Summary
OBJECTIVE
Testosterone therapy for osteoporosis has not been studied extensively in women because of its potential to cause virilization. Female-to-male transsexuals are genetic females who suffer from gender dysphoria and thus take supra-physiologic doses of testosterone to change from the female to male phenotype. The aim of this study is to examine the effects of testosterone treatment on the genetic female skeleton.
PATIENTS AND DESIGN
A group of 15 female-to-male transsexuals was prospectively enrolled for observation over a 2-year period. The subjects had a mean age of 37·0 ± 3·0 years. All of the subjects self-administered testosterone esters intramuscularly at a mean dose of 70·7 ± 4·5 mg weekly.
MEASUREMENTS
The subjects had measurements of bone mineral density (BMD) by dual X-ray absorptiometry (DXA) of the femoral neck and spine (L2–L4) at 12-month intervals. They had determinations of serum oestradiol, testosterone, soluble RANKL (sRANKL), osteoprotegerin (OPG) and urine N-telopeptide (NTX) at the date of enrolment and at the end of 2 years.
RESULTS
There was a significant positive increase in mean BMD of 7·8% at the femoral neck and a nonsignificant increase in mean BMD of 3·1% at the spine over 2 years. The levels of testosterone reached the upper normal range for males and the levels of oestradiol declined to near the postmenopausal range. sRANKL levels decreased significantly in female-to-male transsexuals who newly initiated testosterone therapy. There was no significant change in urine NTX or serum OPG during the study.
CONCLUSIONS
We conclude that supra-physiologic testosterone therapy increases BMD at the hip while maintaining BMD at the spine in female-to-male transsexuals. The effects of testosterone may be the result of testosterone hormone directly acting on the bone or indirectly through aromatization to oestradiol. Lower RANKL levels coupled with unchanged OPG levels results in an increased OPG/RANKL ratio, which may be beneficial to the bone by inhibiting osteoclastogenesis.
Oestrogen is regarded as the dominant sex steroid hormone in maintaining bone mineral density (BMD) in males and females. Oestrogen therapy in postmenopausal women prevents bone loss (Wells et al., 2002), increases BMD (Lindsay & Thome, 1990) and prevents fractures (Women’s Health Initiative, 2002). Oestradiol rather than testosterone in men correlates more strongly with BMD (Greendale et al., 1997; Khosla et al., 1998; Amin et al., 2000; Szulc et al., 2003) and is a stronger determinant of peak bone mass (Khosla et al., 2001). Men who have either defective aromatase (Bilezikian et al., 1998) or oestrogen receptor (Smith et al., 1994) develop severe osteoporosis, which further supports the notion that oestradiol is the more dominant sex steroid in preserving bone density.
Previously, testosterone was considered the more dominant sex steroid in men. With ageing, men experience declining levels of testosterone (Morley et al., 1997) and decreasing BMD (Meier et al., 1984; Jones et al., 1994; Fatayerji et al., 1999; Melton et al., 2000). Testosterone treatment of elderly men with hypogonadism increases BMD (Katznelson et al., 1996; Behre et al., 1997, 1999; Snyder et al., 1999; Amory et al., 2004). However, a study of elderly men rendered hypogonadal with GnRH agonists demonstrated that men receiving oestrogen had significantly lower bone turnover than men receiving testosterone (Falahati-Nini et al., 2000). Thus, it is unclear whether the beneficial effects of testosterone on bone physiology are the direct effect of testosterone or an indirect effect by the aromatization of testosterone to oestradiol, either peripherally or locally by osteoblasts (Shozu & Simpson, 1998).
In the present study, we evaluated the effect of supra-physiologic testosterone administration on BMD and markers of bone turnover in female-to-male (FTM) transsexuals. FTM transsexuals are biologic females who take testosterone in order to change their phenotype to the male gender (Tangpricha et al., 2003). Our initial hypothesis was that testosterone would not be sparing of BMD because oestradiol levels would decline secondary to the antigonadotropic effects of testosterone. We measured BMD at the hip and spine annually over a period of 2 years in addition to levels of sex hormones and markers of bone turnover.
Subjects and methods
Subjects
Eligible participants for the study were FTM transsexuals who had been prescribed testosterone treatment for gender reassignment. Subjects were excluded if they were taking any medications known to impact BMD other than calcium and multivitamins or if they were currently pregnant.
The study was approved by the Institutional Review Board at Boston University School of Medicine and conducted through the General Clinical Research Center (GCRC) at Boston University School of Medicine. All of the study participants gave written informed consent prior to participation in the study.
Study protocol
Subjects attended the GCRC at Boston University School of Medicine for the initial screening and returned to the GCRC every 6 months. Subjects were instructed to return for their visits 1–2 days prior to an injection of testosterone for trough measurements of serum testosterone. The principal investigator performed the initial history and physical examination and interviewed all of the subjects at each subsequent visit.
Bone mineral measurements
BMD at the femoral neck of the hip and spine (L2–L4) were performed at baseline, 12 months and 24 months using a Norland Ellipse dual-energy X-ray absorptiometer. The coefficient of variation (CV) was obtained by daily measurements using a standard phantom. The CV was 1·2% at the femoral neck of the hip and 1·0% at L2–L4 of the spine.
Biochemical measurements
Blood specimens were obtained for serum oestradiol and total testosterone at enrollment, 1 year and 2 years. Serum osteoprotegerin (OPG) and soluble receptor activator of NF-kappaB ligand (sRANKL) were determined at enrollment and at 2 years. A urine sample was collected for determination of urine N-telopeptide (NTX) at each of the annual visits. The levels of serum testosterone and urine NTX were determined by Quest Diagnostics Incorporated (Cambridge, MA, USA). Levels of OPG and sRANKL were determined using a commercially available kit for OPG and sRANKL from ALPCO Diagnostics (Windham, NH, USA) and performed according to the instructions by the manufacturer. Levels of oestradiol were determined using a commercially available kit from Diagnostic Systems Laboratory (Webster, TX, USA) and performed according to protocols supplied by the manufacturer.
Statistical analysis
Descriptive statistics were used to summarize the BMD and biochemical measurements and were expressed as mean ± SEM. Changes in biochemical measurements were evaluated for significance using a paired t-test comparing enrollment baseline and final measurements. Changes in BMD measurements at enrollment, 1 year and 2 years were further analysed with repeated measures by ANOVA. Pair-wise comparisons with adjustment for multiple comparisons (Bonferroni) were performed if the ANOVA was significant. All of the analyses were carried out at the level of a = 0·05. The statistical analyses were performed using Microsoft Excel Office 2003™ (Seattle, WA, USA) and Analyse-It™ (Leeds, UK) statistical software.
Results
Subjects
Twenty-five eligible subjects agreed to participate in the study. Ten subjects failed to complete the 2-year study (three moved from the area and seven failed to return for the 2-year visit). Therefore, 15 subjects completed the 2-year study (Table 1). All of the subjects took intramuscular testosterone esters for their gender reassignment regimen.
Table 1.
Baseline characteristics of female-to-male transsexual study participants
| Testosterone-naïve (n = 8) | Testosterone-treated (n = 7) | All | |
|---|---|---|---|
| Age (years) | 33·1 ± 3·0 | 40·5 ± 4·0 | 37·0 ± 3·0 |
| Years on testosterone | 0 | 4·2 ± 1·0 | |
| Dose of testosterone* (mg testosterone ester/week) | 66·0 ± 6·0 | 76·0 ± 7·0 | 70·7 ± 4·5 |
| Current smokers (%) | 5/8 (63%) | 3/7 (43%) | 8 (53·3%) |
| Prior ovariectomy (%) | 0/8 | 5/7 (71%) | 5/15 (33%) |
| Baseline BMD | |||
| Spine L2–L4 (gm/cm2) | 1·211 ± 0·06 | 1·134 ± 0·06 | 1·172 ± 0·04 |
| Femoral neck (gm/cm2) | 1·072 ± 0·07 | 0·861 ± 0·03 | 0·984 ± 0·05 |
Data expressed as mean ± SEM.
Subjects took either testosterone cypionate or enanthate.
There were no differences between the 15 who completed the study and the 10 who failed to complete the study in regards to initial spine BMD (P = 0·65), initial hip BMD (P = 0·88), oestradiol level (P = 0·49), testosterone level (P = 0·66), testosterone dose (P = 0·60) and urine N-telopeptide (P = 0·14). The only significant difference that was found was in age. The dropouts were statistically younger than the subjects who remained in the study, 27·3 ± 3·0 years old vs. 37·0 ± 3·0 years old, P = 0·03.
Seven subjects were taking testosterone for a mean of 4·1 ± 1·0 years prior to enrolling into the study. An additional eight subjects enrolled in the study prior to starting testosterone therapy. Five out of 15 subjects underwent ovariectomy 2·3 ± 0·5 years prior to enrolment in the study (Table 1). All of these subjects were already receiving testosterone therapy.
Serum oestradiol
Seven out of eight subjects had levels of serum oestradiol levels determined prior to their gender reassignment and before the initiation of testosterone therapy. Their levels of oestradiol significantly decreased by 48% after 2 years of testosterone therapy (Table 2; P = 0·02). There was no statistically significant change in oestradiol levels in subjects who had already been treated with testosterone (Table 2).
Table 2.
Changes in biochemical markers of female-to-male transsexuals over 2 years
| Baseline | 2 years | % | P-value | |
|---|---|---|---|---|
| Previously treated transsexuals | ||||
| Oestradiol* (pmol/l) (n = 4) | 161 ± 15 | 148 ± 20 | 8·0% | 0·67 |
| Testosterone† (nmol/l) (n = 7) | 20·4 ± 3·0 | 26·4 ± 6·0 | +29% | 0·36 |
| OPG (pmol/l) (n = 6) | 5·3 ± 0·7 | 5·5 ± 1·0 | +3·8% | 0·88 |
| sRANKL (pmol/l) (n = 6) | 0·4 ± 0·7 | 0·3 ± 0·7 | 13·0% | 0·58 |
| OPG/RANKL ratio (n = 6) | 17·0 ± 5·0 | 18·4 ± 5·0 | +8·0% | |
| Urine N-telopeptide (n = 7) (BCE/mmol creatinine) | 31·7 ± 7 | 28·3 ± 3 | 10·5% | 0·57 |
| Newly treated transsexuals | ||||
| Oestradiol* (pmol/l) (n = 7) | 400 ± 74 | 206 ± 50 | 48·65% | 0·02 |
| Testosterone† (nmol/l)(n = 7) | 1·52 ± 0·46 | 22·5 ± 4·8 | +1470% | 0·006 |
| OPG (pmol/l) (n = 8) | 5·06 ± 0·89 | 4·30 ± 0·55 | 38·0% | 0·28 |
| sRANKL (pmol/l) (n = 8) | 0·80 ± 0·26 | 0·45 ± 0·18 | 32·0% | 0·048 |
| OPG/RANKL ratio | 12·3 ± 4 | 18·1 ± 5 | +47·0% | |
| Urine N-telopeptide (n = 8) (BCE/mmol creatinine) | 32·9 ± 8 | 30·9 ± 6 | 6·0% | 0·84 |
Reference ranges for oestradiol, males 20·5–185 pmol/l, females, postmenopausal 21–380 pmol/l, females, luteal phase 128–1370 pmol/l.
Reference range for testosterone, males 9·0–34·7 nmol/l, females < 3·5 nmol/l.
Serum testosterone
Seven out eight subjects had levels of serum testosterone determined prior to their gender reassignment and before the initiation of testosterone therapy. The levels of testosterone significantly increased greater than 14-fold after 2 years of testosterone therapy (Table 2; P = 0·006). The subjects who were already being treated with testosterone had no significant change in their serum testosterone level (Table 2).
Markers of bone turnover
The mean urine NTX was low at enrollment for both groups of transsexuals, pre- and posthormone treatment for gender reassignment (Table 2). There was no statistically significant change in urine NTX after 2 years of follow-up.
Osteoprotegerin/RANKL signalling
Levels of OPG decreased by 38% in FTM transsexuals from baseline (prior to gender reassignment) to 2-year measurements (P = 0·28; Table 2). There was no change in OPG levels in subjects who were already taking testosterone at enrollment (Table 2).
Levels of sRANKL significantly decreased by 32% in the first 2 years of testosterone therapy in newly initiated transsexuals (P = 0·048; Table 2). Transsexuals who had previously been treated with testosterone prior to entry had lower mean sRANKL than newly treated transsexuals at enrollment and after 2 years. Their sRANKL levels decreased by a further 13% after the 2-year follow-up period (P = 0·58).
Bone density measurements
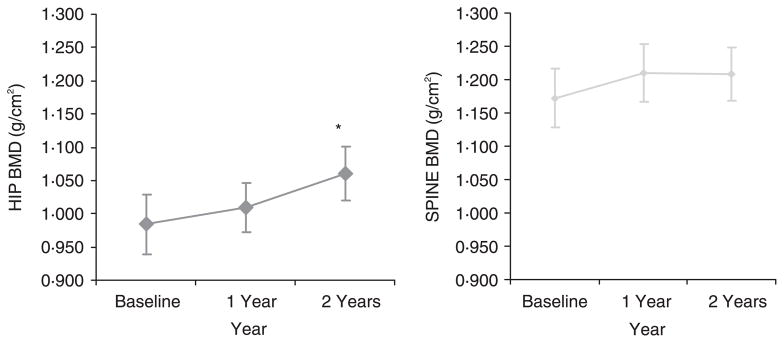
Fourteen out of 15 transsexual subjects had increases in BMD at the hip over the 2-year observation period. Repeated ANOVA measurements were significant (P < 0·001) for changes in hip BMD. There was a significant 7·8% increase after 2 years of observation in mean hip BMD from enrollment (0·984 ± 0·05 g/cm2 −1·060 ± 0·04 g/cm2; t-test with Bonferroni correction, P ≤ 0·01; Fig. 1).
Fig. 1.
Mean bone mineral density (BMD) changes in female-to-male transsexuals on testosterone. The mean hip BMD increased by 7·8% after 2 years from initial enrollment in the study (P < 0·01, t-test with Bonferroni correction for repeated measures). The mean spine BMD increased by 3·1% after 2 years from initial enrollment in the study. *P < 0·05. Error bars represent ± SEM.
Eleven out of 15 transsexual subjects had increases in BMD at the spine over the 2-year observation period. There was a 3·1% increase after 2 years of observation in mean spine BMD from enrollment (1·172 ± 0·04 g/cm2−1·209 ± 0·04 g/cm2; Fig. 1). Post hoc analysis with t-test with Bonferroni correction did not reveal any statistically significant change in enrolment spine BMD measurements to 2-year measurements.
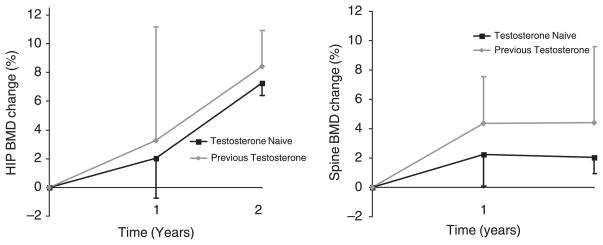
The increases in BMD were analysed in the two subgroups: previously treated with testosterone and testosterone naïve. The previously treated group had a mean increase of 8·4% and 4·4% at the hip and spine, respectively (Fig. 2). The testosterone naïve group had mean increase of 7·2% and 2·0% at the hip and spine, respectively (Fig. 2).
Fig. 2.
Mean individual change (%) in bone mineral density (BMD) in female-to-male transsexuals on testosterone therapy. The mean percentage increase after 2 years in hip BMD was 8·4% and 7·2% in the previously treated and testosterone naïve groups (left panel). The mean percentage increase after 2 years in spine BMD was 4·4% and 2·0% in the previously treated and testosterone naïve groups (left panel).
Discussion
This case series demonstrates that testosterone treatment in FTM transsexuals results in increased hip BMD and maintenance of spinal BMD over an observation period of 2 years. The increase in BMD occurred despite serum oestradiol levels decreasing to near the menopausal range, suggesting a potentially beneficial effect of testosterone. The increase in BMD may be the result of the direct effect of testosterone on bone or an indirect effect of testosterone after aromatization to oestradiol.
One previous study of 19 FTM transsexuals from Holland examined the effects of testosterone administration in regards to spinal BMD only. They demonstrated a loss of BMD at the spine over a 2- to 5-year period (van Kesteren et al., 1998). All of the subjects in this Dutch study underwent ovariectomy within 12–18 months of starting testosterone therapy. They were treated with intramuscular (i.m.) testosterone esters 250 mg every 2 weeks and were continued on a lower dose of parental testosterone postoperatively, testosterone esters 250 mg i.m. every 2–3 weeks, or changed to oral testosterone undeconaote 160 mg/day. The lower dose of testosterone resulted in higher levels of the gonadotropins LH and FSH (21·6 ± 4·10 and 46·2 ± 10·6, respectively) at the end of the study compared to preoperative levels on higher doses of testosterone (1·9 ± 0·4 and 2·7 ± 0·4, respectively). In our study, only five out of 15 subjects underwent ovariectomy prior to enrolling in our study and no subjects had ovariectomy during the study. The subjects in our study did not reduce their dose of testosterone during the 2-year period. We sampled the levels of LH and FSH from 12 of our subjects at the end of our 2-year study and found that they were more uniformly suppressed (5·4 ± 2·6 and 3·2 ± 1·2, respectively). This may explain why our subjects had positive increases in BMD at the hip and preservation of BMD at the spine compared to the study by van Kesteren et al. (1998).
In a cross-sectional analysis of FTM transsexuals in Singapore, a group of FTM transsexuals who were treated for a period of 1–3 years with similar doses of testosterone used in our study had higher spine BMD compared to a group of testosterone-naive FTM transsexuals. Subjects who were noncompliant with testosterone therapy after ovariectomy had significantly lower BMD than testosterone naïve FTM transsexuals. The BMD of five non-compliant subjects improved with re-initiation of testosterone therapy. The authors concluded that testosterone therapy improves BMD in FTM transsexuals especially in a hormone-deficient state such as after ovariectomy (Goh & Ratnam, 1997). These findings are similar to our study. We demonstrated that continuous testosterone therapy in FTM transsexuals significantly increases the BMD at the hip while maintaining BMD at the spine compared to baseline measurements.
Our subjects were receiving supra-physiologic dosages of testosterone, making comparison to other studies using female replacement doses of testosterone more problematic. These studies examining the effects of testosterone replacement in preserving BMD in females have revealed conflicting findings. A randomized 2-year study of 34 postmenopausal women demonstrated that combination therapy of testosterone plus oestrogen pellets was superior in improving BMD at the hip and spine than oestrogen alone (Davis et al., 1995). Another trial of 66 post-menopausal women demonstrated that oral combination therapy of oestrogen and testosterone therapy was not superior to oestrogen alone in improving spine BMD (Watts et al., 1995). A previous study using oestrogen and testosterone pellets showed no additional benefit of testosterone over oestrogen on hip and spine BMD (Garnett et al., 1992).
Our study is consistent with previous studies that androgens inhibit OPG. Hofbauer et al. (2002) demonstrated that androgens inhibit mRNA and protein secretion in osteoblast cultures. Elderly men rendered hypogonadal with GnRH agonists had decreases in OPG after testosterone therapy (Khosla et al., 2002). Oestrogen appears to have the opposite effect on OPG production. When the same elderly men rendered hypogonadal with GnRH agonists were treated with oestrogen, there were increases in OPG (Khosla et al., 2002). Several in vitro studies have demonstrated that oestrogen increases OPG levels in bone marrow stromal and osteoblastic cells (Hofbauer et al., 1999; Chen et al., 2000; Saika et al., 2001). Women who take oral contraceptives containing oestrogen have higher serum OPG levels compared to nonusers (Hofbauer et al., 2004). We noted a decrease in soluble RANKL levels and with no increase in circulating OPG levels in the newly treated testosterone subjects. This would result in an increased OPG/RANKL ratio which might be potentially favourable for bone preservation by decreasing osteoclast formation.
We saw a greater increase in BMD at the hip compared to the spine. Because the hip has more cortical bone than the spine, which has more trabecular bone, one potential explanation for this differential effect could be that testosterone has more positive effects on cortical bone than trabecular bone as suggested by Lips et al. (1996). In his study, he performed iliac crest biopsies on FTM transsexuals and found greater cortical thickness compared to women and healthy young men, whereas there was no difference in trabecular bone thickness.
The increase in BMD occurred with no significant decrease in bone turnover measured by urine NTX. The most plausible explanation for this discrepant finding could be that the subjects already had very low bone turnover by baseline measurements of urine NTX prior to initiating testosterone therapy. This would suggest that testosterone may exert beneficial effects on the bone independent from reducing bone turnover.
In summary, this small case series of genetic FTM transsexuals suggests that testosterone therapy in genetic females in supra-physiologic dosages may improve BMD at the hip and maintain BMD at the spine. This improvement occurs in spite of lowered serum oestradiol levels. Larger controlled studies using this human model of androgen supplementation in genetic females may be difficult to conduct due to its extremely low prevalence. Studies using either lower doses of testosterone or less androgenic testosterone derivatives should be conducted to investigate the role of androgens in female skeletal health.
Acknowledgments
This work was supported by NIH MO1RR00053 and T32DK007201.
References
- Amin S, Zhang Y, Sawin CT, Evans SR, Hannan MT, Kiel DP, Wilson PW, Felson DT. Association of hypogonadism and estradiol levels with bone mineral density in elderly men from the Framingham Study. Annals of Internal Medicine. 2000;133:951–963. doi: 10.7326/0003-4819-133-12-200012190-00010. [DOI] [PubMed] [Google Scholar]
- Amory JK, Watts NB, Easley KA, Sutton PR, Anawalt BD, Matsumoto AM, Bremner WJ, Tenover JL. Exogenous testosterone or testosterone with finasteride increases bone mineral density in older men with low serum testosterone. Journal of Clinical Endocrinology and Metabolism. 2004;89:503–510. doi: 10.1210/jc.2003-031110. [DOI] [PubMed] [Google Scholar]
- Behre HM, Kliesch S, Leifke E, Link TM, Nieschlag E. Long-term effect of testosterone therapy on bone mineral density in hypogonadal men. Journal of Clinical Endocrinology and Metabolism. 1997;82:2386–2390. doi: 10.1210/jcem.82.8.4163. [DOI] [PubMed] [Google Scholar]
- Behre HM, von Eckardstein S, Kliesch S, Nieschlag E. Long-term substitution therapy of hypogonadal men with transscrotal testosterone over 7–10 years. Clinical Endocrinology. 1999;50:629–635. doi: 10.1046/j.1365-2265.1999.00705.x. [DOI] [PubMed] [Google Scholar]
- Bilezikian JP, Morishima A, Bell J, Grumbach MM. Increased bone mass as a result of estrogen therapy in a man with aromatase deficiency. New England Journal of Medicine. 1998;27:339, 599–603. doi: 10.1056/NEJM199808273390905. [DOI] [PubMed] [Google Scholar]
- Chen XW, Garner SC, Anderson JJB. Effects of 17b-estradiol (E2), genistein (GEN) and daidzein (DIZ) on osteoclastogenesis inhibitory factor (OCIF) and osteoclast differentiation factor (ODF) mRNA expressed during MC3T3–E1 cell differentiation. Journal of Bone Mineral Research. 2000;15:S495. [Google Scholar]
- Davis SR, McCloud P, Strauss BJ, Burger H. Testosterone enhances estradiol’s effects on postmenopausal bone density and sexuality. Maturitas. 1995;21:227–236. doi: 10.1016/0378-5122(94)00898-h. [DOI] [PubMed] [Google Scholar]
- Falahati-Nini A, Riggs BL, Atkinson EJ, O’Fallon WM, Eastell R, Khosla S. Relative contributions of testosterone and estrogen in regulating bone resorption and formation in normal elderly men. Journal of Clinical Investigations. 2000;106:1553–1560. doi: 10.1172/JCI10942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatayerji D, Cooper AM, Eastell R. Total and regional bone mineral density in men: effect of age. Osteoporosis International. 1999;10:59–65. doi: 10.1007/s001980050195. [DOI] [PubMed] [Google Scholar]
- Garnett T, Studd J, Watson N, Savvas M, Leather A. The effects of plasma estradiol levels on increases in vertebral and femoral bone density following therapy with estradiol and estradiol with testosterone implants. Obstetrics and Gynecology. 1992;79:968–972. [PubMed] [Google Scholar]
- Goh HHV, Ratnam SS. Effects of hormone deficiency, androgen therapy and calcium supplementation on bone mineral density in female transsexuals. Maturitas. 1997;26:45–52. doi: 10.1016/s0378-5122(96)01073-0. [DOI] [PubMed] [Google Scholar]
- Greendale GA, Edelstein S, Barrett-Connor E. Endogenous sex steroids and bone mineral density in older women and men: the Rancho Bernardo Study. Journal of Bone Mineral Research. 1997;12:1833–1843. doi: 10.1359/jbmr.1997.12.11.1833. [DOI] [PubMed] [Google Scholar]
- Hofbauer LC, Khosla S, Dunstan CR, Lacey DL, Spelsberg TC, Riggs BL. Estrogen stimulates gene expression and protein production of osteoprotegerin in human osteoblastic cells. Endocrinology. 1999;140:4367–4370. doi: 10.1210/endo.140.9.7131. [DOI] [PubMed] [Google Scholar]
- Hofbauer LC, Hicok KC, Chen D, Khosla S. Regulation of osteoprotegerin production by androgens and anti-androgens in human osteoblastic lineage cells. European Journal of Endocrinology. 2002;147:269–273. doi: 10.1530/eje.0.1470269. [DOI] [PubMed] [Google Scholar]
- Hofbauer LC, Schoppet M, Schüller P, Viereck V, Christ M. Effects of oral contraceptives on circulating osteoprotegerin and soluble RANK ligand serum levels in healthy young women. Endocrinology. 2004;60:214–219. doi: 10.1046/j.1365-2265.2003.01969.x. [DOI] [PubMed] [Google Scholar]
- Jones GT, Nguyen V, Sambrook PN, Kelly PJ, Eisman JA. Progressive loss of bone in the femoral neck in elderly people: longitudinal findings from the Dubbo Osteoporosis Epidemiology Study. British Medical Journal. 1994;309:691–695. [PMC free article] [PubMed] [Google Scholar]
- Katznelson L, Finkelstein JS, Schoenfeld DA, Rosenthal DI, Anderson EJ, Klibanski A. Increase in bone density and lean body mass during testosterone administration in men with acquired hypogonadism. Journal of Clinical Endocrinology and Metabolism. 1996;81:4358–4365. doi: 10.1210/jcem.81.12.8954042. [DOI] [PubMed] [Google Scholar]
- van Kesteren P, Lips P, Gooren LJ, Asscheman H, Megens J. Long-term follow-up of bone mineral density and bone metabolism in transsexuals treated with cross-sex hormones. Clinical Endocrinology. 1998;48:347–354. doi: 10.1046/j.1365-2265.1998.00396.x. [DOI] [PubMed] [Google Scholar]
- Khosla S, Melton LJ, III, Atkinson EJ, O’Fallon WM, Klee GG, Riggs BL. Relationship of serum sex steroid levels and bone turnover markers with bone mineral density in men and women: a key role for bioavailable estrogen. Journal of Clinical Endocrinology and Metabolism. 1998;83:2266–2274. doi: 10.1210/jcem.83.7.4924. [DOI] [PubMed] [Google Scholar]
- Khosla S, Melton LJ, III, Atkinson EJ, O’Fallon WM. Relationship of serum sex steroid levels to longitudinal changes in bone density in young versus elderly men. Journal of Clinical Endocrinology and Metabolism. 2001;86:3555–3561. doi: 10.1210/jcem.86.8.7736. [DOI] [PubMed] [Google Scholar]
- Khosla S, Atkinson EJ, Dunstan CR, O’Fallon WM. Effect of estrogen versus testosterone on circulating osteoprotegerin and other cytokine levels in normal elderly men. Journal of Clinical Endocrinology and Metabolism. 2002;87:1550–1554. doi: 10.1210/jcem.87.4.8397. [DOI] [PubMed] [Google Scholar]
- Lindsay R, Thome JF. Estrogen treatment of patients with established osteoporosis. Obstetrics and Gynecology. 1990;76:290–295. [PubMed] [Google Scholar]
- Lips P, van Kesteren PJ, Asscheman H, Gooren LJ. The effect of androgen treatment on bone metabolism in female-to-male transsexuals. Journal of Bone and Mineral Research. 1996;11:1769–1773. doi: 10.1002/jbmr.5650111121. [DOI] [PubMed] [Google Scholar]
- Meier DE, Orwoll ES, Jones JM. Marked disparity between trabecular and cortical bone loss with age in healthy men. Measurement by vertebral computed tomography and radial photon absorptiometry. Annals of International Medicine. 1984;101:605–612. doi: 10.7326/0003-4819-101-5-605. [DOI] [PubMed] [Google Scholar]
- Melton LJ, III, Khosla S, Atkinson EJ, Oconnor MK, Ofallon WM, Riggs BL. Cross-sectional versus longitudinal evaluation of bone loss in men and women. Osteoporosis International. 2000;11:592–599. doi: 10.1007/s001980070080. [DOI] [PubMed] [Google Scholar]
- Morley JE, Kaiser FE, Perry HM, III, Patrick P, Morley PM, Stauber PM, Vellas B, Baumgartner RN, Garry PJ. Longitudinal changes in testosterone, luteinizing hormone, and follicle stimulating hormone in healthy older men. Metabolism Clinical Experiments. 1997;46:410–413. doi: 10.1016/s0026-0495(97)90057-3. [DOI] [PubMed] [Google Scholar]
- Saika M, Inoue D, Kido S. Matsumoto T 17b-Estradiol stimulates expression of osteoprotegerin by a mouse stromal cell line, ST-2, via estrogen receptor-a. Endocrinology. 2001;142:2205–2212. doi: 10.1210/endo.142.6.8220. [DOI] [PubMed] [Google Scholar]
- Shozu M, Simpson ER. Aromatase expression of human osteoblast-like cells. Molecular Cell Endocrinology. 1998;30:139, 117–129. doi: 10.1016/s0303-7207(98)00069-0. [DOI] [PubMed] [Google Scholar]
- Smith EP, Boyd J, Frank GR, Takahashi H, Cohen RM, Specker B, Williams TC, Lubahn DB, Korach KS. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. New England Journal of Medicine. 1994;20, 331(16):1056–1061. doi: 10.1056/NEJM199410203311604. [DOI] [PubMed] [Google Scholar]
- Snyder PJ, Peachey H, Hannoush P, Berlin JA, Loh L, Holmes JH, Dlewati A, Staley J, Santanna J, Kapoor SC, Attie MF, Haddad JG, Jr, Strom BL. Effect of testosterone treatment on bone mineral density in men over 65 years of age. Journal of Clinical Endocrinology and Metabolism. 1999;84:1966–1972. doi: 10.1210/jcem.84.6.5741. [DOI] [PubMed] [Google Scholar]
- Szulc P, Claustrat B, Marchand F, Delmas PD. Increased risk of falls and increased bone resorption in elderly men with partial androgen deficiency: the MINOS study. Journal of Clinical Endocrinology and Metabolism. 2003;88:5240–5247. doi: 10.1210/jc.2003-030200. [DOI] [PubMed] [Google Scholar]
- Tangpricha V, Ducharme SH, Barber TW, Chipkin SR. Endocrinologic treatment of gender identity disorders. Endocrine Practice. 2003;9:12–21. doi: 10.4158/EP.9.1.12. [DOI] [PubMed] [Google Scholar]
- Watts NB, Notelovitz M, Timmons MC, Addison WA, Wiita B, Downey LJ. Comparison of oral estrogens and estrogens plus androgen on bone mineral density, menopausal symptoms, and lipid-lipoprotein profiles in surgical menopause. Obstetrics and Gynecology. 1995;85:529–537. doi: 10.1016/0029-7844(94)00448-m. [DOI] [PubMed] [Google Scholar]
- Wells G, Tugwell P, Shea B, Guyatt G, Peterson J, Zytaruk N, Robinson V, Henry D, O’Connell D, Cranney A Osteoporosis Methodology Group & The Osteoporosis Research Advisory Group. Meta-analyses of therapies for postmenopausal osteoporosis. V. Meta-analysis of the efficacy of hormone replacement therapy in treating and preventing osteoporosis in postmenopausal women. Endocrine Reviews. 2002;23:529–539. doi: 10.1210/er.2001-5002. [DOI] [PubMed] [Google Scholar]
- Writing Group for the Women’s Health Initiative investigators. Risks and benefits of estrogen plus progestin in healthy, postmenopausal women. Principal results from the Women’s Health Initiative randomized controlled trial. Journal of the American Medical Association. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]