Abstract
Kaposi’s sarcoma (KS) remains among the most common causes of oral cancer in HIV-infected individuals. Infection with the KS-associated herpesvirus (KSHV/HHV8) is a necessary event for disease development. Emerging evidence suggests that KSHV infects vascular endothelial (or endothelial progenitor) cells promoting the formation of the KS tumor (or spindle) cell. These cells elaborate angiogenic growth factors and cytokines that promote the dysregulated angiogenesis and profuse edema that characterizes this unusual vascular tumor. Central among these secreted factors is the potent endothelial cell mitogen, vascular endothelial growth factor (VEGF). Indeed, VEGF has proven to be a key player in KSHV pathogenesis and is a molecular hallmark of KS lesions. We have recently shown that a second angiogenic factor, Angiopoietin-like 4 (ANGPTL4), may also play a critical role in KS development. Here we demonstrate that ANGPTL4 is upregulated both directly and indirectly by the KSHV oncogene, vGPCR. We further show that ANGPTL4 is a molecular hallmark of oral KS lesions. Indeed, expression of this protein was observed in more tumor cells and in more biopsies specimens than expression of VEGF (23/25 or 92% vs. 19/25 or 76%, respectively) in oral KS. These surprising results support a key role for ANGPTL4 in Kaposi’s sarcomagenesis and further suggest that this angiogenic factor may provide a novel diagnostic and therapeutic marker for oral KS patients.
Keywords: oral cavity, Kaposi’s sarcoma, Kaposi’s sarcoma associated herpesvirus, KSHV, Human herpesvirus-8, HHV-8, G protein-coupled receptor, vGPCR, Angiopoietin, Angiopoietin-like 4, vascular endothelial growth factor, angiogenesis, vascular permeability
Introduction
KS is a multifocal vascular neoplasm that often affects the oral cavity in immunosuppressed patients1. First described as a skin cancer in older men of Jewish or Mediterranean ancestry, a dramatic change in the epidemiology and clinical course of KS occurred with the emergence of the acquired immune deficiency syndrome (AIDS)2. Today, KS remains as one of the most common malignancies affecting HIV-infected individuals and is the most frequent cancer among children and adult men in countries of sub-Saharan Africa2. Unfortunately, clinical management of KS continues to be a challenge.
A scientific leap in our understanding of the pathogenesis of KS was made possible with the identification of a novel human herpesvirus, HHV8, named Kaposi’s sarcoma associated herpesvirus (KSHV), as the etiological agent for this tumor3. Subsequent work from several groups suggests that endothelial cell infection with KSHV is indeed a prerequisite for KS development, and results in the formation of the KS tumor (or spindle) cell. These KS spindle cells are the driving force of KS lesion, elaborating angiogenic growth factors and cytokines that promote the formation of this vascular tumor2.
Emerging evidence supports a key role for a viral protein, the KSHV G protein-coupled receptor (vGPCR), in the initiation and promotion of KS2. vGPCR is a member of the family of CXC chemokine GPCRs, with closest homology to CXCR2, but with ligand-independent (constitutive) activity. Endothelial cells expressing vGPCR elaborate angiogenic growth factors and cytokines that have been suggested to promote tumor formation through a unique paracrine mechanism2. Indeed, transgenic mice that express vGPCR manifest dermal angioproliferative lesions that closely resemble those seen in KS4–6. These observations have prompted intense investigation into identifying the molecular mechanism(s) whereby vGPCR could play a role in Kaposi’s sarcomagenesis.
Initial work on the contribution of vGPCR to KS development appropriately centered on the upregulation by this viral receptor of the potent endothelial cell mitogen, VEGF, a key player in KSHV pathogenesis7,8. However, we recently identified a novel angiogenic factor, ANGPTL4, which also appears to play an essential role in vGPCR tumorigenesis, promoting angiogenesis and vascular permeability9. Here we set out to examine the prevalence of ANGPTL4 upregulation in oral KS lesions.
Materials and Methods
Cell lines and reagents
pCEFL AU5 vGPCR, pCEFL AU5 GFP, pBIG AU5 vGPCR and pCEFL Tet REV TA have been previously described5,9. HMEC1s were obtained from the CDCs (Atlanta, GA) and grown as described elsewhere9. Cells were transfected with Polyfect (Qiagen). Conditioned media was prepared as previously described9. Recombinant proteins were purchased from Pepro Tech. Concentrations used are: IL-8 (50 ng/ml), GROα (50 ng/ml), PDGF (25 ng/ml), IL-1β (10 ng/ml), IL-10 (25 ng/ml), IL-6 (2 ng/ml), TNFα (25 ng/ml), IP-10 (50 ng/ml), SDF1α (80 ng/ml), VEGF (50 ng/ml), and ANGPTL4 (5 µg/ml).
Additional information can be found in the Supplemental Materials and Methods.
Results
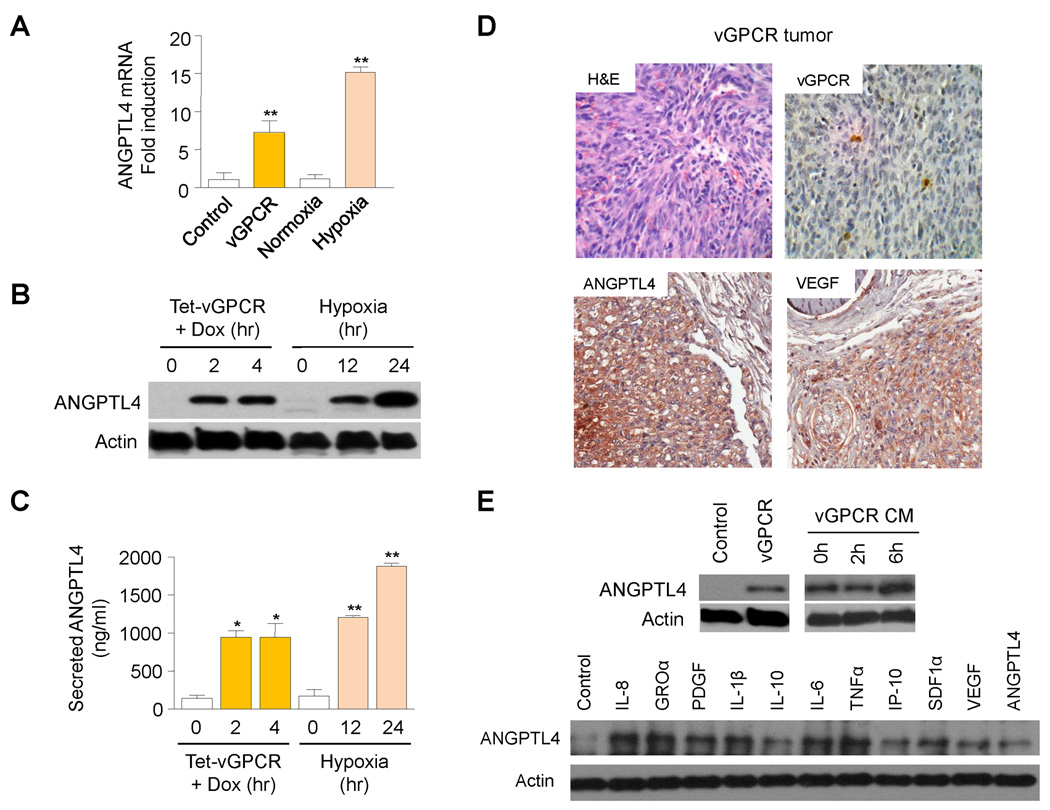
KS is a vascular tumor promoted by KSHV infection and the resultant expression of different viral genes and microRNAs2. Work from several labs has supported a key role for dysregulated expression of the KSHV-encoded GPCR (vGPCR) in the promotion of KS2. Transgenic mice expressing this viral receptor in endothelial cells manifest vascular tumors (vGPCR tumors) histologically similar to human KS, with expression of vGPCR limited to a few cells, suggestive of a paracrine mechanism for vGPCR tumorigenesis5. Indeed, expression of vGPCR in cultured endothelial cells stimulates the release of angiogenic growth factors and pro-inflammatory chemokines and cytokines7,8,10–13. Of interest, expression of vGPCR in immortalized human dermal microvascular endothelial cells (HMEC1s) led to an increase in the mRNA levels of a novel hypoxia-regulated factor, angiopoietin like-4, a member of the family of Angiopoietin-like proteins (ANGPTLs), which has been shown to play an important role in the control of angiogenesis9,14 (Fig. 1A). Indeed, induction of vGPCR expression in HMEC1s using a tetracycline-inducible expression system led to a robust upregulation of ANGPTL4 translation and secretion (Fig. 1B–C).
Figure 1. Direct and Paracrine upregulation of ANGPTL4 by vGPCR.
(A) angptl4 mRNA levels (qRT-PCR), upon transfection of pCEFL AU5 vGPCR (vGPCR) or pCEFL AU5 GFP (Control) in HMEC1. Induction of angptl4 mRNA by hypoxia (1% O2; 24 hr) was used as a control. (B–C) Cellular ANGPTL4 (WB) (B) and secreted ANGPTL4 (ELISA) (C) of HMEC1 transfected with pCEFL Tet REV TA and pBIG AU5 vGPCR (Tet-vGPCR). Cells were left untreated or treated with (1 µg/ml) Dox for 2h or 4h. Induction of ANGPTL4 expression by hypoxia (1% O2; 12hr or 24hr) was used as a control. (D) Representative H&E staining and immunohistochemical detection of (AU5) vGPCR expressing cells as well as ANGPTL4 and VEGF expression in murine vGPCR tumors. (E) Upregulation in HMEC1 of ANGPTL4 upon transfection of pCEFL AU5 vGPCR (vGPCR) or pCEFL AU5 GFP (Control), treatment with conditioned media of vGPCR-expressing cells (vGPCR CM), or exposure to individual recombinant factors present in vGPCR conditioned media.
Immunohistochemical staining of murine vGPCR tumors also demonstrated high levels of expression of ANGPTL4 in most tumor cells (Fig. 1D). These lesions similarly showed elevated levels of another vGPCR upregulated factor, VEGF7,8. However, expression of vGPCR was limited to only a few tumor cells, consistent with a paracrine role for vGPCR in the upregulation of these growth factors (Fig. 1D). Indeed, when we treated HMEC1 with media conditioned by endothelial cells expressing vGPCR, we observed an induction of ANGPTL4 in treated cells (Fig 1E). An increase in ANGPTL4 was also found when HMEC1s were exposed to individual chemokines, cytokines and growth factors found in vGPCR conditioned media10 (Fig. 1E). Collectively, these results suggest that vGPCR upregulates ANGPTL4 by both direct and paracrine mechanisms.
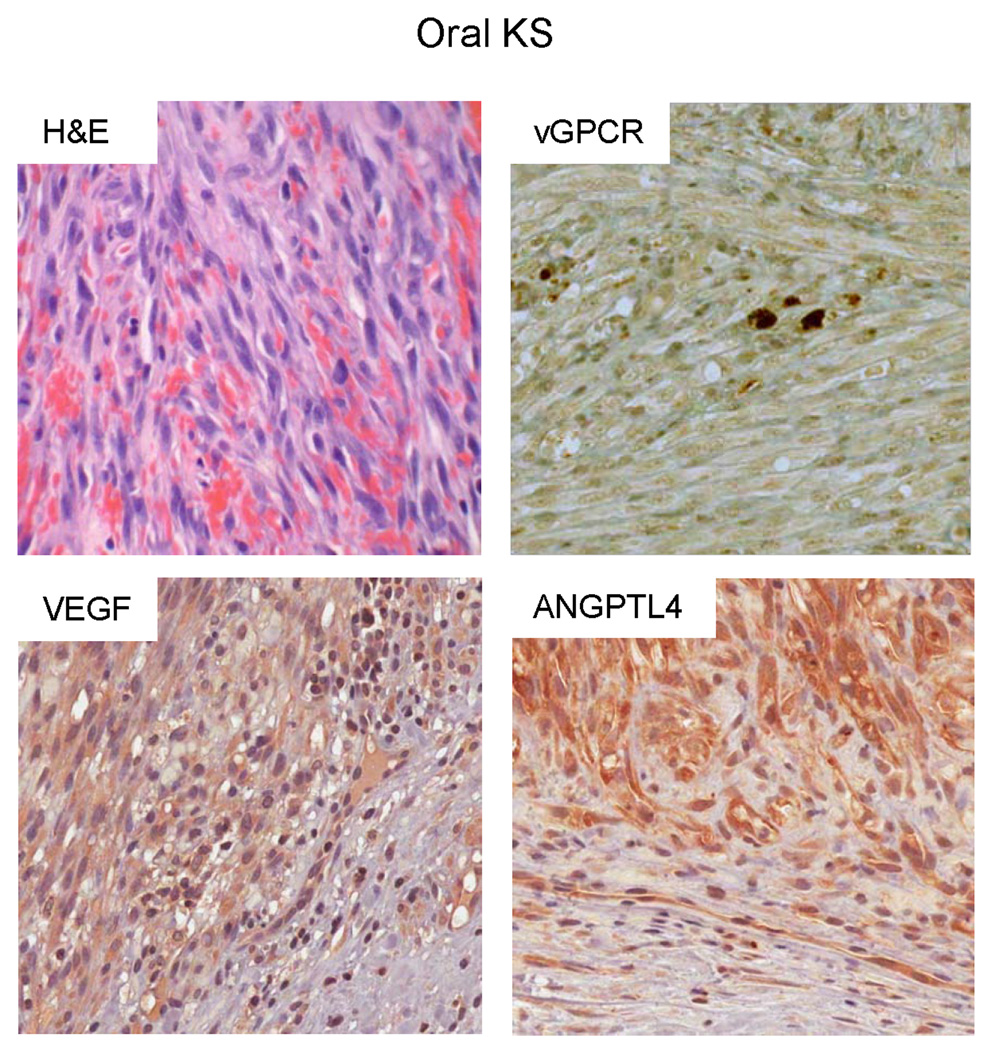
To study the relevance of ANGPTL4 as a potential diagnostic marker in oral KS, we obtained 25 biopsy samples from patients with oral KS tumors. Demographic data of the patients and clinical information of the lesions are included in Table 1. KSHV infection in all the cases was confirmed by the presence of the KSHV Latency-Associated Nuclear Antigen 1 (LANA1) in the tissue. We then performed immunohistochemical analysis on all the biopsies with specific antibodies against ANGPTL4 or VEGF (Fig. 2). Table 2 includes the grading of immunohistochemical reactivity to these antibodies, according to the percentage of positive tumor cells. 23/25 (92%) of the KS lesions tested showed upregulation of ANGPTL4 in tumor cells. This compares to 19/25 (76%) of KS lesions that demonstrated upregulation of VEGF expression. As shown in Table 3, 10/25 (40%) of the KS lesions had high levels of expression of ANGPTL4 in the majority of tumor cells compared to 7/25 (28%) of KS lesions with high levels of VEGF. Collectively, these results support a fundamental role for ANGPTL4 in Kaposi’s sarcomagenesis.
Table 1.
Demographic data of the patients (age, gender, race and HIV status) and clinical information of the oral lesions (location, size, color, clinical presentation) included in our studies. HHV8 infection in all the cases was confirmed by the presence of the latency-associated nuclear antigen 1 (LANA1).
| Case | Age | Sex | Race | Location | Size | Color | Clinical Presentation |
HIV status |
LANA1 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 34 | M | W | Palate | 1.5cm | red | N/A | Positive | + |
| 2 | 29 | M | W | Gingiva | N/A | red/purple | N/A | N/A | + |
| 3 | 44 | M | W | Palate | 3.5×2.5cm | Blue | Swelling | N/A | + |
| 4 | 40 | M | W | Palate | N/A | Blue | N/A | Positive | + |
| 5 | 60 | M | W | Palate | 1 cm | red | Exophytic granulation tissue | N/A | + |
| 6 | 36 | M | W | Palate | 4×3 mm | Purple | Pedunculated mass | N/A | + |
| 7 | 31 | M | W | Gingiva | N/A | Purple | Multiple, spongy lesions | Positive | + |
| 8 | 39 | M | W | Gingiva | N/A | Purple | Soft, multiple | N/A | + |
| 9 | 57 | M | W | Mucobuccal fold | N/A | Dark | Firm, pedunculated | Positive | + |
| 10 | 39 | M | W | Gingiva | N/A | N/A | N/A | Positive | + |
| 11 | 42 | M | W | Gingiva | 1 cm | Purple | Nodular, multiple | Positive | + |
| 12 | 27 | M | W | Tongue | N/A | Blue | Multiple | Positive | + |
| 13 | 32 | M | W | Maxillary tuberosity | N/A | Purple | Exophytic | Positive | + |
| 14 | 38 | M | W | Gingiva | 1×1×2 cm | Blue | Swelling | Positive | + |
| 15 | 33 | M | W | Tongue | N/A | N/A | Verrucous cast | Positive | + |
| 16 | 25 | M | W | Palate | 2.5 cm | N/A | Pedunculated mass | Positive | + |
| 17 | 48 | M | W | N/A | 2×2×.5cm | Red/Purple | N/A | Positive | + |
| 18 | 31 | M | W | Gingiva | 1 cm | N/A | Enlarged operculum | N/A | + |
| 19 | 47 | M | W | Hard palate | N/A | Purple | N/A | Positive | + |
| 20 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | + |
| 21 | 22 | M | W | Lip vestibule | 0.5 × 0.8 | Blue | Swelling | Positive | + |
| 22 | 32 | M | W | Hard palate | 3 × 4 | Red | Exophytic mass | Positive | + |
| 23 | 45 | M | W | Hard palate | 1.5 × 1.2 | Red | Macule | Positive | + |
| 24 | 31 | M | W | Hard palate | 2 cm | Blue | Pedunculated mass | Positive | + |
| 25 | 56 | M | W | Hard palate | Unknown | Red/Blue | Swelling | Positive | + |
N/A: Information not available in chart
Figure 2. Overexpression of ANGPTL4 in oral KS.
Representative H&E and immunohistochemical staining of human oral KS tissue with specific antibodies against vGPCR, ANGPTL4 or VEGF.
Table 2.
Levels of expression of ANGPTL4 or VEGF in oral human KS lesions. Immunohistochemical reactivity was graded in a semiquantitative manner according to the percentage of positive tumor cells (− = 0%; + = <20%; ++ = 20%–50%; +++ = >50%).
| Case | ANGPTL4 | VEGF |
|---|---|---|
| 1 | ++ | + |
| 2 | + | +++ |
| 3 | ++ | − |
| 4 | +++ | − |
| 5 | + | +++ |
| 6 | +++ | ++ |
| 7 | +++ | ++ |
| 8 | − | +++ |
| 9 | +++ | ++ |
| 10 | ++ | + |
| 11 | − | − |
| 12 | + | ++ |
| 13 | ++ | + |
| 14 | + | + |
| 15 | +++ | − |
| 16 | ++ | ++ |
| 17 | ++ | − |
| 18 | +++ | +++ |
| 19 | + | ++ |
| 20 | ++ | +++ |
| 21 | +++ | +++ |
| 22 | +++ | +++ |
| 23 | + | ++ |
| 24 | +++ | − |
| 25 | +++ | + |
Table 3.
Stratification of results of immunohistochemical reactivity according to the percentage of positive tumor cells (− = 0%; + = <20%; ++ = 20%–50%; +++ = >50%).
| ANGPTL4 n (%) |
VEGF n (%) |
|
|---|---|---|
| Negative | 2 (8) | 6 (24) |
| <20% | 6 (24) | 5 (20) |
| 20–50% | 7 (28) | 7 (28) |
| >50% | 10 (40) | 7 (28) |
| Total | 25 (100) | 25 (100) |
Discussion
KS is a multifocal vascular tumor with lesions predominately affecting the skin and oral mucosa1. KS tumorigenesis develops in response to infection by KSHV; indeed, expression of KSHV-encoded latent genes (e.g. LANA1) can be detected in most tumor cells within oral KS lesions. Interestingly, the KSHV lytic protein, vGPCR, expressed only in a few tumor cells, appears to be important in KS development through a unique paracrine mechanism2. The role of vGPCR in KS initiation and maintenance suggest that this viral receptor may provide a new perspective in the search for therapeutic alternatives for KS patients.
vGPCR expression leads to the elaboration of numerous inflammatory and angiogenic factors which are postulated to help promote the proliferation and survival of neighboring endothelial cells15. Among the proteins upregulated by vGPCR, VEGF has been shown to be a key player in KSHV pathogenesis and a molecular hallmark of KS lesions. Interestingly, we have recently shown that vGPCR upregulation of a novel Angiopoietin-like factor, ANGPTL4, may also play an essential role in KS development by the promotion of angiogenesis and vascular permeability9.
ANGPTL4 is known as a gene with upregulated expression in endothelial cells exposed to hypoxia, ischemic tissues, and in hypoxic areas of solid tumors16. Although the precise function of this factor in cancer biology is still unclear, emerging evidence implicates ANGPTL4 in the promotion of tumor progression, angiogenesis, and tumor dissemination. In breast cancer, ANGPTL4 has been recently shown to prime tumor cells for lung metastasis and trigger the disruption of vascular endothelial cell-cell junctions17. Similarly, expression of ANGPTL4 in gastric cancer and in esophageal and oral tongue squamous cell carcinoma correlates with lymphatic and venous invasion, degree of tumor differentiation, and poor survival18–20. ANGPTL4 has also been shown to be a diagnostic marker for primary and metastatic clear cell renal-cell carcinoma21. Conversely, this protein has also been suggested as an anti-angiogenic and anti-metastatic factor14. Collectively, these incongruent data suggest that the contribution of ANGPTL4 to cancer may be dependent on the tumor type and tissue environment.
Here we provide evidence that ANGPTL4 is upregulated both directly and indirectly (through a paracrine mechanism) by the KSHV oncogene, vGPCR. We further demonstrate that ANGPTL4 is a molecular hallmark of oral KS lesions. Indeed, expression of ANGPTL4 was observed in more tumor cells and in more biopsies specimens than expression of VEGF in oral KS. Almost half (12/25) of the KS biopsy specimens had a higher percentage of cells expressing ANGPTL4 compared to VEGF; only 7/25 (28%) of the KS biopsies demonstrated a higher percentage of cells expressing VEGF compared to ANGPTL4. Moreover, only 1/25 (4%) of the KS biopsies tested expressed VEGF but not ANGPTL4. Conversely, 4/25 (16%) of the KS biopsies tested expressed ANGPTL4 but not VEGF. As demonstrated in Table 3, 10/25 (40%) of the KS lesions had high levels of expression of ANGPTL4 in the majority of tumor cells compared to 7/25 (28%) of KS lesions with high levels of VEGF in the majority of tumor cells. Collectively, these results support a fundamental role for vGPCR paracrine upregulation of ANGPTL4 in Kaposi’s sarcomagenesis and further suggest that this angiogenic growth factor may prove to be an essential diagnostic as well as therapeutic target in KS.
Supplementary Material
Acknowledgements
This work was supported by grant R01CA119911 (National Cancer Institute, NIH). BCJ is a recipient of a predoctoral fellowship from the CNPq-Brazil. We thank Dr. Gary S. Hayward (Johns Hopkins University) for kindly providing the specific antibody recognizing KSHV vGPCR. We also thank Dr. Akrit Sodhi (Johns Hopkins University) for insightful suggestions and discussion.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.Feller L, Lemmer J, Wood NH, Jadwat Y, Raubenheimer EJ. HIV-associated oral Kaposi sarcoma and HHV-8: a review. J Int Acad Periodontol. 2007;9:129–136. [PubMed] [Google Scholar]
- 2.Mesri EA, Cesarman E, Boshoff C. Kaposi's sarcoma and its associated herpesvirus. Nat Rev Cancer. 2010;10:707–719. doi: 10.1038/nrc2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, Knowles DM, Moore PS. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science. 1994;266:1865–1869. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- 4.Guo HG, Sadowska M, Reid W, Tschachler E, Hayward G, Reitz M. Kaposi's sarcoma-like tumors in a human herpesvirus 8 ORF74 transgenic mouse. J Virol. 2003;77:2631–2639. doi: 10.1128/JVI.77.4.2631-2639.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Montaner S, Sodhi A, Molinolo A, Bugge TH, Sawai ET, He Y, Li Y, Ray PE, Gutkind JS. Endothelial infection with KSHV genes in vivo reveals that vGPCR initiates Kaposi's sarcomagenesis and can promote the tumorigenic potential of viral latent genes. Cancer Cell. 2003;3:23–36. doi: 10.1016/s1535-6108(02)00237-4. [DOI] [PubMed] [Google Scholar]
- 6.Yang TY, Chen SC, Leach MW, Manfra D, Homey B, Wiekowski M, Sullivan L, Jenh CH, Narula SK, Chensue SW, et al. Transgenic expression of the chemokine receptor encoded by human herpesvirus 8 induces an angioproliferative disease resembling Kaposi's sarcoma. J Exp Med. 2000;191:445–454. doi: 10.1084/jem.191.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bais C, Santomasso B, Coso O, Arvanitakis L, Raaka EG, Gutkind JS, Asch AS, Cesarman E, Gershengorn MC, Mesri EA, et al. G-protein-coupled receptor of Kaposi's sarcoma-associated herpesvirus is a viral oncogene and angiogenesis activator. Nature. 1998;391:86–89. doi: 10.1038/34193. [DOI] [PubMed] [Google Scholar]
- 8.Sodhi A, Montaner S, Patel V, Zohar M, Bais C, Mesri EA, Gutkind JS. The Kaposi's sarcoma-associated herpes virus G protein-coupled receptor up-regulates vascular endothelial growth factor expression and secretion through mitogen-activated protein kinase and p38 pathways acting on hypoxia-inducible factor 1alpha. Cancer Res. 2000;60:4873–4880. [PubMed] [Google Scholar]
- 9.Ma T, Jham BC, Hu J, Friedman ER, Basile JR, Molinolo A, Sodhi A, Montaner S. Viral G protein-coupled receptor up-regulates Angiopoietin-like 4 promoting angiogenesis and vascular permeability in Kaposi's sarcoma. Proc Natl Acad Sci U S A. 2010;107:14363–14368. doi: 10.1073/pnas.1001065107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Montaner S, Sodhi A, Servitja JM, Ramsdell AK, Barac A, Sawai ET, Gutkind JS. The small GTPase Rac1 links the Kaposi sarcoma-associated herpesvirus vGPCR to cytokine secretion and paracrine neoplasia. Blood. 2004;104:2903–2911. doi: 10.1182/blood-2003-12-4436. [DOI] [PubMed] [Google Scholar]
- 11.Couty JP, Geras-Raaka E, Weksler BB, Gershengorn MC. Kaposi's sarcomaassociated herpesvirus G protein-coupled receptor signals through multiple pathways in endothelial cells. J Biol Chem. 2001;276:33805–33811. doi: 10.1074/jbc.M104631200. [DOI] [PubMed] [Google Scholar]
- 12.Pati S, Cavrois M, Guo HG, Foulke JS, Jr, Kim J, Feldman RA, Reitz M. Activation of NF-kappaB by the human herpesvirus 8 chemokine receptor ORF74: evidence for a paracrine model of Kaposi's sarcoma pathogenesis. J Virol. 2001;75:8660–8673. doi: 10.1128/JVI.75.18.8660-8673.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwarz M, Murphy PM. Kaposi's sarcoma-associated herpesvirus G protein-coupled receptor constitutively activates NF-kappa B and induces proinflammatory cytokine and chemokine production via a C-terminal signaling determinant. J Immunol. 2001;167:505–513. doi: 10.4049/jimmunol.167.1.505. [DOI] [PubMed] [Google Scholar]
- 14.Hato T, Tabata M, Oike Y. The role of angiopoietin-like proteins in angiogenesis and metabolism. Trends Cardiovasc Med. 2008;18:6–14. doi: 10.1016/j.tcm.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Jham BC, Montaner S. The Kaposi's sarcoma-associated herpesvirus G protein-coupled receptor: Lessons on dysregulated angiogenesis from a viral oncogene. J Cell Biochem. 2010;110:1–9. doi: 10.1002/jcb.22524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le Jan S, Amy C, Cazes A, Monnot C, Lamandé N, Favier J, Philippe J, Sibony M, Gasc JM, Corvol P, Germain S. Angiopoietin-like 4 is a proangiogenic factor produced during ischemia and in conventional renal cell carcinoma. Am J Pathol. 2003;162:1521–1528. doi: 10.1016/S0002-9440(10)64285-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Padua D, Zhang XH, Wang Q, Nadal C, Gerald WL, Gomis RR, Massagué J. TGFbeta primes breast tumors for lung metastasis seeding through angiopoietin-like 4. Cell. 2008;133:66–77. doi: 10.1016/j.cell.2008.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakayama T, Hirakawa H, Shibata K, Abe K, Nagayasu T, Taguchi T. Expression of angiopoietin-like 4 in human gastric cancer:ANGPTL4 prootes venous invasion. Oncol Rep. 2010;24:599–606. doi: 10.3892/or_00000897. [DOI] [PubMed] [Google Scholar]
- 19.Shibata K, Nakayama T, Hirakawa H, Hidaka S, Nagayasu T. Clinicopathological significance of angiopoietin-like protein 4 expression in oesophageal squamous cell carcinoma. J Clin Pathol. 2010;63:1054–1058. doi: 10.1136/jcp.2010.078600. [DOI] [PubMed] [Google Scholar]
- 20.Wang Z, Han B, Zhang Z, Pan J, Xia H. Expression of angiopoietin-like 4 and tenascin C but not cathepsin C mRNA predicts prognosis of oral tongue squamous cell carcinoma. Biomarkers. 2010;15:39–46. doi: 10.3109/13547500903261362. [DOI] [PubMed] [Google Scholar]
- 21.Verine J, Lehmann-Che J, Soliman H, Feugeas JP, Vidal JS, Mongiat-Artus P, Belhadj S, Philippe J, Lesage M, Wittmer E, Chanel S, Couvelard A, Ferlicot S, Rioux-Leclercq N, Vignaud JM, Janin A, Germain S. Determination of angptl4 mRNA as a diagnostic marker of primary and metastatic clear cell renal-cell carcinoma. PLoS One. 2010;5:e10421. doi: 10.1371/journal.pone.0010421. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.