Abstract
Study Objectives:
Obstructive sleep apnea (OSA) is a common disorder associated with substantially increased cardiovascular risks, reduced quality of life, and increased risk of motor vehicle collisions due to daytime sleepiness. This study evaluates the cost-effectiveness of three commonly used diagnostic strategies (full-night polysomnography, split-night polysomnography, unattended portable home-monitoring) in conjunction with continuous positive airway pressure (CPAP) therapy in patients with moderate-to-severe OSA.
Design:
A Markov model was created to compare costs and effectiveness of different diagnostic and therapeutic strategies over a 10-year interval and the expected lifetime of the patient. The primary measure of cost-effectiveness was incremental cost per quality-adjusted life year (QALY) gained.
Patients or Participants:
Baseline computations were performed for a hypothetical average cohort of 50-year-old males with a 50% pretest probability of having moderate-to-severe OSA (apnea–hypopnea index [AHI] ≥ 15 events per hour).
Measurements and Results:
For a patient with moderate-to-severe OSA, CPAP therapy has an incremental cost-effectiveness ratio (ICER) of $15,915 per QALY gained for the lifetime horizon. Over the lifetime horizon in a population with 50% prevalence of OSA, full-night polysomnography in conjunction with CPAP therapy is the most economically efficient strategy at any willingness-to-pay greater than $17,131 per-QALY gained because it dominates all other strategies in comparative analysis.
Conclusions:
Full-night polysomnography (PSG) is cost-effective and is the preferred diagnostic strategy for adults suspected to have moderate-to-severe OSA when all diagnostic options are available. Split-night PSG and unattended home monitoring can be considered cost-effective alternatives when full-night PSG is not available.
Citation:
Pietzsch JB; Garner A; Cipriano LE; Linehan JH. An integrated health-economic analysis of diagnostic and therapeutic strategies in the treatment of moderate-to-severe obstructive sleep apnea. SLEEP 2011;34(6):695-709.
Keywords: Sleep apnea, obstructive, continuous positive airway pressure, health-economics, Markov model, comparative effectiveness
INTRODUCTION
Obstructive sleep apnea (OSA) is a chronic, significantly underdiagnosed and undertreated condition characterized by recurrent collapse of the upper airway during sleep. Left untreated, OSA is associated with a variety of adverse consequences, including daytime sleepiness, increased cardiovascular morbidity and mortality, impairment of cognitive function, motor vehicle collisions, and reduced quality of life. In light of the large number of untreated patients who would benefit from therapy1 and because of the substantial medical and economic implications of OSA, the choice of appropriate strategies for diagnosis and therapy is widely debated.2–6
Health-economic assessments evaluating various diagnostic and therapeutic approaches have been published.2–6 However, no integrated, end-to-end assessment comprehensively evaluating the overall impact of OSA diagnosis and therapy, including long-term patient outcomes and associated costs, has been conducted to date. Furthermore, several of the previously published studies only evaluated particular aspects of OSA-associated outcomes, such as the relationship of OSA to motor vehicle collisions,7 or relied on aggregate quality-of-life assessments, as opposed to linking quality of life to relevant outcomes observed in OSA.2–6
In this study, we compare 3 commonly employed diagnostic modalities—full-night polysomnography (FN-PSG), split-night polysomnography (SN-PSG), and unattended portable home monitoring (UPHM)—and the established (gold standard) treatment modality of continuous positive airway pressure (CPAP) for moderate-to-severe OSA (apnea-hypopnea index [AHI] ≥ 15 events/h). All input data for the study were compiled from previously published clinical trials and health-economic evaluations.
This study evaluates health-related quality of life (HRQoL) and the impact of treatment on 3 negative health outcomes associated with untreated OSA: strokes, myocardial infarctions, and motor vehicle collisions due to excessive daytime sleepiness.7,8 Cost-effectiveness is assessed through the standard metric of the incremental cost-effectiveness ratio (ICER). Scenario analyses are performed to identify critical parameters that might be directly influenced by patients and physicians, including therapy compliance. To account for the chronic nature of OSA, we investigated 2 time frames: a 10-year time horizon and lifetime.
METHODS
Overview
We constructed a decision-analytic Markov model using TreeAge Pro 2009 Suite (TreeAge Software Inc., Williamstown, MA). The purpose of the model was to evaluate the lifetime cost and health consequences of the diagnosis and treatment of moderate-to-severe OSA. The model compared typical clinical algorithms currently used in the United States, as outlined in previous health-economic studies6 and in clinical practice guidelines.9,10 Health outcomes were expressed in life-years (LY) gained and quality-adjusted life-years (QALYs) gained (see footnote A at end of paper), as well as in the number of myocardial infarctions (MI), strokes, and motor vehicle collisions (MVC) prevented. Costs were considered from the third-party payer perspective and are presented in 2008 US$. Where necessary, costs were converted to 2008 US$ using the U.S. GDP-deflator. Consistent with standard practice in health economic analysis, we discounted costs and benefits at 3% annually to incorporate time preference for money and good health. Model construction and analysis followed the recommendations of the Panel on Cost-Effectiveness in Health and Medicine.11 We define the optimal or preferred strategy to be the non-dominated (see footnote B at end of paper) strategy with the highest QALY benefit that has an incremental cost-effectiveness ratio less than the willingness to pay threshold of $50,000 per QALY-gained.
Model Structure
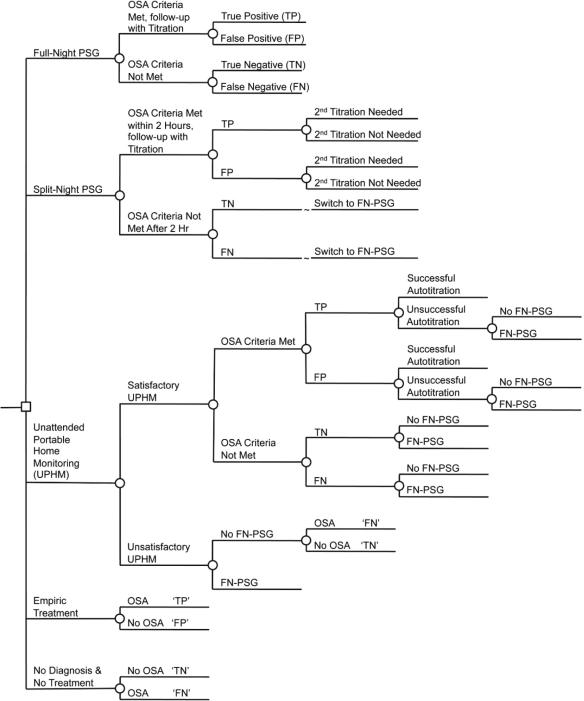
The decision tree is shown in simplified form in Figure 1. The model incorporates three diagnostic choices (full-night polysomnography, split-night polysomnography, portable home monitoring), as well as choices of no use of a diagnostic modality and no treatment, or no diagnosis followed by treatment (defined in this paper as empiric treatment). For each scenario, the decision tree distributes the cohort into one of 4 groups:
True positive diagnosis of OSA (TP)
True negative diagnosis of no OSA (TN)
Incorrect diagnoses of OSA (false positive, FP)
Incorrect diagnosis of no OSA (false negative, FN)
Figure 1.
Simplified illustration of the decision tree structure (diagnosis and titration only). The small square represents the decision to implement a strategy of using a specific diagnostic technology. Circles represent chance events. FN-PSG, Full-night polysomnography; SN-PSG, Split-night polysomnography; UPHM, Unattended Home Monitoring; TN, True Negative; FN, False Negative; TP, True Positive; FP, False Positive.
The proportion in each group depends on the prevalence of OSA in the cohort, and the sensitivity and specificity of the diagnostic procedure and technology.
FN-PSG includes an overnight in-laboratory PSG session, with CPAP titration performed during a subsequent night in the laboratory (as needed if OSA criteria were met).
SN-PSG includes an in-laboratory PSG session of > 2 h during the first part of an overnight stay, with the remainder of the night spent for CPAP titration (for those meeting the OSA criteria). For patients who do not meet OSA criteria during the first part of the night, the protocol changes to FN-PSG (that is, they are evaluated for OSA throughout the remainder of the night, with titration occurring during a separate night). Patients who meet the OSA criteria during the first part of the night but fail to be successfully titrated during the second part of the night, return to the sleep laboratory on a subsequent night for CPAP titration.
Patients undergoing unattended evaluation with a portable home monitor (UPHM) also undergo unattended home CPAP autotitration if they have been positively diagnosed. If a home study is unsatisfactory for technical reasons, if OSA criteria are not met during the home evaluation, or if home autotitration fails, patients are referred for FN-PSG. In all the above cases, a fraction of patients (22%) do not attend the in-laboratory evaluation based on the observed rate in a prospective case study of individuals with suspected OSA.12
In the empiric CPAP treatment strategy, all patients are titrated and subsequently treated with CPAP.
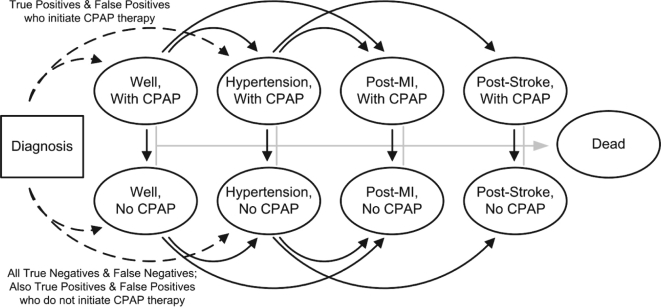
Once classified by diagnosis and disease state, patients enter the Markov component of the model (Figure 2). The Markov model includes a set of mutually exclusive and collectively exhaustive health states. At the end of each cycle, assumed to occur in 1-month intervals, individuals can stay in the health state they are in or move to a new health state based on the probability of that transition. The health state individuals start in is based on their diagnosis status, the prevalence of hypertension in the cohort, and the probability of patient compliance with physician recommendations to initiate CPAP treatment. Patients with a diagnosis of OSA were distributed into one of 4 categories:
Well, no CPAP
Well, using CPAP
Hypertension, no CPAP
Hypertension, using CPAP
Figure 2.
Schematic of the Markov component of the model. Patients are initially distributed based on their diagnosis. Each month, patients can either die, stay in the existing state, or transition into one of the other health states as indicated by the arrows.
We assumed that hypertensive patients were not more (or less) compliant with CPAP therapy than those without hypertension. Patients without a diagnosis of OSA were distributed into “Well, no CPAP” or “Hypertension, no CPAP” based on the prevalence of hypertension in the cohort.
Within each Markov cycle, patients could die from causes other than MVC, MI, or stroke; have a non-fatal or fatal MVC; suffer a non-fatal or fatal myocardial infarction; suffer a non-fatal or fatal stroke; or develop clinical hypertension. Patients compliant with CPAP therapy at the beginning of the cycle could discontinue treatment during the cycle; once treatment was discontinued, the patient remained non-compliant in all future cycles. The probability of transitioning to a different health state at the end of a cycle was calculated using the formula p = 1 - e-rt where p is the probability of the transition, r is the annual rate of the transition, and t is the cycle length of the Markov model in years (t = 1/12 in this model). These probabilities were dependent on the age of the patient, the presence of a chronic condition (OSA or hypertension), therapy compliance, and history of an acute cardiovascular event (MI), or stroke.
Model Inputs
Population characteristics
The base case population was a cohort of 50-year-old male patients with a prevalence of moderate-to-severe OSA (AHI ≥ 15) of 50%. In sensitivity analysis, we considered women, alternative ages, and alternative levels of OSA prevalence.
Test characteristics
The characteristics of the 3 investigated diagnostic modalities were based on the limited evidence available in published clinical trials and meta-analyses, and therefore we varied them widely in sensitivity analysis (see Table 1). We estimated the sensitivity of the second half of the SN-PSG (conditional on not meeting OSA criteria in the first 2 h) by assuming that the total sensitivity of a full night of evaluation would be equal to that of a FN-PSG session. In the absence of data, we assumed that FN-PSG performed after negative or failed unattended portable home monitoring was independent of the previous test result or failure.
Table 1.
Model parameters
| Variable | Base Case | Range | Reference |
|---|---|---|---|
| Cohort Characteristics | |||
| Sex | Male | ||
| Age | 50 | 30–70 | |
| Risks Associated with Events and Modeled States | |||
| Motor vehicle collision causing injury or death | |||
| Annual risk, males | 0.025 | 0.023–0.027 | 21 |
| Annual risk, females | 0.011 | 0.010–0.013 | 21 |
| Probability of death from a MVC with injury or death | 0.0079 | 0.006–0.01 | 39 |
| Hypertension | |||
| Prevalence of hypertension, Males (varies by age, 40-70 y) | 0.239–0.633 | 19 | |
| Prevalence of hypertension, Females (varies by age, 40-70 y) | 0.199–0.788 | 19 | |
| Rate (annual per person, until age 85) for incident hypertension, Males | 0.0195 × ln(Age) – 0.0551 | ± 10% rate | 19 |
| Rate (annual per person, until age 85) for incident hypertension, Females | (2.5 × 10-7) × Age2.948 | ± 10% rate | 19 |
| Hazard ratio for all-cause mortality, life with Hypertension | 1.49 | 1.0–2.2 | 20 |
| Myocardial infarction | |||
| Rate (annual per person, until age 85) for incident MI, Males | (2.0 × 10-12) × Age5.236 | 16 | |
| Rate (annual per person, until age 85) for incident MI, Females | (9.0 × 10-17) × Age7.423 | 16 | |
| Hazard ratio for incident MI, with Hypertension | 2.53 | 2.0–3.5 | 20 |
| Probability of 28-day mortality, males, varies by age | 0.15–0.7 | 50,51 | |
| Probability of 28-day mortality, females, varies by age | 0.20–0.7 | 50,51 | |
| Hazard ratio for all-cause mortality, life after MI | 1.57 | 1.35–1.74 | 52 |
| Stroke | |||
| Rate (annual per person, until age 85) for incident stroke | (3.1 × 10-13) × Age5.569 | 17,18 | |
| Hazard ratio for incident stroke, with Hypertension | 2.78 | 2.0–4.6 | 20 |
| Probability of 28-day mortality, varies by age | 0.09–0.52 | 18 | |
| Hazard ratio for all-cause mortality, life after stroke | 2.30 | 1.1–2.8 | 53 |
| Diagnostic Test Characteristics | |||
| Full night PSG | |||
| Sensitivity | 0.97 | 0.85–1.00 | 6,54 |
| Specificity | 1.00 | 0.85–1.00 | 6,54 |
| Split night PSG | |||
| Sensitivity | 0.89 | 0.76–0.95 | 54 |
| Specificity | 0.94 | 0.72–1.00 | 54 |
| Unattended home monitoring (Type III) | |||
| Sensitivity | 0.91 | 0.83–0.94 | 54,55 |
| Specificity | 0.83 | 0.67–0.94 | 54,55 |
| Technical failure | 0.09 | 0.07–0.12 | 54 |
| Refuse FN-PSG given technical failure | 0.22 | 0–0.44 | 12 |
| CPAP Titration | |||
| Successful titration during split-night PSG | 0.82 | 0.80–0.90 | 6 |
| Successful autotitration during unattended home monitoring | 0.87 | 0.80–0.90 | 56 |
| CPAP Treatment Compliance | |||
| Initial treatment refusal | 0.102 | 0–0.204 | 23 |
| Annual probability of stopping treatment | 0.044 | 0.022–0.086 | 26 |
| Hazard Ratios Associated with OSA | |||
| HR for motor vehicle collision causing injury or death | 3.0 | 2.5–3.5 | 23,24 |
| HR for developing hypertension | 1.8 | 1.3–1.9 | 25 |
| HR for incident MI | 2.8 | 1.9–4.3 | 8 |
| HR for incident stroke | 1.7 | 1.0–3.5 | 22 |
| Costs | |||
| Diagnosis | |||
| Office visits | 89.90 | CPT 99214 | |
| FN-PSG | 810.86 | −10%, +20% | CPT 95810 |
| SN-PSG | 891.14 | −10%, +20% | CPT 95811 |
| UPHM | 209.17 | −10%, +20% | CPT 95806 |
| Titration | |||
| PSG titration | 891.14 | −10%, +20% | CPT 95811 |
| Auto titration | 209.17 | −10%, +20% | CPT 95806 |
| Treatment (monthly) | |||
| CPAP, rental | 111.71 | HCPCS E0601, for 13 months then owned | |
| CPAP replacement time (years) | 5 | 2–10 | |
| CPAP supplies | 114 | 38–380 | 1/3 of maximum allowable from HCPCS A7030-A7039, A7045, A7046 |
| Office visits (each, assumed 2 per year) | 89.90 | CPT 99214 | |
| Acute Events (one-time) | |||
| Non-fatal MVC with injuries | 7,500 | 50–200% | 39 |
| Fatal MVC | 28,100 | 50–200% | 39 |
| Acute MI | 15,110 | 37 | |
| Acute Stroke | 20,419 | 38 | |
| Death from cause other than MI, stroke, MVC; varies (decreases) by age | 24,122–40,801 | 34 | |
| Health state specific costs (monthly) | |||
| Baseline, increases by age | 403–1,543 | 33 | |
| Health state specific care costs | |||
| Hypertension, additive | 56.76 | 25–115 | 35 |
| Post-MI, multiplicative | 1.76 | 1.6–2.2 | Assumed to be the same as stroke |
| Post-Stroke, multiplicative | 1.76 | 1.6–2.2 | 36 |
| Quality of Life Weights | |||
| Baseline, varies (decreases) by age (40-80 years) | 0.871–0.736 | 29 | |
| Health state specific quality of life weights, multiplicative | |||
| Well | 1 | ||
| Hypertension | 0.96 | 0.95–1.0 | 29 |
| Post-MI | 0.86 | 0.80–1.0 | 29 |
| Post-Stroke | 0.81 | 0.78–1.0 | 29 |
| Untreated OSA | 0.84 | 0.80–0.93 | 31,32 |
| Treated OSA | 0.93 | 0.84–0.98 | 31,32 |
| CPAP without OSA | 0.98 | 0.96–1.0 | Estimated |
| Non-fatal MVC (lifetime discounted decrement, subtractive) | 0.036 | 0.031–0.418 | 30 |
Effectiveness of Treatment
Baseline rates of events and risk increase associated with OSA
Age- and gender-specific all-cause mortality was estimated using the 2004 United States life tables.13 To avoid double-counting deaths, age-specific mortality from MI and stroke14 were subtracted from all-cause mortality.15
Age- and gender- specific baseline incidence rates for MI, stroke, and hypertension were estimated by fitting curves to the age- and gender-specific rates reported in large population studies.16–19 Because there is limited information on the risk of MI, stroke, and hypertension in persons over age 85, we assumed that the annual rate of new events was constant after age 85. Based on hazard ratios observed in the Cardiovascular Health Study,20 we assumed that hypertension increases the risk of non-MVC, non-MI, non-stroke mortality, as well as the incidence of MI and stroke. The annual probability of non-fatal and fatal MVC in individuals without OSA was estimated from 2006 data published by the U.S. Department of Transportation.21 The magnitude of the effects of untreated moderate-to-severe OSA (AHI ≥ 15) on the incident rates of MVC, MI, stroke, and hypertension were estimated from the literature.8,22–25
Therapy compliance and effectiveness
Patient compliance with CPAP treatment is critical to treatment success. We assumed that 10.2% of patients would decline therapy, as observed in a study of 353 patients diagnosed with moderate OSA.23 Furthermore, we assumed CPAP compliance would stabilize after 4 years at 68%, as was observed in a study of long-term CPAP compliance.26
Based on the blood pressure reductions observed in moderate-to-severe OSA patients treated with CPAP,27,28 and on the observations of a long-term cohort study investigating cardiovascular risk in untreated OSA, treated OSA, and controls without OSA,8 we assumed further that CPAP use, when the patient is 100% compliant, reduces the risk of MI, stroke, and incident hypertension to general population risk levels. This assumption was investigated in sensitivity analyses to assess the impact of a lower treatment effect.
As in the cases of MI and stroke, we assumed that CPAP treatment reduces the risk of motor vehicle collisions (MVC) to general population levels when the patient was 100% compliant. The magnitude of treatment benefit for MVC risk was evaluated in the sensitivity analyses.
Quality of life parameters
Values for age-specific and health-state specific quality of life (QOL) were derived from the self-reported health of participants in the nationally representative Medical Expenditure Panel Survey (MEPS), as measured by the EQ-5D.29 An individual's age-specific baseline QOL was decreased by a factor of 16% for patients who had suffered an MI, 21% for those who had suffered a stroke, and 4% for patients suffering from hypertension.29 For non-fatal motor vehicle collisions, the lifetime discounted utility decrement was subtracted in the year of the collision.30
Two studies have evaluated QOL with untreated and treated OSA.31,32 Tousignant et al. used the standard gamble method in 19 patients with severe OSA before and after treatment; Chakravoty et al. used the EQ-5D in 32 moderate-to-severe patients. We estimated the age-independent QOL weights from these studies using the expected QOL based on the age of the participant or the average age of the investigated population. The 2 studies were then combined to estimate that baseline QOL of individuals with untreated and treated OSA are 16% and 7% lower than average people of the same age, respectively. In the model, QOL for individuals who do not have OSA but are undergoing CPAP treatment due to a false-positive diagnosis was reduced by 2%.
Costs and resource use
The cost analysis was based on a U.S. third-party payer perspective, considering only direct healthcare costs. Age-specific baseline healthcare costs were based on average U.S. expenditures,33 and a one-time cost of end-of-life care. This cost was incurred in the year of death for individuals whose cause of death was other than fatal MVC, MI, or stroke, using cost estimates based on published Medicare expenditures.34 Incremental costs associated with the treatment of hypertension, an acute cardiovascular event, or life after an acute cardiovascular event were based on literature reports.35–38 Health care costs associated with non-fatal and fatal MVCs were based on a U.S. Department of Transportation study.39
The costs of diagnostic testing and CPAP treatment were based on national average 2008 Medicare reimbursement rates. Diagnostic costs for the 3 investigated diagnostic pathways were based on the respective CPT codes (full-night polysomnography, 95810; split-night polysomnography, 95811; unattended portable home monitoring, 95806). When patients did not meet OSA criteria during the first part of the night during the SN-PSG protocol, the cost incurred is still that of a SN-PSG and a separate cost is incurred for the full-night titration performed at a later date. CPAP titration costs included CPT 95811 for polysomnography-based CPAP titration and CPT 95806 for autotitration. The laboratory-based diagnostic pathways were assumed to be followed by a post-evaluation visit at the doctor's office (CPT 99214) after one month, with biannual office visits afterwards during the duration of CPAP treatment. Based on published practice behaviors,40 we assumed that patients diagnosed by UPHM were followed-up with monthly post-evaluation visits during the first 3 months, visits every 3 months during the following 9 months, and then biannual visits over the duration of their treatment with CPAP.6 Patients who did not meet OSA criteria or rejected initiation of CPAP treatment were assumed to incur the cost of only one additional office visit.
CPAP treatment costs consisted of the cost of the CPAP device, cost of CPAP accessories, and physician visits related to CPAP therapy. Current Medicare procedures reimburse for the cost of the CPAP device through 13 monthly rental charges (HCPCS E0601), after which the patient owns the device. The total cost of the CPAP device over the course of its assumed life of 5 years was amortized into equal monthly charges. Consistent with other health-economic studies,2,4,6 we assumed that most patients do not use the maximum allowable number of CPAP replacement accessories; we estimated the annual cost of CPAP accessories (mask, tubing, headgear, filters, humidifier, and so on) to be 30% of the maximum allowable Medicare reimbursement level for all CPAP accessories. We assumed that individuals who stop treatment do not immediately return equipment; in the base case, we assumed an additional 3 months of CPAP rental charges and 3 months' worth of replacement accessories.
Sensitivity Analysis
Extensive single-variable and multi-variable sensitivity analyses were conducted to evaluate the effects of parameter uncertainty. The parameter ranges (Table 1) for the sensitivity analysis were derived from the literature.
A specific focus of the analysis was on cardiovascular events. In light of a number of randomized trials and systematic reviews that evaluated treatment effects based on the primary measure of reduction in subjective sleepiness as measured on the Epworth Sleepiness Scale (ESS),32,41,42 the chosen assumptions about the effectiveness of CPAP treatment to reduce the increased risk of cardiovascular events in patients with OSA may be optimistic. Additional scenarios were also constructed using lower cardiovascular risk reductions associated with CPAP use—including a scenario in which there was no reduction in cardiovascular risk associated with CPAP treatment.
RESULTS
The results are presented in 2 parts. First, we evaluated the benefits and cost-effectiveness of CPAP therapy in patients with a diagnosis of OSA. Second, we evaluated the benefits and cost-effectiveness of various diagnostic screening technologies.
Benefits and Cost-Effectiveness of CPAP Therapy
In patients with a diagnosis of moderate-to-severe OSA, CPAP therapy reduces the average number of lifetime MVCs and lifetime risk of cardiovascular events (Table 2). We estimate that treatment with CPAP reduces the 10-year risk of MVCs (fatal and non-fatal) by 52%, the 10-year expected number of MIs by 49%, and the 10-year risk of stroke by 31%. The relative risk reduction in MIs is greater than that of in strokes because the OSA has a greater influence on the risk of MI than stroke. The relative risk reduction over the lifetime horizon is less than over the 10-year horizon because the average life expectancy in the treated cohort is longer which increases the number of years they are at risk for an event. Considering a lifetime perspective, we calculate the ICER of CPAP therapy compared to no treatment in a cohort of patients with OSA to be $24,222 per LY gained and $15,915 per QALY gained.
Table 2.
Risk of cardiovascular events, motor vehicle collision, lifetime costs, life expectancy and quality adjusted life expectancy associated with CPAP treatment in a 50-year-old male with moderate-to-severe OSA
| 10 years |
Lifetime |
|||
|---|---|---|---|---|
| No Treatment | Treatment | No Treatment | Treatment | |
| Risk of MI | 11.38% | 5.81% | 56.25% | 38.87% |
| Risk of Stroke | 3.01% | 2.07% | 14.90% | 14.64% |
| Avg. no. of MVC | 0.724 | 0.352 | 1.781 | 0.939 |
| Risk of fatal MVC | 0.57% | 0.28% | 1.41% | 0.74% |
| Undiscounted | ||||
| Cost | $81,743 | $92,442 | $359,919 | $408,845 |
| Life Years | 9.473 | 9.586 | 23.332 | 25.641 |
| QALYs | 6.518 | 7.194 | 15.572 | 18.425 |
| Discounted (3%) | ||||
| Cost | $70,264 | $79,750 | $216,934 | $243,656 |
| Life Years | 8.236 | 8.329 | 16.062 | 17.165 |
| QALYs | 5.672 | 6.259 | 10.814 | 12.493 |
| ICER | ||||
| $/LY-gained | $ 102,585 | $ 24,222 | ||
| $/QALY-gained | $ 16,172 | $ 15,915 | ||
Effects of treatment compliance
Within the range tested, treatment compliance did not have a substantial effect on the cost-effectiveness of CPAP therapy in any population (Table 3). If a patient, from the 50-year-old male cohort, was perfectly compliant, treatment would not be substantially more cost-effective, with an ICER of $15,769 per QALY gained. To illustrate this limited impact when compared to baseline, a worst-case compliance scenario was created in which double the number of patients refused therapy immediately when offered, and twice the number of patients discontinued treatment each month. In this scenario, the ICER of treatment is only marginally higher than in the base case analysis at $16,112 per QALY gained.
Table 3.
Sensitivity analysis: Incremental cost effectiveness ratios of CPAP treatment compared to no treatment under various scenarios (50-year-old males)
| Scenario | ICER ($/QALY-gained) |
|---|---|
| Base case | 15,915 |
| OSA related risks | |
| No increased risk of MVC with OSA | 18,822 |
| Decreased impact of OSA on MVC risks | 16,802 |
| Increased impact of OSA on MVC risks | 15,087 |
| Decreased impact of OSA on hypertension risks | 16,721 |
| Increased impact of OSA on hypertension risks | 15,342 |
| Decreased impact of OSA on MI &stroke risk | 15,107 |
| Increased impact of OSA on MI & stroke risk | 16,307 |
| Efficacy of therapy | |
| Only MVC benefit | 23,595 |
| Only CV benefit | 19,091 |
| 75% of MVC and CV benefit | 17,997 |
| 50% of MVC and CV benefit | 20,841 |
| 25% of MVC and CV benefit | 24,722 |
| 0% of MVC and CV benefit (only benefit is the utility gain from reduced daytime sleepiness) | 30,093 |
| Compliance with Therapy | |
| Perfect compliance | 15,769 |
| Low compliance: double the number of patients who never try therapy, & double the quit rate | 16,112 |
| Costs | |
| Low CPAP treatment cost | 8,640 |
| High CPAP treatment cost | 41,377 |
| Low cost of MVC | 17,275 |
| High cost of MVC | 13,193 |
| Low cost of CV chronic care | 17,027 |
| High cost of CV chronic care | 12,730 |
| Utilities | |
| No utility gain from reduction in daytime sleepiness (only benefit is the utility gain from reduced MVC and CV risks) | 35,277 |
| Large utility gain associated with treated OSA; same as no OSA with CPAP (2% reduction from age-specific HR-QoL) | 12,378 |
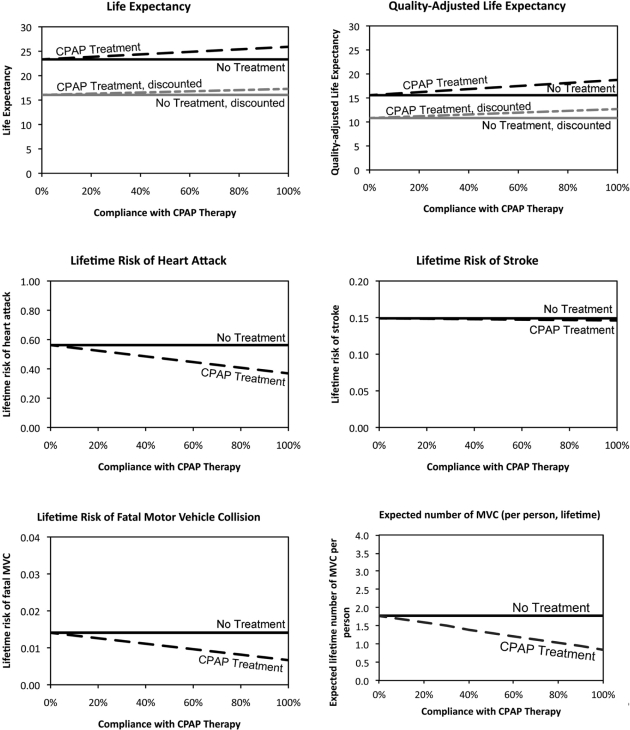
We also calculated the impact of compliance on other outcome measures such as the lifetime risk of injury or death from MVCs, and the risks of MI or stroke, for initial treatment compliance between 0% (no compliance at all) and 100% (full compliance). Figure 3 illustrates the magnitude of health benefits associated with CPAP treatment, compared with treatment forgone by non-compliant patients. For the base case therapy compliance, the model results show that CPAP therapy reduces overall per-person risk of a fatal MVC by approximately 48%, and the total number of MVCs by approximately 47%. Thus, the results suggest that an estimated 670 MVC fatalities can be prevented in a 100,000-patient cohort treated with CPAP.
Figure 3.
Expected life-years, quality-adjusted life years, lifetime risk of MI, stroke, and death from motor vehicle crash (MVC), and expected lifetime number of MVC, varying CPAP therapy compliance (50-year-old male cohort).
Sensitivity analysis
The results were not sensitive to gender or cohort age. However, we observed that men had lower ICERs than women (at 50 years old, men vs. women: $15,915 vs. $18,942 per QALY gained) and younger people had lower ICERs than older people (men at 30 years old vs. 70 years old: $15,478 vs. $20,150; women at 30 vs. 70: $18,836 vs. $22,348 per QALY gained). These small differences are due to differences in near-term baseline cardiovascular risk among the different cohorts.
Sensitivity analyses indicated that the cost-effectiveness of CPAP treatment in patients with OSA was robust (Table 3). Assumptions about the utility gain from reduced daytime sleepiness affected the cost-effectiveness of treatment. Compared to the baseline ICER of $15,915 per QALY-gained, including only benefits associated with reduced daytime sleepiness increased the ICER to $30,093 per QALY gained, whereas including only the benefits associated with reduced occurrences of MVC and cardiovascular events—and excluding the utility gains from reduced daytime sleepiness—results in an ICER of $35,277 per QALY-gained. Increasing the monthly cost of treatment to the Medicare allowable maximum increased the ICER to $41,377 per QALY gained.
Benefits and Cost-Effectiveness of Diagnostic Technology Choices
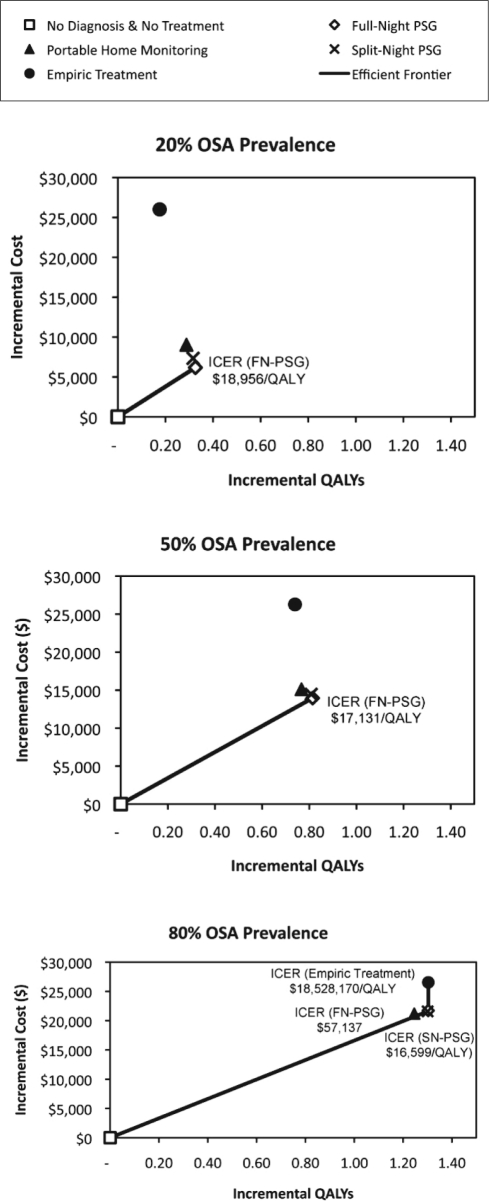
We computed the benefits of diagnosis and subsequent CPAP treatment in terms of the prevention of MVCs and the reduction of cardiovascular events for the base case cohort of 50-year-old male patients with a 50% pretest probability of having OSA (Table 4). The different numbers of false diagnoses (FP and FN) caused by differences in the sensitivity and specificity of the diagnostic modalities investigated result in small but important differences in event rates, costs, and QALYs across strategies. The preferred diagnostic strategy is FN-PSG, with an ICER of $17,131 per QALY gained. Even though FN-PSG is the most expensive technology at diagnosis requiring patients to undergo 2 overnight in-lab assessments (one for diagnosis and the other for titration), the superior diagnostic accuracy of this technology resulted in it costing less and providing more health benefits than any other approach to diagnosis in the long term.
Table 4.
Risk of cardiovascular event, motor vehicle collision, lifetime costs, life expectancy, and quality adjusted life expectancy associated with various diagnostic approaches in a 50-year-old male with a 50% pretest probability of OSA
| No Diagnosis & No Treatment | Full-Night PSG | Split-Night PSG | Unattended Portable Home Monitoring | Empiric Treatment | |
|---|---|---|---|---|---|
| Risk of MI | 44.67% | 36.24% | 36.24% | 36.57% | 35.98% |
| Risk of Stroke | 14.72% | 14.59% | 14.59% | 14.60% | 14.59% |
| Expected number of MVC | 1.227 | 0.818 | 0.818 | 0.834 | 0.806 |
| Risk of fatal MVC | 0.010 | 0.006 | 0.006 | 0.007 | 0.006 |
| Undiscounted | |||||
| Cost | $ 367,159 | $ 391,879 | $ 392,756 | $ 393,574 | $ 410,707 |
| Life Years | 24.8639 | 25.9835 | 25.9835 | 25.9396 | 26.0181 |
| QALYs | 18.1005 | 19.4840 | 19.4753 | 19.4074 | 19.3824 |
| Discounted (3%) | |||||
| Cost | $ 217,602 | $ 231,553 | $ 232,060 | $ 232,716 | $ 243,879 |
| Life Years | 16.7920 | 17.3271 | 17.3271 | 17.3061 | 17.3436 |
| QALYs | 12.3600 | 13.1743 | 13.1683 | 13.1269 | 13.0994 |
| Average CER (compared to No Diagnosis) | |||||
| $/LY-gained | * | 26,073 | 27,023 | 29,400 | 47,637 |
| $/QALY-gained | * | 17,131 | 17,887 | 19,707 | 35,536 |
| ICER (compared to the next best alternative) | |||||
| $/LY-gained | * | 26,073 | Dominated | Dominated | 744,822 |
| $/QALY-gained | * | 17,131 | Dominated | Dominated | Dominated |
“No Diagnosis & No Treatment” was the least costly strategy, therefore it is the comparator for the next strategy on the efficient frontier.
Dominated: Strategies that are not on the efficient frontier are described as dominated. Dominated strategies cost more and provide fewer QALYs than another strategy or a combination of strategies available to the decision-maker.
Effects of Cohort Age, Gender, and Pretest Probability
In the cohort with a prevalence of 50%, the choice of preferred strategy was not affected by age or gender. Men had lower ICERs than women (50-year-old men vs. women: $17,131 vs. $20,184 per QALY gained), and younger people had lower ICERs than older people (men at 30 years old vs. 70 years old: $16,512 vs. $21,835; women at 30 vs. 70: $19,912 vs. $23,893 per QALY gained).
Figure 4 shows the incremental costs and incremental QALYs associated with each of the diagnostic modalities modeled for 3 different pretest probability populations: low OSA prevalence of 20%, base case of 50%, and high OSA prevalence of 80%. In a cohort with low prevalence of 20%, diagnostic evaluation with FN-PSG cost less and provided more QALYs than all other diagnostic alternatives, with an ICER ranging from $18,062 (for a 30-year-old male) to $26,211 (for a 70-year-old female) per QALY gained. In a cohort with prevalence of 80%, for cohorts of men younger than age 50 and women younger than age 60, FN-PSG remains the economically most efficient alternative with ICERs ranging from $16,124 (for a 30-year old male) to $40,513 (for a 50 year old female) per QALY-gained. However, SN-PSG is most efficient for cohorts of men age 50 and older and for cohorts of women age 60 and older, with ICERs ranging from $16,599 (for a 50-year-old male) to $23,103 (for a 70-year-old female) per QALY-gained. At the low (20%) and moderate (50%) pretest probability, the key factor differentiating the diagnostic strategies is the rate of false-positive diagnoses and the long-term costs associated with those misdiagnoses. Because of the higher risk of false-positive diagnosis, the immediate cost-savings from choosing the less expensive, less specific test are overwhelmed by the long-term costs of treating the many false-positive diagnosis patients without benefit. In contrast, at the high pretest probability (80%), the key factors differentiating the diagnostic strategies are the cost of the technology and the long-term QALYs foregone associated with different rates of false-negative diagnoses. Using a less expensive, less sensitive test is not optimal in younger cohorts because of the greater number of years at risk for an event (MVC, MI, or stroke) that can be averted with diagnosis and treatment. It should be noted, however, that at a pretest probability of 80%, the lifetime costs and benefits of FN-PSG and SN-PSG are nearly indistinguishable from each other for all cohorts (differences in expected costs and QALYs are less than $300 and 0.004 QALY).
Figure 4.
Incremental cost-effectiveness of different diagnostic strategies for 3 different levels of OSA prevalence (pretest probability) (50-year-old male cohort).
Sensitivity Analysis
Extensive single-variable and multi-variable sensitivity analyses were performed to assess the robustness of the cost-effectiveness analysis (Table 5). The results were not sensitive to the cost of the diagnostic technologies. However, if FN-PSG and SN-PSG are both assumed to have perfect sensitivity and specificity, then SN-PSG is preferred because it is less expensive for some patients. If FN-PSG and SN-PSG have test characteristics at the lower range of published values, then UPHM becomes the preferred diagnostic strategy with an ICER of $48,206 per QALY gained. However, assuming base case accuracy for FN-PSG, UPHM is outperformed by FN-PSG, even if we assume the best case accuracy for UPHM (sensitivity and specificity of 94%). At a 50% pretest probability of OSA, the cost of the tests had little to no impact on the cost effectiveness of the optimal diagnostic strategy.
Table 5.
Sensitivity analysis: Incremental cost effectiveness ratios compared to the next best diagnostic strategy under various scenarios
| Scenario | No Diagnosis & No Treatment* | Split-Night PSG | Full-Night PSG | Unattended Portable Home Monitoring | Empiric Treatment |
|---|---|---|---|---|---|
| Base case | Dominated | 17,131 | Dominated | Dominated | |
| Test Characteristics | |||||
| Best case, FN | Dominated | 17,095 | Dominated | Dominated | |
| Worst case, FN | 19,876 | Dominated | 516,167 | 2,333,995 | |
| Best case, both FN & SN | 16,777 | Dominated | Dominated | Dominated | |
| Worst case, both FN & SN | Dominated | 20,448 | 48,206 | 2,333,995 | |
| Best case UPHM | Dominated | 17,131 | Dominated | Dominated | |
| Worst case UPHM | Dominated | 17,131 | Dominated | Dominated | |
| No patient refuses FN-PSG after technical failure with UPHM | Dominated | 17,131 | 782,167 | Dominated | |
| No technical failure from UPHM | Dominated | 17,131 | Dominated | Dominated | |
| OSA related risks | |||||
| Decreased impact of OSA on MVC risks | Dominated | 18,036 | Dominated | Dominated | |
| Increased impact of OSA on MVC risks | Dominated | 16,281 | Dominated | Dominated | |
| Decreased impact of OSA on hypertension risks | Dominated | 17,974 | Dominated | Dominated | |
| Increased impact of OSA on hypertension risks | Dominated | 16,551 | Dominated | Dominated | |
| Decreased impact of OSA on MI &stroke risk | Dominated | 23,554 | Dominated | Dominated | |
| Increased impact of OSA on MI & stroke risk | Dominated | 14,821 | Dominated | Dominated | |
| Efficacy of therapy | |||||
| Only MVC benefit | Dominated | 25,986 | Dominated | Dominated | |
| Only CV benefit | Dominated | 20,362 | Dominated | Dominated | |
| 75% of MVC and CV benefit | Dominated | 19,448 | Dominated | Dominated | |
| 50% of MVC and CV benefit | Dominated | 22,582 | Dominated | Dominated | |
| 25% of MVC and CV benefit | Dominated | 26,827 | Dominated | Dominated | |
| 0% of MVC and CV benefit (only benefit is the utility gain from reduced day-time sleepiness) | Dominated | 32,675 | Dominated | Dominated | |
| Compliance with Therapy | |||||
| Perfect compliance | Dominated | 16,696 | Dominated | Dominated | |
| Low compliance: Double the number of patients who never try therapy, & double the quit rate | Dominated | 17,726 | Dominated | Dominated | |
| False Positives self-identify by quitting CPAP therapy at 12 times the rate of True Positives | 17,074 | 51,557 | Dominated | 817,009 | |
| Cost | |||||
| Low FN-PSG cost | Dominated | 17,031 | Dominated | Dominated | |
| High FN-PSG cost | Dominated | 17,330 | Dominated | Dominated | |
| Low SN-PSG cost | Dominated | 17,131 | Dominated | Dominated | |
| Low UPHM cost | Dominated | 17,131 | Dominated | Dominated | |
| Low CPAP treatment cost | Dominated | 9,856 | Dominated | Dominated | |
| High CPAP treatment cost | Dominated | 42,593 | Dominated | Dominated | |
| Low cost of MVC | Dominated | 18,492 | Dominated | Dominated | |
| High cost of MVC | Dominated | 14,409 | Dominated | Dominated | |
| Low cost of CV chronic care | Dominated | 18,243 | Dominated | Dominated | |
| High cost of CV chronic care | Dominated | 13,947 | Dominated | Dominated | |
| Utilities | |||||
| Annualized equivalent of 2 days lost for attending sleep clinic for FN-PSG or SN-PSG | Dominated | 17,304 | Dominated | Dominated | |
| No utility gain from reduction in day-time sleepiness (only benefit is the utility gain from reduced MVC and CV risks) | Dominated | 37,974 | Dominated | Dominated | |
| Large utility gain associated with treated OSA; same as no OSA with CPAP (2% reduction from age-specific HR-QoL) | Dominated | 13,325 | Dominated | Dominated |
“No Diagnosis & No Treatment” was the least costly strategy in all scenarios tested. It is the comparator for the next strategy on the efficient frontier.
Dominated & Strategies that are not on the efficient frontier are described as dominated. Dominated strategies cost more and provide fewer QALYs than another strategy or a combination of strategies available to the decision-maker.
In our base case analysis, we assumed that UPHM had a substantial likelihood of technical failure and that only some patients who had this technical failure would undergo a FN-PSG as recommended. In scenarios in which we assumed that UPHM had no rate of technical failure, FN-PSG continued to be the preferred diagnostic strategy because the comparatively high number of false diagnoses in the UPHM arm resulted in higher costs and fewer QALYs than in the FN-PSG arm. In scenarios in which we continued to assume the base case rate of technical failure but we assumed that all patients would follow-up with the recommended FN-PSG, UPHM provided more QALYs than FN-PSG but with an ICER of $782,167 per QALY-gained and so FN-PSG continued to be the preferred strategy.
The cost effectiveness of OSA diagnosis is a function of the cost effectiveness of OSA treatment. In all scenarios in which treatment was less effective or more costly, diagnosis was also less cost effective and in all scenarios in which treatment was more effective or less costly, diagnosis was also more cost effective. Improved treatment compliance improves treatment effectiveness, but has a very small impact on the ICER of diagnostic modalities.
In our base case, we assumed that false positives would be equally likely to be compliant as true positives. However, it is possible that patients diagnosed as false positives would be more likely to stop CPAP therapy when they perceive no benefit from treatment. We performed a threshold analysis to determine whether this assumption could affect our results. We found that when false positives stop therapy at ≥ 12 times the rate of true positives, SN-PSG becomes preferable to FN-PSG.
Type III vs. Type IV UPHM Devices
In all but a few scenarios, type III unattended portable home monitoring devices were overshadowed by other diagnostic modalities. When FN-PSG and SN-PSG devices were estimated to have low sensitivity (85%), the incremental cost-effectiveness ratio of type III UPHM devices (91% sensitive) was $77,087 per QALY gained. We also considered type IV unattended portable home monitoring devices. These devices have fewer channels than type III devices—and thus lower sensitivity and specificity—but in most instances are currently reimbursed at the same rate. In all cases, diagnosis with type IV devices cost more overall and provided fewer QALYs than type III devices.
DISCUSSION
The objective of this study was to develop a model to evaluate the health impacts and cost-effectiveness of technologies used for the diagnosis and treatment of moderate-to-severe OSA.
Using a mathematical model of OSA, we found that CPAP therapy increases life expectancy and quality-adjusted life expectancy, and reduces the rate of fatal and non-fatal motor vehicle collision, myocardial infarction, and stroke. We also found that CPAP therapy is cost-effective for men and women at all ages considered (30-70 years) who have already been diagnosed with moderate-to-severe OSA with ICERs between $15,478 and $22,348 per QALY gained, which are lower than the ratios for treatments and technologies thought to be of good value and below the commonly used thresholds for determining cost effectiveness in the US.43 Table 6 presents the ICERs of various treatments and technologies for comparison to the ratios presented in this study.
Table 6.
Cost per Quality-Adjusted Life Year (QALY) gained from selected clinical strategies (results of this study are in bold)
| Intervention | ICER ($/QALY) |
|---|---|
| Corticosteroids vs. nonsteroidal anti-inflammatory drugs (NSAIDs) in combination with any disease-modifying anti-rheumatic drug (DMARD) in patients with rheumatoid arthritis (age 50)57 | Cost saving |
| Aspirin vs. no aspirin in combination with usual care in patients at high risk for cardiovascular disease58 | $2,840 |
| Mechanical thrombectomy vs. best medical therapy alone in patients with large-vessel ischemic stroke who are ineligible to receive tissue plasminogen activator (age 67)59 | $12,120 |
| CPAP therapy vs. no treatment in patients with a diagnosis of moderate-to-severe OSA (50-year-old male) | $15,915 |
| Full-night polysomnography and initiation of CPAP therapy vs. no diagnosis and no treatment for the diagnosis of OSA in patients with 50% pretest probability of OSA (50-year-old male) | $17,131 |
| Annual screening for proteinuria and subsequent treatment with ACE inhibitor or ARB therapy vs. routine clinical practice (No Screening) in patients with hypertension at annual physical (age 50)60 | $21,880 |
| Diabetes screening vs. routine clinical practice (no Screening) in patients with hypertension at annual physical (age 55)61 | $44,100 |
| Pioglitazone vs. placebo, in addition to usual care in high-risk patients with type 2 diabetes62 | $47,770 |
| Adult Treatment Panel III (ATP III) guidelines vs. alternate risk and age based strategies for identifying patients for cholesterol lowering statin therapy in the general population (ages 35-85)63 | $48,265 |
| Ileocolonoscopy with follow-up computed tomographic enterography (CTE) vs. ileocolonoscopy only for the diagnosis of small-bowel Crohn disease in patients with 75% pretest probability of small-bowel Crohn disease (30 year old)64 | $55,390 |
| HIV antibody screening every 5 years vs. one-time screening, for the diagnosis of HIV in general population with 1% pretest probability of HIV (age 42)65 | $63,900 |
| Annual mammogram from ages 40 to 80 vs. annual mammogram from ages 45 to 80 for the diagnosis of breast cancer in average risk women in the general population66 | $71,000 |
| Bone densitometry vs. no densitometry for the diagnosis of osteoporosis and fracture risk assessment in men with no history of clinical fracture (age 70)67 | $103,750 |
| One-time whole-body CT scan vs. routine preventive care at age 5068 | $180,500 per LY-gained |
| Dynamic susceptibility-weighted contrast material-enhanced (DSC) magnetic resonance (MR) imaging vs. standard clinical workup for the diagnosis of Alzheimer disease In community dwelling patients with mild to moderate dementia69 | $749,000 |
Values are given in 2008 U.S. dollars, with adjustment using the GDP deflator. Numbers are the ratios of the added cost per person to the gain in QALYs per person.
Comparisons of diagnostic modalities indicate that full-night polysomnography (FN-PSG) is cost-effective and preferred over other diagnostic modalities in almost all of the investigated populations and scenarios. Counter-intuitively, at pretest probabilities of 20% and 50% we found that FN-PSG, which is more expensive up-front, costs less and provides more QALYs than SN-PSG and UPHM over the lifetime of the patient. Over the lifetime horizon, SN-PSG and UPHM are more expensive than FN-PSG because they are less specific tests and therefore result in more false-positive diagnoses. When compared to other therapies that are commonly reimbursed in the United States (Table 6), diagnosis and treatment of OSA can be considered highly cost effective, justifying widespread use of these diagnostic and therapeutic modalities to improve health outcomes in individuals suspected of having or diagnosed with OSA.
The benefits of diagnosis and treatment of OSA are accrued by the patient in the form of increased quality of life associated with reduced daytime sleepiness and reduced risks of hypertension, heart attack, stroke, and motor vehicle collision. In addition, the reduction in the number of fatal and non-fatal motor vehicle collisions has a substantial societal public-safety impact that goes well beyond the benefits captured by the individual patient or by standard health-economic evaluations. Notably, only 2.35% of the total societal costs per MVC fatality, estimated at $1.196 million by the U.S. Department of Transportation,39 are direct medical costs and thus included in health-economic evaluations. The overall impact of OSA diagnosis and treatment can be expected to be substantially higher, especially when considering that accidents caused by OSA sufferers might lead to additional people being injured or killed in OSA-related accidents.
In light of the number of undiagnosed and therefore untreated OSA patients, the results of this study underscore the need to further strengthen current efforts to identify and treat OSA patients. The model results robustly indicate that full-night PSG is the optimal diagnostic choice.
From a health-economic perspective, UPHM, FN-PSG, and SN-PSG have very similar outcomes in populations with a high pretest-probability of OSA (patients with several clinical indicators highly suggestive of having OSA). This result is important, as it indicates that UPHM, despite its lower sensitivity and specificity, can be a cost-effective diagnostic approach (incremental cost-effectiveness ratio of $19,707 per QALY gained compared to no diagnosis) in situations where FN-PSG or SN-PSG are not available, where there are substantial wait times for in-lab diagnosis,44 or when patients are not willing or able to undergo evaluation in a sleep lab.
Prior studies have evaluated the cost effectiveness of CPAP therapy2,4,45,46 and the cost effectiveness of the various diagnosis strategies.6,47,48 Our analysis differs from these analyses because we consider a lifetime analysis horizon in contrast to several analyses that have considered a 5-year time horizon.2,4,6,45 Using a lifetime horizon ensures that all relevant future costs and health effects are captured in the analysis.11 Our analysis of the cost effectiveness of CPAP therapy is consistent with other life-time horizon analyses.46 In addition, a recent study by Ayas et al.49 estimates that, at a pretest prevalence greater than 0.47, a diagnostic protocol starting with UPHM has a lower total cost of diagnosis than FN-PSG.49 This study considers a diagnostic protocol using UPHM to rule-in OSA and then a trial of CPAP and follow-up FN-PSG to separate the true positive from false positive diagnoses. Further, by assuming that all patients who experience a technical failure or initially negative UPHM attend a follow-up FN-PSG, Ayas et al.49 assume that all initially missed cases are found. When we consider this protocol, assuming, as did Ayas, that all false positives would correctly perceive no health benefits from CPAP and that all technical failures and negatives would attend a FN-PSG, we find that the ICER of this modified-UPHM strategy is $29,122 per QALY-gained compared to FN-PSG when the pretest probability of OSA is 50%. However, if attendance at FN-PSG after failed or negative UPHM is imperfect, immediate FN-PSG remains the optimally efficient choice (ICER of $17,131 per QALY-gained). These findings exemplify the need for a lifetime horizon analysis when evaluating the cost-effectiveness of diagnostic strategies for a chronic condition.
Limitations
Our health-economic study has limitations, many of which are common to modeling studies. Our analysis relied on published studies for estimates of the impact of OSA and OSA treatment on event risks, costs, and QOL for modeled states. For some parameters, this literature is limited. Specifically, QOL for untreated and treated OSA has not been assessed in large-scale studies in the past, and our data came from two studies with a limited sample size. The focus of our analysis was the United States healthcare system; some of the results may not be generalizable to other healthcare systems and countries. To mitigate the limitations of existing data, and to expand the generalizability of the study results to settings and assumptions different from our base case, all parameters were assessed in sensitivity analyses.
The study population was limited to men and women of average cardiovascular risk, between the ages of 30 and 70 years. The impact of diagnosing and treating individuals with mild OSA or at higher-than-average baseline risk for cardiovascular events was not evaluated. In this analysis, CPAP was the only treatment considered although other treatments are available. The assumptions of CPAP effectiveness included in the model may not be appropriate for all patients, with additional factors that are currently not included in the model possibly influencing therapy effect.
Finally, our study assumed the baseline cost of CPAP accessories (mask, tubing, headgear, filters, humidifiers, and so on) to be 30% of Medicare's maximum allowable cost for all CPAP accessories. This estimate was based on interviews with practitioners in the field, and is within the range of previously published cost estimates.4 However, such costs may vary depending on specific preferences of patients and physicians, and may vary regionally within the United States.
CONCLUSION
The health-economic evaluation presented in this paper investigates the end-to-end process of OSA diagnosis and therapy, explicitly taking into account OSA-related motor vehicle collisions and important clinical outcome measures. Conclusions that full-night PSG for diagnosis and CPAP treatment of OSA are highly cost-effective are robust within the ranges of input parameter uncertainty. Diagnosis and treatment of OSA contributes to significant increases in patient quality of life and substantial reductions in the risk of motor vehicle collisions, heart attacks, and strokes. Payers and providers should expand access to patient screening and treatment for OSA to capture these large health and public safety benefits.
DISCLOSURE STATEMENT
The authors acknowledge the financial support by InHealth for this project. Northwestern University was the grant recipient, and subcontracted part of the research to Wing Tech Inc. Dr. Pietzsch is President and CEO and shareholder of Wing Tech Inc., a technology consulting firm focusing on early-stage assessment of medical technology. Wing Tech Inc. worked on a subcontract from Northwestern University to perform research on this project. Dr. Pietzsch was funded through this subcontract. Ms. Garner and Ms. Cipriano worked as a consultant for Wing Tech Inc. on this project and received funding through the Wing Tech subcontract from Northwestern University. Dr. Linehan worked as Principal Investigator on this project and received funding through the grant from InHealth to Northwestern University. All authors declare no conflicts of interest.
ACKNOWLEDGMENTS
This paper was written based on research performed by the authors as part of a study “Assessing the Impact of Medical Technology in the Diagnosis and Treatment of Obstructive Sleep Apnea,” funded by the Institute for Health Technology Studies (InHealth). The authors gratefully acknowledge the financial support by InHealth. This work was performed at Northwestern University, Wing Tech Inc., and Stanford University offices.
FOOTNOTE A: The QALY is an effectiveness measure that takes into account both the amount of time a patient stays in a given health state, and the quality of life that is associated with this health state.
FOOTNOTE B: Dominated strategies cost more and provide fewer QALYs than another strategy or a combination of strategies available to the decision-maker.
A commentary on this article appears in this issue on page 691.
REFERENCES
- 1.Kapur V, Strohl KP, Redline S, Iber C, O'Connor G, Nieto J. Underdiagnosis of sleep apnea syndrome in U.S. communities. Sleep Breath. 2002;6:49–54. doi: 10.1007/s11325-002-0049-5. [DOI] [PubMed] [Google Scholar]
- 2.Ayas NT, FitzGerald JM, Fleetham JA, et al. Cost-effectiveness of continuous positive airway pressure therapy for moderate to severe obstructive sleep apnea/hypopnea. Arch Intern Med. 2006;166:977–84. doi: 10.1001/archinte.166.9.977. [DOI] [PubMed] [Google Scholar]
- 3.Weatherly HL, Griffin SC, Mc Daid C, et al. An economic analysis of continuous positive airway pressure for the treatment of obstructive sleep apnea-hypopnea syndrome. Int J Technol Assess Health Care. 2009;25:26–34. doi: 10.1017/S0266462309090047. [DOI] [PubMed] [Google Scholar]
- 4.Sadatsafavi M, Marra CA, Ayas NT, Stradling J, Fleetham J. Cost-effectiveness of oral appliances in the treatment of obstructive sleep apnoea-hypopnoea. Sleep Breath. 2009 doi: 10.1007/s11325-009-0248-4. [DOI] [PubMed] [Google Scholar]
- 5.Mar J, Rueda JR, Duran-Cantolla J, Schechter C, Chilcott J. The cost-effectiveness of nCPAP treatment in patients with moderate-to-severe obstructive sleep apnoea. Eur Respir J. 2003;21:515–22. doi: 10.1183/09031936.03.00040903. [DOI] [PubMed] [Google Scholar]
- 6.Deutsch PA, Simmons MS, Wallace JM. Cost-effectiveness of split-night polysomnography and home studies in the evaluation of obstructive sleep apnea syndrome. J Clin Sleep Med. 2006;2:145–53. [PubMed] [Google Scholar]
- 7.George CF. Sleep apnea, alertness, and motor vehicle crashes. Am J Respir Crit Care Med. 2007;176:954–6. doi: 10.1164/rccm.200605-629PP. [DOI] [PubMed] [Google Scholar]
- 8.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 9.Chesson AL, Jr, Berry RB, Pack A. Practice parameters for the use of portable monitoring devices in the investigation of suspected obstructive sleep apnea in adults. Sleep. 2003;26:907–13. doi: 10.1093/sleep/26.7.907. [DOI] [PubMed] [Google Scholar]
- 10.Morgenthaler TI, Aurora RN, Brown T, et al. Practice parameters for the use of autotitrating continuous positive airway pressure devices for titrating pressures and treating adult patients with obstructive sleep apnea syndrome: an update for 2007. An American Academy of Sleep Medicine report. Sleep. 2008;31:141–7. doi: 10.1093/sleep/31.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gold MR, Siegel JE, Russell LB, Weinstein MC. Cost-effectiveness in Health and Medicine. New York: Oxford University Press; 1996. [Google Scholar]
- 12.Fletcher EC, Stich J, Yang KL. Unattended home diagnosis and treatment of obstructive sleep apnea without polysomnography. Arch Fam Med. 2000;9:168–74. doi: 10.1001/archfami.9.2.168. [DOI] [PubMed] [Google Scholar]
- 13.Arias E. United States Life Tables, 2004. National Vital Statistics Report. 2007;56:1–39. [PubMed] [Google Scholar]
- 14.Center for Disease Control; NCfHS. U.S. Deparment of Health and Human Services. Wahington, DC: DHHS; 2009. Health, United States, 2008. [Google Scholar]
- 15.Kuntz KM, Weinstein MC. Life expectancy biases in clinical decision modeling. Med Decis Making. 1995;15:158–69. doi: 10.1177/0272989X9501500209. [DOI] [PubMed] [Google Scholar]
- 16.British Heart Foundation Statistics Website. HeartStats. British Heart Foundation; 2009. [Google Scholar]
- 17.Brown RD, Whisnant JP, Sicks JD, O'Fallon WM, Wiebers DO. Stroke incidence, prevalence, and survival: secular trends in Rochester, Minnesota, through 1989. Stroke. 1996;27:373–80. [PubMed] [Google Scholar]
- 18.Hollander M, Koudstaal PJ, Bots ML, Grobbee DE, Hofman A, Breteler MM. Incidence, risk, and case fatality of first ever stroke in the elderly population. The Rotterdam Study. J Neurol Neurosurg Psychiatry. 2003;74:317–21. doi: 10.1136/jnnp.74.3.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cutler JA, Sorlie PD, Wolz M, Thom T, Fields LE, Roccella EJ. Trends in hypertension prevalence, awareness, treatment, and control rates in United States adults between 1988-1994 and 1999-2004. Hypertension. 2008;52:818–27. doi: 10.1161/HYPERTENSIONAHA.108.113357. [DOI] [PubMed] [Google Scholar]
- 20.Psaty BM, Furberg CD, Kuller LH, et al. Association between blood pressure level and the risk of myocardial infarction, stroke, and total mortality: the cardiovascular health study. Arch Intern Med. 2001;161:1183–92. doi: 10.1001/archinte.161.9.1183. [DOI] [PubMed] [Google Scholar]
- 21.Chang D Analysis NCfSa. Washington, DC: National Highway Traffic Safety Administration; 2008. Comparison of crash fatalities by sex and age group. [Google Scholar]
- 22.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–41. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 23.George CF. Reduction in motor vehicle collisions following treatment of sleep apnoea with nasal CPAP. Thorax. 2001;56:508–12. doi: 10.1136/thorax.56.7.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barbe F, Sunyer J, de la Pena A, et al. Effect of continuous positive airway pressure on the risk of road accidents in sleep apnea patients. Respiration. 2007;74:44–9. doi: 10.1159/000094237. [DOI] [PubMed] [Google Scholar]
- 25.O'Connor GT, Caffo B, Newman AB, et al. Prospective study of sleep-disordered breathing and hypertension: the Sleep Heart Health Study. Am J Respir Crit Care Med. 2009;179:1159–64. doi: 10.1164/rccm.200712-1809OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McArdle N, Devereux G, Heidarnejad H, Engleman HM, Mackay TW, Douglas NJ. Long-term use of CPAP therapy for sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med. 1999;159:1108–14. doi: 10.1164/ajrccm.159.4.9807111. [DOI] [PubMed] [Google Scholar]
- 27.Becker HF, Jerrentrup A, Ploch T, et al. Effect of nasal continuous positive airway pressure treatment on blood pressure in patients with obstructive sleep apnea. Circulation. 2003;107:68–73. doi: 10.1161/01.cir.0000042706.47107.7a. [DOI] [PubMed] [Google Scholar]
- 28.Haentjens P, Van Meerhaeghe A, Moscariello A, et al. The impact of continuous positive airway pressure on blood pressure in patients with obstructive sleep apnea syndrome: evidence from a meta-analysis of placebo-controlled randomized trials. Arch Intern Med. 2007;167:757–64. doi: 10.1001/archinte.167.8.757. [DOI] [PubMed] [Google Scholar]
- 29.Sullivan PW, Ghushchyan V. Preference-Based EQ-5D index scores for chronic conditions in the United States. Med Decis Making. 2006;26:410–20. doi: 10.1177/0272989X06290495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nyman JA, Barleen NA, Kirdruang P. Quality-adjusted life years lost from nonfatal motor vehicle accident injuries. Med Decis Making. 2008;28:819–28. doi: 10.1177/0272989X08318463. [DOI] [PubMed] [Google Scholar]
- 31.Tousignant P, Cosio MG, Levy RD, Groome PA. Quality adjusted life years added by treatment of obstructive sleep apnea. Sleep. 1994;17:52–60. [PubMed] [Google Scholar]
- 32.Chakravorty I, Cayton R, Szczepura A. Health utilities in evaluating intervention in the sleep apnoea/hypopnoea syndrome. Eur Respir J. 2002;20:1233–8. doi: 10.1183/09031936.00.00014401. [DOI] [PubMed] [Google Scholar]
- 33.Meara E, White C, Cutler DM. Trends in medical spending by age, 1963-2000. Health Aff (Millwood) 2004;23:176–83. doi: 10.1377/hlthaff.23.4.176. [DOI] [PubMed] [Google Scholar]
- 34.Hogan C, Lunney J, Gabel J, Lynn J. Medicare beneficiaries' costs of care in the last year of life. Health Aff (Millwood) 2001;20:188–95. doi: 10.1377/hlthaff.20.4.188. [DOI] [PubMed] [Google Scholar]
- 35.Stuart B, Lloyd J, Zhao L, Kamal-Bahl S. Obesity, disease burden, and prescription spending by community-dwelling Medicare beneficiaries. Curr Med Res Opin. 2008;24:2377–87. doi: 10.1185/03007990802262275. [DOI] [PubMed] [Google Scholar]
- 36.Taylor TN, Davis PH, Torner JC, Holmes J, Meyer JW, Jacobson MF. Lifetime cost of stroke in the United States. Stroke. 1996;27:1459–66. doi: 10.1161/01.str.27.9.1459. [DOI] [PubMed] [Google Scholar]
- 37.Kauf TL, Velazquez EJ, Crosslin DR, et al. The cost of acute myocardial infarction in the new millennium: evidence from a multinational registry. Am Heart J. 2006;151:206–12. doi: 10.1016/j.ahj.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 38.Qureshi AI, Suri MF, Nasar A, et al. Changes in cost and outcome among US patients with stroke hospitalized in 1990 to 1991 and those hospitalized in 2000 to 2001. Stroke. 2007;38:2180–4. doi: 10.1161/STROKEAHA.106.467506. [DOI] [PubMed] [Google Scholar]
- 39.Blincoe LJ, Seay A, Zaloshnja E, et al. Administration NHTS. Washington, DC: U.S. Department of Transportation; 2002. The Economic Impact of Motor Vehicle Crashes, 2000. [Google Scholar]
- 40.Littner M, Hirshkowitz M, Davila D, et al. Practice parameters for the use of auto-titrating continuous positive airway pressure devices for titrating pressures and treating adult patients with obstructive sleep apnea syndrome. An American Academy of Sleep Medicine report. Sleep. 2002;25:143–7. doi: 10.1093/sleep/25.2.143. [DOI] [PubMed] [Google Scholar]
- 41.Campos-Rodriguez F, Pena-Grinan N, Reyes-Nunez N, et al. Mortality in obstructive sleep apnea-hypopnea patients treated with positive airway pressure. Chest. 2005;128:624–33. doi: 10.1378/chest.128.2.624. [DOI] [PubMed] [Google Scholar]
- 42.McDaid C, Duree KH, Griffin SC, et al. A systematic review of continuous positive airway pressure for obstructive sleep apnoea-hypopnoea syndrome. Sleep Med Rev. 2009;13:427–36. doi: 10.1016/j.smrv.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 43.Weinstein MC, Skinner JA. Comparative effectiveness and health care spending--implications for reform. N Engl J Med. 2010;362:460–5. doi: 10.1056/NEJMsb0911104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Flemons WW, Douglas NJ, Kuna ST, Rodenstein DO, Wheatley J. Access to diagnosis and treatment of patients with suspected sleep apnea. Am J Respir Crit Care Med. 2004;169:668–72. doi: 10.1164/rccm.200308-1124PP. [DOI] [PubMed] [Google Scholar]
- 45.Tan MC, Ayas NT, Mulgrew A, et al. Cost-effectiveness of continuous positive airway pressure therapy in patients with obstructive sleep apnea-hypopnea in British Columbia. Can Respir J. 2008;15:159–65. doi: 10.1155/2008/719231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McDaid C, Griffin S, Weatherly H, et al. Continuous positive airway pressure devices for the treatment of obstructive sleep apnoea-hypopnoea syndrome: a systematic review and economic analysis. Health Technol Assess. 2009;13 doi: 10.3310/hta13040. iii-iv, xi-xiv, 1-119, 43-274. [DOI] [PubMed] [Google Scholar]
- 47.Chervin RD, Murman DL, Malow BA, Totten V. Cost-utility of three approaches to the diagnosis of sleep apnea: polysomnography, home testing, and empirical therapy. Ann Intern Med. 1999;130:496–505. doi: 10.7326/0003-4819-130-6-199903160-00006. [DOI] [PubMed] [Google Scholar]
- 48.Epstein LJ, Dorlac GR. Cost-effectiveness analysis of nocturnal oximetry as a method of screening for sleep apnea-hypopnea syndrome. Chest. 1998;113:97–103. doi: 10.1378/chest.113.1.97. [DOI] [PubMed] [Google Scholar]
- 49.Ayas NT, Fox J, Epstein L, Ryan CF, Fleetham JA. Initial use of portable monitoring versus polysomnography to confirm obstructive sleep apnea in symptomatic patients: an economic decision model. Sleep Med. 2010;11:320–4. doi: 10.1016/j.sleep.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 50.Volmink JA, Newton JN, Hicks NR, Sleight P, Fowler GH, Neil HA. Coronary event and case fatality rates in an English population: results of the Oxford myocardial infarction incidence study. The Oxford Myocardial Infarction Incidence Study Group. Heart. 1998;80:40–4. doi: 10.1136/hrt.80.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marshall NS, Wong KK, Liu PY, Cullen SR, Knuiman MW, Grunstein RR. Sleep apnea as an independent risk factor for all-cause mortality: the Busselton Health Study. Sleep. 2008;31:1079–85. [PMC free article] [PubMed] [Google Scholar]
- 52.Roger VL, Jacobsen SJ, Weston SA, et al. Trends in the incidence and survival of patients with hospitalized myocardial infarction, Olmsted County, Minnesota, 1979 to 1994. Ann Intern Med. 2002;136:341–8. doi: 10.7326/0003-4819-136-5-200203050-00005. [DOI] [PubMed] [Google Scholar]
- 53.Dennis MS, Burn JP, Sandercock PA, Bamford JM, Wade DT, Warlow CP. Long-term survival after first-ever stroke: the Oxfordshire Community Stroke Project. Stroke. 1993;24:796–800. doi: 10.1161/01.str.24.6.796. [DOI] [PubMed] [Google Scholar]
- 54.Trikalinos TA, Lau J. Obstructive Sleep Apnea-Hypopnea Syndrome: modeling different diagnostic strategies. [cited 2009];2007 12/04/07. Available from: http://www.cms.hhs.gov/determinationprocess/downloads/id50TA.pdf. [PubMed] [Google Scholar]
- 55.Reichert JA, Bloch DA, Cundiff E, Votteri BA. Comparison of the NovaSom QSG, a new sleep apnea home-diagnostic system, and polysomnography. Sleep Med. 2003;4:213–8. doi: 10.1016/s1389-9457(02)00234-4. [DOI] [PubMed] [Google Scholar]
- 56.Woodson BT, Steward DL, Weaver EM, Javaheri S. A randomized trial of temperature-controlled radiofrequency, continuous positive airway pressure, and placebo for obstructive sleep apnea syndrome. Otolaryngol Head Neck Surg. 2003;128:848–61. doi: 10.1016/S0194-59980300461-3. [DOI] [PubMed] [Google Scholar]
- 57.Bae SC, Corzillius M, Kuntz KM, Liang MH. Cost-effectiveness of low dose corticosteroids versus non-steroidal anti-inflammatory drugs and COX-2 specific inhibitors in the long-term treatment of rheumatoid arthritis. Rheumatology (Oxford) 2003;42:46–53. doi: 10.1093/rheumatology/keg029. [DOI] [PubMed] [Google Scholar]
- 58.Kahn R, Robertson RM, Smith R, Eddy D. The impact of prevention on reducing the burden of cardiovascular disease. Diabetes Care. 2008;31:1686–96. doi: 10.2337/dc08-9022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Patil CG, Long EF, Lansberg MG. Cost-effectiveness analysis of mechanical thrombectomy in acute ischemic stroke. J Neurosurg. 2009;110:508–13. doi: 10.3171/2008.8.JNS08133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Boulware LE, Jaar BG, Tarver-Carr ME, Brancati FL, Powe NR. Screening for proteinuria in US adults: a cost-effectiveness analysis. JAMA. 2003;290:3101–14. doi: 10.1001/jama.290.23.3101. [DOI] [PubMed] [Google Scholar]
- 61.Hoerger TJ, Harris R, Hicks KA, Donahue K, Sorensen S, Engelgau M. Screening for type 2 diabetes mellitus: a cost-effectiveness analysis. Ann Intern Med. 2004;140:689–99. doi: 10.7326/0003-4819-140-9-200405040-00008. [DOI] [PubMed] [Google Scholar]
- 62.Valentine WJ, Tucker D, Palmer AJ, Minshall ME, Foos V, Silberman C. Long-term cost-effectiveness of pioglitazone versus placebo in addition to existing diabetes treatment: a US analysis based on PROactive. Value Health. 2009;12:1–9. doi: 10.1111/j.1524-4733.2008.00403.x. [DOI] [PubMed] [Google Scholar]
- 63.Pletcher MJ, Lazar L, Bibbins-Domingo K, et al. Comparing impact and cost-effectiveness of primary prevention strategies for lipid-lowering. Ann Intern Med. 2009;150:243–54. doi: 10.7326/0003-4819-150-4-200902170-00005. [DOI] [PubMed] [Google Scholar]
- 64.Levesque BG, Cipriano LE, Chang SL, Lee KK, Owens DK, Garber AM. Cost effectiveness of alternative imaging strategies for the diagnosis of small-bowel Crohn's disease. Clin Gastroenterol Hepatol. 2010;8 doi: 10.1016/j.cgh.2009.10.032. 261-7, 7 e1-4. [DOI] [PubMed] [Google Scholar]
- 65.Sanders GD, Bayoumi AM, Sundaram V, et al. Cost-effectiveness of screening for HIV in the era of highly active antiretroviral therapy. N Engl J Med. 2005;352:570–85. doi: 10.1056/NEJMsa042657. [DOI] [PubMed] [Google Scholar]
- 66.Stout NK, Rosenberg MA, Trentham-Dietz A, Smith MA, Robinson SM, Fryback DG. Retrospective cost-effectiveness analysis of screening mammography. J Natl Cancer Inst. 2006;98:774–82. doi: 10.1093/jnci/djj210. [DOI] [PubMed] [Google Scholar]
- 67.Schousboe JT, Taylor BC, Fink HA, et al. Cost-effectiveness of bone densitometry followed by treatment of osteoporosis in older men. JAMA. 2007;298:629–37. doi: 10.1001/jama.298.6.629. [DOI] [PubMed] [Google Scholar]
- 68.Beinfeld MT, Wittenberg E, Gazelle GS. Cost-effectiveness of whole-body CT screening. Radiology. 2005;234:415–22. doi: 10.1148/radiol.2342032061. [DOI] [PubMed] [Google Scholar]
- 69.McMahon PM, Araki SS, Sandberg EA, Neumann PJ, Gazelle GS. Cost-effectiveness of PET in the diagnosis of Alzheimer disease. Radiology. 2003;228:515–22. doi: 10.1148/radiol.2282020915. [DOI] [PubMed] [Google Scholar]