study also showed that MRSA isolates were more likely than MSSA isolates to carry genes for sdrC, sdrD and sdrE [27]. This study was the first time to show the distribution of sdrH gene in S. aureus. Although our initial BLAST results indicated the existence of sdrH in S. aureus, this report confirmed the presence of sdrH genes in the bacteria.
In summary, S. aureus strains from bovine mastitis (RF122 and our isolates), ovine mastitis (ED133), pig, calves and human infections (ST398), poultry with BCO (ED98) and human MRSA (TCH130, MRSA252, Mu3, Mu50, N315, 04-02981, JH1 and JH9) were highly associated with one another. It is proposed that horizontal gene transfer might be responsible for this association. The presence of insertion mutation and deletion mutation in the sdr genes suggested that the sdr genes were variable. These findings are crucial for better understanding the emergence of traits such as increased virulence or antibiotic resistance, together with the forces driving pathogen spread.
Materials and Methods
Bacterial strains and isolates
Eighty four clinical isolates and 134 subclinical bovine mastitis-associated S. aureus isolates from 149 cows were used in this study and different isolates from a same cow were isolated from different sampling points. They were from 4 different regions in Canada, including Western Canada (18 clinical and 38 subclinical isolates), Ontario (16 clinical and 24 subclinical isolates), Quebec (20 clinical and 25 subclinical isolates) and Eastern Canada (30 clinical and 47 subclinical isolates) (Table 2). These were obtained from the Canadian Bovine Mastitis Research Network (CBMRN) and all isolates are MSSA [38]. In addition, published sequences of 21 strains were used as reference strains for comparison analyses. These strains included Mu3 (AP009324), Mu50 (BA000017), TW20 (FN433596), N315 (BA000018), NCTC8325 (CP000253), ED98 (CP001781), COL (CP000046), JKS6159 (CP002114), MRSA252 (BX571856), MSSA476 (BX571857), ED133 (CP001996), MW2 (BA000033), RF122 (AJ938182), USA300 FPR3757 (CP000255), USA300 TCH1516 (CP000730), JH1 (CP000736), JH9 (CP000703), Newman (AP009351), 04-02981 (CP001844), TCH130 (NZ ACHD00000000) and ST398 (AM990992).
Genomic DNA extraction
S. aureus isolates were grown on 3 ml nutrient broth medium overnight (37°C) before being harvested by centrifugation at 10,000 g for 1 min. The pellet was resuspended in 300 ul of the MicroBead solution containing 20 ul of lysostaphin (1 mg/ml; Sigma Aldrich, St. Louis, MO). The genomic DNA was extracted using an UltraClean® Microbial DNA Isolation Kit (Mo Bio Laboratories, Carlsbad, CA) according to manufacturer's instructions. DNA concentration was determined by an ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE). The DNA samples were stored at −20°C until being used for subsequent analyses.
Comparative genome analysis, primer design and PCR amplification
The genomes of 21 strains of S. aureus from different hosts were available in the NCBI nucleotide data base. All of them contained sdrC and sdrH, and nearly all of them contained sdrD and sdrE (strain NCTC8325 and ST398 did not contain sdrE, while strains MRSA252 and RF122 did not have sdrD). Alignment of the sdrC, sdrD, sdrE and sdrH sequence of 21 S. aureus strains showed that both C terminal and N terminal regions in both A region and B repeats of sdrC, sdrD, sdrE and whole sdrH gene were conserved. Consequently, primers were designed for these regions. PCR amplifications were performed in a Mastercycler (Eppendorf AG, Hamburg, Germany) with Crimson Taq DNA polymerase (New England Biolabs Inc. Pickering, ON.). The reaction tubes contained 20 ng of genomic DNA, 0.5 uM of each forward and reverse primer, 2.5 mM MgCl2, 0.2 mM deoxynucleoside triphosphates (dNTPs), 2 U of Crimson Taq DNA polymerase (New England Biolabs Inc) and 5 ul Taq buffer in a total volume of 25 ul. Conditions for the each reaction were as follows: 95°C for 5 min; 32 cycles of 95°C for 30 sec, different annealing temperatures (Table 1) for 30 sec, and 72°C for 200 sec; 72°C for 7 min; and final hold at 4°C. PCR products were analyzed by 1% agarose gel electrophoresis.
Sequence alignment among sdrC, sdrD, sdrE and sdrH genes of bovine mastitis-associated S. aureus produced by PCR
The PCR products for sdrC, sdrD, sdrE and sdrH genes were all submitted to the McGill University and Génome Québec Innovation Centre for sequencing. The Centre offers a Sanger Sequencing Service using Applied Biosystem's 3730xl DNA Analyzer technology. The A region and B repeats of sdrC, sdrD and sdrE genes were amplified separately with different primer pairs (Table 4). All of the mutants found in this research were amplified and sequenced at least twice in order to rule out the possibility of sequence errors. The DNA sequences of different sdr genes were aligned with the DNAMAN software (version 6.0), using the multiple sequence alignment program, to find differences among the sequenced isolates. The full alignment type of the optimal alignment method was used with default parameters. The DNAMAN uses ClustalW algorithm for optimal alignment. Our preliminary result indicated the existence of sdrH in S. aureus isolates, as shown in published sequences in NCBI. Therefore, sdrH was also analyzed in our study.
Table 4. Primers used in PCR amplification of DNA sequence.
| PCR product(size) | Primer | Primers Sequence | Annealing Temperatures |
| sdrC A region (1356 bp) | sdrC -A-F | 5′-GTGGTCATGAAGCTAAAGCGG-3′ | 56°C |
| sdrC -A-R | 5′-TCTTTTGGTCGCCATTAGCAG-3′ | ||
| sdrD A region (1569 bp) | sdrD -A-F | 5′-GGAACCAAGAAGCAAAGGCTG-3′ | 56°C |
| sdrD -A-R | 5′-CTTCTTGACCAGCTCCGCCAC-3′ | ||
| sdrE A region (1663 bp) | sdrE -A-F | 5′-GGAACCAAGAAGCTAAAGCTG-3′ | 56°C |
| sdrE -A-R | 5′-ACTTTTCTTCAGGTTTAACAG-3′ | ||
| sdrC B repeats (691 bp) | sdrC -B-F | 5′-CTGCTAATGGCGACCAAAAGA-3′ | 44°C |
| sdrCDE-R | 5′-TCTGATGTTTCTTCTTC-3′ | ||
| sdrD B repeats (1690 bp) | sdrD -B-F | 5′-GTGGCGGAGCTGGTCAAGAAG-3′ | 44°C |
| sdrCDE-R | 5′-TCTGATGTTTCTTCTTC-3′ | ||
| sdrE B repeats (1027 bp) | sdrE -B-F | 5′-CTGTTAAACCTGAAGAAAAGT-3′ | 44°C |
| sdrCDE-R | 5′-TCTGATGTTTCTTCTTC-3′ | ||
| sdrH full sequence (1272 bp) | sdrH -F | 5′-ATGTCATATCATTGGTTTAAG-3′ | 56°C |
| sdrH -R | 5′-TTATCGTCGCTGTGATTCGTT-3′ |
Construction of gene phylogenetic trees
In order to identify the relationships of sdr genes in S. aureus from different hosts, a phylogenetic approach was used. The DNA sequence of the A region and B repeats of sdrC, sdrD, sdrE genes and the full sequence of sdrH gene from bovine mastitis isolates as well as the genome sequences of 21 S. aureus strains were used to construct their phylogenetic trees. A phylogenetic tree was also constructed for the 21 sequenced S. aureus strains, using the seven MLST loci. The DNA sequences were aligned using the ClastalW2 program with default parameters followed by manual inspection. Phylogenetic trees were constructed with the neighbor-joining (NJ) method and bootstrap values were provided as percents over 1000 replications, utilizing the Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0 on default setting [39]. Alignment gaps were considered complete deletion.
Supporting Information
Bovine mastitis-associated S. aureus isolates was classified according to sdr genes. Alignment of the full DNA sequence of sdrC gene of bovine mastitis isolates classifies S. aureus isolates. sdrC_CQ, sdrC_CE, sdrC_CO and sdrC_CW represent sdrC genes from clinical isolates of Quebec, Eastern Canada, Ontario and Western Canada, respectively. sdrC_Q, sdrC_E, sdrC_O and sdrC_W represent sdrC genes from subclinical isolates of Quebec, Eastern Canada, Ontario and Western Canada, respectively.
(TIF)
Phylogenetic organization of 21 sequenced S. aureus strains was demonstrated using the seven MLST loci. The graph was constructed by using MEGA software (version 4.0) with the NJ method and bootstrap values were provided as percents over 1000 replications. Alignment gaps were treated with the complete deletion option.
(TIF)
Acknowledgments
The authors would like to thank Mr. Lei Guo and Mr. Tsung-Jung Lin for help in preparation of Figures and Tables.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This study was supported by funding from a grant from the National Nature Science Foundation of China (NSFC Grant No. 30828026), a discovery grant from Natural Science and Engineering Research Council of Canada and a grant from Shanghai Municipal Science and Technology Commission (No 9540707400). Mr. Xue is a recipient of a scholarship from the China Scholarship Council. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Kluytmans J, vanBelkum A, Verbrugh H. Nasal carriage of Staphylococcus aureus: Epidemiology, underlying mechanisms, and associated risks. Clinical Microbiology Reviews. 1997;10:505–&. doi: 10.1128/cmr.10.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lowy FD. Medical progress - Staphylococcus aureus infections. New England Journal of Medicine. 1998;339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 3.Baptiste KE, Williams K, Willams NJ, Wattret A, Clegg PD, et al. Methicillin-resistant staphylococci in companion animals. Emerging Infectious Diseases. 2005;11:1942–1944. doi: 10.3201/eid1112.050241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huijsdens XW, van Dijke BJ, Spalburg E, van Santen-Verheuvel MG, Heck MEOC, et al. Community-acquired MRSA and pig-farming. Ann Clin Microbiol Antimicrob. 2006;5:26. doi: 10.1186/1476-0711-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smyth DS, Feil EJ, Meaney WJ, Hartigan PJ, Tollersrud T, et al. Molecular genetic typing reveals further insights into the diversity of animal-associated Staphylococcus aureus. Journal of Medical Microbiology. 2009;58:1343–1353. doi: 10.1099/jmm.0.009837-0. [DOI] [PubMed] [Google Scholar]
- 6.Linares JA, Wigle WL. Staphylococcus aureus pneumonia in turkey poults with gross lesions resembling aspergillosis. Avian Diseases. 2001;45:1068–1072. [PubMed] [Google Scholar]
- 7.Lowder BV, Guinane CM, Ben Zakour NL, Weinert LA, Conway-Morris A, et al. Recent human-to-poultry host jump, adaptation, and pandemic spread of Staphylococcus aureus. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:19545–19550. doi: 10.1073/pnas.0909285106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodgers JD, McCullagh JJ, McNamee PT, Smyth JA, Ball HJ. Comparison of Staphylococcus aureus recovered from personnel in a poultry hatchery and in broiler parent farms with those isolated from skeletal disease in broilers. Veterinary Microbiology. 1999;69:189–198. doi: 10.1016/s0378-1135(99)00112-1. [DOI] [PubMed] [Google Scholar]
- 9.De Lencastre H, Oliveira D, Tomasz A. Antibiotic resistant Staphylococcus aureus: a paradigm of adaptive power. Current Opinion in Microbiology. 2007;10:428–435. doi: 10.1016/j.mib.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nwaneshiudu AI, Mucci T, Pickard DJ, Okeke IN. A second large plasmid encodes conjugative transfer and antimicrobial resistance in O119: H2 and some typical O111 enteropathogenic Eschefichia coli strains. Journal of Bacteriology. 2007;189:6074–6079. doi: 10.1128/JB.00349-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ochman H, Lawrence JG, Groisman EA. Lateral gene transfer and the nature of bacterial innovation. Nature. 2000;405:299–304. doi: 10.1038/35012500. [DOI] [PubMed] [Google Scholar]
- 12.Zaneveld JR, Nemergut DR, Knight R. Are all horizontal gene transfers created equal? Prospects for mechanism-based studies of HGT patterns. Microbiology-Sgm. 2008;154:1–15. doi: 10.1099/mic.0.2007/011833-0. [DOI] [PubMed] [Google Scholar]
- 13.Boyd EF, Davis BM, Hochhut B. Bacteriophage-bacteriophage interactions in the evolution of pathogenic bacteria. Trends in Microbiology. 2001;9:137–144. doi: 10.1016/s0966-842x(01)01960-6. [DOI] [PubMed] [Google Scholar]
- 14.Pallen MJ, Wren BW. Bacterial pathogenomics. Nature. 2007;449:835–842. doi: 10.1038/nature06248. [DOI] [PubMed] [Google Scholar]
- 15.Beres SB, Sylva GL, Sturdevant DE, Granville CN, Liu MY, et al. Genome-wide molecular dissection of serotype M3 group A Streptococcus strains causing two epidemics of invasive infections. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:11833–11838. doi: 10.1073/pnas.0404163101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herron-Olson L, Fitzgerald JR, Musser JM, Kapur V. Molecular Correlates of Host Specialization in Staphylococcus aureus. Plos One. 2007;2 doi: 10.1371/journal.pone.0001120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCarthy AJ, Lindsay JA. Genetic variation in Staphylococcus aureus surface and immune evasion genes is lineage associated: implications for vaccine design and host-pathogen interactions. Bmc Microbiology. 2010;10 doi: 10.1186/1471-2180-10-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Josefsson E, McCrea KW, Ni Eidhin D, O'Connell D, Cox J, et al. Three new members of the serine-aspartate repeat protein multigene family of Staphylococcus aureus. Microbiology-Sgm. 1998;144:3387–3395. doi: 10.1099/00221287-144-12-3387. [DOI] [PubMed] [Google Scholar]
- 19.McCrea KW, Hartford O, Davis S, Eidhin DN, Lina G, et al. The serine-aspartate repeat (Sdr) protein family in Staphylococcus epidermidis. Microbiology-Uk. 2000;146:1535–1546. doi: 10.1099/00221287-146-7-1535. [DOI] [PubMed] [Google Scholar]
- 20.Tung HS, Guss B, Hellman U, Persson L, Rubin K, et al. A bone sialoprotein-binding protein from Staphylococcus aureus: a member of the staphylococcal Sdr family. Biochemical Journal. 2000;345:611–619. [PMC free article] [PubMed] [Google Scholar]
- 21.Barbu EM, Ganesh VK, Gurusiddappa S, Mackenzie RC, Foster TJ, et al. beta-Neurexin Is a Ligand for the Staphylococcus aureus MSCRAMM SdrC. Plos Pathogens. 2010;6 doi: 10.1371/journal.ppat.1000726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arrecubieta C, Lee MH, Macey A, Foster TJ, Lowy FD. SdrF, a Staphylococcus epidermidis surface protein, binds type I collagen. Journal of Biological Chemistry. 2007;282:18767–18776. doi: 10.1074/jbc.M610940200. [DOI] [PubMed] [Google Scholar]
- 23.Hartford O, O'Brien L, Schofield K, Wells J, Foster TJ. The Fbe (SdrG) protein of Staphylococcus epidermidis HB promotes bacterial adherence to fibrinogen. Microbiology-Sgm. 2001;147:2545–2552. doi: 10.1099/00221287-147-9-2545. [DOI] [PubMed] [Google Scholar]
- 24.Peacock SJ, Moore CE, Justice A, Kantzanou M, Story L, et al. Virulent combinations of adhesin and toxin genes in natural populations of Staphylococcus aureus. Infection and Immunity. 2002;70:4987–4996. doi: 10.1128/IAI.70.9.4987-4996.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trad S, Allignet J, Frangeul L, Davi M, Vergassola M, et al. DNA macroarray for identification and typing of Staphylococcus aureus isolates. Journal of Clinical Microbiology. 2004;42:2054–2064. doi: 10.1128/JCM.42.5.2054-2064.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sabat A, Melles DC, Martirosian G, Grundmann H, van Belkum A, et al. Distribution of the serine-aspartate repeat protein-encoding sdr genes among nasal-carriage and invasive Staphylococcus aureus strains. Journal of Clinical Microbiology. 2006;44:1135–1138. doi: 10.1128/JCM.44.3.1135-1138.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Campbell SJ, Deshmukh HS, Nelson CL, Bae IG, Stryjewski ME, et al. Genotypic characteristics of Staphylococcus aureus isolates from a multinational trial of complicated skin and skin structure infections. Journal of Clinical Microbiology. 2008;46:678–684. doi: 10.1128/JCM.01822-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guinane CM, Ben Zakour NL, Tormo-Mas MA, Weinert LA, Lowder BV, et al. Evolutionary Genomics of Staphylococcus aureus Reveals Insights into the Origin and Molecular Basis of Ruminant Host Adaptation. Genome Biology and Evolution. 2010;2:454–466. doi: 10.1093/gbe/evq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schijffelen MJ, Boel CHE, van Strijp JAG, Fluit AC. Whole genome analysis of a livestock-associated methicillin-resistant Staphylococcus aureus ST398 isolate from a case of human endocarditis. Bmc Genomics. 2010;11 doi: 10.1186/1471-2164-11-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holden MTG, Feil EJ, Lindsay JA, Peacock SJ, Day NPJ, et al. Complete genomes of two clinical Staphylococcus aureus strains: Evidence for the rapid evolution of virulence and drug resistance. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:9786–9791. doi: 10.1073/pnas.0402521101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chua K, Seemann T, Harrison PF, Davies JK, Coutts SJ, et al. Complete genome sequence of Staphylococcus aureus strain JKD6159, a unique Australian clone of ST93-IV community methicillin-resistant Staphylococcus aureus. J Bacteriol. 2010;192:5556–5557. doi: 10.1128/JB.00878-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lawrence JG, Roth JR. Selfish operons: Horizontal transfer may drive the evolution of gene clusters. Genetics. 1996;143:1843–1860. doi: 10.1093/genetics/143.4.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brody T, Yavatkar AS, Lin Y, Ross J, Kuzin A, et al. Horizontal Gene Transfers Link a Human MRSA Pathogen to Contagious Bovine Mastitis Bacteria. Plos One. 2008;3 doi: 10.1371/journal.pone.0003074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Archer GL, Thanassi JA, Niemeyer DM, Pucci MJ. Characterization of IS1272, an insertion sequence-like element from Staphylococcus haemolyticus. Antimicrobial Agents and Chemotherapy. 1996;40:924–929. doi: 10.1128/aac.40.4.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bayliss CD, Field D, Moxon ER. The simple sequence contingency loci of Haemophilus influenzae and Neisseria meningitidis. Journal of Clinical Investigation. 2001;107:657–662. doi: 10.1172/JCI12557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moxon R, Bayliss C, Hood D. Bacterial contingency loci: The role of simple sequence DNA repeats in bacterial adaptation. Annual Review of Genetics. 2006;40:307–333. doi: 10.1146/annurev.genet.40.110405.090442. [DOI] [PubMed] [Google Scholar]
- 37.Nubel U, Dordel J, Kurt K, Strommenger B, Westh H, et al. A Timescale for Evolution, Population Expansion, and Spatial Spread of an Emerging Clone of Methicillin-Resistant Staphylococcus aureus. Plos Pathogens. 2010;6 doi: 10.1371/journal.ppat.1000855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Said KB, Ismail J, Campbell J, Mulvey MR, Bourgault AM, et al. Regional Profiling for Determination of Genotype Diversity of Mastitis-Specific Staphylococcus aureus Lineage in Canada by Use of Clumping Factor A, Pulsed-Field Gel Electrophoresis, and spa Typing. Journal of Clinical Microbiology. 2010;48:375–386. doi: 10.1128/JCM.01768-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular evolutionary genetics analysis (MEGA) software version 4.0. Molecular Biology and Evolution. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Bovine mastitis-associated S. aureus isolates was classified according to sdr genes. Alignment of the full DNA sequence of sdrC gene of bovine mastitis isolates classifies S. aureus isolates. sdrC_CQ, sdrC_CE, sdrC_CO and sdrC_CW represent sdrC genes from clinical isolates of Quebec, Eastern Canada, Ontario and Western Canada, respectively. sdrC_Q, sdrC_E, sdrC_O and sdrC_W represent sdrC genes from subclinical isolates of Quebec, Eastern Canada, Ontario and Western Canada, respectively.
(TIF)
Phylogenetic organization of 21 sequenced S. aureus strains was demonstrated using the seven MLST loci. The graph was constructed by using MEGA software (version 4.0) with the NJ method and bootstrap values were provided as percents over 1000 replications. Alignment gaps were treated with the complete deletion option.
(TIF)