Abstract
Cellular X chromosome mosaicism, which is unique to females may be advantageous during pathophysiological challenges as compared to the single X chromosome machinery of males and may also contribute to gender dimorphism in the inflammatory response. We tested the hypothesis whether cellular mosaicism for the X-linked gp91phox (NOX2) deficiency, the catalytic component of the superoxide anion generating NADPH oxidase complex, is advantageous during polymicrobial sepsis. Deficient, WT and heterozygous/mosaic mice were compared following polymicrobial sepsis initiated by cecal ligation and puncture. As compared to WT littermates, sepsis-induced mortality was improved in deficient mice, as well as, in mosaics animals carrying deficient and WT phagocyte subpopulations simultaneously. In contrast, blood bacterial counts were the highest in deficient mice as compared to mosaic or WT animals. Consistent with poor survival, WT mice also showed the worst organ damage following sepsis. In mosaic animals, the deficient neutrophil subpopulations displayed increased organ recruitment and elevated CD11b membrane expression as compared to WT neutrophil subpopulations within the same animal. The dynamics of sepsis-induced blood and organ cytokine content and white blood cell composition changes including lymphocyte subsets in blood and BM showed differences among WT, deficient and mosaic subjects indicating that mosaic mice are not simply the average of the deficient and WT responses. Upon oxidative burst, interchange of oxidants between WT and deficient neutrophil subpopulations occurred in mosaic mice. The study suggests that mice mosaic for gp91phox expression have multiple advantages in comparison to WT as well as deficient mice during the septic course.
Keywords: Gender, sex, X chromosome inactivation, X chromosome skewing, inflammation, polymorphonuclear, organ dysfunction, peritonitis, gp91phox
Introduction
Females show better general health and longer life span than males and they also present improved clinical course under a variety of conditions including infections (1–9). The currently held notion is that sex hormones are responsible for causing gender dimorphic pathophysiological responses and the female advantage in overall health. However, gender dimorphism in pathological processes is also present in young pre-pubertal children as well as in the elderly, which suggest that factors other than sex hormones are also involved (10–14). Recent work supports the possibility that the unique properties of the X-chromosome, including female X-chromosome inactivation and cellular mosaicism for X-linked polymorphic genes may also contribute to gender-associated differences in physiology, pathophysiology and disease progression (12;15–18)
The sex chromosomes differences between males and females not only represent altered gene content but also entail gender-specificity in X chromosome regulation and function. Males carry the Y chromosome passed on from the father and only one X chromosome inherited from the mother. In contrast, females carry two X chromosomes one inherited from the mother (Xm, maternal) one from the father (Xp, paternal). The potential double dose in the expression of X-linked proteins in females is compensated through random X chromosome inactivation in each individual cells, which is achieved by blocking gene expression in one of the parental X-chromosomes. Cellular X chromosome inactivation is complete at an early embryonic stage (19–21) and is maintained through the entire life span of each individual cell resulting in cellular mosaicism for all X-linked mutations. Thus, cellular mosaicism for X-linked polymorphic gene expression is unique to females.
Importantly, whereas the Y chromosome contains only a few genes involved in sex determination during embryonic development, the X chromosome carries a large number of polymorphic genes encoding for proteins with central functions in immunology, signaling, redox processes and metabolic systems (15;22), as well as it expresses polymorphic small regulatory RNAs with potential genome-wide effects (23). Therefore, females carry two cell populations with distinct parental haplotypes, which is expected to manifest differences in regulatory and functional potentials whereby broadening the regulatory and metabolic adaptability to changing environmental conditions.
The notion of the adaptability of mosaicism and its functional relevance is supported by the fact that elderly females frequently display cellular skewing which is characterized by increased representation of circulating blood cells expressing one of the parental X chromosomes. Sustained X chromosome skewing has also been observed in females heterozygous for severe X-linked deficiencies through selective pressure on progenitors (24–27). However, X chromosome mosaicism may also manifest acute skewing towards cell subpopulations that are advantageous in certain patho-physiological conditions through different degrees of gene expression, cell activation, proliferation, migration or apoptosis (15). In support of this hypothesis, we presented a proof of principal study (28) using the X-linked gp91phox deficient mouse strain, where we showed that during endotoxin shock heterozygous mosaic animals convey a phenotype, which is not simply the sum or average of deficient and WT responses. However, endotoxin shock is a fulminant inflammatory condition, which is rarely observed in patients. Thus, it still remains unknown whether female mosaicism could provide a clinical advantage during polymicrobial sepsis, which is a major cause of death in the critically ill. Therefore, in order to test the hypothesis whether female mosaicism represents an advantage during the septic response, we employed a gp91phox deficient mouse strain in combination with cecal ligation and puncture (CLP), which generates a clinical condition reminiscent to human peritonitis and systemic sepsis.
The X-linked gp91phox (NOX2) deficient mouse strain was selected because gp91phox is the catalytic subunit of the NADPH oxidase complex in phagocytes and this complex is an important component of antibacterial defense(29). Superoxide anion generated by gp91phox is dismutated into H2O2, which is utilized by myeloperoxidase to produce HOCl for bacterial killing. Thus, a deficiency for gp91phox is expected to modulate the inflammatory response either through altered bactericidal processes or altered phagocyte activation. Additionally, the deficiency is also expected to alleviate oxidative stress-induced tissue injury, which is an important component of organ dysfunction during sepsis. Thus, studying this X-linked deficient mouse provides the opportunity to test whether the simultaneous presence of normal and deficient cell populations in mosaic females confers an advantage during the septic course over WT or deficient responses. In order to control for the confounding effects of male sex hormone effects, all experiments were performed in female animals. We found that mosaic mice present a clinically improved phenotype with dual advantages in survival and bacterial clearance in comparison with WT or homozygous gp91phox deficient subjects.
Methods
Reagents
Endotoxin-free, cell culture grade buffers, media and reagents were used in the experiments. Fetal bovine serum (FBS) was purchased from Irvine Scientific, (Santa Ana, CA), protein assay kit from Pierce (Rockford, IL). Flourochrome conjugated antibodies, assay diluents, lysing and permeabilizing flow cytometry solutions and kits were purchased from BD Biosiences and BD Pharmingen. All other reagents and chemicals of the highest grade available were purchased from Sigma-Aldrich Co. (St. Louis, MO).
Animals and Cecal Ligation and Puncture (CLP)
Female gp91phox deficient (−/−), heterozygous (+/−) and normal littermate (WT, +/+) mice matched by age (10–16 weeks old, born within two weeks) were used in the experiments. Initial breeders were purchased from the Jackson Laboratory (B6.129S6-Cybbtm1Din/J and WT control C57BL/6). This NOX2 deficient strain is well established on C57BL/6 background. In order to control potential environmental differences between animal facilities, mice were bred at our facility to produce all genotypes including WT. Otherwise healthy, gp91phox deficient and WT animals are phenotypically indistinguishable including growth rate and size. Occasionally, older deficient animals may present chronic granulomatous disease (CGD) like symptoms (enlarged spleen, granuloma formation and marked leukocytosis). The rate of CGD is about 4–6% of hemizygous males, but is rarely observed in homozygous females and has not been found in heterozygous mosaics in our colony. The animals used in this current study displayed no CGD like symptoms. Animals were fed with standard rodent chow.
Polymicrobial septic peritonitis was induced using the cecal ligation and puncture (CLP) model as described earlier (30). Briefly, animals were anesthetized by a subcutaneous injection of Nembutal (5mg/100g bw). A midline abdominal incision was made. The cecum was exposed, ligated and punctured through opposing walls at two sites with a 20-gauge hypodermic needle. Animals were resuscitated by the subcutaneous injection of isotonic, pyrogen-free saline solution (0.025ml/g bw) immediately postoperatively, and also at 20h post-CLP. When animals were followed for longer than 24 hours they received daily saline resuscitations at the same dose. In pilot experiment we compared naïve controls and sham operations (opening the abdomen, moving the intestine but no ligation or puncture) and found no remarkable increase in inflammatory markers (31). Thus, in selected experimental components we used non-treated naïve animals as controls.
The studies were performed in accordance with the Guide for the Care and Use of Laboratory Animals [DHHS Publication No. (NIH) 85–23, Revised 1985, Office of Science and Health Reports, DRR/NIH, Bethesda, MD 20205] and were approved by the Institutional Animal Care and Use Committee of the New Jersey Medical School.
Genotyping
The gp91phox gene in the mutant mouse has a PGKneo gene inserted in exon 3 (32). Total genomic DNA was isolated from tail clippings using the REDExtract-N-Amp Tissue PCR kit (Sigma-Aldrich). DNA was subjected to PCR amplification using forward primers complementary to the PGKneo insert or WT sequences, respectively, and a common downstream primer. Forward primers WT: 5’AAGAGAAACTCCTCTGCTGTGAA3’; Deficient:5’GTTCTAATTCCATCAGAAGCTTATCG 3’; Common reverse primer: 5’ CGCACTGGAACCCCTGAGAAAGG 3’. Gradient PCR reaction was carried out in the presence of 2 mM MgCl2 with the following cycling: 94°C, 3min., 12 cycles of 94°C, 20 sec., 64°C, 30 sec., (0.5C decrease per cycle) 72°C, 35 sec., followed by 25 cycles of 94°C, 20 sec., 58°C, 30 sec., 72°C, 35 sec. with final elongation of 72°C for 2min. PCR amplicons were resolved on 3% agarose gels. WT reaction produces a 240 bp, deficient a 195 bp product, whereas heterozygous samples present both amplicons.
Blood, splenocyte and bone marrow (BM) cell isolation and incubations
Blood was collected into heparinized tubes via cardiac puncture from fully anesthetized animals. Following the exsanguination, femurs were collected from the same animals. Femurs were cut at the diaphyses and BM cells flushed out by repeated injections of phosphate buffered saline (PBS) containing 10 % FBS through the bone channel. BM cells were sedimented and washed by centrifugation and suspended in a final volume to obtain 10-million/ml cells in the same PBS/FBS buffer. Next, the spleen was removed and placed into DMEM containing 10% FBS and penicillin streptomycin solution. Hypodermic needles were used to pull apart the splenic capsule releasing spleen cells into suspension. The cell suspension together with the remaining splenic capsule was squeezed through a 70µm nylon mesh cell strainer. Isolated bone marrow cells or splenocytes were resuspended in DMEM containing 1 % FBS for subsequent analyses or in vitro incubations.
Flow cytometry
BM, blood and spleen flow cytometry analyses and gating strategy has been described in detail previously (33). Briefly, the number of PMNs and lymphocyte subsets in blood and spleen was determinred by the number of total cell counts and the percent distribution of CD3+CD4+, CD3+CD8+ T-cells, CD19+ B-cells and CD11b+ myeloid cells using antibodies against CD markers conjugated with FITC, APC, PERCP or PE (BD Biosciences) in three or four-color incubations. BM cell composition was determined by the cell distribution of CD45+CD19+CD11b− (B-cells), CD11b+CD45+CD19− (myeloid cells). Aliquots of 0.1 ml whole blood, splenocyte or BM cell suspension were incubated with the respective markers for 15 min followed by incubation with BD FACS lysing solution (BD Biosciences) for 7 min at 37°C. Cells were washed twice with BD FACS wash buffer and then fixed with 1% methanol free formaldehyde. FACS acquisitions were performed in a centralized flow cytometry facility. At least 30,000 events were collected for each analysis.
Mosaic PMN subpopulations in blood and tissue samples (whole blood, spleen, BM and peritoneal lavage) from heterozygous mice were identified with anti-mouse gp91phox monoclonal antibody (BD Transduction Laboratories) using the BD “PhosphoFlow” protocol with some modifications and combined with secondary anti-IgG incubations. Cell suspensions (0.1 ml) were incubated with BD Phospho lyse/fix Buffer [BD Pharmingen] at 37°C for 10 min. Cells were washed using PBS and centrifugal sedimentation (300 × g, 5min) which was followed by permeabilization by BD PhoshoFlow Perm Buffer for 30 min. Cells were washed twice with BD Pharmingen stain buffer and then incubated with CD11b-PERCP and purified mouse anti Gp91phox monoclonal antibody at room temperature for 30 min in the dark. Cells were then washed with stain buffer and incubated with PE-conjugated rat anti-mouse IgG1 antibody [BD Pharmingen] for 20 min. Finally, cells were washed twice with stain buffer and analyzed by flow cytometry. Prior to initiating the studies with heterozygous mice, the gp91phox staining method were validated first by parallel incubations with isotype IgG and gp91phox antibody. Validation tests were also carried out by performing the staining protocols on cells from WT and deficient animals, respectively, as well as, on mixed cell suspensions in parallel with mosaic samples. Beside neutrophils, monocytes and macrophages are also positive for CD11b. Pilot studies using macrophage markers and alternative gating showed negligible presence of macrophages or monocytes within gates used for sampling neutrophils.
Blood differentials and cell counts in BM and spleen were determined using a computerized cell counter (Hematrue Veterinary Hematology Analyzer, Heska Corporation, Loveland, CO).
ELISAs, enzyme assays and determination of blood bacterial counts
ELISA kits for IL-10 (CAT # 555252), IL-6 (CAT # 555240), and MCP-1 (CAT # 555260) were purchased from BD biosciences (San Jose, CA), Mip-2 (Dy452E) from R&D Systems (Minneapolis, MN). Plasma from freshly drawn heparinized blood was stored at −85°C till analysis. ELISAS were performed according to the manufacturer’s protocol. All the compared samples from different genotypes were run simultaneously in duplicates on one plate. Values were determined from a calibration curve run parallel with the samples.
MPO activity was measured in frozen tissues homogenized in modified RIPA buffer containing 50mM Tris-HCl, 1% NP-40, 0.25% sodium deoxycholate, 150 mM NaCl, 1mM EDTA, 1mM PMSF, 1 mM NaVO3 and 1mM NaF, pH 7.4. The homogenate was centrifuged at 13400×g for 10 min at 4°C.The supernatant was collected and stored in −80°C. MPO activity was measured as described earlier (34). MPO activity in tissue samples was determined using human leukocyte MPO as a standard (6908 5U/ml from SIGMA-Aldrich) and expressed as units of MPO /mg protein.
Plasma concentrations of lactate dehydrogenase (LDH), aspartate aminotransferase (AST), alanine aminotransferase (ALT), blood urea nitrogen (BUN) and creatine phosphokinase (CK) levels were analyzed using a clinical chemistry analyzer system (VetTest8008, IDEXX Laboratories).
Bacterial counts were determined from a 0.1-ml blood sample collected under sterile conditions and diluted serially in sterile physiological saline. 0.05ml of each dilution was aseptically plated on trypticase blood agar plates (BD Biosciences, San Jose, CA) and after 24h incubation at 37 °C, the number of bacterial colonies was counted.
Statistical analysis
Statistical calculations were performed using JMP software (SAS Institute Inc., Cary, NC). Results were analyzed using ANOVA followed by t-test for pair-wise comparisons or Tukey-Kramer’s test for multiple comparisons. We used the Long Rank Test to assess survival differences among groups. Different study components were performed on 6–8 different animals from each of the in vivo treatment groups unless indicated otherwise. Significant difference was concluded at p<0.05 unless otherwise noted.
Results
Evidence of neutrophil mosaicism for gp91phox expression
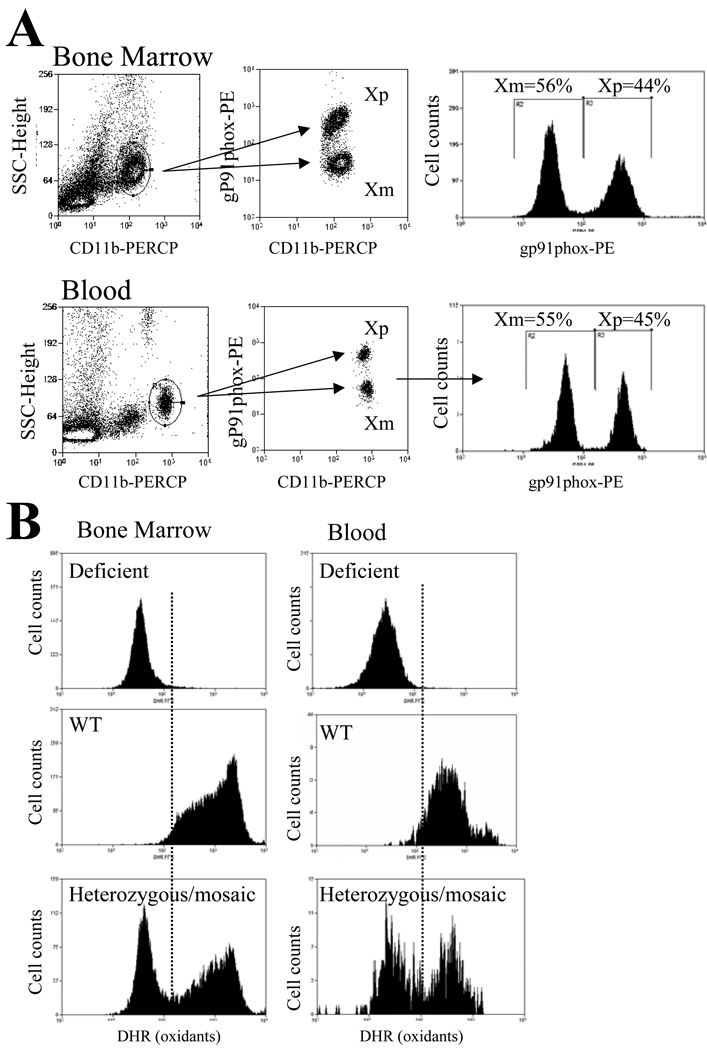
Fig1 presents the flow cytometry method used for tracking mosaic neutrophil subpopulations. Bone marrow or blood neutrophils were stained and gated by CD11b marker and forward scatter. CD11b staining identified a well-defined cell population (Fig 1A left panels). However, simultaneous staining for gp91phox expression resulted in two cell populations with respective low and high staining for gp91phox expression in heterozygous mosaic mice consistent with the presence of mosaic subsets (Fig 1A middle panels). On average, the distribution of the WT and deficient mosaic subpopulations was about 50-50% in blood as well as BM (Fig1A right panels). Gp91phox staining intensities for the two mosaic subpopulations corresponded well with the respective staining intensities of deficient and WT samples (not shown).
Fig 1. Mosaic neutrophil subpopulations in mice heterozygous for gp91phox deficiency.
Part A: Bone marrow cell suspension or whole blood from mice heterozygous for gp91phox deficiency was processed according to the BD phospho-lyse-fix-Perm-III buffer protocol. Samples were incubated with anti-CD11b-PERCP as well as with either anti-gp91phox or the corresponding isotype-IgG followed by incubation with PE-conjugated secondary antibody as described in the materials and methods section. As shown, the well-defined CD11b positive cell population (left panels) separated into two cell populations by the level of gp91phox expression (middle panels). The histograms on the right indicate that the ratio of Xp and Xm expressing mosaic neutrophil subpopulations was approximately one.
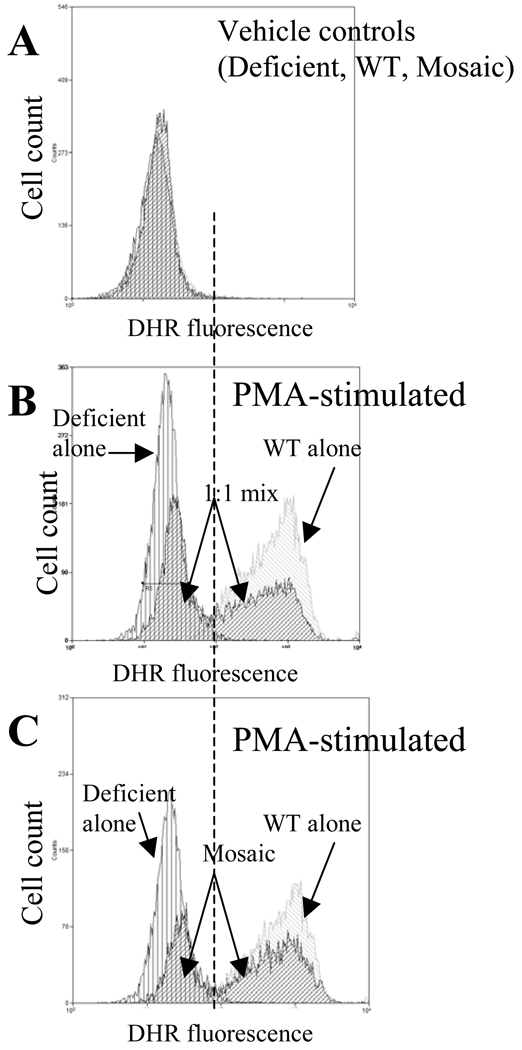
Part B: Bone marrow (left panels) or WBC (right panels) was preincubated with DHR for 20 min followed by the incubation with 1uM phorbol–myristate-acetate (PMA) for 15 min. CD11b positive myeloid cells were gated and analyzed for DHR fluorescence in deficient (top row), WT (middle row) and heterozygous mosaic animals (bottom row). As shown, in WT animals PMA resulted in a marked response whereas in deficient samples there was no increase in DHR fluorescence. In mosaic animals, the presence of two-cell population was evident and the oxidative burst response of mosaic subpopulations corresponded well with the deficient and WT responses, respectively. Representative findings from several experiments with similar observations are shown.
In order to provide additional evidence for the presence of mosaic subpopulations with functional differences in heterozygous animals, blood or BM neutrophils were also stained with dihydrorhodamine (DHR), which is an oxidant sensitive fluorescent marker. Fig 1 B shows that upon initiating oxidative burst by the addition of phorbol myrystate acetate (PMA), homozygous deficient cells showed no response whereas WT presented a marked increase in cellular oxidant content (Fig1B top and middle panels). In contrast, heterozygous animals showed two cell populations one without and one with a marked increase in cell oxidants with corresponding staining intensities to deficient and WT samples (Fig1B bottom panels).
Improved survival and bacterial clearance in septic mosaic animals
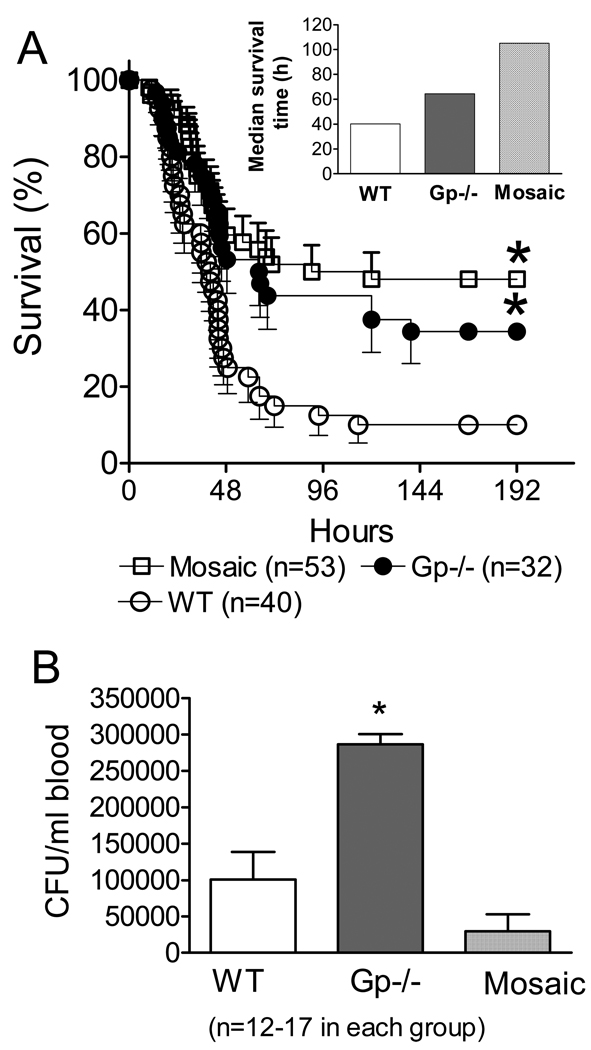
WT, homozygous deficient and mosaic animals were subjected to CLP followed by postoperative and daily fluid resuscitation and mortality was recorded for 8 days (Fig 2A). WT animals showed the highest mortality with a 10% overall survival and 40h median survival time (Fig 2 and insert). Mosaic and deficient animals showed statistically improved survival after CLP compared to WT with 40% (deficient) and 50% (mosaic) overall survival (Fig 2). The longest median survival time (100h) was observed in mosaic subjects. Mean survival time of WT animals was 40 h whereas deficient animals showed an intermediary value (65h) between WT and mosaic mice (Fig 2A insert).
Fig 2. NOX2 mosaicism increases survival and improves bacterial clearance in polymicrobial sepsis.
Part A: WT, gp91phox deficient and gp91phox mosaic animals were made septic by cecal ligation and puncture (CLP). Animals received fluid resuscitation postoperatively and then repeatedly at every 24h and were observed for mortality. Mosaic and deficient animals showed improved survival as compared to WT. *Statistically significant difference compared to WT (Long Rank Test, n=32–53 in individual groups, as indicated).
Part B: In a separate set of experiments, animals were subjected to CLP and resuscitation and 24h later bacterial counts in blood were determined. Deficient animals showed the greatest bacterial counts following sepsis. *Statistically significant difference compared to mosaic animals (Mean ± S.E.M., n=12–17 in each group).
An important component of resolving inflammation is clearing invading bacteria. Thus, in a separate set of experiments, we also tested blood bacterial counts 24h post-CLP (Fig 2B). Deficient subjects showed the greatest numbers of circulating bacteria after CLP, whereas bacterial counts were similar in WT and mosaic animals (Fig 2B).
Sepsis-induced cell composition changes in blood and BM
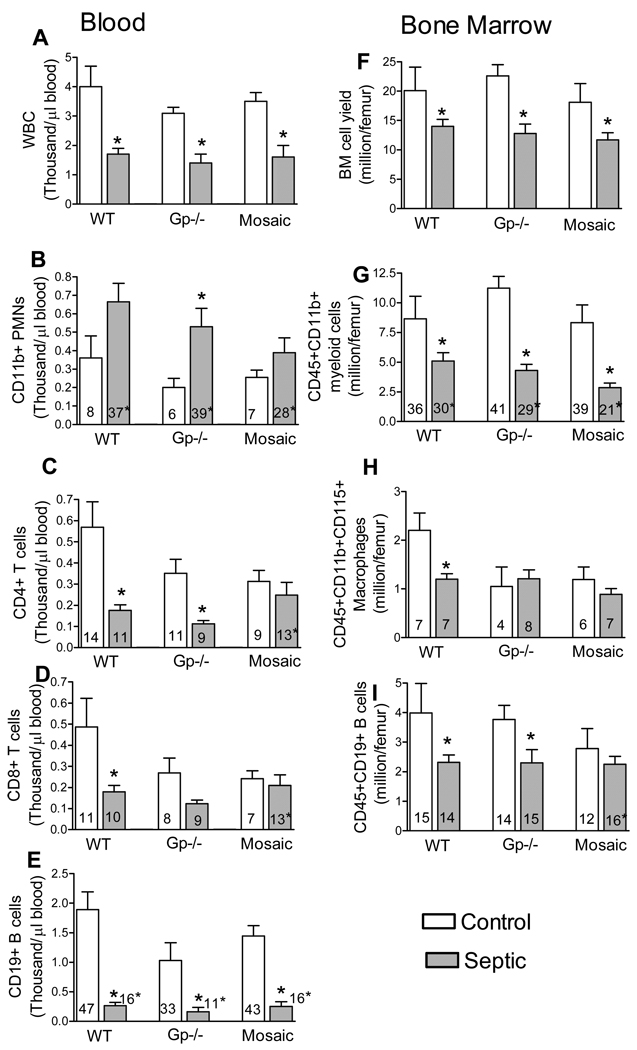
Because differences in redox status are expected to alter cell activation and associated trafficking we tested CLP-induced changes in cell composition in blood and BM (Fig 3). Sepsis depleted circulating WBCs as well as decreased the cellularity of BM (Fig 3 A,F). Deficient subjects showed the greatest relative increase in circulating blood neutrophil numbers (Fig 3B) whereas the decrease in BM myeloid cells was similar among all genotypes (Fig 3G). Interestingly, sepsis did not result in decrease in circulating CD4 or CD8 T-cells from mosaic subjects but caused a significant T cell depletion in WT and deficient mice (Fig 3 C,D). Circulating B-cells were decreased similarly in all three genotypes (Fig 3E). In the BM, sepsis depleted B cell numbers in WT and deficient subject but caused no decrease in mosaic animals (Fig 3I). Sepsis decreased BM macrophage numbers in WT mice but had no effects in deficient and mosaic subjects (Fig 3H).
Fig 3. Sepsis-induced cell composition changes in blood and BM.
From control or CLP-subjected mice, the total numbers of major WBC subtypes as well as percent distribution of cells were determined. Bars represent absolute cell numbers calculated from cell counts and percent distributions, whereas numbers within bars depict values as percent of total cells. BM lymphoid line was identified by double positive staining for CD45 and CD19, neutrophils for CD45, CD11b whereas macrophages by triple staining for CD45, CD11b and CD115. Cell composition in circulating blood was determined by CD11b straining for neutrophils and CD19 for B-cells. Major distribution of helper and cytotoxic T cells were determined by CD3/CD4 or CD3/CD8 dual staining, respectively. *Statistically significant difference between septic and control within the same genotype. Mean ± S.E.M., n=7–8 animals in each group.
Skewed mosaic neutrophil content in blood and spleen following sepsis
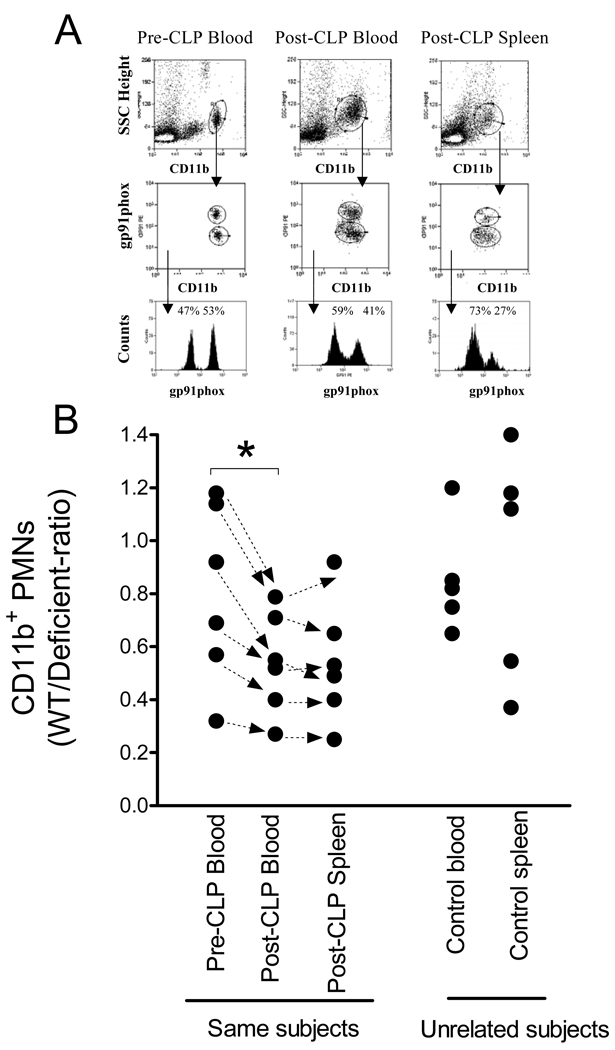
The increase in blood neutrophil numbers in deficient subjects suggested that this might alter phagocyte tissue infiltration. Therefore, we tested in mosaic animals whether the deficient subpopulations respond differently from that of WT subpopulations following sepsis using blood and splenic neutrophil contents as markers. Because untreated mosaic animals show individual variability in the baseline WT/deficient neutrophil ratios (28) we sampled blood from the same animals two days prior and 24h after CLP (Fig 4). The WT/Deficient ratios in the spleen were also determined in these animals after CLP. This approach provided the possibility to test skewing within an individual subject independent of the initial WT/deficient-ratio of circulating neutrophils. As shown naïve circulating neutrophils showed WT/deficient-ratios between 0.4–1.2 in this set of mosaic animals (Fig4B, Pre-CLP blood). However, this ratio decreased after CLP in each individual animal indicating a relative increase in the number of circulating deficient neutrophils (Pre-CLP blood vs. Post-CLP blood). Within the same animal, the WT/deficient-ratio in blood and spleen was similar after sepsis (Post-CLP blood vs. Post-CLP Spleen). Because spleen analysis before and after CLP in the same animal is not feasible we also depict WT/deficient ratios in spleen and blood from an independent control sample (Fig 4B, Unrelated subjects).
Fig 4. Sepsis skews neutrophil ratios towards deficient subpopulation in blood and spleen in mosaic subjects.
Blood was sampled 2 days before CLP and tested for WT/deficient-ratio of CD11b neutrophils by gp91phox expression or lack of. Subsequently, blood and spleen was harvested from the same animals 24h pot-CLP and the analyses repeated. Part A shows a typical flow analysis from an animal. Part B indicates the WT/Deficient-ratios of individual animals. (Arrows connect values derived from the same mouse). Sepsis caused a statistically significant decrease in the WT/deficient ratio of blood neutrophils (p=0.004, Pre-CLP blood versus Post-CLP blood). After CLP, the WT/Deficient-ratios in blood and spleen were similar in the same subject after CLP (Post-CLP Blood versus Post-CLP spleen). Because splenocytes cannot be obtained for analyses before CLP we also depict WT/Deficient neutrophil ratios in spleen and blood from an independent sample of controls (Unrelated subjects).
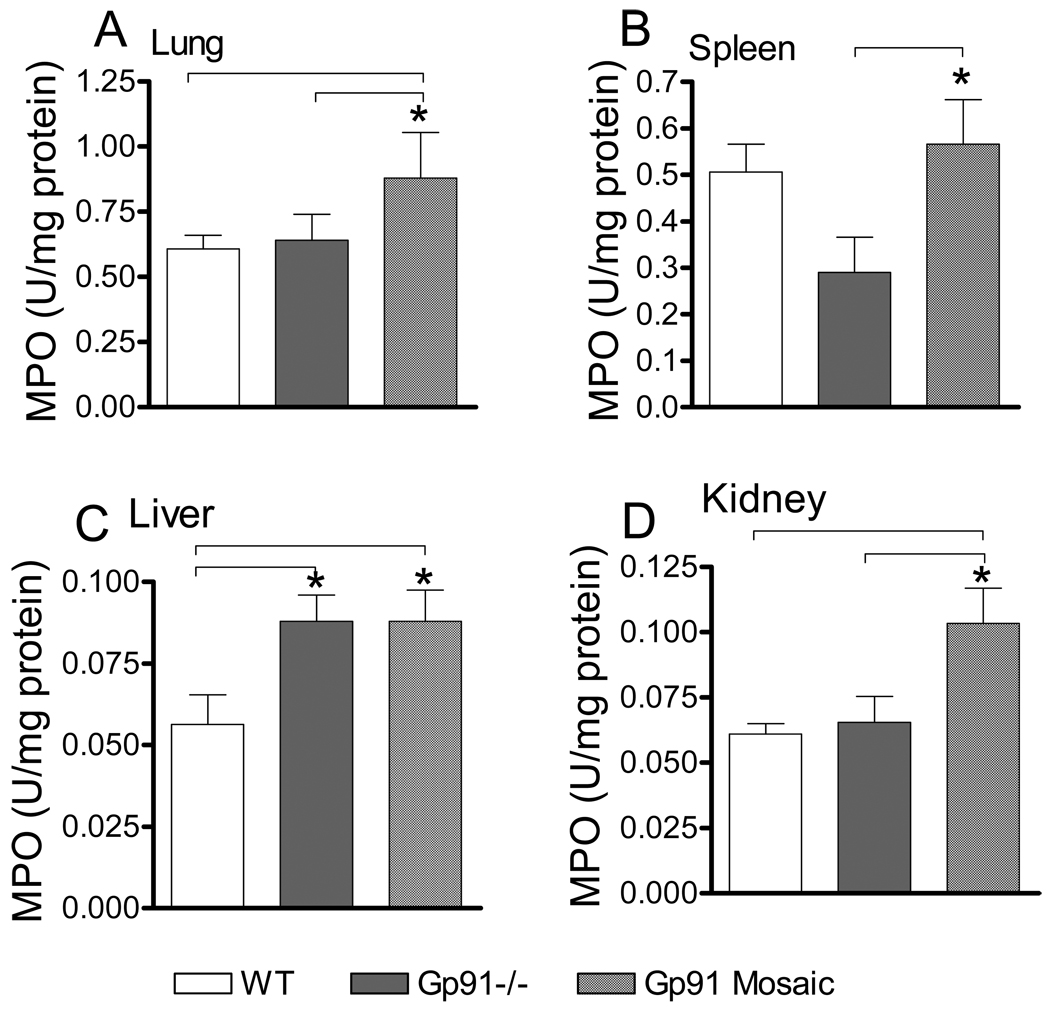
To further gain insight in the status of neutrophil infiltration, we determined MPO activities in various organs, which are known sites of neutrophil accumulation during inflammation. Fig 5 shows that following CLP, neutrophil accumulation was greater in lung and kidney from mosaic animals as compared to WT or deficient subjects. Additionally, whereas liver MPO was increased similarly in mosaic and deficient subjects, splenic MPO was elevated in mosaic animals as compared to deficient mice.
Fig 5. Increased neutrophil tissue infiltration following sepsis in mosaic animals.
Myeloperoxidase (MPO) activity in organs represents a marker for phagocyte infiltration. 24h after CLP animals were sacrificed and MPO activity in lung, spleen, liver and kidney homogenates were determined as described in the materials and methods section. *Statistically significant difference (p<0.05) compared to group as indicated by lines. Mean ± S.E.M., n=6–8 animals in each group.
Elevated CD11b expression in deficient mosaics subsets
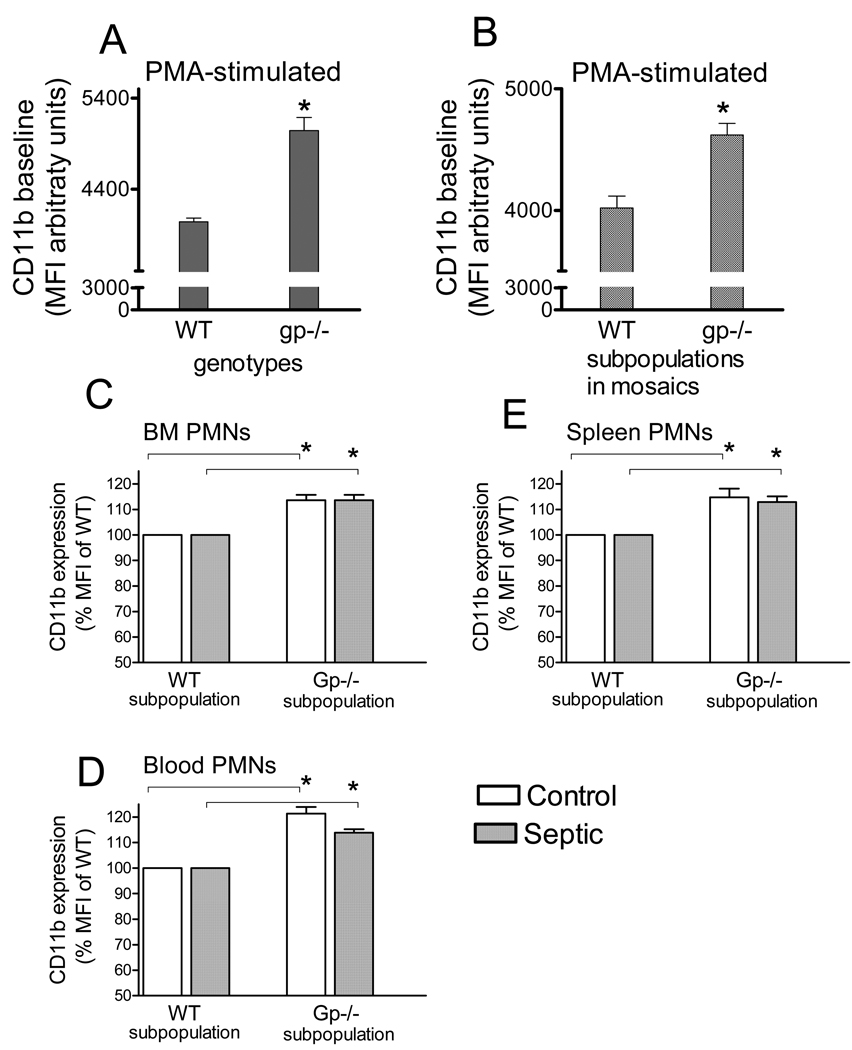
Membrane expression of CD11b is one of the markers of the adhesive potential in neutrophils. Thus, we compared the CD11b membrane expression levels in mosaic subsets separated by gp91phox or DHR staining. PMA treatment caused a 100-fold increase in CD11b membrane expression in neutrophils as compared to non-stimulated controls (not shown). Under these activated conditions, CD11b content was statistically increased in cells from deficient mice compared to WT. CD11b expression was also increased in the deficient subpopulation as compared to WT subpopulation within mosaic animals (Fig 6 A,B). The elevated membrane expression by deficient neutrophil subpopulations as compared to WT subpopulations was evident in blood, BM and spleen from mosaic mice under naïve and septic conditions (Fig 6C,D,E).
Fig 6. Elevated neutrophil CD11b expression in the deficient subpopulations of mosaic animals.
Myeloid cells from bone marrow of WT and deficient animals (A) or mosaic mice (B) were stained for CD11b under PMA-stimulated conditions (AB). Deficient neutrophils or deficient subsets from mosaics showed elevated CD11b expression as compared to WT. In a separate set of experiments neutrophil CD11b membrane expression was also determined in naïve and septic animals from BM, spleen and blood. *Statistically significant difference (p<0.05) as compared to WT. Mean ± S.E.M., n=6–7 animals in each group.
Sepsis induced cytokine response and organ dysfunction in mosaic animals
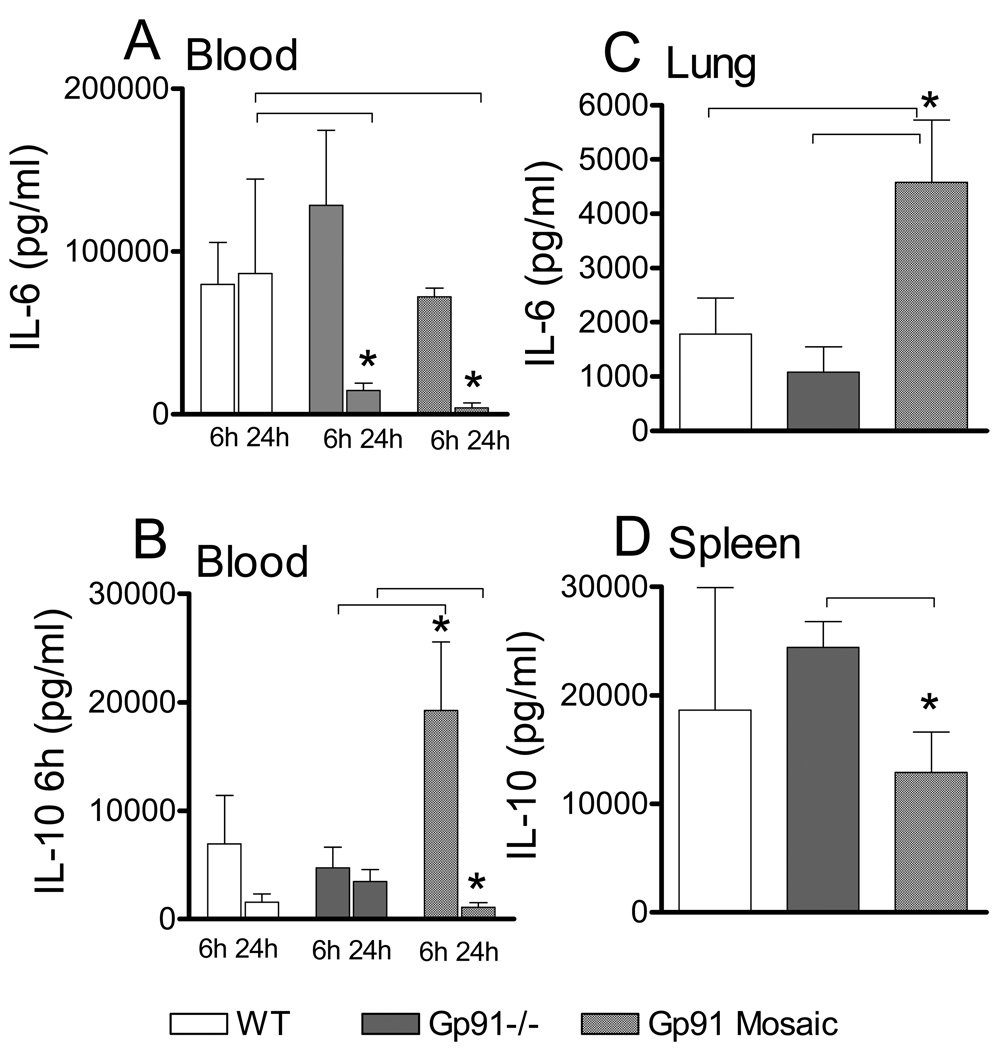
The differences in survival and bacterial clearance among the tested gp91phox genotypes imply differences in the overall course of the inflammatory response. To confirm this, first we compared blood and organ contents of IL-6 and IL-10, which are critically important in orchestrating the septic course. Fig 7 indicates that blood IL-6 at 6h post-CLP was similar in WT, deficient and mosaic subjects however at 24h, blood IL-6 was lower in mosaic and deficient animals than WT (Fig 7A). Blood IL-10 at 6h after CLP was greater in mosaic animals than WT or deficient subjects, whereas at 24h it was similarly low in all mice Fig 7B). In a mirror image with the blood findings, lung IL-6 at 24h post-CLP was increased in mosaic subjects compared to WT or deficient (Fig 7C), whereas splenic IL-10 content showed slightly decreased IL-10 in mosaic animals compared to deficient. IL-6 and IL-10 levels in liver and kidney were not statistically different among genotypes (not shown).
Fig 7. Cytokine levels indicate an altered inflammatory course in mosaic animals as compared to either WT or deficient mice.
Blood was collected at 6h and 24h after CLP and also from lung and spleen 24h after CLP. Cytokine content was determined using ELISA as described in the materials and methods section. Mean ± S.E.M., n=5–8 animals in each group and time. *Statistically significant difference (p<0.05) as indicated by lines.
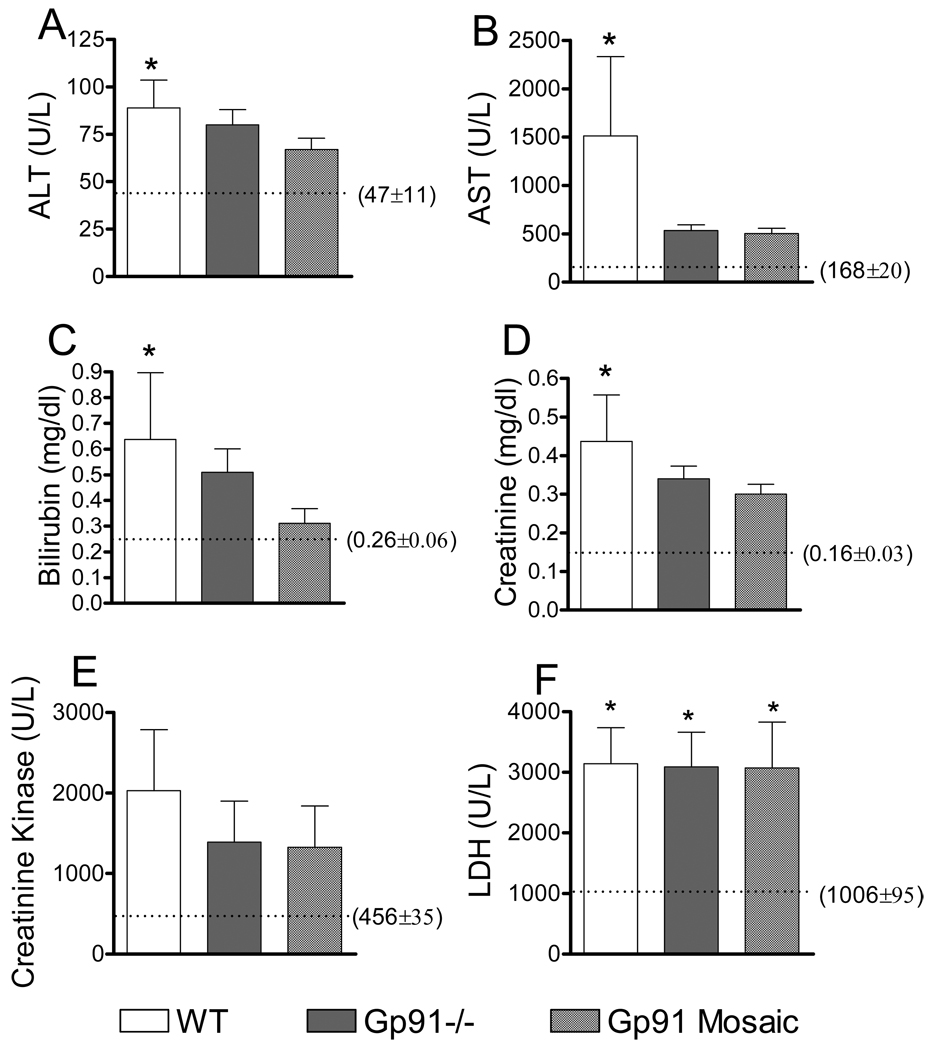
We also determined a set of blood organ dysfunction markers at 24h post-CLP (Fig 8). ALT, AST, billirubin and creatinine concentrations, which are indicators of liver and kidney dysfunction were the most elevated in WT animals consistent with poor WT survival. However, statistical differences were reached only at the p<0.1 levels between WT and unchallenged controls. The slight increases in ALT,AST, bilirubin and creatinine in septic deficient or mosaic animals as compared to controls did not reach statistically significant levels. Creatinine kinase and LDH activity levels, which are indicative of muscle and metabolic dysfunction, were similar among genotypes.
Fig 8. Worsened tissue dysfunction in WT subjects following sepsis.
Blood was collected 24h after CLP and markers of organ dysfunction were measured as described in the material and methods section. Mean ± S.E.M., n=6–8 animals of each genotype in the septic group and n=3 in controls. Control levels are shown with the dotted line with numeric values in brackets. *Statistically significant difference as compared to control (p<0.1).
Intercellular communication between mosaic neutrophil subpopulations
Cellular interplay through reactive oxidant species (ROS) between WT and deficient neutrophil subpopulations may represent a functional advantage in mosaic animals. For example, ROS released during a respiratory burst from the WT subpopulation could reach the NOX2 deficient subpopulation and processed through the downstream enzymatic machinery, which could partially restore redox dependent cellular responses. To test whether oxidant interchange could occur between WT and deficient neutrophils, we measured PMA-induced oxidant content in WT and deficient cells, respectively, as well as, in their experimental 1:1 cell mixtures. In parallel, we compared “natural mixtures” WT and deficient cells obtained from mosaic animals. Under non-stimulated conditions DHR staining was similarly low in deficient, WT and mosaic cells (Fig 9A). After PMA stimulation, when deficient or WT cells were incubated separately, cellular oxidant content was increased in WT but not in deficient samples (Fig 9B vs. Fig9A). However, mixing deficient and WT cells in a 1:1-ratio before PMA stimulation resulted in a slightly increased oxidant content in deficient cells (Fig 9B captions). In mosaic samples, the increase in oxidant content within deficient subpopulation was also observed, which was similar to that of deficient cells observed in the artificial cell mixtures (Fig 9B vs. Fig9C).
Fig 9. Cross talk through ROS between mosaic neutrophil subpopulations.
Part A indicates basal DHR staining in resting neutrophils from WT, deficient and mosaic animals after the administration of vehicle. Part B depicts PMA-induced responses in WT cells alone (marked increase), deficient cells alone (no response) and also in the “artificially mixed” 1:1-ratio of the same deficient and WT cells. PMA stimulation of “artificially mixed” sample resulted in a detectable right shift in fluorescence in deficient cells indicating that oxidants released from WT cells reached the intracellular site of deficient cells. Part C compares the response of a “naturally mixed” mosaic specimen to that of WT and deficient cells, respectively. The right shift by the deficient subpopulation in “naturally mixed” mosaic samples was similar to that observed in the “artificially mixed” sample (Compare parts B and C). The histograms are representative findings from several experiments with similar observations.
Discussion
It is currently a widely held notion that the female advantage over males in health status and clinical outcomes from critically ill conditions is associated with differences in sex hormone milieu. In general, studies have indicated beneficial effects of estrogens and harmful impacts of testosterones and their derivatives (1–9). Likewise, it is well accepted that the gender dimorphic character of the innate immune response or clinical outcome from blood loss and severe injuries is also related to sex hormone effects. However it is surprising that in spite of the fact that the X chromosome displays major differences between males and females in X-linked gene variability, cellular content and regulation, it has not been thoroughly investigated whether female X chromosome mosaicism for parental haplotypes contributes to gender dimorphic physiology or the female advantage (12;15–18). Moreover, whereas multiple studies suggested the potential role of X-chromosome causing gender bias in autoimmune diseases (12;35), it has not been investigated whether X chromosome mosaicism could also impact the non-specific innate immune response. Therefore, our current study represents the first attempt to provide supporting evidence in the broader context of inflammation and indicates that female X chromosome mosaicism could affect the innate immune response with beneficial clinical outcome. Using mosaic animals for X-linked gp91phox expression, we demonstrate for the first time that the simultaneous presence of mosaic phagocyte subpopulations with or without the capacity to produce an oxidative burst is clinically advantageous during severe polymicrobial sepsis.
This clinical benefit in mosaics was manifested despite the fact that, conceptually, the presence of deficient cells could be either advantageous through lessened oxidative stress or disadvantageous through compromised bacterial clearance. A priori, it could also be speculated that mosaicism would simply reflect an intermediary condition between WT and deficient status by the 50-50 % representation of WT and deficient phagocytes in mosaic mice. However, in contrast with these presumptions, our data indicate that gp91phox mosaicism presents its own phenotype with uniquely modulated phagocyte responses presumably due to intercellular communications and feed back mechanisms between WT and deficient subpopulations and a related modulation of the systemic inflammatory response.
Consistent with these propositions, whereas bacterial killing was more efficient in mosaic and WT animals as compared to gp91phox deficient mice, sepsis-induced mortality was worst in WT mice meanwhile deficient and mosaic mice showed similarly improved survival. These observations indicate that excessive neutrophil-mediated oxidative stress is likely more important in causing organ dysfunction and death than a partially compromised bacterial clearance in this sepsis model. Most importantly, gp91phox mosaics seem to have a dual advantage; lessened oxidative stress with improved survival, yet the maintenance of efficient bacterial killing.
Additionally, we provided evidence that oxidant interchange between the WT and deficient neutrophil populations could take place, which may further contribute to the maintenance of efficient bacterial killing in mosaic animals. This is because superoxide anion or H2O2 released from WT subpopulation can enter into the deficient subpopulations and subsequently converted into HOCL through myeloperoxidase, which is a critical step in bacterial killing and expected to be functional in deficient cells. This, oxidant interchange between mosaic subpopulations may also alleviate oxidative stress because at the site of neutrophil infiltration the deficient subpopulation may catabolize some of the ROS released from WT cells thereby alleviating their noxious effects.
An additional important fact is that cellular redox status plays an important role in regulating signaling pathways thereby influencing a variety of cell responses (36–39). An alleviated oxidative stress in mosaic subjects is expected not only to affect the activation of neutrophils per se, but this is also likely to impact redox-dependent responses by the endothelial and parenchymal cells in the target organs of neutrophil infiltration. Thus, it is not surprising that WT, deficient and mosaic animals displayed differences in their overall systemic inflammatory response as reflected in the different kinetics and concentration of blood and tissue cytokines. The associated differences in bone marrow and blood white blood cell counts including a different degree of lymphocyte depletion after sepsis also suggest a uniquely influenced phenotype presented by mosaic animals, which is not simply the sum of the WT and deficient responses.
Variations in redox-dependent signaling and cytokine production are expected to alter neutrophil activation status. CD11b membrane expression reflects changes in neutrophil activation status as CD11b is an important member of adhesion molecules and its membrane content is markedly increased upon administration of inflammatory mediators including phorbol esters mimicking PKC activation. Interestingly, however, the lack of gp91phox alone either in neutrophils from deficient mice or in the deficient neutrophil subpopulation from mosaic animals resulted in an elevated membrane expression of CD11b (Fig 6). This increase in CD11b expression in deficient neutrophils was evident under resting conditions, as well as, after phorbol ester stimulation indicating an elevated cell adhesion potential of deficient neutrophils. Consistent with this possibility, septic mosaic animals displayed increased splenic, lung and kidney neutrophil infiltration as compared to WT and deficient animals. This tissue infiltration in mosaics was likely to be skewed toward the deficient subpopulations similar to that observed in the spleen. These observations together with an unchanged WT/deficient-ratio of the myeloid lineage in the BM (not shown) suggest that sepsis-induced skewing of infiltrating neutrophils towards deficient populations is likely to be the result of increased cell adhesion potential of deficient cells rather than selection of progenitors in the BM. The fact that deficient cells from deficient mice showed a similar level of tissue recruitment as observed in WT suggest that a blunted degree of neutrophil activation in deficient animals may counterbalance their increased adhesion potential. Furthermore, the finding that neutrophil infiltration skewed towards the deficient subpopulation in mosaic animals suggests that the activation of deficient subpopulations could be partially restored by the presence of WT neutrophils. The fact that hepatic neutrophil infiltration was similarly increased in mosaic and deficient mice as compared to WT suggest that the increased adhesion potential of deficient cells can be manifested in this organ even in the absence of neutrophil redox activation. This may be related to the unique structural and cellular characteristics of the liver sinusoids and interstitium.
In summary of our major observations, we propose the following scheme: In WT mice, the presence of fully activated ROS-producing infiltrating neutrophils represents a disadvantage because they are major contributors to multiple organ dysfunction and failure. On the other hand, bacterial clearance functions well in WT mice. The pattern is opposite in deficient subjects. Bacterial clearance is compromised; however, the lack of oxidative burst alleviates neutrophil-mediated stress in tissues and consequently improves mortality compared to WT. However, simultaneous presence of deficient and WT subpopulations in mosaic animals presents multiple advantages. First, bacterial clearance could be maintained not only by the presence of WT subpopulation but also by the possible oxidant interchange between mosaic subpopulations. Secondly, normal redox-dependent signaling and cell activation in the WT subpopulations provides the possibility to repair some of the compromised functions in the deficient subpopulations through paracrine actions. Finally, tissue oxidative stress is also expected to be alleviated in mosaic animals not only by the simple fact that half of the cells lack the capacity to produce an oxidative burst but also by the possibility that the deficient subpopulation may function as a sink of oxidants through their ROS detoxifying machinery especially at sites of neutrophil invasion where deficient and WT cell are in close proximities of each other.
Taken together, our observations using this model support the previously proposed hypothesis (15) that X-linked mutations or common polymorphisms could result in immune cell populations with different activation, functional or regulatory potentials in mosaic females. The presence of mosaic subsets may provide a broadened functional repertoire in accommodating the dynamically changing physiology during inflammation. This is in contrast to males whose X-linked phenotype is driven solely by the maternal haplotype and therefore expected to manifest a more polarized biological response to a particular physiological condition.
Acknowledgments
This study was supported by NIH-NIGMS grants GM-106864 (ZS) and Intramural Research Program of NIH/NIAAA (PP)
Abbreviations
- PMN
polymorphonuclear neutrophils
- BM
Bone marrow
- PMA
Phorbol 12-myristate 13-acetate
- NOX2 (gp91phox)
Catalytic NADPH oxidase subunit
- ROS
Reactive oxygen species
Reference List
- 1.Liu PY, Death AK, Handelsman DJ. Androgens and cardiovascular disease. Endocr.Rev. 2003;24:313–340. doi: 10.1210/er.2003-0005. [DOI] [PubMed] [Google Scholar]
- 2.Chen CC, Parker CR., Jr Adrenal androgens and the immune system. Semin.Reprod.Med. 2004;22:369–377. doi: 10.1055/s-2004-861553. [DOI] [PubMed] [Google Scholar]
- 3.Angele MK, Schwacha MG, Ayala A, Chaudry IH. Effect of gender and sex hormones on immune responses following shock. Shock. 2000;14:81–90. doi: 10.1097/00024382-200014020-00001. [DOI] [PubMed] [Google Scholar]
- 4.Kovacs EJ, Messingham KA, Gregory MS. Estrogen regulation of immune responses after injury. Mol.Cell Endocrinol. 2002;193:129–135. doi: 10.1016/s0303-7207(02)00106-5. [DOI] [PubMed] [Google Scholar]
- 5.Choudhry MA, Bland KI, Chaudry IH. Gender and susceptibility to sepsis following trauma. Endocr.Metab Immune.Disord.Drug Targets. 2006;6:127–135. doi: 10.2174/187153006777442422. [DOI] [PubMed] [Google Scholar]
- 6.George RL, McGwin G, Jr, Windham ST, Melton SM, Metzger J, Chaudry IH, Rue LW., III Age-related gender differential in outcome after blunt or penetrating trauma. Shock. 2003;19:28–32. doi: 10.1097/00024382-200301000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Gannon CJ, Pasquale M, Tracy JK, McCarter RJ, Napolitano LM. Male gender is associated with increased risk for postinjury pneumonia. Shock. 2004;21:410–414. doi: 10.1097/00024382-200405000-00003. [DOI] [PubMed] [Google Scholar]
- 8.De Maio A, Torres MB, Reeves RH. Genetic determinants influencing the response to injury, inflammation, and sepsis. Shock. 2005;23:11–17. doi: 10.1097/01.shk.0000144134.03598.c5. [DOI] [PubMed] [Google Scholar]
- 9.Deitch EA, Livingston DH, Lavery RF, Monaghan SF, Bongu A, Machiedo GW. Hormonally active women tolerate shock-trauma better than do men: a prospective study of over 4000 trauma patients. Ann.Surg. 2007;246:447–453. doi: 10.1097/SLA.0b013e318148566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barrow RE, Herndon DN. Incidence of mortality in boys and girls after severe thermal burns. Surg.Gynecol.Obstet. 1990;170:295–298. [PubMed] [Google Scholar]
- 11.Wells JC. Natural selection and sex differences in morbidity and mortality in early life. J.Theor.Biol. 2000;202:65–76. doi: 10.1006/jtbi.1999.1044. [DOI] [PubMed] [Google Scholar]
- 12.Libert C, Dejager L, Pinheiro I. The X chromosome in immune functions: when a chromosome makes the difference. Nat.Rev.Immunol. 2010;10:594–604. doi: 10.1038/nri2815. [DOI] [PubMed] [Google Scholar]
- 13.Adrie C, Azoulay E, Francais A, Clec'h C, Darques L, Schwebel C, Nakache D, Jamali S, Goldgran-Toledano D, Garrouste-Org, et al. Influence of gender on the outcome of severe sepsis: a reappraisal. Chest. 2007;132:1786–1793. doi: 10.1378/chest.07-0420. [DOI] [PubMed] [Google Scholar]
- 14.Crimmins EM, Hayward MD, Saito Y. Differentials in active life expectancy in the older population of the United States. J.Gerontol.B Psychol.Sci.Soc.Sci. 1996;51:S111–S120. doi: 10.1093/geronb/51b.3.s111. [DOI] [PubMed] [Google Scholar]
- 15.Spolarics Z. The X-files of inflammation: cellular mosaicism of X-linked polymorphic genes and the female advantage in the host response to injury and infection. Shock. 2007;27:597–604. doi: 10.1097/SHK.0b013e31802e40bd. [DOI] [PubMed] [Google Scholar]
- 16.Torres MB, Trentzsch H, Stewart D, Mooney ML, Fuentes JM, Saad DF, Reeves RH, De Maio A. Protection from lethal endotoxic shock after testosterone depletion is linked to chromosome X. Shock. 2005;24:318–323. doi: 10.1097/01.shk.0000177639.22863.99. [DOI] [PubMed] [Google Scholar]
- 17.Migeon BR. The role of X inactivation and cellular mosaicism in women's health and sex-specific diseases. JAMA. 2006;295:1428–1433. doi: 10.1001/jama.295.12.1428. [DOI] [PubMed] [Google Scholar]
- 18.Migeon BR. Why females are mosaics, X-chromosome inactivation, and sex differences in disease. Gend.Med. 2007;4:97–105. doi: 10.1016/s1550-8579(07)80024-6. [DOI] [PubMed] [Google Scholar]
- 19.Heard E, Disteche CM. Dosage compensation in mammals: fine-tuning the expression of the X chromosome. Genes Dev. 2006;20:1848–1867. doi: 10.1101/gad.1422906. [DOI] [PubMed] [Google Scholar]
- 20.Goto T, Monk M. Regulation of X-chromosome inactivation in development in mice and humans. Microbiol.Mol.Biol.Rev. 1998;62:362–378. doi: 10.1128/mmbr.62.2.362-378.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Latham KE. X chromosome imprinting and inactivation in preimplantation mammalian embryos. Trends Genet. 2005;21:120–127. doi: 10.1016/j.tig.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 22.Ross MT, Grafham DV, Coffey AJ, Scherer S, McLay K, Muzny D, Platzer M, Howell GR, Burrows C, Bird CP, et al. The DNA sequence of the human X chromosome. Nature. 2005;434:325–337. doi: 10.1038/nature03440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun G, Yan J, Noltner K, Feng J, Li H, Sarkis DA, Sommer SS, Rossi JJ. SNPs in human miRNA genes affect biogenesis and function. RNA. 2009;15:1640–1651. doi: 10.1261/rna.1560209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abkowitz JL, Taboada M, Shelton GH, Catlin SN, Guttorp P, Kiklevich JV. An X chromosome gene regulates hematopoietic stem cell kinetics. Proc.Natl.Acad.Sci.U.S.A. 1998;95:3862–3866. doi: 10.1073/pnas.95.7.3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hatakeyama C, Anderson CL, Beever CL, Penaherrera MS, Brown CJ, Robinson WP. The dynamics of X-inactivation skewing as women age. Clin.Genet. 2004;66:327–332. doi: 10.1111/j.1399-0004.2004.00310.x. [DOI] [PubMed] [Google Scholar]
- 26.Brown CJ. Skewed X-chromosome inactivation: cause or consequence? J Natl.Cancer Inst. 1999;91:304–305. doi: 10.1093/jnci/91.4.304. [DOI] [PubMed] [Google Scholar]
- 27.Minks J, Robinson WP, Brown CJ. A skewed view of X chromosome inactivation. J.Clin.Invest. 2008;118:20–23. doi: 10.1172/JCI34470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chandra R, Federici S, Hasko G, Deitch EA, Spolarics Z. Female X-chromosome mosaicism for gp91phox expression diversifies leukocyte responses during endotoxemia. Crit Care Med. 2010;38:2003–2010. doi: 10.1097/CCM.0b013e3181eb9ed6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 30.Baker CC, Chaudry IH, Gaines HO, Baue AE. Evaluation of Factors Affecting Mortality-Rate After Sepsis in A Murine Cecal Ligation and Puncture Model. Surgery. 1983;94:331–335. [PubMed] [Google Scholar]
- 31.Nemeth ZH, Csoka B, Wilmanski J, Xu D, Lu Q, Ledent C, Deitch EA, Pacher P, Spolarics Z, Hasko G. Adenosine A2A receptor inactivation increases survival in polymicrobial sepsis. J.Immunol. 2006;176:5616–5626. doi: 10.4049/jimmunol.176.9.5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pollock JD, Williams DA, Gifford MA, Li LL, Du X, Fisherman J, Orkin SH, Doerschuk CM, Dinauer MC. Mouse model of X-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nat.Genet. 1995;9:202–209. doi: 10.1038/ng0295-202. [DOI] [PubMed] [Google Scholar]
- 33.Chandra R, Villanueva E, Feketova E, Machiedo GW, Hasko G, Deitch EA, Spolarics Z. Endotoxemia down-regulates bone marrow lymphopoiesis but stimulates myelopoiesis: the effect of G6PD deficiency. J.Leukoc.Biol. 2008;83:1541–1550. doi: 10.1189/jlb.1207838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu HP, Hsieh YC, Suzuki T, Choudhry MA, Schwacha MG, Bland KI, Chaudry IH. Mechanism of the nongenomic effects of estrogen on intestinal myeloperoxidase activity following trauma-hemorrhage: up-regulation of the PI-3K/Akt pathway. J.Leukoc.Biol. 2007;82:774–780. doi: 10.1189/jlb.0307182. [DOI] [PubMed] [Google Scholar]
- 35.Invernizzi P, Pasini S, Selmi C, Gershwin ME, Podda M. Female predominance and X chromosome defects in autoimmune diseases. J.Autoimmun. 2009;33:12–16. doi: 10.1016/j.jaut.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 36.Jones DP. Redefining oxidative stress. Antioxid.Redox.Signal. 2006;8:1865–1879. doi: 10.1089/ars.2006.8.1865. [DOI] [PubMed] [Google Scholar]
- 37.Zhang WJ, Wei H, Frei B. Genetic deficiency of NADPH oxidase does not diminish, but rather enhances, LPS-induced acute inflammatory responses in vivo. Free Radic.Biol.Med. 2009;46:791–798. doi: 10.1016/j.freeradbiomed.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Piccoli C, D'Aprile A, Ripoli M, Scrima R, Lecce L, Boffoli D, Tabilio A, Capitanio N. Bone-marrow derived hematopoietic stem/progenitor cells express multiple isoforms of NADPH oxidase and produce constitutively reactive oxygen species. Biochem.Biophys.Res.Commun. 2007;353:965–972. doi: 10.1016/j.bbrc.2006.12.148. [DOI] [PubMed] [Google Scholar]
- 39.Yan Z, Banerjee R. Redox remodeling as an immunoregulatory strategy. Biochemistry. 2010;49:1059–1066. doi: 10.1021/bi902022n. [DOI] [PMC free article] [PubMed] [Google Scholar]