Abstract
Purpose
To review findings from major epidemiologic studies regarding risk factors and consequences of elevated markers of inflammation in older adults.
Results
Most of the large, current epidemiologic studies of older adults have included serum interleukin-6 (IL-6), C-reactive protein (CRP) and tumor necrosis factor alpha (TNF-alpha), and some more extensive batteries of measures including soluble receptors. Few risk factors for the modest elevations seen with aging have been defined, including, visceral adiposity, lower sex steroid hormones, smoking, and depression. Of the markers assessed, IL-6 is most robustly associated with disease, disability and mortality.
Conclusion
IL-6 is a non-specific marker of adverse outcomes in older adults.
Keywords: Inflammatory markers, aging, disability, mortality
Introduction
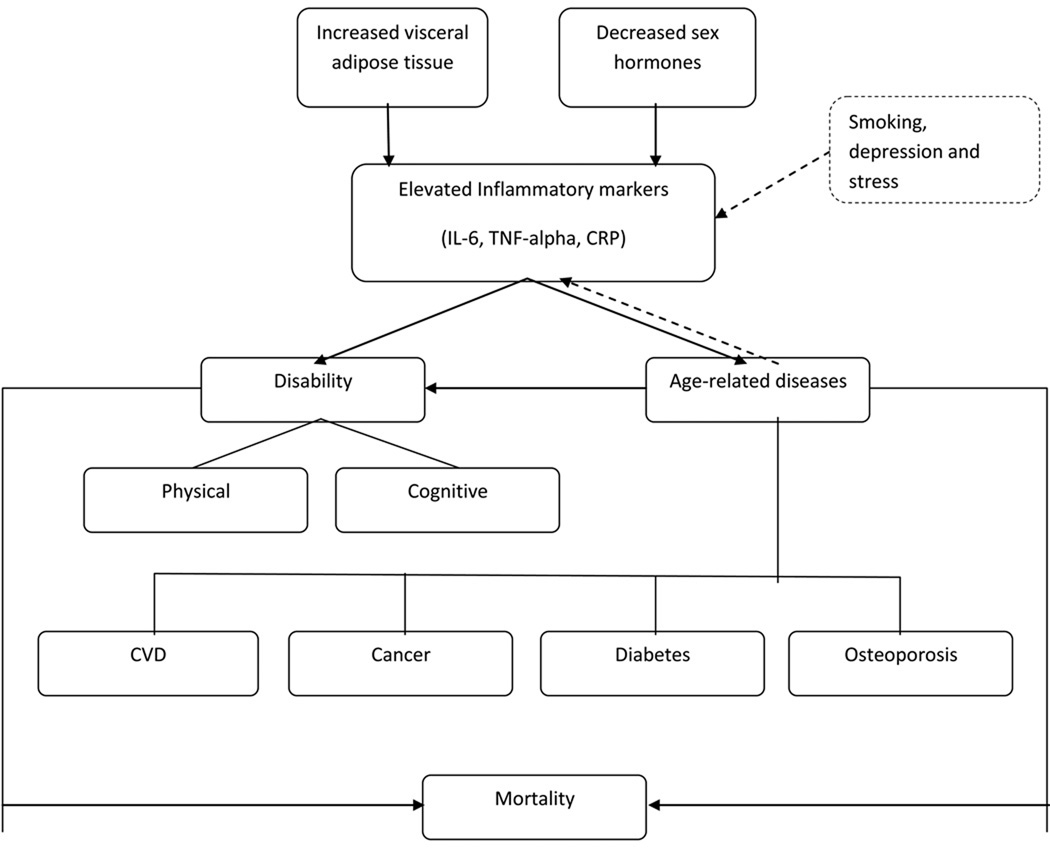
A role for inflammation in the process of aging and age-related disease has been clearly established in several large epidemiologic studies of older adults. While acute inflammation is normally tightly controlled and is a part of the healing process, the low-grade elevation of inflammatory markers seen in older adults has been associated with a number of chronic conditions of aging, such as cardiovascular disease, diabetes, physical disability and cognitive decline. A number of inflammatory markers, especially Interleukin-6 (IL-6), tumor necrosis factor alpha (TNF-alpha) and C-reactive protein (CRP) have the most consistent associations with age-related chronic diseases and disability. IL-6 is a cytokine that is produced by the cells of immune system, vascular endothelial cells, adipocytes and skeletal muscle and has shown to have anti-inflammatory as well as pro-inflammatory properties (DeRijk, Michelson et al. 1997; Xing, Gauldie et al. 1998; Maggio, Guralnik et al. 2006). TNF-alpha, another cytokine, is produced mainly by macrophages and also by some other cells including lymphoid cells, mast cells, vascular endothelial cells, cardiac myocytes, adipocytes, fibroblasts, and neuronal tissue. CRP is an acute phase protein produced by the liver in response to elevations in IL-6.
Numerous studies have shown that levels of several cytokines but especially IL-6 and TNF-alpha increase with age even in apparently healthy individuals and in the absence of acute infection (Wei, Xu et al. 1992; Ershler, Sun et al. 1993; Fagiolo, Cossarizza et al. 1993; Cohen, Pieper et al. 1997; Ferrucci, Corsi et al. 2005). This is in contrast to younger individuals where the levels of cytokines are tightly regulated at very low levels. Levels seen in older adults range from low levels to modest elevations, but are much lower than the levels seen with acute infection. The exact mechanism for the increase with age has not been fully understood. Proposed mechanisms include the known increase in total and visceral adiposity with age and declining levels of sex hormones after menopause and andropause. Oxidative damage with aging, which further invokes an inflammatory response, may be another mechanism leading to an increase in the level of these markers. Evidence shows that TNF-alpha plays an important role in the production of IL-6 through activation of different pathways (Sawada, Suzumura et al. 1992; Sakamoto, Harada et al. 2003; Williams, Lali et al. 2008). IL-6 is a major factor driving chronic elevation of CRP in older adults (Roubenoff, Harris et al. 1998). Thus, it is not surprising that IL-6, CRP and TNF-alpha are correlated in human population studies. Elevated levels of these pro-inflammatory cytokines and CRP have been studied extensively as predictors of disease and disability in older adults.
Even after a number of animal and human studies there is still a debate about whether these markers are direct causes of adverse events or simply summarize the burden of illness in older adults. In this review, we will review some of the larger population studies in older cohorts in order to illustrate the epidemiological significance of these inflammatory markers in population studies of aging.
Major factors associated with elevation of inflammatory markers
Visceral adiposity and high fat diet
Body fat has been shown to increase with age with a shift to more central abdominal or visceral fat depots. Higher levels of total and visceral fat are strongly associated with higher pro-inflammatory cytokines. Adipose tissue acts as an active endocrine organ, capable of secreting a number of cytokines and adipokines, including interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF-alpha) (Trayhurn and Wood 2005). The production of these cytokines is considered to be greater in visceral adipose tissue in comparison to subcutaneous adipose tissue (Fried, Bunkin et al. 1998; Schrager, Metter et al. 2007) and the production rate shows variability during the day (Mohamed-Ali, Goodrick et al. 1997). The production of IL-6 by the adipose tissue is variable and was found to be between 10% and 35% of the systemic IL-6 in two different studies (Mohamed-Ali, Goodrick et al. 1997; Fried, Bunkin et al. 1998). Infiltration of macrophages in the adipose tissue, which in turn are responsible for the local production of TNF-alpha and IL-6, may be a major cause of presence of a low-grade inflammation and insulin resistance in obese subjects (Weisberg, McCann et al. 2003; Xu, Barnes et al. 2003; Curat, Miranville et al. 2004; Zeyda and Stulnig 2009).
In the Health, Aging and Body Composition (Health ABC) Study, visceral fat area and intermuscular thigh fat area on CT scan were found to be significantly associated with higher concentrations of IL-6 and CRP in all race and gender groups, independent of total body fat by DXA (Beasley, Koster et al. 2009). Pou et al. found associations of both subcutaneous adipose tissue (SAT) and visceral adipose tissue (VAT) with elevated IL-6 and CRP in the Framingham Heart Study population, and after adjustment for clinical measures of adiposity (BMI and waist circumference), found VAT to remain associated with multiple markers. Both of these studies suggest that visceral fat in non-obese and obese older adults is a specific contributor to inflammation (Pou, Massaro et al. 2007). IL-6, soluble IL-6 receptor (sIL-6r) and CRP were also found to be associated with overall obesity in the InCHIANTI study population (Schrager, Metter et al. 2007). These studies did not find any significant association of visceral fat with TNF-alpha which is consistent with findings in earlier studies (Mohamed-Ali, Goodrick et al. 1997) (Cartier, Cote et al. 2009). This may be due to the fact that circulating levels of TNF-alpha are very low and it has a very short half life (Kern, Ranganathan et al. 2001).
There is some evidence of high fat diet being associated with an increase in pro-inflammatory markers. Nappo et al. found an increase in the levels of IL-6 and TNF-alpha after a high-fat meal (Nappo, Esposito et al. 2002). Mediterranean diet is believed to be associated with lower levels of IL-6 and CRP and decreased insulin resistance (Esposito, Marfella et al. 2004). These associations were independent body weight changes in a clinical trial of weight loss in middle-aged adults with metabolic syndrome.
Sex hormones
Declining levels of steroid hormones with aging are thought to be another major contributor to the elevation of pro-inflammatory markers. Studies suggest that estrogen and testosterone inhibit the secretion of IL-6 (Pottratz, Bellido et al. 1994; Ray, Prefontaine et al. 1994). Numerous studies have documented an increase in the levels of pro-inflammatory cytokines (mainly IL-6) after the onset of menopause (Jilka, Hangoc et al. 1992; Passeri, Girasole et al. 1993; Kania, Binkley et al. 1995; Pfeilschifter, Koditz et al. 2002; Lee, Carr et al. 2009), indicating the importance of estrogen in regulating IL-6. Data from InCHIANTI study showed a significant inverse relationship between testosterone and the soluble receptor for IL-6, sIL-6r suggesting development of a pro-inflammatory state with declining levels of testosterone (Maggio, Basaria et al. 2005; Maggio, Basaria et al. 2006).
Other risk factors for elevation in inflammatory markers
There are several other factors that have been associated with elevation of these inflammatory markers in human population studies. Acute infection causes a short-term elevation of the pro-inflammatory cytokines and CRP and recent infection cannot always be excluded in observational studies. Smoking has been associated with a rise in these markers especially in the younger population (Das 1985; Arnson, Shoenfeld et al. 2010). Chronic smoking has shown to induce the release of pro-inflammatory markers including IL-6, TNF-alpha and acute phase proteins and decrease the production of anti-inflammatory cytokines (Bermudez, Rifai et al. 2002; Arnson, Shoenfeld et al. 2010). Some studies show that depression and stress may also increase the levels of pro-inflammatory markers (Penninx, Kritchevsky et al. 2003; Tiemeier, Hofman et al. 2003; Howren, Lamkin et al. 2009). IL-6 is also produced by muscle during muscle contraction and physical exercise causes an acute elevation of this marker (Steensberg, van Hall et al. 2000; Pedersen, Steensberg et al. 2001). However, long-term exercise is associated with lower levels of these markers (Geffken, Cushman et al. 2001; Reuben, Judd-Hamilton et al. 2003; Elosua, Bartali et al. 2005; Nicklas, Hsu et al. 2008).
In summary, factors found to be associated with elevated levels of IL-6, TNF-alpha and CRP in community dwelling older adults include greater visceral adiposity and declining levels of sex hormones with age. These factors demonstrate most consistent evidence with elevation of these inflammatory markers, although stress-related factors such as long-term smoking and depression may also play an important role.
Consequences of elevation of inflammatory markers
Age-related Chronic Diseases
Cardiovascular disease
A number of inflammatory markers have been studied for prediction of cardiovascular disease in asymptomatic subjects. While the traditional risk factors have proven to be useful in the younger population, their predictive value has shown to decrease with age (Beckett, Nunes et al. 2000). Studies have shown inflammatory markers to be consistently associated with cardiovascular disease in the older population and these associations are independent of traditional CVD risk factors. CRP has been studied most extensively as a marker and predictor of CVD, but recent longitudinal studies have evaluated the role of other pro-inflammatory markers including IL-6 and TNF-alpha as potential risk factors and predictors of CVD in the elderly.
Looking at CRP in the Cardiovascular Health Study and the Rural Health Promotion Project, Tracy et al. reported CRP to be significantly associated with incident cardiovascular disease even after taking subclinical disease status into account. They concluded that elevations in CRP may be a strong predictor of short-term clinical events (OR=2.67 for incident MI for men and women with subclinical disease for upper vs. lower three quartiles) and incident CVD (OR=2.7 for upper quintile vs. lower four) (Tracy, Lemaitre et al. 1997). Cesari et al. found IL-6, TNF-alpha and CRP to predict the onset of cardiovascular events in the Health ABC study (Cesari, Penninx et al. 2003). They also found IL-6 to be the strongest and most consistent risk factor for cardiovascular events and the risk of events to be the greatest in subjects with high levels of all three markers (Cesari, Penninx et al. 2003). CRP has shown to improve prediction of CVD after accounting for conventional risk factors in other studies as well (Cao, Arnold et al. 2007).
Numerous studies have shown inflammation to be an integral part of the atherosclerotic process (Libby, Ridker et al. 2002). Bruunsgaard et al. found high levels of TNF-alpha to be associated with high prevalence of atherosclerosis (Bruunsgaard, Skinhoj et al. 2000). In Health ABC study, elevated levels of IL-6, TNF-alpha, ankle-arm index (AAI) and CRP were associated with increased coronary heart disease (CHD) risk, but only IL-6 and AAI predicted risk beyond traditional risk factors (Rodondi, Marques-Vidal et al. 2010). CRP was associated with CHD in ≥65 years old men and women in the CHS with 10-year cumulative incidence of CHD being 33% in men and 17% in women with elevated CRP (Cushman, Arnold et al. 2005). The relative risk of CHD for CRP was 1.45 (CRP>3mg/L vs. <1mg/L) after adjustments for conventional risk factors and it appeared to be useful for risk assessment in older population for both men and women (Cushman, Arnold et al. 2005).
As elevated inflammatory markers may accelerate development of atherosclerosis, peripheral artery disease (PAD) is also associated with increased circulating levels of pro-inflammatory markers. The InCHIANTI study showed a significant association of CRP, IL-6, IL-1 receptor antagonist and fibrinogen with PAD (McDermott, Guralnik et al. 2005). Significant association of IL-6 and tumor necrosis factor receptor 2 with PAD was also seen in the Framingham Offspring Study (Murabito, Keyes et al. 2009). This association was independent of other risk factors. These findings are similar to previous longitudinal studies evaluating the association of inflammation and PAD (Ridker, Cushman et al. 1998; Tzoulaki, Murray et al. 2007).
CHS and Health ABC also found inflammatory markers to be associated with heart failure (HF) risk in older population (Suzuki, Katz et al. 2008; Kalogeropoulos, Georgiopoulou et al. 2010). CRP and IL-6 in combination with metabolic syndrome were found to be associated with incident HF and were shown to provide additional information in predicting HF in the CHS subjects (Suzuki, Katz et al. 2008). Health ABC, on the other hand, found CRP to have weaker association with HF and identified IL-6 and TNF-alpha to be independently associated with HF development (Kalogeropoulos, Georgiopoulou et al. 2010). Vasan et al. made similar observations in the Framingham Heart Study subjects where they found increase in risk of heart failure per tertile increment of IL-6 to be 68%, TNF-alpha to be 60% and a serum CRP level ≥5 mg/dL to be associated with a 2.8-fold increased risk of heart failure, but out of the three, identified IL-6 as the best predictor of HF risk (Vasan, Sullivan et al. 2003).
Aviles et al. found CRP to be independently associated with the presence of atrial fibrillation (AF) and to predict patients at increased risk for its future development in CHS with adjusted hazard ratio (HR) for one standard deviation (SD) increase in CRP to be 1.24 (Aviles, Martin et al. 2003). Findings from Intermountain Heart Collaborative Study also showed CRP to independently predict an increased risk of AF and recommended the use of CRP as a risk marker for AF (Anderson, Allen Maycock et al. 2004). Studies have also shown the association of high levels of IL-6 and CRP with low HDL cholesterol in older individuals (Zuliani, Volpato et al. 2007; Cesari, Onder et al. 2009) which may increase the risk of CVD in this population.
Even though results from most of the studies referenced earlier determine that the elevation of pro-inflammatory markers may increase the risk of cardiovascular disease, they only support but do not prove a causal relationship. It is possible that inflammation may be a reflection of the burden of subclinical disease. In older adults, presence of cardiovascular risk factors and prevalent cardiovascular disease may contribute to elevation of pro-inflammatory markers (Ferrucci, Corsi et al. 2005). Elevated inflammatory markers are further shown to increase the risk of development of cardiovascular disease (Tracy, Lemaitre et al. 1997; Aviles, Martin et al. 2003; Cesari, Penninx et al. 2003; Cesari, Penninx et al. 2003; Kalogeropoulos, Georgiopoulou et al. 2010). This can lead to formation of a cause and effect chain with inflammation stimulating development of CVD, and CVD leading to an increase in inflammation. Clinical trials are testing the hypothesis that reduction in CRP may reduce cardiovascular events, which would provide the best evidence for a causal relationship.
Diabetes
There is growing evidence which suggests that pro-inflammatory cytokines are not only produced by visceral adipose tissue, but may also be a major reason for insulin resistance. Circulating levels of IL-6 and TNF-alpha have shown to be higher in type 2 diabetes in numerous studies (Pickup, Chusney et al. 2000). CRP is also thought to be associated with the development of diabetes. Elevated levels of both CRP and IL-6 were found to be independent risk predictors for development diabetes in the Women’s Health Study (WHS) where, after adjustment for confounders, relative risk of diabetes for highest vs. lowest quartiles of CRP and IL-6 was 4.2 and 2.3 respectively (Pradhan, Manson et al. 2001). Similar observations were reported in a multi-ethnic cohort of post-menopausal women in the Women’s Health Initiative Observational Study (WHIOS) (Liu, Tinker et al. 2007), and in the participants of the Multi-Ethnic Study of Atherosclerosis (MESA) where higher levels of IL-6 and CRP predicted short-term incidence of type 2 diabetes (Bertoni, Burke et al. 2010). High levels of IL-6 and CRP were reported in participants with diabetes than those without it in the Health ABC study (Figaro, Kritchevsky et al. 2006). Barzilay et al. found participants with elevated CRP to be twice more likely to have diabetes in CHS (Barzilay, Abraham et al. 2001). Several studies including The Rotterdam Study (Dehghan, Kardys et al. 2007), CHS (Barzilay, Abraham et al. 2001), Nurses’ Health Study (Hu, Meigs et al. 2004), WHIOS (Liu, Tinker et al. 2007) and WHS (Pradhan, Manson et al. 2001) have reported CRP as an independent predictor of diabetes after adjustment for possible confounders including obesity. On the contrary, in studies like European Prospective Investigation of Cancer (EPIC)-Norfolk (Lee, Adler et al. 2009), Insulin Resistance Atherosclerosis Study (IRAS) (Festa, D'Agostino et al. 2002) and Monitoring of Trends and Determinants in Cardiovascular Diseases (MONICA) Augsburg Cohort Study (Thorand, Lowel et al. 2003), even though CRP was associated with diabetes, the association became non-significant after adjustment for body mass index, smoking, and systolic blood pressure.
Therefore, after taking into consideration the complex relationship between inflammation, obesity and diabetes and the inconsistent results of large population studies, it is difficult ascertain whether the associations of inflammatory markers and diabetes in older adults reflect a causal relationship.
Cancer
There has been a major interest on the role of inflammation in the increased risk of cancer with age. Several studies provide evidence of a strong link between chronic inflammation and different types of cancer especially multiple myeloma and lymphatic cancers (Aggarwal, Shishodia et al. 2006; Lu, Ouyang et al. 2006). A systematic review was done by Heikkila et al. to investigate the association of IL-6 with cancer (Heikkila, Ebrahim et al. 2008). They found IL-6 to be associated with some cancers, but, due to the small number of prospective studies, could not confirm the utility of circulating levels of IL-6 for diagnosing or predicting cancer (Heikkila, Ebrahim et al. 2008). Another systematic review was done by the same group to examine the association between CRP and cancer (Heikkila, Harris et al. 2009). They found CRP concentrations to be higher in cancer patients as compared to healthy controls or patients with benign conditions in most of the cross-sectional studies and case-control studies, but found no strong evidence for a causal role of CRP in cancer in the very limited number of prospective studies (Heikkila, Harris et al. 2009). A study on Health ABC population suggested elevated levels of IL-6, TNF-alpha and CRP to be more strongly associated with risk of cancer death than cancer incidence with the hazard ratios for cancer death being 1.63 for IL-6, 1.64 for CRP and 1.82 for TNF-alpha (Il'yasova, Colbert et al. 2005). They found all three markers to be associated with lung cancer, IL-6 and CRP associated with colorectal cancer, CRP associated with breast cancer and none of them associated with prostate cancer (Il'yasova, Colbert et al. 2005). Colorectal cancer was also reported to be positively associated with CRP in the CLUE II cohort (Erlinger, Platz et al. 2004) and with IL-6, TNF-alpha and CRP in the Diet and Health Study (Kim, Keku et al. 2008). On the contrary, no association was found between CRP and colorectal cancer in WHS (Zhang, Buring et al. 2005). Similar to Health ABC, results from CHS and Physicians’ Health Study show no influence of circulating levels of CRP and IL-6 on prostate cancer (Pierce, Biggs et al. 2009; Stark, Li et al. 2009). The Rotterdam Study found high levels of CRP to be associated with an increased risk of incident cancer with the strongest association for lung cancer (Siemes, Visser et al. 2006). IL-6 related gene variation was found to be associated with multiple myeloma in the Nurses' Health Study and the Health Professionals Follow-up Study (Birmann, Tamimi et al. 2009).
Other conditions
A number of other chronic diseases are associated with chronic low-grade inflammation in older populations. In vitro evidence shows chronic inflammation to disrupt bone homeostasis increasing the possibility of development of osteopenia and osteoporosis (Chung, Cesari et al. 2009). Osteoclastogenesis and osteoclast activity is also promoted by IL-6 in in vitro studies, contributing to the process of bone remodeling (Manolagas and Jilka 1995). Several human population studies have shown the association pro-inflammatory markers like IL-6, TNF-alpha and CRP, and osteoporosis in humans (Khosla, Peterson et al. 1994; Zheng, Vrindts et al. 1997), and elevated IL-6 has been found to predict bone loss and resorption (Ding, Parameswaran et al. 2008). Cauley et al. reported that high levels of serum inflammatory markers predict a higher incidence of fractures during a 5.8-year follow-up period in the Health ABC cohort, and the association to be particularly strong in subjects with higher levels of two or three markers (Cauley, Danielson et al. 2007). They found subjects with highest levels of IL-6 to have 39% and with highest levels of CRP to have 37% more risk of fracture (Cauley, Danielson et al. 2007). IL-6 was also found to be associated with rheumatoid arthritis and reflected the levels of disease activity (Madhok, Crilly et al. 1993; Robak, Gladalska et al. 1998; Klimiuk, Sierakowski et al. 2003).
Studies also show that modest elevated pro-inflammatory markers may be related to other conditions like anemia (Ferrucci, Semba et al. 2010), Crohn’s disease (Reinisch, Gasche et al. 1999; Stallmach, Giese et al. 2004) Castleman’s disease (Kishimoto 2010) polymyalgia rheumatica (PMR) and giant cell arteritis (GCA) (Goronzy and Weyand 2002; Martinez-Taboada, Alvarez et al. 2008), though in the latter, levels of these markers are generally much higher than are seen in chronic disease states.
Disability
Physical disability
Frailty has emerged as a very important and useful concept in aging. Frailty is conceptually defined as an increased vulnerability to stress in old age. While there are many operational definitions (Rockwood, Rockwood et al. 2010), criteria from the CHS study have proven useful for defining frailty in human population studies (Fried, Tangen et al. 2001). These criteria include a decline in strength, unintentional weight loss, reported fatigue, low physical activity level, slow walking speed making older adults at risk for frequent falls, hospitalizations, disability and death (Fried, Xue et al. 2009).
Chronic low-grade inflammation has been related to frailty, its components and its outcomes in several studies (Ershler and Keller 2000; Hubbard, O'Mahony et al. 2009; Fulop, Larbi et al. 2010). Sarcopenia (age-related loss of muscle mass, strength and function) is a major component of frailty and a risk factor for disability outcomes (Cruz-Jentoft, Landi et al. 2010; Lang, Streeper et al. 2010). Ferrucci et al. found participants in the highest IL-6 tertiles to be 1.76 times more likely to develop mobility disability in the Iowa Established Populations for Epidemiological Studies of the Elderly (EPESE) (Ferrucci, Harris et al. 1999). The Health ABC population showed higher IL-6, TNF-alpha individually and in combination to be associated with lower muscle mass and muscle strength indicating tendency of older people with high cytokine levels to develop sarcopenia (Visser, Pahor et al. 2002). A more recent paper on the same study population found high serum levels of IL-6, TNF-alpha and CRP to predict an increased incidence of mobility limitation during a 30-month follow-up period with people having elevated levels of all three markers showing the highest incidence of mobility limitation (Penninx, Kritchevsky et al. 2004). TNF-alpha and its soluble receptors also showed strong associations with decline in muscle mass and strength in a study investigating association between inflammatory markers and 5-year change in muscle mass and muscle strength (Schaap, Pluijm et al. 2009). In line with this, Cesari et al. found high levels of IL-6 and CRP significantly and independently associated with poor physical performance and muscle strength in older population in the InCHIANTI Study (Cesari, Penninx et al. 2004). Another paper on the same study found IL-6 to be an independent predictor of handgrip and muscle power especially in participants with highest IL-6 levels (Barbieri, Ferrucci et al. 2003). Data from Women’s Health and Aging Study (WHAS) showed that older women with high IL-6 levels had a higher risk of developing physical disability and experienced a more drastic fall in walking ability than those with lower levels of IL-6 (Ferrucci, Penninx et al. 2002). In a different cohort of Dutch older population in the Longitudinal Aging Study Amsterdam (LASA), high IL-6 and CRP were associated with a 2 to 3 fold greater risk of losing more than 40% of muscle strength after adjustments for confounders, but no consistent associations of these markers with muscle mass were found (Schaap, Pluijm et al. 2006). In the Framingham Heart Study, IL-6 was found to be a significant predictor of loss of fat free mass in the older population (Payette, Roubenoff et al. 2003). Findings from MacArthur Studies of Successful Aging also suggest a relationship between high levels of IL-6 and CRP with poor performance on tests for walking speed and grip strength (Taaffe, Harris et al. 2000). Evidence from several studies suggests the role of inflammation in a number of diseases, which may be another important cause of disability in the older population (Ershler, Sun et al. 1994; Ferrucci, Harris et al. 1999). In summary, the elevation of multiple markers, but especially IL-6 is strongly related to prevent and incident frailty, mobility impairment and disability and the loss of muscle mass and strength with age.
Cognitive decline
Chronic low-grade inflammation in older people has been linked to cognitive decline and dementia, including vascular dementia and Alzheimer’s disease (AD) in a number of studies. In the Health ABC cohort, Yaffe et al. found high levels of IL-6 and CRP to be associated with poor cognitive performance and greater risk of cognitive decline over 2 years of follow-up in older adults (Yaffe, Lindquist et al. 2003). After adjusting for possible confounders, the association decreased but remained significant with the adjusted OR for highest vs. lowest tertiles of IL-6 = 1.23, CRP = 1.24 and TNF-alpha 1.23 (Yaffe, Lindquist et al. 2003). In a separate study in the same cohort, metabolic syndrome was found to contribute to cognitive impairment, but this observation was primarily in the population with high levels of inflammatory markers (Yaffe, Kanaya et al. 2004). In the MacArthur Studies of Successful Aging, high levels of IL-6 were found to be associated with poor cognitive function in older adults and longitudinal analysis showed elevated IL-6 to independently predict increased risk for cognitive decline at 2.5-year (highest tertile OR = 2.03) and 7-year (highest tertile OR = 1.90) follow-up (Weaver, Huang et al. 2002). Similar associations for IL-6 and CRP with dementia, AD and vascular dementia have been observed in the Rotterdam Study (Engelhart, Geerlings et al. 2004) and the Honolulu-Asia Aging Study (Schmidt, Schmidt et al. 2002) and the levels of these markers were found to be elevated before the onset of clinical symptoms indicating possible relevance of their measurement in asymptomatic high-risk individuals. Conselice Study of Brain Ageing (CSBA), an Italian cohort of older population, also found high serum levels of CRP and IL-6 to be associated with risk of vascular dementia (HR 2.56), but did not find any association of these markers with Alzheimer’s Disease (Ravaglia, Forti et al. 2007). Researchers did not find any association of CRP and IL-6 with cognitive decline on older persons in the LASA study population (Comijs, Dik et al. 2004). Although the exact mechanism of how chronic inflammation may lead to dementia is still unclear, the association of these markers with cognitive decline may be partly explained by other conditions related to inflammation. Elevated pro-inflammatory cytokines and CRP are known to be associated with increased risk of atherosclerosis, other vascular disease and diabetes which may further be related to cognitive decline in the elderly.
Mortality
Given the strong associations with multiple chronic diseases and disability in older adults, it is not surprising that elevated inflammatory markers are predictors of all-cause mortality in a number of studies in older cohorts. Elevated levels of circulating IL-6 were reported to be consistently associated with death across multiple causes in the CHS and strongly predict future mortality (Newman, Sachs et al. 2009; Walston, Matteini et al. 2009). In the same study, Jenny et al. found strong association of CRP with early death (Jenny, Yanez et al. 2007). Similar associations were seen in a cohort of 1,293 healthy, non-disabled older adults in the Iowa 65+ Rural Health Study, where the highest quartile of IL-6 was associated with a two times greater risk of death as compared to the lowest quartile and elevation of both IL-6 and CRP was associated with a 2.6 times higher death risk (Harris, Ferrucci et al. 1999). Higher levels of IL-6 and TNF-alpha were associated with increased mortality in older adults in the Framingham Heart Study (Roubenoff, Parise et al. 2003).
Because these markers do not appear to be specific for any one disease or cause of death, these elevations can be viewed as reflecting a fundamental aspect of the aging process.
Possible interventions
The strong associations of inflammatory markers with adverse health outcomes in older adults suggest the potential for targeting inflammation to reduce disability and mortality in old age. Advances have been made in targeting TNF and more recently IL-6 in rheumatic conditions, but whether these are relevant in old age is not yet known. Proteasome inhibitors, IL-6 and IL-6 receptor antibodies are in their initial stages of testing. Indirect evidence for benefit in reducing inflammatory markers in old age comes from statin trials for cardiovascular disease prevention. Studies of physical activity and hormone replacement also suggest that their potential benefits may be achieved in part via an anti-inflammatory pathway. Treatment trials for dementia with non-steroidal anti-inflammatory drugs have not been completed due to adverse cardiovascular events on active treatment.
Statins
Reduction of primary and secondary CVD risk because of the lipid-lowering effect of statins has been documented a number of times in the past. Apart from this, recent studies suggest the effect of statins on lowering the levels of inflammatory markers, especially IL-6 and CRP, which may be a promising approach in the use of these drugs for other chronic inflammatory conditions (Nawawi, Osman et al. 2003; Montecucco and Mach 2009; Ridker, Danielson et al. 2009; Quist-Paulsen 2010). Results from Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER) demonstrated that statin treatment in apparently healthy subjects with elevated CRP and non-elevated LDL cholesterol resulted in significant reduction in both these markers and CVD (Ridker, Danielson et al. 2009; Mora, Glynn et al. 2010). However, the results of this trial have been controversial as it is not clear whether statins actually reduce the levels of CRP and if a decline in CRP explains the benefit by reduction in CVD (de Lorgeril, Salen et al. 2010; Kaul, Morrissey et al. 2010).
Physical activity/ exercise
There is promising evidence suggesting a role of physical activity in reducing the levels of inflammatory markers. Even though several theories have been hypothesized, the exact mechanism for this reduction is not clear. A possible mechanism for this reduction may be related to a preferential decrease in visceral fat with physical activity interventions. Numerous cross-sectional and longitudinal studies have now shown the association of physical activity with a decrease in the levels of these markers especially in the elderly. Reuben et al. reported a beneficial association of high levels of recreational activity on IL-6 and CRP with adjusted odds ratio for highest levels of IL-6 = 0.65 and CRP = 0.70 in the MacArthur Studies of Successful Aging (Reuben, Judd-Hamilton et al. 2003). Similar associations between inflammatory markers and physical activity have been found in other large cohort studies like the CHS and the InCHIANTI study (Geffken, Cushman et al. 2001; Reuben, Judd-Hamilton et al. 2003; Elosua, Bartali et al. 2005; Nicklas, Hsu et al. 2008). One clinical trial specifically showed a lowering of these markers related to participation in a physical activity program in the Lifestyle Interventions for Independence for Elders Pilot (LIFE-P).
Hormones
As observational data shows elevation of pro-inflammatory markers with declining levels of sex hormones, a number of clinical trials have been conducted to look at the beneficial effects of hormone therapy to counter this effect. Testosterone administration was shown to be followed by a marked reduction of inflammatory markers in hypogonadal men in a testosterone replacement crossover trial (Malkin, Pugh et al. 2004). But there is still no consensus on this approach as some studies show contradictory results and hormone supplementation has been linked to a number of severe adverse effects. Another study found a significant inverse correlation between testosterone and inflammatory markers (IL-6, CRP), but did not observe any significant effect of testosterone therapy on these markers (Kapoor, Clarke et al. 2007). A recent trial, Testosterone in Older Men with Mobility limitations (TOM), evaluating the effects of testosterone administration to older population had to be terminated because of safety issues as the proportion of adverse cardiovascular events exceeded too much in the intervention group as compared to the control group (Basaria, Coviello et al. 2010). Similarly, the use of hormone replacement therapy (HRT) in post-menopausal women has shown contradictory results with a number of studies showing no association between HRT and inflammatory markers (Pradhan, Manson et al. 2002), and most of the studies reporting significant association with other diseases (Nelson, Humphrey et al. 2002; Pradhan, Manson et al. 2002).
Other potential interventions
Other possible interventions to decrease the levels of pro-inflammatory markers have been tested or hypothesized. Low-fat diet which may lead to a decline in the total and visceral body fat deposits may be promising in reducing the levels of these markers (Maggio, Guralnik et al. 2006; Chung, Cesari et al. 2009). A few studies have looked at the effect of NSAIDs on inflammatory markers, but till date most of the studies have not found any influence of these drugs on the levels of cytokines and CRP.
Summary
These epidemiologic studies illustrate the significance of inflammatory markers such as IL-6, TNF-alpha and CRP in older populations. These markers have shown to increase with age, potentially as a consequence of declining levels of sex hormones and increase in visceral adipose tissue. Elevation of these markers is associated with a number of age-related chronic diseases making these associations non-specific. Currently, the use of these markers is limited to prediction of disability and identification of individuals at high risk for age-related disease, disability, and mortality.
Elevated inflammatory markers in older adults: risk factors and consequences
Major population studies assessing association between inflammatory markers, aging, disability, chronic diseases, mortality and interventions
| Study, author and year of publication | Measured inflammatory markers | Measures of association | Conclusions |
|---|---|---|---|
|
Health, Aging and Body Composition (HABC) Study cohort study of 3,075 well-functioning, 70–79 years old black and white men and women | |||
| Beasley (Beasley, Koster et al. 2009) | IL-6, TNF-alpha, CRP | β ≈ 0.1/cm2 of visceral fat area in men and black women; β ≈ 0.2/cm2 in white women | Visceral fat significantly and positively associated with IL-6 and CRP. |
| Yaffe (Yaffe, Lindquist et al. 2003) | IL-6, TNF-alpha, CRP | Adjusted OR for cognitive decline for highest vs. lowest tertiles of: IL-6 = 1.23, CRP = 1.24, TNF-alpha = 1.23 | High levels of IL-6, CRP associated with cognitive decline in the elderly. |
| Kalogeropoulos (Kalogeropoulos, Georgiopoulou et al. 2010) | IL-6, TNF-alpha, CRP | Adjusted HR of HF per doubling of: IL- 6=1.24; TNF-alpha= 1.41; CRP=1.01 | IL-6, TNF-alpha, CRP associated with HF risk among older adults and may improve HF risk stratification. |
| Cesari (Cesari, Penninx et al. 2003) | IL-6, TNF-alpha, CRP | RR for IL-6 (per SD increase) with CHD=1.27, stroke=1.45, CHF=1.72; RR for TNF-alpha (per SD increase) with CHD=1.22, CHF=1.59; RR for CRP (per SD increase) with CHF=1.48, | IL-6, TNF-alpha, CRP are independent predictors of cardiovascular events in older adults. |
| Cesari (Cesari, Penninx et al. 2003) | IL-6, TNF-alpha, CRP, sIL-6r, sIL-2r, sTNFR1, sTNFR2 | OR for highest vs. lowest tertile of IL-6 for subclinical CVD=1.58, clinical CVD=2.35; TNF-alpha for subclinical CVD=1.48, clinical CVD=2.05 | IL-6, TNF-alpha significantly associated with clinical and subclinical CVD. CRP had weaker association. |
| Rodondi (Rodondi, Marques-Vidal et al. 2010) | IL-6, TNF-alpha, CRP | HR CHD for highest vs. lowest quartile of IL-6=1.82 | IL-6 associated with future CHD events and improves risk prediction in older adults. |
| Il'yasova (Il'yasova, Colbert et al. 2005) | IL-6, TNF-alpha, CRP | HR for cancer death for: IL-6=1.63, TNF-alpha= 1.82, CRP=1.64 | Elevated levels of inflammatory markers more strongly associated with risk of cancer death than cancer incidence. |
| Hsu (Hsu, Kritchevsky et al. 2009) | IL-6, TNF-alpha, CRP, sIL-6r, sIL-2r, sTNFR1, sTNFR2 | Regression coefficient for knee strength and: TNF-alpha=−2.71, CRP=−0.88; physical performance battery score and: TNF-alpha=−0.05, CRP=−0.02 | TNF-alpha and CRP positively associated with walk time and inversely associated with grip strength. |
| Schaap (Schaap, Pluijm et al. 2009) | IL-6, TNF-alpha, CRP, sIL-6r, sIL-2r, sTNFR1, sTNFR2 | Regression coefficient (per SD change in marker) for 5 –year change in grip strength and TNF-alpha=−0.62; and 5 –year change in knee extensor strength and TNF-alpha=−1.02 | TNF-alpha had strongest and most consistent association with decline in muscle mass and strength. |
| Cauley (Cauley, Danielson et al. 2007) | IL-6, TNF-alpha, CRP, sIL-6r, sIL-2r, sTNFR1, sTNFR2 | RR for fracture with highest quartile vs. lower three quartiles of: CRP=1.34, IL- 6=1.28, TNF-alpha= 1.28 | Elevated inflammatory markers are prognostic for fractures. |
|
Cardiovascular Health Study (CHS) cohort study of 5,888, ≥65 years old black and white men and women | |||
| Tracy (Tracy, Lemaitre et al. 1997) | CRP | OR for incident MI for subjects with subclinical disease (highest vs. lower three quartiles of CRP)=2.67 | CRP associated with incident cardiovascular events in the elderly especially who had subclinical disease at baseline. |
| Cushman (Cushman, Arnold et al. 2005) | CRP | 10-year cumulative CHD incidence with elevated CRP: Men=33%, Women=17%; Adjusted RR of CHD (CRP>3mg/L vs. <1mg/L)=1.45 | Elevated CRP associated with increased 10-year risk of CHD in older adults. |
| Aviles (Aviles, Martin et al. 2003) | CRP | Adjusted HR for AF for highest vs. lowest quartile of CRP=1.31, HR for 1SD increase=1.24 | CRP associated with presence of AF, may also predict future development of AF. |
| Cao (Cao, Arnoldet al. 2007) | CRP | Elevated CRP (>3mg/L) HR CVD events=1.45; CVD deaths=1.72; all-cause mortality=1.52 | CRP associated with increased risk for CVD and all-cause mortality in patients with detectable atherosclerosis. |
| Suzuki (Suzuki, Katz et al. 2008) | IL-6, CRP | Heart Failure HR for CHF for elevated CRP (≥3mg/L)=1.53; IL-6 (≥2.21pg/mL)=1.37 | IL-6 and CRP together with metabolic syndrome (MetS) associated with incident CHF in non-diabetic men and women. |
| Geffken (Geffken, Cushman et al. 2001) | CRP, fibrinogen | Mean values of CRP for lowest vs. highest quartile of physical activity (mg/L)=2.24 vs. 1.82 | Increased exercise is associated with reduced inflammation. |
| Jenny (Jenny, Yanez et al. 2007) | CRP, fibrinogen | HR for early death comparing highest vs. lowest quartile of CRP: men=4.1; women=2.3 | CRP and fibrinogen strongly associated with early death in older adults. |
| Newman (Newman, Sachs et al. 2009) | IL-6, CRP | HR for total 16-year mortality with highest vs. lowest quintile of IL-6=1.66 | IL-6 consistently associated with death across multiple causes. |
| Walston (Walston, Matteini et al. 2009) | IL-6 | HR for total all-cause mortality with highest vs. lowest quartile of IL-6=2.34 | Elevated IL-6 strongly predicted future mortality in European-American and African American adults. |
|
InCHIANTI Study cohort study of 1,453, 20–102 years old men and women | |||
| Schrager (Schrager, Metter et al. 2007) | IL-6, sIL-6r, IL-1ra, TNF-alpha, CRP | Mean value of IL-6 in participants with central obesity with: normal strength=1.6 pg/mL, low strength=2.3 pg/mL | Sarcopenic obesity is associated with elevated IL-6, sIL-6r and CRP. Central obesity may negatively affect muscle strength by increasing cytokine production. |
| McDermott (McDermott, Guralnik et al. 2005) | CRP, fibrinogen, IL-1β, IL-6, IL-10, IL-18, TNF-alpha | Adjusted associations between markers and presence vs. absence of PAD: CRP=3.12 vs. 2.57; IL-6=1.60 vs. 1.37 | PAD is associated with increased IL-6, fibrinogen, CRP. |
| Zuliani (Zuliani, Volpato et al. 2007) | CRP, fibrinogen, IL-1β, IL-6, IL-10, IL-18, TNF-alpha | Adjusted OR low HDL-C and IL-6 (highest vs. lowest tertile)=2.10 | Low HDL-c is associated with high IL-6. |
| Elosua (Elosua, Bartali et al. 2005) | IL-6, sIL-6r, IL-1ra, IL-10, IL-1β, IL-18, TNF-alpha, CRP, fibrinogen | Physical activity (men): log CRP (mg/L) sedentary vs.: light=−0.43; moderate high=−0.73, log IL-6 (pg/mL)=−0.33; women: log CRP (mg/L) sedentary vs.: moderate-high=−0.31; log IL-6 (pg/mL) light=−0.18, moderate high=−0.30 | Physical activity, performance and fitness are inversely associated with pro-inflammatory biomarkers (especially fibrinogen, CRP and IL-6) in older adults. |
| Cesari (Cesari, Penninx et al. 2004) | IL-6, sIL-6r, IL-1ra, IL-10, TNF-alpha, CRP | Correlation (r) of physical performance and: CRP=−0.162, IL-6=−0.251, IL1RA=−0.127 | Elevated IL-6, IL1ra, CRP significantly and independently associated with poorer physical performance and muscle strength in older adults. |
| Barbieri (Barbieri, Ferrucci et al. 2003) | IL-6, sIL-6r | Correlation between IL-6 and: handgrip =−0.17, total power =−0.14 | IL-6 was an independent predictor of handgrip and muscle power (especially in subjects in higher tertile of IL-6). |
| Maggio (Maggio, Basaria et al. 2006) | IL-6, sIL-6r, IL-1β, TNF-alpha | Correlation between sIL-6r and testosterone after adjustments = −0.20 | Significant inverse relationship between testosterone and sIL-6r. |
| Ferrucci (Ferrucci, Corsi et al. 2005) | IL-6, sIL-6r, IL-1ra, IL-10, IL-1β, IL-18,TNF-alpha, CRP, fibrinogen | IL-6 levels in age group 20–39 vs. 85+=0.6 vs. 3.5 (men), 0.6 vs. 2.1 (women); CRP levels in age group 20–39 vs. 85+=1.0 vs. 5.4 (men), 1.1 vs. 3.3 (women) | Older age associated with higher levels of IL-6, IL-1ra, IL-18, CRP and fibrinogen in both men and women. |
|
The Framingham Heart Study cohort study originally consisting of 5,209, 30–62 years old men and women | |||
| Pou (Pou, Massaro et al. 2007) | IL-6, CRP, fibrinogen, TNF-alpha | Pearson correlation coefficients: CRP & SAT women=0.45, men=0.30; CRP & VAT women=0.47, men=0.33; IL-6 & SAT=0.23, IL-6 & VAT=0.23 | Subcutaneous adipose tissue and visceral adipose tissue similarly associated with elevated inflammatory biomarkers. |
| Haider (Haider, Roubenoff et al. 2004) | IL-6, IL-1, CRP, TNF-alpha | Correlation IL-6 and CRP (r)=0.10 | IL-6 correlated with CRP levels. IL-6, IL-1, TNF-alpha not associated with CVD in very elderly population. |
| Vasan (Vasan, Sullivan et al. 2003) | IL-6, CRP, TNF-alpha | HR for CHF risk with highest vs. lowest tertile of IL-6=2.85; TNF-alpha=2.70 | Elevated IL-6, TNF-alpha and CRP associated with increased risk of CHF in people without prior MI. |
| Murabito (Murabito, Keyes et al. 2009) | IL-6, TNF-alpha, TNFR2, fibrinogen, CRP, CD40 ligand, LpPLA2 | OR for change in ABI level per SD change in: IL-6=1.21; TNFR2=1.19 | IL-6 and TNFR2 significantly associated with PAD independent of established risk factors and each other. |
| Other studies | |||
| Weaver (Weaver, Huang et al. 2002) (MacArthur Studies of Successful Aging) | IL-6 | Highest tertile of IL-6 with cognitive decline OR at baseline = 1.46; at 2.5 years = 2.03; at 7 years = 1.90 | Elevated baseline IL-6 associated with poor cognitive function and risk for further cognitive decline. |
| Reuben (Reuben, Judd-Hamilton et al. 2003) (MacArthur Studies of Successful Aging) | IL-6, CRP | Adjusted OR for high levels of recreational activity in highest tertile of: IL-6=0.65, CRP=0.70 | High levels of recreational activity significantly associated with lower levels of IL-6 and CRP in high functioning older people. |
| Taaffe (Taaffe, Harris et al. 2000) (MacArthur Studies of Successful Aging) | IL-6, CRP | Log IL-6 and 6-m walk quartiles: 1.27 ± 0.05, 1.18 ± 0.05, 1.09 ± 0.05, and 1.02 ± 0.05; log CRP and 6-m walk 0.83 ± 0.07, 0.62 ± 0.06, 0.60 ± 0.06, and 0.44 ± 0.07 | High levels of IL-6 and CRP related to poor performance for walking speed and grip strength in high functioning older people. |
| Cartier (Cartier, Cote et al. 2009) (Quebec Family Study) | IL-6, CRP | Correlation of visceral adipose tissue with: CRP=0.39, IL-6=0.32, TNF-alpha=0.14 | IL-6 and CRP are significantly and positively associated with visceral adipose tissue. |
| Cesari (Cesari, Onder et al. 2009) (ilSIRENTE study) | CRP | Spearman’s correlation between CRP and: total cholesterol=−0.169, LDL-C=−0.151, HDL-C=−0.199 | CRP significantly and inversely related to total, LDL and HDL cholesterol. CRP also a strong predictor of mortality. |
| Harris (Harris, Ferrucci et al. 1999) (Iowa 65+Rural Health Study) | IL-6, CRP | RR of death with highest vs. lowest quartile of IL-6=1.9; CRP=1.6; both=2.6 | Elevated levels of IL-6 and CRP associated with mortality in healthy older people. |
| Nicklas (Nicklas, Hsu et al. 2008) (LIFE-P study) | IL-6, CRP | 12 months physical activity resulted in 32% reduction in CRP and 16% reduction in IL-6 levels in comparison to baseline levels | Greater physical activity resulted in lower levels of IL-6 and CRP. |
| Ravaglia (Ravaglia, Forti et al. 2007) (The Conselice Study of Brain Aging) | IL-6, CRP | Combination of high CRP and high IL6 associated with risk of vascular dementia: HR=2.56 | IL-6 and CRP associated with increased risk of vascular dementia. |
| Pradhan (Pradhan, Manson et al. 2001) (The Women’s Health Study) | IL-6, CRP | Multivariate RR of T2DM with (highest vs. lowest quartile of) IL-6=2 .3 CRP=4.2 | Elevated levels of IL-6 and CRP predict development of type 2 diabetes. |
| Schaap (Schaap, Pluijm et al. 2006) (LASA) | IL-6, CRP | OR for loss of muscle strength with IL-6 (highest vs. lowest tertile)=3.65; CRP (highest vs. lowest quartile)=1.90 | Higher levels of IL-6 and CRP increase the risk of muscle strength loss. |
| Ferrucci (Ferrucci, Penninx et al. 2002) (Women’s Health and Aging Study) | IL-6 | Adjusted RR for women in highest vs. lowest tertile of IL-6 with: incident mobility disability=1.50, ADL disability=1.41, severe limitation in walking=1.61 | Higher IL-6 associated with higher risk of developing physical disability and steeper decline in walking ability in older women. |
Acknowledgement
Dr. Singh and Dr. Newman were supported by R01-AG-023629 and R01-AG-030734.
References
- Aggarwal BB, Shishodia S, et al. Inflammation and cancer: how hot is the link? Biochem Pharmacol. 2006;72(11):1605–1621. doi: 10.1016/j.bcp.2006.06.029. [DOI] [PubMed] [Google Scholar]
- Anderson JL, Allen Maycock CA, et al. Frequency of elevation of C-reactive protein in atrial fibrillation. Am J Cardiol. 2004;94(10):1255–1259. doi: 10.1016/j.amjcard.2004.07.108. [DOI] [PubMed] [Google Scholar]
- Arnson Y, Shoenfeld Y, et al. Effects of tobacco smoke on immunity, inflammation and autoimmunity. J Autoimmun. 2010;34(3):J258–J265. doi: 10.1016/j.jaut.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Aviles RJ, Martin DO, et al. Inflammation as a risk factor for atrial fibrillation. Circulation. 2003;108(24):3006–3010. doi: 10.1161/01.CIR.0000103131.70301.4F. [DOI] [PubMed] [Google Scholar]
- Barbieri M, Ferrucci L, et al. Chronic inflammation and the effect of IGF-I on muscle strength and power in older persons. Am J Physiol Endocrinol Metab. 2003;284(3):E481–E487. doi: 10.1152/ajpendo.00319.2002. [DOI] [PubMed] [Google Scholar]
- Barzilay JI, Abraham L, et al. The relation of markers of inflammation to the development of glucose disorders in the elderly: the Cardiovascular Health Study. Diabetes. 2001;50(10):2384–2389. doi: 10.2337/diabetes.50.10.2384. [DOI] [PubMed] [Google Scholar]
- Basaria S, Coviello AD, et al. Adverse events associated with testosterone administration. N Engl J Med. 2010;363(2):109–122. doi: 10.1056/NEJMoa1000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beasley LE, Koster A, et al. Inflammation and race and gender differences in computerized tomography-measured adipose depots. Obesity (Silver Spring) 2009;17(5):1062–1069. doi: 10.1038/oby.2008.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckett N, Nunes M, et al. Is it advantageous to lower cholesterol in the elderly hypertensive? Cardiovasc Drugs Ther. 2000;14(4):397–405. doi: 10.1023/a:1007812232328. [DOI] [PubMed] [Google Scholar]
- Bermudez EA, Rifai N, et al. Relation between markers of systemic vascular inflammation and smoking in women. Am J Cardiol. 2002;89(9):1117–1119. doi: 10.1016/s0002-9149(02)02284-1. [DOI] [PubMed] [Google Scholar]
- Bertoni AG, Burke GL, et al. Inflammation and the incidence of type 2 diabetes: the Multi-Ethnic Study of Atherosclerosis (MESA) Diabetes Care. 2010;33(4):804–810. doi: 10.2337/dc09-1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmann BM, Tamimi RM, et al. Insulin-like growth factor-1- and interleukin-6-related gene variation and risk of multiple myeloma. Cancer Epidemiol Biomarkers Prev. 2009;18(1):282–288. doi: 10.1158/1055-9965.EPI-08-0778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruunsgaard H, Skinhoj P, et al. Ageing, tumour necrosis factor-alpha (TNF-alpha) and atherosclerosis. Clin Exp Immunol. 2000;121(2):255–260. doi: 10.1046/j.1365-2249.2000.01281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao JJ, Arnold AM, et al. Association of carotid artery intima-media thickness, plaques, and C-reactive protein with future cardiovascular disease and all-cause mortality: the Cardiovascular Health Study. Circulation. 2007;116(1):32–38. doi: 10.1161/CIRCULATIONAHA.106.645606. [DOI] [PubMed] [Google Scholar]
- Cartier A, Cote M, et al. Age-related differences in inflammatory markers in men: contribution of visceral adiposity. Metabolism. 2009;58(10):1452–1458. doi: 10.1016/j.metabol.2009.04.025. [DOI] [PubMed] [Google Scholar]
- Cauley JA, Danielson ME, et al. Inflammatory markers and incident fracture risk in older men and women: the Health Aging and Body Composition Study. J Bone Miner Res. 2007;22(7):1088–1095. doi: 10.1359/jbmr.070409. [DOI] [PubMed] [Google Scholar]
- Cesari M, Onder G, et al. C-reactive protein and lipid parameters in older persons aged 80 years and older. J Nutr Health Aging. 2009;13(7):587–593. doi: 10.1007/s12603-009-0168-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesari M, Penninx BW, et al. Inflammatory markers and onset of cardiovascular events: results from the Health ABC study. Circulation. 2003;108(19):2317–2322. doi: 10.1161/01.CIR.0000097109.90783.FC. [DOI] [PubMed] [Google Scholar]
- Cesari M, Penninx BW, et al. Inflammatory markers and cardiovascular disease (The Health, Aging and Body Composition [Health ABC] Study) Am J Cardiol. 2003;92(5):522–528. doi: 10.1016/s0002-9149(03)00718-5. [DOI] [PubMed] [Google Scholar]
- Cesari M, Penninx BW, et al. Inflammatory markers and physical performance in older persons: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2004;59(3):242–248. doi: 10.1093/gerona/59.3.m242. [DOI] [PubMed] [Google Scholar]
- Chung HY, Cesari M, et al. Molecular inflammation: underpinnings of aging and age-related diseases. Ageing Res Rev. 2009;8(1):18–30. doi: 10.1016/j.arr.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen HJ, Pieper CF, et al. The association of plasma IL-6 levels with functional disability in community-dwelling elderly. J Gerontol A Biol Sci Med Sci. 1997;52(4):M201–M208. doi: 10.1093/gerona/52a.4.m201. [DOI] [PubMed] [Google Scholar]
- Comijs HC, Dik MG, et al. The course of cognitive decline in older persons: results from the longitudinal aging study amsterdam. Dement Geriatr Cogn Disord. 2004;17(3):136–142. doi: 10.1159/000076346. [DOI] [PubMed] [Google Scholar]
- Cruz-Jentoft AJ, Landi F, et al. Understanding sarcopenia as a geriatric syndrome. Curr Opin Clin Nutr Metab Care. 2010;13(1):1–7. doi: 10.1097/MCO.0b013e328333c1c1. [DOI] [PubMed] [Google Scholar]
- Curat CA, Miranville A, et al. From blood monocytes to adipose tissue-resident macrophages: induction of diapedesis by human mature adipocytes. Diabetes. 2004;53(5):1285–1292. doi: 10.2337/diabetes.53.5.1285. [DOI] [PubMed] [Google Scholar]
- Cushman M, Arnold AM, et al. C-reactive protein and the 10-year incidence of coronary heart disease in older men and women: the cardiovascular health study. Circulation. 2005;112(1):25–31. doi: 10.1161/CIRCULATIONAHA.104.504159. [DOI] [PubMed] [Google Scholar]
- Das I. Raised C-reactive protein levels in serum from smokers. Clin Chim Acta. 1985;153(1):9–13. doi: 10.1016/0009-8981(85)90133-0. [DOI] [PubMed] [Google Scholar]
- de Lorgeril M, Salen P, et al. Cholesterol lowering, cardiovascular diseases, and the rosuvastatin-JUPITER controversy: a critical reappraisal. Arch Intern Med. 2010;170(12):1032–1036. doi: 10.1001/archinternmed.2010.184. [DOI] [PubMed] [Google Scholar]
- Dehghan A, Kardys I, et al. Genetic variation, C-reactive protein levels, and incidence of diabetes. Diabetes. 2007;56(3):872–878. doi: 10.2337/db06-0922. [DOI] [PubMed] [Google Scholar]
- DeRijk R, Michelson D, et al. Exercise and circadian rhythm-induced variations in plasma cortisol differentially regulate interleukin-1 beta (IL-1 beta), IL-6, and tumor necrosis factor-alpha (TNF alpha) production in humans: high sensitivity of TNF alpha and resistance of IL-6. J Clin Endocrinol Metab. 1997;82(7):2182–2191. doi: 10.1210/jcem.82.7.4041. [DOI] [PubMed] [Google Scholar]
- Ding C, Parameswaran V, et al. Circulating levels of inflammatory markers predict change in bone mineral density and resorption in older adults: a longitudinal study. J Clin Endocrinol Metab. 2008;93(5):1952–1958. doi: 10.1210/jc.2007-2325. [DOI] [PubMed] [Google Scholar]
- Elosua R, Bartali B, et al. Association between physical activity, physical performance, and inflammatory biomarkers in an elderly population: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2005;60(6):760–767. doi: 10.1093/gerona/60.6.760. [DOI] [PubMed] [Google Scholar]
- Engelhart MJ, Geerlings MI, et al. Inflammatory proteins in plasma and the risk of dementia: the rotterdam study. Arch Neurol. 2004;61(5):668–672. doi: 10.1001/archneur.61.5.668. [DOI] [PubMed] [Google Scholar]
- Erlinger TP, Platz EA, et al. C-reactive protein and the risk of incident colorectal cancer. JAMA. 2004;291(5):585–590. doi: 10.1001/jama.291.5.585. [DOI] [PubMed] [Google Scholar]
- Ershler WB, Keller ET. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu Rev Med. 2000;51:245–270. doi: 10.1146/annurev.med.51.1.245. [DOI] [PubMed] [Google Scholar]
- Ershler WB, Sun WH, et al. The role of interleukin-6 in certain age-related diseases. Drugs Aging. 1994;5(5):358–365. doi: 10.2165/00002512-199405050-00005. [DOI] [PubMed] [Google Scholar]
- Ershler WB, Sun WH, et al. Interleukin-6 and aging: blood levels and mononuclear cell production increase with advancing age and in vitro production is modifiable by dietary restriction. Lymphokine Cytokine Res. 1993;12(4):225–230. [PubMed] [Google Scholar]
- Esposito K, Marfella R, et al. Effect of a mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA. 2004;292(12):1440–1446. doi: 10.1001/jama.292.12.1440. [DOI] [PubMed] [Google Scholar]
- Fagiolo U, Cossarizza A, et al. Increased cytokine production in mononuclear cells of healthy elderly people. Eur J Immunol. 1993;23(9):2375–2378. doi: 10.1002/eji.1830230950. [DOI] [PubMed] [Google Scholar]
- Ferrucci L, Corsi A, et al. The origins of age-related proinflammatory state. Blood. 2005;105(6):2294–2299. doi: 10.1182/blood-2004-07-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrucci L, Harris TB, et al. Serum IL-6 level and the development of disability in older persons. J Am Geriatr Soc. 1999;47(6):639–646. doi: 10.1111/j.1532-5415.1999.tb01583.x. [DOI] [PubMed] [Google Scholar]
- Ferrucci L, Penninx BW, et al. Change in muscle strength explains accelerated decline of physical function in older women with high interleukin-6 serum levels. J Am Geriatr Soc. 2002;50(12):1947–1954. doi: 10.1046/j.1532-5415.2002.50605.x. [DOI] [PubMed] [Google Scholar]
- Ferrucci L, Semba RD, et al. Proinflammatory state, hepcidin, and anemia in older persons. Blood. 2010;115(18):3810–3816. doi: 10.1182/blood-2009-02-201087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festa A, D'Agostino R, Jr, et al. Elevated levels of acute-phase proteins and plasminogen activator inhibitor-1 predict the development of type 2 diabetes: the insulin resistance atherosclerosis study. Diabetes. 2002;51(4):1131–1137. doi: 10.2337/diabetes.51.4.1131. [DOI] [PubMed] [Google Scholar]
- Figaro MK, Kritchevsky SB, et al. Diabetes, inflammation, and functional decline in older adults: findings from the Health, Aging and Body Composition (ABC) study. Diabetes Care. 2006;29(9):2039–2045. doi: 10.2337/dc06-0245. [DOI] [PubMed] [Google Scholar]
- Fried LP, Tangen CM, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- Fried LP, Xue QL, et al. Nonlinear multisystem physiological dysregulation associated with frailty in older women: implications for etiology and treatment. J Gerontol A Biol Sci Med Sci. 2009;64(10):1049–1057. doi: 10.1093/gerona/glp076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried SK, Bunkin DA, et al. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab. 1998;83(3):847–850. doi: 10.1210/jcem.83.3.4660. [DOI] [PubMed] [Google Scholar]
- Fulop T, Larbi A, et al. Aging, frailty and age-related diseases. Biogerontology. 2010 doi: 10.1007/s10522-010-9287-2. [DOI] [PubMed] [Google Scholar]
- Geffken DF, Cushman M, et al. Association between physical activity and markers of inflammation in a healthy elderly population. Am J Epidemiol. 2001;153(3):242–250. doi: 10.1093/aje/153.3.242. [DOI] [PubMed] [Google Scholar]
- Goronzy JJ, Weyand CM. Cytokines in giant-cell arteritis. Cleve Clin J Med. 2002;69 Suppl 2:SII91–SII94. doi: 10.3949/ccjm.69.suppl_2.sii91. [DOI] [PubMed] [Google Scholar]
- Haider AW, Roubenoff R, et al. Monocyte cytokine production, systemic inflammation and cardiovascular disease in very elderly men and women: the Framingham Heart Study. Eur J Cardiovasc Prev Rehabil. 2004;11(3):214–215. doi: 10.1097/01.hjr.0000124212.21584.3c. [DOI] [PubMed] [Google Scholar]
- Harris TB, Ferrucci L, et al. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med. 1999;106(5):506–512. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- Heikkila K, Ebrahim S, et al. Systematic review of the association between circulating interleukin-6 (IL-6) and cancer. Eur J Cancer. 2008;44(7):937–945. doi: 10.1016/j.ejca.2008.02.047. [DOI] [PubMed] [Google Scholar]
- Heikkila K, Harris R, et al. Associations of circulating C-reactive protein and interleukin-6 with cancer risk: findings from two prospective cohorts and a meta-analysis. Cancer Causes Control. 2009;20(1):15–26. doi: 10.1007/s10552-008-9212-z. [DOI] [PubMed] [Google Scholar]
- Howren MB, Lamkin DM, et al. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71(2):171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- Hsu FC, Kritchevsky SB, et al. Association between inflammatory components and physical function in the health, aging, and body composition study: a principal component analysis approach. J Gerontol A Biol Sci Med Sci. 2009;64(5):581–589. doi: 10.1093/gerona/glp005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu FB, Meigs JB, et al. Inflammatory markers and risk of developing type 2 diabetes in women. Diabetes. 2004;53(3):693–700. doi: 10.2337/diabetes.53.3.693. [DOI] [PubMed] [Google Scholar]
- Hubbard RE, O'Mahony MS, et al. Inflammation and frailty measures in older people. J Cell Mol Med. 2009;13(9B):3103–3109. doi: 10.1111/j.1582-4934.2009.00733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Il'yasova D, Colbert LH, et al. Circulating levels of inflammatory markers and cancer risk in the health aging and body composition cohort. Cancer Epidemiol Biomarkers Prev. 2005;14(10):2413–2418. doi: 10.1158/1055-9965.EPI-05-0316. [DOI] [PubMed] [Google Scholar]
- Jenny NS, Yanez ND, et al. Inflammation biomarkers and near-term death in older men. Am J Epidemiol. 2007;165(6):684–695. doi: 10.1093/aje/kwk057. [DOI] [PubMed] [Google Scholar]
- Jilka RL, Hangoc G, et al. Increased osteoclast development after estrogen loss: mediation by interleukin-6. Science. 1992;257(5066):88–91. doi: 10.1126/science.1621100. [DOI] [PubMed] [Google Scholar]
- Kalogeropoulos A, Georgiopoulou V, et al. Inflammatory markers and incident heart failure risk in older adults: the Health ABC (Health, Aging, and Body Composition) study. J Am Coll Cardiol. 2010;55(19):2129–2137. doi: 10.1016/j.jacc.2009.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kania DM, Binkley N, et al. Elevated plasma levels of interleukin-6 in postmenopausal women do not correlate with bone density. J Am Geriatr Soc. 1995;43(3):236–239. doi: 10.1111/j.1532-5415.1995.tb07328.x. [DOI] [PubMed] [Google Scholar]
- Kapoor D, Clarke S, et al. The effect of testosterone replacement therapy on adipocytokines and C-reactive protein in hypogonadal men with type 2 diabetes. Eur J Endocrinol. 2007;156(5):595–602. doi: 10.1530/EJE-06-0737. [DOI] [PubMed] [Google Scholar]
- Kaul S, Morrissey RP, et al. By Jove! What is a clinician to make of JUPITER? Arch Intern Med. 2010;170(12):1073–1077. doi: 10.1001/archinternmed.2010.189. [DOI] [PubMed] [Google Scholar]
- Kern PA, Ranganathan S, et al. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab. 2001;280(5):E745–E751. doi: 10.1152/ajpendo.2001.280.5.E745. [DOI] [PubMed] [Google Scholar]
- Khosla S, Peterson JM, et al. Circulating cytokine levels in osteoporotic and normal women. J Clin Endocrinol Metab. 1994;79(3):707–711. doi: 10.1210/jcem.79.3.8077350. [DOI] [PubMed] [Google Scholar]
- Kim S, Keku TO, et al. Circulating levels of inflammatory cytokines and risk of colorectal adenomas. Cancer Res. 2008;68(1):323–328. doi: 10.1158/0008-5472.CAN-07-2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto T. IL-6: from its discovery to clinical applications. Int Immunol. 2010;22(5):347–352. doi: 10.1093/intimm/dxq030. [DOI] [PubMed] [Google Scholar]
- Klimiuk PA, Sierakowski S, et al. Interleukin-6, soluble interleukin-2 receptor and soluble interleukin-6 receptor in the sera of patients with different histological patterns of rheumatoid synovitis. Clin Exp Rheumatol. 2003;21(1):63–69. [PubMed] [Google Scholar]
- Lang T, Streeper T, et al. Sarcopenia: etiology, clinical consequences, intervention, and assessment. Osteoporos Int. 2010;21(4):543–559. doi: 10.1007/s00198-009-1059-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CC, Adler AI, et al. Association of C-reactive protein with type 2 diabetes: prospective analysis and meta-analysis. Diabetologia. 2009;52(6):1040–1047. doi: 10.1007/s00125-009-1338-3. [DOI] [PubMed] [Google Scholar]
- Lee CG, Carr MC, et al. Adipokines, inflammation, and visceral adiposity across the menopausal transition: a prospective study. J Clin Endocrinol Metab. 2009;94(4):1104–1110. doi: 10.1210/jc.2008-0701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P, Ridker PM, et al. Inflammation and atherosclerosis. Circulation. 2002;105(9):1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- Liu S, Tinker L, et al. A prospective study of inflammatory cytokines and diabetes mellitus in a multiethnic cohort of postmenopausal women. Arch Intern Med. 2007;167(15):1676–1685. doi: 10.1001/archinte.167.15.1676. [DOI] [PubMed] [Google Scholar]
- Lu H, Ouyang W, et al. Inflammation, a key event in cancer development. Mol Cancer Res. 2006;4(4):221–233. doi: 10.1158/1541-7786.MCR-05-0261. [DOI] [PubMed] [Google Scholar]
- Madhok R, Crilly A, et al. Serum interleukin 6 levels in rheumatoid arthritis: correlations with clinical and laboratory indices of disease activity. Ann Rheum Dis. 1993;52(3):232–234. doi: 10.1136/ard.52.3.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggio M, Basaria S, et al. Correlation between testosterone and the inflammatory marker soluble interleukin-6 receptor in older men. J Clin Endocrinol Metab. 2006;91(1):345–347. doi: 10.1210/jc.2005-1097. [DOI] [PubMed] [Google Scholar]
- Maggio M, Basaria S, et al. The relationship between testosterone and molecular markers of inflammation in older men. J Endocrinol Invest. 2005;28(11 Suppl Proceedings):116–119. [PubMed] [Google Scholar]
- Maggio M, Guralnik JM, et al. Interleukin-6 in aging and chronic disease: a magnificent pathway. J Gerontol A Biol Sci Med Sci. 2006;61(6):575–584. doi: 10.1093/gerona/61.6.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkin CJ, Pugh PJ, et al. The effect of testosterone replacement on endogenous inflammatory cytokines and lipid profiles in hypogonadal men. J Clin Endocrinol Metab. 2004;89(7):3313–3318. doi: 10.1210/jc.2003-031069. [DOI] [PubMed] [Google Scholar]
- Manolagas SC, Jilka RL. Bone marrow, cytokines, and bone remodeling. Emerging insights into the pathophysiology of osteoporosis. N Engl J Med. 1995;332(5):305–311. doi: 10.1056/NEJM199502023320506. [DOI] [PubMed] [Google Scholar]
- Martinez-Taboada VM, Alvarez L, et al. Giant cell arteritis and polymyalgia rheumatica: role of cytokines in the pathogenesis and implications for treatment. Cytokine. 2008;44(2):207–220. doi: 10.1016/j.cyto.2008.09.004. [DOI] [PubMed] [Google Scholar]
- McDermott MM, Guralnik JM, et al. Patterns of inflammation associated with peripheral arterial disease: the InCHIANTI study. Am Heart J. 2005;150(2):276–281. doi: 10.1016/j.ahj.2004.09.032. [DOI] [PubMed] [Google Scholar]
- Mohamed-Ali V, Goodrick S, et al. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J Clin Endocrinol Metab. 1997;82(12):4196–4200. doi: 10.1210/jcem.82.12.4450. [DOI] [PubMed] [Google Scholar]
- Montecucco F, Mach F. Update on statin-mediated anti-inflammatory activities in atherosclerosis. Semin Immunopathol. 2009;31(1):127–142. doi: 10.1007/s00281-009-0150-y. [DOI] [PubMed] [Google Scholar]
- Mora S, Glynn RJ, et al. Statins for the primary prevention of cardiovascular events in women with elevated high-sensitivity C-reactive protein or dyslipidemia: results from the Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER) and meta-analysis of women from primary prevention trials. Circulation. 2010;121(9):1069–1077. doi: 10.1161/CIRCULATIONAHA.109.906479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murabito JM, Keyes MJ, et al. Cross-sectional relations of multiple inflammatory biomarkers to peripheral arterial disease: The Framingham Offspring Study. Atherosclerosis. 2009;203(2):509–514. doi: 10.1016/j.atherosclerosis.2008.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nappo F, Esposito K, et al. Postprandial endothelial activation in healthy subjects and in type 2 diabetic patients: role of fat and carbohydrate meals. J Am Coll Cardiol. 2002;39(7):1145–1150. doi: 10.1016/s0735-1097(02)01741-2. [DOI] [PubMed] [Google Scholar]
- Nawawi H, Osman NS, et al. Soluble intercellular adhesion molecule-1 and interleukin-6 levels reflect endothelial dysfunction in patients with primary hypercholesterolaemia treated with atorvastatin. Atherosclerosis. 2003;169(2):283–291. doi: 10.1016/s0021-9150(03)00193-x. [DOI] [PubMed] [Google Scholar]
- Nelson HD, Humphrey LL, et al. Postmenopausal hormone replacement therapy: scientific review. JAMA. 2002;288(7):872–881. doi: 10.1001/jama.288.7.872. [DOI] [PubMed] [Google Scholar]
- Newman AB, Sachs MC, et al. Total and cause-specific mortality in the cardiovascular health study. J Gerontol A Biol Sci Med Sci. 2009;64(12):1251–1261. doi: 10.1093/gerona/glp127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicklas BJ, Hsu FC, et al. Exercise training and plasma C-reactive protein and interleukin-6 in elderly people. J Am Geriatr Soc. 2008;56(11):2045–2052. doi: 10.1111/j.1532-5415.2008.01994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passeri G, Girasole G, et al. Increased interleukin-6 production by murine bone marrow and bone cells after estrogen withdrawal. Endocrinology. 1993;133(2):822–828. doi: 10.1210/endo.133.2.8393776. [DOI] [PubMed] [Google Scholar]
- Payette H, Roubenoff R, et al. Insulin-like growth factor-1 and interleukin 6 predict sarcopenia in very old community-living men and women: the Framingham Heart Study. J Am Geriatr Soc. 2003;51(9):1237–1243. doi: 10.1046/j.1532-5415.2003.51407.x. [DOI] [PubMed] [Google Scholar]
- Pedersen BK, Steensberg A, et al. Exercise and interleukin-6. Curr Opin Hematol. 2001;8(3):137–141. doi: 10.1097/00062752-200105000-00002. [DOI] [PubMed] [Google Scholar]
- Penninx BW, Kritchevsky SB, et al. Inflammatory markers and incident mobility limitation in the elderly. J Am Geriatr Soc. 2004;52(7):1105–1113. doi: 10.1111/j.1532-5415.2004.52308.x. [DOI] [PubMed] [Google Scholar]
- Penninx BW, Kritchevsky SB, et al. Inflammatory markers and depressed mood in older persons: results from the Health, Aging and Body Composition study. Biol Psychiatry. 2003;54(5):566–572. doi: 10.1016/s0006-3223(02)01811-5. [DOI] [PubMed] [Google Scholar]
- Pfeilschifter J, Koditz R, et al. Changes in proinflammatory cytokine activity after menopause. Endocr Rev. 2002;23(1):90–119. doi: 10.1210/edrv.23.1.0456. [DOI] [PubMed] [Google Scholar]
- Pickup JC, Chusney GD, et al. Plasma interleukin-6, tumour necrosis factor alpha and blood cytokine production in type 2 diabetes. Life Sci. 2000;67(3):291–300. doi: 10.1016/s0024-3205(00)00622-6. [DOI] [PubMed] [Google Scholar]
- Pierce BL, Biggs ML, et al. C-reactive protein, interleukin-6, and prostate cancer risk in men aged 65 years and older. Cancer Causes Control. 2009;20(7):1193–1203. doi: 10.1007/s10552-009-9320-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pottratz ST, Bellido T, et al. 17 beta-Estradiol inhibits expression of human interleukin-6 promoter-reporter constructs by a receptor-dependent mechanism. J Clin Invest. 1994;93(3):944–950. doi: 10.1172/JCI117100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pou KM, Massaro JM, et al. Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: the Framingham Heart Study. Circulation. 2007;116(11):1234–1241. doi: 10.1161/CIRCULATIONAHA.107.710509. [DOI] [PubMed] [Google Scholar]
- Pradhan AD, Manson JE, et al. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286(3):327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- Pradhan AD, Manson JE, et al. Inflammatory biomarkers, hormone replacement therapy, and incident coronary heart disease: prospective analysis from the Women's Health Initiative observational study. JAMA. 2002;288(8):980–987. doi: 10.1001/jama.288.8.980. [DOI] [PubMed] [Google Scholar]
- Quist-Paulsen P. Statins and inflammation: an update. Curr Opin Cardiol. 2010;25(4):399–405. doi: 10.1097/HCO.0b013e3283398e53. [DOI] [PubMed] [Google Scholar]
- Ravaglia G, Forti P, et al. Blood inflammatory markers and risk of dementia: The Conselice Study of Brain Aging. Neurobiol Aging. 2007;28(12):1810–1820. doi: 10.1016/j.neurobiolaging.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Ray A, Prefontaine KE, et al. Down-modulation of interleukin-6 gene expression by 17 beta-estradiol in the absence of high affinity DNA binding by the estrogen receptor. J Biol Chem. 1994;269(17):12940–12946. [PubMed] [Google Scholar]
- Reinisch W, Gasche C, et al. Clinical relevance of serum interleukin-6 in Crohn's disease: single point measurements, therapy monitoring, and prediction of clinical relapse. Am J Gastroenterol. 1999;94(8):2156–2164. doi: 10.1111/j.1572-0241.1999.01288.x. [DOI] [PubMed] [Google Scholar]
- Reuben DB, Judd-Hamilton L, et al. The associations between physical activity and inflammatory markers in high-functioning older persons: MacArthur Studies of Successful Aging. J Am Geriatr Soc. 2003;51(8):1125–1130. doi: 10.1046/j.1532-5415.2003.51380.x. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Cushman M, et al. Plasma concentration of C-reactive protein and risk of developing peripheral vascular disease. Circulation. 1998;97(5):425–428. doi: 10.1161/01.cir.97.5.425. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Danielson E, et al. Reduction in C-reactive protein and LDL cholesterol and cardiovascular event rates after initiation of rosuvastatin: a prospective study of the JUPITER trial. Lancet. 2009;373(9670):1175–1182. doi: 10.1016/S0140-6736(09)60447-5. [DOI] [PubMed] [Google Scholar]
- Robak T, Gladalska A, et al. Serum levels of interleukin-6 type cytokines and soluble interleukin-6 receptor in patients with rheumatoid arthritis. Mediators Inflamm. 1998;7(5):347–353. doi: 10.1080/09629359890875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockwood K, Rockwood MR, et al. Physiological redundancy in older adults in relation to the change with age in the slope of a frailty index. J Am Geriatr Soc. 2010;58(2):318–323. doi: 10.1111/j.1532-5415.2009.02667.x. [DOI] [PubMed] [Google Scholar]
- Rodondi N, Marques-Vidal P, et al. Markers of atherosclerosis and inflammation for prediction of coronary heart disease in older adults. Am J Epidemiol. 2010;171(5):540–549. doi: 10.1093/aje/kwp428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roubenoff R, Harris TB, et al. Monocyte cytokine production in an elderly population: effect of age and inflammation. J Gerontol A Biol Sci Med Sci. 1998;53(1):M20–M26. doi: 10.1093/gerona/53a.1.m20. [DOI] [PubMed] [Google Scholar]
- Roubenoff R, Parise H, et al. Cytokines, insulin-like growth factor 1, sarcopenia, and mortality in very old community-dwelling men and women: the Framingham Heart Study. Am J Med. 2003;115(6):429–435. doi: 10.1016/j.amjmed.2003.05.001. [DOI] [PubMed] [Google Scholar]
- Sakamoto Y, Harada T, et al. Tumor necrosis factor-alpha-induced interleukin-8 (IL-8) expression in endometriotic stromal cells, probably through nuclear factor-kappa B activation: gonadotropin-releasing hormone agonist treatment reduced IL-8 expression. J Clin Endocrinol Metab. 2003;88(2):730–735. doi: 10.1210/jc.2002-020666. [DOI] [PubMed] [Google Scholar]
- Sawada M, Suzumura A, et al. TNF alpha induces IL-6 production by astrocytes but not by microglia. Brain Res. 1992;583(1–2):296–299. doi: 10.1016/s0006-8993(10)80037-x. [DOI] [PubMed] [Google Scholar]
- Schaap LA, Pluijm SM, et al. Higher inflammatory marker levels in older persons: associations with 5-year change in muscle mass and muscle strength. J Gerontol A Biol Sci Med Sci. 2009;64(11):1183–1189. doi: 10.1093/gerona/glp097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaap LA, Pluijm SM, et al. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am J Med. 2006;119(6):526. doi: 10.1016/j.amjmed.2005.10.049. e529-517. [DOI] [PubMed] [Google Scholar]
- Schmidt R, Schmidt H, et al. Early inflammation and dementia: a 25-year follow-up of the Honolulu-Asia Aging Study. Ann Neurol. 2002;52(2):168–174. doi: 10.1002/ana.10265. [DOI] [PubMed] [Google Scholar]
- Schrager MA, Metter EJ, et al. Sarcopenic obesity and inflammation in the InCHIANTI study. J Appl Physiol. 2007;102(3):919–925. doi: 10.1152/japplphysiol.00627.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemes C, Visser LE, et al. C-reactive protein levels, variation in the C-reactive protein gene, and cancer risk: the Rotterdam Study. J Clin Oncol. 2006;24(33):5216–5222. doi: 10.1200/JCO.2006.07.1381. [DOI] [PubMed] [Google Scholar]
- Stallmach A, Giese T, et al. Cytokine/chemokine transcript profiles reflect mucosal inflammation in Crohn's disease. Int J Colorectal Dis. 2004;19(4):308–315. doi: 10.1007/s00384-003-0554-4. [DOI] [PubMed] [Google Scholar]
- Stark JR, Li H, et al. Circulating prediagnostic interleukin-6 and C-reactive protein and prostate cancer incidence and mortality. Int J Cancer. 2009;124(11):2683–2689. doi: 10.1002/ijc.24241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steensberg A, van Hall G, et al. Production of interleukin-6 in contracting human skeletal muscles can account for the exercise-induced increase in plasma interleukin-6. J Physiol. 2000;529(Pt 1):237–242. doi: 10.1111/j.1469-7793.2000.00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Katz R, et al. Metabolic syndrome, inflammation, and incident heart failure in the elderly: the cardiovascular health study. Circ Heart Fail. 2008;1(4):242–248. doi: 10.1161/CIRCHEARTFAILURE.108.785485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taaffe DR, Harris TB, et al. Cross-sectional and prospective relationships of interleukin-6 and C-reactive protein with physical performance in elderly persons: MacArthur studies of successful aging. J Gerontol A Biol Sci Med Sci. 2000;55(12):M709–M715. doi: 10.1093/gerona/55.12.m709. [DOI] [PubMed] [Google Scholar]
- Thorand B, Lowel H, et al. C-reactive protein as a predictor for incident diabetes mellitus among middle-aged men: results from the MONICA Augsburg cohort study, 1984–1998. Arch Intern Med. 2003;163(1):93–99. doi: 10.1001/archinte.163.1.93. [DOI] [PubMed] [Google Scholar]
- Tiemeier H, Hofman A, et al. Inflammatory proteins and depression in the elderly. Epidemiology. 2003;14(1):103–107. doi: 10.1097/00001648-200301000-00025. [DOI] [PubMed] [Google Scholar]
- Tracy RP, Lemaitre RN, et al. Relationship of C-reactive protein to risk of cardiovascular disease in the elderly. Results from the Cardiovascular Health Study and the Rural Health Promotion Project. Arterioscler Thromb Vasc Biol. 1997;17(6):1121–1127. doi: 10.1161/01.atv.17.6.1121. [DOI] [PubMed] [Google Scholar]
- Trayhurn P, Wood IS. Signalling role of adipose tissue: adipokines and inflammation in obesity. Biochem Soc Trans. 2005;33(Pt 5):1078–1081. doi: 10.1042/BST0331078. [DOI] [PubMed] [Google Scholar]
- Tzoulaki I, Murray GD, et al. Inflammatory, haemostatic, and rheological markers for incident peripheral arterial disease: Edinburgh Artery Study. Eur Heart J. 2007;28(3):354–362. doi: 10.1093/eurheartj/ehl441. [DOI] [PubMed] [Google Scholar]
- Vasan RS, Sullivan LM, et al. Inflammatory markers and risk of heart failure in elderly subjects without prior myocardial infarction: the Framingham Heart Study. Circulation. 2003;107(11):1486–1491. doi: 10.1161/01.cir.0000057810.48709.f6. [DOI] [PubMed] [Google Scholar]
- Visser M, Pahor M, et al. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: the Health ABC Study. J Gerontol A Biol Sci Med Sci. 2002;57(5):M326–M332. doi: 10.1093/gerona/57.5.m326. [DOI] [PubMed] [Google Scholar]
- Walston JD, Matteini AM, et al. Inflammation and stress-related candidate genes, plasma interleukin-6 levels, and longevity in older adults. Exp Gerontol. 2009;44(5):350–355. doi: 10.1016/j.exger.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver JD, Huang MH, et al. Interleukin-6 and risk of cognitive decline: MacArthur studies of successful aging. Neurology. 2002;59(3):371–378. doi: 10.1212/wnl.59.3.371. [DOI] [PubMed] [Google Scholar]
- Wei J, Xu H, et al. Increase of plasma IL-6 concentration with age in healthy subjects. Life Sci. 1992;51(25):1953–1956. doi: 10.1016/0024-3205(92)90112-3. [DOI] [PubMed] [Google Scholar]
- Weisberg SP, McCann D, et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LM, Lali F, et al. Rac mediates TNF-induced cytokine production via modulation of NF-kappaB. Mol Immunol. 2008;45(9):2446–2454. doi: 10.1016/j.molimm.2007.12.011. [DOI] [PubMed] [Google Scholar]
- Xing Z, Gauldie J, et al. IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. J Clin Invest. 1998;101(2):311–320. doi: 10.1172/JCI1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Barnes GT, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112(12):1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K, Kanaya A, et al. The metabolic syndrome, inflammation, and risk of cognitive decline. JAMA. 2004;292(18):2237–2242. doi: 10.1001/jama.292.18.2237. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Lindquist K, et al. Inflammatory markers and cognition in well-functioning African-American and white elders. Neurology. 2003;61(1):76–80. doi: 10.1212/01.wnl.0000073620.42047.d7. [DOI] [PubMed] [Google Scholar]
- Zeyda M, Stulnig TM. Obesity, inflammation, and insulin resistance--a mini-review. Gerontology. 2009;55(4):379–386. doi: 10.1159/000212758. [DOI] [PubMed] [Google Scholar]
- Zhang SM, Buring JE, et al. C-reactive protein levels are not associated with increased risk for colorectal cancer in women. Ann Intern Med. 2005;142(6):425–432. doi: 10.7326/0003-4819-142-6-200503150-00008. [DOI] [PubMed] [Google Scholar]
- Zheng SX, Vrindts Y, et al. Increase in cytokine production (IL-1 beta, IL-6, TNF-alpha but not IFN-gamma, GM-CSF or LIF) by stimulated whole blood cells in postmenopausal osteoporosis. Maturitas. 1997;26(1):63–71. doi: 10.1016/s0378-5122(96)01080-8. [DOI] [PubMed] [Google Scholar]
- Zuliani G, Volpato S, et al. High interleukin-6 plasma levels are associated with low HDL-C levels in community-dwelling older adults: the InChianti study. Atherosclerosis. 2007;192(2):384–390. doi: 10.1016/j.atherosclerosis.2006.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]