Abstract
Th17 play a central role in autoimmune inflammatory responses. Th1 are also necessary for autoimmune disease development. The interplay of Th1 signals and how they coordinate with Th17 during inflammatory disease pathogenesis are incompletely understood. In this study, by adding Stat4 deficiency to Stat6/T-bet double knockout, we further dissected the role of Stat4 in Th1 development and colitis induction. We showed that in the absence of the strong Th2 mediator Stat6, neither Stat4 nor T-bet is required for IFN-γ production and Th1 development. However, addition of Stat4 deficiency abolished colitis induced by Stat6/T-bet double-knockout cells, despite Th1 and Th17 responses. The failure of colitis induction by Stat4/Stat6/T-bet triple-knockout cells is largely due to elevated Foxp3+ regulatory T cell (Treg) development. These results highlight the critical role of Stat4 Th1 signals in autoimmune responses in suppressing Foxp3+ Treg responses and altering the balance between Th17 and Tregs to favor autoimmune disease.
Mice deficient in the Th1 transcription factors Stat4 or T-bet are severely impaired in their ability to produce IFN-γ and Th1 cells and are resistant to the development of experimental autoimmune encephalomyelitis or colitis (1–4). Thus IL-12, T-bet, and Th1 effector cells were initially suggested to be important in the molecular pathogenesis of inflammatory responses and autoimmune diseases (2, 5, 6). Later studies demonstrated that colitis is due more to the effects of IL-23 and Th17 cells than to Th1 cells and cytokines (7, 8). However, there are increased levels of IL-17 when T-bet is absent (9–11), which fail to induce colitis (2, 11), suggesting that Th1 responses may still be involved in the pathogenesis of autoimmunity. It is not clear how Stat4, T-bet, and Th1 signals coordinate with IL-17 and Th17 signals during autoimmune disease pathogenesis.
Regulation of IFN-γ production and Th1 development of CD4 T cells is mainly mediated by IFN-γ/Stat1/T-bet– and IL-12/Stat4–signaling pathways (12–17). We and other investigators showed that in addition to directly regulating IFN-γ and Th1 differentiation, T-bet is critical to prevent the IL-4/Stat6/GATA-3 Th2 signal cascade from suppressing IL-12Rβ2 expression and, thus, maintain the IL-12/Stat4–signaling pathway for IFN-γ regulation (11, 14). Restoration of IL-12/Stat4 signals by adding Stat6 deficiency to T-bet deficiency allows Th1 development and colitis induction (11), pointing to an important role for IL-12/Stat4 in Th1 and autoimmune pathogenesis. The direct role of Stat4 in Th1 development and autoimmunity requires further clarification.
Foxp3+ regulatory T cells (Tregs) are central in immune regulation (18, 19), and TGF-β plays a critical role in inducing and maintaining Tregs and controlling autoimmune disease pathogenesis (20–25). Treg and Th17 developmental pathways are reciprocally regulated (26–29), so that TGF-β plus IL-6 drive Th17 pathways (9, 10, 26–28, 30). Tregs were suggested to be one of the main sources for TGF-β in Th17 differentiation (25), and the Th17 cytokines IL-21 and IL-23 suppress Foxp3 induction and Treg generation (31, 32). Th1 signals were shown to negatively regulate Th17 and Treg development. Mice defective in Th1 development have decreased Th1 but increased numbers of Th17 cells (9–11, 33). IL-12/Stat4 Th1 signals inhibit TGF-β from inducing Treg, through the indirect effects of IFN-γ/T-bet (34) and the direct effects of Stat4 binding to the Foxp3 locus (35). It has not been fully determined whether and how IL-12/Stat4 signals affect the balance of Th17 and Treg and autoimmune pathogenesis.
To dissect the precise role of IL-12/Stat4 in Th1, Th17, Treg development, and autoimmune colitis, we generated Stat4/Stat6/T-bet triple-knockout (TKO) mice. Our data revealed that neither Stat4 nor T-bet is necessary for IFN-γ production and Th1 development in an adoptive-transfer model of colitis. However, Stat4 is essential for CD4 T cells to be pathogenic. Without Stat4, Th17 development decreased, whereas Treg development increased. Our data indicated a critical role for Stat4 in skewing Th17 and Treg responses and autoimmune disease.
Materials and Methods
Mice and reagents
Stat4/Stat6/T-bet TKO mice were generated from Stat4-deficient (BALB/c background, The Jackson Laboratory, Bar Harbor, ME) and Stat6/T-bet double-knockout (DKO) (BALB/c background) (11) mice. C.B-17 SCID mice were purchased from Taconic Farms (Hudson, NY). Recombinant mouse IL-2, IL-4, IL-6, IL-12, IFN-γ, PE–anti–IL-17A, allophycocyanin–anti-CD11c, and PE–anti-CD103 mAbs were purchased from BD Pharmingen (San Diego, CA). rTGF-β was purchased from R&D Systems (Minneapolis, MN). Recombinant mouse IL-23, IL-27, anti-CD3ε, anti-CD28, anti–IFN-γ, anti–IL-12, allophycocyanin–anti-CD4, PE–anti-CD4, FITC–anti-CD45RB, PE–anti–IL-13, FITC–anti–IL-10, PE–anti–IL-4, PE–anti–IFN-γ, FITC–anti–IFN-γ, FITC–anti-Foxp3 mAbs, intracellular staining kit, monesin solution, and cell proliferation dye eFluor 670 were purchased from eBioscience (San Diego, CA). PMA and ionomycin were purchased from Sigma-Aldrich (St. Louis, MO).
T cell purification, activation, intracellular staining, and ELISA
CD4+CD25− T cells were selected by flow cytometric sorting and seeded in 96-well round-bottom plates (5 × 104 cells/well). Cells were stimulated with plate-bound anti-CD3ε (5 µg/ml) and soluble anti-CD28 (1 µg/ml), along with various cytokines. After 3 d of culture, ELISA for IFN-γ was performed using the mouse IFN-γ ELISA Kit (eBioscience). Intracellular staining for Foxp3 expression was performed following the manufacturer’s protocol (eBioscience). Intracellular cytokine staining was performed after restimulation with PMA (100 ng/ml) and ionomycin (500 ng/ml) in the presence of monesin (1/1000 dilution) for 4 h. Analytical flow cytometry was performed with a FACSCanto (BD Biosciences).
Induction of colitis in SCID mice by adoptive cell transfer
CD4 T cells were enriched from spleens using a CD4 negative isolation kit (Invitrogen, Carlsbad, CA) and labeled with allophycocyanin–anti-CD4, PE–anti-CD25, and FITC–anti-CD45RB mAbs. CD4+CD25−CD45RBhigh cells were sorted by MoFlo (Dako Cytomation, Carpinteria, CA). A total of 4 × 105 cells in 200 µl PBS was injected i.p. into each recipient.
Histology and fluorescent immunohistochemistry
Colons were cut into small portions, fixed in 10% formalin in PBS (Fisher Scientific, Pittsburgh, PA), embedded in paraffin wax, and stained with H&E. Histologic sections were examined and scored using the following scale: grade 0, no evidence of inflammation; grade 1, low level of leukocyte infiltration, minimal structural changes; grade 2, moderate leukocyte infiltration, crypt elongation, bowel wall thickening but not extending beyond the mucosal layer; grade 3, high level of leukocyte infiltration, infiltration beyond the mucosal layer, and superficial ulcerations; and grade 4, marked degree of transmural leukocyte infiltration, elongated and distorted crypts, and extensive ulcerations. Frozen tissue sections of 7–8 µm thickness from colon, mesenteric lymph node (MLN), or thymus were collected onto glass slides in a cryostat. The slides were air dried, fixed in acetone at −20°C, blocked with 5% normal goat serum, and stained with primary Abs: rat anti-mouse CD4, Armenian hamster anti-mouse CD11c (BD Pharmingen), or rat anti-mouse Foxp3 (eBioscience), followed by fluorophore-conjugated secondary Abs (Cy3-goat anti-rat IgG, Cy5-goat anti-Armenian hamster IgG) (Jackson ImmunoResearch Laboratories, West Grove, PA) in the dark at room temperature. For additional direct immunofluorescence staining, the slides were further blocked with 20% normal rat serum and stained with FITC-rat anti-mouse CD68 (AbD Serotec, Raleigh, NC) or allophycocyanin-rat anti-mouse CD4 (eBioscience). The slides were mounted with Fluoro Gel II with DAPI (Electron Microscopy Sciences, Hatfield, PA), and coverslips were applied. Images were acquired by a Leica DMRA2 fluorescence microscope with a Hamamatsu digital charge-coupled device camera and analyzed by Volocity software.
T cell proliferation and migration assay
CD4 T cells were enriched and labeled with PE–anti-CD4, FITC–anti-CD45RB, and cell-proliferation dye eFluor 670. eFluor 670-labeled CD4+CD45RBhigh cells were sorted and injected i.p. into each SCID mouse. Proliferation analysis was performed on gated CD4 cells from MLN, spleen, and thymus by flow cytometry 5 or 15 d later. Frozen tissue sections were viewed under a fluorescence microscope using a Cy5 filter set for eFluor 670.
Quantitative real-time RT-PCR
Total RNA was isolated using an RNeasy Protect Mini Kit (Qiagen, Valencia, CA) and treated with DNAse I (Invitrogen), and cDNA was reverse transcribed using an Omniscript RT kit (Qiagen) with random primers. The primers for PCR were: cyclophilin A: 5′-AGG GTG GTG ACT TTA CAC GC-3′ and 5′-ATC CAG CCA TTC AGT CTT GG-3′; c-Maf: 5′-AGC AGT TGG TGA CCATGT CG-3′ and 5′-TGG AGATCT CCT GCT TGA GG-3′; IL-10: 5′-AAC TGC ACC CAC TTC CCA GTC-3′ and 5′-CAT TAA GGA GTC GGT TAG CAG-3′; IL-4: 5′-GAA GCC CTA CAG ACG AGC TCA-3′ and 5′-ACA GGA GAA GGG ACG CCAT-3′; IFN-γ: 5′-TGG CTC TGC AGG ATT TTC ATG-3′ and 5′-TCA AGT GGC ATA GAT GTG GAA GAA-3′; IL-17A: 5′-CTC CAG AAG GCC CTC AGA CTA-3′ and 5′-AGC TTT CCC TCC GCA TTG ACA-3′; and Eomes: 5′-TAC GGC CAG GGT TCT CCG CTC TAC-3′ and 5′-GGG CCG GTT GCA CAG GTA GAC GTG-3′. Each reaction was performed with the LightCycler system (Roche, Indianapolis, IN) and the SYBR Green PCR kit (Qiagen). All experiments were performed at least three times. Amplification of the cyclophilin A gene was used as a housekeeping gene.
Coculture of CD4 T cells and CD11chigh subsets
Wild-type MLN dendritic cells (DCs) were first enriched using pan T (Thy1.2) and pan B (B220) Dynabeads (Invitrogen) to deplete Tand B cells. CD103− or CD103+ CD11chigh DCs were then sorted by a MoFlo sorter gated on CD3− cells. A total of 1 × 104 CD103− or CD103+ MLN CD11chigh DCs and 5 × 104 CD4+CD25− T cells from Stat6/T-bet DKO or Stat4/Stat6/T-bet TKO mice were seeded in 96-well round-bottom plates and cultured in the presence of anti-CD3 mAb (1 µg/ml) and IL-2 (1 ng/ml). On day 3, intracellular staining for Foxp3, IL-17, and IFN-γ expression was performed and analyzed on gated CD4 cells. ELISA for IL-12 (p70) and IL-27 was performed using the mouse IL-12 p70 Platinum and IL-27 ELISA Kits (eBioscience).
Statistical analysis
Statistical significances between groups were determined by the Student t test; p < 0.05 was considered to indicate a significant difference.
Results
Stat4/Stat6/T-bet TKO model for dissecting the role of IL-12/Stat4 in Th cell development
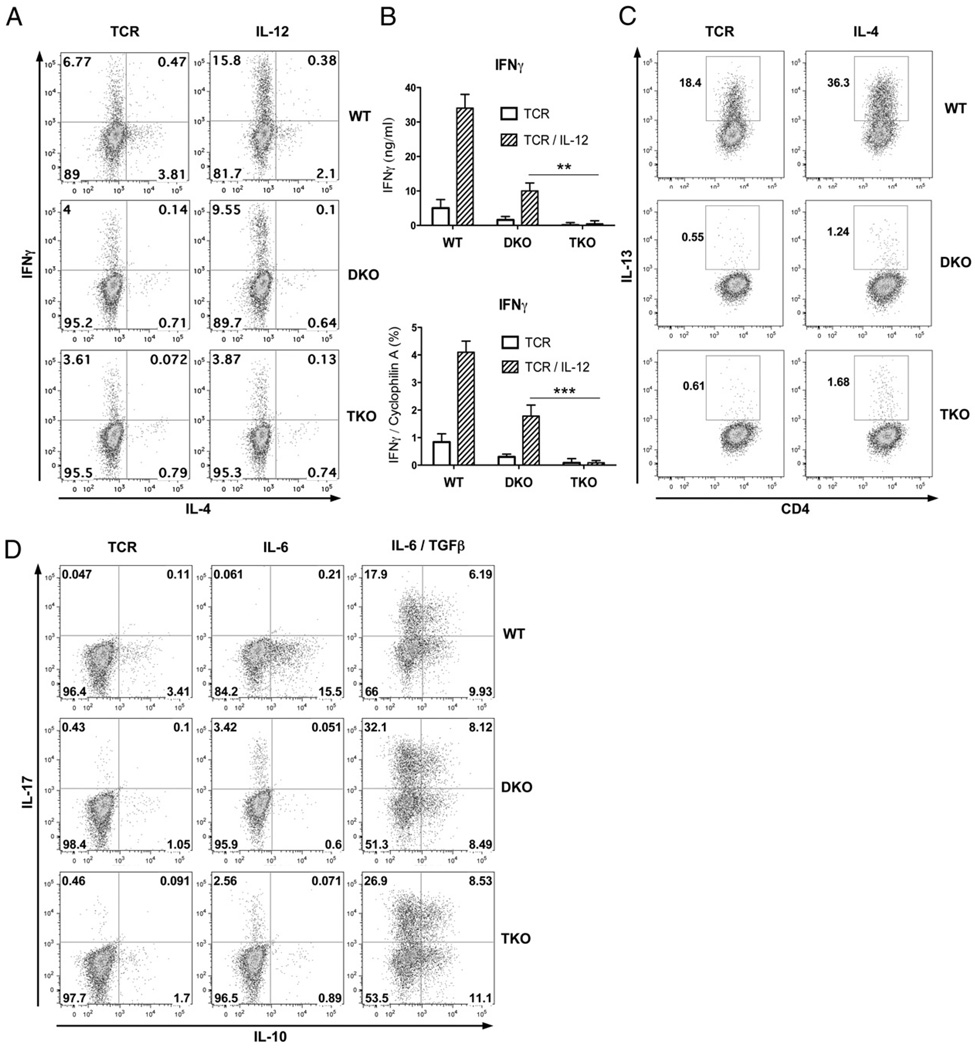
Our recent studies showed that adding Stat6 deficiency to T-bet deficiency to limit interference from elevated levels of IL-4/Stat6/GATA-3 Th2-polarizing signals restored the IL-12/Stat4 Th1-development pathway (11). To further determine the precise role of IL-12 signals in IFN-γ production and Th1 development, we added Stat4 deficiency to Stat6/T-bet DKO and generated Stat4/Stat6/T-bet TKO mice. Stat4/Stat6/T-bet TKO mice are healthy, and the distribution of CD4 and CD8 T cells in thymus, spleen, and lymph nodes, as well as CD4 T cell surface markers CD62L, CD25, CD44, CD45RB, and CD69, were comparable to wild-type mice (data not shown). CD4+CD25− T cells from wild-type, Stat6/T-bet DKO, and Stat4/Stat6/T-bet TKO mice were purified and stimulated with plate-bound anti-CD3 plus soluble anti-CD28 mAbs, with or without IL-12 or IL-4. After 3 d of culture, intracellular staining was performed to measure IFN-γ (Th1) or IL-13 (Th2) production. As shown in Fig. 1A, addition of Stat4 deficiency abolished IL-12–mediated IFN-γ production. Similar results were confirmed by ELISA and quantitative RT-PCR (Fig. 1B). There was very limited IL-13 expression after IL-4 stimulation in DKO or TKO cells (Fig. 1C), indicating that even relieving Stat4 and T-bet Th1-inhibitory signals did not result in Th2 development, demonstrating the essential role of IL-4/Stat6 in Th2 development. We next examined whether Stat4 deficiency affected Th17 development. As shown in Fig. 1D, IL-6 alone induced similar IL-17 production in DKO and TKO cells. Similar IL-10 expression was observed in IL-6/TGF-β DKO or TKO treated cells, demonstrating that addition of Stat4 deficiency did not alter Th17 development or their capacity for IL-10 expression. These data indicated that Stat4/Stat6/T-bet TKO cells provided a viable model for dissecting the roles of IL-12/Stat4 in Th1, Th2, and Th17 effector development and inflammatory responses.
FIGURE 1.
Stat4/Stat6/T-bet TKO have Th17 but not Th1 or Th2 responses. A and B, Stat4 deficiency abolished IL-12–mediated IFN-γ production. Wild-type, Stat6/T-bet DKO, and Stat4/Stat6/T-bet TKO CD4+CD25− T cells were stimulated with plate-bound anti-CD3 (5 µg/ml) and soluble anti-CD28 (1 µg/ml), with or without IL-12 (10 ng/ml). Quantitative RT-PCR for IFN-γ expression was performed on day 2; ELISA for IFN-γ and intracellular staining for IFN-γ and IL-4 were performed on day 3. **p < 0.01, p < 0.001. C, Stat4 deficiency did not alter Th2 differentiation. Intracellular staining for IL-13 in CD4+CD25− T cells after anti-CD3 plus anti-CD28 stimulation, with or without IL-4 (20 ng/ml) for 3 d. D, Stat4 deficiency did not alter composition of IL-10 expression in Th17 cells. Intracellular IL-10 and IL-17 staining in CD4+CD25− T cells after 3 d of stimulation with anti-CD3 and anti-CD28 combined with IL-6 (10 ng/ml) or IL-6 plus TGF-β (5 ng/ml).
Stat4 is essential for colitis induction
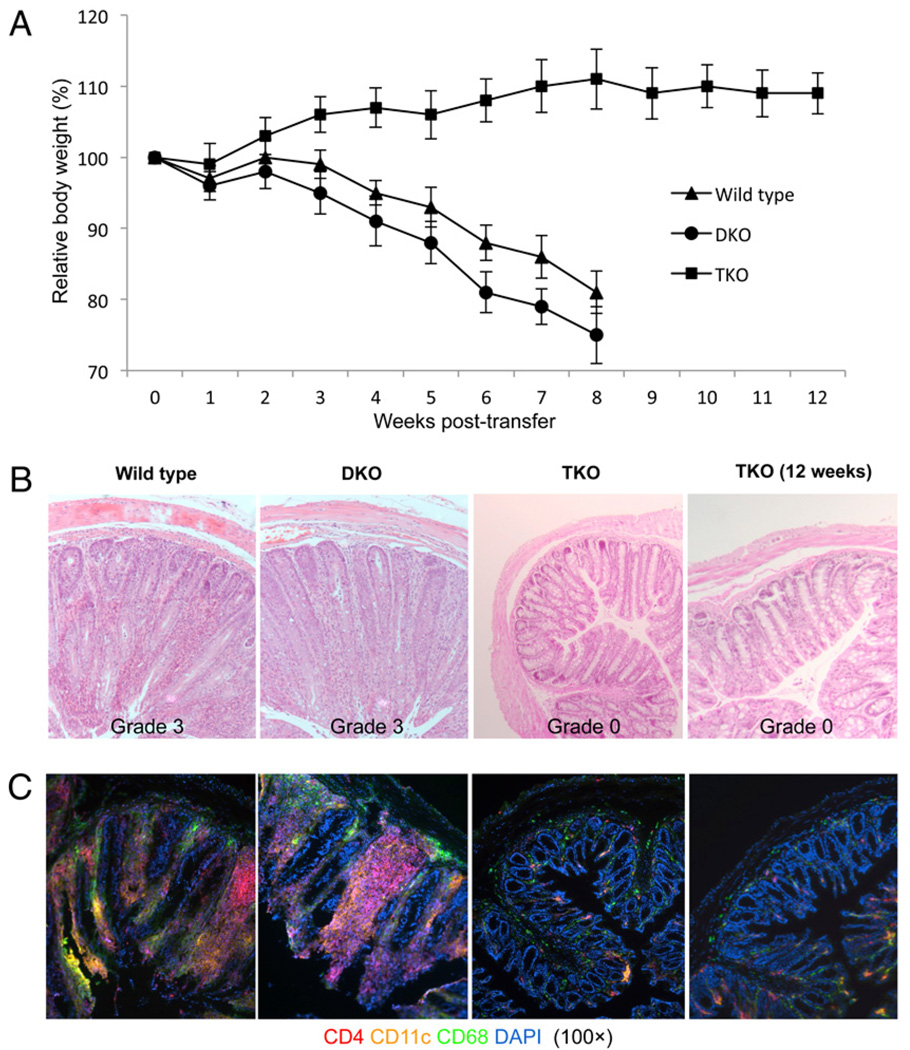
We next examined the role of IL-12/Stat4 signals in autoimmune inflammatory disease pathogenesis in vivo. We chose the adoptive-transfer colitis model, in which CD4+CD25−CD45RBhigh cells were transferred into SCID mice, thus focusing on naive CD4 T cells and eliminating the potential confounding effects of Stat4, Stat6, or T-bet deficiency on APCs, and the pathological features of this model resemble those observed in human Crohn’s disease. We first examined how Stat4 deficiency affected colitis development. Wild-type, DKO, and TKO CD4+CD25−CD45RBhigh T cells were purified and transferred into SCID mice, and body weights were monitored. As shown in Fig. 2A, recipients of wild-type and DKO CD4 T cells had progressive weight loss over 8 wk, whereas Stat4 deficiency in TKO T cells abolished wasting disease. Histologic analysis (Fig. 2B) showed that Stat4 deficiency resulted in diminished colonic inflammation. Immunohistochemistry for CD4, CD11c, and CD68 (Fig. 2C) showed decreased numbers of CD4 T cells, DCs, and macrophages in the recipients of TKO cells. No colitis was observed in mice receiving TKO cells for up to 12 wk (Fig. 2A–C), indicating that failure of colitis development was probably not due to a delayed response. These data indicated that Stat4 signals are critical for determining whether CD4 T cells are pathologically active.
FIGURE 2.
Addition of Stat4 deficiency prevents colitis by Stat6/T-bet DKO cells. A, Relative body weight of SCID mice after transfer of 4 × 105 wildtype, DKO, or TKO CD4+CD25−CD45RBhigh cells. H&E staining (B) and fluorescent immunohistochemistry (C) for CD4, CD11c, and CD68 in the distal colons of SCID mice 8 or 12 wk after T cell reconstitution (original magnification ×100). Representative sections are shown from 10 mice in each group. Histological scoring of colitis was described in Materials and Methods.
Neither Stat4 nor T-bet is required for Th1 development in vivo
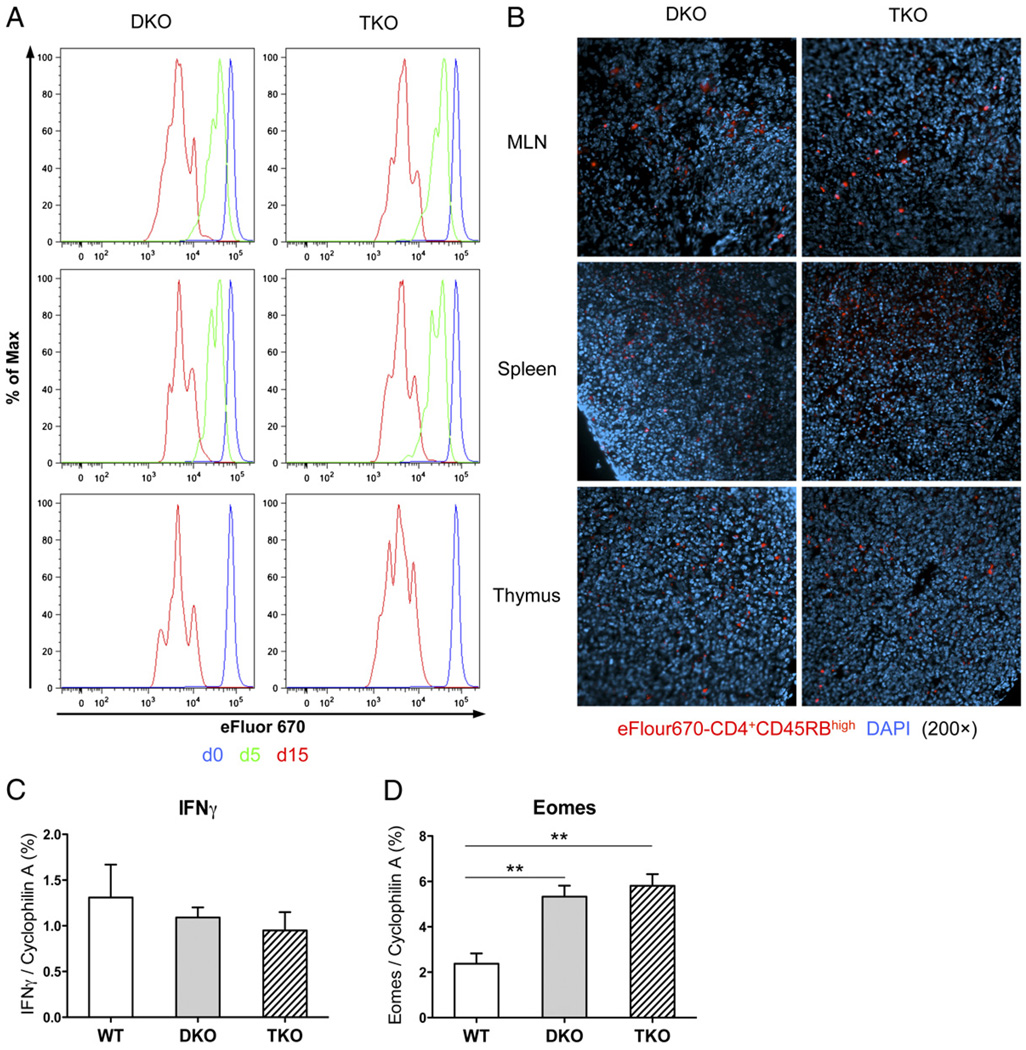
To determine whether Stat4 deficiency affected CD4 T cell distribution, migration, or proliferation in vivo, which consequently resulted in their different pathogenic capacities, eFluor 670-labeled DKO or TKO CD4+CD45RBhigh cells were sorted and transferred into SCID mice. Five or 15 d later, flow cytometry was performed on CD4 T cells from MLN, spleen, and thymus. As shown in Fig. 3A, there were no differences in CD4 expression or proliferation between mice receiving DKO and TKO cells. DKO and TKO cells were equally distributed in MLN, spleen, or thymus (Fig. 3B). These results showed that Stat4 deficiency did not affect migratory or proliferative properties of TKO CD4 T cells.
FIGURE 3.
Neither Stat4 nor T-bet is required for Th1 development. A, Cell distribution, migration, and proliferation after adoptive transfer. eFluor 670-labeled CD4+CD45RBhigh cells from DKO or TKO mice were injected into SCID mice and harvested 5 or 15 d later, and proliferation analysis was performed on gated CD4 cells from MLN, spleen, and thymus. B, Distribution of eFluor 670-labeled CD4+CD45RBhigh cells in MLN, spleen and thymus 5 d after T cell reconstitution (original magnification ×200). Representative sections from five mice in each group are shown. C and D, Quantitative RT-PCR for IFN-γ and Eomes expression in MLN CD4 T cells purified 8 wk after adoptive transfer. **p < 0.01.
We next determined whether failure of colitis induction by TKO CD4 T cells was due to diminished IFN-γ production and Th1 development. MLN CD4 T cells were purified, and IFN-γ expression was examined by quantitative RT-PCR. Surprisingly, there was similar IFN-γ expression by recipients of TKO or DKO cells (Fig. 3C), indicating that abolishing IL-12/Stat4 signals did not affect IFN-γ production in vivo. We and other investigators showed that Eomes plays a complementary role for T-bet in IFN-γ regulation (11, 36–39). Eomes expression was examined in MLN CD4 T cells (Fig. 3D), and significantly higher levels were observed in recipients of DKO or TKO cells compared with wild-type cells, supporting a role for Eomes in IFN-γ production and Th1 development. Overall, the results showed equal IFN-γ and Th1 in DKO and TKO cells; therefore, failure to induce colitis was not due to changes in Th1 development.
Stat4 is required for optimal Th17 response in vivo
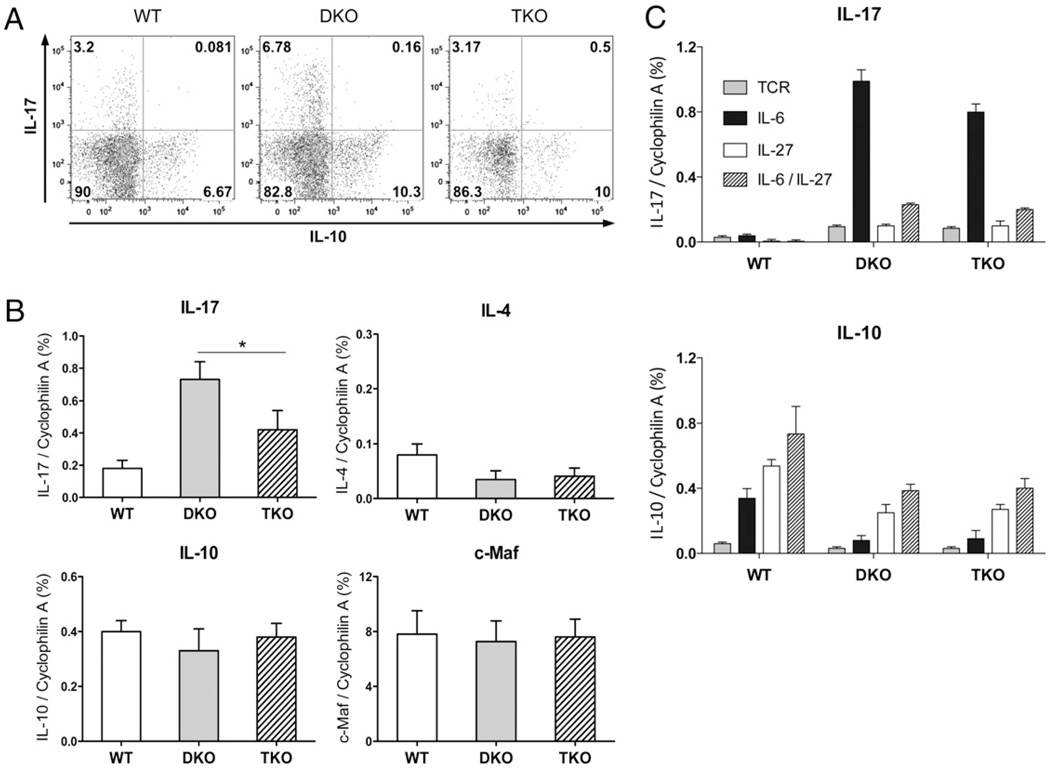
Although Fig. 1D showed similar IL-17 expression by DKO and TKO cells in vitro, we next determined how Stat4 deficiency affected Th17 development in vivo. Intracellular staining and quantitative RT-PCR of MLN CD4 T cells (Fig. 4A, 4B) showed significant IL-17 expression in wild-type cells. DKO cells expressed more IL-17, confirming that Stat6/T-bet double deficiency favored Th17 development (11). Stat4 deficiency did not abolish Th17 responses; however, there was 50% less IL-17 in TKO cells compared with DKO cells. This suggested that Stat4 played a positive regulatory role in IL-17 and Th17 responses for colitis induction. There was very little IL-4 expression (Fig. 4B) in wild-type, DKO, or TKO T cells, indicating that Stat4 deficiency did not alter IL-4/Th2 responses, and reduced IL-17 expression was not due to suppressive effects from IL-4 Th2 signals.
FIGURE 4.
Stat4 is required for optimal IL-17 but not IL-4 or IL-10 expression. A, Eight weeks after cell transfer, T cells were isolated from MLN and restimulated with PMA and ionomycin in the presence of monesin for 4 h, and IL-17 and IL-10 expression on gated CD4 cells was measured by intracellular staining. B, Real-time RT-PCR for IL-17, IL-4, IL-10, and c-Maf expression in MLN CD4 T cells. *p < 0.05. C, Real-time RT-PCR for IL-17 and IL-10 expression in wild-type, DKO, and TKO CD4+CD25− T cells after 2 d of culture with anti-CD3 and anti-CD28, combined with IL-6, IL-27, or IL-6 plus IL-27.
IL-10 was demonstrated to be critical for suppressing autoimmunity and inflammatory responses (40–43), and whether Th17 express IL-10 is crucial in determining their protective or pathological consequences (44, 45). We examined whether Stat4 affected IL-10 production in vivo. There was similar IL-10 expression in DKO and TKO CD4 T cells, and no IL-17 cells produced IL-10, indicating that Stat4 deficiency did not alter total IL-10 or the composition of IL-17+IL-10+ nonpathogenic versus IL-17+IL-10− colitogenic Th17 cells (Fig. 4A, 4B). c-Maf was recently shown to play an important regulatory role in IL-10 and IL-17 expression (46–48). There was no difference in c-Maf expression between DKO and TKO T cells, indicating that reduced IL-17 expression was not due to altered c-Maf expression (Fig. 4B).
IL-27 shares the common gp130 signal receptor chain with IL-6, and similar to IL-6, it activates Stat3 and Stat1. However, IL-27 suppresses Th17 development. We examined whether Stat4 affected the response to IL-27. In vitro stimulation showed that IL-27 suppressed Th17 while promoting IL-10 expression in wild-type, DKO, and TKO CD4+CD25− T cells, and the responses of DKO and TKO cells were similar to each other (Fig. 4C). Thus, Stat4 deficiency did not alter the IL-17 or IL-10 responses to IL-27.
Stat4 deficiency results in higher levels of Foxp3+ Tregs
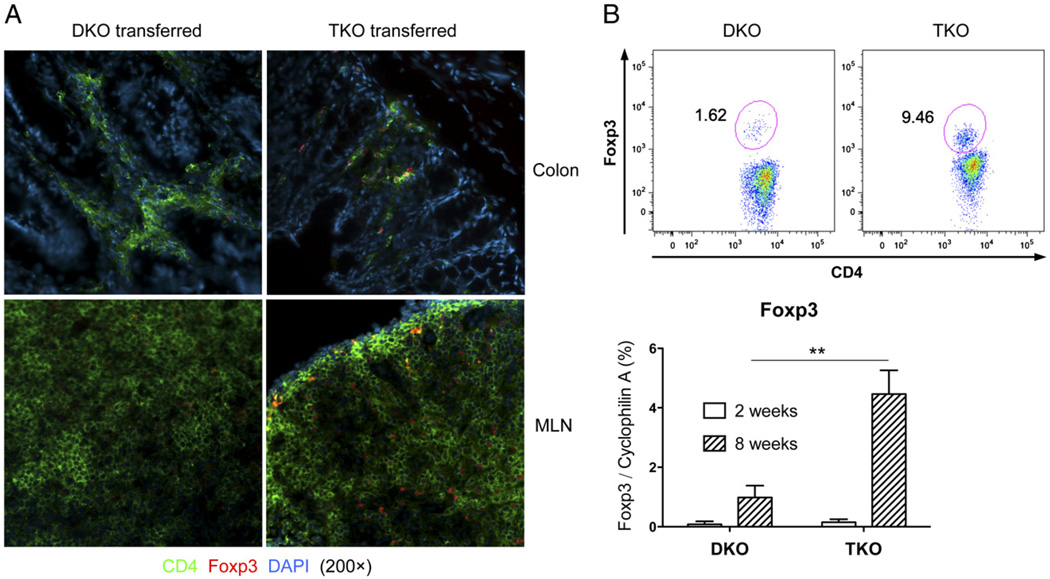
Foxp3+ Tregs play a critical role in controlling immune responses, and IL-12/Stat4 was implicated in promoting Th1 and suppressing Treg development (34, 35). We next determined whether Stat4 affected the Treg response. Immunohistochemistry was performed on colons and MLNs of mice receiving TKO or DKO cells. There were striking differences in the numbers of CD4+Foxp3+ Tregs in the two groups (Fig. 5A). Flow cytometry and quantitative RT-PCR further confirmed greater numbers of Foxp3+ cells and levels of Foxp3 expression in TKO CD4 T cells than in DKO MLN CD4 T cells (Fig. 5B), indicating that Stat4 deficiency allowed a more robust Foxp3+ Treg response. Very low levels of Foxp3 were observed in MLNs 2 wk after transfer (Fig. 5B), and IL-12Rβ2–deficient CD4+CD25+ Tregs had less proliferation capacity than wild-type Tregs (49), indicating that the high numbers of Foxp3+ cells observed in TKO recipients were probably not due to contamination of Foxp3+ cells during sorting, or TKO cells intrinsically favored Foxp3+ Treg expansion.
FIGURE 5.
Stat4 deficiency results in higher levels of Foxp3+ Tregs. A, Immunohistochemistry for CD4 and Foxp3 in the distal colons and MLN in SCID mice 8 wk after T cell reconstitution (original magnification ×200). Representative sections are shown from 10 mice in each group. B, Intracellular staining for Foxp3 on gated MLN CD4 cells 8 wk after adoptive transfer (upper panels) and real-time RT-PCR for Foxp3 expression on MLN CD4 T cells 2 or 8 wk after adoptive transfer (lower panel). **p < 0.01.
IL-12/Stat4 signals alter the balance between Th17 and Treg development
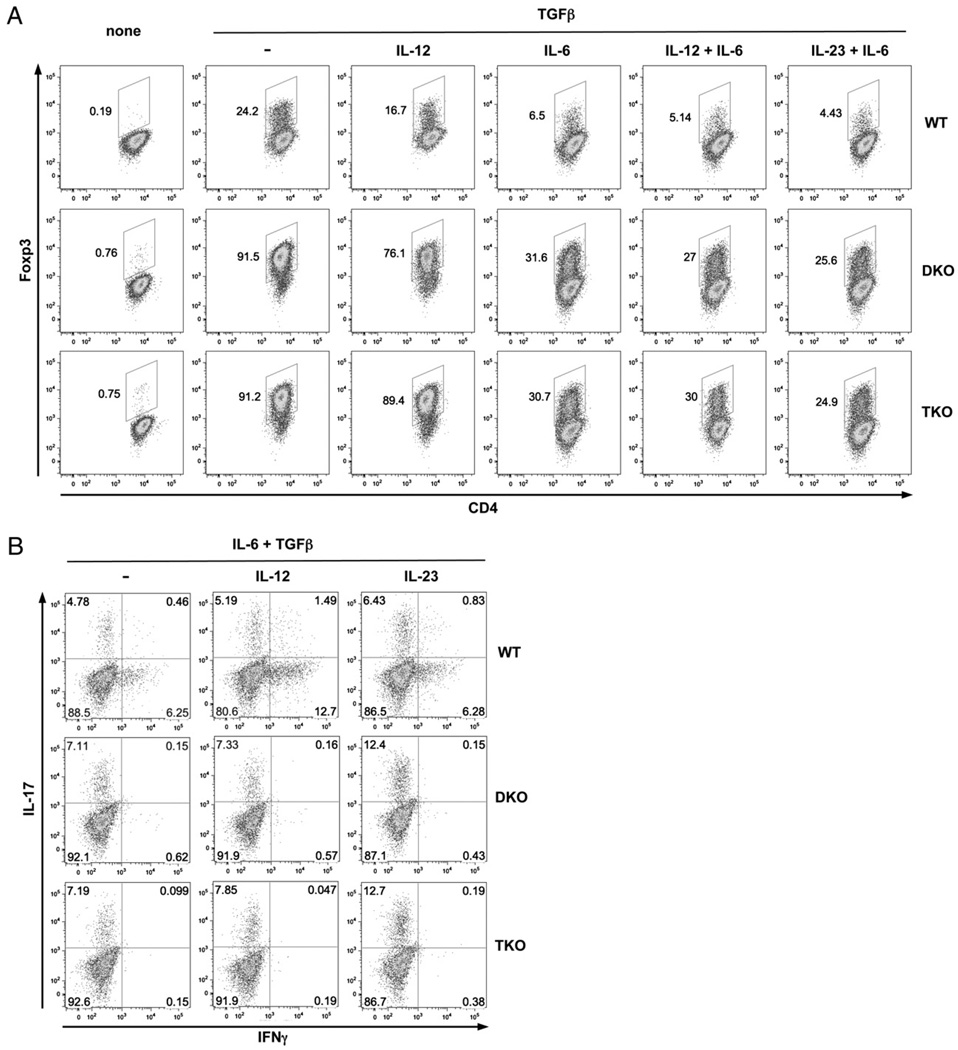
The selective effects of Stat4 in the suppression of Tregs while promoting Th17 in vivo prompted us to examine how IL-12/Stat4 signals affected the balance of Th17 and Treg development. Because IL-12 also influences APC cytokine production, which, in turn, influences induction of Tregs and Th17, we used an APC-free culture system. Because resting CD4 T cells express little IL-12Rβ2, and IFN-γ is critical for IL-12Rβ2 induction (50), it was possible that differential IFN-γ production by various cells could lead to differential IL-12R expression and, thus, cloud the interpretation of direct effects of IL-12/Stat4. Therefore, we also added exogenous IFN-γ to the cultures. As shown in Fig. 6A, TGF-β induced very high numbers of Foxp3+ cells in DKO and TKO cells compared with wild-type CD4 T cells. These results confirmed that Th1 and Th2 signals suppress Foxp3 expression and demonstrated that Stat4 did not regulate the intrinsic responses of CD4 T cells to TGF-β for Foxp3 expression. Exogenous IL-12 suppressed Foxp3 expression in wild-type (31% inhibition) or DKO (17% inhibition) cells but not in TKO (2% inhibition) cells. IL-6 profoundly suppressed Foxp3 expression (Fig. 6A) in wild-type (73%), DKO (65%), and TKO (66%) cells, indicating that Stat4 deficiency did not alter the response to IL-6. The combination of IL-12 plus IL-6 resulted in additive suppression of Foxp3 in wild-type (79%) and DKO (70%) cells but not in TKO (67%) cells (Fig. 6A), pointing to different mechanisms for Foxp3 suppression by IL-12/Stat4 signals and IL-6 signals. The combination of IL-23 plus IL-6 inhibited Foxp3 in all cells, with DKO and TKO having similar responses (Fig. 6A), also indicating that IL-12–mediated Foxp3 suppression was not due to secondary effects of IL-23. Intracellular staining for IL-17 on the same cells showed that IL-12 had little effect on IL-17 expression by IL-6 plus TGF-β or on the ability of IL-23 to promote Th17 development (Fig. 6B). Thus, changes in Foxp3 expression were not directly linked to changes in IL-17 expression. Together, these results demonstrated that IL-12/Stat4 Th1 signals specifically suppressed Foxp3.
FIGURE 6.
IL-12/Stat4 signals alter the balance between Th17 and Tregs. A, IL-12/Stat4 suppresses Foxp3 expression. Wild-type, DKO, and TKO CD4+CD25− T cells were stimulated with anti-CD3 and anti-CD28 in the presence of low-dose IFN-γ (1 ng/ml) for 3 d. Intracellular staining for Foxp3 was performed under Treg conditions (TGF-β), with or without IL-12, IL-6, IL-12 plus IL-6, or IL-23 plus IL-6. B, IL-12 does not affect Th17 development. Intracellular IL-17 and IFN-γ expression were measured under Th17 conditions (IL-6 + TGF-β), with or without IL-12 or IL-23. Data are representative of three independent experiments with similar results.
Stat4 differentially affects Treg and Th17 development by mesenteric DCs
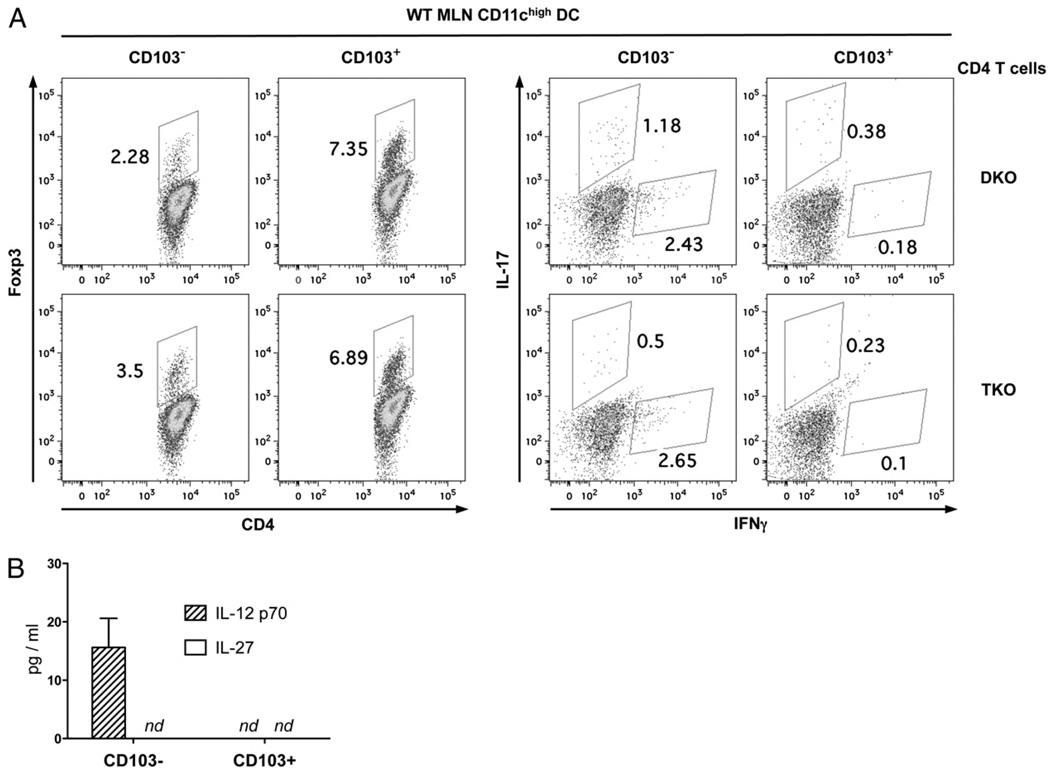
It was demonstrated that two functionally distinct CD11chigh DC subsets reside in the MLNs: CD103+CD11chigh DCs drive Treg development, whereas CD103−CD11chigh DCs promote the inflammatory response (51–53). To further investigate how IL-12/ Stat4 signals affect Treg and Th17 responses, CD103+ or CD103− MLN CD3−B220−NK1.1−CD11chigh DCs were isolated from wild-type BALB/c mice and cultured with DKO or TKO CD4+ CD25− T cells in the presence of anti-CD3 mAb plus IL-2, and intracellular staining for Foxp3, IL-17, and IFN-γ was performed after 5 d. As shown in Fig. 7A, CD103+CD11chigh DCs induced two to three times more Foxp3+ cells than did CD103−CD11chigh DCs, confirming that CD103+CD11chigh DCs favor Treg development (51–53). In contrast to previous observations with wild-type CD4 T cells (51–53), we also observed that CD103−CD11chigh DCs induced some Foxp3 in DKO and TKO cells, supporting observations that limiting Th1 and Th2 signals favored Treg development. Importantly, Stat4 deficiency resulted in higher levels of Foxp3 expression (54% increase) by CD103−, but not CD103+, CD11chigh DCs, suggesting that Stat4 activation by CD103−CD11chigh DCs negatively regulates Foxp3+ Treg development. CD103−, but not CD103+, CD11chigh DCs induced significant IFN-γ and IL-17 expression in DKO cells, and Stat4 deficiency resulted in reduced levels of IL-17 but not IFN-γ expression (Fig. 7A). Thus, Stat4 was not required for IFN-γ and Th1 development, but it played a role in optimal Th17 development driven by inflammatory CD11chigh DCs. Measurements of IL-27 showed that differential IL-17 production was not due to differential IL-27 production (Fig. 7B). Together, these data demonstrated that Stat4 activation altered the balance between Th17 and Tregs driven by mesenteric CD11chigh DCs.
FIGURE 7.
Stat4 inhibits Tregs while promoting Th17 development driven by CD103− CD11chigh MLN DCs. CD4+CD25− T cells from DKO and TKO mice were cultured with wild-type CD103− or CD103+ MLN CD11chigh DCs in the presence of anti-CD3 (1 µg/ml) and IL-2 (1 ng/ml) for 3 d. A, Intracellular staining for Foxp3, IL-17, and IFN-γ expression was performed and analyzed on gated CD4 cells. B, The amounts of IL-12 p70 and IL-27 in the culture supernatants were measured by ELISA. Data are representative of three independent experiments with similar results. nd, not detected.
Discussion
IL-12/Stat4 signals have long been associated with Th1 development, inflammatory responses, and autoimmune disease pathogenesis, although their precise roles remain incompletely understood. By generating and studying Stat4/Stat6/T-bet TKO cells, we further dissected IL-12/Stat4 in Th1, Th2, Th17, and Treg responses. We showed that IL-12/Stat4 did not necessarily play a direct role in Th1/Th2 polarization; rather, it selectively suppressed Tregs, skewing the balance between Th17 and Treg responses and consequently favoring inflammatory colitis development.
IL-12/Stat4–driven IFN-γ participates in the IFN-γ/Stat1/T-bet Th1-developmental signal cascade, whereas T-bet is required for maintaining IL-12Rβ2 expression and IL-12/Stat4 signal transduction (11, 14). This reciprocal interaction complicates the ability to examine the independent roles of these pathways. Our finding that, after eliminating the inhibitory effects from Th2-development signals by Stat6 deficiency, IFN-γ production was observed in cells deficient in T-bet (Stat6/T-bet DKO) or Stat4 and T-bet (Stat4/Stat6/T-bet TKO), indicated that neither Stat4 nor T-bet is necessary for directly regulating IFN-γ expression and Th1 development. Their functions in Th1 development are mainly for amplifying existing IFN-γ expression and for preventing inhibition by Stat6/GATA-3 Th2 signals. IFN-γ production observed in vivo (Fig. 3C) but not in vitro (Fig. 1A, 1B) implied that, in addition to IL-12, other cytokine(s) may regulate IFN-γ expression. Eomes was shown to be involved in IL-21–mediated CD4 IFN-γ regulation (39), and the observation that IFN-γ production corresponded with elevated Eomes expression (Fig. 3C), suggests a possible role for IL-21/Eomes in IFN-γ production and Th1 development (11, 39). Because it is too difficult to add Eomes deficiency to Stat4/Stat6/T-bet deficiency, our data do not exclude the possibility that additional signal pathways may be involved in IFN-γ and Th1 development in vivo. In contrast to Th1 responses, relieving T-bet and Stat4 Th1 signals did not result in significantly higher levels of Th2 development when Stat6 was absent (Figs. 1C, 4B), indicating a fundamental difference between Th1 and Th2 differentiation; although Th1 responses are driven by multiple pathways, Th2 responses are driven almost exclusively through Stat6.
Stat4 is required for IL-17 production driven by IL-23 plus IL-18 (54), and decreased IL-17 production is observed following Bacteroides fragilis infection (55) and during allergic airway inflammation (56) in Stat4-deficient mice. We found that Stat4 deficiency also resulted in reduced IL-17 production in an inflammatory colitis model. Because similar IFN-γ Th1 and IL-4 Th2 responses were observed in DKO and TKO cells (Figs. 3C, 4B), the reduced IL-17 expression observed in TKO cells was unlikely to be due to secondary effects from Th1 or Th2 signals. Similar to IL-6, IL-27 activates Stat3 and Stat1 (57, 58); however, IL-27 does not induce IL-17 but rather inhibits Th17 development (44, 59, 60). Because IL-27 is produced mostly by APCs, and our model used adoptive transfer of CD4 T cells into the same SCID mice, it is unlikely that reduced IL-17 expression was due to differential IL-27 production by APCs. In addition, similar IL-27 inhibition of IL-17 responses was observed in DKO and TKO cells (Fig. 4C), excluding the possibility of differential responses of DKO and TKO to IL-27. IL-12/Stat4 signals were shown to negatively regulate Th17 development (11, 61, 62). However, we showed that when exogenous IFN-γ was provided and T-bet was absent, IL-12 did not inhibit IL-17 production (Fig. 6B). These data revealed that, in contrast to IFN-γ or T-bet Th1 signals, IL-12/Stat4 have a limited role in directly suppressing IL-17 expression. Overall, IL-12/Stat4 signals do not directly affect IL-17 expression and Th17 development.
IL-10 plays a critical role in suppressing autoimmunity and inflammatory responses, and its production by Th17 cells restrains the pathologic effects of Th17 (44, 45). Stat4 has been implicated in IL-10 regulation by the Notch pathway (63). Similar IL-10 production was observed in mice receiving Stat6/T-bet DKO and Stat4/Stat6/T-bet TKO cells (Fig. 4A, 4B), suggesting that Stat4 does not play a significant role in IL-10 regulation during colitis induction. Our results also showed that neither Th17 IL-10 nor IL-27–induced IL-10 played a role in the failure of colitis induction by Stat4 deficiency.
By examining Stat6/T-bet DKO and Stat4/Stat6/T-bet TKO cells, we were able to show direct inhibitory effects of IL-12/Stat4 on Foxp3 expression (Fig. 6A). IL-23 activates Stat3 and Stat4 and was shown to skew the balance between Th17 and Treg response (32). Because IL-23 suppressed Foxp3 expression equally efficiently in DKO and TKO cells (Fig. 6A), this indicated that IL-23 inhibition was not through Stat4 activation, and the higher levels of Foxp3-expressing cells observed in TKO compared with DKO recipients and cells (Figs. 5, 7) were due to impaired IL-12/Stat4 signals rather than IL-23/Stat4 signals. Therefore, our data showed that, in addition to IL-23/Stat3 signals, IL-12/Stat4 signals skew the balance between Th17 and Tregs. Together, the opposing effects of Th17 and Tregs in autoimmune colitis demonstrated that the balance between Th17 and Tregs is critical in determining the outcome of disease pathogenesis. Because we used cells deficient in multiple transcription factors (Stat4, Stat6, and T-bet), these multiple genetic-deficient cells may have limitations with regard to reflecting or predicting human disease development. Nevertheless, our data showed an important function for IL-12/Stat4 Th1 signals in skewing the balance between Th17 and Tregs by suppressing Tregs, demonstrating the necessity to suppress IL-12/Stat4 Th1 signals for immunotherapy designed to engage the protective functions of Tregs in inflammatory responses.
Acknowledgments
This work was supported by National Institutes of Health Grants R01 AI 62855 (to Y.D.) and R0I AI 41428 (to J.S.B.).
Abbreviations used in this article
- DC
dendritic cell
- DKO
double knockout
- MLN
mesenteric lymph node
- TKO
triple knockout
- Treg
regulatory T cell
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Chitnis T, Najafian N, Benou C, Salama AD, Grusby MJ, Sayegh MH, Khoury SJ. Effect of targeted disruption of STAT4 and STAT6 on the induction of experimental autoimmune encephalomyelitis. J. Clin. Invest. 2001;108:739–747. doi: 10.1172/JCI12563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neurath MF, Weigmann B, Finotto S, Glickman J, Nieuwenhuis E, Iijima H, Mizoguchi A, Mizoguchi E, Mudter J, Galle PR, et al. The transcription factor T-bet regulates mucosal T cell activation in experimental colitis and Crohn’s disease. J. Exp. Med. 2002;195:1129–1143. doi: 10.1084/jem.20011956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bettelli E, Sullivan B, Szabo SJ, Sobel RA, Glimcher LH, Kuchroo VK. Loss of T-bet, but not STAT1, prevents the development of experimental autoimmune encephalomyelitis. J. Exp. Med. 2004;200:79–87. doi: 10.1084/jem.20031819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lovett-Racke AE, Rocchini AE, Choy J, Northrop SC, Hussain RZ, Ratts RB, Sikder D, Racke MK. Silencing T-bet defines a critical role in the differentiation of autoreactive T lymphocytes. Immunity. 2004;21:719–731. doi: 10.1016/j.immuni.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 5.Strober W, Fuss IJ, Blumberg RS. The immunology of mucosal models of inflammation. Annu. Rev. Immunol. 2002;20:495–549. doi: 10.1146/annurev.immunol.20.100301.064816. [DOI] [PubMed] [Google Scholar]
- 6.Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat. Rev. Immunol. 2003;3:521–533. doi: 10.1038/nri1132. [DOI] [PubMed] [Google Scholar]
- 7.Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat. Immunol. 2007;8:345–350. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- 8.Neurath MF. IL-23: a master regulator in Crohn disease. Nat. Med. 2007;13:26–28. doi: 10.1038/nm0107-26. [DOI] [PubMed] [Google Scholar]
- 9.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 10.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang Y, Xu J, Niu Y, Bromberg JS, Ding Y. T-bet and eomesodermin play critical roles in directing T cell differentiation to Th1 versus Th17. J. Immunol. 2008;181:8700–8710. doi: 10.4049/jimmunol.181.12.8700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–669. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 13.Mullen AC, High FA, Hutchins AS, Lee HW, Villarino AV, Livingston DM, Kung AL, Cereb N, Yao TP, Yang SY, Reiner SL. Role of T-bet in commitment of TH1 cells before IL-12-dependent selection. Science. 2001;292:1907–1910. doi: 10.1126/science.1059835. [DOI] [PubMed] [Google Scholar]
- 14.Usui T, Preiss JC, Kanno Y, Yao ZJ, Bream JH, O’Shea JJ, Strober W. T-bet regulates Th1 responses through essential effects on GATA-3 function rather than on IFNG gene acetylation and transcription. J. Exp. Med. 2006;203:755–766. doi: 10.1084/jem.20052165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaplan MH, Sun YL, Hoey T, Grusby MJ. Impaired IL-12 responses and enhanced development of Th2 cells in Stat4-deficient mice. Nature. 1996;382:174–177. doi: 10.1038/382174a0. [DOI] [PubMed] [Google Scholar]
- 16.Magram J, Connaughton SE, Warrier RR, Carvajal DM, Wu CY, Ferrante J, Stewart C, Sarmiento U, Faherty DA, Gately MK. IL-12-deficient mice are defective in IFN gamma production and type 1 cytokine responses. Immunity. 1996;4:471–481. doi: 10.1016/s1074-7613(00)80413-6. [DOI] [PubMed] [Google Scholar]
- 17.Thierfelder WE, van Deursen JM, Yamamoto K, Tripp RA, Sarawar SR, Carson RT, Sangster MY, Vignali DA, Doherty PC, Grosveld GC, Ihle JN. Requirement for Stat4 in interleukin-12-mediated responses of natural killer and T cells. Nature. 1996;382:171–174. doi: 10.1038/382171a0. [DOI] [PubMed] [Google Scholar]
- 18.Shevach EM, DiPaolo RA, Andersson J, Zhao DM, Stephens GL, Thornton AM. The lifestyle of naturally occurring CD4+ CD25+ Foxp3+ regulatory T cells. Immunol. Rev. 2006;212:60–73. doi: 10.1111/j.0105-2896.2006.00415.x. [DOI] [PubMed] [Google Scholar]
- 19.Sakaguchi S, Ono M, Setoguchi R, Yagi H, Hori S, Fehervari Z, Shimizu J, Takahashi T, Nomura T. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol. Rev. 2006;212:8–27. doi: 10.1111/j.0105-2896.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- 20.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD252− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J. Exp. Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY, et al. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453:236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kulkarni AB, Ward JM, Yaswen L, Mackall CL, Bauer SR, Huh CG, Gress RE, Karlsson S. Transforming growth factor-beta 1 null mice. An animal model for inflammatory disorders. Am. J. Pathol. 1995;146:264–275. [PMC free article] [PubMed] [Google Scholar]
- 23.Gorelik L, Flavell RA. Transforming growth factor-beta in T-cell biology. Nat. Rev. Immunol. 2002;2:46–53. doi: 10.1038/nri704. [DOI] [PubMed] [Google Scholar]
- 24.Powrie F, Carlino J, Leach MW, Mauze S, Coffman RL. A critical role for transforming growth factor-beta but not interleukin 4 in the suppression of T helper type 1-mediated colitis by CD45RB(low) CD4+ T cells. J. Exp. Med. 1996;183:2669–2674. doi: 10.1084/jem.183.6.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li MO, Wan YY, Flavell RA. T cell-produced transforming growth factor-beta1 controls T cell tolerance and regulates Th1- and Th17-cell differentiation. Immunity. 2007;26:579–591. doi: 10.1016/j.immuni.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 26.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 27.Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 28.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 29.Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, Blank RB, Meylan F, Siegel R, Hennighausen L, et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 30.Ivanov II, Zhou L, Littman DR. Transcriptional regulation of Th17 cell differentiation. Semin. Immunol. 2007;19:409–417. doi: 10.1016/j.smim.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fantini MC, Rizzo A, Fina D, Caruso R, Becker C, Neurath MF, Macdonald TT, Pallone F, Monteleone G. IL-21 regulates experimental colitis by modulating the balance between Treg and Th17 cells. Eur. J. Immunol. 2007;37:3155–3163. doi: 10.1002/eji.200737766. [DOI] [PubMed] [Google Scholar]
- 32.Izcue A, Hue S, Buonocore S, Arancibia-Cárcamo CV, Ahern PP, Iwakura Y, Maloy KJ, Powrie F. Interleukin-23 restrains regulatory T cell activity to drive T cell-dependent colitis. Immunity. 2008;28:559–570. doi: 10.1016/j.immuni.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mathur AN, Chang HC, Zisoulis DG, Kapur R, Belladonna ML, Kansas GS, Kaplan MH. T-bet is a critical determinant in the instability of the IL-17-secreting T-helper phenotype. Blood. 2006;108:1595–1601. doi: 10.1182/blood-2006-04-015016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wei J, Duramad O, Perng OA, Reiner SL, Liu YJ, Qin FX. Antagonistic nature of T helper 1/2 developmental programs in opposing peripheral induction of Foxp3+ regulatory T cells. Proc. Natl. Acad. Sci. USA. 2007;104:18169–18174. doi: 10.1073/pnas.0703642104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Malley JT, Sehra S, Thieu VT, Yu Q, Chang HC, Stritesky GL, Nguyen ET, Mathur AN, Levy DE, Kaplan MH. Signal transducer and activator of transcription 4 limits the development of adaptive regulatory T cells. Immunology. 2009;127:587–595. doi: 10.1111/j.1365-2567.2008.03037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pearce EL, Mullen AC, Martins GA, Krawczyk CM, Hutchins AS, Zediak VP, Banica M, DiCioccio CB, Gross DA, Mao CA, et al. Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science. 2003;302:1041–1043. doi: 10.1126/science.1090148. [DOI] [PubMed] [Google Scholar]
- 37.Townsend MJ, Weinmann AS, Matsuda JL, Salomon R, Farnham PJ, Biron CA, Gapin L, Glimcher LH. T-bet regulates the terminal maturation and homeostasis of NK and Valpha14i NKT cells. Immunity. 2004;20:477–494. doi: 10.1016/s1074-7613(04)00076-7. [DOI] [PubMed] [Google Scholar]
- 38.Intlekofer AM, Takemoto N, Wherry EJ, Longworth SA, Northrup JT, Palanivel VR, Mullen AC, Gasink CR, Kaech SM, Miller JD, et al. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat. Immunol. 2005;6:1236–1244. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- 39.Suto A, Wurster AL, Reiner SL, Grusby MJ. IL-21 inhibits IFN-gamma production in developing Th1 cells through the repression of Eomesodermin expression. J. Immunol. 2006;177:3721–3727. doi: 10.4049/jimmunol.177.6.3721. [DOI] [PubMed] [Google Scholar]
- 40.Rennick DM, Fort MM, Davidson NJ. Studies with IL-10−/− mice: an overview. J. Leukoc. Biol. 1997;61:389–396. doi: 10.1002/jlb.61.4.389. [DOI] [PubMed] [Google Scholar]
- 41.Spencer SD, Di Marco F, Hooley J, Pitts-Meek S, Bauer M, Ryan AM, Sordat B, Gibbs VC, Aguet M. The orphan receptor CRF2–4 is an essential subunit of the interleukin 10 receptor. J. Exp. Med. 1998;187:571–578. doi: 10.1084/jem.187.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barbara G, Xing Z, Hogaboam CM, Gauldie J, Collins SM. Interleukin 10 gene transfer prevents experimental colitis in rats. Gut. 2000;46:344–349. doi: 10.1136/gut.46.3.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Deventer SJ, Elson CO, Fedorak RN Crohn’s Disease Study Group. Multiple doses of intravenous interleukin 10 in steroid-refractory Crohn’s disease. Gastroenterology. 1997;113:383–389. doi: 10.1053/gast.1997.v113.pm9247454. [DOI] [PubMed] [Google Scholar]
- 44.Stumhofer JS, Silver JS, Laurence A, Porrett PM, Harris TH, Turka LA, Ernst M, Saris CJ, O’Shea JJ, Hunter CA. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat. Immunol. 2007;8:1363–1371. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- 45.McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T, Cua DJ. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat. Immunol. 2007;8:1390–1397. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- 46.Xu J, Yang Y, Qiu G, Lal G, Wu Z, Levy DE, Ochando JC, Bromberg JS, Ding Y. c-Maf regulates IL-10 expression during Th17 polarization. J. Immunol. 2009;182:6226–6236. doi: 10.4049/jimmunol.0900123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pot C, Jin H, Awasthi A, Liu SM, Lai CY, Madan R, Sharpe AH, Karp CL, Miaw SC, Ho IC, Kuchroo VK. Cutting edge: IL-27 induces the transcription factor c-Maf, cytokine IL-21, and the costimulatory receptor ICOS that coordinately act together to promote differentiation of IL-10-producing Tr1 cells. J. Immunol. 2009;183:797–801. doi: 10.4049/jimmunol.0901233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bauquet AT, Jin H, Paterson AM, Mitsdoerffer M, Ho IC, Sharpe AH, Kuchroo VK. The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH-17 cells. Nat. Immunol. 2009;10:167–175. doi: 10.1038/ni.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao Z, Yu S, Fitzgerald DC, Elbehi M, Ciric B, Rostami AM, Zhang GX. IL-12R beta 2 promotes the development of CD4+CD25+ regulatory T cells. J. Immunol. 2008;181:3870–3876. doi: 10.4049/jimmunol.181.6.3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Szabo SJ, Dighe AS, Gubler U, Murphy KM. Regulation of the interleukin (IL)-12R β 2 subunit expression in developing T helper 1 (Th1) and Th2 cells. J. Exp. Med. 1997;185:817–824. doi: 10.1084/jem.185.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Laffont S, Siddiqui KR, Powrie F. Intestinal inflammation abrogates the tolerogenic properties of MLN CD103+ dendritic cells. Eur. J. Immunol. 2010;40:1877–1883. doi: 10.1002/eji.200939957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Coombes JL, Siddiqui KR, Arancibia-Cárcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J. Exp. Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Annacker O, Coombes JL, Malmstrom V, Uhlig HH, Bourne T, Johansson-Lindbom B, Agace WW, Parker CM, Powrie F. Essential role for CD103 in the T cell-mediated regulation of experimental colitis. J. Exp. Med. 2005;202:1051–1061. doi: 10.1084/jem.20040662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mathur AN, Chang HC, Zisoulis DG, Stritesky GL, Yu Q, O’Malley JT, Kapur R, Levy DE, Kansas GS, Kaplan MH. Stat3 and Stat4 direct development of IL-17-secreting Th cells. J. Immunol. 2007;178:4901–4907. doi: 10.4049/jimmunol.178.8.4901. [DOI] [PubMed] [Google Scholar]
- 55.Chung DR, Kasper DL, Panzo RJ, Chitnis T, Grusby MJ, Sayegh MH, Tzianabos AO. CD4+ T cells mediate abscess formation in intra-abdominal sepsis by an IL-17-dependent mechanism. J. Immunol. 2003;170:1958–1963. doi: 10.4049/jimmunol.170.4.1958. [Published erratum appears in 2003 J. Immunol. 170: 4411.] [DOI] [PubMed] [Google Scholar]
- 56.Furuta S, Kagami S, Tamachi T, Ikeda K, Fujiwara M, Suto A, Hirose K, Watanabe N, Saito Y, Iwamoto I, Nakajima H. Overlapping and distinct roles of STAT4 and T-bet in the regulation of T cell differentiation and allergic airway inflammation. J. Immunol. 2008;180:6656–6662. doi: 10.4049/jimmunol.180.10.6656. [DOI] [PubMed] [Google Scholar]
- 57.Pflanz S, Timans JC, Cheung J, Rosales R, Kanzler H, Gilbert J, Hibbert L, Churakova T, Travis M, Vaisberg E, et al. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4(+) T cells. Immunity. 2002;16:779–790. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 58.Pflanz S, Hibbert L, Mattson J, Rosales R, Vaisberg E, Bazan JF, Phillips JH, McClanahan TK, de Waal Malefyt R, Kastelein RA. WSX-1 and glycoprotein 130 constitute a signal-transducing receptor for IL-27. J. Immunol. 2004;172:2225–2231. doi: 10.4049/jimmunol.172.4.2225. [DOI] [PubMed] [Google Scholar]
- 59.Batten M, Li J, Yi S, Kljavin NM, Danilenko DM, Lucas S, Lee J, de Sauvage FJ, Ghilardi N. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat. Immunol. 2006;7:929–936. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- 60.Stumhofer JS, Laurence A, Wilson EH, Huang E, Tato CM, Johnson LM, Villarino AV, Huang Q, Yoshimura A, Sehy D, et al. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat. Immunol. 2006;7:937–945. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- 61.Lee YK, Turner H, Maynard CL, Oliver JR, Chen D, Elson CO, Weaver CT. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009;30:92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mukasa R, Balasubramani A, Lee YK, Whitley SK, Weaver BT, Shibata Y, Crawford GE, Hatton RD, Weaver CT. Epigenetic instability of cytokine and transcription factor gene loci underlies plasticity of the T helper 17 cell lineage. Immunity. 2010;32:616–627. doi: 10.1016/j.immuni.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rutz S, Janke M, Kassner N, Hohnstein T, Krueger M, Scheffold A. Notch regulates IL-10 production by T helper 1 cells. Proc. Natl. Acad. Sci. USA. 2008;105:3497–3502. doi: 10.1073/pnas.0712102105. [DOI] [PMC free article] [PubMed] [Google Scholar]