Abstract
A biofilm-forming strain of sulfate-reducing bacteria (SRB), isolated from a naturally occurring mixed biofilm and identified by 16S rDNA analysis as a strain of Desulfomicrobium norvegicum, rapidly removed 200 μM selenite from solution during growth on lactate and sulfate. Elemental selenium and elemental sulfur were precipitated outside SRB cells. Precipitation occurred by an abiotic reaction with bacterially generated sulfide. This appears to be a generalized ability among SRB, arising from dissimilatory sulfide biogenesis, and can take place under low redox conditions and in the dark. The reaction represents a new means for the deposition of elemental sulfur by SRB under such conditions. A combination of transmission electron microscopy, environmental scanning electron microscopy, and cryostage field emission scanning electron microscopy were used to reveal the hydrated nature of SRB biofilms and to investigate the location of deposited sulfur-selenium in relation to biofilm elements. When pregrown SRB biofilms were exposed to a selenite-containing medium, nanometer-sized selenium-sulfur granules were precipitated within the biofilm matrix. Selenite was therefore shown to pass through the biofilm matrix before reacting with bacterially generated sulfide. This constitutes an efficient method for the removal of toxic concentrations of selenite from solution. Implications for environmental cycling and the fate of sulfur and selenium are discussed, and a general model for the potential action of SRB in selenium transformations is presented.
Sulfate-reducing bacteria (SRB) are a phylogenetically and physiologically diverse group of bacteria, characterized by their common capacity to conserve energy for growth by linking the oxidation of various substrates to the dissimilatory reduction of sulfate (S6+) to sulfide (S2−). As such, SRB comprise a functional group within a sulfuretum, linking broad-scale cycling between sulfate and sulfide by ecological communities of SRB and sulfide-oxidizing bacteria (12, 28). Biological reoxidation of reduced sulfur species typically occurs at oxic-anoxic transition zones and is attributed largely to phototrophs and chemolithotrophs. Small-scale cycling through elemental sulfur (S0) also occurs and is generally attributed to syntrophic associations of sulfide oxidizers and sulfur reducers (3, 17).
The biological cycling of selenium is receiving increasing attention, due not only to the biological importance of selenium as an essential trace element but also to the potential for selenium pollution to cause significant ecological damage (42). Selenium is a group 16 metalloid element possessing several stable oxidation states. Under oxic conditions, selenium is present mostly as the oxyanions selenite (SeO32−, Se4+ oxidation state) and selenate (SeO42−, Se6+ oxidation state), whereas under anoxic conditions, selenide (Se2−) and elemental selenium (Se0) appear predominant (5, 6). Selenium is incorporated by organisms through selenide, is important in some enzyme systems, and may substitute for sulfur in amino acids and other organic molecules (2). The (bio)chemical similarities of selenium to sulfur have also led workers to focus on the biological cycling of selenium by organisms involved in the sulfur cycle (46). More recently, work has focused on the separate cycling of selenium and sulfur, particularly emphasizing the dissimilatory reduction of selenium by microorganisms as an important biogeochemical process in its own right (35).
SRB have the capacity to enzymatically reduce small amounts of selenium in a number of ways. Selenate may be reduced to selenide in nanomolar amounts via the dissimilatory sulfate-reducing pathway, resulting in the production of volatile hydrogen selenide (46). Assimilatory reduction of selenium by SRB is also required for the incorporation of selenide as an essential trace nutrient, and selenide may be released as the volatile alkylated species dimethyl selenide and dimethyl diselenide (24). A separate pathway by which SRB enzymatically reduce selenium oxyanions to elemental selenium has also been demonstrated (40). SRB do not appear to be able to couple this to growth, however, and the range of environmental circumstances under which this might take place is poorly understood.
Here we report on the precipitation of significant quantities of selenium and sulfur by SRB, growing as attached biofilm, under environmentally relevant conditions. This represents a further means for the removal of selenium from aqueous solution by SRB and demonstrates, for the first time, the capacity to precipitate elemental sulfur during sulfate-reducing growth. The microbially induced chemical reaction is detailed and the location and nature of the precipitate within intact biofilms is shown.
MATERIALS AND METHODS
Organisms, media, and culture conditions.
A biofilm-adapted strain of Desulfomicrobium norvegicum (Dundee isolate 1) was used, originally isolated from a mixed culture obtained from an estuarine sediment of the river Tay (43) and identified by metabolic characterization and 16S rRNA analysis (C. Boothman, D. E. Holmes, S. L. Hockin, C. White, G. M. Gadd, and J. R. Lloyd, unpublished data). Selection for biofilm growth was maintained by repeated batch culturing to late stationary phase in the presence of a polystyrene coupon, to which the bacteria preferentially attached. The coupon was removed, aseptically cut into pieces, and used to inoculate further subcultures (43). Cultures were maintained on a defined medium, modified from that of Widdel and Pfennig (45). Lactate was the sole carbon/energy source, and sulfate was the terminal electron acceptor. The medium contained the following (amounts are indicated in parentheses): Na lactate (2.24 g liter−1), Na2SO4 (4.00 g liter−1), KH2PO4 (0.20 g liter−1), NH4Cl (0.25 g liter−1), NaCl (1.00 g liter−1), MgCl2 · 6H2O (0.40 g liter−1), KCl (0.50 g liter−1), CaCl2 · 2H2O (0.15 g liter−1), FeSO4 (39.5 mg liter−1), NaOH (500.0 μg liter−1), Na2SeO3 · 5H2O (6.0 μg liter−1), Na2WO4 · 2H2O (8.0 μg liter−1), MnCl2 · 4H2O (100.0 μg liter−1), CoCl2 · 6H2O (190.0 μg liter−1), ZnSO47H2O (144.0 μg liter−1), CuCl22H2O (2.0 μg liter−1), and Na2MoO4 (36.0 μg liter−1). Vitamins were filter sterilized (0.45-μm pore size, cellulose nitrate) and added to final concentrations (amounts [in micrograms per liter] are indicated in parentheses) of para-aminobenzoic acid (4.0), D(+)-biotin (1.0), nicotinic acid (10.0), Ca-pantothenate (5.0), pyridoxine-HCl (15.0), and thiamine-HCl (10.0). The medium was thoroughly deoxygenated by sparging with high-purity nitrogen, and sodium sulfide, at a final concentration of 60 mg of Na2S · 9H2O liter−1, was used as a redox-poising agent, unless otherwise indicated. The medium was adjusted to an initial pH of 7.0 by the addition of HCl or NaOH.
Experimental conditions.
The experimental medium was as described above, with the addition of 200 μM sodium selenite to positive treatments (a concentration found to be subinhibitory to biofilm-grown SRB in preliminary experiments). The potential for abiotic interactions between selenium oxyanions and sulfide meant that the addition of sodium sulfide was unsuitable for experimental cultures. However, SRB are able to exert a degree of redox control over their environment, enabling the initiation of active sulfate reduction (9), and at the relatively high inoculum densities used in these experiments, active sulfate reduction began without any appreciable lag time.
Biofilms were pregrown on 80- by 250-mm Thermanox coupons in 25-ml borosilicate glass vials fitted with butyl rubber bungs. The headspace was filled with high-purity nitrogen under positive pressure, and samples were taken by inserting a hypodermic needle through the bung. Before aseptic transfer to selenium-containing medium, biofilms were washed overnight in anaerobic sulfate-free salt solution to remove traces of free sulfate/sulfide. Media were adjusted to pH 7.0 and were thoroughly deoxygenated by sparging with high-purity N2. Manipulations were carried out in a nitrogen stream, and incubations were carried out under a nitrogen headspace at positive pressure at 30°C in the dark.
Measurement of selenite and sulfate.
All samples were centrifuged (12,000 × g, 8 min) to remove suspended and colloidal material before analysis. Supernatants were diluted with 0.1 M ZnCl2 solution as appropriate, recentrifuged to remove resulting sulfidic precipitates, and membrane filtered (0.2-μm pore size) directly into autosampler vials. Sulfate measurements were determined by ion chromatography with a PRP-X100 anion-exchange column in a Metrohm 733 unit with a 732 conductance detector linked to a 750 autosampler through Metrohm IC-Net software. The eluant was 5 mM potassium hydrogen phthalate in 2% (vol/vol) acetonitrile, adjusted to pH 4.6 with 1 M NaOH. The flow rate was set at 2.0 ml min−1, the sample loop was 200 μl, and the standard was sodium sulfate. Selenite was measured by anodic stripping voltammetry, with a dropping mercury electrode and potassium chloride reference electrode. The equipment comprised a Metrohm 633 VA stand with Autolab/Eco-chemie current recorder and voltage generator and Autolab GPES manager software. Samples were run in 16 ml of 2 M ammonium sulfate, with the addition of 1.6 ml of 0.1 M Na2 EDTA as a chelating agent and 0.4 ml of 1.0 M CuSO4 · 5H2O. The solution was adjusted to pH 2.2 with concentrated H2SO4. All reagents were analytical grade. Using this method, sensitivities into the nanomolar range were attained.
Electron microscopy.
A Philips XL30 environmental scanning electron microscope (ESEM) with EDX facility, in dry operating mode, was used for elemental mapping and point analysis. Unfixed biofilms were carefully removed from culture media, equilibrated overnight in degassed anaerobic deionized water to remove sodium and potassium salts from the saline culture medium, and placed under an argon atmosphere to prevent oxidation before drying under a vacuum and coating with 5 nm of carbon. Analysis was carried out immediately after coating. Semiquantitative EDX analysis was carried out by using several replicate measurements standardized against powder mixtures of the elements of concern in a range of relative molar concentrations. Elements used as standards were at least Analar grade. Cryoelectron microscopy was carried out with a Hitachi S-4700 high-resolution field emission scanning electron microscope (FESEM) fitted with a low-temperature stage and Oxford Alto 2500 cryopreparation chamber. Biofilm samples were fixed in 2.5% (vol/vol aqueous) glutaraldehyde to prevent dehydration during freezing, snap-frozen in liquid nitrogen, fractured in the Oxford cryochamber, and sublimated at −90°C to reveal internal features before coating with 15 nm of carbon. For transmission electron microscopy, biofilms were prepared by rinsing in salt solution and fixing with 2.5% glutaraldehyde, as described above. A hydrophilic melamine resin (Nanoplast) was used to prepare blocks by part-curing for 2 days at 4°C under a nitrogen atmosphere, before being poured into a butyl-rubber mold, leaving a convex meniscus. Biofilms were carefully removed from cultures, and excess water was removed by wicking with a small piece of filter paper. A section of biofilm was then cut, carefully placed facedown over the mold, and allowed to settle into the resin. This was allowed to impregnate the biofilm and cure for a further 2 days at room temperature, under nitrogen, before raising the temperature to 40°C for the final cure. In this way, the hydrated structure of the biofilm was maintained. Thin sections (120 nm) were then prepared in the standard way, with a microtome fitted with a glass knife. Sections were mounted on copper grids for viewing and poststained with 1% lead citrate where appropriate.
Abiotic incubations.
Reaction tests with spent culture medium and abiotic reaction mixtures were carried out in sealed 100-ml Wheaton bottles with nitrogen in the headspace. All solutions were sparged with nitrogen before use, and manipulations were carried out anaerobically. Spent culture medium was taken from late-stationary/decline-phase cultures of free cells. Cultures were lactate limited, and at the end of growth, no carbon or energy source remained available for active metabolism, except that from slow biomass turnover. Spent cultures contained ca. 10 mM total sulfide and had a pH of ≈8.5. Two hundred micromolar Na2SeO3 was added directly to the cultures. Abiotic tests were carried out in 10 mM NaCl solution adjusted to pH 7.5, and all of these incubations were adjusted to pH 7.0 to 7.5 following the addition of reactants. Medium was 10 mM NaCl in deionized water, kept anaerobic by bubbling nitrogen, and adjusted to pH 7.5 before and after the addition of further elements by using HCl or NaOH as appropriate. Additions were 5.0 mM Na2S plus 250 μM Na2SeO3; 5.0 mM Na2S plus 250 μM Na2SeO3 plus 250 μM FeCl2 · 7H2O; 250 μM Na2S plus 5.0 mM Na2SeO3 plus 250 μM FeCl2 · 7H2O; 250 μM Na2S plus 250 μM Na2SeO3 plus 5.0 mM FeCl2 · 7H2O; and 250 μM Na2SeO3 added to extant FeS precipitate. The precipitates that formed were collected by shaking the bottle and drawing off the medium plus suspended precipitate with a syringe and hypodermic needle inserted through the seal. The suspension was passed through a 0.2-μm-pore-size cellulose nitrate membrane filter and washed with two syringe volumes of anaerobic sodium chloride solution. Filters were mounted directly onto the microscope stage for analysis. These experiments were carried out over a number of days, and pH changes and precipitates were allowed to equilibrate overnight.
RESULTS
Selenite removal from solution.
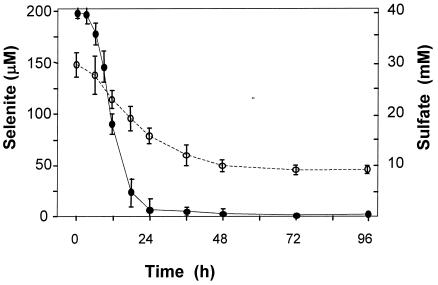
When preformed biofilms of D. norvegicum were used to inoculate batch cultures containing 200 μM selenite, rapid removal of selenite from solution took place (Fig. 1). Selenite disappearance began shortly following the commencement of sulfate-reducing growth and was complete before cultures reached the point of substrate (lactate) limitation (about 48 h). No significant removal of selenite occurred in abiotic control incubations containing polystyrene coupons (data not shown). An orange-red color developed in the biofilm within 24 h, and subsequently, an orange color also developed in the medium.
FIG. 1.
Removal of selenite from solution by SRB biofilms in batch culture. Twenty-five-milliliter cultures were inoculated with pregrown biofilms on 25- by 8-mm polystyrene coupons. •, selenite concentration (micromolar); ○, sulfate concentration (millimolar). Error bars represent standard deviations (n = 4).
Location and nature of the precipitate within the biofilm.
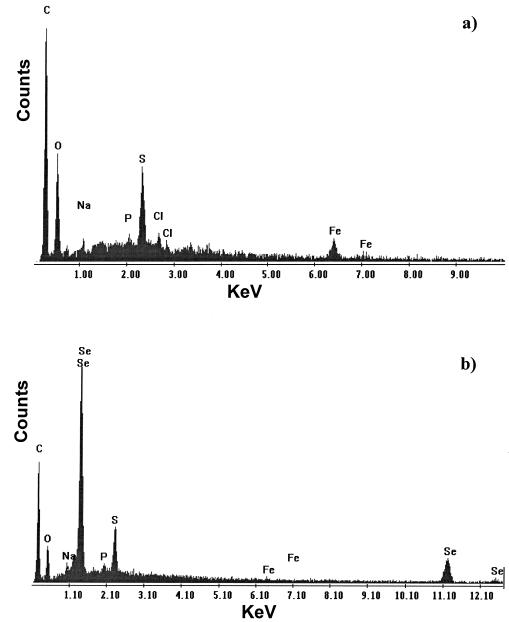
After washing, biofilms retained their coloration. EDX maps of washed, selenite-exposed biofilms showed strong peaks for the presence of both selenium and sulfur while detectable quantities of transition metals were not present, in contrast to control biofilms (Fig. 2). Apart from carbon and oxygen peaks (from the carbon coating and from the organic biofilm matrix) and sodium (sodium chloride remaining within the matrix), no other elements were present in detectable quantities.
FIG. 2.
Raster-generated EDX spectra for small areas of biofilm from control biofilm (a) and selenite-containing culture (b). Typical spectra are shown from one of many determinations.
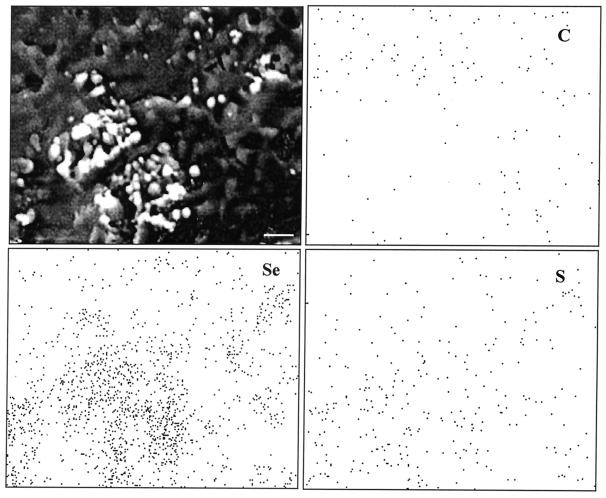
Examination of washed, selenite-exposed biofilms by ESEM showed that abundant spherical nanometer-to-micrometer-sized particles were associated with the biofilm (Fig. 3a). This was in contrast to the much smaller and less abundant amorphous metal sulfides associated with control biofilms. Raster-generated EDX maps showed that the distribution of both selenium and sulfur near the biofilm surface was associated with the presence of the granules, whereas carbon distribution was inversely related to that of the granules (Fig. 3). No associations were found for other elements. Point EDX analysis of aggregated granules (spot size, ca. 1 μm) showed that the granules contained selenium and sulfur in the approximate relative atomic Se/S ratio of 1:1.5.
FIG. 3.
Shown are (clockwise from top left-hand side) a plan view of a typical selenite-exposed D. norvegicum biofilm generated by ESEM in wet mode (upper left) and EDX elemental maps of the same area of the biofilm with signal-density distributions of carbon (C), sulfur (S), and selenium (Se). Abundant granular precipitates (light color) and rod-shaped bacteria, partially obscured by the extracellular matrix, are visible near the contoured surface of the hydrated biofilm. Selenium and sulfur are clearly associated with the areas where granules are present, whereas carbon is less abundant. Typical results are shown from 1 of about 20 determinations from several biofilms. Bar, 1 μm.
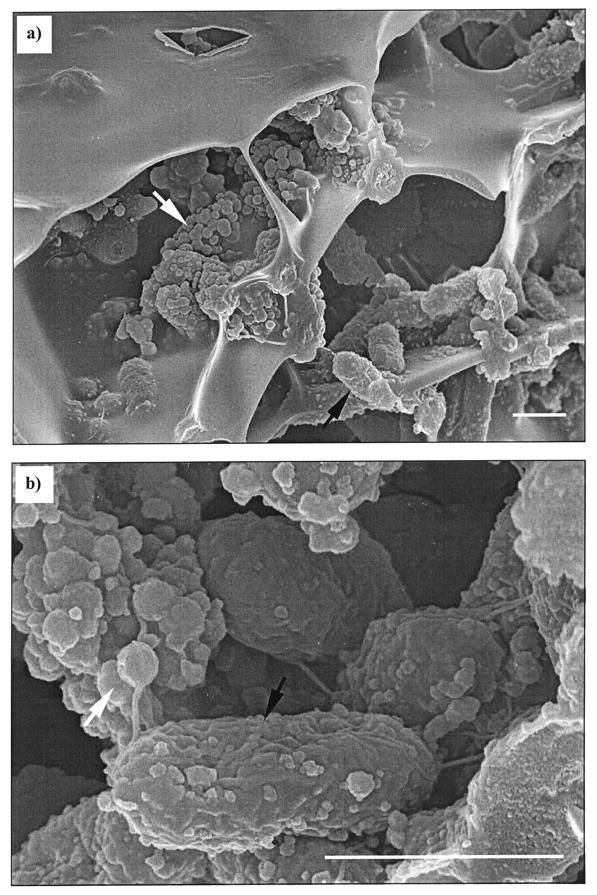
Using cryofracturing in conjunction with low-temperature FESEM, it was possible to image sections of biofilms that retained their hydrated form. Selenite-exposed biofilms that were prepared in this way clearly showed the presence of granules precipitated beneath the biofilm canopy and abundantly present within the biofilm matrix (Fig. 4 ). The polygonal voids within the matrix were caused by the formation of ice crystals during plunge-freezing which were then sublimated in the microscope prechamber. Although this may have led to some short-distance movement of elements from the center to the edge of the crystal, the effect is fortuitous in allowing a three-dimensional view through the interior of the biofilm section. A typical example is shown, taken from a region near the biofilm surface, to aid the orientation of the viewer (Fig. 4). However, similar granules could be seen throughout the biofilm profile. These were not seen in control biofilms.
FIG.4.
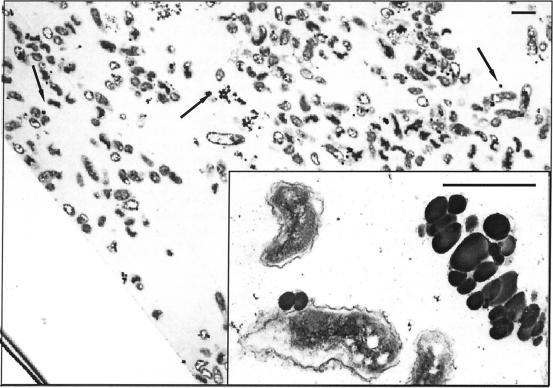
Cryosectioned FESEM images of hydrated biofilm exposed to 200 μM Na selenite and inoculated for 96 h at 30°C. (a) An isometric view shows a biofilm section beneath the surface canopy of the extracellular matrix. Individual cells of D. norvegicum (black arrow) form colonies within the matrix. Abundant Se and S granules (white arrow) are clearly seen precipitated beneath the biofilm canopy. (b) An enlargement of a small area, taken from another region of the same biofilm, shows some granules associated with the surface of an individual bacterium (black arrow), with more abundant precipitation in the extracellular matrix (white arrow). Polar flagella can also be seen. The bacterium at bottom right has been sectioned during freeze fracture. Typical examples are shown from two of many areas observed in several biofilms. Bar, 1 μm.
Further investigation of the location of the precipitated granules, with a hydrophilic melamine resin, preserved the colocation of cells and precipitated elements within the hydrated matrix structure (Fig. 5). The section clearly shows that electron-dense (dark colored) granules are distributed right through the biofilm section, although they are more abundant in the upper portion of the biofilm. The granules were associated with cell surfaces, or were remote from cells, within the extracellular polymer (EPS) matrix (Fig. 5, inset). It can be seen that some dehydration of cells occurred during the postcure, causing a crinkled appearance. However, the cell membranes remained intact and granules were always observed outside the cell wall and never in the cytoplasm, or periplasm, as might be expected had direct enzymatic reduction of selenium been involved in precipitation.
FIG. 5.
Transmission electron microscopy section of hydrated biofilm. The section is mounted diagonally, with the substrate running from the top left to the bottom center, and the biofilm surface, with some sloughed cells, is towards the top right hand edge of the image. Electron-dense granules can be seen throughout the section (arrows and inset). The section shown here was poststained with lead citrate-uranyl acetate; unstained sections showed similar results. Bars, 1 μm.
Abiotic tests.
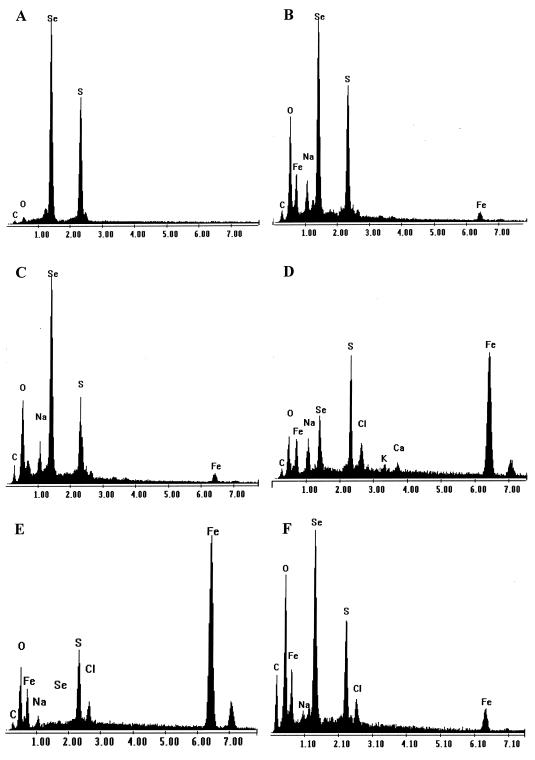
Tests with spent cultures showed that an orange-colored precipitate, similar to that seen in growing, developed within minutes of adding sodium selenite. EDX scans of washed precipitates (data not shown) confirmed that sulfur and selenium were the only two significant elements present. Using 0.2-μm-pore-size-filtered, cell-free medium made no difference to this. The results of abiotic tests with simple reaction mixtures showed that it was possible to recreate the precipitation reaction seen in SRB biofilm cultures. Selenium and sulfur were precipitated under all conditions (Fig. 6) where sulfide was present in solution. However, the addition of increasing concentrations of iron when sulfide was limiting inhibited selenium deposition. Where iron and sulfur were only present as extant FeS, this inhibited selenium deposition completely over the time during which observations were made. Acidification of this medium caused dissolution of the acid-labile FeS, and on raising the pH to above neutral, selenium and sulfur precipitated as for the other treatments.
FIG. 6.
EDX scans of washed precipitates from a series of abiotic reaction mixtures. Additions to 10 mM NaCl were 5.0 mM Na2S plus 250 μM Na2SeO3 (A), 5.0 mM Na2S plus 250 μM Na2SeO3 plus 250 μM FeCl2 · 7H2O (B), 250 μM Na2S plus 5.0 mM Na2SeO3 plus 250 μM FeCl2 · 7H2O (C), 250 μM Na2S plus 250 μM Na2SeO3 plus 5.0 mM FeCl2 · 7H2O (D), and 250 μM Na2SeO3 (E) added to extant FeS precipitate (F) as in E following acidification of the medium and readjustment to pH 7.5. The vertical scale is indicative only. Carbon and oxygen peaks are from the cellulose filters.
DISCUSSION
Selenite removal began early during SRB biofilm incubation and was largely complete within 24 h (Fig. 1). The pronounced orange-red color that developed was in marked contrast to control biofilms that developed a grey-black color due to the precipitation of transition metal sulfides. Most of the selenium initially present in cultures was found to be precipitated within the biofilm matrix, but outside SRB cells, as nanometer-sized spherical granules containing both selenium and sulfur.
Electron microscopy revealed the form and content of the deposited selenium-containing granules (Fig. 3 and 4). Although cell growth occurred during experimental incubations and free cells were released into the medium, new growth has consistently been shown to be from the surface of bacterial biofilms, with extant cells remaining irreversibly embedded within the EPS matrix (13, 21). The appearance of granules deep within the matrix therefore demonstrates that these solids form within the biofilm structure. Entrapment of granules precipitated in the bulk medium also takes place at the biofilm surface, enhancing the ability of the biofilm to accumulate these elements from the surrounding environment. These results differ from those for the deposition of copper and cadmium sulfides, which appeared to be limited to surface layers of the biofilm (43, 44). While SRB biofilm matrix components include moieties with the capacity to bind metal cations (1, 11), less is understood about the interactions of EPS components with (oxy)anions. These results show that extracellular components of the SRB biofilm do not play a fundamental role in preventing the diffusion of selenite through the biofilm, but rapid extracellular precipitation of selenium may constitute an effective defense mechanism. Such information is useful when considering the potential use of these organisms for bioremediation and in developing an understanding of the ability of microbial biofilms to sequester metals and metalloids and so influence biogeochemical cycles.
Had selenium adsorption and coprecipitation with metal sulfides, or the precipitation of metal selenides, contributed significantly to selenium removal from solution, then a significant iron content would be expected in the granules, which was not the case. Enzymatic reduction of selenite to Se0 would have resulted in a selenium signal greatly exceeding those of other elements. However, sulfur was also present in large amounts. Previous observations of enzymatic seleno-oxyanion reduction also suggest that selenium deposition is intracellular (40). An apparent abiotic reaction of selenite in SRB culture has been noted by previous workers (27, 40). The results described here are best explained by such an abiotic interaction and are consistent with an exothermic oxidation-reduction reaction between selenite and sulfide.
A two-step model is therefore proposed for the dual-precipitation of selenium and sulfur from solution by an abiotic, but biologically mediated, pathway. Sulfate acts as a terminal electron acceptor for anaerobic respiration, resulting in the production of sulfide, which then participates in an abiotic reaction with selenite. Using published log K values (19, 22, 32, 36), the reductions of sulfate to sulfide and of selenite to elemental selenium at pH >7.5 can be represented by the half reactions:
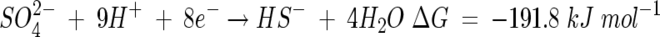 |
(1) |
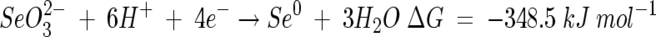 |
(2) |
while the oxidation of sulfide to elemental sulfur can be represented by:
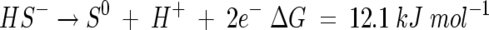 |
(3) |
Reactions 2 and 3 can be combined to represent the oxidation of sulfide by selenite:
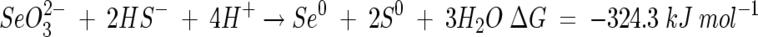 |
(4) |
This is a strongly exothermic reaction and is thermodynamically favored over competing reactions such as the formation of amorphous iron sulfide:
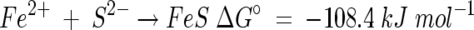 |
(5) |
The reaction as given in equation 4 predicts a 1:2 ratio for Se to S, but results of semiquantitative EDX analysis gave a range of ratios that were somewhat lower than this, at ca. 1:1.5. Although such data must be interpreted with caution, results were consistent between replicates and repeat measurements.
There may be a number of biological and chemical explanations to account for the lower Se/S ratio (20, 22, 36), and experiments were carried out to investigate this. The rapid precipitation of selenium and sulfur in cell-free spent medium showed that the reaction did not require the presence of cells. Tests with simple chemical reactants (Fig. 6) showed that an abiotic reaction took place between selenite and sulfide under reducing conditions. Precipitation still occurred in the presence of Fe2+, but very high concentrations of iron inhibited selenium precipitation. Where all iron and sulfur were present as extant FeS, Se precipitation was inhibited, but when Fe2+ and S2− ions were released by acidification of this medium and the mixture was brought back to circumneutral pH, selenium and sulfur precipitated as before.
It was in fact possible to control the precipitate fractions by manipulating the equilibrium pH. When only sulfide and selenite were present (in addition to Na+ and Cl−), sulfur was the sole precipitate at pH <3.0. When pH was >10.0, only selenium was precipitated, and at values between these two limits, mixtures of sulfur and selenium were precipitated in various fractions. Where Fe2+ was present in excess, the above situation was modified, with the exclusive formation of FeS above a pH ≈10.5. This can be interpreted in terms of the relative stability fields of elemental sulfur, selenium, and FeS plus the very low solubility of FeS at high pH (21, 30, 32). Under low redox and low pH conditions, sulfur is precipitated while selenium is replaced by selenite and selenide, and at high pH, the reverse is true. The formation of soluble polysulfides and polyselenides appears to be a possibility under such circumstances (18, 34). At moderate pH values and under reducing conditions, both elements are well within their stability fields and a mixture of the two is precipitated. This interpretation adequately explains why the precipitates from the biofilm incubations were relatively enriched for selenium; at the pH value of about 8.5 reached during incubation, selenium is more stable than sulfur and so makes up a higher proportion of the precipitate. It is possible that somewhat higher pH values also develop within the biofilm during active metabolism, further favoring selenium precipitation.
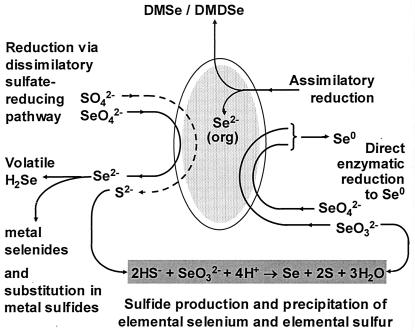
This implies that selenium and sulfur can be precipitated by SRB during application in bioreactors containing mixed metal and metalloid liquors, as well as under a wide range of environmental conditions, but that formation of FeS (or other transition metal sulfides) may inhibit the reaction where Me2+ ≫ S2− and at a pH above neutral. However, in ocean surface and estuarine sediments, free iron is frequently in the subpicomolar range while sulfide and selenium are widely present in surface waters at nanomolar to micromolar concentrations (6, 7, 8, 15, 23, 41). This ratio may be further enhanced within SRB biofilms that contain locally elevated sulfide concentrations but where transition metals are partially excluded by adsorption and metal sulfide precipitation. The above reaction serves to extend the generalized model for the potential transformations of selenium by SRB (Fig. 7).
FIG. 7.
Model of the currently understood transformations of selenium by SRB. The diagram is illustrative and does not imply a cellular location for the individual pathways. DMSe, dimethyl selenide; DMDSe, dimethyl diselenide.
Dissimilatory bacterial selenium reduction has been proposed as a major source of elemental selenium (10, 25, 26), and reduction of Se6+ by SRB is probably not important in selenate-impacted environments (25). However, while microbial selenate reduction in sediments is widespread (35), the dissimilatory reduction of selenite (Se4+) appears to be much less so (37). In contrast, SRB are ubiquitous in anoxic microniches within more oxidized environments, where concentrations of dissolved selenium species are generally higher (14, 23, 25, 29, 33, 38, 39).
The presence of terms to simulate rapid reduction and sequestration of selenium within sediments is critical to models predicting the removal and fate of selenium from selenite-impacted waters (4). Dissimilatory reduction of selenite has been assumed as the mechanism, but this may be questionable, as SRB activity has the potential to drive the removal of selenite from pore waters even in relatively sulfate-deprived freshwater environments (16, 31). This would depend on the ability of the process to compete with other chemical reactions and biological transformations. The evidence from the in vitro experiments described above is that the reaction is rapid and can take place in preference to the formation of transition metal sulfides. Nevertheless, studies to investigate the production of elemental selenium from biologically generated sulfide under environmental conditions would be necessary to assess the relative contribution of this mechanism to selenium cycling.
Acknowledgments
S.H. gratefully acknowledges the receipt of a BBSRC industrial CASE postgraduate studentship supported by BNFL.
We also thank Martin Kierans, of the Center for High Resolution Imaging and Processing, University of Dundee, for technical advice and assistance with electron microscopy and H. Eccles (BNFL) for scientific advice and useful discussions.
REFERENCES
- 1.Beech, I. B., and C. W. S. Cheung. 1995. Interactions of exopolymers produced by sulfate-reducing bacteria with metal ions. Int. Biodeterior. Biodegradation 35:59-72. [Google Scholar]
- 2.Bock, A. K., K. Forchhammer, J. Heider, W. Leinfelder, G. Sawers, B. Veprek, and F. Zinoni. 1991. Selenocysteine: the 21st amino acid. Mol. Microbiol. 5:515-520. [DOI] [PubMed] [Google Scholar]
- 3.Bottcher, M. E., and B. Thamdrup. 2001. Anaerobic sulfide oxidation and stable isotope fractionation associated with bacterial sulfur disproportionation in the presence of MnO2. Geochim. Cosmochim. Acta 65:1573-1581. [Google Scholar]
- 4.Bowie, G. L. 1996. Assessing selenium cycling and accumulation in aquatic ecosystems. Water Air Soil Pollut. 90:93-104. [Google Scholar]
- 5.Cutter, G. A. 1982. Selenium in reducing waters. Science 217:829-831. [DOI] [PubMed] [Google Scholar]
- 6.Cutter, G. A., and L. S. Cutter. 1995. Behaviour of dissolved antimony, arsenic and selenium in the Atlantic Ocean. Mar. Chem. 49:295-306. [Google Scholar]
- 7.Cutter, G. A., and L. S. Cutter. 1998. Metalloids in the high latitude North Atlantic Ocean: sources and internal cycling. Mar. Chem. 61:25-36. [Google Scholar]
- 8.Cutter, G. A., R. Walsh, and C. de Echols. 1999. Production and speciation of hydrogen sulfide in surface waters of the high latitude north Atlantic ocean. Deep Sea Res. II 46:991-1010. [Google Scholar]
- 9.Cypionka, H. 2000. Oxygen respiration by Desulfovibrio species. Annu. Rev. Microbiol. 54:827-848. [DOI] [PubMed] [Google Scholar]
- 10.Dowdle, P. R., and R. S. Oremland. 1998. Microbial oxidation of elemental selenium in soil slurries and bacterial culture. Environ. Sci. Technol. 1:3749-3755. [Google Scholar]
- 11.Flemming, H. K. 1995. Sorption sites in biofilms. Water Sci. Technol. 32:27-33. [Google Scholar]
- 12.Fuseler, K., D. Krekeler, U. Sydow, and H. Cypionka. 1996. A common pathway of sulfide oxidation by sulfate-reducing bacteria. FEMS Microbiol. Lett. 144:129-134. [Google Scholar]
- 13.Gilbert, P., P. J. Collier, and M. R. W. Brown. 1990. Influence of growth rate on susceptibility to antimicrobial agents; biofilm, cell cycle, dormancy and stringent responses. Antimicrob. Agents Chemother. 34:1856-1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hardy, J. A., and W. A. Hamilton. 1981. The oxygen tolerance of sulfate-reducing bacteria isolated from North Sea Waters. Curr. Microbiol. 6:259-262. [Google Scholar]
- 15.Jia-Zhong, Z., and F. Millero. 1994. Investigation of metal-sulfide complexes in sea water by cathodic stripping square wave voltammetry. Anal. Chim. Acta 284:497-504. [Google Scholar]
- 16.Jin, H. Y., D. H. Lee, Y. G. Zo, C. S. Kang, and S. J. Kim. 1996. Distribution and activity of sulfate-reducing bacteria in Lake Soyang sediments. J. Microbiol. 34:131-136. [Google Scholar]
- 17.Jorgensen, B. B. 1982. Ecology of the bacteria of the sulfur cycle with special reference to anoxic-oxic interface environments. Philos. Trans. R. Soc. 298:543-561. [DOI] [PubMed] [Google Scholar]
- 18.Licht, D. 1995. Speciation and analysis of aqueous polyselenide solutions. J. Electrochem. Soc. 142:1546-1551. [Google Scholar]
- 19.Lide, D. R. 2002. CRC handbook of chemistry and physics, 83rd ed. CRC Press, London, United Kingdom.
- 20.Lovley, D. R., and E. J. P. Phillips. 1994. Novel processes for anaerobic sulfate production from elemental sulfur by sulfate-reducing bacteria. Appl. Environ. Microbiol. 60:2394-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marshall, K. C. 1988. Adhesion and growth of bacteria at surfaces in oligotrophic habitats. Can. J. Microbiol. 34:503-506. [Google Scholar]
- 22.Masscheleyn, P. H., R. Delaune, and W. Patrick. 1991. Biogeochemical behaviour of selenium, in soils and sediments: an equilibrium and thermodynamics approach. J. Environ. Sci. Health 26:555-573. [Google Scholar]
- 23.Measures, C. I., and J. D. Burton. 1980. The vertical distribution and oxidation states of dissolved selenium in the northeast Atlantic Ocean and their relationship to biological processes. Earth Planet. Sci. Lett. 46:385-396. [Google Scholar]
- 24.Michalke, K., E. B. Wickenheiser, M. Mehring, A. V. Hirner, and R. Hensel. 2000. Production of volatile derivatives of metal(loid)s by microflora involved in anaerobic digestion of sewage sludge. Appl. Environ. Microbiol. 66:2791-2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oremland, R. S., J. T. Hollibaugh, A. S. Maest, T. S. Presser, L. G. Miller, and C. W. Culbertson. 1989. Selenate reduction to elemental selenium by anaerobic bacteria in sediments and culture: biogeochemical significance of a novel, sulfate-independent respiration. Appl. Environ. Microbiol. 55:2333-2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oremland, R. S., N. A. Steinberg, A. S. Maest, L. G. Miller, and J. T. Hollibaugh. 1990. Measurement of in situ rates of selenate removal by dissimilatory bacterial reduction in sediments. Environ. Sci. Technol. 24:1157-1164. [Google Scholar]
- 27.Postgate, J. R. 1952. Competitive and non-competitive inhibitors of bacterial sulfate reduction. J. Gen. Microbiol. 6:128-142. [DOI] [PubMed] [Google Scholar]
- 28.Postgate, J. R. 1984. The sulphate-reducing bacteria, 2nd ed. Cambridge University Press, Cambridge, United Kingdom.
- 29.Ramsing, N. B., H. Fossing, T. G. Ferdelman, F. Andersen, and B. Thamdrup. 1996. Distribution of bacterial populations in a stratified fjord (Mariager Fjord, Denmark) quantified by in situ hybridization and related to chemical gradients in the water column. Appl. Environ. Microbiol. 62:1391-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rickard, D., M. A. A. Schoonen, and G. W. Luther III. 1995. Chemistry of iron sulfides in sedimentary environments. Geochem. Trans. Sed. Sulfur 612:168-193.
- 31.Sass, H., H. Cypionka, and H. D. Babenzien. 1997. Vertical distribution of sulfate-reducing bacteria at the oxic-anoxic interface in sediments of the oligotrophic Lake Stechlin. FEMS Microbiol. Ecol. 22:245-255. [Google Scholar]
- 32.Seby, F., M. Potin-Gautier, E. Giffaut, G. Borge, and O. F. X. Donard. 2001. A critical review of thermodynamic data for selenium species at 25oC. Chem. Geol. 171:173-194. [Google Scholar]
- 33.Seiburth, J. M. 1993. C1 bacteria in the water column of Chesapeake Bay, USA 1, distribution of subpopulations of O2-tolerant, obligately anaerobic, methylotrophic methanogens that occur in microniches reduced by their bacterial consorts. Mar. Ecol. Prog. 95:67-80. [Google Scholar]
- 34.Steudel, R. 1996. Mechanism for the formation of elemental sulfur from aqueous sulfide in chemical and microbiological desulfurisation processes. Ind. Eng. Chem. Res. 35:1417-1423. [Google Scholar]
- 35.Stolz, J. F., and R. S. Oremland. 1999. Bacterial respiration of arsenic and selenium. FEMS Microbiol. Rev. 23:615-627. [DOI] [PubMed] [Google Scholar]
- 36.Stumm, W., and J. Morgan. 1996. Aquatic chemistry: chemical rates and equilibria in natural waters, 3rd ed. John Wiley & Sons, Chichester, United Kingdom.
- 37.Switzer Blum, J., A. B. Bindi, and J. Buzzelli. 1978. Bacillus arsenicoselenatis sp. nov.: two haloalkophiles from Mono Lake, California that respire oxyanions of selenium and arsenic. Arch. Microbiol. 171:19-30. [DOI] [PubMed] [Google Scholar]
- 38.Teske, A., C. Wawer, G. Muyzer, and N. B. Ramsing. 1996. Distribution of sulfate-reducing bacteria in a stratified fjord (Mariager Fjord, Denmark) as evaluated by most probable number counts and denaturing gradient gel electrophoresis of PCR-amplified ribosomal DNA fragments. Appl. Environ. Microbiol. 62:1405-1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Teske, A., N. B. Ramsing, K. Habicht, and C. Wawer. 1998. Sulfate-reducing bacteria and their activities in cyanobacterial mats of Solar Lake (Sinai, Egypt). Appl. Environ. Microbiol. 64:2943-2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tomei, F. A., L. L. Barton, C. L. Lemanski, T. G. Zocco, N. H. Fink, and L. O. Sillerud. 1995. Transformation of selenate and selenite to elemental selenium by Desulfovibrio desulfuricans. J. Ind. Microbiol. 14:329-336. [Google Scholar]
- 41.Walsh, R. S., G. A. Cutter, W. M. Dunstan, J. Radfordknoery, and J. T. Elder. 1994. The biogeochemistry of hydrogen sulfide: phytoplankton production in the surface ocean. Limonol. Oceanog. 39:941-948. [Google Scholar]
- 42.White, A. F., S. M. Benson, A. W. Yee, H. A. Wollenberg, and S. Flexser. 1991. Groundwater contamination at the Kesterton Reservoir; 2 geochemical parameters influencing selenium mobility. Water Resour. Res. 27:1085-1098. [Google Scholar]
- 43.White, C., and G. M. Gadd. 1998. Accumulation and effects of cadmium on sulphate-reducing bacterial biofilms. Microbiology 144:1407-1415. [DOI] [PubMed] [Google Scholar]
- 44.White, C., and G. M. Gadd. 2000. Copper accumulation by sulfate-reducing bacterial biofilms. FEMS Microbiol. Lett. 183:313-318. [DOI] [PubMed] [Google Scholar]
- 45.Widdel, F., and N. Pfennig. 1981. Studies on dissimilatory sulfate-reducing bacteria that decompose fatty acids. 1. Isolation of new sulfate-reducing bacteria enriched with acetate from saline environments-description of Desulfobacter postgatei gen. nov., sp. nov. Arch. Microbiol. 129:395-400. [DOI] [PubMed] [Google Scholar]
- 46.Zehr, J. P., and R. S. Oremland. 1987. Reduction of selenate to selenide by sulfate-respiring bacteria: experiments with cell suspensions and estuarine sediments. Appl. Environ. Microbiol. 53:1365-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]