Abstract
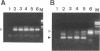
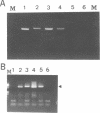
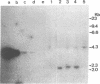
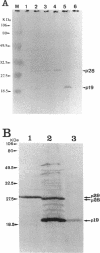
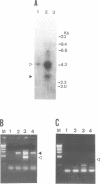
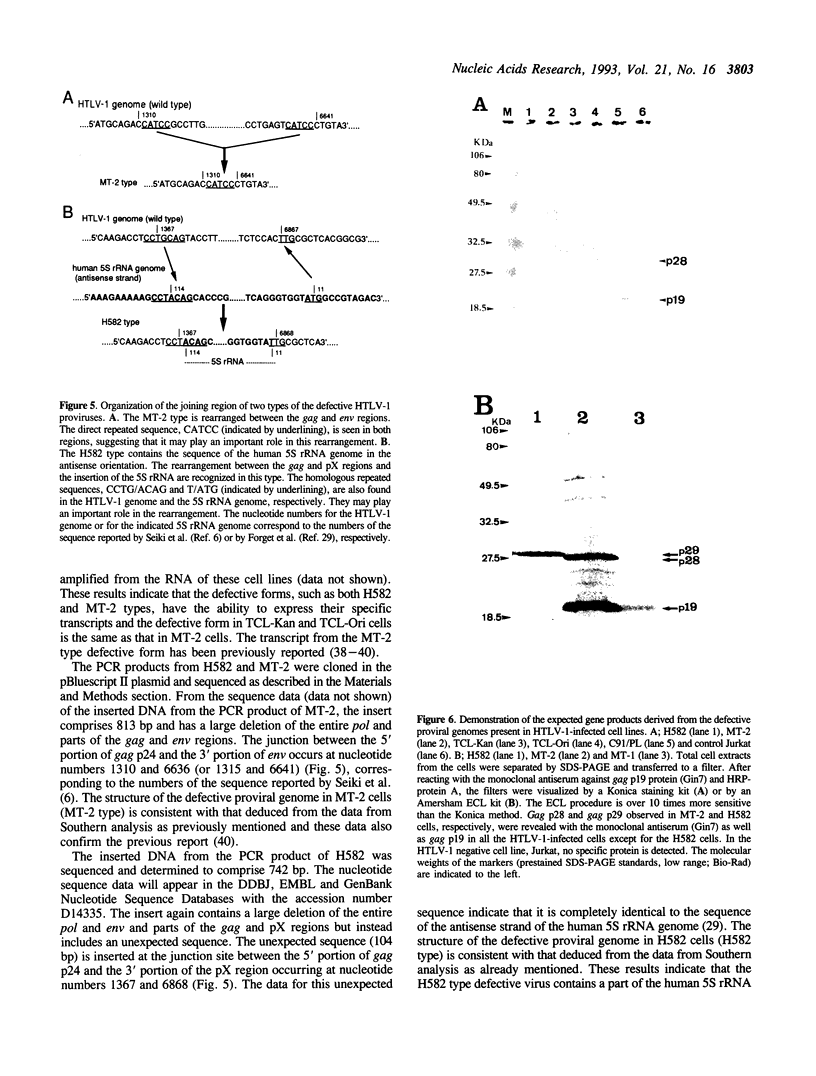
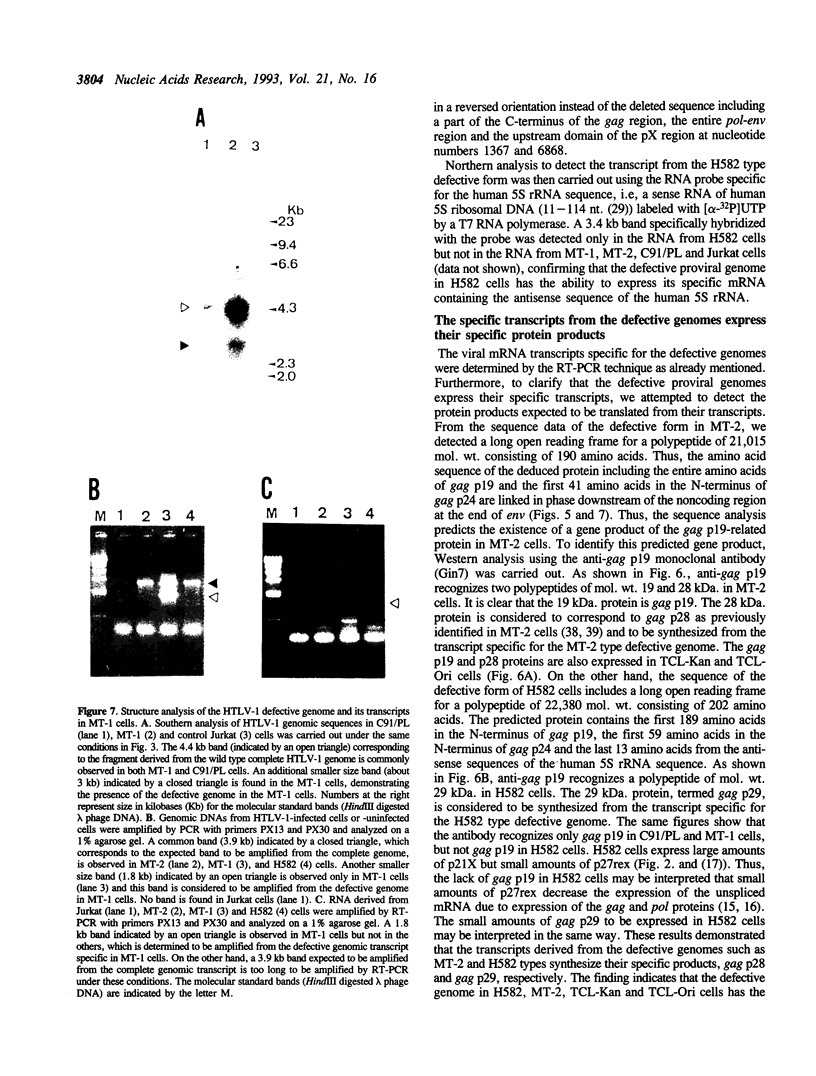
In addition to the three typical transcripts such as genomic/gag-pol mRNA, env mRNA and tax/rex mRNA, we previously found the singly spliced pX mRNA, termed p21X mRNA, responsible for producing the p21X protein in human T-cell leukemia virus type 1 (HTLV-1)-infected cells. Our finding of the p21X mRNA being constitutively expressed in the fresh peripheral blood mononuclear cells (PBMCs) from patients with ATL has suggested that the expression mechanism is quite different from that of the others. In this paper, the expression mechanism of p21X mRNA was investigated by analyzing the organization of the proviral genomes present in the representative HTLV-1-infected cell lines which are positive or negative for the expression of p21X mRNA. Southern and PCR analyses show that most of the analyzed cell lines contain both one complete and one defective genome each. However, one cell line without the p21X mRNA expression, C91/PL, contains only the complete genome, suggesting that the complete HTLV-1 has no ability to express p21X mRNA in spite of having the ability to produce the infectious virus. The defective genomes of the p21X mRNA positive cell lines, MT-2 and H582, have a large deletion of the entire pol and parts of the gag and env regions including the common domain of the second exon of the doubly spliced tax/rex mRNA, while another defective genome of the p21X mRNA negative cell line, MT-1, has a deletion within the gag-pol gene. We show that these defective genomes have the ability to express their distinct, defective genomic mRNA, suggesting they are active. The defective genomic mRNAs in MT-2 and H582 cells retain the first splice donor and the second splice acceptor sites, suggesting the possibility of synthesizing p21X mRNA by splicing singly with these sites. These findings assume that defective HTLV-1 genomes deleting the second exon region acquire the ability to express p21X mRNA but no ability to express tax/rex mRNA. Such a deletion may explain the difference between the expression mechanisms in the p21X mRNA transcript and those in the other viral transcripts.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Forget B. G., Weissman S. M. The nucleotide sequence of ribosomal 5 S ribonucleic acid from KB cells. J Biol Chem. 1969 Jun 25;244(12):3148–3165. [PubMed] [Google Scholar]
- Fujisawa J., Seiki M., Kiyokawa T., Yoshida M. Functional activation of the long terminal repeat of human T-cell leukemia virus type I by a trans-acting factor. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2277–2281. doi: 10.1073/pnas.82.8.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessain A., Barin F., Vernant J. C., Gout O., Maurs L., Calender A., de Thé G. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet. 1985 Aug 24;2(8452):407–410. doi: 10.1016/s0140-6736(85)92734-5. [DOI] [PubMed] [Google Scholar]
- Hidaka M., Inoue J., Yoshida M., Seiki M. Post-transcriptional regulator (rex) of HTLV-1 initiates expression of viral structural proteins but suppresses expression of regulatory proteins. EMBO J. 1988 Feb;7(2):519–523. doi: 10.1002/j.1460-2075.1988.tb02840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinuma Y., Nagata K., Hanaoka M., Nakai M., Matsumoto T., Kinoshita K. I., Shirakawa S., Miyoshi I. Adult T-cell leukemia: antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6476–6480. doi: 10.1073/pnas.78.10.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino T., Takeuchi K., Nam S. H., Siomi H., Sabe H., Kobayashi N., Hatanaka M. Structural analysis of p28 adult T-cell leukaemia-associated antigen. J Gen Virol. 1986 Jul;67(Pt 7):1373–1379. doi: 10.1099/0022-1317-67-7-1373. [DOI] [PubMed] [Google Scholar]
- Inoue J., Yoshida M., Seiki M. Transcriptional (p40x) and post-transcriptional (p27x-III) regulators are required for the expression and replication of human T-cell leukemia virus type I genes. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3653–3657. doi: 10.1073/pnas.84.11.3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannagi M., Sugamura K., Sato H., Okochi K., Uchino H., Hinuma Y. Establishment of human cytotoxic T cell lines specific for human adult T cell leukemia virus-bearing cells. J Immunol. 1983 Jun;130(6):2942–2946. [PubMed] [Google Scholar]
- Kaplan J., Tilton J., Peterson W. D., Jr Identification of T cell lymphoma tumor antigens on human T cell lines. Am J Hematol. 1976;1(2):219–223. doi: 10.1002/ajh.2830010206. [DOI] [PubMed] [Google Scholar]
- Katoh I., Yasunaga T., Yoshinaka Y. Bovine leukemia virus RNA sequences involved in dimerization and specific gag protein binding: close relation to the packaging sites of avian, murine, and human retroviruses. J Virol. 1993 Apr;67(4):1830–1839. doi: 10.1128/jvi.67.4.1830-1839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz R. A., Terry R. W., Skalka A. M. A conserved cis-acting sequence in the 5' leader of avian sarcoma virus RNA is required for packaging. J Virol. 1986 Jul;59(1):163–167. doi: 10.1128/jvi.59.1.163-167.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyokawa T., Seiki M., Iwashita S., Imagawa K., Shimizu F., Yoshida M. p27x-III and p21x-III, proteins encoded by the pX sequence of human T-cell leukemia virus type I. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8359–8363. doi: 10.1073/pnas.82.24.8359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi N., Koyanagi Y., Yamamoto N., Hinuma Y., Sato H., Okochi K., Hatanaka M. 28,000-dalton polypeptide (p28) of adult T-cell leukemia-associated antigen encoded by 24 S mRNA of human T-cell leukemia virus has an associated protein kinase activity. J Biol Chem. 1984 Sep 25;259(18):11162–11164. [PubMed] [Google Scholar]
- Konishi H., Kobayashi N., Hatanaka M. Defective human T-cell leukemia virus in adult T-cell leukemia patients. Mol Biol Med. 1984 Aug;2(4):273–283. [PubMed] [Google Scholar]
- Korber B., Okayama A., Donnelly R., Tachibana N., Essex M. Polymerase chain reaction analysis of defective human T-cell leukemia virus type I proviral genomes in leukemic cells of patients with adult T-cell leukemia. J Virol. 1991 Oct;65(10):5471–5476. doi: 10.1128/jvi.65.10.5471-5476.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T. H., Coligan J. E., Sodroski J. G., Haseltine W. A., Salahuddin S. Z., Wong-Staal F., Gallo R. C., Essex M. Antigens encoded by the 3'-terminal region of human T-cell leukemia virus: evidence for a functional gene. Science. 1984 Oct 5;226(4670):57–61. doi: 10.1126/science.6089350. [DOI] [PubMed] [Google Scholar]
- Lever A., Gottlinger H., Haseltine W., Sodroski J. Identification of a sequence required for efficient packaging of human immunodeficiency virus type 1 RNA into virions. J Virol. 1989 Sep;63(9):4085–4087. doi: 10.1128/jvi.63.9.4085-4087.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann R., Mulligan R. C., Baltimore D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell. 1983 May;33(1):153–159. doi: 10.1016/0092-8674(83)90344-6. [DOI] [PubMed] [Google Scholar]
- Manzari V., Wong-Staal F., Franchini G., Colombini S., Gelmann E. P., Oroszlan S., Staal S., Gallo R. C. Human T-cell leukemia-lymphoma virus (HTLV): cloning of an integrated defective provirus and flanking cellular sequences. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1574–1578. doi: 10.1073/pnas.80.6.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi I., Kubonishi I., Yoshimoto S., Akagi T., Ohtsuki Y., Shiraishi Y., Nagata K., Hinuma Y. Type C virus particles in a cord T-cell line derived by co-cultivating normal human cord leukocytes and human leukaemic T cells. Nature. 1981 Dec 24;294(5843):770–771. doi: 10.1038/294770a0. [DOI] [PubMed] [Google Scholar]
- Nagashima K., Yoshida M., Seiki M. A single species of pX mRNA of human T-cell leukemia virus type I encodes trans-activator p40x and two other phosphoproteins. J Virol. 1986 Nov;60(2):394–399. doi: 10.1128/jvi.60.2.394-399.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohshima K., Kikuchi M., Masuda Y., Kobari S., Sumiyoshi Y., Eguchi F., Mohtai H., Yoshida T., Takeshita M., Kimura N. Defective provirus form of human T-cell leukemia virus type I in adult T-cell leukemia/lymphoma: clinicopathological features. Cancer Res. 1991 Sep 1;51(17):4639–4642. [PubMed] [Google Scholar]
- Orita S., Higashi T., Kawasaki Y., Harada A., Igarashi H., Monden T., Morimoto H., Shimano T., Mori T., Miyoshi J. A novel point mutation at codon 146 of the K-ras gene in a human colorectal cancer identified by the polymerase chain reaction. Virus Genes. 1991 Jan;5(1):75–79. doi: 10.1007/BF00571733. [DOI] [PubMed] [Google Scholar]
- Orita S., Saiga A., Takagi S., Tanaka T., Okumura K., Aono Y., Hinuma Y., Igarashi H. A novel alternatively spliced viral mRNA transcribed in cells infected with human T cell leukemia virus type 1 is mainly responsible for expressing p21X protein. FEBS Lett. 1991 Dec 16;295(1-3):127–134. doi: 10.1016/0014-5793(91)81402-t. [DOI] [PubMed] [Google Scholar]
- Orita S., Takagi S., Saiga A., Minoura N., Araki K., Kinoshita K., Kondo T., Hinuma Y., Igarashi H. Human T cell leukaemia virus type 1 p21X mRNA: constitutive expression in peripheral blood mononuclear cells of patients with adult T cell leukaemia. J Gen Virol. 1992 Sep;73(Pt 9):2283–2289. doi: 10.1099/0022-1317-73-9-2283. [DOI] [PubMed] [Google Scholar]
- Osame M., Usuku K., Izumo S., Ijichi N., Amitani H., Igata A., Matsumoto M., Tara M. HTLV-I associated myelopathy, a new clinical entity. Lancet. 1986 May 3;1(8488):1031–1032. doi: 10.1016/s0140-6736(86)91298-5. [DOI] [PubMed] [Google Scholar]
- Poiesz B. J., Ruscetti F. W., Reitz M. S., Kalyanaraman V. S., Gallo R. C. Isolation of a new type C retrovirus (HTLV) in primary uncultured cells of a patient with Sézary T-cell leukaemia. Nature. 1981 Nov 19;294(5838):268–271. doi: 10.1038/294268a0. [DOI] [PubMed] [Google Scholar]
- Popovic M., Sarin P. S., Robert-Gurroff M., Kalyanaraman V. S., Mann D., Minowada J., Gallo R. C. Isolation and transmission of human retrovirus (human t-cell leukemia virus). Science. 1983 Feb 18;219(4586):856–859. doi: 10.1126/science.6600519. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiki M., Hattori S., Hirayama Y., Yoshida M. Human adult T-cell leukemia virus: complete nucleotide sequence of the provirus genome integrated in leukemia cell DNA. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3618–3622. doi: 10.1073/pnas.80.12.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiki M., Hikikoshi A., Taniguchi T., Yoshida M. Expression of the pX gene of HTLV-I: general splicing mechanism in the HTLV family. Science. 1985 Jun 28;228(4707):1532–1534. doi: 10.1126/science.2990031. [DOI] [PubMed] [Google Scholar]
- Siomi H., Nosaka T., Saida T., Miwa H., Hinuma Y., Shirakawa S., Miyamoto N., Kondo T., Araki K., Ichimaru M. Two major subgroups of human T-cell leukemia virus-1 in Japan. Virus Genes. 1988 Jul;1(4):377–383. doi: 10.1007/BF00257100. [DOI] [PubMed] [Google Scholar]
- Siomi H., Shida H., Nam S. H., Nosaka T., Maki M., Hatanaka M. Sequence requirements for nucleolar localization of human T cell leukemia virus type I pX protein, which regulates viral RNA processing. Cell. 1988 Oct 21;55(2):197–209. doi: 10.1016/0092-8674(88)90043-8. [DOI] [PubMed] [Google Scholar]
- Slamon D. J., Shimotohno K., Cline M. J., Golde D. W., Chen I. S. Identification of the putative transforming protein of the human T-cell leukemia viruses HTLV-I and HTLV-II. Science. 1984 Oct 5;226(4670):61–65. doi: 10.1126/science.6089351. [DOI] [PubMed] [Google Scholar]
- Sodroski J. G., Rosen C. A., Haseltine W. A. Trans-acting transcriptional activation of the long terminal repeat of human T lymphotropic viruses in infected cells. Science. 1984 Jul 27;225(4660):381–385. doi: 10.1126/science.6330891. [DOI] [PubMed] [Google Scholar]
- Takeuchi K., Kobayashi N., Nam S. H., Yamamoto N., Hatanaka M. Molecular cloning of cDNA encoding gp68 of adult T-cell leukaemia-associated antigen: evidence for expression of the pX IV region of human T-cell leukaemia virus. J Gen Virol. 1985 Aug;66(Pt 8):1825–1829. doi: 10.1099/0022-1317-66-8-1825. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Koyanagi Y., Chosa T., Yamamoto N., Hinuma Y. Monoclonal antibody reactive with both p28 and p19 of adult T-cell leukemia virus-specific polypeptides. Gan. 1983 Jun;74(3):327–330. [PubMed] [Google Scholar]
- Watanabe S., Temin H. M. Encapsidation sequences for spleen necrosis virus, an avian retrovirus, are between the 5' long terminal repeat and the start of the gag gene. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5986–5990. doi: 10.1073/pnas.79.19.5986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto N., Okada M., Koyanagi Y., Kannagi M., Hinuma Y. Transformation of human leukocytes by cocultivation with an adult T cell leukemia virus producer cell line. Science. 1982 Aug 20;217(4561):737–739. doi: 10.1126/science.6980467. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Miyoshi I., Hinuma Y. Isolation and characterization of retrovirus from cell lines of human adult T-cell leukemia and its implication in the disease. Proc Natl Acad Sci U S A. 1982 Mar;79(6):2031–2035. doi: 10.1073/pnas.79.6.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M., Seiki M. Recent advances in the molecular biology of HTLV-1: trans-activation of viral and cellular genes. Annu Rev Immunol. 1987;5:541–559. doi: 10.1146/annurev.iy.05.040187.002545. [DOI] [PubMed] [Google Scholar]