Abstract
The possibility of an inverse association between vitamin D and risk of cancer and, in particular, of cutaneous malignant melanoma has been suggested, but results of epidemiologic studies are still conflicting.
We examined the relation between dietary vitamin D intake and melanoma risk through a population-based case-control study (380 cases, 719 controls) in a northern region of Italy, a country with average vitamin D intake lower than in northern Europe or the US. We assessed average daily intake of vitamin D from foodstuffs using the European Prospective Investigation into Cancer and Nutrition (EPIC) semiquantitative food frequency questionnaire.
In this population, levels of vitamin D intake were considerably lower than those observed in recent US studies. We found an inverse relation between dietary vitamin D and melanoma risk in the sample as a whole, in both crude and adjusted analyses. In sex and age-specific analyses, this association appeared to be stronger among males and among older subjects.
These findings suggest that, at the relatively low levels of intake observed in this sample, an inverse relation between dietary vitamin D and risk of cutaneous malignant melanoma may exist.
Keywords: Vitamin D, melanoma, diet, case-control study, epidemiology
Introduction
Attention has been devoted in recent years to the possibility that fat-soluble vitamin D may decrease the risk of cancer (1,2) and the risk of cutaneous malignant melanoma (CMM) in particular (3–5). Evidence for a possible preventive role comes from both epidemiologic investigations (6) and laboratory studies (2,7), which have suggested several biologic pathways through which vitamin D might counteract cancer risk. However, the few studies so far conducted on dietary vitamin D and CMM have reported inconsistent results, with some studies reporting a reduced risk associated with high levels and others finding no association (8) and even a positive relation (9). These studies have suffered in some cases from methodological limitations such as low numbers of CMM cases (10) and the use of hospital-referred controls (11,12), a potential major source of bias (13). Moreover, the inherent limitations of dietary assessment methods might also explain the inconsistencies among these studies. Thus studies with methodological improvements are needed to resolve the scientific uncertainties. Similarly conflicting evidence regarding the vitamin D-cancer relationship has arisen in observational and experimental studies on other site-specific neoplasms (14–19) and caution has been correctly advocated to deal with this issue from public health and clinical perspectives (20–22).
We carried out a population-based case-control study in a northern region of Italy, designed to test the hypothesis that diet composition is related to the risk of CMM, as suggested by some epidemiologic studies (10,12,23,24). The population of Italy has been reported to have a low vitamin D status, as expected on the basis of its inverse relation with southern latitude (25); elderly women might be at high risk of hypovitaminosis D Isaia (26). We here report the results of this study concerning the relation between vitamin D intake and CMM risk.
Methods
Subjects
We carried out a population-based case-control study focusing on the association between dietary habits and melanoma risk in five provinces, Parma, Reggio Emilia, Modena, Bologna and Ferrara, of the Emilia Romagna region in northern Italy (population about 4,000,000). We attempted to include in the study all patients with newly-diagnosed CMM referred during the years 2005 and 2006 to the Departments of Dermatology of the Parma, Modena, Bologna and Ferrara University Hospitals and to the Unit of Dermatology of the Reggio Emilia Santa Maria Nuova Hospital, and residing in one of the five provinces at time of diagnosis. Patients who appeared to meet the eligibility criteria were approached by their dermatologist about participation in the study. Of the patients approached, 394/572 (68.9%) agreed to participate, giving their informed consent and returning questionnaires. Fourteen participating patients were eventually excluded, 7 due to actual residence outside the study provinces and 7 due to extreme values derived from the food frequency questionnaire (FFQ; see below), leaving a total of 380 cases for the analysis. Of these patients, 175 were males (86 aged less than 60) and 205 were females (132 younger than 60).
After enrollment of cases, we identified 2825 potential controls from the Emilia-Romagna region general population, through random selection of individuals enrolled in the Emilia-Romagna region National Health Service directory (mandatory for all residents). We identified six referents for each CMM case, matched for gender, year of birth (±5 years) and province of residence. Potential controls were mailed an envelope containing a description of the study, lifestyle and food frequency questionnaires and a pre-paid return envelope. We also informed the family doctors of the potential controls by mail, providing information about study methodology and purposes and asking them to encourage the involvement of the potential participant. Over a two-year period, 747 potential controls (26.4%) gave their informed consent and returned the questionnaires. Twenty-eight of these individuals were excluded from the study, 24 due to incompleteness of their FFQ and 4 due to extreme values (see below), leaving 719 controls for the analysis. Of these subjects, 319 were males (148 <60 years) and 400 females ((266 <60 years).
Data Collection
For cases, the dermatologists collected clinical data on CMM (Breslow tumor thickness, Clark invasion level, histological type, localization, degree of ulceration and regression), though these data were not available for all patients.
All subjects were administered a lifestyle questionnaire and FFQ. The questionnaires were given to cases during a routine visit to their dermatological center, completed at home and returned to the center. If the subject was no longer followed at the center, he/she was sent the questionnaires by mail and returned it using a pre-paid envelope. Controls returned the questionnaires to the Department of Public Health Sciences of University of Modena and Reggio Emilia. For both cases and controls we provided phone and email contact information in order to offer assistance when completing and returning the questionnaires.
The lifestyle questionnaire included items on demographic characteristics (place and date of birth, province of residence, marital status, education, weight and height), phenotypic characteristics (eye, hair and skin color), sunburn history (never, only before or only after 18 years of age) and skin sun reaction (speed of tan and tendency to burn). Eye color was classified into three categories: light (blue/green), light brown or dark brown/blacks. Hair color was classified as blond, red, light brown or dark brown/black at 20 years, and skin color was classified as white, light brown, brown/olive or dark brown/ebony. Each subject was assigned to the corresponding phototype using the Fitzpatrick phototyping scale (27).
Dietary habits were assessed using the FFQ used in the European Prospective Investigation into Cancer and Nutrition (EPIC). This questionnaire was specifically designed and validated for the central-northern Italy population (28,29) and has been previously used in epidemiologic research, including by our group in a small earlier study on diet and melanoma risk (10,23). The EPIC questionnaire is a self-administered semiquantitative FFQ encompassing 248 questions about frequency and quantity of consumption of 188 food items over the previous year, and includes illustrations of sample dishes of particular sizes and references to standard portion sizes. Participants can specify frequency of consumption of items by day, week or month. Questions on seasoning and food preparation are also included. The questionnaire responses were checked and study subjects were recontacted to complete missing information.
Completed FFQs were processed by the Nutritional Epidemiology Unit of Milan National Cancer Institute using specially-developed software. The software calculates frequency and quantity of consumption of foodstuffs and of overall intake of nutrients, using a reference database for food chemical composition (30).
We excluded from the analysis 11 subjects (7 cases and 4 controls) for whom the ratio of total energy intake (determined from the questionnaire) to calculated basal metabolic rate was at either extreme of the distribution (cut-offs were the first and last half percentiles), to reduce the impact of implausible extreme values on the analysis.
Multivitamin and mineral supplement consumption is not assessed by the EPIC FFQ. To estimate the prevalence and levels of consumption among the study subjects, we conducted a telephone survey in a random subsample of 80 subjects (40 cases and 40 controls) residing in the Modena and Reggio Emilia provinces.
Statistical Analysis
We used conditional logistic regression models to compute odds ratios (OR) and 95% confidence intervals (CI), adjusting for potential confounders. We matched the available controls to cases according to province of residence, sex and age (± 5 years). All cases but one could be retained in the study due to availability of one or more matched controls. For these analyses, dietary intake of vitamin D was categorized into quintiles based on the intake distribution in controls, and the reference group was the lowest quintile. We also tested for trend by entering quintile indicator (1, 2, 3, 4, 5) as a linear variable.
We obtained a nonparametric estimate of the relationship between dietary vitamin D intake and CMM risk using a generalized additive model (31). This model consisted of a logistic regression model that included potential confounders and modeled the relationship between the log odds of being a case and dietary vitamin D intake using a natural cubic smoothing spline. As a sensitivity analysis, we used a range of smoothing parameters (degrees of freedom); results obtained using 3 degrees of freedom are presented. These analyses were conducted using the gam package in R version 2.9.2 (32).
Results
Characteristics of study subjects and their dietary vitamin D intake are reported in Table 1. There were more females than males in the sample, and the median age was 57 years (range 17-94). Education levels and marital status were similar in the two groups. Cases tended to have more fair skin types and were more likely to report a history of sunburns. Median daily dietary vitamin D intake was higher in controls compared with cases, and it tended to be higher in males than in females, and higher in younger subjects compared to older subjects. Table 2 compares the distribution of intake in the study subjects to distributions reported in the studies of Weinstock et al. (11), Millen et al. (12), and Asgari et al. (8), which involved populations in the United States. These results show that intakes among these residents of northern Italy were considerably lower. Concerning intake of vitamin D-containing supplements, through the telephone survey carried out in a random sample of the study population we identified only five consumers of these products, three among cases (7.5%, 3/40) and two among controls (5%, 2/40), during the five years before the interview. For all five subjects, additional vitamin D intake was due to occasional consumption of multivitamin/mineral supplements particularly during the summer. When we examined dietary vitamin D intake according to Breslow tumor thickness, we noted a tendency towards lower values in patients with the highest degrees of thickness among males and to some extent also in females, though in the latter group patients with in situ melanoma exhibited low values of intake (data not shown).
Table 1.
Characteristics of study subjects
| Cases |
Controls |
|||
|---|---|---|---|---|
| n | (%) | n | (%) | |
| All subjects | 380 | (34.6) | 719 | (65.4) |
| Gender | ||||
| Male | 175 | (46.1) | 319 | (44.4) |
| Female | 205 | (53.9) | 400 | (55.6) |
| Age | ||||
| <20 | 1 | (0.1) | 2 | (0.4) |
| 20–39 | 107 | (14.9) | 62 | (12.6) |
| 40–59 | 306 | (42.5) | 170 | (34.4) |
| 60–79 | 278 | (38.7) | 235 | (47.6) |
| ≥80 | 27 | (3.8) | 25 | (5.6) |
| Province | ||||
| Bologna | 122 | (32.1) | 198 | (27.5) |
| Ferrara | 33 | (8.7) | 93 | (12.9) |
| Modena | 87 | (22.9) | 234 | (32.6) |
| Parma | 32 | (8.4) | 57 | (7.9) |
| Reggio Emilia | 106 | (27.9) | 137 | (19.1) |
| Educational attainment | ||||
| Less than primary school | 7 | (1.8) | 10 | (1.4) |
| Primary school | 84 | (22.1) | 160 | (22.2) |
| Middle school | 95 | (25.0) | 176 | (24.5) |
| High school | 136 | (35.8) | 266 | (37.0) |
| University | 52 | (14.8) | 103 | (14.3) |
| Unknown | 2 | (0.5) | 4 | (0.6) |
| Marital status | ||||
| Married | 257 | (67.6) | 493 | (68.7) |
| Unmarried/single | 68 | (17.9) | 103 | (14.3) |
| Divorced | 23 | (6.0) | 48 | (6.6) |
| Widowed | 31 | (8.2) | 74 | (10.3) |
| Unknown | 1 | (0.3) | 1 | (0.1) |
| Phototype | ||||
| I | 105 | (27.6) | 109 | (15.2) |
| II | 136 | (35.8) | 238 | (33.1) |
| III | 122 | (32.1) | 312 | (43.4) |
| IV | 17 | (4.5) | 60 | (8.3) |
| V | 0 | (0.0) | 0 | (0.0) |
| VI | 0 | (0.0) | 0 | (0.0) |
| Sunburns history | ||||
| Never | 182 | (47.9) | 452 | (62.9) |
| Before 18 years | 108 | (28.4) | 164 | (22.8) |
| After 18 years | 90 | (23.7) | 103 | (14.3) |
| Vitamin D intake (μg/day) | Median | Range | Median | Range |
| All subjects | 2.3 | (0.3–9.4) | 2.4 | (0.3–13.4) |
| Males | 2.4 | (0.6–7.3) | 2.4 | (0.5–13.4) |
| Females | 2.2 | (0.3–9.4) | 2.4 | (0.3–11.8) |
| All subjects <60 years | 2.5 | (0.4–9.4) | 2.6 | (0.3–11.8) |
| Males <60 years | 2.6 | (0.9–7.3) | 2.6 | (0.6–8.4) |
| Females <60 years | 2.3 | (0.4–9.4) | 2.5 | (0.3–11.8) |
| All subjects ≥60 years | 2.0 | (0.3–6.1) | 2.2 | (0.5–13.4) |
| Males ≥60 years | 2.1 | (0.6–5.3) | 2.3 | (0.5–13.4) |
| Females ≥60 years | 2.0 | (0.3–6.1) | 2.0 | (0.6–7.1) |
Table 2.
Distribution of vitamin D intake (μg/day) in the study subjects compared to US populations.
| Quintiles range | |||||
|---|---|---|---|---|---|
| I | II | III | IV | V | |
| Present study (control subjects) | <1.6 | 1.6–2.1 | 2.1–2.7 | 2.7–3.7 | ≥3.7 |
| Weinstock et al.(10),a | 2.6 | 2.6–4.7 | 4.7–6.6 | 6.7–9.9 | >9.9 |
| Millen et al.(11) | ≤1.5 | 1.5–2.8 | 2.8–4.7 | 4.7–8.0 | >8.0 |
| Quartiles range | |||||
| I | II | III | IV | ||
| Present study (control subjects) | <1.8 | 1.8–2.4 | 2.4–3.4 | >3.4 | |
| Asgari et al.(7) | <3.0 | 3.0–4.7 | 4.7–7.1 | >7.1 | |
Including dietary supplements
The risk of CMM was inversely associated with vitamin D intake in men and in all subjects combined in unadjusted analyses for trend conducted using conditional logistic regression, and this association remained in multivariate analysis controlling for total energy and calcium intake, phototype, skin sun reaction, history of sunburns and education (Table 3). In this adjusted analysis with vitamin D intake categorized by quintiles, the odds of CMM were consistently lower in the highest quintile for men and for all subjects. After stratification by sex and by age, an inverse relation between CMM risk and vitamin D intake was stronger in males than females, and in older subjects than in the younger groups.
Table 3.
Odds ratios (with 95% confidence intervals in parentheses) for melanoma by quintile of dietary intake of vitamin D, Emilia Romagna region, Italy
| Quintile of vitamin D dietary intake (μg/day)a |
|||||||
|---|---|---|---|---|---|---|---|
| Case-control matched sets | I (<1.62)b | II (1.62 – 2.13) | III (2.13–2.72) | IV (2.72–3.67) | V (≥3.67) | PC | |
| All subjects | 380 | 1.00 | 0.96 (0.63 – 1.45) | 0.82 (0.54 – 1.26) | 0.62 (0.39 – 0.98) | 0.53 (0.31 – 0.88) | 0.005 |
| Men | 175 | 1.00 | 0.80 (0.41 – 1.56) | 0.84 (0.45 – 1.58) | 0.72 (0.36 – 1.45) | 0.38 (0.17 – 0.87) | 0.044 |
| Women | 205 | 1.00 | 1.12 (0.64 – 1.94) | 0.80 (0.44 – 1.44) | 0.56 (0.30 – 1.04) | 0.69 (0.34 – 1.39) | 0.057 |
| All subjects < 60 years | 218 | 1.00 | 0.97 (0.54 – 1.74) | 0.88 (0.48 – 1.61) | 0.57 (0.29 – 1.13) | 0.63 (0.32 – 1.27) | 0.078 |
| All subjects ≥ 60 years | 162 | 1.00 | 0.91 (0.49 – 1.69) | 0.70 (0.37 – 1.32) | 0.71 (0.36 – 1.37) | 0.37 (0.13 – 0.87) | 0.032 |
| Men < 60 years | 86 | 1.00 | 0.83 (0.26 – 2.63) | 0.96 (0.35 – 2.63) | 0.92 (0.29 – 2.84) | 0.76 (0.14 – 1.50) | 0.216 |
| Men ≥ 60 years | 89 | 1.00 | 0.87 (0.37 – 2.05) | 0.76 (0.31 – 1.85) | 0.75 (0.30 – 1.93) | 0.33 (0.09 – 1.16) | 0.148 |
| Women < 60 years | 132 | 1.00 | 1.03 (0.50 – 2.13) | 0.84 (0.38 – 1.84) | 0.46 (0.19 – 1.09) | 0.76 (0.30 – 1.88) | 0.169 |
| Women ≥ 60 years | 73 | 1.00 | 1.09 (0.41 – 2.93) | 0.53 (0.20 – 1.43) | 0.69 (0.25 – 1.85) | 0.33 (0.08 – 1.40) | 0.110 |
Based on dietary vitamin D intake distribution in controls
Referent category
P for trend using quintiles of vitamin D intake in a conditional logistic regression model adjusting for energy and calcium intake, phototype, history of sunburns, skin sun reaction and education
We also estimated the association between vitamin D intake and CMM risk when entering in the multivariate model the intakes of other vitamins and of minerals, namely iron, calcium, potassium, zinc, phosphorus, thiamine, niacin, riboflavin, vitamin B6, pyridoxine, retinol, carotenoids, vitamin C, folate and vitamin E, in addition to energy intake (results not shown). In this analysis, an increase of 1 μg/day of vitamin D intake was associated with an OR for melanoma of 0.85 (95% CI 0.74–0.97, P=0.020), compared with the OR of 0.87 (95% CI 0.78–0.99, P=0.029) in the unadjusted analysis. None of the dietary factors entered in the regression model showed any association with disease risk with the exception of a slight positive association with calcium intake, which however disappeared in the unadjusted analysis limited to this variable.
As a sensitivity analysis, we conducted a series of analyses in which we omitted one province of residence at a time and re-ran the models. These results indicated that no single province had an undue influence on the estimates (results not provided).
We also carried out subgroup analyses in order to evaluate possible modifying effects of phototype, tumor Breslow thickness, sunburn history and skin sun reaction, calculating the OR for continuous increase in vitamin D intake. Most odds ratios were consistent with an inverse relation between vitamin D and CMM risk, across all phototype, Breslow thickness, sunburn history and skin sun reaction groups, although precision was low due to small sample sizes. The strongest suggestion of a modifying effect was seen in men with phototype IV, who had a particularly strong inverse association, with phototype II, and with a Breslow thickness between 1 and 2 mm. Most ORs were consistent with a stronger relation among men than among women. Females exhibiting a high tendency to burn and slowly tan showed little evidence of a vitamin D-melanoma risk relation.
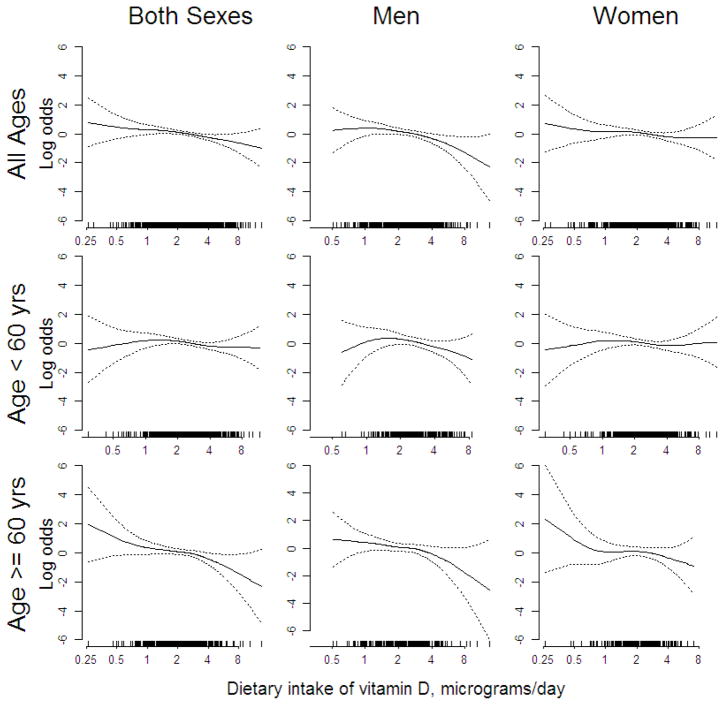
Figure 1 presents nonparametric estimates of the relationship between dietary vitamin D intake and CMM risk obtained using a generalized additive model, controlling for age, sex (when both sexes were included), energy and calcium intake, phototype, skin sun reaction, history of sunburns and education. These results indicate a decrease in CMM risk with increasing dietary intake among older men and women, and limited evidence of such a relation in females younger than 60.
Figure 1.
Nonparametric estimates of the association between melanoma risk and dietary vitamin D intake obtained from a generalized additive model, with adjustment for age, energy and calcium intake, phototype, skin sun reaction, history of sunburns and education. Outer dotted lines represent 95% confidence limits. Rugplots at the bottom of each panel indicate the vitamin D intake values among the subjects in the analysis.
Discussion
This study has important strengths and limitations. One strength is the validity and completeness of the EPIC food frequency questionnaire, which had been specifically developed and validated in the northern Italy population and which should have allowed in the study population a very accurate estimate of the intake of vitamin D and more generally of nutrients. Furthermore, the sample size of this study yielded statistically stable risk estimates and was sufficient to support stratified analyses, which allowed us to investigate the potential role of sex and age as effect modifiers in the vitamin D-melanoma relation as well as of phototype, melanoma Breslow thickness, sunburn history and skin sun reaction, albeit with more limited precision. Finally, we consider it unlikely that recall bias occurred with regard to assessment of vitamin D intake, since there seems to be little if any awareness among this population about the efficacy of vitamin D in preventing melanoma or more generally cancer, thus making it highly unlikely that the consumption of foods with high vitamin D content was incorrectly reported.
In this study, we did not systematically collect information about consumption of dietary supplements containing vitamin D, and therefore we could not exclude the occurrence of measurement error in the assessment of dietary intake. However, the use of vitamin D supplements is exceptionally rare in Italy compared with the US (8,12), as also confirmed by the survey we carried out among 40 cases and 40 controls randomly sampled among the study subjects residing in the Modena and Reggio Emilia provinces. Therefore, in the present investigation substantial misclassification of dietary vitamin D intake due to missing information about this additional source of intake is most unlikely to have occurred.
Due to the study design, we were unable to evaluate the role of some important risk factors for CMM, such as number and characteristics of atypical nevi and overall sun exposure. However, there is little basis to hypothesize that these nevi were more frequent in subjects with low vitamin D intake, and that in that case they were not a consequence of this reduced intake.
This study focused on vitamin D dietary intake and not on other indicators of vitamin D status, such as the blood levels of 25-hydroxyvitamin D, whose relation with dietary intake correlates well in some studies but not so well in others (33). However, recent studies appear to confirm that dietary habits are an important determinant of serum or plasma 25-hydroxyvitamin D levels (34–37), though this relation might be weaker in US populations (38,39). Moreover, a validation of the ability of dietary vitamin D assessment methodology to reflect circulating 25-hydroxyvitamin D levels has been done within the EPIC study framework in the ‘Oxford cohort’, showing a strong direct association between these two indicators of vitamin D status (40). The questionnaire used in that validation study was based on frequency of consumption of 130 foods over the previous 12 month, while the questionnaire we administered investigated consumption of 188 food items, and it assessed both frequency and quantity of consumption. Therefore, despite the potential differences between these English and Italian populations and the lack of a direct validation study in our setting, we consider it very likely that the assessment method we used could reliably estimate vitamin D status.
We experienced a low participation rate among controls, and therefore we cannot entirely rule out the possible occurrence of some selection bias in this population. We attempted to further investigate this issue in a small subgroup, the residents in one of the study municipalities (Reggio Emilia, around 160,000 inhabitants), collecting information about educational attainment level and income in the year 2005, with a methodology recently used and limiting the analysis to males due to the very high level of missing information found in females for annual income (41). The distribution of educational attainment and income was substantially similar among responders and non-responders, suggesting that selection bias may have been minimal (data not shown), and we consider these indications as generalizable to the entire study control population due to the homogeneity of the Emilia-Romagna population as far as ethnic, cultural and socioeconomic characteristics are concerned.
Overall, our results suggest an inverse relation between vitamin D intake and melanoma risk, across all study subgroups though more evident in older subjects and in males. The possibility that age and gender influence the vitamin D - melanoma risk relation is of interest but is not supported by previous results from the US studies (8,9,11,12), and our observations might reflect selective confounding or differential measurement error, i.e., a reduced ability of dietary assessment in estimating vitamin D status in females and in the youngest subjects, thus slightly biasing the risk estimates towards the null.
The inverse association we observed between vitamin D intake and disease risk is consistent with results reported from a US hospital-based case-control study (12) but not with the results of the two smaller investigations on a nearly identical population from the Massachusetts General Hospital (9,11), showing a direct association between melanoma risk and overall vitamin D intake. Furthermore, a recent large cohort study found an excess disease risk associated with high vitamin D intake from foods (but not from supplements) (8), though this investigation examined a population with an age range at baseline of 50–76, and no separate analysis for the older age group was reported. Moreover, in all of the US investigations, average vitamin D intakes of the study populations were considerably higher than in our population, and therefore the possibility of a biphasic relation between vitamin D and CMM risk according to the range of exposure, i.e. of an increased risk at both low and high levels of intake within a relatively low range, should be considered. It should also be noted that melanoma risk was found to be directly associated with consumption of vitamin D-containing dietary supplements (9,11), though such association was not confirmed in two other studies (8,12). A small cohort study carried out in a US population showed an increased melanoma risk (1.6, 95% CI 0.5–6.1) in the lower tertile of serum 1,25 dihydroxyvitamin D levels (42).
We did not identify clear evidence of a differential relation between CMM risk and vitamin D according to tumor thickness, with the exception of the 1–2 mm Breslow score category. Evaluation of these results is however hampered by the low number of cases in the >2 mm thickness category, which also makes it difficult to compare these findings with the recent observations of an inverse association between Breslow thickness and vitamin D serum concentrations among US melanoma patients (43).
In this study, we collected information about two indicators related to sun exposure (subject to recall bias due to the case-control design of the study), sunburn history and tanning habits-skin sun reaction, which were independently related to melanoma risk (data not shown). Stratified analyses yielded little evidence of any relation between these two factors and the vitamin D-melanoma inverse association generally observed across all subgroups.
In conclusion, our study provides observational evidence supporting a beneficial effect of higher vitamin D intake from foods on melanoma risk in this well-nourished Italian population with average levels of intake generally lower than the US population and little evidence of any supplementation. However, due to the conflicting results of the epidemiologic literature on the vitamin D-melanoma relation (6), some indications of excess cancer risk associated with higher vitamin D status (17,44), and the still incomplete risk assessment of vitamin D (20), further careful investigation of this issue and of the potential risks associated with vitamin D supplementation is clearly needed before advocating any public health measures in this area.
Acknowledgments
We acknowledge the cooperation of all patients and controls who agreed to participate in the study and made it possible. We appreciated the assistance of Masanao Yajima with the figures.
Funding: The study was supported by a grant by Lega Contro i Tumori – LILT, Section of Reggio Emilia (M. Vinceti); by Modena Policlinico Hospital and Local Health Unit (M. Vinceti and G. Pellacani); and by NIH P30 CA16042 (C. Crespi).
Footnotes
Conflict of interest statements: The authors declare that they have no competing interest.
References
- 1.Davis CD. Vitamin D and cancer: current dilemmas and future research needs. Am J Clin Nutr. 2008;88:565S–569S. doi: 10.1093/ajcn/88.2.565S. [DOI] [PubMed] [Google Scholar]
- 2.Peterlik M, Grant WB, Cross HS. Calcium, vitamin D and cancer. Anticancer Res. 2009;29:3687–3698. [PubMed] [Google Scholar]
- 3.Egan KM. Vitamin D and melanoma. Ann Epidemiol. 2009;19:455–461. doi: 10.1016/j.annepidem.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Weinstock MA, Moses AM. Skin cancer meets vitamin D: the way forward for dermatology and public health. J Am Acad Dermatol. 2009;61:720–724. doi: 10.1016/j.jaad.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 5.Godar DE, Landry RJ, Lucas AD. Increased UVA exposures and decreased cutaneous Vitamin D(3) levels may be responsible for the increasing incidence of melanoma. Med Hypotheses. 2009;72:434–443. doi: 10.1016/j.mehy.2008.09.056. [DOI] [PubMed] [Google Scholar]
- 6.Gandini S, Raimondi S, Gnagnarella P, Dore JF, Maisonneuve P, et al. Vitamin D and skin cancer: a meta-analysis. Eur J Cancer. 2009;45:634–641. doi: 10.1016/j.ejca.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Osborne JE, Hutchinson PE. Vitamin D and systemic cancer: is this relevant to malignant melanoma? Br J Dermatol. 2002;147:197–213. doi: 10.1046/j.1365-2133.2002.04960.x. [DOI] [PubMed] [Google Scholar]
- 8.Asgari MM, Maruti SS, Kushi LH, White E. A cohort study of vitamin D intake and melanoma risk. J Invest Dermatol. 2009;129:1675–1680. doi: 10.1038/jid.2008.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stryker WS, Stampfer MJ, Stein EA, Kaplan L, Louis TA, et al. Diet, plasma levels of beta-carotene and alpha-tocopherol, and risk of malignant melanoma. Am J Epidemiol. 1990;131:597–611. doi: 10.1093/oxfordjournals.aje.a115544. [DOI] [PubMed] [Google Scholar]
- 10.Vinceti M, Pellacani G, Malagoli C, Bassissi S, Sieri S, et al. A population-based case-control study of diet and melanoma risk in northern Italy. Public Health Nutr. 2005;8:1307–1314. doi: 10.1079/phn2005754. [DOI] [PubMed] [Google Scholar]
- 11.Weinstock MA, Stampfer MJ, Lew RA, Willett WC, Sober AJ. Case-control study of melanoma and dietary vitamin D: implications for advocacy of sun protection and sunscreen use. J Invest Dermatol. 1992;98:809–811. doi: 10.1111/1523-1747.ep12499962. [DOI] [PubMed] [Google Scholar]
- 12.Millen AE, Tucker MA, Hartge P, Halpern A, Elder DE, et al. Diet and melanoma in a case-control study. Cancer Epidemiol Biomarkers Prev. 2004;13:1042–1051. [PubMed] [Google Scholar]
- 13.Malagoli C, Vinceti M, Pellacani G, Sieri S, Krogh V, et al. Diet and melanoma risk: effects of choice of hospital versus population controls. Tumori. 2008;94:669–673. doi: 10.1177/030089160809400504. [DOI] [PubMed] [Google Scholar]
- 14.Chlebowski RT, Johnson KC, Kooperberg C, Pettinger M, Wactawski-Wende J, et al. Calcium plus vitamin D supplementation and the risk of breast cancer. J Natl Cancer Inst. 2008;100:1581–1591. doi: 10.1093/jnci/djn360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahn J, Peters U, Albanes D, Purdue MP, Abnet CC, et al. Serum vitamin D concentration and prostate cancer risk: a nested case-control study. J Natl Cancer Inst. 2008;100:796–804. doi: 10.1093/jnci/djn152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelly JL, Friedberg JW, Calvi LM, van Wijngaarden E, Fisher SG. Vitamin D and non-Hodgkin lymphoma risk in adults: a review. Cancer Invest. 2009;27:942–951. doi: 10.3109/07357900902849632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stolzenberg-Solomon RZ, Hayes RB, Horst RL, Anderson KE, Hollis BW, et al. Serum vitamin D and risk of pancreatic cancer in the prostate, lung, colorectal, and ovarian screening trial. Cancer Res. 2009;69:1439–1447. doi: 10.1158/0008-5472.CAN-08-2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jenab M, Bueno-de-Mesquita HB, Ferrari P, van Duijnhoven FJ, Norat T, et al. Association between pre-diagnostic circulating vitamin D concentration and risk of colorectal cancer in European populations:a nested case-control study. BMJ. 2010;340:b5500. doi: 10.1136/bmj.b5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woolcott CG, Wilkens LR, Nomura AM, Horst RL, Goodman MT, et al. Plasma 25–hydroxyvitamin D levels and the risk of colorectal cancer: the multiethnic cohort study. Cancer Epidemiol Biomarkers Prev. 2010;19:130–134. doi: 10.1158/1055-9965.EPI-09-0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Costello RB. Vitamin D and health in the 21st century: federal initiatives to advance research. Am J Med Sci. 2009;338:34–39. doi: 10.1097/MAJ.0b013e3181aaee78. [DOI] [PubMed] [Google Scholar]
- 21.Goodwin PJ. Vitamin D in cancer patients: above all, do no harm. J Clin Oncol. 2009;27:2117–2119. doi: 10.1200/JCO.2008.20.8629. [DOI] [PubMed] [Google Scholar]
- 22.Chlebowski RT. Caution regarding 25-hydroxyvitamin D monitoring in women with breast cancer. J Clin Oncol. 2009;27:e72–73. doi: 10.1200/JCO.2009.23.8576. [DOI] [PubMed] [Google Scholar]
- 23.Vinceti M, Bonvicini F, Pellacani G, Sieri S, Malagoli C, et al. Food intake and risk of cutaneous melanoma in an Italian population. Eur J Clin Nutr. 2008;62:1351–1354. doi: 10.1038/sj.ejcn.1602850. [DOI] [PubMed] [Google Scholar]
- 24.Fortes C, Mastroeni S, Melchi F, Pilla MA, Antonelli G, et al. A protective effect of the Mediterranean diet for cutaneous melanoma. Int J Epidemiol. 2008;37:1018–1029. doi: 10.1093/ije/dyn132. [DOI] [PubMed] [Google Scholar]
- 25.Lips P. Vitamin D status and nutrition in Europe and Asia. J Steroid Biochem Mol Biol. 2007;103:620–625. doi: 10.1016/j.jsbmb.2006.12.076. [DOI] [PubMed] [Google Scholar]
- 26.Isaia G, Giorgino R, Rini GB, Bevilacqua M, Maugeri D, et al. Prevalence of hypovitaminosis D in elderly women in Italy: clinical consequences and risk factors. Osteoporos Int. 2003;14:577–582. doi: 10.1007/s00198-003-1390-7. [DOI] [PubMed] [Google Scholar]
- 27.Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124:869–871. doi: 10.1001/archderm.124.6.869. [DOI] [PubMed] [Google Scholar]
- 28.Pasanisi P, Berrino F, Bellati C, Sieri S, Krogh V. Validity of the Italian EPIC questionnaire to assess past diet. IARC Sci Publ. 2002;156:41–44. [PubMed] [Google Scholar]
- 29.Pala V, Sieri C, Palli D, Salvini S, Berrino F, et al. Diet in the Italian EPIC cohorts: presentation of data and methodological issues. Tumori. 2003;89:594–607. doi: 10.1177/030089160308900603. [DOI] [PubMed] [Google Scholar]
- 30.Salvini S, Saieva C, Sieri S, Vineis P, Panico S, et al. Physical activity in the EPIC cohort in Italy. IARC Sci Publ. 2002;156:267–269. [PubMed] [Google Scholar]
- 31.Hastie TJ, Tibshirani RJ. Generalized Additive Models. Boca Raton: Chapman & Hall/CRC; 1990. [Google Scholar]
- 32.R Development Core Team. A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2005. [Google Scholar]
- 33.Millen AE, Bodnar LM. Vitamin D assessment in population-based studies: a review of the issues. Am J Clin Nutr. 2008;87:1102S–1105S. doi: 10.1093/ajcn/87.4.1102S. [DOI] [PubMed] [Google Scholar]
- 34.Lym YL, Joh HK. Serum 25-hydroxyvitamin D3 is related to fish intake and exercise in Korean adult men. Asia Pac J Clin Nutr. 2009;18:372–376. [PubMed] [Google Scholar]
- 35.Hintzpeter B, Mensink GB, Thierfelder W, Muller MJ, Scheidt-Nave C. Vitamin D status and health correlates among German adults. Eur J Clin Nutr. 2008;62:1079–1089. doi: 10.1038/sj.ejcn.1602825. [DOI] [PubMed] [Google Scholar]
- 36.Hill TR, Cotter AA, Mitchell S, Boreham CA, Dubitzky W, et al. Vitamin D status and its determinants in adolescents from the Northern Ireland Young Hearts 2000 cohort. Br J Nutr. 2008;99:1061–1067. doi: 10.1017/S0007114507842826. [DOI] [PubMed] [Google Scholar]
- 37.Brock KE, Graubard BI, Fraser DR, Weinstein SJ, Stolzenberg-Solomon RZ, et al. Predictors of vitamin D biochemical status in a large sample of middle-aged male smokers in Finland. Eur J Clin Nutr. 2010 doi: 10.1038/ejcn.2009.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shea MK, Benjamin EJ, Dupuis J, Massaro JM, Jacques PF, et al. Genetic and non-genetic correlates of vitamins K and D. Eur J Clin Nutr. 2009;63:458–464. doi: 10.1038/sj.ejcn.1602959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Millen AE, Wactawski-Wende J, Pettinger M, Melamed ML, Tylavsky FA, et al. Predictors of serum 25-hydroxyvitamin D concentrations among postmenopausal women: the Women’s Health Initiative Calcium plus Vitamin D clinical trial. Am J Clin Nutr. 2010;91:1324–1335. doi: 10.3945/ajcn.2009.28908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roddam AW, Neale R, Appleby P, Allen NE, Tipper S, et al. Association between plasma 25-hydroxyvitamin D levels and fracture risk: the EPIC-Oxford study. Am J Epidemiol. 2007;166:1327–1336. doi: 10.1093/aje/kwm210. [DOI] [PubMed] [Google Scholar]
- 41.Malagoli C, Fabbi S, Teggi S, Calzari M, Poli M, et al. Risk of hematological malignancies associated with magnetic fields exposure from power lines: a case-control study in two municipalities of northern Italy. Environ Health. 2010;9:16. doi: 10.1186/1476-069X-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cornwell ML, Comstock GW, Holick MF, Bush TL. Prediagnostic serum levels of 1,25-dihydroxyvitamin D and malignant melanoma. Photodermatol Photoimmunol Photomed. 1992;9:109–112. [PubMed] [Google Scholar]
- 43.Newton-Bishop JA, Beswick S, Randerson-Moor J, Chang YM, Affleck P, et al. Serum 25-hydroxyvitamin D3 levels are associated with breslow thickness at presentation and survival from melanoma. J Clin Oncol. 2009;27:5439–5444. doi: 10.1200/JCO.2009.22.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Freedman DM, Looker AC, Abnet CC, Linet MS, Graubard BI. Serum Vitamin D and Cancer Mortality in the NHANES III Study (1988–2006) Cancer Res. 2010 doi: 10.1158/0008-5472.CAN-10-1420. [DOI] [PMC free article] [PubMed] [Google Scholar]