Abstract
Aging is associated with oxidative damage and an imbalance in redox signaling in a variety of tissues, yet little is known about the extent of age-induced oxidative stress in the sympathoadrenal system. Lifelong caloric restriction has been shown to lower levels of oxidative stress and slow the aging process. Therefore, the aims of this study were two-fold: 1) to investigate the effect of aging on oxidative stress in the adrenal medulla and hypothalamus and 2) determine if lifelong 40% caloric restriction (CR) reverses the adverse effects of age-induced oxidative stress in the sympathetic adrenomedullary system. Adult (18 months) and very old (38 months) male Fischer 344 x Brown Norway rats were divided into ad libitum or 40% CR groups and parameters of oxidative stress were analyzed in the adrenal medulla and the hypothalamus. A significant age-dependent increase in lipid peroxidation (+20%, P<0.05) and tyrosine nitration (+111%, P<0.001) were observed in the adrenal medulla while age resulted in a reduction in the protein expression of key antioxidant enzymes, CuZnSOD (−27%, P<0.01) and catalase (−27%, P<0.05) in the hypothalamus. Lifelong CR completely prevented the age-induced increase in lipid peroxidation in the adrenal medulla and restored the age-related decline in antioxidant enzymes in the hypothalamus. These data indicate that aging results in a significant increase in oxidative stress in the sympathoadrenal system. Importantly, lifelong CR restored the age-related changes in oxidative stress in the adrenal medulla and hypothalamus. Caloric restriction could be a potential non-pharmacological intervention to prevent increased oxidative stress in the sympathetic adrenomedullary system with age.
Keywords: aging, caloric restriction, oxidative stress, antioxidant enzymes, adrenal medulla, hypothalamus
INTRODUCTION
While the fundamental causes of aging are still a matter of debate, oxidative stress seems to play a role in the process [30]. Oxidative stress represents an imbalance between the production of reactive species and a biological system’s ability to readily detoxify the reactive molecules or to repair the resulting damage. Reactive species like superoxide, hydrogen peroxide, nitric oxide and peroxynitrite can damage all components of a cell if they are not actively buffered by an antioxidant defense system. Key antioxidant enzymes in the cell include superoxide dismutase (SOD), catalase, and gluthathione peroxidase (GPx).
The process of aging is linked with the accumulation of oxidatively damaged molecules including proteins, lipids, and DNA that compromise cellular function. This is considered the “Oxidative Stress Theory of Aging” whereby age-related loss of physiological function is due to the build up of oxidative damage [27]. The accumulation of such damage occurs either by an increase in reactive species production, a decrease in reactive species scavenging, or via a reduction in repair/turnover of the oxidatively damaged biomolecules. While it has been shown that the aging brain is especially prone to oxidative stress [20], it is unclear if the sympathoadrenal system is oxidatively challenged with age.
The adrenal glands and the hypothalamus are closely related structures that play a role in the sympathetic nervous system (SNS). Upon stimulation, catecholamines, like norepinephrine and epinephrine, are released from the sympathoadrenal system in response to stress and cause general physiological changes that prepare the body for activity [11]. Catecholamines are formed from their amino acid precursor tyrosine in the brain, chromaffin cells of the adrenal medulla, and sympathetic nerves. Tyrosine hydroxylase (TH) catalyzes the hydroxylation of tyrosine, producing dopamine, whereas dopamine β-hydroxylase (DβH) catalyzes the conversion of dopamine to norepinephrine [13]. We and others have shown that the levels of catecholamine biosynthetic enzymes increase with age in the adrenal medulla [2, 4, 5, 16].
Lifelong caloric restriction (CR), where total caloric intake is reduced by 10–40% but adequate nutrition is otherwise maintained, has consistently been shown to lower levels of oxidative stress, slow the aging process, and extend lifespan in mammals [8, 14]. Specifically, studies have shown that CR decreases lipid peroxidation, prevents protein carbonylation and up-regulates antioxidant enzyme expression. Although there have been extensive studies on the ability of CR to reduce age-related oxidative damage in the brain [14, 18, 25, 28], little is known about the beneficial effects of CR on age-associated oxidative stress specifically within the sympathoadrenal system.
In this study, we hypothesized that aging would result in a significant increase in oxidative stress in the adrenal medulla and the hypothalamus. Furthermore, we believed that lifelong CR would prevent the adverse effects of age-induced oxidative stress in the sympathoadrenal system. We found that a lifelong CR diet decreases markers of oxidative damage in the adrenal medulla while concomitantly increasing the level of antioxidant enzymes in the hypothalamus.
METHODS
Experimental animals and design
Ad libitum-fed (AL) and calorie-restricted (CR) male Fischer 344 x Brown Norway rats of ages 18 months (adult) and 38 months (very old) were obtained from the National Institute on Aging colony at Harlan Sprague Dawley Inc. (Indianapolis, IN). This strain was chosen they live longer than the Wistar or the F344 strain in the absence of disease-specific anomalies [29]. Experiments were conducted according to the Guiding Principles in the Care and Use of Laboratory Animals, and procedures were approved by the local Institutional Animal Care and Use Committee.
At the NIA, all rats were fed ad libitum (NIH-31 diet) until 14 weeks of age. The CR regime was initiated by incremental caloric reduction of 10% per week over 4 weeks, reaching full 40% CR by week 17. The vitamin-fortified NIH-31 diet fed to CR rats provided 60% of the calories and 100% of the vitamins consumed by ad libitum rats. Rats were housed in a specific pathogen-free facility throughout the experiments and were fed this same diet 1 hour prior to the onset of the dark cycle in an attempt to synchronize circadian rhythms to that of the ad libitum rats [21, 24].
Rats were maintained on a 12:12 hour light-dark cycle with water available ad libitum and they were assessed on a weekly basis for signs of overt health problems. The animals were also palpated to monitor for symptoms of disease and gross tumors.
Tissue harvesting and preparation
Animals were over-anesthetized with pentobarbital (120 mg/kg ip) and the adrenal glands and the hypothalamus were rapidly removed, immediately frozen in liquid nitrogen and stored at −80°C until subsequent analyses. Prior to homogenization, the adrenal glands were decapsulated and the medullae were separated from the cortex.
Western Blot Analysis
The adrenal medullae and the hypothalamus were homogenized and assayed to quantitatively determine protein levels of the catecholaminergic enzymes, markers of oxidative stress and protein levels of antioxidant enzymes. Tissue samples were homogenized in 50 mM Tris (pH 7.0) with leupeptin and protein content was assessed by the DC protein assay (Bio-Rad, Hercules, CA). An equal amount of protein for each sample was separated by polyacrylamide gel electrophoresis via 12.5% gradient polyacrylamide gels containing 0.1% sodium dodecyl sulfate for ~1 h at 100 mA. After electrophoresis, the proteins were transferred to nitrocellulose membranes at 90 V for 1:30. To control for protein loading and transfer differences, membranes were stained with Ponceau S and analyzed. The membranes were washed and subsequently blocked with 5% skimmed milk in Tris-buffered saline containing 0.1% Tween 20 for 1 hr at room temperature and subsequently incubated overnight at 4°C with a primary antibody. This step was followed by incubation at room temperature with a secondary antibody directed against the primary for 1 hr. All bound antibodies were detected by chemifluorescence (ECL Plus Western Blotting Detection System; GE Healthcare), scanned (Storm 860 Phosphorimager Scanner; GE Healthcare) and analyzed using Image Quant software.
Primary Antibodies
Tyrosine hydroxylase (Anti-TH; Pel Freez Biologicals, Rogers, AR) and dopamine beta hydroxylase (Anti-DβH; Novus Biologicals, Littleton, CO) were probed as a measurement indicative of sympathetic nervous system (SNS) activity.
4-hydroxynonenal (Anti-HNE; EMD Chemicals) and nitrotyrosine (Anti-3-nitrotyrosine; AbCam) were measured to ascertain lipid peroxidation and tyrosine nitration, respectively.
Copper zinc superoxide dismutase (Anti-CuZnSOD; EMD Chemicals, Gibbstown, NJ), manganese superoxide dismutase (Anti-MnSOD; Millipore, Billerica, MA), catalase (Anti-Catalase; EMD Chemicals), and Glutathione Peroxidase (Anti-GPx; AbCam, Cambridge, MA) were analyzed to determine antioxidant capacity in both tissues.
Statistical Analysis
Data are presented as means ± SE. Comparisons between groups for each dependent variable were made by a one-way analysis of variance (ANOVA) and, when appropriate, a Tukey HSD (honestly significant difference) test was performed post-hoc. Significance was established at p < 0.05.
RESULTS
Body weight
Lifelong CR (40%) significantly lowered animal body weight in both age groups. Rat body weight was reduced by 33% (P<0.001) in the adult (18 month old) CR (301 ± 4 g) animals when compared with adult AL (446 ± 14 g) rats. Similarly, animal body weight was reduced by 44% (P<0.001) in the very old (38 month old) CR (245 ± 5 g) animals when compared with very old AL (435 ± 24 g) rats.
Catecholaminergic enzyme expression in the adrenal medulla
As previously reported, TH expression increased significantly in the adrenal medulla with age. TH protein levels were elevated by 19 ± 4% (P<0.01) in the very old AL animals (119 ± 4%) when compared with the adult AL rats (100 ± 2%). CR completely reversed elevated TH protein levels with age by 54% (65 ± 5%; P<0.001). Similar to TH expression, protein levels of DβH were significantly raised 28 ± 3% (P<0.001) in the very old AL rats in the adrenal medulla when compared to the adult AL animals. CR significantly lowered DβH levels in the very old rats as DβH protein levels were 128 ± 3% in the very old AL animals versus 117 ± 3% in the very old CR rats.
Oxidative damage in the adrenal medulla
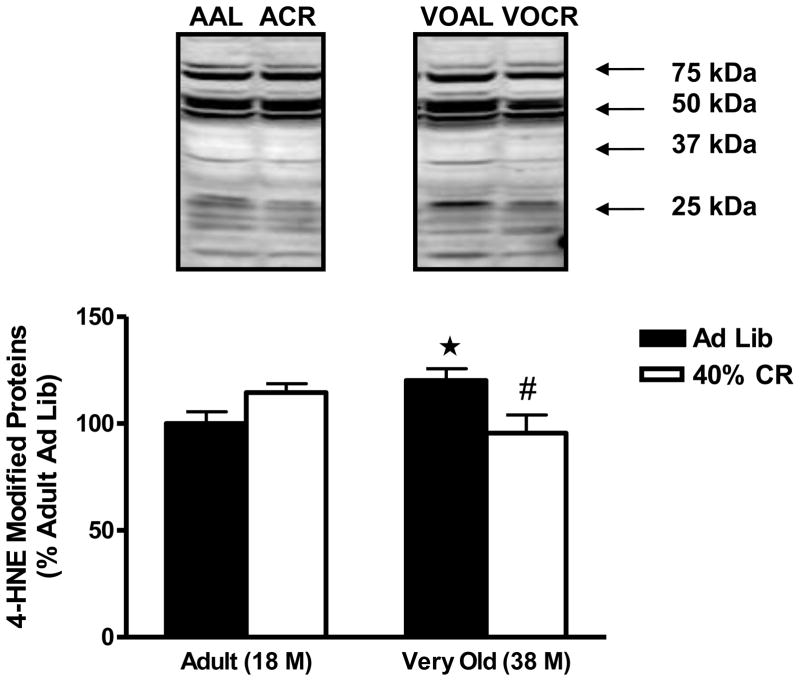
4-hydroxynonenal (4-HNE) is the primary adduct formed during the lipid peroxidation cascade and is commonly used to assess oxidative damage. Age significantly increased 4-HNE in the adrenal medulla as 4-HNE modified proteins were elevated by 20 ± 5% (P<0.05) in the very old AL animals when compared with the adult AL rats (Figure 1). Lifelong 40% CR significantly attenuated the accumulation of 4-HNE protein adducts as very old CR animals had basal levels of adrenal medullae 4-HNE (P<0.05).
Figure 1.
Effects of age and lifelong 40% caloric restriction on 4-hydroxynoneal (4-HNE) modified proteins (at 75, 50, 37 and 25 kDa) in the adrenal medulla. Data represent means expressed as percentage of adult ad libitum group ± SEM. *Significantly increased versus Adult Ad Lib (P<0.05). #Significantly decreased versus Very Old Ad Lib (P<0.05). AAL = Adult Ad Libitum (n=9); ACR = Adult 40% Caloric Restriction (n=9); VOAL = Very Old Ad Libitum (n=6); VOCR = Very Old 40% Caloric Restriction (n=9).
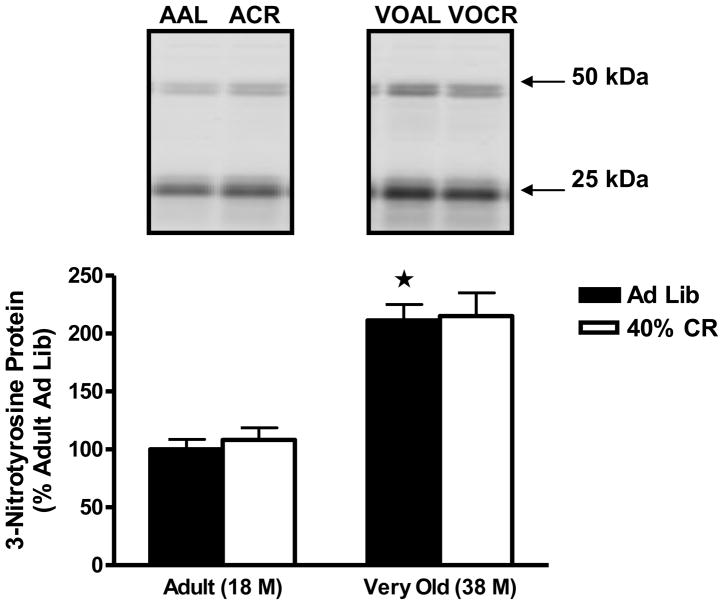
3-nitrotyrosine is a product of tyrosine nitration mediated by reactive nitrogen species and is used to measure nitric oxide (NO)-dependent oxidative stress. There was a significant increase in 3-nitrotyrosine in the adrenal medulla with age, as 3-nitrotyrosine levels were elevated by 111 ± 14% (P<0.001) in the very old AL animals when compared with the adult AL rats (Figure 2). Surprisingly, 40% CR failed to reduce 3-nitrotyrosine levels in the adrenal medulla of very old rats.
Figure 2.
Effects of age and lifelong 40% caloric restriction on 3-nitrotyrosine proteins (at 50 and 25 kDa) in the adrenal medulla. Data represent means expressed as percentage of adult ad libitum group ± SEM. *Significantly increased versus Adult Ad Lib (P<0.05).
Antioxidant capacity in the adrenal medulla
There were very few changes in antioxidant enzyme expression in the adrenal medulla with age or 40% CR (Table 1). Neither copper zinc superoxide dismutase (CuZnSOD) nor manganese superoxide dismutase (MnSOD) protein expression changed with age or 40% CR. Interestingly, catalase protein levels were significantly decreased (26%, P<0.05) in the adult rats with CR. Lastly, GPx levels were decreased by 16 ± 4% (P<0.05) with age and CR failed to attenuate the decrease in GPx in the adrenal medulla.
Table 1.
Antioxidant enzyme expression in the adrenal medulla
| CuZnSOD | MnSOD | Catalase | GPx | |
|---|---|---|---|---|
| AAL | 100 ± 7 | 100 ± 5 | 100 ± 5 | 100 ± 3 |
| ACR | 94 ± 4 | 103 ± 4 | 74 ± 4* | 85 ± 5* |
| VOAL | 92 ± 5 | 109 ± 6 | 96 ± 5 | 84 ± 4* |
| VOCR | 91 ± 7 | 92 ± 4 | 81 ± 3 | 75 ± 4 |
Data represent means expressed as percentage of adult ad libitum group ± SEM.
Significantly different versus Adult Ad Lib (P<0.05).
Lipid peroxidation in the hypothalamus
Hypothalamic 4-HNE modified proteins were increased in the very old AL animals (119 ± 7%) when compared with the adult AL rats (100 ± 8%) and lifelong 40% CR reduced 4-HNE protein adducts in the very old rats (99 ± 11%), however, neither reached statistical significance.
Antioxidant capacity in the hypothalamus
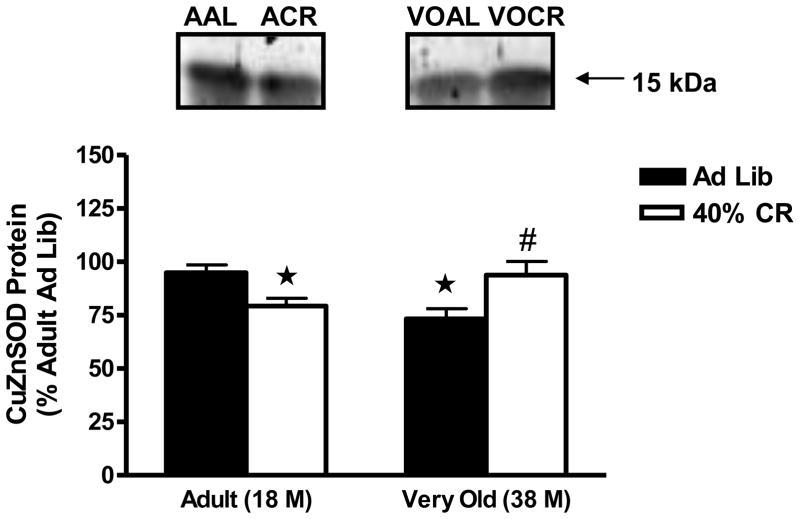
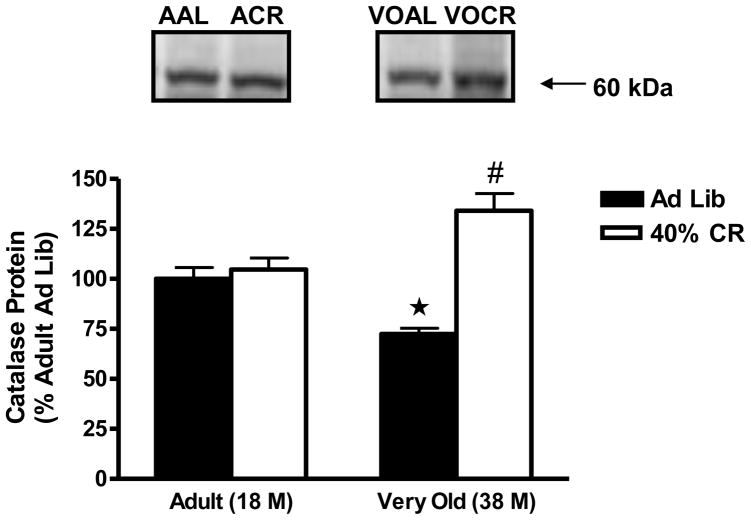
CuZnSOD protein levels decreased significantly in the hypothalamus with age, as CuZnSOD levels were reduced by 27 ± 5% (P<0.01) in the very old AL rats when compared with the adult AL animals (Figure 3). CR reversed CuZnSOD protein levels by 21% in the very old animals (P<0.05). Whereas age and 40% CR had no effect on MnSOD protein levels (AAL, 100± 7%; ACR, 134 ± 7% vs. VOAL, 109 ± 10; VOCR, 105 ± 10%), similar to CuZnSOD expression, protein levels of catalase were significantly lowered (27 ± 3%, P<0.05) (Figure 4) in the very old AL rats. CR significantly increased catalase protein levels in the hypothalamus of very old animals as catalase levels were 73 ± 3% in the very old AL rats versus 134 ± 9% in the very old CR rats.
Figure 3.
Effects of age and lifelong 40% caloric restriction on copper zinc superoxide dismutase (CuZnSOD) protein (15 kDa) in the hypothalamus. Data represent means expressed as percentage of adult ad libitum group ± SEM. *Significantly decreased versus Adult Ad Lib (P<0.05). #Significantly increased versus Very Old Ad Lib (P<0.05).
Figure 4.
Effects of age and lifelong 40% caloric restriction on catalase protein (60 kDa) in the hypothalamus. Data represent means expressed as percentage of adult ad libitum group ± SEM. *Significantly decreased versus Adult Ad Lib (P<0.05). #Significantly increased versus Very Old Ad Lib (P<0.05).
DISCUSSION
Overview of Principal Findings
These experiments provide important information regarding the effect of lifelong CR on age-induced oxidative stress in the sympathoadrenal system. Importantly, this is the first study examining oxidative stress in the adrenal medulla and the hypothalamus of very old animals. Twenty four months is considered old for the Fischer 344 x Brown Norway rat strain so this study went 14 months beyond with is considered senescent. Here we showed that lifelong CR prevented the accumulation of oxidatively damaged proteins in the adrenal medulla and attenuated the reduction in antioxidant enzymes in the hypothalamus of aged animals. While lifelong 40% CR might not be practical for humans, this study clearly shows the strength of this non-pharmacological intervention in preventing age-associated oxidative damage in the sympathoadrenal system. Based on our findings, we believe that the sympathetic adrenomedullary system would also benefit from an acute, short-term CR diet or a long-term, mild CR diet.
Lipid peroxidation occurs as a response to oxidative stress, and among the end-products it produces 4-hydroxynonenal (4-HNE). The high levels of 4-HNE modified proteins found in our very old animals could tentatively be explained by an increase in oxidant production in the adrenals coupled with the high polyunsaturated fatty acid composition of the organ [1]. While we did not measure actual oxidant production in this study, the observation that the aging adrenal medulla resulted in increased lipid peroxidation coincides with a study by Siqueira et al. (2005) that measured increased levels of free radicals in the adrenals of 21-month old Wistar rats [12]. Given the fact that the majority of the antioxidant enzymes of the adrenal medulla remained unchanged with age, even a small enhancement of free radical production would exceed the ability of the organ to defend itself against oxidative damage. In fact, the antioxidant capacity of the very old animals was slightly diminished via the age-associated reduction in GPx.
The accumulation of 4-HNE protein adducts in the adrenal medulla with age may also be due to a lack of proteolytic degradation. Numerous investigators have shown that the removal of oxidized proteins through proteolytic cleavage is diminished with age [6–7]. Furthermore, several proteolytic enzymes responsible for degrading oxidized proteins decline with age [6–7]. Taken together, oxidized proteins are likely to accumulate with aging. Luckily, CR was effective in preventing the increase in 4-HNE modified proteins in the adrenal medulla with age. This effect could be due to less lipid peroxidation throughout the life span, and/or sustained proteasomal degradation of oxidized molecules, and/or their removal by an alternative mechanism. Due to small sample volumes, we were unable to identify the proteins that were modified with 4-HNE but their identification merits further examination.
While we found a significant increase in 4-HNE modified proteins in the adrenal medulla of aged animals, the increase in 4-HNE protein adducts in the hypothalamus did not reach statistical significance. However, we observed a significant reduction in both CuZnSOD and catalase in the hypothalamus of the very old animals. Our findings that the antioxidant capacity of the hypothalamus decreases with age agrees with a study by Semsei et al. (1991) that demonstrated a 21–27% decrease in brain SOD and catalase activities in the aging Fischer 344 rat [10]. The generalized decrease in CuZnSOD and catalase levels indicates a highly reduced enzyme capacity to scavenge reactive species produced in the hypothalamus with age. Lifelong CR worked sufficiently to attenuate the age-related reduction in antioxidant enzyme expression in the hypothalamus. Maintenance of antioxidant capacity is crucial in the aging brain since it is especially prone to oxidative damage [20].
3-nitrotyrosine is used to measure nitric oxide (NO)-dependent oxidative stress and it is primarily formed when peroxynitrite (from NO and superoxide) reacts with tyrosine in proteins. We observed a significant increase in 3-nitrotyrosine in the adrenal medullae of very old rats. Nitric oxide synthase (NOS) is responsible for NO production and it is well established that NO plays a large role in adrenomedullary production of catecholamines. Kim et al. (2003) showed that NO leads to long-term up-regulation of catecholamine synthesis via induction of TH and DβH gene expression in bovine chromaffin cells [15]. While Iwai and colleagues illustrated that nNOS mRNA and protein expression levels are elevated in the adrenal medulla of rats treated with resperpine (a catecholamine biosynthetic enzyme inducer) [17]. In this study and in our previous ones, we have consistently shown that age results in a significant increase in both TH and DβH in the adrenal medulla [2, 4, 5, 16]. Therefore, it was not surprising to find elevated levels of 3-nitrotyrosine in our very old animals. A significant increase in NO production from nNOS coupled with an increase in superoxide with age likely contributes to the elevated levels of tyrosine nitration in the adrenal medullae.
Unexpectedly, lifelong CR failed to prevent the age-induced increase in 3-nitrotyrosine. The nitration of tyrosine residues by peroxynitrate is critical because it can compromise one of the most important mechanisms of cellular regulation, the cyclic interconversion between phosphorylated and unphosphorylated forms of tyrosine [19]. It was postulated that the nitration of tyrosine residues is an irreversible process and may lock enzymes into inactive forms [26]. It is unknown why lifelong CR failed to attenuate 3-nitrotyrosine in this study. Clearly more research needs to be performed to better understand the role of 3-nitrotyrosine production and clearance with age and CR.
One of the well-established physiological changes associated with normal aging is an increase in SNS activity accompanied by an increase in catecholamine biosynthesis and secretion. As mentioned above, we found that TH and DβH are significantly elevated in the adrenal medullae with age. In the present study, lifelong CR significantly attenuated the age-induced increase in TH and DβH expression in the adrenal medulla. This observation coincides with our previous work that mild (8%) CR prevents the age-related increase in adrenomedullary TH and DβH expression in F344 rats [22].
The exact mechanism by which lifelong CR attenuates catecholamine biosynthesis in the adrenal medulla is unclear. Our previous studies have ruled out the possibilities that decreased body weight [22] and/or increased physical activity [23] play a role in the CR attenuation of age-induced TH and DβH expression in the adrenal medulla. Briefly, we have shown that while TH and DβH expression decrease with mild CR, body weight remains unchanged [22] and physical activity via submaximal endurance training actually increases TH and DβH expression in the adrenal medullae [23] instead of decreasing it. Perhaps, the role of CR on attenuating age-induced catecholamine biosynthetic enzymes involves leptin signaling. Leptin has been shown to stimulate the function of adrenal chromaffin cells and enhance catecholamine synthesis [3] and CR has been shown to reduce concentrations of plasma leptin [9]. Clearly the mechanism(s) by which lifelong CR prevents the age-induced induction of catecholaminergic biosynthetic enzymes in the adrenal medulla warrants further investigation.
In summary, we report clear age-associated changes in oxidative damage, antioxidant defense, and catecholaminergic enzymes in the adrenal medulla and hypothalamus of male Fischer 344 x Brown Norway rats. Moreover, our findings indicate that lifelong CR reduces the age-associated induction of oxidative stress in the system and attenuates catecholaminergic enzyme induction. We suggest that the increase in oxidative damage, paralleled by the decrease in antioxidant expression, are important factors contributing to the aging process in the sympathetic adrenomedullary system. Lifelong CR may be a potential non-pharmacological intervention to thwart aging and protect function of the sympathoadrenal system.
Acknowledgments
This work was supported by the Medical Research Service of the Department of Veterans Affairs (NT), the University of Florida Institute on Aging and the Claude D. Pepper Older Americans Independence Center NIH P30 AG028740 (NT), an Evelyn F. McKnight Brain Research Grant (TCF) and grants from the National Institute of Aging, AG14979 (TCF) and T32 AG000196 (MAW).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Winkler H, Smith AD. Lipids of adrenal chromaffin granules: fatty acids composition of phospholipids, in particular lysolecithin. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1968;261:379–388. doi: 10.1007/BF00537182. [DOI] [PubMed] [Google Scholar]
- 2.Voogt JL, Arbogast LA, Quadri SK, Andrews G. Tyrosine hydroxylase messenger RNA in the hypothalamus, substantia nigra and adrenal medulla of old female rats. Brain Res Mol Brain Res. 1990;8:55–62. doi: 10.1016/0169-328x(90)90009-3. [DOI] [PubMed] [Google Scholar]
- 3.Utsunomiya K, Yanagihara N, Tachikawa E, Cheah TB, Kajiwara K, Toyohira Y, Ueno S, Izumi F. Stimulation of catecholamine synthesis in cultured bovine adrenal medullary cells by leptin. J Neurochem. 2001;76:926–934. doi: 10.1046/j.1471-4159.2001.00123.x. [DOI] [PubMed] [Google Scholar]
- 4.Tumer N, Larochelle JS. Tyrosine hydroxylase expression in rat adrenal medulla: influence of age and cold. Pharmacol Biochem Behav. 1995;51:775–780. doi: 10.1016/0091-3057(95)00030-z. [DOI] [PubMed] [Google Scholar]
- 5.Tumer N, Hale C, Lawler J, Strong R. Modulation of tyrosine hydroxylase gene expression in the rat adrenal gland by exercise: effects of age. Brain Res Mol Brain Res. 1992;14:51–56. doi: 10.1016/0169-328x(92)90009-z. [DOI] [PubMed] [Google Scholar]
- 6.Starke-Reed PE, Oliver CN. Protein oxidation and proteolysis during aging and oxidative stress. Arch Biochem Biophys. 1989;275:559–567. doi: 10.1016/0003-9861(89)90402-5. [DOI] [PubMed] [Google Scholar]
- 7.Stadtman ER. Protein oxidation and aging. Science. 1992;257:1220–1224. doi: 10.1126/science.1355616. [DOI] [PubMed] [Google Scholar]
- 8.Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shimokawa I, Higami Y. Leptin signaling and aging: insight from caloric restriction. Mech Ageing Dev. 2001;122:1511–1519. doi: 10.1016/s0047-6374(01)00284-6. [DOI] [PubMed] [Google Scholar]
- 10.Semsei I, Rao G, Richardson A. Expression of superoxide dismutase and catalase in rat brain as a function of age. Mech Ageing Dev. 1991;58:13–19. doi: 10.1016/0047-6374(91)90116-h. [DOI] [PubMed] [Google Scholar]
- 11.Seals DR, Esler MD. Human ageing and the sympathoadrenal system. J Physiol. 2000;528:407–417. doi: 10.1111/j.1469-7793.2000.00407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodrigues Siqueira I, Fochesatto C, da Silva Torres IL, Dalmaz C, Alexandre Netto C. Aging affects oxidative state in hippocampus, hypothalamus and adrenal glands of Wistar rats. Life Sci. 2005;78:271–278. doi: 10.1016/j.lfs.2005.04.044. [DOI] [PubMed] [Google Scholar]
- 13.Nagatsu T, Levitt M, Udenfriend S. Tyrosine Hydroxylase. The Initial Step in Norepinephrine Biosynthesis. J Biol Chem. 1964;239:2910–2917. [PubMed] [Google Scholar]
- 14.Martin B, Mattson MP, Maudsley S. Caloric restriction and intermittent fasting: two potential diets for successful brain aging. Ageing Res Rev. 2006;5:332–353. doi: 10.1016/j.arr.2006.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim D, Choi HJ, Kim SW, Cho SW, Hwang O. Upregulation of catecholamine biosynthetic enzymes by nitric oxide. J Neurosci Res. 2003;72:98–104. doi: 10.1002/jnr.10557. [DOI] [PubMed] [Google Scholar]
- 16.Kedzierski W, Porter JC. Quantitative study of tyrosine hydroxylase mRNA in catecholaminergic neurons and adrenals during development and aging. Brain Res Mol Brain Res. 1990;7:45–51. doi: 10.1016/0169-328x(90)90072-l. [DOI] [PubMed] [Google Scholar]
- 17.Iwai N, Hanai K, Tooyama I, Kitamura Y, Kinoshita M. Regulation of neuronal nitric oxide synthase in rat adrenal medulla. Hypertension. 1995;25:431–436. doi: 10.1161/01.hyp.25.3.431. [DOI] [PubMed] [Google Scholar]
- 18.Hyun DH, Emerson SS, Jo DG, Mattson MP, de Cabo R. Calorie restriction up-regulates the plasma membrane redox system in brain cells and suppresses oxidative stress during aging. Proc Natl Acad Sci U S A. 2006;103:19908–19912. doi: 10.1073/pnas.0608008103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hunter T. Protein kinases and phosphatases: the yin and yang of protein phosphorylation and signaling. Cell. 1995;80:225–236. doi: 10.1016/0092-8674(95)90405-0. [DOI] [PubMed] [Google Scholar]
- 20.Halliwell B. Oxidants and the central nervous system: some fundamental questions. Is oxidant damage relevant to Parkinson’s disease, Alzheimer’s disease, traumatic injury or stroke? Acta Neurol Scand Suppl. 1989;126:23–33. doi: 10.1111/j.1600-0404.1989.tb01779.x. [DOI] [PubMed] [Google Scholar]
- 21.Feuers RJ, Leakey JE, Duffy PH, Hart RW, Scheving LE. Effect of chronic caloric restriction on hepatic enzymes of intermediary metabolism in aged B6C3F1 female mice. Prog Clin Biol Res. 1990;341B:177–185. [PubMed] [Google Scholar]
- 22.Erdos B, Broxson CS, Landa T, Scarpace PJ, Leeuwenburgh C, Zhang Y, Tumer N. Effects of life-long caloric restriction and voluntary exercise on age-related changes in levels of catecholamine biosynthetic enzymes and angiotensin II receptors in the rat adrenal medulla and hypothalamus. Exp Gerontol. 2007;42:745–752. doi: 10.1016/j.exger.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Erdem SR, Demirel HA, Broxson CS, Nankova BB, Sabban EL, Tumer N. Effect of exercise on mRNA expression of select adrenal medullary catecholamine biosynthetic enzymes. J Appl Physiol. 2002;93:463–468. doi: 10.1152/japplphysiol.00627.2001. [DOI] [PubMed] [Google Scholar]
- 24.Duffy PH, Feuers R, Nakamura KD, Leakey J, Hart RW. Effect of chronic caloric restriction on the synchronization of various physiological measures in old female Fischer 344 rats. Chronobiol Int. 1990;7:113–124. doi: 10.3109/07420529009056963. [DOI] [PubMed] [Google Scholar]
- 25.Dubey A, Forster MJ, Lal H, Sohal RS. Effect of age and caloric intake on protein oxidation in different brain regions and on behavioral functions of the mouse. Arch Biochem Biophys. 1996;333:189–197. doi: 10.1006/abbi.1996.0380. [DOI] [PubMed] [Google Scholar]
- 26.Drew B, Leeuwenburgh C. Aging and the role of reactive nitrogen species. Ann N Y Acad Sci. 2002;959:66–81. doi: 10.1111/j.1749-6632.2002.tb02084.x. [DOI] [PubMed] [Google Scholar]
- 27.Bokov A, Chaudhuri A, Richardson A. The role of oxidative damage and stress in aging. Mech Ageing Dev. 2004;125:811–826. doi: 10.1016/j.mad.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 28.Bendlin BB, Canu E, Willette A, Kastman EK, McLaren DG, Kosmatka KJ, Xu G, Field AS, Colman RJ, Coe CL, Weindruch RH, Alexander AL, Johnson SC. Effects of aging and calorie restriction on white matter in rhesus macaques. Neurobiol Aging. doi: 10.1016/j.neurobiolaging.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baylis C, Corman B. The aging kidney: insights from experimental studies. J Am Soc Nephrol. 1998;9:699–709. doi: 10.1681/ASN.V94699. [DOI] [PubMed] [Google Scholar]
- 30.Abrass IB. The biology and physiology of aging. West J Med. 1990;153:641–645. [PMC free article] [PubMed] [Google Scholar]