Abstract
Background
Waterpipe tobacco smoking usually involves heating flavored tobacco with charcoal and inhaling the resulting smoke after it has passed through water. Waterpipe tobacco smoking increases heart rate and produces subjective effects similar to those reported by cigarette smokers. These responses are thought to be nicotine-mediated, though no placebo-control studies exist. Accordingly, this double-blind, placebo-control study compared the acute physiological and subjective effects of waterpipe tobacco smoking to those produced when participants used a waterpipe to smoke a flavor-matched, tobacco-free preparation.
Methods
Occasional waterpipe tobacco smokers (N=37; 2–5 monthly smoking episodes for ≥ 6 months) completed two double-blind, counterbalanced sessions that differed by product: preferred brand/flavor of waterpipe tobacco or flavor-matched, tobacco-free preparation. For each 45-minute, ad lib smoking episode blood and expired air CO were sampled, cardiovascular and respiratory response were measured, and subjective response was assessed.
Results
Waterpipe tobacco smoking significantly increased mean (±SEM) plasma nicotine concentration (3.6±0.7 ng/ml) and heart rate (8.6±1.4 bpm) while placebo did not (0.1±0.0 ng/ml; 1.3±0.9 bpm). For carboxyhemoglobin (COHb) and expired air CO, significant increases were observed for tobacco (3.8±0.4%; 27.9±2.6 ppm) and for placebo (3.9±0.4%; 27.7±3.3 ppm) with no differences across condition. Independent of condition, symptoms of nicotine/tobacco abstinence (e.g., “urges to smoke”, “anxious”) were reduced and direct effects (e.g., “dizzy”, “satisfy”) increased.
Discussion
These results from the first placebo-control study of waterpipe tobacco smoking demonstrate that waterpipe-induced heart rate increases are almost certainly mediated by nicotine though the subjective effects observed in these occasional smokers were not.
Keywords: waterpipe, nicotine, carbon monoxide, placebo, double-blind
1. Introduction
For centuries, millions of people have smoked tobacco using a waterpipe (a.k.a. hookah, narghile, shisha): inhalation of charcoal-heated air passes through tobacco, travels down the body, and bubbles through water in the bowl before reaching smokers’ lungs (World Health Organization, 2005). While often associated with southwest Asia, waterpipe tobacco smoking is now seen worldwide (e.g., Pärna et al., 2008; Jensen et al., 2010). In the U.S., for example, past 30-day waterpipe tobacco smoking has been reported by 9–20% of some college samples (Cobb et al., 2010). A survey of 8,745 students from 8 universities revealed that 7.2% reported past-30 day use and 29.5% reported “ever use” (Primack et al., 2010). Past 30-day use among 14–18 year old Arab Americans may be as high as 16% and non-Arab Americans as high as 11% (Weglicki et al., 2007).
One reason for the global spread of waterpipe tobacco smoking may involve the oft-reported belief that waterpipes are less risky than cigarettes (Aljarrah et al., 2009; Smith-Simone et al., 2008). This belief seemingly is contradicted by demonstrations that various constituents of waterpipe smoke are known to cause cancer (e.g., polycyclic aromatic hydrocarbons [PAH]; Sepetdjian et al, 2008), lung disease (e.g., volatile aldehydes; Al Rashidi et al., 2008), cardiovascular disease (e.g., carbon monoxide [CO]; Shihadeh and Saleh, 2005), and dependence (i.e., nicotine; Shihadeh, 2003). At least some of these smoke toxicants have been found in waterpipe tobacco smokers during smoking, including CO and nicotine (El-Nachef and Hammond, 2008; Shafagoj and Mohammed, 2002).
While there is a growing literature investigating waterpipe smoke toxicant content and exposure, relatively few studies have examined the acute effects of waterpipe tobacco smoking. In terms of cardiovascular response, two laboratory studies demonstrate that waterpipe tobacco smoking produces clear cardiovascular effects. A single 45-minute waterpipe smoking episode has been shown to increase average heart rate (HR) by 6 (Eissenberg and Shihadeh, 2009) or 16 bpm (Shafagoj and Mohammed, 2002), as well as to increase systolic blood pressure (SBP) by 6.7 mmHG, diastolic blood pressure (DBP) by 4.4 mmHG and mean arterial pressure (MAP) by 5.2 mmHG (Shafagoj and Mohammed, 2002). In both studies these cardiovascular effects were attributed to waterpipe-induced increases in plasma nicotine (see also Shafagoj et al., 2002). Subjective effects of waterpipe tobacco smoking have also been observed; the suppression of tobacco abstinence symptoms commonly reported in cigarette smokers (e.g., urges to smoke, craving) were suppressed following a single waterpipe use episode (Maziak et al., 2009). These subjective effects are also thought to be mediated by waterpipe-delivered nicotine.
Importantly, the role of nicotine as a causal factor in the acute effects of waterpipe smoking is speculative, as no study has included a nicotine-free placebo condition using double-blind administration procedures. Without such a study, several non-nicotine factors might explain some effects observed during waterpipe tobacco smoking, including CO intoxication (e.g., Lim et al., 2009), expectancy, or activity associated with the use episode. Therefore, the purpose of this double-blind, placebo-control, within-subject study of waterpipe use was to determine the extent to which the acute effects of waterpipe tobacco smoking were due to nicotine exposure. We hypothesized that cardiovascular and subjective effects reported elsewhere would also be observed when participants used a waterpipe to smoke tobacco that delivered nicotine, but not when participants smoked a tobacco-free herbal waterpipe preparation that did not deliver nicotine.
2. Materials and Methods
2.1 Participants
Eight women and 29 men were recruited for this university IRB-approved study. These individuals (three African American, seven Asian, 20 Caucasian, one Hawaiian/Pacific Islander and six mixed/other ethnicity) were healthy, between the ages of 18–50 (mean ± standard error of the mean [SEM] = 20.5±2.1 years) and reported smoking waterpipe tobacco 2–5 times/month (3.8±1.0) for ≥ six months (20.2±12.9). Participants’ average expired air CO level at screening was 2.5±1.6 ppm. Exclusion criteria included self-reported history of chronic health problems or psychiatric conditions, regular use of prescription medications (other than vitamins or birth control), and current pregnancy (verified by urinalysis) or breastfeeding, as well as self-reported current use of >5 cigarettes/month, other tobacco products, marijuana (> 5 days in past month) or other illicit drugs (past 30-day use of cocaine, benzodiazepines, opioids, or methamphetamine; confirmed by urinalysis).
2.2 Materials
The waterpipe consisted of a chrome body (43cm) screwed into an acrylic base (24cm; volume 1230mL; www.myasaray.com). Approximately 2.5cm of the body’s conduit was submerged by 870mL water poured into the base. The glazed ceramic head (7.6cm; five, 6mm holes in bottom) was covered with a circular sheet of aluminum foil, perforated by a screen pincher (www.smoking-hookah.com). A 33mm, quicklighting charcoal briquette (Three Kings, Holland) was placed on top of the foil. The leather hose was fitted with topography measurement hardware and included a wooden mouthpiece capped with a sterile plastic tip (www.hookahcompany.com).
During the active waterpipe condition, participants smoked their preferred brand and flavor of product. The most popular tobacco brand was Starbuzz (U.S.; n=18), followed by Nakhla (Egypt; n=2) and Al Fakher (United Arab Emirates; n=2); Nakhla was used as the default brand for participants who did not report a preference (n=15). The most popular flavors were fruit-based: apple/double apple (n=8), strawberry (n=6), mango (n=4), peach (n=3), cherry (n=2), watermelon (n=2), as well as grape, mixed fruit, orange, and guava (each n=1). Other preferred flavors were mint (n=6), rose (n=1), and vanilla (n=1). During the placebo waterpipe condition, participants smoked a flavor-matched, tobacco-free herbal product (Soex; Soex India Pvt. Ltd; Mumbai, India). According to the manufacturer, Soex is “100% tobacco-free and nicotine-free”, primarily composed of chopped “sugar cane, molasses, and flavor”.
2.3 Study Design and Procedures
Participants completed two counterbalanced, 2-hour sessions that differed by product used: active waterpipe tobacco or flavor-matched tobacco-free placebo. Participants were notified during the informed consent process that they would be a smoking a product that “may or may not contain tobacco” during each session. Once overnight tobacco abstinence was verified (CO levels ≤10ppm), a catheter was inserted into a forearm vein and physiological recording commenced. Thirty minutes later, breath, blood, and subjective response were measured and session-specific product was administered: a waterpipe containing 10g of product in the foil-covered head with a lit charcoal briquette placed on top (additional half briquettes available upon request). The waterpipe head was always packed and emptied by a researcher who had no participant contact. A foil covering obscured head contents, and neither participant nor study staff was informed of the product to be used on a particular day. Participants were given a minimum of 45 minutes to smoke the waterpipe ad lib while watching a video of their choice. Blood, breath, and subjective response were measured periodically during and/or after the smoking episode. The laboratory was ventilated and during session mean peak ambient CO level was 4.0±1.0 ppm (collapsed across condition, N=64 samples). Payment for completing both sessions was $175.
2.4 Physiological Measures
Expired- and ambient-air CO were assessed with a BreathCO monitor (Vitalograph; Lenexa, KS). Carboxyhemoglobin (COHb) concentration was analyzed within 2 minutes after venous blood sampling (NPT7 blood gas analyzer, Radiometer America); 10 mL of the blood sample was centrifuged, plasma stored at −70°C, and analyzed for nicotine level (limit of quantitation [LOQ] 2.0 ng/mL; modified LC-MS/MS version of that reported by Naidong et al., 2001; see Breland et al., 2006). HR was measured every 20 seconds and BP every 5 minutes (Model 507E, Criticare Systems).
Cigarette smoking has been shown to alter expired air nitric oxide (NO) acutely (Kharitonov et al., 1995; Chambers et al., 1998), and NO is related to pulmonary disease (Louhelainen et al., 2008); changes in NO due to waterpipe tobacco smoking are unclear. Thus, expired NO was analyzed using a Nitric Oxide Analyzer (280i, Ionics Inst); the average of three satisfactory measurements for each time point was used in analyses. Previous work suggests that waterpipe smoking may impair lung function (Aydin et al., 2004; Kiter et al., 2000); thus, pulmonary function testing (PFT) was performed with a spirometer (Vitalograph, Lenaxa, KS) to measure forced expiratory volume in 1 second (FEV1), forced expiratory vital capacity (FVC), and FEV1/FVC ratio (National Collaborating Centre for Chronic Conditions, 2003). The better of two satisfactory PFT maneuvers (based on FEV1 results) was used in analyses.
2.5 Subjective Measures
All subjective measures were computerized (as in Breland et al., 2006). Individual items for each measure are outlined in Table 1.
Table 1.
Statistical analysis results for all outcome measures (N = 37)
| Conditiona | Timeb | Cond × Time | ||||
|---|---|---|---|---|---|---|
| F | p | F | p | F | p | |
| Physiological Measures | ||||||
| Nicotinec | 34.70 | <.001 | 15.5 | <.001 | 20.1 | <.001 |
| Heart Rated | 17.70 | <.001 | 11.3 | <.001 | 13.7 | <.001 |
| Systolic Blood Pressuree | 5.20 | <.05 | 1.3 | n.s. | 1.1 | n.s. |
| Diastolic Blood Pressuree | 1.20 | n.s. | 1.3 | n.s. | 2.0 | n.s. |
| Mean Arterial Pressuref | 4.60 | <.05 | 1.1 | n.s. | 0.1 | n.s. |
| COHbc | 0.4 | n.s. | 95.7 | <.001 | 0.7 | n.s. |
| COc | 0.0 | n.s. | 104.2 | <.001 | 0.1 | n.s. |
| Nog | 0.7 | n.s. | 0.47 | n.s. | 0.8 | n.s. |
| FEV1h | 0.0 | n.s. | 8.1 | <.01 | 0.0 | n.s. |
| FVCh | 0.2 | n.s. | 5.7 | <.05 | 0.8 | n.s. |
| FEV1/FVCh | 0.5 | n.s. | 0.1 | n.s. | 3.0 | n.s. |
| Hughes and Hatsukamii | ||||||
| Urges to smoke a waterpipe | 2.0 | n.s | 6.7 | <.05 | 0.1 | n.s. |
| Irritability/Frustration/Anger | 2.8 | n.s | 0.8 | n.s | 0.9 | n.s. |
| Anxious | 1.4 | n.s | 5.9 | <.05 | 0.0 | n.s. |
| Difficulty concentrating | 0.0 | n.s | 0.0 | n.s | 0.4 | n.s |
| Restlessness | 2.4 | n.s | 0.1 | n.s | 0.1 | n.s |
| Hunger | 0.7 | n.s | 2.8 | n.s | 0.0 | n.s |
| Impatient | 0.0 | n.s | 0.0 | n.s | 1.4 | n.s |
| Craving a waterpipe/Nicotine | 0.3 | n.s | 1.6 | n.s | 0.2 | n.s |
| Drowsiness | 2.5 | n.s | 1.7 | n.s | 0.0 | n.s |
| Depression/Feeling blue | 0.3 | n.s | 0.7 | n.s | 2.0 | n.s |
| Desire for sweets | 2.6 | n.s | 0.2 | n.s | 2.3 | n.s |
| Tiffany-Drobes Questionnaire of Smoking Urges (QSU)i | ||||||
| Factor 1 | 0.3 | n.s | 38.5 | <.001 | 3.3 | n.s |
| Factor 2 | 2.3 | n.s | 4.1 | n.s. | 0.0 | n.s |
| Direct Effects Of Nicotinei | ||||||
| Nauseous | 0.8 | n.s | 2.2 | n.s | 4.0 | n.s |
| Dizzy | 0.5 | n.s. | 9.8 | <.01 | 1.8 | n.s |
| Lightheaded | 0.0 | n.s | 17.6 | <.001 | 4.0 | n.s. |
| Nervous | 0.8 | n.s | 2.2 | n.s | 4.0 | n.s |
| Sweaty | 7.3 | <.05 | 2.3 | n.s | 1.3 | n.s |
| Headache | 0.2 | n.s | 1.7 | n.s | 0.6 | n.s |
| Excessive Salivation | 1.0 | n.s | 8.5 | <.05 | 0.4 | n.s |
| Heart Pounding | 3.2 | n.s | 9.8 | <.01 | 0.3 | n.s |
| Confused | 1.9 | n.s | 0.0 | n.s | 0.2 | n.s |
| Weak | 0.3 | n.s | 3.0 | n.s | 2.0 | n.s |
| Direct Effects of Tobacco | ||||||
| Was the waterpipe satisfying?i | 5.9 | <.05 | 103.4 | <.001 | 2.7 | n.s |
| Was the waterpipe pleasant?i | 3.2 | n.s | 144.1 | <.001 | 0.2 | n.s |
| Did the waterpipe taste good?j | 2.2 | n.s | 139.2 | <.001 | 1.2 | n.s |
| Did the waterpipe taste bad?j | 0.4 | n.s | 25.3 | <.001 | 3.5 | n.s |
| Did the waterpipe make you dizzy?i | 9.9 | n.s | 53.8 | <.001 | 2.0 | n.s |
| Did the waterpipe calm you down?i | 0.2 | n.s | 63.2 | <.001 | 1.1 | n.s |
| Did the waterpipe make you feel confused?i | 3.2 | n.s | 6.7 | <.05 | 0.2 | n.s |
| Did the waterpipe help you concentrate?i | 1.1 | n.s | 20.4 | <.001 | 0.4 | n.s |
| Did the waterpipe make you feel more awake?i | 0.0 | n.s | 22.0 | <.001 | 2.4 | n.s |
| Did the waterpipe reduce your hunger for food?i | 1.6 | n.s | 13.2 | <.01 | 0.4 | n.s |
| Did the waterpipe make you sick?i | 6.6 | <.05 | 10.6 | <.01 | 3.3 | n.s |
| Did the waterpipe make you sleepy?j | 7.6 | <.01 | 42.6 | <.001 | 4.5 | <.05 |
| Would you like to smoke another waterpipe RIGHT NOW?i | 2.1 | n.s | 16.9 | <.001 | 1.2 | n.s |
Condition factors: 2 (active, placebo)
Time factors: levels vary according to measure
dfcondition = (1, 36); dftime = (3, 108); dfconditionxtime = (3, 108)
dfcondition = (1, 36); dftime = (9, 324); dfconditionxtime = (9, 324)
dfcondition = (1, 35); dftime = (9, 315); dfconditionxtime = (9, 315)
dfcondition = (1, 34); dftime = (9, 306); dfconditionxtime = (9, 306)
dfcondition = (1, 33); dftime = (2, 66); dfconditionxtime = (2, 66)
dfcondition = (1, 33); dftime = (1, 33); dfconditionxtime = (1, 33)
dfcondition = (1, 36); dftime = (1, 36); dfconditionxtime = (1, 36)
dfcondition = (1, 35); dftime = (1, 35); dfconditionxtime = (1, 35)
The Hughes and Hatsukami (1986) questionnaire consists of 11 Visual Analog Scale (VAS) items. Items are presented as a word or phrase centered above a horizontal line that ranges from 0 (“Not at all”) to 100 (“Extremely”). Participants used a computer mouse to place a vertical mark anywhere along the horizontal line, and the score is the distance of the vertical mark from the left anchor, expressed as a percentage of total line length.
The Tiffany-Drobes Questionnaire of Smoking Urges (QSU): Brief Form (Cox et al., 2001) consists of 10 smoking-related items (e.g., “ I crave a cigarette right now”) that participants rate on a 7-point scale (“Strongly disagree” to “Strongly agree”). The items were collapsed into two previously-defined factors: ‘intention to smoke’ (Factor 1) and ‘anticipation of relief from withdrawal’ (Factor 2).
The Direct Effects of Nicotine Scale (DENS) consists of 15 VAS items developed to assess the incidence of nicotine-related side effects (Evans et al., 2006), and the Direct Effects of Tobacco Scale (DETS) consists of 13 VAS items developed to asses commonly reported cigarette smoking effects (Foulds et al., 1992; items modified so that the word “cigarette” was replaced by “waterpipe”).
2.6 Smoking Topography Measures
Topography was measured via a pressure transducer integrated into the waterpipe hose (Shihadeh et al., 2005), whereby inhalation-induced pressure changes are amplified, digitized, and sampled. Software converts signals to air flow (ml/sec) and integrates the flow data, producing measures of puff volume, duration, number, and interpuff interval (IPI).
2.7 Data Analysis
HR and BP values were averaged for 5-minute periods beginning with the 5 minutes preceding product administration (missing values replaced with average of value before and after; <2% of data). Due to human error or device malfunctioning, DETS data are based on n=36 participants, topography data are based on n=33 participants, and NO and PFT tests are based on n=34 participants. Twelve of the 37 completers chose to smoke for longer than the minimum 45-minute smoking period (for these twelve participants, mean = 51.7±9.4 minutes) during the tobacco condition only (n=5), the placebo condition only (n=4), or both conditions (n=3). Participants who smoked 45 or > 45 minutes did not differ significantly on any demographic variable (F’s < 3.5 and X2 < 6.1; P’s >.05).
Initially, a mixed repeated measures analysis of variance (ANOVA) was performed for all outcomes where smoking session duration was the between-subjects factor (45 or > 45 minutes). For all physiologic and subjective data, the two within-subjects factors were condition (active or placebo) and time (levels varied by measure). Topography data were analyzed using two different approaches (see Eissenberg & Shihadeh, 2009; Maziak et al., 2009). First, data were averaged within each session to obtain a single value for each variable and analyzed using a univariate (condition) within-subject ANOVA. Second, data for each variable were averaged into 5-minute bins for the first 40 minutes of the 45-minute smoking bouts (data from the last bin, 40–45 minutes, are excluded due to the few number of puffs taken by some participants during this time period). These binned data were then entered into a two-factor (condition by time) ANOVA. Of 158 main effects and interactions involving the between-subjects factor, only 6 were significant (P<0.05; none involved nicotine, HR, COHb, CO, or topography). Because these few significant results may reflect Type I error rather than a real difference due to the effects of waterpipe smoking duration, the analysis was repeated without the between-subjects factor; the results from this completely within-subjects analysis are reported below. Huynh-Feldt corrections were used to adjust for violations of the sphericity assumption (Huynh and Feldt, 1976). Differences between means were examined using Tukey’s Honestly Significant Difference (HSD; Keppel, 1991). Comparisons for which P <.05 are reported as significant.
3. Results
Statistical analysis results for all measures except topography are displayed in Table 1. The results of primary interest involve significant condition by time interactions, meaning that changes in outcome measures across time depended upon product smoked.
3.1 Physiological Effects
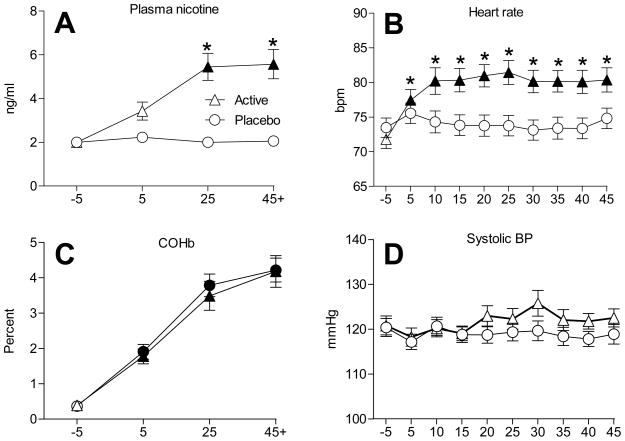
A significant interaction was observed for plasma nicotine (Figure 1A). For the placebo product condition, mean plasma nicotine concentration (±SEM) observed at pre-smoking (2.0±0.0 ng/ml) did not differ relative to data collected 5 minutes (2.2±0.1 ng/ml), 25 minutes (2.0±0.0 ng/ml) and immediately after smoking (2.1±0.0 ng/ml; all n.s., Tukey’s HSD). For the active tobacco product, average plasma nicotine levels increased from 2.0±0.0 ng/ml at baseline to 3.4±0.4 ng/ml at 5 minutes (n.s., Tukey’s HSD) to 5.5±0.6 ng/ml at 25 minutes (P<.05; Tukey’s HSD), and to 5.6±0.7 ng/ml immediately post-smoking (P<.05; Tukey’s HSD). As the figure shows, average plasma nicotine levels at the 25-minute and post-smoking timepoints were significantly different between active and placebo (P<.05, Tukey’s HSD).
Figure 1.
Means (±1 SEM) for plasma nicotine (A), HR (B), COHb (C), and systolic BP (D) for active and placebo waterpipe tobacco conditions. The timepoint “45+” refers to the fact that some participants (n=12) smoked longer than the minimum 45 minute bout. Filled symbols indicate a significant difference from baseline and asterisks (*) indicate a significant difference between active and placebo conditions at that timepoint (Tukey’s HSD; P<.05).
A significant interaction was observed for HR, as is demonstrated in Figure 1B. For placebo, HR remained stable relative to baseline (73.5±1.4 bpm) such that no significant differences were observed at 5 (75.6±1.5 bpm), 10 (74.3±1.6 bpm), 15 (73.8±1.5 bpm), 20 (73.8±1.5 bpm), 25 (73.8±1.5 bpm), 30 (73.1±1.5 bpm), 35 (73.4±1.6 bpm), 40 (73.4±1.5 bpm), or 45 minutes (74.8±1.5 bpm) post-administration (all n.s., Tukey’s HSD). In contrast, when smoking tobacco in the waterpipe, HR increased relative to baseline (71.8±1.3 bpm), such that faster HR was observed at 5 (77.4±1.6 bpm), 10 (80.2±1.9 bpm), 15 (80.3±1.7 bpm), 20 (81.0±1.6 bpm), 25 (81.4±1.7 bpm), 30 (80.2±1.6 bpm), 35 (80.2±1.5 bpm), 40 (80.1±1.7), and 45 minutes (80.4±1.8) post-administration (all P <.05, Tukey’s HSD). Relative to placebo, HR observed during the tobacco smoking session was significantly faster at every post-administration timepoint (P<.05; Tukey’s HSD).
No significant main or interaction effects were observed for DBP and a main effect of condition was observed for SBP and MAP. For SBP (see Figure 1D), average pre-smoking values were similar for placebo (120.4±2.0 mmHg) and active conditions (120.9±2.1 mmHg) and this similarity was also observed at 5 (placebo=117.2±1.2 mmHg; active=118.3±2.1 mmHg), 10 (placebo=120.7±2.0 mmHg; active=120.2±1.9 mmHg), 15 (placebo=118.8±1.7 mmHg; active=119.1±1.7 mmHg) and 45 minutes (placebo=118.9±2.2 mmHg; active=122.6±2.0 mmHg) post-product administration (all n.s., Tukey’s HSD). No significant between-condition differences for SBP were observed (n.s., Tukey’s HSD). A similar pattern of results was observed for MAP.
Table 1 shows that a significant effect of time (but no significant main effect of condition or interaction) was observed for COHb. COHb concentration increased for both conditions after product administration, relative to pre-smoking values (Figure 1C). For placebo, mean COHb concentration increased significantly from 0.4±0.1% pre-smoking to 1.9±0.2% at 5 minutes, 3.8±0.3% at 25 minutes, and 4.2±0.5% immediately post-smoking (P<.05, Tukey’s HSD). For active tobacco, mean COHb concentration increased significantly from 0.4±0.1% pre-smoking to 1.8±0.2% at 5 minutes, 3.5±0.4% at 25 minutes, and 4.2±0.3% immediately post-smoking (P<.05; Tukey’s HSD).
A significant main effect of time was revealed for CO, where average levels increased significantly from pre- to post-smoking within each condition. For placebo, mean CO concentration was 2.2±0.2 ppm at pre-smoking, 30.1±2.6 ppm at 5 minutes post-smoking and 28.1±2.4 ppm at 15 minutes post-smoking (P<.05; Tukey’s HSD). Similarly, for active waterpipe tobacco, mean CO concentration was 2.2±0.2 ppm at pre-smoking, and increased significantly to 29.8±3.4 ppm at 5 minutes post-smoking and 29.0±3.4 ppm at 15 minutes post-smoking (P<.05; Tukey’s HSD). No differences between active and placebo conditions were observed for COHb or CO.
No main or interaction effects were observed for NO (F’s< 1.0, P’s>.05). For FEV1/FVC, no significant main effects or interactions were observed (F’s<3.0, P’s>.05). For FEV1 and FVC the main effect of condition and the interactions were not significant, although a significant main effect of time was observed. For FEV1, levels decreased significantly from 3.7±0.1 pre-smoking to 3.6±0.1 at 20 minutes post-smoking. Levels for FVC also significantly decreased from pre- (4.0±0.1) to 20 minutes post-smoking (3.9±0.1).
3.2 Suppression of nicotine/tobacco withdrawal effects
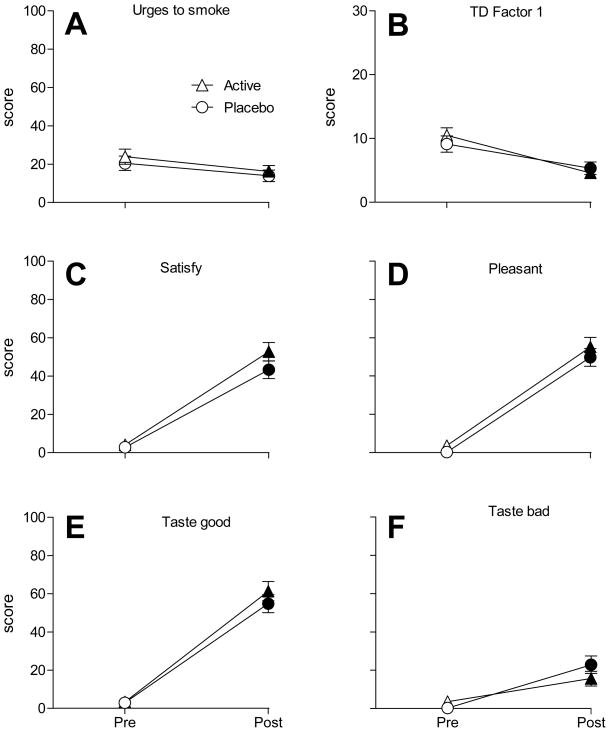
No significant main effects of condition or condition by time interactions (F’s≤2.8, P’s >.05), and only two main effects of time (‘urges to smoke’ and ‘anxious’; F’s≥5.9, P’s<.05), were observed for the Hughes-Hatsukami items. As displayed in Figure 2A, ‘urges to smoke’ ratings did not differ between waterpipe conditions. Collapsed across condition, however, mean ratings decreased significantly from pre- (22.2±2.7) to post-smoking (15.1±2.1). Likewise, mean ratings of ‘anxious’ decreased significantly from pre (8.1±1.6) to post-smoking (4.4±1.1; collapsed across condition).
Figure 2.
Means (±1 SEM) for Hughes-Hatsukami item “Urges to smoke a waterpipe” (A), Tiffany-Drobes QSU Brief Factor 1 (intention to smoke) (B), and DETS items “Was the waterpipe satisfying?” (C), “Was the waterpipe pleasant?” (D), “Did the waterpipe taste good?” (E), and “Did the waterpipe taste bad?” (F). All items significant for main effect of time (F’s> 6.7; P’s<.05). Item “Was the waterpipe satisfying?” significant for main effect of condition (F = 5.9; P<.05). Filled symbols indicate a significant difference from baseline and asterisks (*) indicate a significant difference between active and placebo at that timepoint (Tukey’s HSD; P<.05).
No significant main effects or interactions were observed for QSU Factor 2 (F’s≤4.1, P’s>.05), though for Factor 1 the main effect of time was significant (other F’s≤3.3, P’s>.05). As Figure 2B shows, Factor 1 mean scores did not differ between conditions, but decreased from pre- (9.8±0.9) to post-smoking (5.0±0.7; collapsed across condition).
3.3 Direct Effects of Nicotine and Tobacco
No significant condition by time interaction was observed for any DENS item (Table 1). A main effect of condition was observed for ‘sweaty’ and average ratings collapsed across time were 3.3±1.3 for placebo versus 7.8±1.9 for active. A main effect of time was observed for four DENS items (‘dizzy’, ‘lightheaded’, ‘excessive salivation’, and ‘heart pounding’; F’s≥8.5, P’s<.05). All four items revealed the same pattern of results: increased ratings from pre- to post-smoking. For example, mean ‘lightheaded’ (item with largest F value) ratings were 4.6±0.8 pre-smoking and 16.0±1.7 post-smoking.
Only one of 13 DETS items, ‘Did the waterpipe make you sleepy?’, revealed a significant interaction effect. For this item, mean ratings increased significantly from pre- to post smoking for both placebo (absolute mean difference = 20.9±0.2; Tukey’s HSD, P<.05) and active (absolute mean difference = 30.7±1.7; P<.05, Tukey’s HSD) conditions. Additionally, at the post-smoking timepoint, mean ratings were significantly higher for active (34.8±4.6) than for placebo (21.3±4.6; P<.05, Tukey’s HSD). A significant main effect of condition was observed for the DETS item ‘Did the waterpipe make you sick?’, with significantly higher mean ratings for active (7.1±1.8) than for placebo (2.3±0.6).
For every DETS item, results revealed a significant main effect of time in that scores increased from pre- to post-waterpipe smoking. For instance, mean ratings for ‘Was the waterpipe pleasant?’ (item with largest F value) increased significantly from pre- (1.1±1.0) to post-smoking (52.5±3.4; Tukey’s HSD, P<.05). This same pattern was observed for all DETS items, including those shown in Figure 2. As is also apparent from these figures, no significant differences between active and placebo were observed (P’s>.05).
3.4 Smoking topography
When topography data were averaged to obtain a single value for each session (as in Eissenberg & Shihadeh, 2009), results did not reveal any significant differences between conditions for any topography measure (F’s<1.8, P’s>.05). Means (SD) for all waterpipe smoking topography variables for active versus placebo conditions (collapsed across time) are displayed in Table 2.
Table 2.
Puff topography when participants smoked active or placebo waterpipe products ad libitum
| Topography Measure | Active | Placebo | P* | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Puff Number | 66.3 | 42.2 | 71.2 | 48.6 | n.s. |
| Total puff volume (l) | 57.0 | 45.6 | 55.7 | 32.0 | n.s. |
| Mean puff volume (ml) | 906.1 | 517.4 | 873.0 | 366.7 | n.s. |
| Puff duration (sec) | 3.9 | 1.5 | 3.7 | 1.4 | n.s. |
| Inter-puff-interval (sec) | 47.5 | 21.4 | 45.8 | 26.4 | n.s. |
N = 33 due to missing data from four participants
F(1,32) <1.8
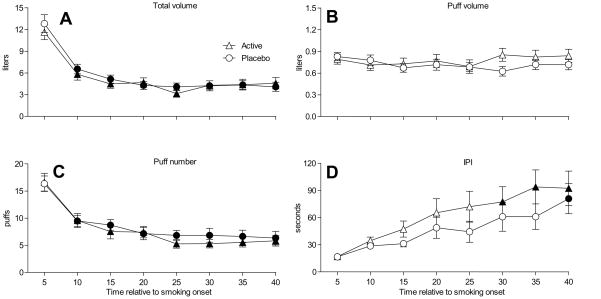
When topography data were examined as a function of condition and time (as in Maziak et al., 2009), results revealed a significant interaction effect for puff volume [F(7,224) = 2.5, P<.05], a significant main effect of time for total volume, puff number, and IPI [F’s(7,224) > 6.9, P’s<.001], and a significant main effect of condition for IPI [F(1,32) = 9.6, P<.01]. Figure 3 shows the time course data for each topography measure and demonstrates that total volume (Panel A) and puff number (Panel C) decreased significantly over time, while IPI (Panel D) increased significantly over time. As Figure 3 also shows, Tukey’s HSD failed to reveal any time points at which any topography measures differed by condition.
Figure 3.
Means (±1 SEM) for topography variables total puff volume (A), puff volume (B), puff number (C), and IPI (D) Filled symbols indicate a significant difference from baseline (Tukey’s HSD; P<.05). Post-hoc tests failed to reveal any time points at which any topography measure differed by condition.
4. Discussion
This report details the first double-blind, placebo-control study of the acute effects of waterpipe tobacco smoking with the goal of determining the extent to which the acute effects of a waterpipe smoking episode are due to nicotine exposure. Many results observed when participants smoked tobacco in the waterpipe were similar to those reported elsewhere (Eissenberg and Shihadeh, 2009; Shafagoj et al., 2002; Shafagoj and Mohammed, 2002): significant increases in plasma nicotine (mean pre- to post-smoking increases of 3.6±0.7 ng/ml) and HR (mean pre- to post-smoking boost of 8.6±1.4 bpm), as well as evidence of substantial CO exposure (mean expired air CO pre- to post-smoking boost of 26.8±3.4 ppm). In contrast, when participants smoked a non-tobacco product in the waterpipe there was neither a significant increase in plasma nicotine (mean pre- to post-smoking increase of 0.1±0.0 ng/ml) nor in HR (mean pre- to post-smoking increase of 1.3±0.9 bpm), though similar CO exposure was apparent (mean expired air CO pre- to post-smoking boost of 26.0±2.5 ppm). Results of this study are therefore clear: HR increases observed during the smoking episode are almost certainly due to tobacco-delivered nicotine rather than CO exposure or the act of smoking.
Contrary to previous work (Shafagoj et al., 2002), significant BP increases associated with waterpipe tobacco smoking were not observed (see Figure 1D). This discrepancy between reports with respect to waterpipe-induced BP increases may reflect the 10-fold greater level of nicotine exposure reported by Shafagoj et al (2002) relative to the current report (mean post-smoking plasma concentration of 60.3 versus 5.6 ng/ml, respectively). Other than Shafagoj et al (2002), no other controlled study has suggested that waterpipe tobacco smoking can produce such a dramatic increase in plasma nicotine concentration, but additionally, no other study in which plasma nicotine concentration has been measured during waterpipe smoking has sampled from a highly experienced population of waterpipe smokers using a large amount of tobacco (20g, twice the amount used here; Shafogoj et al., 2002). We also did not observe any waterpipe-induced changes in NO, a finding that is inconsistent with at least one study where waterpipe tobacco smokers were found to have higher serum NO concentrations relative to non-smokers (Ghasemi et al., 2010). For pulmonary function, results demonstrated that FEV1 and FVC significantly decreased from pre- to post-smoking. Unfortunately, no study has examined acute changes in these lung function parameters as a function of waterpipe tobacco smoking, though cross-sectional reports (Al-Fayez et al., 1988; Mohammad et al., 2008) reveal that FEV1, FVC, and FEV1/FVC are lower in waterpipe smokers than in non-smokers.
While nicotine clearly mediates the cardiovascular effects reported here, this study provides no evidence that the subjective effects of waterpipe tobacco smoking are due to nicotine self-administration, at least among the population of 2–5 times/month users sampled. That is, across the subjective effect measures reported here, many main effects of time were observed (i.e., significant differences from pre- to post-smoking) indicating that smoking produced an effect; however, there were few interactions of time and condition (see Figure 2). A similar pattern of results has been reported when cigarette smokers use cigarettes that do not deliver measurable doses of nicotine (e.g., denicotinized tobacco cigarettes): on many subjective measures, the products that do not deliver nicotine are as effective as those that do (Buchhalter et al., 2005; Pickworth et al., 1999). These results support the notion that non-nicotine factors (associative factors, expectancy, etc.) can mediate smoking-induced subjective effects. In a broader context, stimuli that have been paired with drug administration are well known to have powerful and long-lasting effects (e.g., Siegel et al., 1982; Falk and Lau, 1995; Donny et al., 2007). The fact that these stimuli when presented alone can elicit compensatory responses, control self-administration, and suppress craving in no way downplays the importance of the drug with which they have been paired. Rather, the responses these stimuli come to elicit highlight yet another aspect of a drug’s addictive potential. Thus, in the present study, the observation that product nicotine delivery was largely unrelated to subjective effect on many measures may be an indicator that previous pairings of the drug with waterpipe-associated taste, smell, and other stimuli have already produced a conditional effect in our study population.
The results of this study must be considered in the context of some important limitations. First, participants included in this study could be considered occasional users (2–5 smoking episodes/month). Waterpipe smokers with a more extensive use history (e.g., daily use) might present a different effect profile for nicotine containing and tobacco-free products. Indeed, topography data collected in this study of occasional U.S. waterpipe tobacco smokers differs from that collected in a study of more frequent Syrian waterpipe tobacco smokers (Maziak et al., 2009): for example, the mean total smoke inhaled in the current study was 56 liters, as compared to 79 liters in the Syrian sample. Moreover, topography results reported here suggest that, for participants in this study, smoking behavior was independent of product used (i.e., nicotine-delivering tobacco versus non-tobacco product). More experienced users may smoke the waterpipe more intensively, thus receiving more nicotine and experiencing greater nicotine-related effects. More experienced users may also be able to discriminate between tobacco and non-tobacco products and alter their topography accordingly. In addition, participants’ average scores for most withdrawal-related items before smoking were observed to be on the low end of the scale (e.g., average baseline scores on all but two Hughes-Hatsukami items did not exceed 17 out of a possible 100); thus, the failure to observe smoking-induced score suppression may likely be attributable to a floor effect. More experienced users may report higher levels of withdrawal symptoms after a period of nicotine/tobacco abstinence (e.g., Maziak et al., 2009). The laboratory setting necessarily differs from a typical waterpipe smoking environment, although laboratory furnishings and activities (reclining chairs, participant-selected movies) were chosen to approximate features observed in local waterpipe cafés. Participants also smoked waterpipe in solitary, rather in a group setting as is typically observed. Convenience samples of U.S. waterpipe tobacco smokers suggest that over 90% share a waterpipe when they smoke (e.g., Ward et al., 2007), and the influence of sharing a waterpipe on its acute effects has not yet been assessed.
Conclusions
Overall, results from this double-blind, placebo-control study demonstrate that waterpipe tobacco smoking produces some effects likely due to nicotine (e.g., cardiovascular response) and some effects likely due to other factors (e.g., subjective experience). Importantly, nicotine- and non-nicotine factors may be involved in the development of tobacco dependence in cigarette smokers (e.g., Eissenberg, 2004; Brandon et al., 2004), thus waterpipe tobacco smokers may also be at risk for dependence (Maziak et al., 2004). Future work is needed to delineate these factors in waterpipe smokers and understand better their role in waterpipe dependence. Also notable is the observation that using a waterpipe to smoke a non-tobacco product results in a substantial level of CO exposure that did not differ from that observed when smoking tobacco under identical conditions. Some waterpipe smokers may believe that non-tobacco products can be used to reduce exposure to smoke toxicants (Roskin and Aveyard, 2009). However, while nicotine exposure is clearly eliminated, CO exposure is not. Moreover, charcoal is the source of CO and carcinogenic PAHs (Monzer et al., 2008) in waterpipe smoke. Thus, aside from dependence, the health risks of using a waterpipe to smoke non-tobacco preparations may be similar to those of smoking tobacco whenever charcoal is the heat source.
Acknowledgments
Role of Funding Source
This work was funded by PHS Grants R01CA120142, R01DA024876 and F31DA028102. The NIH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Portions of this work were presented at the 16th Annual Meeting of the Society for Research on Nicotine and Tobacco, Baltimore, MD, February 24–27th, 2010.
Footnotes
Contributors
Author MB assisted with IRB approval, study set-up, and led the data analysis and manuscript preparation processes. Author BK managed all aspects of study execution (set-up, recruitment, and screening and session completion) and assisted with drafts of select manuscript sections. Author JA assisted with all aspects of study execution (set-up, recruitment, and screening and session completion), maintained study data, and completed a portion of the data analyses. Author MW served as the medical monitor for this study. Author CC assisted with aspects of study execution and manuscript preparation. Author AS managed all aspects of topography measurement, clean-up, and analysis. Author TE was the primary investigator who oversaw all aspects of study design and execution, data analysis and manuscript preparation. Authors have read multiple drafts of this manuscript and approved the final version.
Conflict of Interest
The authors declare no conflicts of interest.
All work performed at Virginia Commonwealth University
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Melissa D. Blank, Virginia Commonwealth University, Department of Psychology, PO Box 980205, Richmond, VA 23298-0205, USA
Caroline O. Cobb, Virginia Commonwealth University, Department of Psychology, PO Box 980205, Richmond, VA 23298-0205, USA
Barbara Kilgalen, Virginia Commonwealth University, Department of Psychology, PO Box 980205, Richmond, VA 23298-0205, USA.
Janet Austin, Virginia Commonwealth University, Department of Psychology, PO Box 980205, Richmond, VA 23298-0205, USA.
Michael F. Weaver, Virginia Commonwealth University, Department of Internal Medicine, PO Box 980109, Richmond, VA 23298-0109, USA
Alan Shihadeh, American University of Beirut, Department of Mechanical Engineering, Beirut, Lebanon 1107 2020, Syrian Center for Tobacco Studies, Aleppo, Syria.
Thomas Eissenberg, Virginia Commonwealth University, Department of Psychology and Institute for Drug and Alcohol Studies, PO Box 980205, Richmond, VA 23298-0205, USA, Syrian Center for Tobacco Studies, Aleppo, Syria.
References
- Aljarrah K, Ababneh ZQ, Al-Delaimy WK. Perceptions of hookah smoking harmfulness: Predictors and characteristics among current hookah users. Tob Induced Dis. 2009;5:16. doi: 10.1186/1617-9625-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Fayez SF, Salleh M, Ardawi M, Zahran FM. Effects of sheesha and cigarette smoking on pulmonary function of Saudi males and females. Trop Geogr Med. 1988;40:115–123. [PubMed] [Google Scholar]
- Al Rashidi M, Shihadeh A, Saliba NA. Volatile aldehydes in the mainstream smoke of the narghile waterpipe. Food Chem Toxicol. 2008;46:3546–3549. doi: 10.1016/j.fct.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aydin A, Kiter G, Durak H, Ucan ES, Kaya GC, Ceylan E. Water-pipe smoking effects on pulmonary permeability using technetium-99m DTPA inhalation scintigraphy. Ann Nucl Med. 2004;18:285–289. doi: 10.1007/BF02984465. [DOI] [PubMed] [Google Scholar]
- Brandon TH, Herzog TA, Irvin JE, Gwaltney CJ. Cognitive and social learning models of drug dependence: implications for the assessment of tobacco dependence in adolescents. Addiction Suppl. 2004;1:51–77. doi: 10.1111/j.1360-0443.2004.00737.x. [DOI] [PubMed] [Google Scholar]
- Breland AB, Kleykamp BA, Eissenberg T. Clinical laboratory evaluation of potential reduced exposure products for smokers. Nicotine Tob Res. 2006;8:727–738. doi: 10.1080/14622200600789585. [DOI] [PubMed] [Google Scholar]
- Buchhalter AR, Acosta MC, Evans SE, Breland AB, Eissenberg T. Tobacco abstinence symptom suppression: the role played by the smoking-related stimuli that are delivered by denicotinized cigarettes. Addiction. 2005;100:550–559. doi: 10.1111/j.1360-0443.2005.01030.x. [DOI] [PubMed] [Google Scholar]
- Chambers DC, Tunnicliffe WS, Ayres JG. Acute inhalation of cigarette smoke increases lower respiratory tract nitric oxide concentrations. Thorax. 1998;53:677–679. doi: 10.1136/thx.53.8.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb C, Ward KD, Maziak W, Shihadeh AL, Eissenberg T. Waterpipe tobacco smoking: An emerging health crisis in the united states. Am J Health Behav. 2010;34:275– 285. doi: 10.5993/ajhb.34.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob Res. 2001;3:7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- Donny EC, Houtsmuller E, Stitzer ML. Smoking in the absence of nicotine: behavioral, subjective and phsyiological effects over 11 days. Addiction. 2007;102:324–34. doi: 10.1111/j.1360-0443.2006.01670.x. [DOI] [PubMed] [Google Scholar]
- Eissenberg T. Measuring the emergence of tobacco dependence: the contribution of negative reinforcement models. Addiction. 2004;99(Suppl 1):5–29. doi: 10.1111/j.1360-0443.2004.00735.x. [DOI] [PubMed] [Google Scholar]
- Eissenberg T, Shihadeh A. Waterpipe and cigarette smoking: a direct comparison of toxicant effects. Am J Prev Med. 2009;37:518–523. doi: 10.1016/j.amepre.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Nachef WN, Hammond SK. Exhaled carbon monoxide with waterpipe use in US students. JAMA. 2008;299:36–38. doi: 10.1001/jama.2007.6. [DOI] [PubMed] [Google Scholar]
- Evans SE, Blank M, Sams C, Weaver MF, Eissenberg T. Transdermal nicotine-induced tobacco abstinence symptoms suppression: nicotine dose and smokers’ gender. Exp Clin Psychopharmacol. 2006;14:121–135. doi: 10.1037/1064-1297.14.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk JL, Lau CE. Stimulus control of addictive behavior: persistence in the presence and absence of a drug. Pharmacol Biochem Behav. 1995;50:71–5. doi: 10.1016/0091-3057(94)00256-i. [DOI] [PubMed] [Google Scholar]
- Foulds J, Stapleton J, Feyerabend C, Vesey C, Jarvis M, Russell MAH. Effects of transdermal nicotine patches on cigarette smoking: a double blind crossover study. Psychopharmacology. 1992;106:421–427. doi: 10.1007/BF02245429. [DOI] [PubMed] [Google Scholar]
- Ghasemi A, Syedmoradi L, Momenan AA, Zahediasl S, Azizi F. The influence of cigarette and qalyan (hookah) smoking on serum nitric oxide metabolite concentration. Scand J Clin Lab Invest. 2010;70:116–21. doi: 10.3109/00365511003611282. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Huynh H, Feldt LS. Estimation of the box correction for degrees of freedom from sample data in a randomized block and split-plot designs. J Educ Stat. 1976;1:69–82. [Google Scholar]
- Jensen PD, Cortes R, Engholm G, Kremers S, Gislum M. Waterpipe use predicts progression to regular cigarette smoking among Danish youth. Subst Use Misuse. 2010;45:1245–1261. doi: 10.3109/10826081003682909. [DOI] [PubMed] [Google Scholar]
- Keppel G. Design and analysis: A researcher’s handbook. Englewood Cliffs, NJ: Prentice Hall; 1991. [Google Scholar]
- Kharitonov SA, Robbins RA, Yates D, Keatings V, Barnes PJ. Acute and chronic effects of cigarette smoking on exhaled nitric oxide. Am J Respir Crit Care Med. 1995;152:609–612. doi: 10.1164/ajrccm.152.2.7543345. [DOI] [PubMed] [Google Scholar]
- Kiter G, Ucan ES, Ceylan E, Kilinc O. Water-pipe smoking and pulmonary functions. Respir Med. 2000;94:891–894. doi: 10.1053/rmed.2000.0859. [DOI] [PubMed] [Google Scholar]
- Lim BL, Lim GH, Seow E. Case of carbon monoxide poisoning after smoking shisha. Int J Emerg Med. 2009;2:121–122. doi: 10.1007/s12245-009-0097-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maziak W, Rastam S, Ibrahim I, Ward KD, Shihadeh A, Eissenberg T. CO exposure, puff topography, and subjective effects in waterpipe tobacco smokers. Nicotine Tob Res. 2009;11:806–811. doi: 10.1093/ntr/ntp066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maziak W, Ward KD, Soweid RA, Eissenberg T. Tobacco smoking using a waterpipe: a re-emerging strain in a global epidemic. Tob Control. 2004;13:327–333. doi: 10.1136/tc.2004.008169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammad Y, Kakah M, Mohammad Y. Chronic respiratory effect of narguileh smoking compared with cigarette smoking in women from the East Mediterranean region. Int J Chron Obstruct Pulmon Dis. 2008;3:405–414. doi: 10.2147/copd.s1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monzer B, Sepetdjian E, Saliba N, Shihadeh A. Charcoal emissions as a source of CO and carcinogenic PAH in mainstream narghile waterpipe smoke. Food Chem Toxicol. 2008;46:2991–2995. doi: 10.1016/j.fct.2008.05.031. [DOI] [PubMed] [Google Scholar]
- Naidong W, Shou W, Chen YL, Jiang X. Novel liquid chromatographic-tandem mass spectrometric methods using silica columns and aqueous-organic mobile phases for quantitative analysis of polar ionic analytes in biological fluids. J Chromatogr, B: Biomed Sci Appl. 2001;754:387–399. doi: 10.1016/s0378-4347(01)00021-4. [DOI] [PubMed] [Google Scholar]
- National Collaborating Centre for Chronic Conditions. Chronic obstructive pulmonary disease: national clinical guideline on management of chronic obstructive pulmonary disease in adults in primary and secondary care. Thorax. 2003;59:1–232. [PMC free article] [PubMed] [Google Scholar]
- Pärna K, Usin J, Ringmets I. Cigarette and waterpipe smoking among adolescents in Estonia: HBSC survey results, 1994–2006. BMC Public Health. 2008;8:392. doi: 10.1186/1471-2458-8-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickworth WB, Fant RV, Nelson RA, Rohrer MS, Henningfield JE. Pharmacodynamic effects of new de-nicotinized cigarettes. Nicotine Tob Res. 1999;1:357–364. doi: 10.1080/14622299050011491. [DOI] [PubMed] [Google Scholar]
- Primack BA, Fertman CI, Rice KR, Adachi-Mejia AM, Fine MJ. Waterpipe and cigarette smoking among college athletes in the United States. J Adolesc Health. 2010;46:45–51. doi: 10.1016/j.jadohealth.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roskin J, Aveyard P. Canadian and English students’ beliefs about waterpipe smoking: a qualitative study. BMC Public Health. 2009;9:10. doi: 10.1186/1471-2458-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seigel S, Hinson RE, Krank MD, McCully J. Heroin “overdose” death: contribution of drug-associated environmental cues. Science. 1982;216:436–437. doi: 10.1126/science.7200260. [DOI] [PubMed] [Google Scholar]
- Sepetdjian E, Shihadeh A, Saliba NA. Measurement of 16 polycyclic aromatic hydrocarbons in narghile waterpipe tobacco smoke. Food Chem Toxicol. 2008;46:1582–1590. doi: 10.1016/j.fct.2007.12.028. [DOI] [PubMed] [Google Scholar]
- Shafagoj YA, Mohammed FI. Levels of maximum end-expiratory carbon monoxide and certain cardiovascular parameters following hubble-bubble smoking. SMJ. 2002;23:953–958. [PubMed] [Google Scholar]
- Shafagoj YA, Mohammed FI, Hadidi KA. Hubble-bubble (water pipe) smoking: levels of nicotine and cotinine in plasma, saliva and urine. Int J Clin Pharmacol Ther. 2002;40:249–255. doi: 10.5414/cpp40249. [DOI] [PubMed] [Google Scholar]
- Shihadeh A. Investigation of mainstream smoke aerosol of the argileh water pipe. Food Chem Toxicol. 2003;41:143–152. doi: 10.1016/s0278-6915(02)00220-x. [DOI] [PubMed] [Google Scholar]
- Shihadeh A, Antonios C, Azar S. A portable, low-resistance puff topography instrument for pulsating, high-flow smoking devices. Behav Res Methods. 2005;37:186–191. doi: 10.3758/bf03206414. [DOI] [PubMed] [Google Scholar]
- Shihadeh A, Saleh R. Polycyclic aromatic hydrocarbons, carbon monoxide, “tar”, and nicotine in the mainstream smoke aerosol of the narghile waterpipe. Food Chem Toxicol. 2005;43(5):655–661. doi: 10.1016/j.fct.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Smith-Simone S, Maziak W, Ward KD, Eissenberg T. Waterpipe tobacco smoking: knowledge, attitudes, beliefs, and behaviors in two U.S. samples. Nicotine Tob Res. 2008;10:393–398. doi: 10.1080/14622200701825023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward KD, Eissenberg T, Gray JN, Srinivas V, Wilson N, Maziak W. Characteristics of U.S. waterpipe users: A preliminary report. Nicotine Tob Res. 2007;9:1339–1346. doi: 10.1080/14622200701705019. [DOI] [PubMed] [Google Scholar]
- Weglicki LS, Templin T, Hammad A, Jamil H, Abou-Mediene S, Farroukh M, Rice VH. Health issues in the Arab American community. Tobacco use patterns among high school students: do Arab American youth differ? Ethn Dis. 2007;17:S3-22–S3-24. [PubMed] [Google Scholar]
- World Health Organization. Waterpipe tobacco smoking: health effects, research needs and recommended actions by regulators. Geneva, Switzerland: World Health Organization; 2005. Retrieved from http://www.who.int/tobacco/global_interaction/tobreg/Waterpipe%20recommendation_Final.pdf. [Google Scholar]