Summary
Proteolytic cleavage of huntingtin (Htt) is known to be a key event in the pathogenesis of Huntington’s disease (HD). Our understanding of proteolytic processing of Htt has thus far focused on the cysteine protease families of caspases and calpains. Identifying critical protease families involved in Htt proteolysis and toxicity using an unbiased approach has not been reported. To accomplish this, we designed a high-throughput western blot-based screen to examine the generation of the smallest N-terminal polyglutamine-containing Htt fragment. Using this approach, we screened a set of 514 siRNAs targeting the repertoire of human protease genes. This screen identified 11 proteases that, when inhibited, reduced Htt fragment accumulation. Three of these belonged to the matrix metalloproteinase (MMP) family. One family member, MMP-10, directly cleaves Htt, and prevents cell death when knocked down in striatal Hdh111Q/111Q cells. Correspondingly, we found MMPs are activated in mouse models of HD, and loss of function of Drosophila homologs of MMPs and four other proteases suppress Htt-induced neuronal dysfunction in vivo.
Keywords: Polyglutamine diseases, protease, metallomatrix protease, RNAi, modifier, mouse models
Introduction
Huntington’s disease (HD) is a dominantly inherited neurodegenerative disorder that primarily affects neurons in the striatum and cortex (Albin, 1995; Cudkowicz and Kowall, 1990; Hedreen et al., 1991). HD is caused by a polyglutamine (polyQ) encoding CAG expansion in the gene for the huntingtin protein (Htt) (Group, 1993). Expression of mutant Htt leads to selective neuronal dysfunction and degeneration despite its ubiquitous expression pattern (Bhide et al., 1996; Gourfinkel-An et al., 1997). Truncated mutant Htt, in contrast to full-length mutant Htt, induces apoptosis (Martindale et al., 1998) and mutant N-terminal Htt fragments have been observed in human HD tissue and pre-symptomatic HD mouse models, suggesting that proteolysis is required for disease progression (Mende-Mueller et al., 2001; Wang et al., 2008; Wellington et al., 2002). Inhibition of mutant Htt cleavage reduces and in some cases prevents toxicity in vitro and in vivo, indicating a causative role for Htt proteolysis in HD pathogenesis (Gafni et al., 2004; Graham et al., 2006; Kim et al., 1999; Wellington et al., 2000).
A number of proteases have been shown to cleave mutant Htt. Caspases and calpains are the most widely studied and cleave Htt within the N-terminal region between amino acids 469 and 586 (Gafni et al., 2004; Wellington et al., 2000). Recent work has identified Htt cleavage sites closer to the N-terminus, between amino acids 105 and 167 (Lunkes et al., 2002; Ratovitski et al., 2007; Tanaka et al., 2006), producing smaller and potentially more toxic mutant polyQ-containing fragments. While these studies have suggested that cathepsins and/or calpains are the proteases responsible for producing these smaller fragments, the exact cleavage sites and identity of the proteases involved have not been unequivocally identified (Kim et al., 2006; Lunkes et al., 2002; Ratovitski et al., 2007).
Historically, Htt was the first neurodegenerative disease protein identified as a caspase substrate, and our understanding of proteolytic processing of Htt has thus far focused on the cysteine protease families of caspases and calpains (Gafni et al., 2004; Goldberg et al., 1996; Graham et al., 2006; Hermel et al., 2004; Wellington et al., 2002; Wellington et al., 1998; Yanai et al., 2006). In this study, we used an unbiased approach to identify other protease families involved in Htt proteolysis and toxicity. From a drug development perspective, this is a particularly attractive approach given that there are 514 known and predicted protease genes present in the human genome, and many of these have existing inhibitory compounds developed as therapeutics for a number of human diseases. One such class of proteases, the matrix metalloproteinases (MMPs) is involved in a number of diverse pathological processes. MMPs are zinc-containing proteolytic enzymes secreted to degrade extracellular matrix proteins (Hornebeck and Lafuma, 1991). An imbalance in MMP/TIMP (tissue inhibitor metalloproteinase) activity is implicated in conditions such as rheumatoid arthritis, kidney disease, cardiovascular disease, and cancer (Baker et al., 2002; Konttinen et al., 1999; Ronco et al., 2007). Recent evidence has linked inhibition of MMPs to reduced neuronal damage after transient cerebral ischemia (Lee et al., 2009). MMP-3 and MMP-9 knockout mice have significantly decreased striatal neuronal cell death after intracerebral hemorrhage (Xue et al., 2009). Further, intraperitoneal injection of the MMP-3/MMP-9 inhibitor, SB-3CT, showed a decrease in MMP activity and infarct size in the ischemic cortex in a mouse model of stroke (Gu et al., 2005). While the importance of MMPs in disease has led to the development of a few highly specific MMP inhibitors, further studies are needed to characterize the contribution of MMP family members to normal cell function and disease.
Given that smaller Htt fragments generally yield greater cellular toxicity (Hackam et al., 1998; Martindale et al., 1998), we designed a high-throughput western blot-based screen to examine the generation of the smallest N-terminal polyQ-containing fragment from full-length Htt. We screened a set of 514 small interfering RNA (siRNAs) pools targeting the repertoire of all known and predicted protease genes encoded in the human genome to identify those proteases that when inhibited reduced Htt proteolysis. Our primary screen identified 41 proteases that alter Htt fragment accumulation, and 11 of these were confirmed in retesting. Nine of the 11 proteases are expressed in striatal cells, and their knockdown significantly reduced Htt-mediated striatal cell death in a secondary cellular toxicity screen. Furthermore, decreasing the levels of five of these proteases suppressed mutant Htt induced toxicity in a Drosophila HD model.
Of the nine proteases validated by the secondary screen, three are matrix metalloproteinase family members, suggesting that these enzymes play a role in Htt proteolysis and toxicity. We demonstrate that MMP-10 directly cleaves Htt and reduces cell death when knocked down in striatal Hdh111Q/111Q cells. We also find that MMP activity is significantly elevated in mouse models of HD, and reduced MMP activity suppresses Htt-induced neuronal dysfunction in Drosophila. These data show that MMPs affect expanded polyQ-Htt proteolysis and toxicity, suggesting this family of enzymes may be relevant therapeutic targets in the disease.
Results
Identification of Proteases Involved in the Generation of the Small N-terminal Mutant Htt Fragment
Given that smaller Htt fragments generally yield greater cellular toxicity (Hackam et al., 1998; Martindale et al., 1998), we designed a high-throughput western blot-based screen to examine the generation of a small N-terminal polyQ-containing fragment from full-length Htt in a 96-well format (Figure 1A). As shown in Figure 1B, this screen allows the analysis of multiple proteolytic cleavage products generated from full-length mutant Htt. Mutant Htt and cleavage products were detected with a monoclonal antibody that recognizes the expanded polyQ stretch in the N-terminus of Htt (1C2). We focused our analysis on the generation of the smallest N-terminal polyQ containing Htt fragment observed, which migrates at 55 kDa (Figure 1B, indicated by arrowhead). siRNAs directed against all 514 known or predicted human proteases were screened in duplicate for effects on proteolysis of expanded full-length Htt in HEK293T cells using the western blot assay. In this primary screen, forty-one initial hits were identified that reduced the levels of the smallest N-terminal Htt fragment (possible aspartyl Htt cleavage product (Lunkes et al., 2002)) with at least a 30% reduction in expression of the Htt fragment relative to the full-length protein. This analysis was done using densitometry as shown in Figure 1C. The conditions of this assay result in significant knockdown of specific protease targets as demonstrated by western blot analysis of caspases (Supplementary Figure 1).
Figure 1.
Western blot screen for protease siRNAs that reduce the abundance of the Htt 55 kDa fragment. (A) siRNA/DNA co-transfection of cells was done in 96-well format, and transfected cells were incubated 48h before the addition of epoxomicin to stabilize Htt fragments. Cells were lysed and crude lysates transferred to SDS-PAGE loading buffer for subsequent western blot analysis. The first two columns of each plate contained control transfections- antibody specificity control: non-targeting (NT) siRNA/empty vector (grey wells); negative control: NT siRNA/Htt 138Q full-length (FL) (lavender wells)- and the rest of the plate contained an individual protease siRNA w/Htt138Q FL DNA (green wells). (B) Each of the rows of the 96-well plate transfections in (A) were loaded onto a single SDS-PAGE gel and subjected to western blotting. The antibody 1C2 (MAB1574) detects two robust non-specific bands (*) in vector only lanes, while the remaining bands in Htt138Q FL lanes are either unique or dramatically enriched. The bands range from the full-length protein (FL) to the 55 kDa fragment of interest (arrowhead). (C) Densitometry spectra of the MMP-10 and NT lanes from the western blot shown in panel B. Each gel was subjected to densitometric analysis of bands, and the volume ratio of the 55 kDa to FL was compared to the ratio in the NT control lane to identify candidate proteases.
Retesting of these 41 primary hits resulted in 11 of the hits being confirmed by their consistent decrease in the production of the 55 kDa putative aspartyl Htt cleavage product (Table 1). The proteases identified include members of the calpain family (CAPN5 and CAPN7); the signal peptide protease-like IMP5 and an amino-terminal signal peptide protease (SPC18); members of the secreted serine-protease kallikrein family (KLK10 and KLK11); the transmembrane-E3-ubiquitin ligase, RNF128; the MMP-2 interacting integrin, ITGA2B (Choi et al., 2008b); and three members of the matrix metalloproteinase family (MMP-10, MMP-14, MMP-23B).
Table 1.
Proteases whose knockdown reduced 55kDa Htt fragment in primary western blot screen.
| Gene Symbol | GeneID Number | Description |
|---|---|---|
| CAPN5 | 726 | calpain 5 |
| CAPN7 | 23473 | calpain 7 |
| IMP5 | 162540 | intramembrane protease 5 |
| ITGA2B | 3674 | integrin, alpha 2b (platelet glycoprotein IIb of IIb/IIIa complex, antigen CD41) |
| KLK10 | 5655 | kallikrein-related peptidase 10 |
| KLK11 | 11012 | kallikrein-related peptidase 11 |
| MMP10 | 116869 | matrix metallopeptidase 10 |
| MMP14 | 4323 | matrix metallopeptidase 14 (membrane-inserted) |
| MMP23B | 8510 | matrix metallopeptidase 23B |
| RNF128 | 79589 | ring finger protein 128 |
| SPC18 | 23478 | SEC11 homolog A (S.cerevisiae) |
Secondary Screen for Modifiers of Htt Toxicity in Immortalized Mouse Striatal Cells
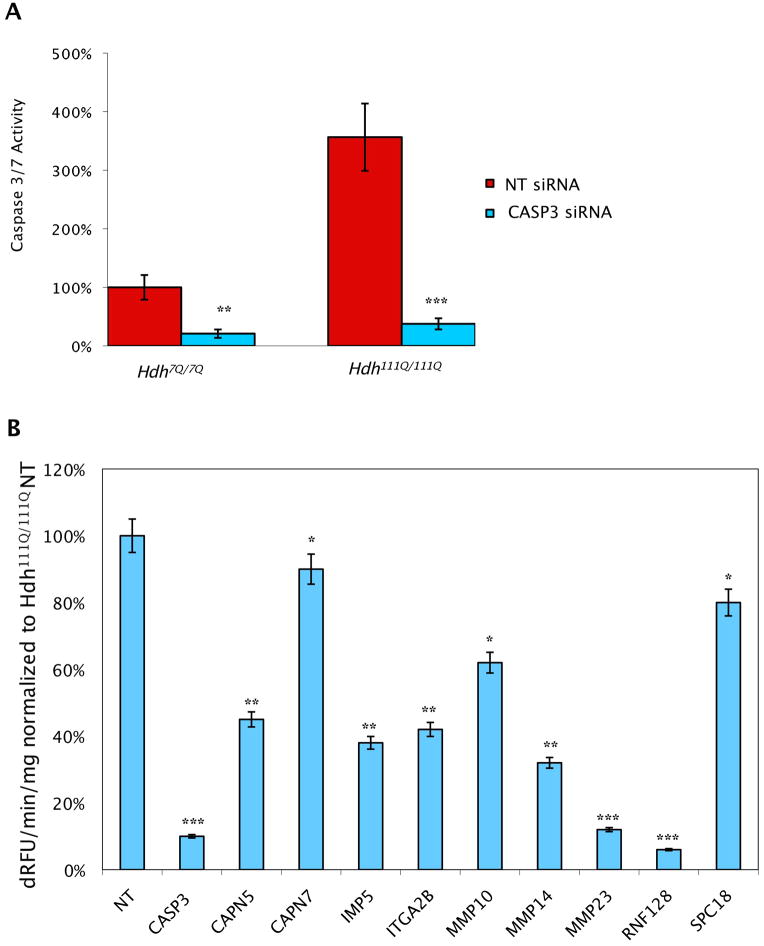
There is a body of literature suggesting that truncation of the full-length protein is cytotoxic in all polyQ diseases. For Htt protein, the smaller N-terminal truncation products are associated with increased toxicity. Since the 55 kDa product is the smallest detectable Htt cleavage product in our western blot analysis, we predict that blocking production of this fragment will prevent Htt-mediated cell death. To analyze the effect of the 11 initial hits identified in our western blot screen, we developed a Htt-mediated cell death assay relevant to HD. Since HD primarily affects striatal and cortical neurons we used immortalized mouse striatal cell Hdh7Q/7Q and Hdh111Q/111Q lines for our secondary screen of cellular toxicity, as well as subsequent genetic and biochemical studies (Trettel et al., 2000). Previous work in our laboratory has demonstrated that striatal cells from mutant Htt knock-in mice have altered calcium handling (Oliveira et al., 2006) and other groups have reported that they are selectively vulnerable to mitochondrial complex II inhibitor-induced cell death through a non-apoptotic pathway (Mao et al., 2006; Milakovic and Johnson, 2005). To develop an assay relevant to cell death, we cultured Hdh7Q/7Q and Hdh111Q/111Q cells and measured caspase activation 24 h after serum withdrawal (Figure 2A). We found that Hdh111Q/111Q cells have a 3.7 fold increase in caspase activation compared to control Hdh7Q/7Q. Further, siRNA against caspase-3, which serves as a positive control in our assay, reduced caspase activity by 8.7 fold in Hdh111Q/111Q cells (Figure 2A).
Figure 2.
Secondary screen of striatal cell-based toxicity assay. (A) PolyQ expansion in mouse knock-in striatal cells produces robust caspase activation when compared to wild-type controls. Caspase activity was measured in striatal Hdh7Q/7Q and Hdh111Q/111Q cells undergoing serum withdrawal for 24 h. Fold change in activity normalized to non-targeting siRNA (NT) in Hdh7Q/7Q or Hdh111Q/111Q cells (ANOVA, N=5, **p < 0.01 or ***p < 0.005). (B) siRNAs targeting 9 of the 11 proteases found as hits in the primary screen result in suppression of caspase activation in Hdh111Q/111Q cells. siRNAs to the indicated protease or a NT siRNA control were electroporated into Hdh111Q/111Q cells and incubated for 48 h. Caspase 3/7 activity was then measured 24 h after serum withdrawal. Fold change in activity normalized to NT siRNA in Hdh111Q/111Q, ANOVA analysis, N=5, *p < 0.05, **p < 0.01, ***p < 0.001.
Having established a robust striatal cellular toxicity assay, we evaluated whether the 11 proteases identified in our primary screen are active in this assay. Intriguingly, siRNA-mediated knockdown of 9 of the 11 proteases results in significant reduction of toxicity in this cell model (Figure 2B). KLK10 and KLK11, the two original hits that were negative in this secondary assay, are not expressed in striatal cells. We used KLK cDNA transfected Hdh111Q/111Q cells as a positive control to verify KLK antibodies (data not shown).
These results suggest that enzymes that promote the production of small N-terminal Htt cleavage products in our western blotting assay are likely to modulate HD toxicity.
MMP-10 and MMP-14 are Expressed in Striatal Hdh111Q/111Q cells
Since three of the nine validated hits (MMP-10, MMP-14, MMP-23B) are members of the matrix metalloproteinase family and one (ITGA2B) interacts with MMP-2 (Choi et al., 2008b), we chose to further characterize the potential role of this family of enzymes in HD. Most zinc-containing metalloproteases contain a characteristic HEXXH consensus sequence that coordinates to the zinc. Matrix metalloproteinases are a subfamily of the metzincins, which share the extended zinc-binding XEXXHXXGXXH consensus motif and a conserved methionine adjacent to the catalytic zinc. MMPs were discovered by their ability to “dissolve” collagen fibers in tadpole skin. There are 25 human MMPs (Supplementary Figure 2) that are structurally related zinc- and calcium- dependent enzymes. Some are activated intracellularly by furin proteases and many have been implicated in cancer, stroke and arthritis.
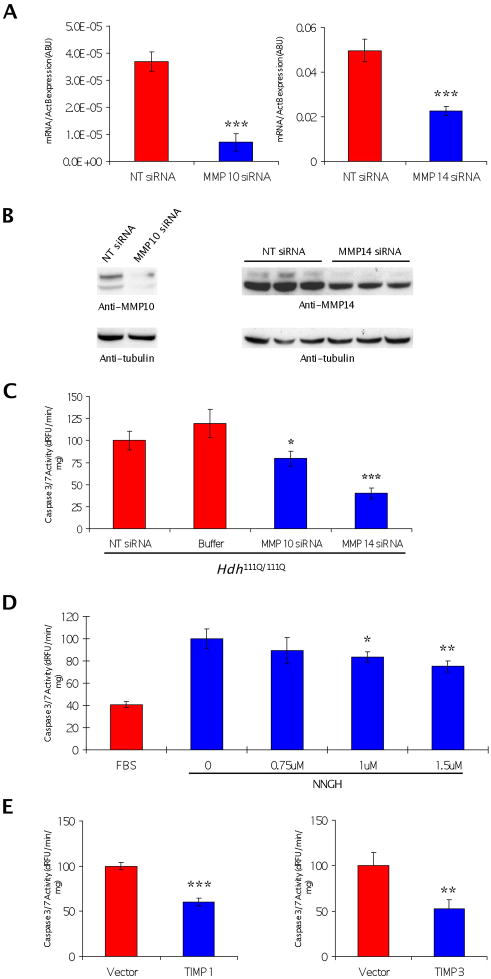
To further analyze the effect of MMP knockdown in striatal cells, we evaluated whether these proteases were expressed in Hdh111Q/111Q cells and if the knockdown occurred in response to the siRNA. Western blotting for MMP-10 and MMP-14 demonstrated that they are present in Hdh111Q/111Q cells at detectable levels, and introduction of siRNAs specific to their messenger RNAs does cause a significant reduction in MMP mRNA and protein expression (Figure 3A, B). We found the level of knockdown correlated with the reduction in caspase activity as titration of siRNA to MMP-10 (or deconvoluted siRNA pools) further reduced protein levels correlating with a reduced caspase-3 activity (data not shown). As shown in Figure 3C, siRNA directed against MMP-10 or MMP-14 significantly reduced caspase activation in Hdh111Q/111Q cells. We also found that MMP-23B was knocked down by the siRNA treatment and reduced caspase activation in Hdh111Q/111Q cells (data not shown). The MMP-23B mRNA level of expression in mouse striatal cells and tissue is quite low when compared to MMP-14 (0.00663 +/− 0.001 vs 0.5304 +/−.006; 0.00026 +/− 0.00006 vs 0.0078 +/−0.0003).
Figure 3.
Knockdown of MMP-10 and MMP-14 and inhibitors of MMP activity block caspase activation in striatal Hdh111Q/111Q cells. (A) Quantitative RT-PCR validation of siRNA knockdown. t-test was performed (p = 0.0004 and p = 0.7E-9, respectively) (B) Western blot analysis of siRNA targeting on the expression of the indicated MMP. (C) Effect of siRNA to MMP-10, MMP-14 or non-targeting on activation of caspase-3 in Hdh111Q/111Q cells during 24 h serum withdrawal. Caspase activity is in units dRFU/min/mg (ANOVA, n= 5, *p < 0.05 ***, p < 0.001). (D) Inhibition of MMP’s with NNGH lowers caspase activation in striatal cells (ANOVA analysis, p = 0.027 and 0.005). (E) TIMP1 and TIMP3 overexpression of protein reduces caspase activation in HD striatal cells ( t-test was performed, p = 0.3E-6 and 0.007).
Next, we tested a pharmacologic inhibitor, NNGH, which is a non-peptidic, potent inhibitor of MMPs. The crystal structure of the catalytic domain of human matrix metalloproteinase 10 has been solved with this molecule (Bertini et al., 2004). In addition to pharmacologic inhibitors, we tested known endogenous inhibitors of MMPs-TIMP1 and TIMP3 in Hdh111Q/111Q cells. As shown in Figure 3D, E, we found that these inhibitors blocked Htt-mediated toxicity in Hdh111Q/111Q cells.
MMP-10 is Processed in Striatal Hdh111Q/111Q Cells and MMP Activity is Increased
Given the involvement of MMPs in blocking Htt-mediated toxicity, we investigated whether these proteins were processed to their active forms in striatal cells containing mutant Htt. Strikingly, we found in striatal Hdh111Q/111Q cells cultured with serum, the levels of MMP-10 and MMP-14 were altered when compared to Hdh7Q/7Q (Figure 4A). Further, MMP-10 was processed suggesting activation of the protease in the striatal Hdh111Q/111Q cells as analyzed by western blotting (Figure 4A; 5.7-fold increase in active form in Hdh111Q/111Q vs. Hdh7Q/7Q cells, **p < 0.01). The specificity of the MMP antibodies was confirmed by overexpressing MMP-10 and MMP-14 in striatal cells (Figure 4B). MMP-23B levels did not change in striatal Hdh111Q/111Q cells when compared to Hdh7Q/7Q (data not shown).
Figure 4.

Activation of MMP in striatal Hdh111Q/111Q cells relative to Hdh7Q/7Q. (A) Striatal cells were electroporated with the indicated construct and cell lysates were analyzed by western blotting with the indicated MMP antibody. Cells were cultured in the presence of serum. (B) Western blotting of Hdh7Q/7Q and Hdh111Q/111Q cell lystates probed with MMP antibodies. (C) Activity of MMP fluorogenic substrate Mca-Pro-Leu-Dpa-Ala-Arg-NH2 [Mca=(7-methoxycoumarin-4-yl)-acetyl; Dpa=N-3(2,3-dinitrophenyl)-L-α-β-diaminopropionyl] in striatal Hdh7Q/7Q and Hdh111Q/111Q cell lysates. The activity is expressed in RFU/μg protein (n =5, t-test, **p < 0.01).
The proteolytic processing of MMP-10 does not necessarily indicate that levels of active MMP have increased in the mutant Htt cells. We, therefore, evaluated the MMP activity in striatal Hdh7Q/7Q and Hdh111Q/111Q cells. Figure 4C shows that MMP enzymatic activity is significantly increased in striatal Hdh111Q/111Q cells when compared to Hdh7Q/7Q using a fluorogenic substrate assay.
MMPs are Activated in the R6/2 and YAC128 Mouse Model of HD
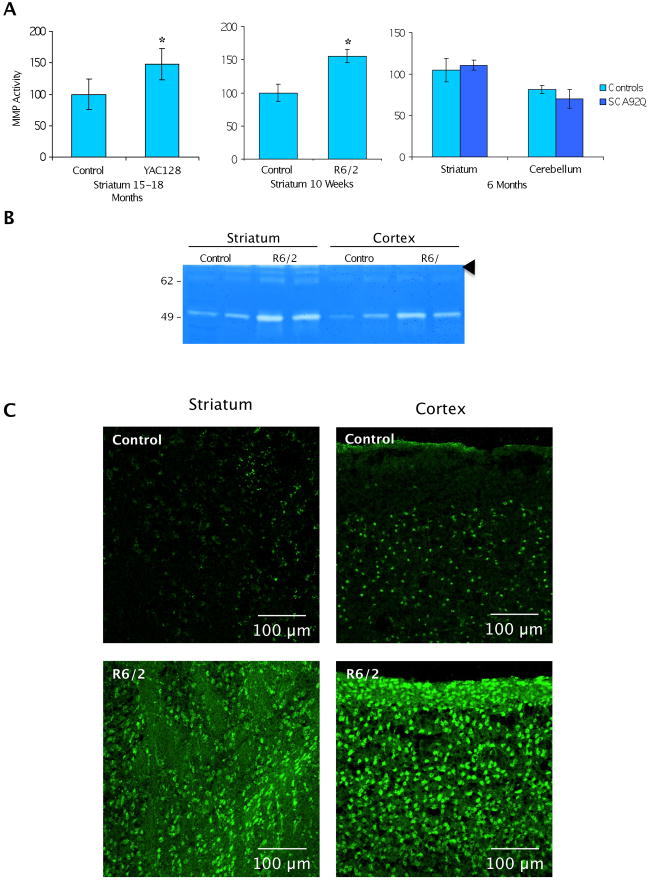
We next examined whether our cell culture results could be recapitulated in mouse models of the disease. We used a full-length Htt (YAC128) and a fragment mouse model (R6/2) of HD. The full-length HD model provides information about the natural history of proteolysis of full-length Htt and the proteases activated. The fragment model can provide information about the amplification and feedback of proteases once a relevant fragment is formed. The MMP enzymatic activity was assayed in the sub-regions of the brain in the YAC128 and R6/2 mouse model of HD. Figure 5A shows that MMP enzymatic activity is indeed increased in 16 month-old YAC128 or 10 week-old R6/2 striatum, with a 1.6 to 1.8-fold increase in MMP activity relative to the control striatum. We also examined the MMP enzymatic activity in another polyQ disease, SCA7 (Mookerjee et al., 2009). As shown in 5A (right panel), MMP enzymatic activity does not increase in SCA7 mice expressing ataxin-7-92Q. This suggests MMP activation is relevant in HD, but may not be a feature of other polyQ diseases.
Figure 5.
MMP activity is elevated in YAC128 and R6/2 striatal tissue but not in a spinocerebellar ataxin-7 poly-Q mouse model. (A) MMP activity assay with fluorogenic substrate activity measured in units of RFU/μg protein (t-test, N =3, *p < .05). (B) Striatal and cortex tissue immunoprecipitates using gelatin sepharose 4B beads were resolved on a 10% zymogram gelatin gel and assayed for gelatinase activity. Arrowhead indicates 90 kDa activity. (C) In situ zymography on 12–13 week control and R6/2 mouse brain tissue. MMP enzyme activity shown by a DG gelatin fluorescein conjugate MMP substrate. Images are shown at 20× magnification.
We used zymography to analyze the specific activity of the various MMPs in HD mouse brain, after MMP affinity precipitation (using gelatin beads). As shown in Figure 5B, we found elevated MMP activity detected in several bands ranging from ~50 kDa to ~90 kDa in lysates of the cortex and striatum of R6/2 mouse when compared to controls. The most robust increase in R6/2 lysate activity corresponds to the ~50 kDa bands (Figure 5B). Since MMP-10 migrates at around 50 kDa in its active form, this result is in agreement with our striatal cell line studies. We cannot exclude the possibility that other co-migrating MMPs may contribute to the observed activity. The band at 90 kDa may represent MMP-9 (MW 92 kDa, arrowhead), a protease activated by MMP-10.
In situ Gelatinase Activity
Since the MMP activity assay and zymography indicated increased levels of MMP activity in R6/2 and YAC128 lysates, we employed an in situ gelatinase assay to evaluate the levels of MMP directly in R6/2 and control tissue sections. In sections from R6/2 mice, we observed increased fluorescence when compared to control in both the striatum and cortex (Figure 5C). Inhibition with the MMP inhibitor 1,10-phenanthroline abolished the fluorescence signal, confirming that the activity was attributable to MMPs (Supplementary Figure 3). Since MMP family members can be expressed in both glial and neuronal populations, we determined the sub-population that contained MMP activity by co-staining with GFAP (glial-specific) or NeuN (neuronal specific). The majority of MMP staining is present in neurons, although some MMP activity does co-label GFAP-positive cells (Supplementary Figure 3).
Direct vs. Indirect Effects of MMPs on HD-mediated Toxicity
Knockdown of MMPs suppresses mutant Htt toxicity and decreases the amount of small Htt fragment accumulation. This indicates that MMPs may either cleave mutant Htt directly, or modulate events upstream or downstream of Htt cleavage. For example, in HIV, MMP-2 cleaves stromal cell-derived factor (SDF)-1α to activate neuronal cell death (Vergote et al., 2006). To determine whether Htt is a direct substrate for MMP-10 or MMP-14, we incubated cell lysates expressing Htt with the recombinant MMPs. As a control, we included MMP-2, an MMP family member not found to alter HD cellular toxicity or proteolysis in our original screen. As shown in Figure 6A, full-length wild-type or mutant Htt expressed in cellular lysates is a substrate for MMP-10, but not MMP-14 or MMP-2. A cleavage product appears at 48 kDa originating from normal Htt, and 70 kDa for mutant Htt148Q. In vitro translation of Htt15Q (1-1212) also confirmed that Htt is a substrate for MMP-10 but not MMP-14 or MMP-2 (Figure 6B). All three MMP family members were equally active against the fluorescent substrate, Mca-Pro-Leu-Dpa-Ala-Arg-NH2, suggesting that Htt is a preferred substrate for MMP-10 (data not shown). These data indicate that MMP10 knockdown suppresses Htt toxicity through its direct effect on Htt cleavage while the effect of MMP14 knockdown is indirect.
Figure 6.
MMP10 directly cleaves Htt. (A) Full-length Htt23Q and Htt148Q cellular 293T lysates (with protease inhibitors) were treated with recombinant MMP-2, MMP-10 or MMP-14 and western blot was probed with Htt N-terminal antibody. (B) In vitro translated Htt15Q (1–1212) and Htt15Q (1–1212) Δ167–170 mutant were treated with recombinant MMP-2, MMP-10 or MMP-14. A Htt cleavage product of ~45 kDa appears when MMP-10 is added and is not affected by deletion of amino acids 167–170. (C) Htt23Q (1–450) and Htt57Q (1–450) cellular lysates were treated with recombinant MMP-10 and western blot was probed with anti-6HisGS (tag at N-terminus of Htt). (D) In vitro translation of Htt15Q (1–469), Htt15Q (1–414) and Htt15Q (1–378) demonstrates that cleavage occurs between amino acids 378 and 414. (E) In vitro translation of a Htt15Q (1–469) deletion mutant (Δ402–403 or ΔGI) reduces the MMP10 product. (F) Control and HD postmortem tissue lysates from caudate were analyzed by western blotting. Blots were probed with anti-huntingtin 115–129 antibody. The size of the fragment is similar to that generated by MMP10 treatment of HD lysates (with protease inhibitors) shown in lane 3 and 4. TBP was utilized as a loading control. (G) Immunofluorescence of striatal Hdh111Q/111Q cells labeled with anti-huntingtin 115–129 antibody (green) and anti-MMP10 antibody (red). Left panel cultured in serum; right cultured in serum-free media. Cells are shown at 126× magnification and arrows indicate cell analyzed and colocalization. Arrowheads point to MMP-10 products produced. Quantitative analysis of immunofluorescence via Imaris software indicates significant colocalization in cells as shown in right panel (t-test, **p < 0.01).
MMP-10 Cleaves Htt at Amino Acid 402
MMP-10 appears to cleave Htt in a region closer to the N-terminus than the caspase and calpain cleavage products (55–72 kDa) (Gafni et al., 2004; Hermel et al., 2004; Wellington et al., 1998) producing a smaller 48 kDa product. Recent in vitro studies suggest Htt is also cleaved at amino acid 167 (Ratovitski et al., 2009). Deletion of the residues at this site has no effect on the proteolysis of Htt by MMP-10 (Figure 6B). Therefore the site of MMP-10 cleavage is likely between amino acid 167 (Cp2 site) and 469 (calpain site). Consistent with this, Htt (1–450) is still a substrate for MMP-10 (Figure 6C).
To locate the approximate site of cleavage, Htt15Q (1–469) and Htt138Q (1–469) were in vitro translated and incubated with MMP-10. As shown in Figure 6D, MMP-10 addition to Htt15Q (1–469) and Htt138Q (1–469) generates a 48 kDa and 70 kDa fragment, respectively. Htt15Q (1–167), Htt15Q (1–329), Htt15Q (1–378), Htt15Q (1–414) and Htt138Q (1–167) serve as size controls. Analysis of the molecular mass of the Htt15Q cleavage product suggests cleavage occurs around amino acid 414 of Htt. Examination of the amino acid sequence of Htt in this region, and comparison to known MMP substrates, suggests a possible cleavage site at amino acid 402. To test this possibility, we carried out deletion analysis on this site. As shown in Figure 6E, deletion of amino acids 402–403 (ΔGI) of Htt results in a protein resistant to proteolysis by MMP-10. Htt15Q (1–414), Htt15Q (1–402) and Htt15Q (1–378) serve as size controls. Htt15Q (1–402) matches the molecular mass of the MMP10-generated Htt cleavage product.
To confirm that the cleavage product is generated in vivo, HD post-mortem cellular lysate were compared to control lysate and analyzed by western blotting. As shown in Figure 6F, a Htt cleavage product around 45–55 kDa is evident in the HD tissue and is consistent with the size of the products in our in vitro assays (accounting for a CAG repeat in the patient of ~42). Consistent with this, when we treated HD post-mortem cellular lysates with MMP-10 a cleavage product increased at 50 kDa (Figure 6F). Further, recent analysis of the HD150 knock-in mice suggests Htt fragments of this size are present in striatal and cortical lysates (Landles et al., 2010).
Since Htt is likely to be a substrate via intracellular cleavage by MMP-10, we evaluated localization of endogenous Htt and MMP-10. As shown in Figure 6G, Htt and MMP-10 co-localize in discrete punctate structures (left panel). Further, in cells undergoing cell death Htt and MMP-10 show enhanced colocalization (right panel). Quantification of colocalization is shown in the bar graph of Figure 6G. The colocalization does not result form a direct protein-protein interaction as we did not find MMP-10 and Htt coimmunoprecipitate (Supplementary Figure 4).
Reduced Activities of MMP and Other Proteases Identified in the siRNA Screens Improve Neuronal Function in a Drosophila model of HD
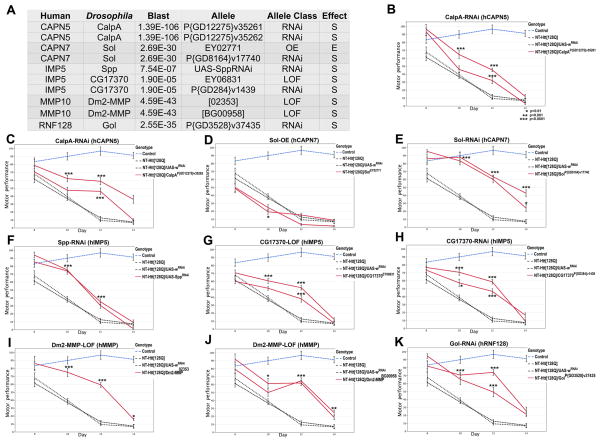
The data presented above suggest that reducing the activity of MMPs, and perhaps other proteases, may ameliorate Htt-induced neuronal dysfunction in vivo. To test this idea, we used a behavioral readout in a HD Drosophila model expressing the first 336 amino acids of the human protein including 128 glutamines in exon 1. In this model system, neuron-specific expression of expanded Htt leads to quantifiable progressive motor deficits (Kaltenbach et al., 2007). In contrast to the mammalian genome that contains 25 MMPs with partially overlapping functions, Drosophila only has two MMP family members, Dm1-MMP and Dm2-MMP. Although there are no clear orthologous relationships between the Drosophila and mammalian genes, they are clearly evolutionarily conserved as evidenced by their domain structure, amino acid sequence similarities, and functional interactions (Llano et al., 2000; Page-McCaw, 2008; Page-McCaw et al., 2003; Wei et al., 2003). Of the two Drosophila genes Dm2-MMP is expressed in the postembryonic brain (Page-McCaw et al., 2003), while Dm1-MMP expression was not detected in the adult (Llano et al., 2000). Thus, we asked whether genetically reducing the activity of Dm2-MMP leads to improvement of Htt-induced motor dysfunction. We found that heterozygous loss-of-function alleles of Dm2-MMP show robust and significant effects in improving the motor performance of HD flies. Importantly, this observation was reproduced with two different Dm2-MMP alleles indicating that the effect is caused by reduced function of Dm2-MMP, and not by genetic background effects (Figures 7A, 7I and 7J). Figure 7 shows as well that partial loss of function in Drosophila homologues of CAPN5, CAPN7, IMP5 and RNF128 also ameliorate motor deficits in the HD Drosophila model. These data support the idea that MMPs and perhaps other proteases characterized here may be therapeutic targets in HD.
Figure 7.
Reduced activity of proteases improves motor dysfunction in HD Drosophila model. (A) Table summarizing the effects caused by modulating the levels of the indicated proteases on NT-Htt[128Q]-induced motor performance in Drosophila. Columns 1 and 2 list the human proteases and Drosophila homologs; column 3 shows the amino acid sequence similarity as the blast E-value; column 4 indicates the specific allele tested; column-5 shows the allele class: inducible RNAi constructs (RNAi), loss of function caused by insertion of transposable element (LOF) or overexpression caused by an activating transposable element (OE); column-6 reveals the suppressor (S) or enhancer (E) effect of each allele on NT-Htt[128Q]-induced motor deficits. (B-K) Quantification of motor performance as a function of age in flies of the indicated genotypes using a climbing assay. Control animals expressing just the Elav-Gal4 driver perform well in the climbing assay beyond 14 days (Control-blue dashed line). Flies expressing NT-Htt[128Q] in the nervous system either alone or together with a control RNAi, show progressive motor dysfunction when compared with controls (NT-Htt[128Q] and NT-Htt[128Q]/UAS-whiteRNAi, respectively. Black dashed lines). Flies expressing NT-Htt[128Q] in the nervous system but with decreased levels of the proteases: CalpA (CAPN5 homolog, B-C); Sol (CAPN7 homolog, E); Spp (IMP5 homolg, F); CG17370 (IMP5 homolg, G-H); Dm2-MMP (MMP homolog, I-J) or Gol (RNF18 homolog, K) show improved motor performance (Red solid line in B-C and E-H). In contrast, flies expressing NT-Htt[128Q] with increased levels of the protease Sol (CAPN7 homolog) display a worse motor performance (red solid line in D). Data was analyzed using ANOVA followed by Tukey’s hsd. Error bars represent s.e.m. * p<0.01, ** p<0.001, ***p<0.0001. Flies were raised at 26.5°C.
In addition to the behavioral readout, we also tested for photoreceptor degeneration in these flies (Supplementary Figure 5). Expression of NT-Htt[128Q] in the eye causes severe photoreceptor degeneration, tissue loss and significant shortening in the thickness of the retina when compared to controls (Supplementary Figure 4). Animals expressing NT-Htt[128Q] and with decreased levels of CalpA, Sol and Dm2-MMP showed improved retinal integrity and improved thickness when compared to animals expressing NT-Htt[128Q] alone.
Discussion
We have carried out an unbiased RNAi screen to identify proteases that when knocked down reduced the production of toxic N-terminal Htt proteolytic fragments. We screened 514 siRNAs targeting all known and predicted human proteases and found 11 proteases that altered the level of the smallest N-terminal mutant Htt protein detected by the 1C2 anti-polyQ antibody. The proteases identified include members of the calpain family (CAPN5 and CAPN7); the signal peptide protease-like IMP5 and an amino-terminal signal peptide protease (SPC18); members of the secreted serine-protease kallikrein family (KLK10 and KLK11); the transmembrane-E3-ubiquitin ligase, RNF128; the MMP-2 interacting integrin, ITGA2B (Choi et al., 2008b); and three members of the matrix metalloproteinase family (MMP-10, MMP-14, MMP-23B).
Our results are consistent with the prior observations demonstrating that calpain family members are involved in the proteolysis of Htt and pathogenesis of HD (Gafni and Ellerby, 2002; Gafni et al., 2004; Kim et al., 2001). Both CAPN5 and CAPN7 levels are increased in HD mouse models and post-mortem tissue. Several other interesting proteases were identified in our studies. For example, IMP5, a signal peptide peptidase (SPP) is an unusual aspartyl protease that mediates clearance of signal peptides by proteolysis within the endoplasmic reticulum (ER) (Krawitz et al., 2005). Like presenilins, the proteolytically active subunit of the gamma-secretase complex, SPP contains a critical GXGD motif in its C-terminal catalytic center. Htt is associated with the ER, and perhaps IMP5 plays a role in Htt processing in this organelle (Atwal and Truant, 2008). Currently the substrates and mechanism for these proteases are unknown. Another identified protease, RNF128, is a ubiquitin E3 ligase, that promotes proteasomal degradation via Lys-48 linkages by capture of transmembrane substrates (Lineberry et al., 2008). Further studies will be required to determine if Htt is a substrate for RNF128 and how this enzyme alters proteolysis of Htt as observed in our screen. Decreasing the levels of these four proteases (CAPN5, CAPN7, IMP5 and RNF18) suppresses Htt-induced toxicity in mouse striatal cells. Furthermore, knockdown of their Drosophila homologs suppresses neuronal dysfunction in a fly HD model. This underlines their potential as therapeutic targets for HD.
Over 36% of the proteases identified in our screen were matrix metalloproteinase family members, and therefore we focused our analysis on these proteases in HD. In humans, there are 187 metalloproteases, and this represents the largest of the five protease classes (Hornebeck and Lafuma, 1991; Malemud, 2006). There are 25 human MMPs and four TIMPs. MMPs are traditionally thought to degrade the extracellular matrix, but emerging technology suggests there are many other substrates for MMPs such as chemokines, cell receptors, cyotokines, growth factors and fas ligands. We found that the knockdown of the MMP-2 interacting integrin ITGA2B (Choi et al., 2008b) and three members of the matrix metalloproteinase family (MMP-10, MMP-14, MMP-23) suppressed toxicity in immortalized striatal cell line harboring a knock-in of mutant Htt. We show that MMP activity is increased in cell culture and animal models of HD. Furthermore, MMP-10 is proteolytically processed and activated in these models. Relevant to our studies, MMP-10 is known to be upregulated in stroke and is present in neurons (Cuadrado et al., 2009).
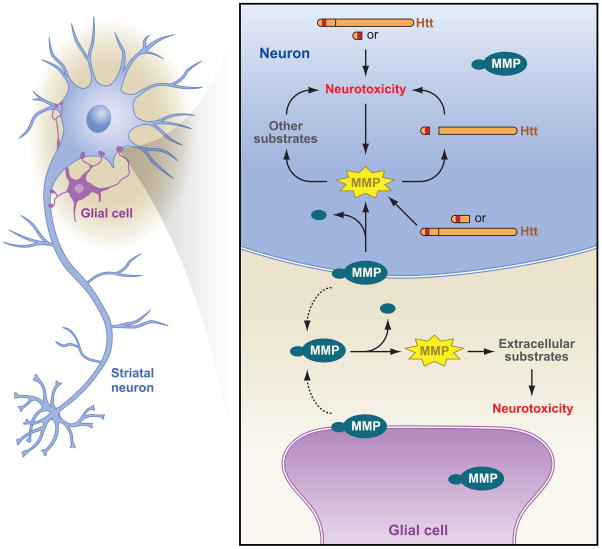
The mechanism by which MMPs suppress HD-mediated toxicity could be through the known function of MMPs in targeting the extracellular matrix or cytokines (See Figure 8 for potential mechanisms). However, our screen was designed to identify proteases that when knocked down would reduce the proteolysis of mutant Htt. In order to determine the mechanism for MMPs in HD, we evaluated whether Htt is a substrate for particular MMP family members. We found that MMP-10 cleaves Htt directly whereas MMP-14 and MMP-2 do not. Inhibition of MMP-10 by siRNA-mediated knockdown reduces the generation of the smallest detectable N-terminal polyQ-containing fragment of Htt in cell culture. We mapped the cleavage site of Htt generated by MMP-10 to amino acid 402. It is noteworthy that the MMP-10 cleavage site in Htt is highly conserved, with (S/T)XXGG(I/L) being present from human to Fugu. These results suggest that knockdown of MMP-10 reduces HD toxicity through directly effecting the proteolysis of Htt. For the other MMP family members, known mechanisms of cleavage of extracellular matrix or cytokines is likely to play a significant role in reducing HD-mediated toxicity.
Figure 8.
MMP-10 is unprocessed in wild-type striatal cells. In HD striatal cells the MMP-10 and MMP-14 are activated. Upon activation MMPs may cause neurotoxicity different cellular locations/substates in our model. In striatal neurons, MMP10 may process mutant Htt into a toxic fragment intracellularly and possibly processing other cellular substates. Proteolysis of these substrates would activate cell death. On the outside of the cell (MMP is membrane bound) proteolysis of extracellular substrates could result in processing of substrates involved in inflammation. This could also in glia as depicted by our model.
Recent evidence suggests that some matrix metalloproteinase family members such as MMP-3 function inside the cell during neuronal cell death (Choi et al., 2008a). While MMPs are generally thought to be secreted as pro-enzymes and processed to their active forms outside the cell, we found that MMP-10 was activated inside the cell. This has been reported for other MMPs (Luo et al., 2002; Pei and Weiss, 1995, 1996). Furthermore, we found that MMP-10 and Htt colocalize, suggesting the possibility that cleavage occurs intracellularly. Htt is known to associate with membranes and has a site of palmitoylation that would allow proteolysis to occur at the membrane surface (Kegel et al., 2009a; Kegel et al., 2009b; Yanai et al., 2006).
Activation of MMPs is known to occur in diseases including stroke, cancer and HIV (Malemud, 2006). In our study, we found MMPs to be activated in cellular and animal HD models. Knockdown of particular MMP family members reduced mutant Htt-mediated cell death in an immortalized striatal cell line, and significantly improved motor deficits caused by neuronal expression of polyQ-expanded Htt in Drosophila. These data suggest that selective inhibition of the implicated MMP family members should be considered for developing novel therapeutics for HD. For example, MMP-9 knockout mice show improved outcomes after cerebral ischemia (Asahi et al., 2000). This may be directly relevant to HD as a recent analysis of postmortem HD revealed MMP-9 is upregulated in HD brain and absent in age-matched controls (Silvestroni et al., 2009). AG3340 (prinomastat), a small molecule hydroxamate-based inhibitor of MMPs, was examined in a model of chronic cerebral hypoperfusion and is neuroprotective in adult rats and mice when administered just before insult (Cai et al., 2006). Reduced activation of astrocytes and microglia was also associated with retained blood-brain barrier integrity in these studies. In addition, in a mouse model of middle cerebral artery occlusion, the gelatinase-selective compound SB-3CT reduced infarct volume when administered starting at either 2 or 6 h after insult (Gu et al., 2005).
Taken together, our results suggest that general inhibition of MMPs may be of therapeutic benefit in Huntington’s disease, and that specific inhibitors of MMP-10 may be particularly relevant to disease treatment.
Experimental Procedures
Plasmid Constructs
The full-length expanded polyQ Htt expression construct, Htt138Q and pcDNA 3.1 (Invitrogen), were used for the western blotting screen (Martindale et al., 1998). For evaluating MMP proteolysis of Htt with recombinant enzymes, human Htt constructs expressing the first 469, 414, 402, 378, 329 and 167 amino acids were made by site-directed mutagenesis of the Htt 15Q and 138Q (1–1212) constructs (Wellington et al., 2000). Amino acids 167 to 170 were deleted in Htt 15Q (1–1212) and 402 to 403 were deleted in Htt 15Q (1–469) to help map the MMP cleavage site. Primers for the 469 stop construct were F: 5′-CAGCAGCTCTGCCTTAACATAGTCAGTGAAGGATGAGATC-3′ and R: 5′-GATCTCATCCTTCAC TGACTATGTTAAGGCAGAGCTGCTG-3′, 414 stop construct were F: 5′-GCTAAGGAGGA GTCTGGTTGACGAAGCCGTAGTGGGAG-3′ and R: 5′-CTCCCACTACGGCTTCGTC AACCAGACTCCTCCTTAGC-3′, 402 stop construct were F: 5′-CTGACCGCAGTCGGGG GCTAGGGGCAGCTCACCGCTGCTAAG-3′ and R: 5′-CTTAGCAGCGGTGAGCTGCCCCTAGCC CCCGACTGCGGTCAG-3′, 378 stop construct were F: 5′-CAATGTTGTGACCGGAGCCTAGGAG CTGTTGCAGCAGCTC-3′ and R: 5′-GAGCTGCTGCAACAGCTCC-TAGGCTCCGGTCACAACATTG-3′, 329 stop construct were F: 5′-GGTGCCCTTGCTGCAGCAGTAGGTCAAGGACACAAGCCTG-3′ and R: 5′-CAGGCTTGTGTCCTTGACCTACTGCTGCAGCAAGGGCACC-3′, 167 stop construct were F: 5′-GATTCTAATCTTCCAAGGTAGCAGCTCGAGCTCTATAAGG-3′ and R: 5′-CCTTAT AGAGCTCGAGCT-GCTACCTTGGAAGATTAGAATC-3′, Δ167–170 were F: 5′-CAAAGCTTTGATGGA-TTCTAATCTTCCAGAGCTCTATAAGG-3′ and R: 5′-CCTTATAGAGCTCTGG-AAGATTAGAATCCATCAAAGCTTTG-3′ and Δ402–403 were F: 5′-CCCTGACCGCAGTCGGCGGCCAGCTCACCGCTGCTAAGGAG-3′ and R: 5′-CTCCTTAGCAGCGGTGAGCTG GCCGCCGACTGCGGTCAGGG-3′. Polymerase chain reactions used 100 ng of DNA, 5.0 μl of 10× Pfu buffer (Stratagene), 0.2 mM dNTPs (Promega), 125 ng each of forward and reverse primers (Integrated DNA Technologies), 5% Me2SO, and 1 μl Pfu polymerase (Stratagene) for 18 cycles at 96°C for 1 min, 55°C for 1 min, and 68°C for 24 min, then 68°C for 7 min. Plasmids were DpnI (Promega)-treated, transformed into XL1-Blue supercompetent cells (Stratagene), and purified using the QIAprep Spin Miniprep Kit (Qiagen). Mutations and CAG length were confirmed by DNA sequencing. Constructs containing 6his-Htt23Q (1–450) and 6his-Htt55Q (1–450) were used for western analysis (Kaltenbach et al., 2007).
Htt Proteolysis Western Blot Primary Screen
We screened Dharmacon’s siGENOME SMARTPOOL Protease Set consisting of 514 protease siRNA pools of four duplexes for their ability to reduce the abundance of a mutant Htt N-terminal fragment. Human embryonic kidney 293T (HEK293T) cells were cultured in Dulbecco’s modified eagle medium (DMEM; Mediatech) containing penicillin/streptomycin (100 units/mL/100 g/ml) and 10% heat-inactivated fetal bovine serum (FBS) (Gibco). Cells were seeded in DMEM with 10% serum without penicillin/streptomycin at 5 × 104 cells/well in collagen-coated 96-well plates (BD, Biocoat) and incubated at 37°C for 24 h. Lipofectamine 2000 (Invitrogen) was used for transient transfections according to manufacturer’s instructions. Cells were transfected with 80 ng of DsRed DNA, 0.32 μg pcI-Htt138Q DNA, 136 nM siRNA and 1 μg Lipofectamine 2000 in 25 μL DMEM lacking serum or penicillin/streptomycin. After a 48 h incubation at 37°C, media was replaced with DMEM containing 10% FBS and 1 μM epoxomicin and incubated for an additional 24 h. Cells were lysed directly in wells with 25 μL M-PER (Thermo Scientific) containing protease inhibitors (1 tablet/10 mL, Complete Mini, EDTA-free, Roche) and RQ1 DNase (Promega) and 1mM MgCl2. Plates were shaken at 700 rpm for 10 min. To 10 μl of lysate, 6 μl of 4× LsDS loading buffer (Invitrogen) and 1 μl of 1M DTT was added and heated to 99°C for 5 min. Lysate proteins were resolved on 4–12% bis-tris gels, transferred to nitrocellulose membrane, and probed with monoclonal antibody MAB1574 (1C2, 1:2000, Chemicon). Multiple film exposures were collected for each blot, and non-saturated films were scanned and analyzed by densitometry using ImageQuant TL. Hits were identified as those siRNAs which reduced the amount of the ~55 kDa band (relative to the FL Htt) by 30% or more in duplicate. The 41 siRNAs that met these criteria were then retested in triplicate.
Secondary Screen for Caspase 3/7 Activity Suppression in Striatal Hdh7Q/7Q and Hdh111Q/111Q Cells
Striatal cell lines cells were nucleofected with siRNA as described below, plated at 5 × 104 cells/well on collagen-coated 96-well plates, and incubated for 48 h in DMEM containing 10% FBS and 100U/ml/100μg/ml penicillin/streptomycin, followed by serum-deprivation for 24 h before assaying. Cells were lysed in 50 μl of a 50/50 mixture of DMEM/Apo Lysis Buffer (Cell Technology Inc). Aliquots (10 μl) were taken for protein quantification by the BCA method (Pierce) before the addition of DMEM/Apo Lysis Buffer with 20 mM DTT and 2% APO 3/7 HTS Substrate (Cell Technology, Inc.). The substrate is a fluorescence-quenched Rhodamine-DEVD conjugate ((zDEVD)2-Rhodamine 110) that fluoresces (Ex485nm/Em530nm) upon cleavage of DEVD by caspase 3/7. Fluorescence was read for 1 h at 37 °C using a Fusion-Alpha FP HT (PerkinElmer). Toxicity is represented as caspase 3/7 activity in dRFU/min/mg protein.
Western Blotting of Striatal Hdh7Q/7Q and Hdh111Q/111Q Using siRNA Directed Against Target Proteases
Dharmacon siGENOME SMARTpools (Thermo Scientific) were used for siRNA disruption of CASP3 (M-043042), CAPN5 (M-042053), CAPN7 (M-043031), IMP5 (M-054596), ITGA2B (M-046584), KLK10 (M-062688), KLK11 (M-043814), MMP-10 (M-049762), MMP-14 (M-062241), MMP-23 (M-047760), RNF128 (M-060871), SPC18 (M-056742), and non-targeting (D-001206). Striatal Hdh7Q/7Q and Hdh111Q/111Q cells were maintained at 33°C in a humidified atmosphere of 95% air and 5% CO2, in DMEM containing 10% FBS, 100 U/mL penicillin and 100 μg/mL streptomycin. RNAi treatment of cells was as follows: 1 x 106 cells per siRNA treatment were collected and nucleofected using Kit L (Amaxa) with 3 μg of siRNA, and then 6 × 105 cells/well were plated on 6-well plates. After 48 h incubation, cells were serum starved for 24 h followed by harvesting for analysis by western blotting. For western blotting without siRNAs the cells were treated with and without serum.
Cells were resuspended in M-PER with protease inhibitors (1 tablet/10 mL, Complete Mini, EDTA-free, Roche). Whole-cell lysates were sonicated 5 × 5 sec pulses at 40 mA. Samples were centrifuged at 22K rpm and supernatant was collected for western blotting. 4× LDS sample buffer (Invitrogen) and 1 μl of 1M DTT were added to 20–40 μg total protein and boiled for 10 min. Samples were resolved by SDS-PAGE using 4–12% NuPage Bis-Tris gels under reducing conditions in MES running buffer. Gels were run at a constant 200V for 1 h, transferred to 0.45 μm nitrocellulose membranes in 1× NuPage transfer buffer for 8 h at 20V. Membranes were incubated in TBS with 0.1% Tween 20 (TBS-T), 5% non-fat milk for 1 h. Primary antibodies anti-MMP-10 (1:5000, Abcam ab28205), MMP-14 (1:2000, Abcam ab51074), MMP-23 (1: 500, Abcam ab5314); anti-polyQ (1:2000, Chemicon, MAB1574); and anti-Htt (1:500, MAB2166; 1:1000, MAB5490; 1:1000, MAB5492; 1:100 MAB5374, all from Chemicon); anti-tubulin (1:1000, Sigma); and anti-β-actin (1:1000, Cell Signaling) were diluted into TBS with 0.1% Tween 20 (TBS-T), 5% non-fat milk for 1 h. Blots were developed using Pierce ECL (Thermo Scientific). Band intensity was quantified using ImageQuant TL v2005.
RT-PCR of Striatal Cell Knockdown and Mouse Tissue mRNA
Total RNA was isolated from electroporated striatal cells (1 well of a 6-well plate) or striatal mouse tissue with the RNeasy Mini Kit (Qiagen) according to the manufacturer’s instructions. 1μg of RNA was converted to cDNA by using the Message Sensor RT kit (Applied Biosystems). Real time quantitative PCR (qPCR) was performed with either Universal Probe Library dye (UPL from Roche) or SYBR Green (Applied Biosystems) on the LightCycler 480 system (Roche). For quantification the threshold cycle Cp of each amplification was determined by the 2nd derivative analysis provided by the LightCycler 480 software and the 2−ΔΔCp method was used to determine the relative expression level of each gene normalized against the house-keeping gene β-actin. The specificity of each pair of primers was tested by comparing to a negative control sample of water on both quantification analysis and high resolution melting curve analysis. The primers used are: MMP10 F: 5′-ATGTTCTGTGGCTCCGGATGAG-3′, R: 5′-TGTGCTCAGGTGATGCTTTGTG-3′; MMP14 F: 5′-AACTTCGTGTTGCCTGATGA-3′, R: 5′-TTTGTGGGTGACCCTGACTT-3′; MMP23 F: 5′-GCACTGTCCCAGGATGAACT-3′, R: 5′-CCCAGGATGCACACACAA-3′.
MMP Cell Activity Assays
Striatal Hdh7Q/7Q and Hdh111Q/111Q cell pellets or mouse striatal tissue were lysed in M-PER or T-PER (Thermo Scientific), respectively. MMP enzyme activity was measured using the MMP assay kit (Biomol) per the manufacture’s protocol with the fluorogenic substrate Mca-Pro-Leu-Dpa-Ala-Arg-NH2 [Mca=(7-methoxycoumarin-4-yl)-acetyl; Dpa=N-3(2,3-dinitrophenyl)-L-α-β-diaminopropionyl]. Fluorescence was normalized to total protein in the lysate.
Gelatinase Gel Zymography
Gelatinase activity from cortex and striatum of R6/2 and control mouse tissue lysates (T-PER) was measured following affinity purification. Briefly, 500 μg of total protein was added to 500 μl lysis buffer (50 mM Tris-HCl, pH 7.6, 150 mM NaCl, 5 mM CaCl2, 0.05% Brij-35, 1% Triton X-100, and 0.02% NaN3) and affinity purified over gelatin Sepharose (GE Healthcare). MMP protein was eluted with 10% DMSO in PBS. 150 μg of purified lysate was added to 3 μl of 6× SDS buffer and resolved on a 10% zymogram gel (Invitrogen) containing 0.1% gelatin. Following electrophoresis, gels were incubated in 2.7% Triton X-100 at room temperature for 30 min then placed in developing buffer, 50 mM Tris-HCl, pH 7.6, 200 mM NaCl, 5 mM CaCl2, and 0.05% Brij-35, for 72 h at 37°C. After developing, gels were stained in SimplyBlue (Invitrogen) for 1 h at room temperature and destained in ddH2O overnight. Gelatinase activity was detected as a clearing of the blue-stained gelatin.
In situ Zymography, Immunohistochemistry of R6/2 and Control Brain Sections
R6/2 and control mice were perfused with ice cold PBS, pH 7.4 and brain tissue was collected and immediately frozen on dry ice. Frozen 20 μm sagittal sections were mounted on slides and quickly exposed to zymography solution of pH 7.4 containing 50 mM Tris-HCL, 150 mM NaCl, 5 mM CaCl2, 0.3 mM NaN3, and 40 μg DQ gelatin (Molecular Probes, Eugene, OR), overnight at 37°C. Control slides included 1 μM 1–10-phenanthroline MMP inhibitor. Reaction products were visualized on a confocal microscope (LSM510, Zeiss).
In situ zymography sections were fixed in 4% PFA in PBS, permeabilized in 1% Tween in 1 × TBS, blocked in 10% normal donkey serum with chicken anti-mouse IgG at 1:500. Primary antibodies were diluted in 1% BSA in 1× TBS and incubated 24 h at 4°C. All washes were done 3x10 min in 1× TBS. Secondary antibody was diluted in 1% BSA in 1× TBS and applied to slices for 1 h. MAB5374 Htt(82–150) antibody (1:50, Chemicon) was labeled with Alexa 647 donkey anti-mouse (Molecular Probes); and GFAP (1:500, Sigma) was labeled with Alexa 555 donkey anti-rabbit (Molecular Probes). Following incubation in secondary antibody, slices were washed and mounted in Prolong Gold (Invitrogen) with DAPI.
In vitro Protein Synthesis and Cleavage
Htt15Q (1–1212), Htt15Q (1–469), Htt138Q (1–469), Htt 15Q (1–414), Htt 15Q (1–402), Htt 15Q (1–378), Htt 15Q (1–329), Htt15Q (1–167), Htt138Q (1–167), Htt15Q (1–1212) Δ167–170 and Htt 15Q(1–469) Δ402–403 constructs were translated with TNTCoupled Reticulocyte Lysate Systems kit (Promega). 3 μl of translation product was diluted in M-PER and 10 U of the catalytic domain of MMP-2, MMP-10, or MMP-14 (Biomol) were added and incubated for 2 h at 37°C. Following addition of SDS and DTT, samples were heated to 90°C for 10 min and run on 12% Bis-Tris gels. Gels were fixed and dried before exposing to film.
Immunocytochemistry of Striatal Hdh111Q/111Q Cells
Immunocytochemistry was performed on Hdh111Q/111Q cells cultured with serum or 24 h serum depravation. Cells were fixed in 4% PFA in PBS for 20 min and washed 5 min in 1× PBS and 2× 5 min in 1× TBS. Blocking, primary antibodies, secondary antibodies and washes were applied according to In situ procedure described above. MAB5490 antibody Htt (115–129) (1:500, Chemicon) was labeled with Alexa 555 donkey anti-mouse (Molecular Probes) and MMP10 antibody (1:2000, Abcam) was labeled with Alexa 488 donkey anti-rabbit. Cells were washed 3× 5 min in 1× TBS and mounted in Prolong Gold (Invitrogen) with DAPI.
Drosophila Motor Performance Assay
Strains were obtained from either the Bloomington Stock Center (http://flystocks.bio.indiana.edu), or the Vienna Drosophila RNAi Center (http://stockcenter.vdrc.at/control/main). Motor performance test are described in the Supplemental Methods.
Supplementary Material
Acknowledgments
This work was supported by NIH (NS40251 to LME), (NS42179 to JB), CHDI (to REH, LME and JB), T32 training grant AG000266 (JPM) and Nathan Shock P30AG025708. We thank Danielle Crippen for training in immunohistochemistry and Espen Walker, University of New Mexico, for assistance in providing in situ zymography protocol. Hdh7Q/7Q and Hdh111Q/111Q cells were generously provided by Dr. Marcy MacDonald (Massachusetts General Hospital).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albin RL. Selective neurodegeneration in Huntington’s disease. Annals of neurology. 1995;38:835–836. doi: 10.1002/ana.410380602. [DOI] [PubMed] [Google Scholar]
- Asahi M, Asahi K, Jung JC, del Zoppo GJ, Fini ME, Lo EH. Role for matrix metalloproteinase 9 after focal cerebral ischemia: effects of gene knockout and enzyme inhibition with BB-94. J Cereb Blood Flow Metab. 2000;20:1681–1689. doi: 10.1097/00004647-200012000-00007. [DOI] [PubMed] [Google Scholar]
- Atwal RS, Truant R. A stress sensitive ER membrane-association domain in Huntingtin protein defines a potential role for Huntingtin in the regulation of autophagy. Autophagy. 2008;4:91–93. doi: 10.4161/auto.5201. [DOI] [PubMed] [Google Scholar]
- Baker AH, Edwards DR, Murphy G. Metalloproteinase inhibitors: biological actions and therapeutic opportunities. J Cell Sci. 2002;115:3719–3727. doi: 10.1242/jcs.00063. [DOI] [PubMed] [Google Scholar]
- Bertini I, Calderone V, Fragai M, Luchinat C, Mangani S, Terni B. Crystal structure of the catalytic domain of human matrix metalloproteinase 10. J Mol Biol. 2004;336:707–716. doi: 10.1016/j.jmb.2003.12.033. [DOI] [PubMed] [Google Scholar]
- Bhide PG, Day M, Sapp E, Schwarz C, Sheth A, Kim J, Young AB, Penney J, Golden J, Aronin N, DiFiglia M. Expression of normal and mutant huntingtin in the developing brain. J Neurosci. 1996;16:5523–5535. doi: 10.1523/JNEUROSCI.16-17-05523.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Z, Lin S, Fan LW, Pang Y, Rhodes PG. Minocycline alleviates hypoxic-ischemic injury to developing oligodendrocytes in the neonatal rat brain. Neuroscience. 2006;137:425–435. doi: 10.1016/j.neuroscience.2005.09.023. [DOI] [PubMed] [Google Scholar]
- Choi DH, Kim EM, Son HJ, Joh TH, Kim YS, Kim D, Flint Beal M, Hwang O. A novel intracellular role of matrix metalloproteinase-3 during apoptosis of dopaminergic cells. J Neurochem. 2008a;106:405–415. doi: 10.1111/j.1471-4159.2008.05399.x. [DOI] [PubMed] [Google Scholar]
- Choi WS, Jeon OH, Kim HH, Kim DS. MMP-2 regulates human platelet activation by interacting with integrin alphaIIbbeta3. J Thromb Haemost. 2008b;6:517–523. doi: 10.1111/j.1538-7836.2007.02871.x. [DOI] [PubMed] [Google Scholar]
- Cuadrado E, Rosell A, Penalba A, Slevin M, Alvarez-Sabin J, Ortega-Aznar A, Montaner J. Vascular MMP-9/TIMP-2 and neuronal MMP-10 up-regulation in human brain after stroke: a combined laser microdissection and protein array study. J Proteome Res. 2009;8:3191–3197. doi: 10.1021/pr801012x. [DOI] [PubMed] [Google Scholar]
- Cudkowicz M, Kowall NW. Degeneration of pyramidal projection neurons in Huntington’s disease cortex. Annals of neurology. 1990;27:200–204. doi: 10.1002/ana.410270217. [DOI] [PubMed] [Google Scholar]
- Gafni J, Ellerby LM. Calpain activation in Huntington’s disease. J Neurosci. 2002;22:4842–4849. doi: 10.1523/JNEUROSCI.22-12-04842.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gafni J, Hermel E, Young JE, Wellington CL, Hayden MR, Ellerby LM. Inhibition of calpain cleavage of huntingtin reduces toxicity: accumulation of calpain/caspase fragments in the nucleus. The Journal of biological chemistry. 2004;279:20211–20220. doi: 10.1074/jbc.M401267200. [DOI] [PubMed] [Google Scholar]
- Goldberg YP, Nicholson DW, Rasper DM, Kalchman MA, Koide HB, Graham RK, Bromm M, Kazemi-Esfarjani P, Thornberry NA, Vaillancourt JP, Hayden MR. Cleavage of huntingtin by apopain, a proapoptotic cysteine protease, is modulated by the polyglutamine tract. Nat Genet. 1996;13:442–449. doi: 10.1038/ng0896-442. [DOI] [PubMed] [Google Scholar]
- Gourfinkel-An I, Cancel G, Trottier Y, Devys D, Tora L, Lutz Y, Imbert G, Saudou F, Stevanin G, Agid Y, et al. Differential distribution of the normal and mutated forms of huntingtin in the human brain. Annals of neurology. 1997;42:712–719. doi: 10.1002/ana.410420507. [DOI] [PubMed] [Google Scholar]
- Graham RK, Deng Y, Slow EJ, Haigh B, Bissada N, Lu G, Pearson J, Shehadeh J, Bertram L, Murphy Z, et al. Cleavage at the caspase-6 site is required for neuronal dysfunction and degeneration due to mutant huntingtin. Cell. 2006;125:1179–1191. doi: 10.1016/j.cell.2006.04.026. [DOI] [PubMed] [Google Scholar]
- Group HsDCR. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell. 1993;72:971–983. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- Gu Z, Cui J, Brown S, Fridman R, Mobashery S, Strongin AY, Lipton SA. A highly specific inhibitor of matrix metalloproteinase-9 rescues laminin from proteolysis and neurons from apoptosis in transient focal cerebral ischemia. J Neurosci. 2005;25:6401–6408. doi: 10.1523/JNEUROSCI.1563-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackam AS, Singaraja R, Wellington CL, Metzler M, McCutcheon K, Zhang T, Kalchman M, Hayden MR. The influence of huntingtin protein size on nuclear localization and cellular toxicity. J Cell Biol. 1998;141:1097–1105. doi: 10.1083/jcb.141.5.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedreen JC, Peyser CE, Folstein SE, Ross CA. Neuronal loss in layers V and VI of cerebral cortex in Huntington’s disease. Neuroscience letters. 1991;133:257–261. doi: 10.1016/0304-3940(91)90583-f. [DOI] [PubMed] [Google Scholar]
- Hermel E, Gafni J, Propp SS, Leavitt BR, Wellington CL, Young JE, Hackam AS, Logvinova AV, Peel AL, Chen SF, et al. Specific caspase interactions and amplification are involved in selective neuronal vulnerability in Huntington’s disease. Cell Death Differ. 2004;11:424–438. doi: 10.1038/sj.cdd.4401358. [DOI] [PubMed] [Google Scholar]
- Hornebeck W, Lafuma C. Matrix metalloproteinase (MMP) C R Seances Soc Biol Fil. 1991;185:127–134. [PubMed] [Google Scholar]
- Kaltenbach LS, Romero E, Becklin RR, Chettier R, Bell R, Phansalkar A, Strand A, Torcassi C, Savage J, Hurlburt A, et al. Huntingtin interacting proteins are genetic modifiers of neurodegeneration. PLoS Genet. 2007;3:e82. doi: 10.1371/journal.pgen.0030082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kegel KB, Sapp E, Alexander J, Valencia A, Reeves P, Li X, Masso N, Sobin L, Aronin N, Difiglia M. Polyglutamine expansion in huntingtin alters its interaction with phospholipids. J Neurochem. 2009a doi: 10.1111/j.1471-4159.2009.06255.x. [DOI] [PubMed] [Google Scholar]
- Kegel KB, Schewkunow V, Sapp E, Masso N, Wanker EE, DiFiglia M, Goldmann WH. Polyglutamine expansion in huntingtin increases its insertion into lipid bilayers. Biochem Biophys Res Commun. 2009b;387:472–475. doi: 10.1016/j.bbrc.2009.07.039. [DOI] [PubMed] [Google Scholar]
- Kim M, Lee HS, LaForet G, McIntyre C, Martin EJ, Chang P, Kim TW, Williams M, Reddy PH, Tagle D, et al. Mutant huntingtin expression in clonal striatal cells: dissociation of inclusion formation and neuronal survival by caspase inhibition. J Neurosci. 1999;19:964–973. doi: 10.1523/JNEUROSCI.19-03-00964.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YJ, Sapp E, Cuiffo BG, Sobin L, Yoder J, Kegel KB, Qin ZH, Detloff P, Aronin N, DiFiglia M. Lysosomal proteases are involved in generation of N-terminal huntingtin fragments. Neurobiol Dis. 2006;22:346–356. doi: 10.1016/j.nbd.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Kim YJ, Yi Y, Sapp E, Wang Y, Cuiffo B, Kegel KB, Qin ZH, Aronin N, DiFiglia M. Caspase 3-cleaved N-terminal fragments of wild-type and mutant huntingtin are present in normal and Huntington’s disease brains, associate with membranes, and undergo calpain-dependent proteolysis. Proc Natl Acad Sci U S A. 2001;98:12784–12789. doi: 10.1073/pnas.221451398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konttinen YT, Ainola M, Valleala H, Ma J, Ida H, Mandelin J, Kinne RW, Santavirta S, Sorsa T, Lopez-Otin C, Takagi M. Analysis of 16 different matrix metalloproteinases (MMP-1 to MMP-20) in the synovial membrane: different profiles in trauma and rheumatoid arthritis. Ann Rheum Dis. 1999;58:691–697. doi: 10.1136/ard.58.11.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawitz P, Haffner C, Fluhrer R, Steiner H, Schmid B, Haass C. Differential localization and identification of a critical aspartate suggest non-redundant proteolytic functions of the presenilin homologues SPPL2b and SPPL3. J Biol Chem. 2005;280:39515–39523. doi: 10.1074/jbc.M501645200. [DOI] [PubMed] [Google Scholar]
- Landles C, Sathasivam K, Weiss A, Woodman B, Moffitt H, Finkbeiner S, Sun B, Gafni J, Ellerby LM, Trottier Y, et al. Proteolysis of mutant huntingtin produces an exon 1 fragment that accumulates as an aggregated protein in neuronal nuclei in Huntington disease. The Journal of biological chemistry. 2010;285:8808–8823. doi: 10.1074/jbc.M109.075028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Park JW, Kim SP, Lo EH, Lee SR. Doxycycline inhibits matrix metalloproteinase-9 and laminin degradation after transient global cerebral ischemia. Neurobiol Dis. 2009;34:189–198. doi: 10.1016/j.nbd.2008.12.012. [DOI] [PubMed] [Google Scholar]
- Lineberry N, Su L, Soares L, Fathman CG. The single subunit transmembrane E3 ligase gene related to anergy in lymphocytes (GRAIL) captures and then ubiquitinates transmembrane proteins across the cell membrane. J Biol Chem. 2008;283:28497–28505. doi: 10.1074/jbc.M805092200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llano E, Pendas AM, Aza-Blanc P, Kornberg TB, Lopez-Otin C. Dm1-MMP, a matrix metalloproteinase from Drosophila with a potential role in extracellular matrix remodeling during neural development. J Biol Chem. 2000;275:35978–35985. doi: 10.1074/jbc.M006045200. [DOI] [PubMed] [Google Scholar]
- Lunkes A, Lindenberg KS, Ben-Haiem L, Weber C, Devys D, Landwehrmeyer GB, Mandel JL, Trottier Y. Proteases acting on mutant huntingtin generate cleaved products that differentially build up cytoplasmic and nuclear inclusions. Molecular cell. 2002;10:259–269. doi: 10.1016/s1097-2765(02)00602-0. [DOI] [PubMed] [Google Scholar]
- Luo D, Mari B, Stoll I, Anglard P. Alternative splicing and promoter usage generates an intracellular stromelysin 3 isoform directly translated as an active matrix metalloproteinase. J Biol Chem. 2002;277:25527–25536. doi: 10.1074/jbc.M202494200. [DOI] [PubMed] [Google Scholar]
- Malemud CJ. Matrix metalloproteinases (MMPs) in health and disease: an overview. Front Biosci. 2006;11:1696–1701. doi: 10.2741/1915. [DOI] [PubMed] [Google Scholar]
- Mao Z, Choo YS, Lesort M. Cystamine and cysteamine prevent 3-NP-induced mitochondrial depolarization of Huntington’s disease knock-in striatal cells. Eur J Neurosci. 2006;23:1701–1710. doi: 10.1111/j.1460-9568.2006.04686.x. [DOI] [PubMed] [Google Scholar]
- Martindale D, Hackam A, Wieczorek A, Ellerby L, Wellington C, McCutcheon K, Singaraja R, Kazemi-Esfarjani P, Devon R, Kim SU, et al. Length of huntingtin and its polyglutamine tract influences localization and frequency of intracellular aggregates. Nat Genet. 1998;18:150–154. doi: 10.1038/ng0298-150. [DOI] [PubMed] [Google Scholar]
- Mende-Mueller LM, Toneff T, Hwang SR, Chesselet MF, Hook VY. Tissue-specific proteolysis of Huntingtin (htt) in human brain: evidence of enhanced levels of N- and C-terminal htt fragments in Huntington’s disease striatum. J Neurosci. 2001;21:1830–1837. doi: 10.1523/JNEUROSCI.21-06-01830.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milakovic T, Johnson GV. Mitochondrial respiration and ATP production are significantly impaired in striatal cells expressing mutant huntingtin. J Biol Chem. 2005;280:30773–30782. doi: 10.1074/jbc.M504749200. [DOI] [PubMed] [Google Scholar]
- Mookerjee S, Papanikolaou T, Guyenet SJ, Sampath V, Lin A, Vitelli C, DeGiacomo F, Sopher BL, Chen SF, La Spada AR, Ellerby LM. Posttranslational modification of ataxin-7 at lysine 257 prevents autophagy-mediated turnover of an N-terminal caspase-7 cleavage fragment. J Neurosci. 2009;29:15134–15144. doi: 10.1523/JNEUROSCI.4720-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira JM, Chen S, Almeida S, Riley R, Goncalves J, Oliveira CR, Hayden MR, Nicholls DG, Ellerby LM, Rego AC. Mitochondrial-dependent Ca2+ handling in Huntington’s disease striatal cells: effect of histone deacetylase inhibitors. J Neurosci. 2006;26:11174–11186. doi: 10.1523/JNEUROSCI.3004-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page-McCaw A. Remodeling the model organism: matrix metalloproteinase functions in invertebrates. Semin Cell Dev Biol. 2008;19:14–23. doi: 10.1016/j.semcdb.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page-McCaw A, Serano J, Sante JM, Rubin GM. Drosophila matrix metalloproteinases are required for tissue remodeling, but not embryonic development. Dev Cell. 2003;4:95–106. doi: 10.1016/s1534-5807(02)00400-8. [DOI] [PubMed] [Google Scholar]
- Pei D, Weiss SJ. Furin-dependent intracellular activation of the human stromelysin-3 zymogen. Nature. 1995;375:244–247. doi: 10.1038/375244a0. [DOI] [PubMed] [Google Scholar]
- Pei D, Weiss SJ. Transmembrane-deletion mutants of the membrane-type matrix metalloproteinase-1 process progelatinase A and express intrinsic matrix-degrading activity. J Biol Chem. 1996;271:9135–9140. doi: 10.1074/jbc.271.15.9135. [DOI] [PubMed] [Google Scholar]
- Ratovitski T, Gucek M, Jiang H, Chighladze E, Waldron E, D’Ambola J, Hou Z, Liang Y, Poirier MA, Hirschhorn RR, et al. Mutant huntingtin N-terminal fragments of specific size mediate aggregation and toxicity in neuronal cells. J Biol Chem. 2009;284:10855–10867. doi: 10.1074/jbc.M804813200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratovitski T, Nakamura M, D’Ambola J, Chighladze E, Liang Y, Wang W, Graham R, Hayden MR, Borchelt DR, Hirschhorn RR, Ross CA. N-terminal proteolysis of full-length mutant huntingtin in an inducible PC12 cell model of Huntington’s disease. Cell Cycle. 2007;6:2970–2981. doi: 10.4161/cc.6.23.4992. [DOI] [PubMed] [Google Scholar]
- Ronco P, Lelongt B, Piedagnel R, Chatziantoniou C. Matrix metalloproteinases in kidney disease progression and repair: a case of flipping the coin. Semin Nephrol. 2007;27:352–362. doi: 10.1016/j.semnephrol.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Silvestroni A, Faull RL, Strand AD, Moller T. Distinct neuroinflammatory profile in post-mortem human Huntington’s disease. Neuroreport. 2009;20:1098–1103. doi: 10.1097/WNR.0b013e32832e34ee. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Igarashi S, Nakamura M, Gafni J, Torcassi C, Schilling G, Crippen D, Wood JD, Sawa A, Jenkins NA, et al. Progressive phenotype and nuclear accumulation of an amino-terminal cleavage fragment in a transgenic mouse model with inducible expression of full-length mutant huntingtin. Neurobiol Dis. 2006;21:381–391. doi: 10.1016/j.nbd.2005.07.014. [DOI] [PubMed] [Google Scholar]
- Trettel F, Rigamonti D, Hilditch-Maguire P, Wheeler VC, Sharp AH, Persichetti F, Cattaneo E, MacDonald ME. Dominant phenotypes produced by the HD mutation in STHdh(Q111) striatal cells. Hum Mol Genet. 2000;9:2799–2809. doi: 10.1093/hmg/9.19.2799. [DOI] [PubMed] [Google Scholar]
- Vergote D, Butler GS, Ooms M, Cox JH, Silva C, Hollenberg MD, Jhamandas JH, Overall CM, Power C. Proteolytic processing of SDF-1alpha reveals a change in receptor specificity mediating HIV-associated neurodegeneration. Proc Natl Acad Sci U S A. 2006;103:19182–19187. doi: 10.1073/pnas.0604678103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CE, Tydlacka S, Orr AL, Yang SH, Graham RK, Hayden MR, Li S, Chan AW, Li XJ. Accumulation of N-terminal mutant huntingtin in mouse and monkey models implicated as a pathogenic mechanism in Huntington’s disease. Hum Mol Genet. 2008;17:2738–2751. doi: 10.1093/hmg/ddn175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei S, Xie Z, Filenova E, Brew K. Drosophila TIMP is a potent inhibitor of MMPs and TACE: similarities in structure and function to TIMP-3. Biochemistry. 2003;42:12200–12207. doi: 10.1021/bi035358x. [DOI] [PubMed] [Google Scholar]
- Wellington CL, Ellerby LM, Gutekunst CA, Rogers D, Warby S, Graham RK, Loubser O, van Raamsdonk J, Singaraja R, Yang YZ, et al. Caspase cleavage of mutant huntingtin precedes neurodegeneration in Huntington’s disease. J Neurosci. 2002;22:7862–7872. doi: 10.1523/JNEUROSCI.22-18-07862.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellington CL, Ellerby LM, Hackam AS, Margolis RL, Trifiro MA, Singaraja R, McCutcheon K, Salvesen GS, Propp SS, Bromm M, et al. Caspase cleavage of gene products associated with triplet expansion disorders generates truncated fragments containing the polyglutamine tract. J Biol Chem. 1998;273:9158–9167. doi: 10.1074/jbc.273.15.9158. [DOI] [PubMed] [Google Scholar]
- Wellington CL, Singaraja R, Ellerby L, Savill J, Roy S, Leavitt B, Cattaneo E, Hackam A, Sharp A, Thornberry N, et al. Inhibiting caspase cleavage of huntingtin reduces toxicity and aggregate formation in neuronal and nonneuronal cells. The Journal of biological chemistry. 2000;275:19831–19838. doi: 10.1074/jbc.M001475200. [DOI] [PubMed] [Google Scholar]
- Xue M, Fan Y, Liu S, Zygun DA, Demchuk A, Yong VW. Contributions of multiple proteases to neurotoxicity in a mouse model of intracerebral haemorrhage. Brain. 2009;132:26–36. doi: 10.1093/brain/awn215. [DOI] [PubMed] [Google Scholar]
- Yanai A, Huang K, Kang R, Singaraja RR, Arstikaitis P, Gan L, Orban PC, Mullard A, Cowan CM, Raymond LA, et al. Palmitoylation of huntingtin by HIP14 is essential for its trafficking and function. Nat Neurosci. 2006;9:824–831. doi: 10.1038/nn1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.