Abstract
Background and Aims
The extrinsic death receptor-mediated pathway of apoptosis is involved in nonalcoholic steatohepatitis (NASH) development. Our aims were to create and validate a noninvasive prediction model for NASH diagnosis based on specific circulating markers of apoptosis.
Methods
Our initial cohort consisted of 95 consecutive patients undergoing a liver biopsy for clinically suspected NASH. Blood was obtained from each patient at the time of liver biopsy. Plasma caspase 3 generated cytokeratin-18 fragments (CK-18), soluble Fas (sFas) and soluble Fas ligand (sFasL) were measured. Histology was assessed by an experienced hepatopathologist. The validation cohort consisted of 82 consecutive patients that underwent liver biopsy at the time of bariatric surgery.
Results
Patients with NASH had significantly higher levels of CK-18 and sFas than patients in the “not NASH” group [median (25th, 75th percentile): 508 (280, 846) U/L versus 176 (131, 224) U/L (P< 0.001), and 11.8 (7.8, 12.5) ng/ml versus 5.9 (4.8, 8.3) ng/ml (P< 0.001), respectively]. A significant positive correlation was revealed between the apoptosis markers and liver histopathology independent of other metabolic factors. A prediction model was generated including CK-18 fragments and sFas levels that showed an AUC of 0.93 and 0.79 in the initial and validation cohorts, respectively. A cutoff value using this model predicted NASH with a sensitivity and specificity of 88% and 89%, respectively.
Conclusions
Quantification of circulating levels of two apoptotic markers accurately predicts the presence of NASH, supporting the potential usefulness of these markers in clinical practice for noninvasive diagnosis of NASH.
Keywords: Nonalcoholic Fatty Liver Disease, Nonalcoholic Steatohepatitis, Apoptosis, Noninvasive diagnosis
Introduction
Nonalcoholic fatty liver disease (NAFLD) has become the most common form of chronic liver disease, currently affecting 20 to 30% of adults and 10% of children in the United States [1]. The spectrum of NAFLD is wide ranging from hepatic steatosis to nonalcoholic steatohepatitis (NASH) and cirrhosis [2]. Patients with hepatic steatosis appear to have a non-progressive course with more benign prognosis. Patients with NASH may progress to cirrhosis in as many as 25% of cases and suffer from its complications including portal hypertension, liver failure and hepatocellular carcinoma [3, 4]. The liver biopsy remains the gold standard for differentiating between hepatic steatosis and NASH in addition to providing information regarding the degree of steatosis, severity of inflammatory activity and stage of fibrosis [5]. However, the liver biopsy is an invasive procedure that carries possible significant risks. There are several clinical trials investigating therapies for NASH; the results of which will hopefully provide physicians with treatment options for this condition. This underscores the importance of a screening test that identifies NASH in patients with NAFLD. Such screening test should be simple, noninvasive, reproducible, and accurately differentiates NASH from hepatic steatosis.
Hepatocyte apoptosis plays a critical role in liver injury and NASH development [6–8]. Increase in hepatocyte apoptosis is typically present in humans as well as animal models of NASH but absent in those with hepatic steatosis [8]. Increasing evidence suggests a role of both the so called extrinsic (death receptor mediated) pathway and the intrinsic (organelle-initiated) pathway of apoptosis. Fas, a death receptor member of the TNFR family, appears to have a prominent role. Fas protein expression is increased in liver samples from NASH patients [6]. Expression of this receptor increases in experimental models of NASH and results in increased sensitivity to Fas mediated apoptosis [9]. Accumulation of free fatty acids in liver cells results in upregulation of Fas in the cell surface [9]. Although the relative importance of the two main apoptotic pathways in human NASH remains to be elucidated, in hepatocytes, both pathways tend to converge at the level of the mitochondria resulting in permeabilization of the mitochondrial outer membrane and release of multiple proteins from the mitochondrial inter-membrane space into the cytosol [10]. This results in activation of effector caspases (mainly caspase 3) which will then cleave a number of different substrates inside the cell including cytokeratin 18 (CK-18), the major intermediate filament protein in the liver, resulting in the characteristic morphologic changes of apoptosis [10]. We have previously demonstrated that caspase generated CK-18 fragments are significantly elevated in NASH patients [7]. Since the initial report, we and others have confirmed the utility of quantification of this marker for NASH diagnosis [11–14]. The aim of the present study was to test a panel of circulating apoptotic markers for diagnosis of NASH.
Patients and Methods
Patients Characteristics
The study was approved by the Cleveland Clinic Institutional Review Board, and all patients gave written informed consent prior to participation. Our initial cohort consisted of 95 consecutive patients undergoing a baseline liver biopsy for clinical suspicion of NASH by their treating hepatologists. Up to date, there are no established guidelines for performing a liver biopsy in patients with suspected NAFLD. Thus, the decision to perform the biopsy was individualized, and mostly performed due to persistently abnormal liver enzymes (mainly serum ALT) in a patient with clinically suspected NASH. The validation cohort included 82 consecutive patients who underwent liver biopsy at the time of bariatric surgery as part of their standard of care. Demographic, clinical, and laboratory data were collected. The absence of past or current excessive alcohol use was defined by an average daily consumption of alcohol of < 20 g/day for men and < 10 g/day for women. Absence of other liver diseases was confirmed via serological testing, imaging studies or histological findings. Blood was obtained from each patient at the time of liver biopsy. Patients were subsequently divided into two groups according to their histological diagnosis (see Liver Histology): “NASH” and “not NASH”.
Liver Histology
The histological diagnosis was established using hematoxylin-eosin and Masson trichrome stains of formalin-fixed paraffin-embedded liver and graded by an experienced hepatopathologist (L.Y.) who was blinded to the clinical characteristics of the patients including levels of serum biomarkers. The hepatopathologist provided an overall diagnostic interpretation based on the criteria reported by Brunt et al. [15] and also reported a NAFLD Activity Score (NAS) for each liver biopsy based on the NAFLD scoring system recently proposed by the National Institute of Diabetes and Digestive and Kidney Diseases NASH Clinical Research Network [16]. According to this scoring system, the degree of steatosis and inflammatory activity is measured using a zero to 8 scale (steatosis = 0–3; lobular inflammation = 0–3; ballooning = 0–2). The NAS is the unweighted sum of steatosis, lobular inflammation, and hepatocellular ballooning scores. The stage of fibrosis was assessed using a zero to 4 scale (0 = no fibrosis; 1 = mild/moderate zone 3 perisinusoidal fibrosis, or portal/periportal fibrosis only; 2 = perisinusoidal and portal/periportal fibrosis; 3 = bridging fibrosis; 4 = cirrhosis). Patients were divided into two groups according to their histological diagnosis (hepatopathologist’s overall diagnostic interpretation based on Brunt’s criteria): “NASH” and “not NASH”.
Measurement of Caspase-Generated CK-18 Fragments, soluble Fas, and soluble Fas Ligand in the Blood
Blood samples obtained from patients at the time of their liver biopsies were initially processed to plasma then stored frozen at − 80 °C. Plasma caspase 3 generated CK-18 fragments, soluble Fas (sFas) and soluble Fas ligand (sFasL) were quantitatively measured using specific sandwich ELISA based immunoassays for each. The M30-Apoptosense ELISA kit (PEVIVA; Alexis, Grünwald, Germany) was used for quantitative measurement of CK-18 fragments by selectively recognizing the caspase cleavage-generated neoepitope in the C-terminal domain of CK-18. The Human Fas/TNFRSF6 Quantikine ELISA Kit (R&D systems, Minneapolis, MN) was used for quantitative measurement of sFas and the Human Fas Ligand/TNFSF6 Quantikine ELISA Kit (R&D systems, Minneapolis, MN) was used for quantitative measurement of sFasL. All assays were performed in duplicate, and the absorbance was determined by using a microplate reader (Molecular Devices M2, Sunnyvale, CA).
Statistical Analysis
Descriptive statistics were computed for all factors. These included means, standard deviations and percentiles for continuous variables and frequencies for categorical factors. Univariable analysis was done to compare subjects with NASH to those without. Student’s t-tests and Wilcoxon rank sum tests were used to compare continuous variables and Pearson’s chi-square tests were used for categorical variables. Receiver operating characteristic (ROC) analysis was performed to assess the role of CK-18 fragments, sFas and sFasL levels in the diagnosis of NASH. The area under the ROC curves (AUC) and corresponding 95% confidence intervals were estimated. In addition, multivariable logistic regression analysis was performed to assess the combinations of the three hepatocyte apoptotic biomarkers and whether addition of age, BMI, gender, race, ALT, AST, insulin, glucose, HOMA, presence of diabetes, hypertension, metabolic syndrome or hyperlipidemia improved NASH prediction; DeLong’s method [17] was used to compare AUCs. A P< 0.05 was considered statistically significant. SAS version 9.2 software (The SAS Institute, Cary, NC) and R version 2.4.1 software (The R Foundation for Statistical Computing, Vienna, Austria) were used to perform all analyses.
Results
Patients Characteristics
The main clinical and serological characteristics of the initial cohort patients are described in Table 1. The mean age of patients was 50 (± 11.6) years. The patients’ gender (50.5% male) and race (83.2% Caucasian) did not statistically differ between the two histologic groups. Patients with NASH were significantly older and had significantly higher body mass index. They also had significantly higher prevalence of hypertension, clinical diabetes and metabolic syndrome, and significantly higher serum ALT and AST levels and HOMA index compared with patients in the “not NASH” group (all P< 0.05). Triglyceride level was significantly higher and HDL level was lower in patients with NASH, though did not reach statistically significant difference. Table 2 lists the histologic characteristics of the liver biopsies of the initial cohort.
Table 1.
Clinical and serological characteristics of the patient population (initial cohort).
| Characteristics | All (N=95) | NASH (N=41) | Not NASH (N=54) | P- Value* |
|---|---|---|---|---|
| Sex, % male | 50.5 | 41.5 | 57.4 | 0.12 |
| Race, % Caucasian | 83.2 | 90.2 | 77.8 | 0.11 |
| Age (years) | 50 (11.6) | 52.8 (10.1) | 47.8 (12.3) | 0.033 |
| BMI (kg/m2) | 31.4 (5.1) | 33.5 (4.6) | 29.7 (4.9) | <0.001 |
| ALT (IU/L) | 53.5 (32, 87) | 73 (42, 101) | 39 (26, 71) | 0.005 |
| AST (IU/L) | 54 (38, 75) | 59 (46, 106) | 49 (34, 65) | 0.023 |
| HOMA | 5 (1.3, 13.3) | 7.5 (4.2, 14.7) | 1.3 (0.6, 5.3) | <0.001 |
| Cholesterol (mg/dL) | 209 (164, 238) | 212 (167, 243) | 209 (161, 236) | 0.56 |
| Triglycerides (mg/dL) | 169.5 (127.5, 219) | 197.5 (150, 241) | 156 (100, 189) | 0.019 |
| HDL (mg/dL) | 46 (40, 53) | 44 (40, 51) | 47 (43, 60) | 0.075 |
| LDL (mg/dL) | 109.4 (77.6, 138) | 108.4 (92.5, 149.7) | 111 (77, 134) | 0.31 |
| Hyperlipidemia, % | 53.3 | 58.8 | 46.2 | 0.33 |
| Diabetes, % | 27.4 | 39.0 | 18.5 | 0.026 |
| Hypertension, % | 45.2 | 57.5 | 35.9 | 0.038 |
| Metabolic syndrome, % | 50.0 | 74.4 | 31.4 | <0.001 |
Values are presented as mean (standard deviation) for age and BMI, median (25th, 75th ercentile) for all other continuous variables and percentage (%) otherwise.
P- values correspond to t-tests for age and BMI, Wilcoxon rank sum tests for other continuous ariables, and Pearson's chi-square tests otherwise.
Table 2.
Histologic characteristics of the liver biopsies of the initial cohort.
| Characteristics | NASH (N=41) | Not NASH (N=54) | P- Value* |
|---|---|---|---|
| Steatosis | <0.001 | ||
| 0= < 5% | 0 (0.0) | 18 (33.3) | |
| 1= 5–33% | 5 (12.2) | 24 (44.4) | |
| 2= 34–66% | 24 (58.5) | 10 (18.5) | |
| 3= >66% | 12 (29.3) | 2 (3.7) | |
| Ballooning | <0.001 | ||
| 0= None | 2 (5.0) | 54 (100.0) | |
| 1= Few balloon cells | 14 (35.0) | 0 (0.0) | |
| 2= Many/ prominent ballooning | 24 (60.0) | 0 (0.0) | |
| Lobular inflammation | <0.001 | ||
| 0= No foci | 0 (0.0) | 50 (92.6) | |
| 1= <2 foci per 200X field | 20 (48.8) | 4 (7.4) | |
| 2= 2–4 foci per 200X field | 19 (46.3) | 0 (0.0) | |
| 3= >4 foci per 200X field | 2 (4.9) | 0 (0.0) | |
| Fibrosis | <0.001 | ||
| 0 | 4 (9.8) | 52 (96.3) | |
| 1 | 14 (34.2) | 1 (1.9) | |
| 2 | 6 (14.6) | 0 (0.0) | |
| 3 | 13 (31.7) | 0 (0.0) | |
| 4 | 4 (9.8) | 1 (1.9) | |
| NAS | 5.0 (5.0, 6.0) | 1.0 (0.0, 2.0) | <0.001 |
Values are presented as median (25th, 75th percentile) for NAS and N (%) otherwise.
Scores were determined according to Kleiner et al. [16]
P- values correspond to Wilcoxon rank sum tests.
Cytokeratin-18 Fragments and sFas are Markedly Increased in Patients with NASH
Plasma levels of CK-18 fragments ranged from 60 to 2,306 U/L [median (25th, 75th percentile): 224 (151, 431) U/L]. Patients with NASH had significantly higher levels of CK-18 fragments than patients with “not NASH” [median (25th, 75th percentile): 508 (280, 846) U/L versus 176 (131, 224) U/L; P< 0.001]. Plasma levels of sFas ranged from 3.3 to 17.5 ng/ml [median (25th, 75th percentile): 7.6 (5.4, 10.3) ng/ml] and plasma levels of sFasL ranged from 5 to 200 pg/ml [median (25th, 75th percentile): 78 (65, 91) pg/ml]. Plasma sFas levels were significantly higher in patients with NASH compared with those with “not NASH” [median (25th, 75th percentile): 11.8 (7.8, 12.5) ng/ml versus 5.9 (4.8, 8.3) ng/ml; P< 0.001]. Patients with NASH had higher levels of sFasL than patients with “not NASH”, but did not reach statistically significant difference [median (25th, 75th percentile): 80 (66, 92) pg/ml versus 76 (65, 85) pg/ml, P= 0.2].
Both CK-18 fragments levels and sFas levels in the plasma showed a significant positive correlation with the liver histopathology characteristics, mainly with the presence of lobular inflammation and hepatocyte ballooning (Table 3). Though there was a positive correlation between sFasL and the same histopathology characteristics, it was weaker than the correlation shown with each of CK-18 fragments and sFas.
Table 3.
Correlation between hepatocyte apoptotic markers in the plasma and liver histologic characteristics (initial cohort).
| CK-18 fragments | sFas | |||
|---|---|---|---|---|
| Characteristics | rho (95% CI) | P- Value* | rho (95% CI) | P- Value* |
| Fibrosis | 0.51 (0.33,0.69) | <0.001 | 0.57 (0.40,0.74) | <0.001 |
| Lobular inflammation | 0.61 (0.45,0.77) | <0.001 | 0.59 (0.42,0.75) | <0.001 |
| Steatosis | 0.46 (0.28,0.65) | <0.001 | 0.48 (0.30,0.66) | <0.001 |
| Ballooning | 0.66 (0.50,0.81) | <0.001 | 0.60 (0.43,0.76) | <0.001 |
| NAS | 0.60 (0.43,0.76) | <0.001 | 0.59 (0.42,0.76) | <0.001 |
Abbreviations: rho, Spearman's correlation coefficient; CI, confidence interval.
P- values correspond to H0: rho= 0
Prediction Model for NASH
Cytokeratin-18 Fragments and sFas as Independent Predictors of NASH
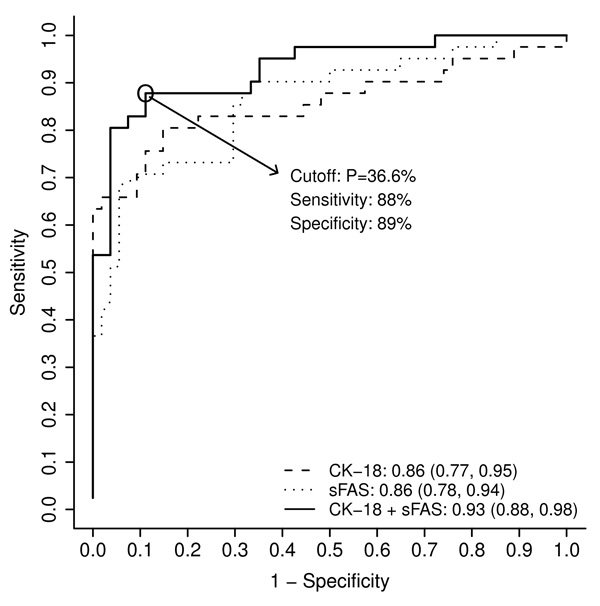
In order to generate a noninvasive model for prediction of NASH utilizing hepatocyte apoptotic biomarkers, the ROC analysis was performed. The AUC (95% CI) for each of CK-18 fragments, sFas, and sFasL was 0.86 (0.77, 0.95), 0.86 (0.78, 0.94), and 0.58 (0.46, 0.70); respectively. The combination of plasma CK-18 fragments and sFas levels had an AUC estimated at 0.93 [(95% CI): 0.88, 0.98] and was found to be significantly higher than 0.5 (chance assignment) and significantly higher than the AUC of either CK-18 fragments (P= 0.027) or sFas (P= 0.034) alone (Figure 1). Adding sFasL level to this combination did not increase the AUC so it was not included in the final model. Age, BMI, gender, race, ALT, AST, insulin, glucose, HOMA, presence of diabetes, hypertension, metabolic syndrome and hyperlipidemia were considered for inclusion in the model. None of these factors significantly increased the AUC, so the final model consisted of plasma CK-18 fragments and sFas levels. The regression formula for prediction of presence of NASH based on these 2 variables was: Risk score = −6.4894 + 0.0078 × CK-18 fragments (U/L) + 0.4668 × sFas (ng/ml). The best cutoff point was a probability of 36.6% (corresponded to score of −0.5509) that accurately predicted NASH with a sensitivity of 88%, a specificity of 89%, and positive and negative predictive values of 86% and 91%, respectively (Figure 1). This model correctly classified 88% (84/95) of the patients.
Figure 1. An apoptosis panel consisting of plasma CK-18 fragments and sFas levels accurately diagnoses NASH in patients with suspected NAFLD (initial cohort).
The area under the ROC curve is shown for the performance of this panel and its individual components for discriminating NASH from “not NASH”. The panel has an AUC that is significantly higher than the AUC of either CK-18 fragments or sFas alone. The regression formula for prediction of presence of NASH based on this 2-factor model is: Risk score = −6.4894 + 0.0078 × CK-18 fragments (U/L) + 0.4668 × sFas (ng/mL). The best cutoff point is a probability of 36.6% (corresponds to score of −0.5509) that accurately predicts NASH with a sensitivity of 88% and a specificity of 89%. Abbreviation: P, probability.
On multivariable logistic regression analysis, each of CK-18 and sFas remained an independent predictor of NASH. The risk of having NASH on liver biopsy increased with increasing CK-18 fragments levels. For every 50 U/L increase in the plasma level of CK-18, the likelihood of having NASH increased 50% [OR (95% CI): 1.5 (1.2, 1.9), P= 0.0009]. The risk of NASH also increased with increasing sFas levels. For every 1 ng/ml increase in the plasma sFas levels, the likelihood of having NASH increased 60% [OR (95% CI): 1.6 (1.2, 2.06), P= 0.0004].
The diagnostic accuracy of the prediction model was next examined in an independent cohort of 82 consecutive patients with morbid obesity that underwent liver biopsy at the time of bariatric surgery as part of a standard clinical procedure. Patients were subsequently divided into two groups according to their histological findings: “NASH” (n = 20) and “not NASH” (n = 62). The main clinical and serological characteristics of the patients are described in Table 4. Within our validation cohort, patients with NASH had significantly higher levels of CK-18 fragments and sFas than patients in the “not NASH” group [median (25th, 75th percentile): 339 (202, 780) U/L versus 207 (162, 265) U/L (P< 0.001), and 5.6 (4.5, 6.2) ng/ml versus 4.1 (3.4, 5.2) ng/ml (P= 0.004), respectively].
Table 4.
Clinical and serological characteristics of the bariatric surgery patients (validation cohort).
| Characteristics | All (N=82) | NASH (N=20) | Not NASH (N=62) | P-Value* |
|---|---|---|---|---|
| Sex, % female | 80.5 | 80.0 | 80.7 | 0.99 |
| Race, % Caucasian | 80.5 | 100.0 | 74.2 | 0.009 |
| Age (years) | 50.1 (9.8) | 50.8 (10.2) | 49.9 (9.7) | 0.74 |
| BMI (kg/m2) | 48.5 (7.2) | 46.5 (6.3) | 49.2 (7.4) | 0.12 |
| ALT (IU/L) | 20.5 (16.0, 33.0) | 30.5 (22.0, 45.0) | 19.0 (15.5, 30.5) | 0.018 |
| AST (IU/L) | 23.0 (18.0, 29.0) | 25.5 (21.0, 37.0) | 22.5 (16.5, 28.0) | 0.055 |
| Cholesterol (mg/dL) | 193.5 (164.0, 213.5) | 178.5 (168.0, 212.0) | 194.5 (163.0, 214.0) | 0.94 |
| Triglycerides (mg/dL) | 133.0 (100.0, 185.0) | 260.5 (120.0, 343.0) | 127.5 (97.0, 154.0) | 0.023 |
| HDL (mg/dL) | 50.0 (42.0, 57.5) | 45.5 (40.0, 53.0) | 52.0 (42.0, 60.0) | 0.16 |
| LDL (mg/dL) | 110.0 (83.5, 133.0) | 93.5 (75.0, 118.0) | 117.0 (85.0, 135.0) | 0.2 |
| Hyperlipidemia, % | 57.3 | 75.0 | 51.6 | 0.066 |
| Diabetes, % | 40.2 | 55.0 | 35.5 | 0.12 |
| Hypertension, % | 67.1 | 60.0 | 69.4 | 0.44 |
| Metabolic syndrome, % | 77.3 | 87.5 | 74.0 | 0.33 |
Values are presented as mean (standard deviation) for age and BMI, median (25th, 75th percentile) for all other continuous variables and percentage (%) otherwise.
P- values correspond to t-tests for age and BMI, Wilcoxon rank sum tests for other continuous variables and Pearson's chi-square or Fisher's Exact (F) tests otherwise.
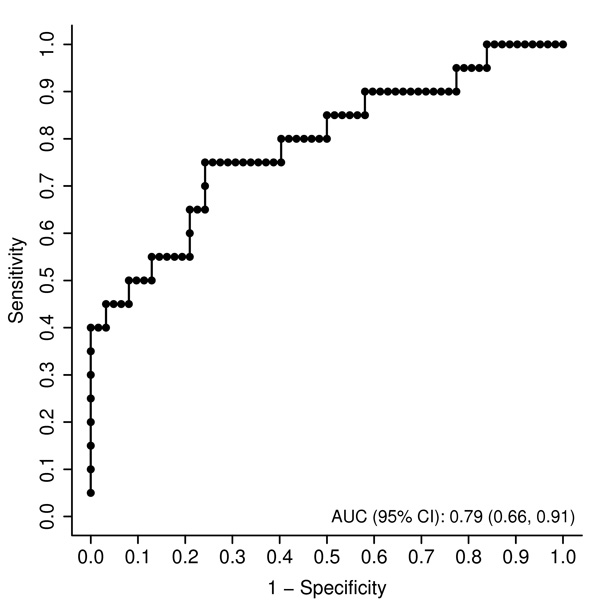
The 2-factor model consisting of plasma CK-18 fragments and sFas levels applied on the initial cohort was examined on the validation cohort for diagnosis of NASH. The AUC remained high in the validation group [AUC (95% CI): 0.79 (0.66, 0.91)] (Figure 2). This AUC was higher than the AUC for either CK-18 fragments (0.77) or sFas (0.72) alone; however this was not statistically significant. This model correctly classified 83% (68/82) of the patients.
Figure 2. An apoptosis panel consisting of plasma CK-18 fragments and sFas levels accurately diagnoses NASH in morbidly obese patients undergoing bariatric surgery (validation cohort).
The area under the ROC curve is shown for the performance of this panel for discriminating NASH from “not NASH”.
Discussion
Obesity and type 2 diabetes have reached epidemic proportions in most of the western world, and both conditions are strongly associated with NAFLD [2, 18, 19]. NAFLD encompasses a wide spectrum of conditions associated with over-accumulation of lipids in the liver ranging from hepatic steatosis in which there is evidence for fat accumulation without signs of liver cell injury or inflammation, to nonalcoholic steatohepatitis characterized by the accumulation of fat in the liver along with evidence of liver cell damage, inflammation and different degrees of scarring or fibrosis [2]. While most patients with steatosis tend to have a benign non-progressive clinical course, a significant proportion of those with NASH have a progressive disease with significant associated risk of developing cirrhosis and its feared complications [3, 4]. In light of the dramatic increase in the prevalence of NAFLD in conjunction with the significant research effort in developing novel therapies targeted to patients with NASH, non invasive, simple, reproducible and reliable biomarkers are greatly needed. They will not only help in the diagnosis of NASH, but also be useful for assessment of treatment response and prognosis and remain a research priority in the NAFLD field.
Hepatocyte apoptosis is a prominent pathologic feature of human NASH and the magnitude of apoptosis present correlates with the degree of liver damage and stage of fibrosis [6, 8, 20]. Experimental studies suggest that the extrinsic pathways of apoptosis and in particular activation of the Fas/FasL system may be a central mechanism triggering liver injury and NASH development [6]. Expression of this receptor increases in experimental models of NASH and this results in increased sensitivity to Fas mediated apoptosis [9], and exposure of human liver cells to free fatty acids (FFA) results in upregulation of Fas expression and increased sensitivity to Fas mediated apoptosis [6]. The precise mechanism by which FFA promotes Fas generation remains unknown. A recent report demonstrated that the hepatocyte growth factor receptor, Met, associates directly with Fas in normal liver tissues preventing Fas activation, while Fas sequestration by Met is abrogated in both human and experimental NASH resulting in increased Fas-Fas Ligand complex and apoptosis [21]. Thus, noninvasive quantification of hepatocellular apoptosis represents a rational approach to assess the extent of liver damage.
Our current data provides a noninvasive model for diagnosis of NASH which includes two hepatocyte apoptotic biomarkers; plasma CK-18 fragments and sFas. The results demonstrate that this panel appears to be more accurate for diagnosis of NASH than using CK-18 fragments level alone. The AUC for our 2-factor model including plasma CK-18 fragments and sFas (0.93) was significantly higher than utilizing CK-18 fragments level alone (0.86) or sFas alone (0.86). Moreover, our cutoff point using this model provided sensitivity and specificity for prediction of NASH (88% and 89%, respectively) that are higher than any single cutoff value for CK-18 fragments alone reported in our multi-center validation study [11]. The high AUC in the validation group (0.79) indicates that this panel provides reproducible results even for a highly distinct group of patients that represent those undergoing bariatric surgery in whom the prevalence and severity of NASH is typically lower than in patients undergoing liver biopsy for suspected NASH diagnosis [22, 23].
Our results show that eighteen patients (19%) of the initial cohort had normal or minimal changes on liver biopsies. In a separate analysis (data not shown), we compared these patients with those who had evidence of NAFLD on biopsy. There was no statistically significant difference between the two groups in race, gender, age, BMI, AST and ALT levels, and clinical prevalence of diabetes, hyperlipidemia and hypertension. Though, patients with biopsy proven NAFLD had significantly higher prevalence of fatty liver on abdominal imaging done prior to the liver biopsy compared to patients with normal liver biopsy. We also performed further analysis (data not shown) where we excluded patients with normal liver biopsy and compared patients with NASH with patients with hepatic steatosis. CK-18 fragments and sFas levels remained significantly higher in patients with NASH compared to patients with hepatic steatosis. However, we decided to keep patients with normal liver biopsy in the “not NASH” group as they represent part of the spectrum of NAFLD and thus a more realistic use of the apoptotic markers for noninvasive diagnosis of NASH in the practical clinical setting.
The current study has several strengths. We included a large group of consecutive well-characterized NAFLD patients and cross-validated our results in a separate highly distinct set of patients. Add to this, the biological plausibility of the markers tested based on extensive evidence of their role in NASH development. The limitations of our study include the fact that we did not account for the possibility of sampling error as well as intra-observer and inter-observer variability in regards to liver biopsies [24]. However, a series of studies looking at sampling error in NAFLD have demonstrated that this is more of an issue for the individual histological findings of necroinflammatory activity and hepatocyte ballooning, but much less so for the diagnosis of NASH and staging of fibrosis [25, 26].
Thus, our apoptosis panel appears to have several features that fulfill many of the requirements for an ideal test for diagnosis of NASH including that the test is simple, easy to measure and handle, reproducible, and mechanism-based. In summary, our findings identifies a diagnostic apoptosis panel including plasma CK-18 fragments and sFas for diagnosis of NASH, supporting the potential usefulness of these markers in clinical practice for noninvasive diagnosis of NASH.
Acknowledgments
Financial disclosure: Dr. Feldstein reports that he is named as co-inventor on pending patents filed by the Cleveland Clinic that refer to the use of biomarkers in fatty liver disorders. This work was supported by NIH grants (DK076852) and (DK082451) to AEF and National Center for Research Resources, Clinical Research Unit Grant M01 RR-018390.
Abbreviations
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- TNFR
tumor necrosis factor receptor
- CK-18
cytokeratin-18
- NAS
NAFLD activity score
- sFas
soluble Fas
- sFasL
soluble Fas ligand
- ELISA
enzyme-linked immunosorbent assay
- ROC
receiver operating characteristic
- AUC
area under the receiver operating characteristic curve
- BMI
body mass index
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- HOMA
homeostatic model assessment
- HDL
high-density lipoprotein
- LDL
low-density lipoprotein
- CI
confidence interval
- OR
odds ratio
- FFA
free fatty acids
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wieckowska A, Feldstein AE. Diagnosis of nonalcoholic fatty liver disease: invasive versus noninvasive. Semin Liver Dis. 2008;28:386–395. doi: 10.1055/s-0028-1091983. [DOI] [PubMed] [Google Scholar]
- 2.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 3.Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–121. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 4.Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–1419. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 5.Brunt EM, Tiniakos DG. Pathological features of NASH. Front Biosci. 2005;10:1475–1484. doi: 10.2741/1632. [DOI] [PubMed] [Google Scholar]
- 6.Feldstein AE, Canbay A, Angulo P, Taniai M, Burgart LJ, Lindor KD, et al. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology. 2003;125:437–443. doi: 10.1016/s0016-5085(03)00907-7. [DOI] [PubMed] [Google Scholar]
- 7.Wieckowska A, Zein NN, Yerian LM, Lopez AR, McCullough AJ, Feldstein AE. In vivo assessment of liver cell apoptosis as a novel biomarker of disease severity in nonalcoholic fatty liver disease. Hepatology. 2006;44:27–33. doi: 10.1002/hep.21223. [DOI] [PubMed] [Google Scholar]
- 8.Feldstein AE, Gores GJ. Apoptosis in alcoholic and nonalcoholic steatohepatitis. Front Biosci. 2005;10:3093–3099. doi: 10.2741/1765. [DOI] [PubMed] [Google Scholar]
- 9.Feldstein AE, Canbay A, Guicciardi ME, Higuchi H, Bronk SF, Gores GJ. Diet associated hepatic steatosis sensitizes to Fas mediated liver injury in mice. J Hepatol. 2003;39:978–983. doi: 10.1016/s0168-8278(03)00460-4. [DOI] [PubMed] [Google Scholar]
- 10.Malhi H, Gores GJ. Cellular and molecular mechanisms of liver injury. Gastroenterology. 2008;134:1641–1654. doi: 10.1053/j.gastro.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feldstein AE, Wieckowska A, Lopez AR, Liu YC, Zein NN, McCullough AJ. Cytokeratin-18 fragment levels as noninvasive biomarkers for nonalcoholic steatohepatitis: A multicenter validation study. Hepatology. 2009;50:1072–1078. doi: 10.1002/hep.23050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diab DL, Yerian L, Schauer P, Kashyap SR, Lopez R, Hazen SL, et al. Cytokeratin 18 fragment levels as a noninvasive biomarker for nonalcoholic steatohepatitis in bariatric surgery patients. Clin Gastroenterol Hepatol. 2008;6:1249–1254. doi: 10.1016/j.cgh.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Younossi ZM, Jarrar M, Nugent C, Randhawa M, Afendy M, Stepanova M, et al. A novel diagnostic biomarker panel for obesity-related nonalcoholic steatohepatitis (NASH) Obes Surg. 2008;18:1430–1437. doi: 10.1007/s11695-008-9506-y. [DOI] [PubMed] [Google Scholar]
- 14.Malik R, Chang M, Bhaskar K, Nasser I, Curry M, Schuppan D, et al. The clinical utility of biomarkers and the nonalcoholic steatohepatitis CRN liver biopsy scoring system in patients with nonalcoholic fatty liver disease. J Gastroenterol Hepatol. 2009;24:564–568. doi: 10.1111/j.1440-1746.2008.05731.x. [DOI] [PubMed] [Google Scholar]
- 15.Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467–2474. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 16.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 17.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 18.Bedogni G, Miglioli L, Masutti F, Tiribelli C, Marchesini G, Bellentani S. Prevalence of and risk factors for nonalcoholic fatty liver disease: the Dionysos nutrition and liver study. Hepatology. 2005;42:44–52. doi: 10.1002/hep.20734. [DOI] [PubMed] [Google Scholar]
- 19.Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37:917–923. doi: 10.1053/jhep.2003.50161. [DOI] [PubMed] [Google Scholar]
- 20.Feldstein AE, Werneburg NW, Li Z, Bronk SF, Gores GJ. Bax inhibition protects against free fatty acid-induced lysosomal permeabilization. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1339–G1346. doi: 10.1152/ajpgi.00509.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zou C, Ma J, Wang X, Guo L, Zhu Z, Stoops J, et al. Lack of Fas antagonism by Met in human fatty liver disease. Nat Med. 2007;13:1078–1085. doi: 10.1038/nm1625. [DOI] [PubMed] [Google Scholar]
- 22.Machado M, Marques-Vidal P, Cortez-Pinto H. Hepatic histology in obese patients undergoing bariatric surgery. J Hepatol. 2006;45:600–606. doi: 10.1016/j.jhep.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 23.Mathurin P, Gonzalez F, Kerdraon O, Leteurtre E, Arnalsteen L, Hollebecque A, et al. The evolution of severe steatosis after bariatric surgery is related to insulin resistance. Gastroenterology. 2006;130:1617–1624. doi: 10.1053/j.gastro.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 24.Ratziu V, Charlotte F, Heurtier A, Gombert S, Giral P, Bruckert E, et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128:1898–1906. doi: 10.1053/j.gastro.2005.03.084. [DOI] [PubMed] [Google Scholar]
- 25.Merriman RB, Ferrell LD, Patti MG, Weston SR, Pabst MS, Aouizerat BE, et al. Correlation of paired liver biopsies in morbidly obese patients with suspected nonalcoholic fatty liver disease. Hepatology. 2006;44:874–880. doi: 10.1002/hep.21346. [DOI] [PubMed] [Google Scholar]
- 26.Janiec DJ, Jacobson ER, Freeth A, Spaulding L, Blaszyk H. Histologic variation of grade and stage of non-alcoholic fatty liver disease in liver biopsies. Obes Surg. 2005;15:497–501. doi: 10.1381/0960892053723268. [DOI] [PubMed] [Google Scholar]