Abstract
Objectives
We evaluated the epidemiologic evidence that vitamin D may be related to human autoimmune disease risk.
Methods
PubMed limited to English from inception through April 2010 was searched using keywords: “vitamin D”, “autoimmune” and autoimmune disease names. We summarized in vitro, animal, and genetic association studies of vitamin D in autoimmune disease pathogenesis. We sorted studies by design and disease and performed a systematic review of: a) cross-sectional data concerning vitamin D level and autoimmune disease; b) interventional data on vitamin D supplementation in autoimmune diseases and c) prospective data linking vitamin D level or intake to autoimmune disease risk.
Results
Vitamin D has effects on innate and acquired immune systems and vitamin D receptor polymorphisms have been associated with various autoimmune diseases. In experimental animal models, vitamin D supplementation can prevent or forestall autoimmune disease. We identified 76 studies in which vitamin D levels were studied in autoimmune disease patients, particularly with active disease, and compared to controls. Nineteen observational or interventional studies assessed the effect of vitamin D supplementation as therapy for various autoimmune diseases (excluding psoriasis and vitiligo) with a range of study approaches and results. The few prospective human studies performed conflict as to whether vitamin D level or intake is associated with autoimmune disease risk. No interventional trials have investigated whether vitamin D affects human autoimmune disease risk.
Conclusions
Cross-sectional data point to a potential role of vitamin D in autoimmune disease prevention, but prospective interventional evidence in humans is still lacking.
Keywords: vitamin D, autoimmune disease, intake, risk factor, epidemiology, systematic review
Introduction
The cause of the breakdown in immune tolerance that allows for the development of immunity to self-targets in autoimmune diseases such as rheumatoid arthritis (RA), inflammatory bowel disease (IBD) and multiple sclerosis (MS) is unknown. It is hypothesized that environmental exposures, including factors that stimulate endogenous inflammation, trigger the development of autoimmunity in genetically susceptible individuals(1). Autoimmune diseases cluster within families and within individuals, with many individuals developing more than one autoimmune disease(2-4). Polymorphisms in several genes have been associated with increased susceptibility to multiple autoimmune diseases(2, 5-8). Autoimmune diseases also share epidemiologic risk factors such as cigarette smoking and crystalline silica exposure(9, 10). Many autoimmune diseases are characterized by activation of the adaptive immune system with associated innate immune cell activation leading to inappropriately elevated levels of widespread systemic inflammation, in particular tumor necrosis factor-α (TNFα) and interleukins-1 and -6 (IL-1 and IL-6), potent cytokines produced by macrophages and monocytes among other cell types.
The Autoimmune Diseases Coordinating Committee of the National Institutes of Health estimated that 23.5 million Americans were affected by one or more autoimmune diseases in 2005(11). This number appears to be growing and is almost certainly an underestimate(12). For unknown reasons, most autoimmune diseases are more common among women than men, although this is less true after menopause(13). Together, autoimmune diseases are the third leading cause of morbidity in the industrialized world and are a leading cause of mortality among women(14, 15). With the exceptions of celiac sprue and pernicious anemia, there are no current means for the prevention or cure of most autoimmune diseases. Treatment of autoimmunity often consists of corticosteroids, immunosuppressant agents and biologic agents that target anti-tumor necrosis factor and other inflammatory cytokines. The 2005 NIH report estimated that the annual direct and indirect costs of autoimmune diseases in the U.S. far exceed $100 billion annually.
Vitamin D, obtained from diet, supplements, or conversion of 7-dehydrocholesterol in the skin by ultraviolet-B radiation, is hydroxylated in the liver to 25-hydroxyvitamin D [25(OH)D], the major circulating vitamin D metabolite(16, 17), which is further synthesized by 1α-hydroxylase to the active form, 1,25-dihydroxyvitamin D [1,25(OH)2D]. Current dietary recommendations are geared only to prevent quite low vitamin D levels. Circulating 1,25(OH)2D has a very short half life and is tightly regulated by parathyroid hormone (PTH). Fibroblast growth factor 23 (FGF23), which is produced in osteoblasts, is also important in regulating 1,25 (OH)2 production in the kidney(18). “Normal” levels for 25(OH) D at most laboratories are >20ng/ml. However, at 25(OH)D levels below 30ng/ml, PTH secretion is increased, suggesting that the current “normal” levels for vitamin D are inadequate.(19-21) Vitamin D deficiency using the current standard for serum25(OH)D level is relatively common. In one study, over 50% of inpatients at Massachusetts General Hospital had low 25(OH)D levels(22).
Vitamin D is an inexpensive and relatively safe nutritional supplement, widely held to have anti-inflammatory and immunomodulating effects. The purported health benefits of this inexpensive and available dietary supplement have received enormous attention in both the medical literature and the popular press(23-26). It has been widely hypothesized that vitamin D deficiency acts as an environmental trigger for the induction of autoimmunity, and that high-dose vitamin D supplementation could be preventive(27-34), yet the scientific evidence appears to conflict. The Agency for Healthcare Research and Quality recently performed a systematic review entitled, “Vitamin D and Calcium: A Systematic Review of Health Outcomes”, published in August 2009(35). The report investigated the published literature regarding serum 25 (OH) D or 1,25 (OH)2 D concentrations and multiple disease outcomes, including autoimmune diseases. The review did not include studies investigating sunlight exposure as a source of vitamin D intake, nor did it include studies in which dietary intake of vitamin D was assessed without measurement of serum levels, as nutrient composition tables for vitamin D were thought to be inadequate. The report also excluded cross-sectional and retrospective case-control studies where the measure of exposure occurred after or concurrent with the outcome. After these exclusions, no studies were identified that addressed the relationship between vitamin D and incident autoimmune disease. We sought to perform an extensive systematic review of the published literature to evaluate the strength of all types of evidence linking vitamin D intake to the risk of incident autoimmune diseases.
Methods
We searched the PubMed database from inception through April 2010 restricted to English language and human studies with the following search terms “vitamin D AND”: “autoimmune disease”; “autoimmunity”, “rheumatoid arthritis”; “spondylitis”: “spondyloarthropathy”; “psoriatic arthritis”; “systemic lupus erythematosis”; “scleroderma”; “systemic sclerosis”; “myositis”; “dermatomyositis”; “polymyositis”; “vasculitis”; “polymyalgia rheumatica”; “type 1 diabetes”; “multiple sclerosis; “autoimmune thyroiditis“; “Graves”; “Hashimoto's”; “inflammatory bowel disease”; “Crohn's disease”; “ulcerative colitis”; “vitiligo”; “autoimmune hepatitis”; “Behcet's”; “uveitis”; “Addison's”. We excluded review articles, case reports and studies primarily related to bone metabolism or osteoporosis (unless they included relevant data on vitamin D levels). Studies on the topical and parenteral treatment of psoriasis and vitiligo with vitamin D analogs were beyond the scope of this review and treatment with vitamin D-related compounds has been extensively reviewed in a systematic manner (e.g. (36). In addition, primary biliary cirrhosis and celiac disease were not included in the PubMed search since loss of fat-soluble vitamins is common in these diseases and vitamin D deficiency is highly prevalent in cirrhotics(37).We also conducted hand searches of reference lists to add to our list of manuscripts.
We aimed to address the following questions: 1) What are the cross-sectional data linking vitamin D to autoimmune disease?; 2) What are the prospective data linking vitamin D to the risk of future autoimmune disease? 3) What are the interventional data concerning dietary and/or supplemental vitamin D and risk of autoimmune disease? We carefully reviewed the abstracts of the studies identified and selected those relevant to our questions to perform a systematic review of the strengths and weaknesses of this literature. We included and qualitatively summarized background basic science studies, both in vitro and in animal models, genetic studies, studies of vitamin D's effects upon biomarkers of systemic inflammation, as well as ecologic/geographic epidemiologic studies of the incidence or prevalence of autoimmune diseases according to latitude (potentially related to vitamin D from UV light).
To explore the potential for publication bias among studies of vitamin D intake and risk of developing autoimmune disease, a funnel plot was created for these studies by plotting the risk estimates (odds ratios or relative risks) of developing an autoimmune disease in each study (x axis) against the number of autoimmune cases in each study (y axis). (A pattern resembling a symmetrical inverted funnel is generally interpreted as showing no significant publication bias, whereas the absence of studies in the lower sections of an inverted funnel, where small studies would lie, implies the presence of publication bias.)(38)
Results
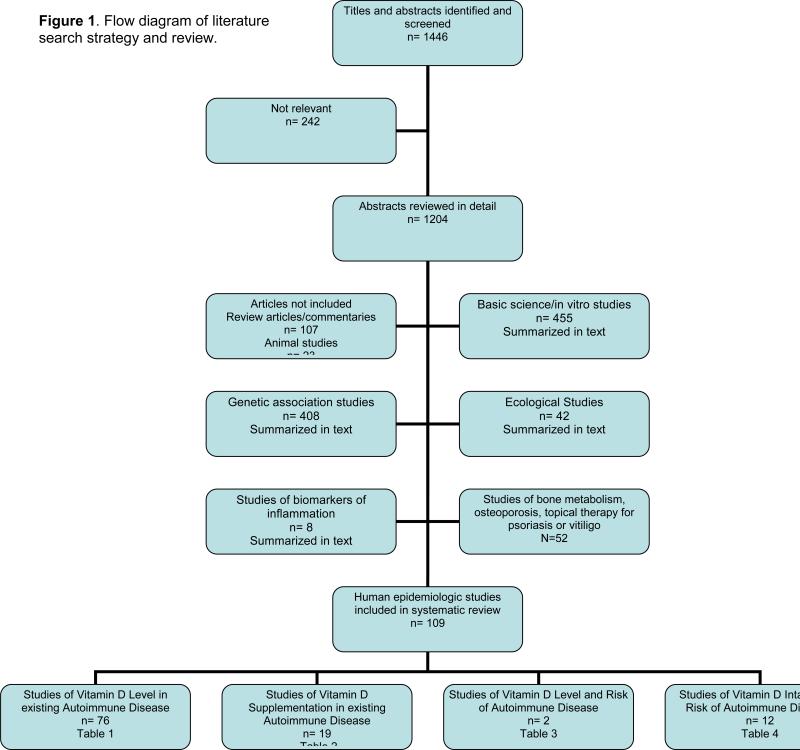
We identified 1446 potentially relevant studies through our literature search and sorted and reviewed them as in Figure 1. Two hundred and forty-two were immediately excluded as irrelevant to this review, leaving 1204 that were reviewed in further detail. Basic science and in vitro studies, genetic studies, ecological studies, and studies of biomarkers of inflammation, were reviewed and summarized in the text. The human epidemiologic studies were sorted into epidemiologic studies of vitamin D levels in existing autoimmune disease (Table 1), studies and trials of vitamin D supplementation in existing autoimmune disease (Table 2), epidemiologic studies of vitamin D level and risk of autoimmune disease (Table 3) and epidemiologic studies of vitamin D intake and risk of autoimmune disease (Table 4). We reviewed the evidence in each of the studies these tables in detail and summarized it in the text.
Figure 1.
Flow diagram of literature search strategy and review.
Table 1.
Epidemiologic Studies of Vitamin D Levels in Existing Autoimmune Diseases
| Autoimmune Disease | Geographic | Study Design | Subjects | Controls | Hormones studied | Association | Results | Year | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Rheumatoid Arthritis | Turkey | Case-control | 65 RA | 40 Healthy controls | 25(OH) D | No | P = 0.94 No difference in RA subjects vs. healthy controls, but significant decrease in subgroup with highest disease activity | 2010 | Turnahoglu et al, (116) |
| Rheumatoid Arthritis | North America | Case-only | 266 early RA | - | 25(OH) D | No | No multivariate associations of 25(OH) D with any disease measures with the exception of borderline association with rheumatoid factor positivity at enrollment (p = 0.05) No significant associations with disease activity after multivariate analysis | 2010 | Craig et al, (117) |
| Rheumatoid Arthritis, Psoriatic Arthritis, Ankylosing Spondylitis | Israel | Case-control | 85 RA, 22 psoriatic arthritis, 14 AS | - | 25(OH) D and PTH | No | Association between vitamin D level and ethnic origin but not disease activity (among other factors) | 2009 | Braun-Moscovici et al, (210) |
| Rheumatoid Arthritis | North America | Case-control (Nested in high risk cohort) | 76 RA autoantibody positive, asymptomatic “at-risk” subjects | 154 RA autoantibody negative “at risk” controls | 25(OH) D | No | No association of autoantibody status and vitamin D level in individuals at high risk RA. | 2009 | Feser et al, (122) |
| Rheumatoid Arthritis | Italy, Estonia | Case-control | 64 female Estonian RA, 53 female Italian RA | 30 Estonian, 35 Italian age- and sex-matched | 25(OH) D | Yes | Inverse correlation between levels and DAS28 scores among Italian patients in summer (r = -0.57, P< 0.0001) and Estonian patients in winter (r = -0.40, p< 0.05) | 2007 | Cutolo et al, (211) |
| Inflammatory Polyarthritis | Great Britain | Case-only | 183 consecutive inflammatory polyarthritis < 6 months | - | 25(OH)D, 1,25(OH)2 D | Yes | Signficant inverse relationship between 25(OH)D level at baseline and tender joint count, DAS28*, CRP, and HAQ* and between baseline 1,25(OH)(2)D and HAQ*. | 2007 | Patel et al, (121) |
| Rheumatoid Arthritis | Germany | Case-only | 96 RA | - | 25(OH) D and PTH | Yes | P <0.001 (with glucocorticoids), P < 0.01 (without glucocorticoids) Inverse correlation between vitamin D level and disease activity | 1998 | Oelzner et al, (212) |
| Rheumatoid Arthritis | Denmark | Case-control | 29 RA, 21 SLE, 12 OA | 72 Healthy controls | 1,25(OH)2 D and 25(OH) D | No | No difference in vitamin D levels compared to controls | 1995 | Muller et al, (118) |
| Rheumatoid Arthritis | Finland | Case-only | 143 female RA patients | - | 1,25(OH)2 D 25(OH)D | Yes | 63% patients had levels below normal limit during summer | 1993 | Kroger et al, (213) |
| Rheumatoid Arthritis | Denmark | Case-control | 102 RA | 38 Healthy subjects | 25(OH) D; 24,25(OH) D; 25,26-OH D; 1,25(OH)2 D | Yes | P <0.01-0.001 (for 25(OH) D) 25(OH) D levels lower than in controls and significant inverse relation between level and functional class | 1987 | Als et al, (115) |
| Rheumatoid Arthritis | Great Britain | Case-control | 30 RA | 30 OA | 1,25(OH)2 D | No | No difference between RA and OA and 1,25(OH)2 D levels did not correlate either with articular index or with sedimentation rate | 1982 | Bird et al, (119) |
| Rheumatoid Arthritis | Great Britain | Case-control | 30 RA | 30 OA | 25(OH) D | No | No significant correlations between 25(OH) D and duration of arthritis or articular index | 1980 | Bird et al, (120) |
| Ankylosing Spondylitis and Psoriatic Arthritis | Germany | Case-only | 76 AS, 120 PsoA | - | 25(OH) D; 1,25(OH)2 D | Yes | P < 0.0005 for negative correlation between CRP and 25-OH D when combining AS and PsoA; P < 0.0005 for 25-OH level in PsoA versus AS | 2009 | Teichmann et al, (141) |
| Ankylosing Spondylitis | Turkey | Case-control | 100 AS | 58 Healthy controls | 25(OH) D and PTH | No | P < 0.05 for 25(OH) D lower in cases than controls | 2010 | Mermerci Baskan et al, (137) |
| Ankylosing Spondylitis | Germany | Case-control | 58 AS | 58 matched healthy controls | 25(OH) D; 1,25(OH)2 D; PTH | Yes | P < 0.05 for negative correlation between 1,25(OH)2 D and disease activity and TNF-alpha | 2005 | Lange et al, (140) |
| Ankylosing Spondylitis | Germany | Case-control | 70 AS | 45 matched healthy controls | 25(OH) D; 1,25(OH)2 D; PTH | Yes | P < 0.01 for negative correlation between 1,25(OH)2 D and disease activity | 2001 | Lange et al, (139) |
| Ankylosing Spondylitis | Austria | Case-only | 73 AS | - | 25(OH) D and PTH | - | 18% with 25(OH) D < 8 ng/ml, 73% with 25(OH) D less than 20 ng/ml | 2001 | Falkenbach et al, (214) |
| Ankylosing Spondylitis | Germany | Case-only | 14 AS at entry and 15 months later | - | 25(OH) D; 1,25(OH)2 D and PTH | No | Vitamin D levels did not differ significantly between baseline and follow-up in AS patients | 1997 | Lee et al, (215) |
| Ankylosing Spondylitis | Germany | Case-control | 38 AS | 52 controls | 1,25(OH)2 D and PTH | No | No significant difference | 1993 | Franck et al, (138) |
| Systemic Lupus Erythematosus | Poland | Case-control | 45 SLE | 49 controls | 25(OH) D | Yes | Lower 25(OH)D in cases than controls p = 0.0005, and antibodies to 1,25 (OH) D detected in 4 pts (8.9%, NS). | 2010 | Bogaczewicz et al, (127) |
| Systemic Lupus Erythematosus | Europe and Israel | Case-only | 378 SLE | - | 25(OH) D | Yes | R= -0.12, P = 0.018 for vitamin D levels and disease activity scores Negative correlation between 25(OH) D levels and disease activity in SLE patients | 2010 | Amital et al, (129) |
| Systemic Lupus Erythematosus | South Korea | Case-control | 104 SLE | 49 controls | 25(OH) D | Yes | P = 0.03 for vitamin D insufficiency in SLE compared to controls, but did not correlate with SLE disease activity. | 2010 | Kim et al, (123) |
| Systemic Lupus Erythematosus | North America | Case-only | 198 SLE | - | 25(OH) D | Yes | R = -0.234, P = 0.002 for inverse correlation of vitamin D with disease activity | 2010 | Ben-Zvi et al, (128) |
| Systemic Lupus Erythematosus | Canada | Case - only | 124 female SLE | - | 25(OH) D and 1,25(OH)2 D | No | No significant association between low vitamin D levels and disease activity; 25(OH) D levels associated with season, glucocorticoid exposure, and serum creatinine | 2010 | Toloza et al, (132) |
| Systemic Lupus Erythematosus | Saudi Arabia | Case-control | 165 SLE | 214 volunteers | 25(OH) D | Yes | P < 0.0001 for vitamin D deficiency in SLE versus controls | 2009 | Damanhouri, (124) |
| Systemic Lupus Erythematosus | North America | Case-only | 181 SLE | - | 25(OH) D | Yes | P = 0.018 for negative correlation between low 25(OH) D and SLE disease activity index (adjusted for age, season and white race) | 2009 | Wu et al, (130) |
| Systemic Lupus Erythematosus | North America | Case-only | 38 pediatric SLE | 207 healthy controls | 25(OH) D; 1,25(OH)2 D; iPTH | Yes | P = 0.01 for low 25(OH) D low level and disease activity index scores | 2009 | Wright et al, (125) |
| Systemic Lupus Erythematosus | Brazil | Case-control | 36 SLE | 26 controls | 25(OH) D; 1,25(OH)2 D; PTH | Yes | R = -0.65; P < 0.001 for 25(OH) D level and negative correlation with disease activity index | 2009 | Borba et al, (126) |
| Systemic Lupus Erythematosus | Spain | Case-only | 92 SLE | - | 25(OH) D | No | P = 0.08 for fatigue and vitamin D deficiency; no significant association with disease activity | 2008 | Ruiz-Irastorza et al, (133) |
| Systemic Lupus Erythematosus | U.S. | Case-only | 37 SLE | - | 25(OH)D | Yes | 65% < 80 nmol/L and 20% <47.7 nmol/L. Above normal level correlated with low disease activity, but also with significantly higher dsDNA antibodies. | 2008 | Thudi et al, (131) |
| Systemic Lupus Erythematosus | Canada | Case-only | 25 SLE | - | 25(OH) D; 1,25(OH)2 D; PTH | No | 1,25-OH lower in SLE patients using hydroxychloroquine compared to nonusers | 2001 | Huisman et al, (135) |
| Systemic Lupus Erythematosus | Denmark | Case-control | 21 SLE, 29 RA, 12 OA | 72 Healthy controls | 1,25(OH)2 D and 25(OH) D | Yes | P = 0.0008 for decreased 25(OH) levels in SLE versus controls; no correlation with anti-DNA antibodies, sedimentation rate, or blood counts. | 1995 | Muller et al, (118) |
| Systemic Lupus Erythematosus and Dermatomyositis and Juvenile RA | North America | Case-only | 17 pediatric SLE, 13 juvenile dermatomyositis, 83 JRA | - | 25(OH) D; 1,25(OH)2 D; PTH | No | No significant differences between active and inactive stages of pediatric SLE with regards to vitamin D levels | 1990 | Reed et al, (134) |
| Undifferentiated Connective Tissue Disease | Hungary | Case-only | 161 UCTD | 59 controls | 25(OH)D | Yes | 25(OH)D significantly lower than in controls in summer and winter. Significant associations of low 25(OH)D with active manifestations and with evolution to diagnosed connective tissue disease within 2.3 years. | 2008 | Zold et al, (136) |
| Scleroderma | Italy | Case-only | 108 scleroderma | - | 25(OH) D | Yes | Vitamin D deficiency is associated with more severe disease P = 0.026 for longer disease duration, P = 0.014 for lower diffusing lung capacity, P = 0.037 for higher pulmonary artery pressure | 2010 | Carameschi et al, (147) |
| Scleroderma | Brazil | Case-control | 10 juvenile scleroderma | 10 matched controls | 25(OH) D, iPTH | Yes | P = 0.04 for lower vitamin D levels compared with controls | 2010 | Shinjo et al, (143) |
| Scleroderma | Italy | Case-control | 60 Scleroderma | 60 controls | 25(OH) D | Yes | P < 0.001 for lower vitamin D levels compared with controls, but no associations with disease features or skin score | 2009 | Calzolari et al, (142) |
| Scleroderma | France and Italy | Case-only | 90 scleroderma | - | 25(OH) D and iPTH | Yes | R = -0.17 (P = 0.04) for negative correlation between low vitamin D and disease activity score | 2009 | Vacca et al, (148) |
| Scleroderma | Israel | Case-only | 60 scleroderma | - | 25(OH) D, PTH | - | 46% of scleroderma patients are vitamin D deficient | 2008 | Braun-Moscovici et al, (179) |
| Scleroderma | North America | Case-control | 8 scleroderma | 8 matched healthy controls | 25(OH) D; 1,25(OH)2 D | No | Similar levels in cases and controls. | 1991 | Matsuoka et al, (144) |
| Scleroderma | Holland | Case-control | 20 scleroderma | - | 25(OH) D; 1,25(OH)2 D; 24-25(OH) D | No | Normal 25(OH) D and 24,25(OH) D levels in scleroderma; lower 1,25(OH)2 D in a subgroup with calcinosis | 1985 | Serup et al, (145) |
| Scleroderma | Holland | Case-control | 25 scleroderma | 92 controls | 1,25(OH)2 D | No | P < 0.001 for higher 1,25(OH)2 D in scleroderma compared to controls | 1984 | Serup et al, (146) |
| Type I Diabetes Mellitus | India | Case-control | 50 children within 1 week of diagnosis of T1D* | 50 healthy children | 25(OH) D | Yes | P < 0.009 for lower vitamin D in new onset diabetics | 2009 | Borkar et al, 8 (149) |
| Type I Diabetes Mellitus | North America | Case-control | 46 new-onset T1D*, 110 established T1D, | 153 control subjects; 106 first-degree relatives | 25(OH) D | No | P = 0.87 No significant associations of reduced vitamin D level and T1D | 2009 | Bierschenk et al, (158) |
| Type I Diabetes Mellitus | Great Britain | Case-control | 40 T1D,* 40 T2D* | 41 non-diabetic controls | 1,25(OH)2 D, PTH, Erythropoietin | Yes | P = 0.001 for median vitamin D level in cases vs. controls. Tubulointerstitial damage associated with low 1,25(OH)2 D | 2009 | Singh et al, (150) |
| Type I Diabetes Mellitus | North America | Case-only | 128 Pediatric T1D* | - | 25(OH) D | - | 61% Vitamin D insufficient; 15% Vitamin D deficient | 2009 | Svoren et al, (216) |
| Type I Diabetes Mellitus | Qatar | Case-control | 170 pediatric T1D* | 170 healthy controls | 25(OH) D, PTH | Yes | P = 0.009 for mean vitamin D level; 28.8% versus 17.1% severe vitamin D deficiency | 2008, 2009 | Bener et al, (217, 151) |
| Type I Diabetes Mellitus | Australia | Case-only | 64 pediatric new-onset T1D* | - | 25(OH) D | - | P = 0.001 (for associate acidosis) Low vitamin D in 42% with acidosis versus 5.6% without acidosis Acidosis may alter vitamin D metabolism or low vitamin D may contribute to presenting with ketoacidosis | 2009 | Huynh et al, (160) |
| Type I Diabetes Mellitus | Australia | Case-control | 47 pediatric T1D* | 94 Healthy controls | 25(OH) D, 1,25(OH)2 D | Yes | P = 0.002 for 25(OH) D deficiency | 2007 | Greer et al, (152) |
| Type I Diabetes Mellitus | Sweden | Case-control | 459 T1D* at diagnosi, 138 8 years later | 208 - matched controls | 25(OH) D | Yes | P < 0.0001 for vitamin D level at diagnosis; P = 0.04 for vitamin D level 8 years later | 2006 | Littorin et al, (155) |
| Type I Diabetes Mellitus | North America | Case-control | 50 T1D* | 63 T2D* | 25(OH) D | No | P = 0.01 for lower 25-OH-D levels in T2D versus T1D* (adjusted for body mass index and age) | 2006 | Di Cesar et al, (156) |
| Type I Diabetes Mellitus | Italy | Case-control | 46 T1D* | 24 healthy controls | 25(OH) D; 1,25(OH)2 D; PTH | Yes | P < 0.01 for vitamin D levels Lower vitamin D level in incipient nephropathy/microalbuminuria | 1999 | Verrotti et al, (157) |
| Type I Diabetes Mellitus | Germany | Case-control | 49 new onset T1D* | 42 healthy controls | 25(OH) D; 1,25(OH)2 D | Yes | P < 0.01 for 1,25(OH)2 D at onset of T1D | 1991 | Baumgartl et al, (153) |
| Type I Diabetes Mellitus | Mexico | Case-only | 22 T1D* | - | 25(OH) D | Yes | P < 0.001 for low 25(OH) D in poorly controlled T1D* | 1990 | Arreola et al, PMID 2103709 (159) |
| Type I Diabetes Mellitus | Norway | Case-control | 46 pubertal T1D* | 191 Healthy controls | 25(OH) D; 1,25(OH)2 D; 24,25(OH) D | Yes | P < 0.05 for 1,25(OH)2 D Relative decrease in 1,25(OH)2 D and increased 24,25(OH) D levels in T1D at puberty | 1985 | Rodland et al, (154) |
| Type I Diabetes Mellitus | Denmark | Case-only | 74 T1D* | - | 25(OH) D; 1,25(OH)2 D; 24,25(OH) D | - | P < 0.02 for 1,25-OH level during ketoacidosis; P < 0.01 for 25(OH) D in T1D groups with diabetic nephropathy | 1983 | Storm et al, (161) |
| Multiple Sclerosis | Holland | Case-control | 36 MS | 20 Healthy controls | 25(OH) D | - | R= -0.359, P = 0.048 25(OH) D negative correlation with IgG index in MS | 2010 | Vogt et al, (162) |
| Multiple Sclerosis | Norway | Case-control | 36 MS | 38 other neurologic diseases | Serum and cerebrospinal fluid 25(OH) D | Yes | P = 0.0012 and 0.041 for cerebrospinal fluid-to-vitamin D serum ratio (lower in MS compared with other neurological diseases) | 2010 | Holmoy et al, (164) |
| Multiple Sclerosis | North America | Case-control | 173 MS +9 transverse myelitis, | 16 other neurologic diseases | 25(OH) D | - | 84% of all patients had insufficient levels Large numbers of patients with MS and transverse myelitis are deficient in vitamin D | Hiremath et al, (165) | |
| Multiple Sclerosis | Argentina | Case-control | 132 MS-various forms | 60 Healthy controls | 1,25(OH)2 D, 25(OH) D | Yes | P < 0.00001 for 25(OH) D and 1,25(OH)2 D levels (lower in relapsing-remitting MS during exacerbation compared with remission | 2009 | Correale et al, (167) |
| Multiple Sclerosis | Holland | Case-control | 103 MS | 110 Healthy controls | 1,25(OH)2 D, 25(OH) D | Yes | Among women: for every 10 nmol/L increase in serum 25(OH)D level the odds of MS decreased 19% (OR = 0.81; 95% CI 0.69-0.95) for dose-dependent decreased odds of MS among women); r= -0.29; P = 0.02 for negative correlation between disability status and 25(OH) D levels in women only | 2009 | Kragt et al, (166) |
| Multiple Sclerosis | Finland | Case-control | 23 MS | 23 Healthy controls | 25(OH) D and iPTH every 3 months for 1 year | Yes | P = 0.012 for inverse relationship between serum vitamin D level and MS clinical activity | 2008 | Soilu-Hanninen et al, (218) |
| Multiple Sclerosis | Great Britain | Case-control twin study | 40 monozygotic and 59 dizygotic twins with MS | 40 monozygotic and 59 dizygotic twins without MS | 25(OH) D | No | No association with having MS (P = 0.4) | 2008 | Orton et al, (170) |
| Multiple Sclerosis | Holland | Case-control | 267 MS | - | 25(OH) D, 1,25(OH)2 D | Yes | P = 0.043 for high 25(OH) D and chance of remaining relapse-free | 2008 | Smolders et al,(172) |
| Multiple Sclerosis | Ireland | Case-control | 29 MS | 22 age- and sex-matched controls | 25(OH) D; 1,25(OH)2 D; PTH | No | No differences between cases and controls | 2007 | Barnes et al, (169) |
| Multiple Sclerosis | Australia | Case-control | 136 MS | 272 controls | 25(OH) D | Yes | OR = 3.07 (95% CI 1.37-6.90) for disability and vitamin D insufficiency | 2007 | van der Mei et al, (163) |
| Multiple Sclerosis | Finland | Case-control | 40 MS | 40 Controls | 25(OH) D | Yes | P = 0.03 for lower vitamin D level during relapse versus remission | 2005 | Soilu-Hanninen et al, (171) |
| Multiple Sclerosis | Germany | Case-control | 53 MS | 415 Controls | 25(OH) D | Yes | R2 = 0.8491 for vitamin D level; R2 = 0.7931 for brain lesions (two-fitted, third-order polynomial curves corresponding closely when 25(OH) D data lagged 2 months) Inverse correlation of gadolinium-enhancing lesions on MRI and vitamin D levels following seasonal fluctuations | 2000 | Embry et al, (168) |
| Hashimoto's Thyroiditis | India | Case-only | 642 healthy individuals | - | 25(OH) D | - | r - 0.08, P = 0.04 for vitamin D level inverse correlation with anti-thyroid antibodies | 2009 | Goswami et al, (177) |
| Crohn's disease | India | Case-control | 34 Crohn's | 34 irritable bowel syndrome controls | 25(OH) D | Yes | Correlation coefficient -0.484, significance P<0.004 Lower vitamin D levels in Crohn's disease and association with severe disease activity | 2009 | Joseph et al, (173) |
| Crohn's disease and Ulcerative colitis | America | Case-only | 130 young Crohn's, and UC* | - | 25(OH) D, iPTH | No | P =0.97 in multiple regression for vitamin D level and disease activity | 2006 | Pappa et al, (176) |
| Crohn's disease | Japan | Case-control | 33 Crohn's | 15 healthy controls | 25(OH) D, iPTH | Yes | P = 0.04 for vitamin D and disease activity in logistic regression | 2004 | Tajika et al(174) |
| Crohn's disease | Great Britain | Case-only | 40 Crohn's | - | 25(OH) D, 24,25(OH) D, 1,25(OH)2 D, PTH | Yes | P < 0.05 for low 25(OH) D levels in active disease | 1985 | Harries et al (175) |
| Vitiligo | Great Britain | Case-only | 45 Vitiligo | - | 25(OH)D | Yes | 55.6% were insufficient (<30 ng/mL), and 13.3% were very low (<15 ng/mL). | 2007 | Silverberg, et al.(178) |
DAS28= Disease Activity Score 28 joint assessment; HAQ= Health Assessment Questionnaire; PsOA= psoriatic arthritis; T1D= type 1 diabetes; T2D= type 2 diabetes; UC= ulcerative colitis
Table 2.
Studies and Trials of Vitamin D Supplementation in Existing Autoimmune Disease
| Autoimmune Disease | Population | Study Design | Subjects | Controls | Vitamin D analog | Follow-up Period/Dura tion of Study | Results | Year | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Rheumatoid Arthritis | Yugoslavia | Open-label trial | 19 RA on DMARDs | - | oral alphacalcidiol (2 ug/d) | 3 months | Clinical improvement in the majority of 89% | 1999 | Andjelkovic et al, (182) |
| Rheumatoid Arthritis | Sweden | Double-blind trial | 25 RA treated | 24 RA placebo | oral calciferol (100,000 IU/d) | 1 year | 67% Objective and subjective improvement; lower sedimentation rates and higher hemoglobin levels | 1973 | Brohult et al, (181) |
| Psoriatic Arthritis | Hungary | Open-label trial | 10 PsoA | 9 PsoA placebo | oral alphacalcidol (0.25 ug twice/d) | 6 months | P = 0.048 for disease activity at 6 months | 2009 | Gaal et al(183) |
| Psoriatic Arthritis | North America | Open-label study | 10 PsoA | - | Oral 1,25(OH)D 2ug/d | 6 months | P < 0.01 for tender joint count | 1990 | Huckins et al, (184) |
| Systemic Lupus Erythematosus | Spain | Open-label; observational | 60 SLE | 20 SLE | various doses cholecalciferol or calcidiol liquid | 2 years | P = 0.87 and P = 0.63 for disease activity and damage indices; P = 0.001 for fatigue based on a visual analogue scale. No effect on SLE activity. | 2010 | Ruiz-Irastorza et al, (185) |
| Scleroderma | Holland | Randomized, double-blind, placebo controlled trial | 7 scleroderma, 20 morphea | - | Oral calcitriol 0.75 ug/d for 6 months plus 1.25 ug/d for 3 months | 9 months with 6 months followup | No significant difference between placebo and calcitriol with regards to skin score | 2000 | Hulshof et al, (190) |
| Scleroderma | Macedonia | Open-label trial | 3 morphea | - | Oral calcitriol 0.5-0.75 ug/day | 4-6 months with 6 months followup | Significant clinical improvement with regards to joint mobility, skin flexibility, skin induration | 1999 | Caca-Biljanovska et al, (188) |
| Scleroderma | Holland | Open-label trial | 7 pediatric linear scleroderma | - | Oral calcitriol 0.25-0.75 microgram/day per dose-escalating protocol | 3-10.5 months | 5 out of 7 patients with good to excellent improvement of skin lesions | 1999 | Elst et al, (189) |
| Scleroderma | Holland | Open-label trial | 3 generalized morphea | - | Oral calcitriol 0.5-0.75 ug/day | 3-7 months | Improved mobility of joints and increased skin extensibility | 1994 | Hulshof et al, (187) |
| Scleroderma | France | Open-label trial | 11 scleroderma p | - | Oral calcitriol at a mean dose of 1.75 ug/day | 6 months to 3 years | Significant improvement of clinical findings | 1993 | Humbert et al, (186) |
| Type I Diabetes Mellitus | China | Randomized controlled trial | 17 latent autoimmune diabetes (LADA) | 18 LADA | 1 alpha-OH D3 0.5 ug/day (+/- Insulin) | 1 year | P < 0.01; 70% versus 22% maintained fasting C-peptide 1 alpha-OH D3 plus insulin therapy can preserve beta-cell function in patients with LADA | 2009 | Li et al, PMID 19488999 (191) |
| Type I Diabetes Mellitus | Italy | Randomized controlled trial | 34 recent-onset T1D* receiving calcitriol plus insulin | 33 recent-onset T1D receiving nicotinamide plus insulin | Calcitriol 0.25 ug on alternate days | 1 year | P < 0.03 for reduction in insulin requirement at 3 and 6 months but not 9 and 12 months Calcitriol has a modest effect on residual beta-cell function and only temporarily reduces the amount of insulin required | 2006 | Pitocco et al, (192) |
| Multiple Sclerosis | Canada | Randomized controlled trial | 25 MS | 24 MS | up to 40,000 IU/day over 28 weeks, then 10,000 IU/day for 12 weeks | 52 weeks (Phase I/II dose-escalation trial) | P = 0.09 for annualized relapse rate (0.26 versus 0.45) treatment group patients appeared to have fewer relapse events although not statistically significant | 2010 | Burton et al,(195) |
| Multiple Sclerosis | North America | Observational study | 40 MS on either low dose cholecalciferol or high dose ergocalciferol | 9 MS without vitamin D | cholecalciferol (< 800 IU/day) or ergocalciferol (50,000 IU/day) for 7-10 d then weekly or biweekly doses) | 4 to 12 months | P = 0.01 for 25(OH) D increase in patients switching from low to high dose supplementation; effect on relapse rate not studied | 2009 | Hiremath et al (165) |
| Multiple Sclerosis | Canada | Open-label trial | 12 MS patients in an active phase | - | Daily calcium + increasing doses of vitamin D3 (from 28,000 to 280,000 IU/week) | 28 weeks | P = 0.03 for decreased number of gadolinium-enhancing lesions per patient Vitamin D did not affect disease progression or activity but the number of gadolinium-enhancing lesions per patient decreased | 2007 | Kimball et al(194) |
| Multiple Sclerosis | North America | Open-label trial | 15 cases of relapsing-remitting MS | - | Oral calcitriol 2.5 ug/day | 48 weeks | 33% at baseline versus 29% at both 24 and 48 weeks (for gadolinium-enhancing lesions). On-study exacerbation rate (27%) was less than baseline. | 2005 | Wingerchuk et al, (219) |
| Multiple Sclerosis | Israel | Open-label trial | 5 relapsing-remitting MS | - | Alphacalcidol 1.5 ug/day | 6 months | 3 patients stable, 1 improved, 1 relapsed | 2003 | Achiron et al, (220) |
| Multiple Sclerosis | U.S. | Observational | 16 MS | - | Calcium, Magnesium and vitamin D as cod liver oil cod liver oil at the rate of 5,000 i.u./day | 1-2 years | Fewer exacerbations than expected historically p<0.01 | 1986 | Goldberg et al,(193) |
| Inflammatory Bowel Disease | Eastern Europe | Open-label trial | 17 Crohn's taking 1,25(OH) 2 D | 19 Crohn's taking 25(OH) D | Supplemental (1,25 OH versus 25(OH) D) | 12 months | P < 0.05 (for disease activity) 1,25(OH)2 D has a more prominent beneficial effect on disease activity compared with 25(OH) D | 2009 | Miheller et al, (196) |
DMARDs= disease-modifying anti-rheumatic drugsPsOA= psoriatic arthritis; T1D= type 1 diabetes; LADA= latent autoimmune diabetes
Table 3.
Epidemiologic Studies of Vitamin D Level and Risk of Developing an Autoimmune Disease
| Autoimmune Disease | Population | Study Design | Subjects | Controls | Hormone studied | Confounders considered | Results | Year | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Rheumatoid Arthritis | Holland | Case-control | 79 subjects who donated blood and later developed RA. Samples from 3 time points: 1, 2, and 5 years before symptoms | 79 controls matched for age, sex, and time of sample donation | 25(OH) D | Age, sex, time sample donation | No association between vitamin D level and preclinical RA (1, 2, 5 and more years before symptoms) | 2006 | Nielen et al, (197) |
| Multiple Sclerosis | North America | Case-control | 257 military recruits with banked blood who later developed MS | 514 controls matched for age, sex, race/ethnicity, and dates of blood collection | 25(OH) D | Age, sex, race/ethnicity, and dates of blood collection | OR 0.59, 95% CI 0.36-0.97, for the risk of MS among whites. Inverse relation is particularly strong before age 20. No association among Blacks and Hispanics | 2006 | Munger et al, (198) |
Table 4.
Epidemiologic Studies of Vitamin D Intake and Risk of Developing Autoimmune Disease
| Autoimmune Disease | Population | Study Design | Subjects | Controls | Vitamin D Intake | Follow-up Period/Duration of Study | Confounders Considered | Results | Year | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Rheumatoid Arthritis | North America (Nurses’ Health Study) | Prospective cohort | 722 who developed RA | 186,389 | Dietary and supplement intake | Up to 22 years | Age, all women, race, age at menarche, oral contraceptive use, parity, duration of breastfeeding, menopausal status, postmenopausal hormone use, cigarette smoking, latitude of residence at age 15 (North, Middle or South U.S.), physical activity, body mass index | No association; highest quintile intake RR 1.0 (95%CI 0.8, 1.3) | 2008 | Costenbader et al, (200) |
| Rheumatoid Arthritis | North America (Iowa Women's Health Study) | Prospective Cohort | 152 who developed RA | 29,386 | Diet and supplement intake | 11 years | Age, all women, all postmenopausal, | Higher intake at baseline associated with lower risk up to 11 years later: RR 0.67 (95%CI 0.44,1.00) | 2004 | Merlino et al, (199) |
| Systemic Lupus Erythematosus | North America (Nurses’ Health Study) | Prospective Cohort | 190 who developed SLE | 186,389 | Diet and supplement intake | Up to 22 years | Age, all women, race, age at menarche, oral contraceptive use, parity, duration of breastfeeding, menopausal status, postmenopausal hormone use, cigarette smoking, latitude of residence at age 15 (North, Middle or South U.S.), physical activity, body mass index | No association; highest quintile intake RR 1.4 (95%CI 0.8, 2.3) | 2008 | Costenbader et al, (200) |
| Type I Diabetes Mellitus | Finland (Diabetes Prediction and Prevention Study) | Birth Cohort | 165 children who developed TID or advanced beta-cell autoimmunity | 3723 infants | Maternal vitamin D intake and at 1 and 3 months post partum. | Mean of 4.3 years | familial diabetes and genetic risk factors for T1D, sex, gestational age, maternal age, maternal education, delivery hospital, route of delivery, number of earlier deliveries and smoking during pregnancy | Maternal intake of vitamin D from food RR 1.25 (95%CI 0.80,1.95), and from supplements RR 1.05 (95%CI 0.95-1.16) for risk of advanced beta cell autoimmunity/type 1 diabetes in offspring | 2010 | Marjamaki et al, (208) |
| Type I Diabetes Mellitus | Italy | Case-control | 83 T1D ages 0-14 | 166 age and sex-matched, same region | Vitamin D administration during lactation as baby (recall) | Recall as adults | medical history of severe infections nor history of surgical operations | OR 0.31 (95% CI 0.11, 0.8) for vitamin D administration during lactation and risk type 1 DM | 2007 | Tenconi et al, (202) |
| Type I Diabetes Mellitus | Sweden | Cohort-retrospective and prospective | 8.7% at 1 year and 8.9% at 2.5 years + for glutamic acid decarboxylase or islet antigen-2 autoantibodies | 18,886 infants | Questionnaire: Vitamin D supplementation during pregnancy, at 1 year and 2.5 years | 2.5 years | familial type 1 diabetes, maternal education, maternal age, delivery type, weight increase from birth, breastfeeding duration, introduction of cow's-milk protein, fish intake | OR 0.71 (95% CI 0.52-0.96) for reduced islet-cell autoimmunity at 1 year, but OR 1.25 (95%CI 0.91, 1.73) at 2.5 years. Surrogate measure for type 1 diabetes used. | 2007 | Brekke et al, (221) |
| Type I Diabetes Mellitus | North America (Diabetes Autoimmunity Study in the Young) | High-risk cohort | 16 children who developed islet-cell autoimmunity | 206 children without islet-cell autoimmunity | Maternal dietary and supplemental vitamin D intake during pregnancy-recalled after birth | Average 4 years | HLA genotype, family history of type 1 diabetes, presence of GDM, and ethnicity | HR 0.37 (95% CI 0.17-0.78) for increased maternal vitamin D intake and risk for islet-cell autoimmunity. Surrogate measure for type 1 diabetes used. | 2003 | Fronczak et al, (207) |
| Type I Diabetes Mellitus | Norway | Case-control | 545 cases (children mean age 10.9) | 1668 controls population (children mean age 9.3) | Questionnaire: vitamin D supplement use during pregnancy and the first year of life (recalled) | maternal use of cod liver oil during pregnancy, child's use of cod liver oil during the first year of life, duration of exclusive breastfeeding, child's age at introduction of solid foods, maternal education, maternal smoking during pregnancy, maternal age at delivery, child's number of siblings, type 1 diabetes among child's siblings or parents, and the child's age and sex | OR 0.98 (95% CI 0.73, 1.31) for maternal use of vitamin D ≥ 5 times/week vs. none during pregnancy and OR 0.97 (0.73, 1.29) for vitamin D use ≥ 5 times/week during first year of life | 2003 | Stene et al, (203) | |
| Type I Diabetes Mellitus | Finland | Birth cohort | 81 developed T1D | 10366 newborns | Vitamin D supplementation during first year of life | 31 years | Sex, maternal parity, gestational and maternal age, maternal education, social status, birth weight, and growth rate in infancy and suspected rickets | RR 0.12 (95% CI 0.03-0.51) for regular versus no supplementation | 2001 | Hypponen et al, (206) |
| Type I Diabetes Mellitus | Norway | Case-control | 78 cases | 980 controls | Questionnaire: Maternal itamin D intake during pregnancy (recall) | - | Age, sex, breastfeeding and maternal education | OR 1.27 (95 % CI 0.70, 2.31) for vitamin D supplementation in 1st year life | 2000 | Stene et al, (204) |
| Type I Diabetes Mellitus | Europe (7 centers) | Case-control | 820 cases | 2335 controls-population-based, age-group matched | Questionnaire or interview: recalled infancy vitamin D intake | - | Age, birth weight, duration of breast feeding, maternal age and study center | OR 0.67 (95% CI 0.53, 0.86) for vitamin D supplementation in infancy | 1999 | EURODIAB Substudy 2 study group, (222) |
| Multiple Sclerosis | North America (Nurses’ Health Study) | Prospective Cohort study | 173 MS cases | 92,253 women | Dietary and supplemental vitamin D | Up to 20 years | Age, all women, smoking and latitude at birth | RR 0.67 (95% CI 0.40,1.12); p trend 0.006; for highest quintile | 2004 | Munger et al,(201) |
Basic Science in vitro and in vivo studies
Through binding to the vitamin D receptor (VDR), the lipid-soluble active 1,25(OH)2D regulates an array of genes, many involved in inflammation and acquired and innate immune responses(27, 39-42). VDRs are found at high levels on dendritic cells, T and B lymphocytes and macrophages. The function of these cells is profoundly affected by binding of activated 1,25 (OH)2D(43-47). The 1α-hydroxylase that converts 25(OH)D to its active form is expressed in the kidney, in activated macrophages, dendritic cells and other tissues(29, 48, 49). This form of the enzyme, unlike that in the kidney, is not regulated by parathyroid hormone, but rather inducible by factors including interferon gamma and is downregulated with dendritic cell maturation(50). The expression of VDRs on resting CD4+ T cells increases 5 fold with T cell activation(51). 1,25(OH)2D inhibits the expression of interleukin(IL)-2, an important growth factor for T lymphocytes, and suppresses the secretion of Th1 cytokines IL-12, interferon gamma, and tumor necrosis factor, while increasing IL-4, IL-5 and IL-10, leading to the development of a Th2-skewed T cell population.(52-58) The addition of 1,25(OH)2D to CD4+ T cells also inhibits the expression of IL-6, a co-factor stimulating Th17 cells, important in the development of autoimmunity(59, 60). When added to SLE-patient derived B cells in vitro, 1,25(OH)2D inihibits autoantibody production(61).
In vitro, 1,25(OH)2D inhibits the differentiation of monocytes into dendritic cells and blocks the stimulatory effects that T cells have on them(58, 62, 63). Instead, 1,25(OH)2D promotes monocyte differentiation into macrophages, prevents them from releasing inflammatory cytokines and chemokines(64), and reduces their capacity to present antigens to lymphocytes by decreasing MHC-II molecule cell surface expression(39, 65). 1,25(OH)2D-VDR transcriptional signaling also exerts anti-inflammatory effects through the down-regulation of the prostaglandin (PG) pathway and cyclooxygenase-2 (COX-2)(66) and tolerizing effects through capacity to convert CD4 T cells into IL-10-secreting T regulatory cells, suppressing the proliferation of responder T cells.(67). 1,25(OH)2D interacts with VDRs on osteoblasts, stimulating expression of the receptor activator of nuclear factor kB (NFkB) ligand (RANKL)(27). Immature dendritic cells that have differentiated from monocytes in the presence of 1,25(OH)2D respond poorly to inflammatory chemokines that regulate dendritic cell maturation and migration to lymph nodes(45). Experimental data show that 25(OH)D can inhibit pro-inflammatory cytokines such as IL-6 and TNF-α, decrease serum levels of C- reactive peptide (CRP), and upregulate production of the anti-inflammatory cytokine interleukin-10 (IL-10)(44). 1,25(OH)2D downregulates dendritic cell production of IL-12 and augments IL-10, important in the development of T regulatory (Treg) cells(27, 58). As dendritic cells are central to the maintenance of both protective immunity and self-tolerance(68, 69), vitamin D's influence on their maturation and function could have consequences on autoimmune disease risk and/or progression. However, as the pathogenesis of autoimmune disease itself is still unclear, many of pathways implicated suggest a potential role of vitamin D insufficiency in disease progression, but it is not clear to what role in the triggering of autoimmune disease vitamin D intake or deficiency play.
In vivo, supplementation with 1,25(OH)2D forestalls the development of inflammatory arthritis, autoimmune encephalomyelitis (a model for MS), type I diabetes and autoimmune thyroiditis in experimental animal models(70-74). Treatment with a low calcemic vitamin D analog had a prophylactic as well as therapeutic effect on a murine model of Th1-like colitis(75). Administration of 1,25(OH)2D or its analogs to non-obese diabetic mice modulates the expression of chemokines and cytokines and prevents diabetes(76). Vitamin D receptor knock-out mice develop severe diarrhea, rectal bleeding, and marked body weight loss, leading to death in 2 weeks. Thus, vitamin D deficiency, it is thought to compromise the mucosal barrier and increase susceptibility to mucosal damage and potentially the risk of inflammatory bowel disease(77).
Evidence in Humans: Associations of Vitamin D with Circulating Biomarkers of Systemic Inflammation
Cross-sectional studies in healthy and ill populations suggest potential favorable effects of vitamin D—as measured by circulating 25(OH)D, sun exposure, or dietary or supplement intake—on inflammatory biomarkers(78-80). Inverse associations between serum vitamin D levels and serum CRP concentrations have been found in patients with diabetes mellitus, atherosclerotic vascular disease, inflammatory polyarthritis,and prolonged chronic illness(35, 81, 82). Increased TGF-β serum levels were observed when vitamin D was given for a 6 month period to 16 subjects with MS(83). TNF-α, IFN-γ, and IL-13 levels, however, did not change after supplementation. In a trial involving 200 overweight subjects enrolled in a weight loss program, participants were randomized to vitamin D (83 ug/d) or placebo in a double-blind manner for 12 months and serum TNF- α levels did decrease more in the vitamin D group than in the placebo group marker (10.2% compared with 3.2% decrease; p 0.049)(84). In a recent interventional study, 324 adults were assigned to 20,000 IU of vitamin D per week, 40,000 IU vitamin D per week or placebo. Multiple cytokines, including interleukin (IL)-2, -4, -5, -10, -12, -13, -17, intercellular adhesion molecule-1, interferon-gamma, monocyte chemotactic protein-1, and high CRP, were measured at the start and end of one year. No significance differences in changes levels of any of these cytokines was detected, nor was there any indication of a polarization of the T cells towards a Th2 dominant type(85).
Ecologic Associations implicating Vitamin D in Autoimmune Disease
Evidence comes from ecologic observations that several autoimmune diseases, including inflammatory bowel disease, MS, and type I diabetes and RA are more prevalent at Northern latitudes where sun exposure is reduced(86-92). The strongest ecologic evidence linking vitamin D with autoimmune disease risk is for MS (93). An increased prevalence of MS at Northern, compared to Southern, latitudes has long been observed and a strong inverse correlation of MS incidence with UV light exposure is also seen(91, 94). MS has also been associated with birth during the winter compared to other seasons of the year and it is hypothesized that this could reflect low maternal vitamin D during pregnancy(95). Lastly, seasonal variation in MS relapses detected by MRI has been observed, with increased flares occurring during the winter compared to summer months(96).
Genetic Polymorphisms in Vitamin D Pathway Genes Associated with Autoimmune Diseases
Polymorphisms in the VDR gene have been associated with increased risk of multiple autoimmune diseases, including Hashimoto's thyroiditis, IBD, Graves’ disease, RA, SLE, primary biliary cirrhosis, autoimmune hepatitis, Addison's disease, vitiligo, celiac disease and type I diabetes, as well as MS in humans(97-112). A vitamin D response element (VDRE) is found in the promoter region of the HLA DRB1*1501 allele, an allele strongly associated with MS susceptibility pathogenesis in Caucasians(112). However, not all of the polymorphisms associated with autoimmune diseases in past studies have known functional consequences and the strengths of the associations vary. Moreover, not all of these associations have been replicated.
Cross-sectional Studies of Vitamin D in Existing Autoimmune Diseases (Table 1- supplemental material)
Circulating 25(OH)D, reflecting all sources of vitamin D exposure with a half-life of 2-3 weeks, has been used in epidemiologic studies as a comprehensive and stable indicator of vitamin D status(17, 113). Some studies have assessed 1,25(OH)2D levels, but as the half-life of 1,25(OH)D is only 4 hours and this metabolite dependent on fluctuating calcium need, these results are harder to interpret (113). Studies of vitamin D levels comparing populations with and without existent autoimmune diseases have been conducted around the world and with somewhat conflicting results. In RA, for example, two past studies revealed lower levels of 25(OH)D in RA patients than in healthy matched controls(114, 115), but five studies did not find such a difference (116-119, 120 ). Patel and colleagues found a strong inverse association between baseline levels of serum 25(OH)D in patients with newly diagnosed early inflammatory polyarthritis (45%of whom were classified as having RA at 1 year) and baseline disease activity, as assessed by tender joint counts, RA disease activity scores (DAS28) and health assessment questionnaires (HAQ) scores(121). For each 10-ng/ml increase in 25(OH)D, they found a decrease in the DAS28 of 0.3 and in the C-reactive peptide level of approximately 25%. At 1 year, only significant inverse association between higher baseline vitamin D levels lower HAQ scores. In a large group of individuals at increased risk for RA, however, plasma 25(OH) D concentrations were not associated with the presence of RA-related autoantibodies(122).
In SLE, at least six case-control studies have now demonstrated a lower level of 25(OH) D in SLE cases than in matched controls(118, 123-127). Several studies have found that lower levels of vitamin D correlate with more active SLE (125, 126, 128-131), but several others have not confirmed this(118, 123, 132-134). One study showed that 25(OH)D level was lower in hydroxychloroquine users than non-users and hydroxychloroquine is known to inhibit the synthesis of vitamin D(135). A case-control study from Hungary reported lower 25(OH)D levels in individuals with undifferentiated connective tissue disease (before starting medications) than controls in summer and winter seasons, and demonstrated that levels were lower in those with more active manifestations and among the 35 who developed into a diagnosed connective tissue disease within 2.3 years of follow-up(136). A study involving 113 children with SLE, juvenile RA or juvenile dermatomyositis did not find an abnormal prevalence of 25(OH)D or 1,25(OH)D deficiency, although the study's primary goal was to assess osteocalcin levels(134).
There have been four case-control studies comparing 25(OH) D and 1,25 (OH) D levels in patients with ankylosing spondylitis to healthy controls. A study of 100 AS patients and 58 controls in Turkey found that 25(OH) D levels were lower in patients than controls(137), but an earlier study of 38 AS patients and 52 controls in Germany had not detected a significant difference(138). Two studies by Lange have found that 1,25(OH)D levels are lower in ankylosing spondylitis patients and negatively correlated with disease activity, thought possibly related to the high prevalence of osteoporosis seen in this disease(139, 140). Seventy-six ankylosing spondylitis patients had significantly lower 25(OH) levels than did 120 psoriatic arthritis patients, and a significant negative correlation between C-reactive peptide levels and 25(OH) D levels was reported in the combined cohort of these patients in a study by Teichmann(141).
We found eight past studies addressing vitamin D levels in patients with scleroderma. Of the five case-control studies, two demonstrated lower 25(OH) D levels in patients with scleroderma than controls(142, 143), and three did not(144-146). In two case-only analyses, it was found that 25(OH)D levels were, not surprisingly, lower in those with more severe underlying scleroderma, more longstanding disease, more disease activity, more pulmonary hypertension and lower diffusing lung capacity(147, 148).
In type I DM, there have been at least nine studies documenting lower 25(OH) D levels in established or new onset type I diabetes patients, compared to matched controls(149-157), and only one published study that did not confirm this(158). It has also been shown that 25(OH)D levels are lower in individuals with poorly controlled type I diabetes(159), ketoacidosis(160, 161), incipient nephropathy or tubulointerstitial damage(150, 157).
In MS as well, there have now been several studies reporting that 25(OH) D levels are lower in cases with MS than in healthy controls (162-168), and two that have not(169, 170). Lower vitamin D levels have been found in MS patients especially during the summer, compared to winter months(171), in those with progressive as opposed to relapsing-remitting forms of the disease(172), and associated with increased disability in MS(163, 166), and clinical activity and risk of relapse(162, 167, 168, 172).
Two case-control studies have reported lower 25(OH)D levels in patients with Crohn's disease compared to controls(173 , 174), and a three of four studies have found that levels are lower in those with active inflammatory bowel disease(173-176). The only study of the association between vitamin D level and thyroid autoimmunity was conducted in India where a large group of over 600 healthy individuals were screened for anti-thyroid antibodies. A weak but significant inverse correlation was found between vitamin D level and titer of anti-thyroid peroxidase autoantibodies(177). One cross-sectional case-only study of 25(OH) D levels in patients with vitiligo reported apparently low levels, without controlling for season(178). We found no epidemiologic studies of vitamin D levels in existing pernicious anemia, vasculitis, adult inflammatory myositis, polymylagia rheumatica, Addison's disease, uveitis, or Behcet's disease.
In sum, many, but not all case-control studies, circulating levels of 25(OH)D and occasionally 1,25 (OH)2D have been found to be lower in subjects with various autoimmune diseases than in matched healthy controls. The evidence pointing to lower vitamin D levels in existing disease is not equally strong for all autoimmune diseases and some diseases have yet to be studied. The differences between the many similar studies of a specific disease may relate to their different sizes and the heterogeneity in their statistical power, as well as to adjustment for confounding factors. Taken together, it is also apparent from these studies that ethnic origin (179), season of the year (180), and disability (163, 166), have strong effects on circulating vitamin D levels and it is not clear that all of these cross-sectional studies controlled for these important confounders. Additionally, disease activity and disability, glucocorticoid use(132), serum creatinine(132), microalbuminuria(157), ketoacidosis(160, 161), and use of hydroxychloroquine(135) influence circulating vitamin D levels. Hydroxychloroquine prescribed in several autoimmune diseases may inhibit the conversion of 25(OH) D to 1,25(OH)2D(135). Thus, while interesting, the major limitation of these cross-sectional studies is that reverse causation is very likely. Low vitamin D level may be the consequence, not the cause, of active autoimmune disease.
Vitamin D Supplementation and Effects on Existing Disease Activity (Table 2)
Several small open-label studies and interventional trials have tested the effects of supplementation with vitamin D, or its analogs, on the activity of established autoimmune diseases. In a double-blind trial conducted in 1973, oral calciferol (100,000 IU/d) for one year was administered to 25 subjects who were compared to 24 subjects who received a placebo. The intervention group had improved hand strength, decreased morning stiffness and need for analgesics/anti-inflammatory medications and decreased erythrocyte sedimentation rate (ESR). (181) In a more recent study, high dose 1-alpha (OH) D3 (alphacalcidiol) reduced pain and CRP levels in 19 RA subjects in a 3 month open-label trial(182).
Two small open-label studies of 6 months each have shown some promise for oral alphacalcidol (0.25 ug twice/d and 1,25(OH)D in reducing disease activity and tender joint counts in psoriatic arthritis(183 , 184). In an open-label observational study of 60 SLE patients in Spain who took vitamin D3 supplementation for two years, significant improvement was seen in subject fatigue, as measure by a visual analog scale, but not SLE disease activity measures(185). Ongoing NIH-sponsored trial is investigating vitamin D supplementation on disease activity and interferon-related cytokine activation (“the interferon signature”) in SLE.
Five small trials have now studied the effects of oral calcidiol supplementation on scleroderma, morphea or linear scleroderma(186-190). While earlier open-label studies had shown some benefit, in particular for morphea, the most recent randomized trial found that calcitriol was not more effective than placebo in patients with morphea, and the scleroderma group in that trial (n=7) was too small to any draw conclusions(190). On the other hand, randomized trials in Italy and China have now shown that vitamin D supplementation in individuals with latent autoimmune diabetes (autoantibodies highly associated with the development of type 1 diabetes) can forestall the development of type 1 diabetes, although it only temporarily reduces the amount of insulin required(191, 192).
We found five small studies or trials of vitamin D supplementation for MS. Fewer exacerbations of disease were seen among 16 MS patients given daily cod liver oil vitamin D for a year in an open-label study in 1986(193). In another open-label study of calcium and escalating doses of vitamin D supplementation in 12 MS patients, there did not appear to be an effect on disease progression or activity but the number of gadolinium-enhancing lesions per patient appeared decreased after just 12 weeks(194). In the largest randomized controlled trial yet, 25 MS subjects were randomized to doses of up to 40,000 IU/day over 28 weeks, followed by 10,000 IU/day for 12 weeks over 52 weeks and 24 MS subjects were randomized to placebo. While the treatment group appeared to have fewer relapse events, the difference was not statistically significant (p=0.09) possibly due to insufficient statistical power(195). Thus far, the studies have been small and inconclusive and larger scale trials are called for.
Lastly, one open-label trial in Eastern Europe has compared the effects of supplementation with 25(OH)D to those of 1,25(OH)D for Crohn's disease and found that 1,25 D supplementation was associated with a significantly greater decrease in disease activity (assessed by Crohn's Disease Activity Index) (196).
Studies to date have mainly been small and most open-label, and have include a range of vitamin D preparations, doses and durations. However, it appears that vitamin D may have an adjunctive role in amelioration of RA, MS, type I diabetes, and perhaps SLE and Crohn's disease. This evidence addresses the question of whether vitamin D has an effect upon established disease, but not upon the risk of developing an autoimmune disease de novo.
Epidemiologic Studies of Vitamin D Level and Risk of Developing Autoimmune Disease (Table 3)
In the hierarchy of epidemiologic studies, stronger data for association with susceptibility is afforded by prospective studies. Additionally, prospective studies are able to address the potential role of vitamin D in the pathogenesis of autoimmune disease. The relation between vitamin D serum or plasma level—measured by circulating 25(OH)D——and the incidence of autoimmune diseases has been examined in only two prospective observational epidemiologic studies of RA and MS, but not in other autoimmune diseases. Serum 25(OH)D level was not related to future RA risk in a Dutch case-control study examining blood bank samples from 79 individuals who developed RA compared to 79 healthy controls, matched on age, sex, and time of blood donation(197). To address the question in MS, Munger and colleagues used banked blood samples from Department of Defense military recruits, 257 of whom had later developed MS. Higher serum levels of 25(OH)D were associated with significantly lower risk of incident MS, but only among whites, not Blacks nor Hispanics, and the effect was most pronounced for individuals under age 20(198).
Epidemiologic Studies of Vitamin D Intake and Risk of Developing Autoimmune Disease (Table 4)
Prospective epidemiologic data on vitamin D intake and the risk of developing autoimmune diseases have been systematically reviewed and summarized in Table 4. The diseases that have been studied include RA, SLE, type 1 DM and MS. For RA risk, the data have been somewhat conflicting. In the Iowa Women's Health Study, higher baseline vitamin D intake was associated with decreased risk of subsequent RA(199). In the Nurses’ Health Study, a prospective cohort study involving over 180,000 women with multiple dietary assessments over 20 years, intake of vitamin D was not related to risk of developing either SLE or RA(200). However, in the same cohort, an inverse relationship was discovered between higher intake of vitamin D from supplements, but not overall dietary intake and lower risk of incident MS up to 20 years later(201).
Several observational studies have addressed the association between maternal vitamin D intake in pregnancy, lactation, or intake in infancy and early childhood and the risk of developing type 1 diabetes. A meta-analysis of four case-control (202-204) was performed in 2008(205)omitted from table 4 due to redundancy). Pooled data from the case-control studies showed that the risk of type 1 diabetes was significantly reduced in infants who were supplemented with vitamin D compared to those who were not supplemented (pooled odds ratio 0.71, 95% CI 0.60 to 0.84). One large birth cohort study prospectively assessed vitamin D supplementation during the first year of life (as part of a ricket's prevention study), and confirmed cases of type 1 diabetes in the children up to 31 years later. They found a strongly protective effect of regular vitamin D supplementation in the first year of life: RR 0.12 (95% CI 0.03-0.510 (206).
Surrogate markers for the development of type 1 diabetes, glutamic acid decarboxylase or islet antigen-2 autoantibodies, which are highly associated with type 1 diabetes risk, have been used in a few prospective studies. Two birth cohorts of infants at high genetic risk of type 1 diabetes have investigated relationships between maternal intake of vitamin D during pregnancy and the development of this islet cell autoimmunity. A high maternal dietary intake of vitamin D in foods, but not supplements, assessed after pregnancy by questionnaire, was with associated with increased risk of islet cell autoantibodies among the children followed for an average of four years in an American birth cohort(207). A larger prospective birth cohort in Finland has reported no association between maternal vitamin D intake (also assessed post-partum by questionnaire) and risk of type 1 diabetes or islet cell autoantibodies in their high genetic risk offspring, followed up to 4.3 years, however(208).
Our funnel plot for the studies examining the relationship of vitamin D dietary intake and the risk of the four autoimmune diseases that have been studied, is shown in Figure 2. Given the dearth of studies, we have included studies of all four autoimmune diseases with substantial heterogeneity in design, exposure assessment and timing in relation to onset of disease/autoantibodies and outcomes. The plot appears slightly asymmetric, displaying potential publication bias with few small non-protective studies. However, given the small number of studies, no conclusions can be drawn.
Figure 2. Funnel plot of the risk estimates (odds ratios or relative risks) of developing autoimmune disease associated with vitamin D intake (none vs. supplementation or highest vs. lowest level of intake).
Sample size of the study refers to the number of autoimmune disease cases in each study. The appearance of an inverted funnel, where small studies of positive and negative studies have been published argues against a potential publication bias.
Potential Causes of Conflicting Results
While the development of an autoimmune response to self-antigens underlies all of these diseases, it is likely that vitamin D is not uniformly involved in the pathogenesis of each and every autoimmune disease and is more important in the etiology of some versus others among them. It is clear that not all autoimmune diseases have been equally studied and for most there is a dearth of evidence addressing the potential effects of vitamin D on autoimmune disease susceptibility. Past case-control studies may have been biased by subject recall, cohort studies may have been underpowered for small effects, and potential confounders such as socioeconomic status may not have been consistently well addressed. Prospective observational studies that rely on dietary intake are hampered by subjects’ ability to accurately recall food intake and by inadequate means of assessing vitamin D content of all foods. The inconsistency in epidemiologic data regarding vitamin D intake and risk of autoimmune disease has led to some debate and may result in part from the fact that oral vitamin D intakes in many of the studied populations were too low to produce significant effects, as well as limited within-population variability in intakes. The difference between high and low oral intakes in observational studies is generally only 300-400 IU/d. Given that a vitamin D3 intake of 1 μg [40 IU] increases circulating 25(OH)D by ~1 nmol/L,(209) such an increment would be expected to raise 25(OH)D by ≤10 nmol/L.
Misclassification of vitamin D status due to incomplete or single exposure assessments could also contribute to null or inconsistent findings. The critical window for vitamin D intake or level to affect risk of developing an autoimmune disease is not known and may vary according to disease: maternal intake during pregnancy, early life intake and intake during different phases of adulthood may not be equally relevant to different diseases. Possible health benefits of vitamin D may be offset by other components in dairy products, which are main dietary sources of vitamin D. Disentangling independent effects of vitamin D and calcium on risk of autoimmune disease in observational studies is difficult due to their high correlation in countries—including the U.S.—where milk is fortified with vitamin D. The use of vitamin D supplements in a trial setting would eliminate this problem.
Conclusions
Understanding of the pluripotent immunomodulating and anti-inflammatory effects of vitamin D is advancing. Despite the in vitro and animal evidence for vitamin D's potential to decrease systemic inflammation and prevent autoimmune disease in humans, there remains insufficient human data to firmly support the hypothesis that vitamin D intake is related to the risk of developing autoimmune disease. Data from laboratory studies and cross-sectional and observational epidemiologic investigations suggest a potential protective effect for vitamin D in autoimmune disease susceptibility.
The human epidemiologic and interventional evidence, however, is weak. Despite the multitude of cross-sectional and case-control studies that demonstrate lower levels of vitamin D among individuals with autoimmune diseases than healthy controls, these studies are not able to address causality. Reverse causality, that the disease process of an autoimmune disease, even in early disease, could lower circulating vitamin D concentration is a likely possibility in these studies. Prospective epidemiologic data on vitamin D status and whether intake level has any effect on the incidence of autoimmune diseases are limited or conflicting. There have been no large randomized trials of high-dose vitamin D supplements for the primary prevention of autoimmune diseases in a general population.
Confirming or refuting that elevated dietary vitamin D intake reduces systemic inflammation and/or the risk of incident autoimmune disease is critical, as no prophylactic therapy currently exists for these diseases. The growing enthusiasm for vitamin D supplementation, however, underscores the need for timely and rigorous testing of this hypothesis before use becomes so prevalent as to render it impossible. To understand the effects of moderate-to-high dose vitamin D supplementation, a high-quality, double-blind randomized controlled trial with a large sample size and long duration and designed specifically to include an assessment of these outcomes in a general population is necessary.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: The VITamin D and OmegA-3 TriaL (VITAL) is being conducted by our research group at Brigham and Women's Hospital, Harvard Medical School (PI: JoAnn E. Manson, MD, DrPH). VITAL is funded by the National Institutes of Health (National Cancer Institute, National Heart Lung and Blood Institute, and other institutes and agencies are co-sponsors). The VITAL ancillary study on autoimmune disease incidence is funded by the National Institute of Arthritis and Musculoskeletal Diseases and is being conducted by Karen H. Costenbader, MD, MPH (PI) and colleagues at Brigham and Women's Hospital, Harvard Medical School. Study vitamin D supplements are donated by Pharmavite LLC of Northridge, California.
References
- 1.Majka DS, Holers VM. Can we accurately predict the development of rheumatoid arthritis in the preclinical phase? Arthritis Rheum. 2003;48(10):2701–5. doi: 10.1002/art.11224. [DOI] [PubMed] [Google Scholar]
- 2.Criswell LA, Pfeiffer KA, Lum RF, Gonzales B, Novitzke J, Kern M, et al. Analysis of Families in the Multiple Autoimmune Disease Genetics Consortium (MADGC) Collection: the PTPN22 620W Allele Associates with Multiple Autoimmune Phenotypes. Am J Hum Genet. 2005;76(4):561–71. doi: 10.1086/429096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin JP, Cash JM, Doyle SZ, Peden S, Kanik K, Amos CI, et al. Familial clustering of rheumatoid arthritis with other autoimmune diseases. Hum Genet. 1998;103(4):475–82. doi: 10.1007/s004390050853. [DOI] [PubMed] [Google Scholar]
- 4.Cohen R, Robinson D, Jr., Paramore C, Fraeman K, Renahan K, Bala M. Autoimmune disease concomitance among inflammatory bowel disease patients in the United States, 2001-2002. Inflamm Bowel Dis. 2008;14(6):738–43. doi: 10.1002/ibd.20406. [DOI] [PubMed] [Google Scholar]
- 5.Becker KG. The common genetic hypothesis of autoimmune/inflammatory disease. Curr Opin Allergy Clin Immunol. 2001;1(5):399–405. doi: 10.1097/01.all.0000011052.77127.a6. [DOI] [PubMed] [Google Scholar]
- 6.Becker KG, Simon RM, Bailey-Wilson JE, Freidlin B, Biddison WE, McFarland HF, et al. Clustering of non-major histocompatibility complex susceptibility candidate loci in human autoimmune diseases. Proc Natl Acad Sci U S A. 1998;95(17):9979–84. doi: 10.1073/pnas.95.17.9979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kristiansen OP, Larsen ZM, Pociot F. CTLA-4 in autoimmune diseases--a general susceptibility gene to autoimmunity? Genes Immun. 2000;1(3):170–84. doi: 10.1038/sj.gene.6363655. [DOI] [PubMed] [Google Scholar]
- 8.Vaidya B, Pearce S. The emerging role of the CTLA-4 gene in autoimmune endocrinopathies. Eur J Endocrinol. 2004;150(5):619–26. doi: 10.1530/eje.0.1500619. [DOI] [PubMed] [Google Scholar]
- 9.Costenbader KH, Karlson EW. Cigarette smoking and autoimmune disease: what can we learn from epidemiology? Lupus. 2006;15(11):737–45. doi: 10.1177/0961203306069344. [DOI] [PubMed] [Google Scholar]
- 10.Parks CG, Conrad K, Cooper GS. Occupational exposure to crystalline silica and autoimmune disease. Environ Health Perspect. 1999;107(Suppl 5):793–802. doi: 10.1289/ehp.99107s5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. [November 2008]; http://www3.niaid.nih.gov/topics/autoimmune/PDF/ADCCFinal.pdf.
- 12.Jacobson DL, Gange SJ, Rose NR, Graham NM. Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clin Immunol Immunopathol. 1997;84(3):223–43. doi: 10.1006/clin.1997.4412. [DOI] [PubMed] [Google Scholar]
- 13.Beeson PB. Age and sex associations of 40 autoimmune diseases. Am J Med. 1994;96(5):457–62. doi: 10.1016/0002-9343(94)90173-2. [DOI] [PubMed] [Google Scholar]
- 14.Notkins AL, Lernmark A, Leslie D. Preface. Autoimmunity. 2004;37:251–2. [Google Scholar]
- 15.Cooper GS, Stroehla BC. The epidemiology of autoimmune diseases. Autoimmun Rev. 2003;2(3):119–25. doi: 10.1016/s1568-9972(03)00006-5. [DOI] [PubMed] [Google Scholar]
- 16.Holick MF. Vitamin D for health and in chronic kidney disease. Semin Dial. 2005;18(4):266–75. doi: 10.1111/j.1525-139X.2005.18402.x. [DOI] [PubMed] [Google Scholar]
- 17.Giovannucci E. The epidemiology of vitamin D and cancer incidence and mortality: a review (United States). Cancer Causes Control. 2005;16(2):83–95. doi: 10.1007/s10552-004-1661-4. [DOI] [PubMed] [Google Scholar]
- 18.Jurutka PW, Bartik L, Whitfield GK, Mathern DR, Barthel TK, Gurevich M, et al. Vitamin D receptor: key roles in bone mineral pathophysiology, molecular mechanism of action, and novel nutritional ligands. J Bone Miner Res. 2007;22(Suppl 2):V2–10. doi: 10.1359/jbmr.07s216. [DOI] [PubMed] [Google Scholar]
- 19.Hollis BW. Circulating 25-hydroxyvitamin D levels indicative of vitamin D sufficiency: implications for establishing a new effective dietary intake recommendation for vitamin D. J Nutr. 2005;135(2):317–22. doi: 10.1093/jn/135.2.317. [DOI] [PubMed] [Google Scholar]
- 20.Kinyamu HK, Gallagher JC, Rafferty KA, Balhorn KE. Dietary calcium and vitamin D intake in elderly women: effect on serum parathyroid hormone and vitamin D metabolites. Am J Clin Nutr. 1998;67(2):342–8. doi: 10.1093/ajcn/67.2.342. [DOI] [PubMed] [Google Scholar]
- 21.Drinka PJ, Krause PF, Nest LJ, Goodman BM. Determinants of vitamin D levels in nursing home residents. J Am Med Dir Assoc. 2007;8(2):76–9. doi: 10.1016/j.jamda.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 22.Thomas MK, Lloyd-Jones DM, Thadhani RI, Shaw AC, Deraska DJ, Kitch BT, et al. Hypovitaminosis D in medical inpatients. N Engl J Med. 1998;338(12):777–83. doi: 10.1056/NEJM199803193381201. [DOI] [PubMed] [Google Scholar]
- 23.Gosnell M. [March 25, 2008];Top 100 science stories of 2007: #8: Can vitamin D save your life? Discover 2007 December 12, 2007;Sect. Available at http://discovermagazine.com/2008/jan/year-in-science-2007/can-vitamin-d-save-your-life.
- 24.Guthrie C. [March 25, 2008];The 10 biggest medical breakthroughs. #10: Benefits of vitamin D. Time 2007 December 24, 2007;Sect. Available at: http://www.time.com/time/specials/2007/top10/article/0,30583,1686204_1686252_1690393,00.html.
- 25.Harvard Health Letter The top 10 health stories of 2006. Harv Health Lett. 2006;32(2):1–3. [PubMed] [Google Scholar]
- 26.Harvard Health Letter A more D-manding diet. Some experts say we should have a lot more vitamin D in our diets because it's protective against several diseases. Harv Health Lett. 2007;32(8):3. [PubMed] [Google Scholar]
- 27.Arnson Y, Amital H, Shoenfeld Y. Vitamin D and autoimmunity: new aetiological and therapeutic considerations. Ann Rheum Dis. 2007;66(9):1137–42. doi: 10.1136/ard.2007.069831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cantorna MT. Vitamin D and autoimmunity: is vitamin D status an environmental factor affecting autoimmune disease prevalence? Proc Soc Exp Biol Med. 2000;223(3):230–3. doi: 10.1046/j.1525-1373.2000.22333.x. [DOI] [PubMed] [Google Scholar]
- 29.Holick MF. Vitamin D: importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr. 2004;79(3):362–71. doi: 10.1093/ajcn/79.3.362. [DOI] [PubMed] [Google Scholar]
- 30.Adorini L, Penna G. Control of autoimmune diseases by the vitamin D endocrine system. Nat Clin Pract Rheumatol. 2008;4(8):404–12. doi: 10.1038/ncprheum0855. [DOI] [PubMed] [Google Scholar]
- 31.Schwalfenberg G. Not enough vitamin D: health consequences for Canadians. Can Fam Physician. 2007;53(5):841–54. [PMC free article] [PubMed] [Google Scholar]
- 32.Wolff AE, Jones AN, Hansen KE. Vitamin D and musculoskeletal health. Nat Clin Pract Rheumatol. 2008;4(11):580–8. doi: 10.1038/ncprheum0921. [DOI] [PubMed] [Google Scholar]
- 33.Pelajo CF, Lopez-Benitez JM, Miller LC. Vitamin D and Autoimmune Rheumatologic Disorders. Autoimmun Rev. 2010 doi: 10.1016/j.autrev.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 34.Maruotti N, Cantatore FP. Vitamin D and the Immune System. J Rheumatol. 2010 doi: 10.3899/jrheum.090797. [DOI] [PubMed] [Google Scholar]
- 35.Chung M, Balk EM, Brendel M, Ip S, Lau J, Lichtensteon A, et al. Vitamin D and Calcium: A Systematic Review of Health Outcomes. Agency for Healthcare Research and Quality; Rockville, MD: Aug, 2009. 2009. [Google Scholar]
- 36.Mason AR, Mason J, Cork M, Dooley G, Edwards G. Topical treatments for chronic plaque psoriasis. Cochrane Database Syst Rev. 2009;(2):CD005028. doi: 10.1002/14651858.CD005028.pub2. [DOI] [PubMed] [Google Scholar]
- 37.Arteh J, Narra S, Nair S. Prevalence of Vitamin D Deficiency in Chronic Liver Disease. Dig Dis Sci. 2009 doi: 10.1007/s10620-009-1069-9. [DOI] [PubMed] [Google Scholar]
- 38.Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–63. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 39.Cantorna MT, Mahon BD. Mounting evidence for vitamin D as an environmental factor affecting autoimmune disease prevalence. Exp Biol Med (Maywood) 2004;229(11):1136–42. doi: 10.1177/153537020422901108. [DOI] [PubMed] [Google Scholar]
- 40.Hayes CE, Nashold FE, Spach KM, Pedersen LB. The immunological functions of the vitamin D endocrine system. Cell Mol Biol (Noisy-le-grand) 2003;49(2):277–300. [PubMed] [Google Scholar]
- 41.Griffin MD, Xing N, Kumar R. Vitamin D and its analogs as regulators of immune activation and antigen presentation. Annu Rev Nutr. 2003;23:117–45. doi: 10.1146/annurev.nutr.23.011702.073114. [DOI] [PubMed] [Google Scholar]
- 42.Nagpal S, Na S, Rathnachalam R. Noncalcemic actions of vitamin D receptor ligands. Endocr Rev. 2005;26(5):662–87. doi: 10.1210/er.2004-0002. [DOI] [PubMed] [Google Scholar]
- 43.Jones G, Strugnell SA, DeLuca HF. Current understanding of the molecular actions of vitamin D. Physiol Rev. 1998;78(4):1193–231. doi: 10.1152/physrev.1998.78.4.1193. [DOI] [PubMed] [Google Scholar]
- 44.Canning MO, Grotenhuis K, de Wit H, Ruwhof C, Drexhage HA. 1-alpha,25-dihydroxyvitamin D3 (1,25(OH)(2)D(3)) hampers the maturation of fully active immature dendritic cells from monocytes. Eur J Endocrinol. 2001;145(3):351–7. doi: 10.1530/eje.0.1450351. [DOI] [PubMed] [Google Scholar]
- 45.Gauzzi MC, Purificato C, Donato K, Jin Y, Wang L, Daniel KC, et al. Suppressive effect of 1alpha,25-dihydroxyvitamin D3 on type I IFN-mediated monocyte differentiation into dendritic cells: impairment of functional activities and chemotaxis. J Immunol. 2005;174(1):270–6. doi: 10.4049/jimmunol.174.1.270. [DOI] [PubMed] [Google Scholar]
- 46.Piemonti L, Monti P, Sironi M, Fraticelli P, Leone BE, Dal Cin E, et al. Vitamin D3 affects differentiation, maturation, and function of human monocyte-derived dendritic cells. J Immunol. 2000;164(9):4443–51. doi: 10.4049/jimmunol.164.9.4443. [DOI] [PubMed] [Google Scholar]
- 47.van Halteren AG, Tysma OM, van Etten E, Mathieu C, Roep BO. 1alpha,25-dihydroxyvitamin D3 or analogue treated dendritic cells modulate human autoreactive T cells via the selective induction of apoptosis. J Autoimmun. 2004;23(3):233–9. doi: 10.1016/j.jaut.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 48.Fritsche J, Mondal K, Ehrnsperger A, Andreesen R, Kreutz M. Regulation of 25-hydroxyvitamin D3-1 alpha-hydroxylase and production of 1 alpha,25-dihydroxyvitamin D3 by human dendritic cells. Blood. 2003;102(9):3314–6. doi: 10.1182/blood-2002-11-3521. [DOI] [PubMed] [Google Scholar]
- 49.Monkawa T, Yoshida T, Hayashi M, Saruta T. Identification of 25-hydroxyvitamin D3 1alpha-hydroxylase gene expression in macrophages. Kidney Int. 2000;58(2):559–68. doi: 10.1046/j.1523-1755.2000.00202.x. [DOI] [PubMed] [Google Scholar]
- 50.Hewison M, Freeman L, Hughes SV, Evans KN, Bland R, Eliopoulos AG, et al. Differential regulation of vitamin D receptor and its ligand in human monocyte-derived dendritic cells. J Immunol. 2003;170(11):5382–90. doi: 10.4049/jimmunol.170.11.5382. [DOI] [PubMed] [Google Scholar]
- 51.Mahon BD, Wittke A, Weaver V, Cantorna MT. The targets of vitamin D depend on the differentiation and activation status of CD4 positive T cells. J Cell Biochem. 2003;89(5):922–32. doi: 10.1002/jcb.10580. [DOI] [PubMed] [Google Scholar]
- 52.Takeuchi A, Reddy GS, Kobayashi T, Okano T, Park J, Sharma S. Nuclear factor of activated T cells (NFAT) as a molecular target for 1alpha,25-dihydroxyvitamin D3-mediated effects. J Immunol. 1998;160(1):209–18. [PubMed] [Google Scholar]
- 53.Overbergh L, Decallonne B, Waer M, Rutgeerts O, Valckx D, Casteels KM, et al. 1alpha,25-dihydroxyvitamin D3 induces an autoantigen-specific T-helper 1/T-helper 2 immune shift in NOD mice immunized with GAD65 (p524-543). Diabetes. 2000;49(8):1301–7. doi: 10.2337/diabetes.49.8.1301. [DOI] [PubMed] [Google Scholar]
- 54.Boonstra A, Barrat FJ, Crain C, Heath VL, Savelkoul HF, O'Garra A. 1alpha,25-Dihydroxyvitamin d3 has a direct effect on naive CD4(+) T cells to enhance the development of Th2 cells. J Immunol. 2001;167(9):4974–80. doi: 10.4049/jimmunol.167.9.4974. [DOI] [PubMed] [Google Scholar]
- 55.Lemire J. 1,25-Dihydroxyvitamin D3--a hormone with immunomodulatory properties. Z Rheumatol. 2000;59(Suppl 1):24–7. doi: 10.1007/s003930070034. [DOI] [PubMed] [Google Scholar]
- 56.Lemire JM, Archer DC, Beck L, Spiegelberg HL. Immunosuppressive actions of 1,25-dihydroxyvitamin D3: preferential inhibition of Th1 functions. J Nutr. 1995;125(6 Suppl):1704S–1708S. doi: 10.1093/jn/125.suppl_6.1704S. [DOI] [PubMed] [Google Scholar]
- 57.van Etten E, Mathieu C. Immunoregulation by 1,25-dihydroxyvitamin D3: basic concepts. J Steroid Biochem Mol Biol. 2005;97(1-2):93–101. doi: 10.1016/j.jsbmb.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 58.Penna G, Adorini L. 1 Alpha,25-dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J Immunol. 2000;164(5):2405–11. doi: 10.4049/jimmunol.164.5.2405. [DOI] [PubMed] [Google Scholar]
- 59.Xue ML, Zhu H, Thakur A, Willcox M. 1 alpha,25-Dihydroxyvitamin D3 inhibits pro-inflammatory cytokine and chemokine expression in human corneal epithelial cells colonized with Pseudomonas aeruginosa. Immunol Cell Biol. 2002;80(4):340–5. doi: 10.1046/j.1440-1711.80.4august.1.x. [DOI] [PubMed] [Google Scholar]
- 60.Stockinger B. Th17 cells: An orphan with influence. Immunol Cell Biol. 2007;85(2):83–4. doi: 10.1038/sj.icb.7100035. [DOI] [PubMed] [Google Scholar]
- 61.Linker-Israeli M, Elstner E, Klinenberg JR, Wallace DJ, Koeffler HP. Vitamin D(3) and its synthetic analogs inhibit the spontaneous in vitro immunoglobulin production by SLE-derived PBMC. Clin Immunol. 2001;99(1):82–93. doi: 10.1006/clim.2000.4998. [DOI] [PubMed] [Google Scholar]
- 62.Griffin MD, Lutz W, Phan VA, Bachman LA, McKean DJ, Kumar R. Dendritic cell modulation by 1alpha,25 dihydroxyvitamin D3 and its analogs: a vitamin D receptor-dependent pathway that promotes a persistent state of immaturity in vitro and in vivo. Proc Natl Acad Sci U S A. 2001;98(12):6800–5. doi: 10.1073/pnas.121172198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Berer A, Stockl J, Majdic O, Wagner T, Kollars M, Lechner K, et al. 1,25-Dihydroxyvitamin D(3) inhibits dendritic cell differentiation and maturation in vitro. Exp Hematol. 2000;28(5):575–83. doi: 10.1016/s0301-472x(00)00143-0. [DOI] [PubMed] [Google Scholar]
- 64.Helming L, Bose J, Ehrchen J, Schiebe S, Frahm T, Geffers R, et al. 1alpha,25-Dihydroxyvitamin D3 is a potent suppressor of interferon gamma-mediated macrophage activation. Blood. 2005;106(13):4351–8. doi: 10.1182/blood-2005-03-1029. [DOI] [PubMed] [Google Scholar]
- 65.Lemire JM. Immunomodulatory role of 1,25-dihydroxyvitamin D3. J Cell Biochem. 1992;49(1):26–31. doi: 10.1002/jcb.240490106. [DOI] [PubMed] [Google Scholar]
- 66.Moreno J, Krishnan AV, Swami S, Nonn L, Peehl DM, Feldman D. Regulation of prostaglandin metabolism by calcitriol attenuates growth stimulation in prostate cancer cells. Cancer Res. 2005;65(17):7917–25. doi: 10.1158/0008-5472.CAN-05-1435. [DOI] [PubMed] [Google Scholar]
- 67.Unger WW, Laban S, Kleijwegt FS, van der Slik AR, Roep BO. Induction of Treg by monocyte-derived DC modulated by vitamin D3 or dexamethasone: differential role for PD-L1. Eur J Immunol. 2009;39(11):3147–59. doi: 10.1002/eji.200839103. [DOI] [PubMed] [Google Scholar]
- 68.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 69.Lanzavecchia A, Sallusto F. Regulation of T cell immunity by dendritic cells. Cell. 2001;106(3):263–6. doi: 10.1016/s0092-8674(01)00455-x. [DOI] [PubMed] [Google Scholar]
- 70.Lemire JM, Archer DC. 1,25-dihydroxyvitamin D3 prevents the in vivo induction of murine experimental autoimmune encephalomyelitis. J Clin Invest. 1991;87(3):1103–7. doi: 10.1172/JCI115072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cantorna MT, Hayes CE, DeLuca HF. 1,25-Dihydroxycholecalciferol inhibits the progression of arthritis in murine models of human arthritis. J Nutr. 1998;128(1):68–72. doi: 10.1093/jn/128.1.68. [DOI] [PubMed] [Google Scholar]
- 72.Zella JB, McCary LC, DeLuca HF. Oral administration of 1,25-dihydroxyvitamin D3 completely protects NOD mice from insulin-dependent diabetes mellitus. Arch Biochem Biophys. 2003;417(1):77–80. doi: 10.1016/s0003-9861(03)00338-2. [DOI] [PubMed] [Google Scholar]
- 73.Cantorna MT, Hayes CE, DeLuca HF. 1,25-Dihydroxyvitamin D3 reversibly blocks the progression of relapsing encephalomyelitis, a model of multiple sclerosis. Proc Natl Acad Sci U S A. 1996;93(15):7861–4. doi: 10.1073/pnas.93.15.7861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fournier C, Gepner P, Sadouk M, Charreire J. In vivo beneficial effects of cyclosporin A and 1,25-dihydroxyvitamin D3 on the induction of experimental autoimmune thyroiditis. Clin Immunol Immunopathol. 1990;54(1):53–63. doi: 10.1016/0090-1229(90)90005-b. [DOI] [PubMed] [Google Scholar]
- 75.Daniel C, Radeke HH, Sartory NA, Zahn N, Zuegel U, Steinmeyer A, et al. The new low calcemic vitamin D analog 22-ene-25-oxa-vitamin D prominently ameliorates T helper cell type 1-mediated colitis in mice. J Pharmacol Exp Ther. 2006;319(2):622–31. doi: 10.1124/jpet.106.107599. [DOI] [PubMed] [Google Scholar]
- 76.Gysemans CA, Cardozo AK, Callewaert H, Giulietti A, Hulshagen L, Bouillon R, et al. 1,25-Dihydroxyvitamin D3 modulates expression of chemokines and cytokines in pancreatic islets: implications for prevention of diabetes in nonobese diabetic mice. Endocrinology. 2005;146(4):1956–64. doi: 10.1210/en.2004-1322. [DOI] [PubMed] [Google Scholar]
- 77.Kong J, Zhang Z, Musch MW, Ning G, Sun J, Hart J, et al. Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am J Physiol Gastrointest Liver Physiol. 2008;294(1):G208–16. doi: 10.1152/ajpgi.00398.2007. [DOI] [PubMed] [Google Scholar]
- 78.Shea MK, Booth SL, Massaro JM, Jacques PF, D'Agostino RB, Sr., Dawson-Hughes B, et al. Vitamin K and vitamin D status: associations with inflammatory markers in the Framingham Offspring Study. Am J Epidemiol. 2008;167(3):313–20. doi: 10.1093/aje/kwm306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Timms PM, Mannan N, Hitman GA, Noonan K, Mills PG, Syndercombe-Court D, et al. Circulating MMP9, vitamin D and variation in the TIMP-1 response with VDR genotype: mechanisms for inflammatory damage in chronic disorders? Qjm. 2002;95(12):787–96. doi: 10.1093/qjmed/95.12.787. [DOI] [PubMed] [Google Scholar]
- 80.Schleithoff SS, Zittermann A, Tenderich G, Berthold HK, Stehle P, Koerfer R. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr. 2006;83(4):754–9. doi: 10.1093/ajcn/83.4.754. [DOI] [PubMed] [Google Scholar]
- 81.Targher G, Bertolini L, Padovani R, Zenari L, Scala L, Cigolini M, et al. Serum 25-hydroxyvitamin D3 concentrations and carotid artery intima-media thickness among type 2 diabetic patients. Clin Endocrinol (Oxf) 2006;65(5):593–7. doi: 10.1111/j.1365-2265.2006.02633.x. [DOI] [PubMed] [Google Scholar]
- 82.Brown DJ, Milroy R, Preston T, McMillan DC. The relationship between an inflammation-based prognostic score (Glasgow Prognostic Score) and changes in serum biochemical variables in patients with advanced lung and gastrointestinal cancer. J Clin Pathol. 2007;60(6):705–8. doi: 10.1136/jcp.2005.033217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mahon BD, Gordon SA, Cruz J, Cosman F, Cantorna MT. Cytokine profile in patients with multiple sclerosis following vitamin D supplementation. J Neuroimmunol. 2003;134(1-2):128–32. doi: 10.1016/s0165-5728(02)00396-x. [DOI] [PubMed] [Google Scholar]
- 84.Zittermann A, Frisch S, Berthold HK, Gotting C, Kuhn J, Kleesiek K, et al. Vitamin D supplementation enhances the beneficial effects of weight loss on cardiovascular disease risk markers. Am J Clin Nutr. 2009;89(5):1321–7. doi: 10.3945/ajcn.2008.27004. [DOI] [PubMed] [Google Scholar]
- 85.Jorde R, Sneve M, Torjesen PA, Figenschau Y, Goransson LG, Omdal R. No effect of supplementation with cholecalciferol on cytokines and markers of inflammation in overweight and obese subjects. Cytokine. doi: 10.1016/j.cyto.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 86.Ponsonby AL, McMichael A, van der Mei I. Ultraviolet radiation and autoimmune disease: insights from epidemiological research. Toxicology. 2002;181-182:71–8. doi: 10.1016/s0300-483x(02)00257-3. [DOI] [PubMed] [Google Scholar]
- 87.Sonnenberg A, Wasserman IH. Epidemiology of inflammatory bowel disease among U.S. military veterans. Gastroenterology. 1991;101(1):122–30. doi: 10.1016/0016-5085(91)90468-z. [DOI] [PubMed] [Google Scholar]
- 88.Cantorna MT. Vitamin D and its role in immunology: multiple sclerosis, and inflammatory bowel disease. Prog Biophys Mol Biol. 2006;92(1):60–4. doi: 10.1016/j.pbiomolbio.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 89.Hernan MA, Olek MJ, Ascherio A. Geographic variation of MS incidence in two prospective studies of US women. Neurology. 1999;53(8):1711–8. doi: 10.1212/wnl.53.8.1711. [DOI] [PubMed] [Google Scholar]
- 90.Staples JA, Ponsonby AL, Lim LL, McMichael AJ. Ecologic analysis of some immune-related disorders, including type 1 diabetes, in Australia: latitude, regional ultraviolet radiation, and disease prevalence. Environ Health Perspect. 2003;111(4):518–23. doi: 10.1289/ehp.5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Handel AE, Handunnetthi L, Giovannoni G, Ebers GC, Ramagopalan SV. Genetic and environmental factors and the distribution of multiple sclerosis in Europe. Eur J Neurol. doi: 10.1111/j.1468-1331.2010.03003.x. [DOI] [PubMed] [Google Scholar]
- 92.Vieira VM, Hart JE, Webster TF, Weinberg J, Puett R, Laden F, et al. Association between Residences in U.S. Northern Latitudes and Rheumatoid Arthritis: A Spatial Analysis of the Nurses’ Health Study. Environ Health Perspect. 2010 doi: 10.1289/ehp.0901861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ascherio A, Munger K. Epidemiology of multiple sclerosis: from risk factors to prevention. Semin Neurol. 2008;28(1):17–28. doi: 10.1055/s-2007-1019126. [DOI] [PubMed] [Google Scholar]
- 94.Beretich BD, Beretich TM. Explaining multiple sclerosis prevalence by ultraviolet exposure: a geospatial analysis. Mult Scler. 2009;15(8):891–8. doi: 10.1177/1352458509105579. [DOI] [PubMed] [Google Scholar]
- 95.Salzer J, Svenningsson A, Sundstrom P. Season of birth and multiple sclerosis in Sweden. Acta Neurol Scand. 121(1):20–3. doi: 10.1111/j.1600-0404.2009.01181.x. [DOI] [PubMed] [Google Scholar]
- 96.Auer DP, Schumann EM, Kumpfel T, Gossl C, Trenkwalder C. Seasonal fluctuations of gadolinium-enhancing magnetic resonance imaging lesions in multiple sclerosis. Ann Neurol. 2000;47(2):276–7. [PubMed] [Google Scholar]
- 97.Ban Y, Taniyama M, Ban Y. Vitamin D receptor gene polymorphism is associated with Graves’ disease in the Japanese population. J Clin Endocrinol Metab. 2000;85(12):4639–43. doi: 10.1210/jcem.85.12.7038. [DOI] [PubMed] [Google Scholar]
- 98.Ban Y, Taniyama M, Ban Y. Vitamin D receptor gene polymorphisms in Hashimoto's thyroiditis. Thyroid. 2001;11(6):607–8. doi: 10.1089/105072501750302967. [DOI] [PubMed] [Google Scholar]
- 99.Birlea S, Birlea M, Cimponeriu D, Apostol P, Cosgarea R, Gavrila L, et al. Autoimmune diseases and vitamin D receptor Apa-I polymorphism are associated with vitiligo in a small inbred Romanian community. Acta Derm Venereol. 2006;86(3):209–14. doi: 10.2340/00015555-0093. [DOI] [PubMed] [Google Scholar]
- 100.Boraska V, Skrabic V, Zeggini E, Groves CJ, Buljubasic M, Peruzovic M, et al. Family-based analysis of vitamin D receptor gene polymorphisms and type 1 diabetes in the population of South Croatia. J Hum Genet. 2008;53(3):210–4. doi: 10.1007/s10038-007-0234-2. [DOI] [PubMed] [Google Scholar]
- 101.Fan L, Tu X, Zhu Y, Zhou L, Pfeiffer T, Feltens R, et al. Genetic association of vitamin D receptor polymorphisms with autoimmune hepatitis and primary biliary cirrhosis in the Chinese. J Gastroenterol Hepatol. 2005;20(2):249–55. doi: 10.1111/j.1440-1746.2005.03532.x. [DOI] [PubMed] [Google Scholar]
- 102.Fukazawa T, Yabe I, Kikuchi S, Sasaki H, Hamada T, Miyasaka K, et al. Association of vitamin D receptor gene polymorphism with multiple sclerosis in Japanese. J Neurol Sci. 1999;166(1):47–52. doi: 10.1016/s0022-510x(99)00112-4. [DOI] [PubMed] [Google Scholar]
- 103.Lin WY, Wan L, Tsai CH, Chen RH, Lee CC, Tsai FJ. Vitamin D receptor gene polymorphisms are associated with risk of Hashimoto's thyroiditis in Chinese patients in Taiwan. J Clin Lab Anal. 2006;20(3):109–12. doi: 10.1002/jcla.20110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Maalej A, Petit-Teixeira E, Michou L, Rebai A, Cornelis F, Ayadi H. Association study of VDR gene with rheumatoid arthritis in the French population. Genes Immun. 2005;6(8):707–11. doi: 10.1038/sj.gene.6364260. [DOI] [PubMed] [Google Scholar]
- 105.Pani MA, Seissler J, Usadel KH, Badenhoop K. Vitamin D receptor genotype is associated with Addison's disease. Eur J Endocrinol. 2002;147(5):635–40. doi: 10.1530/eje.0.1470635. [DOI] [PubMed] [Google Scholar]
- 106.Ramos-Lopez E, Jansen T, Ivaskevicius V, Kahles H, Klepzig C, Oldenburg J, et al. Protection from type 1 diabetes by vitamin D receptor haplotypes. Ann N Y Acad Sci. 2006;1079:327–34. doi: 10.1196/annals.1375.050. [DOI] [PubMed] [Google Scholar]
- 107.Sakulpipatsin W, Verasertniyom O, Nantiruj K, Totemchokchyakarn K, Lertsrisatit P, Janwityanujit S. Vitamin D receptor gene BsmI polymorphisms in Thai patients with systemic lupus erythematosus. Arthritis Res Ther. 2006;8(2):R48. doi: 10.1186/ar1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.San-Pedro JI, Bilbao JR, Perez de Nanclares G, Vitoria JC, Martul P, Castano L. Heterogeneity of vitamin D receptor gene association with celiac disease and type 1 diabetes mellitus. Autoimmunity. 2005;38(6):439–44. doi: 10.1080/08916930500288455. [DOI] [PubMed] [Google Scholar]
- 109.Shimada A, Kanazawa Y, Motohashi Y, Yamada S, Maruyama T, Ikegami H, et al. Evidence for association between vitamin D receptor BsmI polymorphism and type 1 diabetes in Japanese. J Autoimmun. 2008;30(4):207–11. doi: 10.1016/j.jaut.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 110.Stefanic M, Papic S, Suver M, Glavas-Obrovac L, Karner I. Association of vitamin D receptor gene 3'-variants with Hashimoto's thyroiditis in the Croatian population. Int J Immunogenet. 2008;35(2):125–31. doi: 10.1111/j.1744-313X.2008.00748.x. [DOI] [PubMed] [Google Scholar]
- 111.Vogel A, Strassburg CP, Manns MP. Genetic association of vitamin D receptor polymorphisms with primary biliary cirrhosis and autoimmune hepatitis. Hepatology. 2002;35(1):126–31. doi: 10.1053/jhep.2002.30084. [DOI] [PubMed] [Google Scholar]
- 112.Ramagopalan SV, Maugeri NJ, Handunnetthi L, Lincoln MR, Orton SM, Dyment DA, et al. Expression of the multiple sclerosis-associated MHC class II Allele HLA-DRB1*1501 is regulated by vitamin D. PLoS Genet. 2009;5(2):e1000369. doi: 10.1371/journal.pgen.1000369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zerwekh JE. Blood biomarkers of vitamin D status. Am J Clin Nutr. 2008;87(4):1087S–91S. doi: 10.1093/ajcn/87.4.1087S. [DOI] [PubMed] [Google Scholar]
- 114.Cutolo M, Otsa K, Uprus M, Paolino S, Seriolo B. Vitamin D in rheumatoid arthritis. Autoimmun Rev. 2007;7(1):59–64. doi: 10.1016/j.autrev.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 115.Als OS, Riis B, Christiansen C. Serum concentration of vitamin D metabolites in rheumatoid arthritis. Clin Rheumatol. 1987;6(2):238–43. doi: 10.1007/BF02201030. [DOI] [PubMed] [Google Scholar]
- 116.Turhanoglu AD, Guler H, Yonden Z, Aslan F, Mansuroglu A, Ozer C. The relationship between vitamin D and disease activity and functional health status in rheumatoid arthritis. Rheumatol Int. 2010 Mar 19; doi: 10.1007/s00296-010-1393-6. [DOI] [PubMed] [Google Scholar]
- 117.Craig SM, Yu F, Curtis JR, Alarcon GS, Conn DL, Jonas B, et al. Vitamin D status and its associations with disease activity and severity in African Americans with recent-onset rheumatoid arthritis. J Rheumatol. 2010;37(2):275–81. doi: 10.3899/jrheum.090705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Muller K, Kriegbaum NJ, Baslund B, Sorensen OH, Thymann M, Bentzen K. Vitamin D3 metabolism in patients with rheumatic diseases: low serum levels of 25-hydroxyvitamin D3 in patients with systemic lupus erythematosus. Clin Rheumatol. 1995;14(4):397–400. doi: 10.1007/BF02207671. [DOI] [PubMed] [Google Scholar]
- 119.Bird HA, Wright V, Hennes U, Theiss E. Comparison of serum 1,25-dihydroxycholecalciferol concentrations in rheumatoid arthritis and osteoarthrosis. Ann Rheum Dis. 1982;41(3):257–8. doi: 10.1136/ard.41.3.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bird HA, Peacock M, Storer JH, Wright V. Comparison of serum 25-OH vitamin D concentrations in rheumatoid arthritis and osteoarthrosis. Br Med J. 1980;280(6229):1416. doi: 10.1136/bmj.280.6229.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Patel S, Farragher T, Berry J, Bunn D, Silman A, Symmons D. Association between serum vitamin D metabolite levels and disease activity in patients with early inflammatory polyarthritis. Arthritis Rheum. 2007;56(7):2143–9. doi: 10.1002/art.22722. [DOI] [PubMed] [Google Scholar]
- 122.Feser M, Derber LA, Deane KD, Lezotte DC, Weisman MH, Buckner JH, et al. Plasma 25,OH vitamin D concentrations are not associated with rheumatoid arthritis (RA)-related autoantibodies in individuals at elevated risk for RA. J Rheumatol. 2009;36(5):943–6. doi: 10.3899/jrheum.080764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kim HA, Sung JM, Jeon JY, Yoon JM, Suh CH. Vitamin D may not be a good marker of disease activity in Korean patients with systemic lupus erythematosus. Rheumatol Int. 2010 doi: 10.1007/s00296-010-1442-1. [DOI] [PubMed] [Google Scholar]
- 124.Damanhouri LH. Vitamin D deficiency in Saudi patients with systemic lupus erythematosus. Saudi Med J. 2009;30(10):1291–5. [PubMed] [Google Scholar]
- 125.Wright TB, Shults J, Leonard MB, Zemel BS, Burnham JM. Hypovitaminosis D is associated with greater body mass index and disease activity in pediatric systemic lupus erythematosus. J Pediatr. 2009;155(2):260–5. doi: 10.1016/j.jpeds.2009.02.033. [DOI] [PubMed] [Google Scholar]
- 126.Borba VZ, Vieira JG, Kasamatsu T, Radominski SC, Sato EI, Lazaretti-Castro M. Vitamin D deficiency in patients with active systemic lupus erythematosus. Osteoporos Int. 2009;20(3):427–33. doi: 10.1007/s00198-008-0676-1. [DOI] [PubMed] [Google Scholar]
- 127.Bogaczewicz J, Sysa-Jedrzejowska A, Arkuszewska C, Zabek J, Kontny E, Wozniacka A. [Prevalence of autoantibodies directed against 1,25(OH)2D3 in patients with systemic lupus erythematosus]. Pol Merkur Lekarski. 2010;28(164):103–7. [PubMed] [Google Scholar]
- 128.Ben-Zvi I, Aranow C, Mackay M, Stanevsky A, Kamen DL, Marinescu LM, et al. The impact of vitamin D on dendritic cell function in patients with systemic lupus erythematosus. PLoS One. 2010;5(2):e9193. doi: 10.1371/journal.pone.0009193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Amital H, Szekanecz Z, Szucs G, Danko K, Nagy E, Csepany T, et al. Serum concentrations of 25-OH vitamin D in patients with systemic lupus erythematosus (SLE) are inversely related to disease activity: is it time to routinely supplement patients with SLE with vitamin D? Ann Rheum Dis. 2010 doi: 10.1136/ard.2009.120329. [DOI] [PubMed] [Google Scholar]
- 130.Wu PW, Rhew EY, Dyer AR, Dunlop DD, Langman CB, Price H, et al. 25-hydroxyvitamin D and cardiovascular risk factors in women with systemic lupus erythematosus. Arthritis Rheum. 2009;61(10):1387–95. doi: 10.1002/art.24785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Thudi A, Yin S, Wandstrat AE, Li QZ, Olsen NJ. Vitamin D levels and disease status in Texas patients with systemic lupus erythematosus. Am J Med Sci. 2008;335(2):99–104. doi: 10.1097/MAJ.0b013e318134eeb6. [DOI] [PubMed] [Google Scholar]
- 132.Toloza SM, Cole DE, Gladman DD, Ibanez D, Urowitz MB. Vitamin D insufficiency in a large female SLE cohort. Lupus. 2010;19(1):13–9. doi: 10.1177/0961203309345775. [DOI] [PubMed] [Google Scholar]
- 133.Ruiz-Irastorza G, Egurbide MV, Olivares N, Martinez-Berriotxoa A, Aguirre C. Vitamin D deficiency in systemic lupus erythematosus: prevalence, predictors and clinical consequences. Rheumatology (Oxford) 2008;47(6):920–3. doi: 10.1093/rheumatology/ken121. [DOI] [PubMed] [Google Scholar]
- 134.Reed A, Haugen M, Pachman LM, Langman CB. Abnormalities in serum osteocalcin values in children with chronic rheumatic diseases. J Pediatr. 1990;116(4):574–80. doi: 10.1016/s0022-3476(05)81605-1. [DOI] [PubMed] [Google Scholar]
- 135.Huisman AM, White KP, Algra A, Harth M, Vieth R, Jacobs JW, et al. Vitamin D levels in women with systemic lupus erythematosus and fibromyalgia. J Rheumatol. 2001;28(11):2535–9. [PubMed] [Google Scholar]
- 136.Zold E, Szodoray P, Gaal J, Kappelmayer J, Csathy L, Gyimesi E, et al. Vitamin D deficiency in undifferentiated connective tissue disease. Arthritis Res Ther. 2008;10(5):R123. doi: 10.1186/ar2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mermerci Baskan B, Pekin Dogan Y, Sivas F, Bodur H, Ozoran K. The relation between osteoporosis and vitamin D levels and disease activity in ankylosing spondylitis. Rheumatol Int. 2010;30(3):375–81. doi: 10.1007/s00296-009-0975-7. [DOI] [PubMed] [Google Scholar]
- 138.Franck H, Keck E. Serum osteocalcin and vitamin D metabolites in patients with ankylosing spondylitis. Ann Rheum Dis. 1993;52(5):343–6. doi: 10.1136/ard.52.5.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lange U, Jung O, Teichmann J, Neeck G. Relationship between disease activity and serum levels of vitamin D metabolites and parathyroid hormone in ankylosing spondylitis. Osteoporos Int. 2001;12(12):1031–5. doi: 10.1007/s001980170013. [DOI] [PubMed] [Google Scholar]
- 140.Lange U, Teichmann J, Strunk J, Muller-Ladner U, Schmidt KL. Association of 1.25 vitamin D3 deficiency, disease activity and low bone mass in ankylosing spondylitis. Osteoporos Int. 2005;16(12):1999–2004. doi: 10.1007/s00198-005-1990-5. [DOI] [PubMed] [Google Scholar]
- 141.Teichmann J, Voglau MJ, Lange U. Antibodies to human tissue transglutaminase and alterations of vitamin D metabolism in ankylosing spondylitis and psoriatic arthritis. Rheumatol Int. 2009 doi: 10.1007/s00296-009-1186-y. [DOI] [PubMed] [Google Scholar]
- 142.Calzolari G, Data V, Carignola R, Angeli A. Hypovitaminosis D in systemic sclerosis. J Rheumatol. 2009;36(12):2844. doi: 10.3899/jrheum.090439. author reply 2845. [DOI] [PubMed] [Google Scholar]
- 143.Shinjo SK, Bonfa E, de Falco Caparbo V, Pereira RM. Low bone mass in juvenile onset sclerosis systemic: the possible role for 25-hydroxyvitamin D insufficiency. Rheumatol Int. 2010 doi: 10.1007/s00296-010-1421-6. [DOI] [PubMed] [Google Scholar]
- 144.Matsuoka LY, Dannenberg MJ, Wortsman J, Hollis BW, Jimenez SA, Varga J. Cutaneous vitamin D3 formation in progressive systemic sclerosis. J Rheumatol. 1991;18(8):1196–8. [PubMed] [Google Scholar]
- 145.Serup J, Hagdrup H. Vitamin D metabolites in generalized scleroderma. Evidence of a normal cutaneous and intestinal supply with vitamin D. Acta Derm Venereol. 1985;65(4):343–5. [PubMed] [Google Scholar]
- 146.Serup J, Hagdrup HK. Increased 1,25-dihydroxyvitamin D in patients with generalized scleroderma and no aberrant calcifications. Arch Dermatol Res. 1984;276(3):205–6. doi: 10.1007/BF00414023. [DOI] [PubMed] [Google Scholar]
- 147.Caramaschi P, Dalla Gassa A, Ruzzenente O, Volpe A, Ravagnani V, Tinazzi I, et al. Very low levels of vitamin D in systemic sclerosis patients. Clin Rheumatol. 2010 doi: 10.1007/s10067-010-1478-3. [DOI] [PubMed] [Google Scholar]
- 148.Vacca A, Cormier C, Piras M, Mathieu A, Kahan A, Allanore Y. Vitamin D deficiency and insufficiency in 2 independent cohorts of patients with systemic sclerosis. J Rheumatol. 2009;36(9):1924–9. doi: 10.3899/jrheum.081287. [DOI] [PubMed] [Google Scholar]
- 149.Borkar VV, Verma S, Bhalla A. Low levels of vitamin D in North Indian children with newly diagnosed type 1 diabetes. Pediatr Diabetes. 2009 doi: 10.1111/j.1399-5448.2009.00589.x. [DOI] [PubMed] [Google Scholar]
- 150.Singh DK, Winocour P, Summerhayes B, Viljoen A, Sivakumar G, Farrington K. Are low erythropoietin and 1,25-dihydroxyvitamin D levels indicative of tubulo-interstitial dysfunction in diabetes without persistent microalbuminuria? Diabetes Res Clin Pract. 2009;85(3):258–64. doi: 10.1016/j.diabres.2009.06.022. [DOI] [PubMed] [Google Scholar]
- 151.Bener A, Alsaied A, Al-Ali M, Al-Kubaisi A, Basha B, Abraham A, et al. High prevalence of vitamin D deficiency in type 1 diabetes mellitus and healthy children. Acta Diabetol. 2009;46(3):183–9. doi: 10.1007/s00592-008-0071-6. [DOI] [PubMed] [Google Scholar]
- 152.Greer RM, Rogers MA, Bowling FG, Buntain HM, Harris M, Leong GM, et al. Australian children and adolescents with type 1 diabetes have low vitamin D levels. Med J Aust. 2007;187(1):59–60. doi: 10.5694/j.1326-5377.2007.tb01130.x. [DOI] [PubMed] [Google Scholar]
- 153.Baumgartl HJ, Standl E, Schmidt-Gayk H, Kolb HJ, Janka HU, Ziegler AG. Changes of vitamin D3 serum concentrations at the onset of immune-mediated type 1 (insulin-dependent) diabetes mellitus. Diabetes Res. 1991;16(3):145–8. [PubMed] [Google Scholar]
- 154.Rodland O, Markestad T, Aksnes L, Aarskog D. Plasma concentrations of vitamin D metabolites during puberty of diabetic children. Diabetologia. 1985;28(9):663–6. doi: 10.1007/BF00291972. [DOI] [PubMed] [Google Scholar]
- 155.Littorin B, Blom P, Scholin A, Arnqvist HJ, Blohme G, Bolinder J, et al. Lower levels of plasma 25-hydroxyvitamin D among young adults at diagnosis of autoimmune type 1 diabetes compared with control subjects: results from the nationwide Diabetes Incidence Study in Sweden (DISS). Diabetologia. 2006;49(12):2847–52. doi: 10.1007/s00125-006-0426-x. [DOI] [PubMed] [Google Scholar]
- 156.Di Cesar DJ, Ploutz-Snyder R, Weinstock RS, Moses AM. Vitamin D deficiency is more common in type 2 than in type 1 diabetes. Diabetes Care. 2006;29(1):174. doi: 10.2337/diacare.29.1.174. [DOI] [PubMed] [Google Scholar]
- 157.Verrotti A, Basciani F, Carle F, Morgese G, Chiarelli F. Calcium metabolism in adolescents and young adults with type 1 diabetes mellitus without and with persistent microalbuminuria. J Endocrinol Invest. 1999;22(3):198–202. doi: 10.1007/BF03343541. [DOI] [PubMed] [Google Scholar]
- 158.Bierschenk L, Alexander J, Wasserfall C, Haller M, Schatz D, Atkinson M. Vitamin D levels in subjects with and without type 1 diabetes residing in a solar rich environment. Diabetes Care. 2009;32(11):1977–9. doi: 10.2337/dc09-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Arreola F, Paniagua R, Diaz-Bensussen S, Urquieta B, Lopez-Montano E, Partida-Hernandez G, et al. Bone mineral content, 25-hydroxycalciferol and zinc serum levels in insulin-dependent (type I) diabetic patients. Arch Invest Med (Mex) 1990;21(2):195–9. [PubMed] [Google Scholar]
- 160.Huynh T, Greer RM, Nyunt O, Bowling F, Cowley D, Leong GM, et al. The association between ketoacidosis and 25(OH)-vitamin D levels at presentation in children with type 1 diabetes mellitus. Pediatr Diabetes. 2009;10(1):38–43. doi: 10.1111/j.1399-5448.2008.00439.x. [DOI] [PubMed] [Google Scholar]
- 161.Storm TL, Sorensen OH, Lund B, Christiansen JS, Andersen AR, Lumholtz IB, et al. Vitamin D metabolism in insulin-dependent diabetes mellitus. Metab Bone Dis Relat Res. 1983;5(3):107–10. doi: 10.1016/0221-8747(83)90010-3. [DOI] [PubMed] [Google Scholar]
- 162.Vogt MH, ten Kate J, Drent RJ, Polman CH, Hupperts R. Increased osteopontin plasma levels in multiple sclerosis patients correlate with bone-specific markers. Mult Scler. 2010;16(4):443–9. doi: 10.1177/1352458509359723. [DOI] [PubMed] [Google Scholar]
- 163.van der Mei IA, Ponsonby AL, Dwyer T, Blizzard L, Taylor BV, Kilpatrick T, et al. Vitamin D levels in people with multiple sclerosis and community controls in Tasmania, Australia. J Neurol. 2007;254(5):581–90. doi: 10.1007/s00415-006-0315-8. [DOI] [PubMed] [Google Scholar]
- 164.Holmoy T, Moen SM, Gundersen TA, Holick MF, Fainardi E, Castellazzi M, et al. 25-hydroxyvitamin D in cerebrospinal fluid during relapse and remission of multiple sclerosis. Mult Scler. 2009;15(11):1280–5. doi: 10.1177/1352458509107008. [DOI] [PubMed] [Google Scholar]
- 165.Hiremath GS, Cettomai D, Baynes M, Ratchford JN, Newsome S, Harrison D, et al. Vitamin D status and effect of low-dose cholecalciferol and high-dose ergocalciferol supplementation in multiple sclerosis. Mult Scler. 2009;15(6):735–40. doi: 10.1177/1352458509102844. [DOI] [PubMed] [Google Scholar]
- 166.Kragt J, van Amerongen B, Killestein J, Dijkstra C, Uitdehaag B, Polman C, et al. Higher levels of 25-hydroxyvitamin D are associated with a lower incidence of multiple sclerosis only in women. Mult Scler. 2009;15(1):9–15. doi: 10.1177/1352458508095920. [DOI] [PubMed] [Google Scholar]
- 167.Correale J, Ysrraelit MC, Gaitan MI. Immunomodulatory effects of Vitamin D in multiple sclerosis. Brain. 2009;132(Pt 5):1146–60. doi: 10.1093/brain/awp033. [DOI] [PubMed] [Google Scholar]
- 168.Embry AF, Snowdon LR, Vieth R. Vitamin D and seasonal fluctuations of gadolinium-enhancing magnetic resonance imaging lesions in multiple sclerosis. Ann Neurol. 2000;48(2):271–2. [PubMed] [Google Scholar]
- 169.Barnes MS, Bonham MP, Robson PJ, Strain JJ, Lowe-Strong AS, Eaton-Evans J, et al. Assessment of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D3 concentrations in male and female multiple sclerosis patients and control volunteers. Mult Scler. 2007;13(5):670–2. doi: 10.1177/1352458506072666. [DOI] [PubMed] [Google Scholar]
- 170.Orton SM, Morris AP, Herrera BM, Ramagopalan SV, Lincoln MR, Chao MJ, et al. Evidence for genetic regulation of vitamin D status in twins with multiple sclerosis. Am J Clin Nutr. 2008;88(2):441–7. doi: 10.1093/ajcn/88.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Soilu-Hanninen M, Airas L, Mononen I, Heikkila A, Viljanen M, Hanninen A. 25-Hydroxyvitamin D levels in serum at the onset of multiple sclerosis. Mult Scler. 2005;11(3):266–71. doi: 10.1191/1352458505ms1157oa. [DOI] [PubMed] [Google Scholar]
- 172.Smolders J, Menheere P, Kessels A, Damoiseaux J, Hupperts R. Association of vitamin D metabolite levels with relapse rate and disability in multiple sclerosis. Mult Scler. 2008;14(9):1220–4. doi: 10.1177/1352458508094399. [DOI] [PubMed] [Google Scholar]
- 173.Joseph AJ, George B, Pulimood AB, Seshadri MS, Chacko A. 25 (OH) vitamin D level in Crohn's disease: association with sun exposure & disease activity. Indian J Med Res. 2009;130(2):133–7. [PubMed] [Google Scholar]
- 174.Tajika M, Matsuura A, Nakamura T, Suzuki T, Sawaki A, Kato T, et al. Risk factors for vitamin D deficiency in patients with Crohn's disease. J Gastroenterol. 2004;39(6):527–33. doi: 10.1007/s00535-003-1338-x. [DOI] [PubMed] [Google Scholar]
- 175.Harries AD, Brown R, Heatley RV, Williams LA, Woodhead S, Rhodes J. Vitamin D status in Crohn's disease: association with nutrition and disease activity. Gut. 1985;26(11):1197–203. doi: 10.1136/gut.26.11.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Pappa HM, Gordon CM, Saslowsky TM, Zholudev A, Horr B, Shih MC, et al. Vitamin D status in children and young adults with inflammatory bowel disease. Pediatrics. 2006;118(5):1950–61. doi: 10.1542/peds.2006-0841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Goswami R, Marwaha RK, Gupta N, Tandon N, Sreenivas V, Tomar N, et al. Prevalence of vitamin D deficiency and its relationship with thyroid autoimmunity in Asian Indians: a community-based survey. Br J Nutr. 2009;102(3):382–6. doi: 10.1017/S0007114509220824. [DOI] [PubMed] [Google Scholar]
- 178.Silverberg JI, Silverberg AI, Malka E, Silverberg NB. A pilot study assessing the role of 25 hydroxy vitamin D levels in patients with vitiligo vulgaris. J Am Acad Dermatol. 62(6):937–41. doi: 10.1016/j.jaad.2009.11.024. [DOI] [PubMed] [Google Scholar]
- 179.Braun-Moscovici Y, Furst DE, Markovits D, Rozin A, Clements PJ, Nahir AM, et al. Vitamin D, parathyroid hormone, and acroosteolysis in systemic sclerosis. J Rheumatol. 2008;35(11):2201–5. doi: 10.3899/jrheum.071171. [DOI] [PubMed] [Google Scholar]
- 180.Cutolo M, Otsa K, Laas K, Yprus M, Lehtme R, Secchi ME, et al. Circannual vitamin d serum levels and disease activity in rheumatoid arthritis: Northern versus Southern Europe. Clin Exp Rheumatol. 2006;24(6):702–4. [PubMed] [Google Scholar]
- 181.Brohult J, Jonson B. Effects of large doses of calciferol on patients with rheumatoid arthritis. A double-blind clinical trial. Scand J Rheumatol. 1973;2(4):173–6. doi: 10.3109/03009747309097085. [DOI] [PubMed] [Google Scholar]
- 182.Andjelkovic Z, Vojinovic J, Pejnovic N, Popovic M, Dujic A, Mitrovic D, et al. Disease modifying and immunomodulatory effects of high dose 1 alpha (OH) D3 in rheumatoid arthritis patients. Clin Exp Rheumatol. 1999;17(4):453–6. [PubMed] [Google Scholar]
- 183.Gaal J, Lakos G, Szodoray P, Kiss J, Horvath I, Horkay E, et al. Immunological and clinical effects of alphacalcidol in patients with psoriatic arthropathy: results of an open, follow-up pilot study. Acta Derm Venereol. 2009;89(2):140–4. doi: 10.2340/00015555-0555. [DOI] [PubMed] [Google Scholar]
- 184.Huckins D, Felson DT, Holick M. Treatment of psoriatic arthritis with oral 1,25-dihydroxyvitamin D3: a pilot study. Arthritis Rheum. 1990;33(11):1723–7. doi: 10.1002/art.1780331117. [DOI] [PubMed] [Google Scholar]
- 185.Ruiz-Irastorza G, Gordo S, Olivares N, Egurbide MV, Aguirre C. Changes in vitamin D levels in patients with systemic lupus erythematosus: Effects on fatigue, disease activity and damage. Arthritis Care Res (Hoboken) 2010 doi: 10.1002/acr.20186. [DOI] [PubMed] [Google Scholar]
- 186.Humbert P, Dupond JL, Agache P, Laurent R, Rochefort A, Drobacheff C, et al. Treatment of scleroderma with oral 1,25-dihydroxyvitamin D3: evaluation of skin involvement using non-invasive techniques. Results of an open prospective trial. Acta Derm Venereol. 1993;73(6):449–51. doi: 10.2340/0001555573449451. [DOI] [PubMed] [Google Scholar]
- 187.Hulshof MM, Pavel S, Breedveld FC, Dijkmans BA, Vermeer BJ. Oral calcitriol as a new therapeutic modality for generalized morphea. Arch Dermatol. 1994;130(10):1290–3. [PubMed] [Google Scholar]
- 188.Caca-Biljanovska NG, Vlckova-Laskoska MT, Dervendi DV, Pesic NP, Laskoski DS. Treatment of generalized morphea with oral 1,25-dihydroxyvitamin D3. Adv Exp Med Biol. 1999;455:299–304. doi: 10.1007/978-1-4615-4857-7_44. [DOI] [PubMed] [Google Scholar]
- 189.Elst EF, Van Suijlekom-Smit LW, Oranje AP. Treatment of linear scleroderma with oral 1,25-dihydroxyvitamin D3 (calcitriol) in seven children. Pediatr Dermatol. 1999;16(1):53–8. doi: 10.1046/j.1525-1470.1999.99016.x. [DOI] [PubMed] [Google Scholar]
- 190.Hulshof MM, Bouwes Bavinck JN, Bergman W, Masclee AA, Heickendorff L, Breedveld FC, et al. Double-blind, placebo-controlled study of oral calcitriol for the treatment of localized and systemic scleroderma. J Am Acad Dermatol. 2000;43(6):1017–23. doi: 10.1067/mjd.2000.108369. [DOI] [PubMed] [Google Scholar]
- 191.Li X, Liao L, Yan X, Huang G, Lin J, Lei M, et al. Protective effects of 1-alpha-hydroxyvitamin D3 on residual beta-cell function in patients with adult-onset latent autoimmune diabetes (LADA). Diabetes Metab Res Rev. 2009;25(5):411–6. doi: 10.1002/dmrr.977. [DOI] [PubMed] [Google Scholar]
- 192.Pitocco D, Crino A, Di Stasio E, Manfrini S, Guglielmi C, Spera S, et al. The effects of calcitriol and nicotinamide on residual pancreatic beta-cell function in patients with recent-onset Type 1 diabetes (IMDIAB XI). Diabet Med. 2006;23(8):920–3. doi: 10.1111/j.1464-5491.2006.01921.x. [DOI] [PubMed] [Google Scholar]
- 193.Goldberg P, Fleming MC, Picard EH. Multiple sclerosis: decreased relapse rate through dietary supplementation with calcium, magnesium and vitamin D. Med Hypotheses. 1986;21(2):193–200. doi: 10.1016/0306-9877(86)90010-1. [DOI] [PubMed] [Google Scholar]
- 194.Kimball SM, Ursell MR, O'Connor P, Vieth R. Safety of vitamin D3 in adults with multiple sclerosis. Am J Clin Nutr. 2007;86(3):645–51. doi: 10.1093/ajcn/86.3.645. [DOI] [PubMed] [Google Scholar]
- 195.Burton JM, Kimball S, Vieth R, Bar-Or A, Dosch HM, Cheung R, et al. A phase I/II dose-escalation trial of vitamin D3 and calcium in multiple sclerosis. Neurology. 2010 doi: 10.1212/WNL.0b013e3181e1cec2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Miheller P, Muzes G, Hritz I, Lakatos G, Pregun I, Lakatos PL, et al. Comparison of the effects of 1,25 dihydroxyvitamin D and 25 hydroxyvitamin D on bone pathology and disease activity in Crohn's disease patients. Inflamm Bowel Dis. 2009;15(11):1656–62. doi: 10.1002/ibd.20947. [DOI] [PubMed] [Google Scholar]
- 197.Nielen MM, van Schaardenburg D, Lems WF, van de Stadt RJ, de Koning MH, Reesink HW, et al. Vitamin D deficiency does not increase the risk of rheumatoid arthritis: comment on the article by Merlino et al. Arthritis Rheum. 2006;54(11):3719–20. doi: 10.1002/art.22191. [DOI] [PubMed] [Google Scholar]
- 198.Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. Jama. 2006;296(23):2832–8. doi: 10.1001/jama.296.23.2832. [DOI] [PubMed] [Google Scholar]
- 199.Merlino LA, Curtis J, Mikuls TR, Cerhan JR, Criswell LA, Saag KG. Vitamin D intake is inversely associated with rheumatoid arthritis: results from the Iowa Women's Health Study. Arthritis Rheum. 2004;50(1):72–7. doi: 10.1002/art.11434. [DOI] [PubMed] [Google Scholar]
- 200.Costenbader KH, Feskanich D, Holmes M, Karlson EW, Benito-Garcia E. Vitamin D intake and risks of systemic lupus erythematosus and rheumatoid arthritis in women. Ann Rheum Dis. 2008;67(4):530–5. doi: 10.1136/ard.2007.072736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Munger KL, Zhang SM, O'Reilly E, Hernan MA, Olek MJ, Willett WC, et al. Vitamin D intake and incidence of multiple sclerosis. Neurology. 2004;62(1):60–5. doi: 10.1212/01.wnl.0000101723.79681.38. [DOI] [PubMed] [Google Scholar]
- 202.Tenconi MT, Devoti G, Comelli M, Pinon M, Capocchiano A, Calcaterra V, et al. Major childhood infectious diseases and other determinants associated with type 1 diabetes: a case-control study. Acta Diabetol. 2007;44(1):14–9. doi: 10.1007/s00592-007-0235-9. [DOI] [PubMed] [Google Scholar]
- 203.Stene LC, Joner G. Use of cod liver oil during the first year of life is associated with lower risk of childhood-onset type 1 diabetes: a large, population-based, case-control study. Am J Clin Nutr. 2003;78(6):1128–34. doi: 10.1093/ajcn/78.6.1128. [DOI] [PubMed] [Google Scholar]
- 204.Stene LC, Ulriksen J, Magnus P, Joner G. Use of cod liver oil during pregnancy associated with lower risk of Type I diabetes in the offspring. Diabetologia. 2000;43(9):1093–8. doi: 10.1007/s001250051499. [DOI] [PubMed] [Google Scholar]
- 205.Zipitis CS, Akobeng AK. Vitamin D supplementation in early childhood and risk of type 1 diabetes: a systematic review and meta-analysis. Arch Dis Child. 2008;93(6):512–7. doi: 10.1136/adc.2007.128579. [DOI] [PubMed] [Google Scholar]
- 206.Hypponen E, Laara E, Reunanen A, Jarvelin MR, Virtanen SM. Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. Lancet. 2001;358(9292):1500–3. doi: 10.1016/S0140-6736(01)06580-1. [DOI] [PubMed] [Google Scholar]
- 207.Fronczak CM, Baron AE, Chase HP, Ross C, Brady HL, Hoffman M, et al. In utero dietary exposures and risk of islet autoimmunity in children. Diabetes Care. 2003;26(12):3237–42. doi: 10.2337/diacare.26.12.3237. [DOI] [PubMed] [Google Scholar]
- 208.Marjamaki L, Niinisto S, Kenward MG, Uusitalo L, Uusitalo U, Ovaskainen ML, et al. Maternal intake of vitamin D during pregnancy and risk of advanced beta cell autoimmunity and type 1 diabetes in offspring. Diabetologia. 2010 doi: 10.1007/s00125-010-1734-8. [DOI] [PubMed] [Google Scholar]
- 209.Vieth R. Why the optimal requirement for Vitamin D3 is probably much higher than what is officially recommended for adults. J Steroid Biochem Mol Biol. 2004;89-90(1-5):575–9. doi: 10.1016/j.jsbmb.2004.03.038. [DOI] [PubMed] [Google Scholar]
- 210.Braun-Moscovici Y, Toledano K, Markovits D, Rozin A, Nahir AM, Balbir-Gurman A. Vitamin D level: is it related to disease activity in inflammatory joint disease? Rheumatol Int. 2009 doi: 10.1007/s00296-009-1251-6. [DOI] [PubMed] [Google Scholar]
- 211.Cutolo M, Otsa K, Yprus M, Seriolo B. Vitamin D and rheumatoid arthritis: comment on the letter by Nielen et al. Arthritis Rheum. 2007;56(5):1719–20. doi: 10.1002/art.22569. [DOI] [PubMed] [Google Scholar]
- 212.Oelzner P, Muller A, Deschner F, Huller M, Abendroth K, Hein G, et al. Relationship between disease activity and serum levels of vitamin D metabolites and PTH in rheumatoid arthritis. Calcif Tissue Int. 1998;62(3):193–8. doi: 10.1007/s002239900416. [DOI] [PubMed] [Google Scholar]
- 213.Kroger H, Penttila IM, Alhava EM. Low serum vitamin D metabolites in women with rheumatoid arthritis. Scand J Rheumatol. 1993;22(4):172–7. doi: 10.3109/03009749309099266. [DOI] [PubMed] [Google Scholar]
- 214.Falkenbach A, Tripathi R, Sedlmeyer A, Staudinger M, Herold M. Serum 25-hydroxyvitamin D and parathyroid hormone in patients with ankylosing spondylitis before and after a three-week rehabilitation treatment at high altitude during winter and spring. Wien Klin Wochenschr. 2001;113(9):328–32. [PubMed] [Google Scholar]
- 215.Lee YS, Schlotzhauer T, Ott SM, van Vollenhoven RF, Hunter J, Shapiro J, et al. Skeletal status of men with early and late ankylosing spondylitis. Am J Med. 1997;103(3):233–41. doi: 10.1016/s0002-9343(97)00143-5. [DOI] [PubMed] [Google Scholar]
- 216.Svoren BM, Volkening LK, Wood JR, Laffel LM. Significant vitamin D deficiency in youth with type 1 diabetes mellitus. J Pediatr. 2009;154(1):132–4. doi: 10.1016/j.jpeds.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Bener A, Alsaied A, Al-Ali M, Hassan AS, Basha B, Al-Kubaisi A, et al. Impact of lifestyle and dietary habits on hypovitaminosis D in type 1 diabetes mellitus and healthy children from Qatar, a sun-rich country. Ann Nutr Metab. 2008;53(3-4):215–22. doi: 10.1159/000184439. [DOI] [PubMed] [Google Scholar]
- 218.Soilu-Hanninen M, Laaksonen M, Laitinen I, Eralinna JP, Lilius EM, Mononen I. A longitudinal study of serum 25-hydroxyvitamin D and intact parathyroid hormone levels indicate the importance of vitamin D and calcium homeostasis regulation in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2008;79(2):152–7. doi: 10.1136/jnnp.2006.105320. [DOI] [PubMed] [Google Scholar]
- 219.Wingerchuk DM, Lesaux J, Rice GP, Kremenchutzky M, Ebers GC. A pilot study of oral calcitriol (1,25-dihydroxyvitamin D3) for relapsing-remitting multiple sclerosis. J Neurol Neurosurg Psychiatry. 2005;76(9):1294–6. doi: 10.1136/jnnp.2004.056499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Achiron A, Barak Y, Miron S, Izhak Y, Faibel M, Edelstein S. Alfacalcidol treatment in multiple sclerosis. Clin Neuropharmacol. 2003;26(2):53. doi: 10.1097/00002826-200303000-00001. [DOI] [PubMed] [Google Scholar]
- 221.Brekke HK, Ludvigsson J. Vitamin D supplementation and diabetes-related autoimmunity in the ABIS study. Pediatr Diabetes. 2007;8(1):11–4. doi: 10.1111/j.1399-5448.2006.00223.x. [DOI] [PubMed] [Google Scholar]
- 222.The EURODIAB Substudy 2 Study Group Vitamin D supplement in early childhood and risk for Type I (insulin-dependent) diabetes mellitus. Diabetologia. 1999;42(1):51–4. doi: 10.1007/s001250051112. [DOI] [PubMed] [Google Scholar]