Abstract
The main objectives of current work were (1) to compare the effects of monophasic or biphasic electrical field stimulation on structure and function of engineered cardiac organoids based on enriched cardiomyocytes (CM) and (2) to determine if electrical field stimulation will enhance electrical excitability of cardiac organoids based on multiple cell types. Organoids resembling cardiac myofibers were cultivated in Matrigel-coated microchannels fabricated of poly(ethylene glycol)-diacrylate. We found that field stimulation using symmetric biphasic square pulses at 2.5 V/cm, 1 Hz, 1 ms (per pulse phase) was an improved stimulation protocol, as compared to no stimulation and stimulation using monophasic square pulses of identical total amplitude and duration (5 V/cm, 1 Hz, 2 ms). This was supported by the highest success rate for synchronous contractions, low excitation threshold, the highest cell density, and the highest expression of Connexin-43 in the biphasic group. Subsequently, enriched CM were seeded on the networks of (1) cardiac fibroblasts (FB), (2) D4T endothelial cells (EC), or (3) a mixture of FB and EC that were precultured for 2 days prior to the addition of enriched CM. Biphasic field stimulation was also effective at improving electrical excitability of these cardiac organoids by improving the three-dimensional organization of the cells, increasing cellular elongation and enhancing Connexin-43 presence.
Introduction
The goal of cardiac tissue engineering is to create tissue constructs of similar properties to the native myocardium that will ultimately be used as replacement tissues for myocardial infarction or congestive heart failure.1 Flexibility, mechanical stability, and contractile functionality are amongst the most important characteristics of engineered cardiac constructs.
Native myocardium consists of multiple cell types, including cardiomyocytes (CM), and up to 70% of nonmyocytes (fibroblasts [FB] and endothelial cells [EC]), which are actively involved in cross talk.2 We demonstrated that cultivation of enriched CM on synthetic scaffolds pretreated with FB improved electrical excitability compared to cultivation of enriched CM alone.3 Similarly, Narmoneva et al. showed that the cultivation of CM on preformed networks of EC inhibited CM apoptosis and necrosis.4 Endothelial cells also improved the three-dimensional organization of CM by forming capillary-like networks that promoted CM reorganization.4 This is consistent with other reports showing that coculture of ventricular nonmyocytes and myocytes prevented doxorubicin-induced myocyte apoptosis by the activation of cAMP response element-binding protein (CREB), which was mediated by the secretion of endothelin-1 by nonmyocytes.5
Ventricular CM beat in response to electrical impulses whose rapid propagation requires high cell density and formation of gap junction between adjoining CM. Our previous studies demonstrated that the use of suprathreshold monophasic pulses on enriched CM cultured on scaffolds could improve the structural and functional properties of engineered myocardium.6 Yet biphasic electrical field stimulation may be more beneficial for tissue engineering due to the decreased current required for excitation compared to monophasic stimulation7 and improved efficacy especially at higher pulse durations (≥ 2.5 ms).8 In addition, it is known that biphasic pulses improve defibrillation efficacy compared to monophasic pulses.9,10
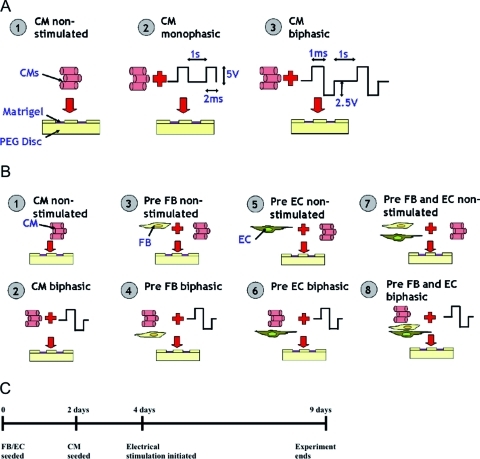
The main objectives of current work were (1) to compare the effect of electrical field stimulation using monophasic and biphasic pulses of identical amplitude and duration on the structure and function of engineered cardiac organoids based on enriched CM (Fig. 1A), and (2) to determine if electrical field stimulation will enhance electrical excitability of cardiac organoids based on multiple cell types (Fig. 1B).
FIG. 1.
Experimental design. (A) Experimental groups for the first part of the study included enriched CM with (1) no stimulation, (2) monophasic square pulses at 5 V/cm, 1 Hz, and duration of 2 ms, and (3) biphasic square pulses at 2.5V/cm, 1 Hz, and duration of 1 ms. (B) Experimental groups for the second part of the study included (1) enriched CM, (2) enriched CM with biphasic stimulation, (3) enriched CM seeded on precultured networks of FB, (4) enriched CM seeded on precultured networks of FB followed by biphasic stimulation, (5) enriched CM seeded on precultured networks of EC, (6) enriched CM seeded on precultured networks of EC followed by biphasic stimulation, (7) enriched CM seeded on precultured networks of FB and EC, and (8) enriched CM seeded on precultured networks of FB and EC followed by biphasic stimulation. (C) Experimental timeline for the stimulated preculture groups. CM, cardiomyocytes; FB, fibroblasts; EC, endothelial cells. Color images available online at www.liebertonline.com/tea
Materials and Methods
Microfabrication of poly(ethylene glycol)-diacrylate discs
Poly(ethylene glycol) (PEG) discs were prepared according to our previously developed method.11 A setup (Supplementary Fig. 1A, available online at www.liebertonline.com/tea) consisting of a fine polypropylene mesh (Part No. 9265T41, McMaster-Carr, Aurora, OH) with a silicone rubber gasket mold on top was first prepared. Poly(ethylene glycol)-diacrylate (PEG-DA) discs were then fabricated by pipetting 75 μL of liquid solution containing PEG-DA (700 MW, Sigma-Aldrich 455008, Oakville, Canada) and 0.5% v/v 2-hydroxy- 2-methyl-propiophenone photoinitiator (Sigma, Oakville, Canada) into each of the circular holes of the mold. A microscope glass slide was placed onto the mold, and the PEG-DA was crosslinked by exposure to ultraviolet light for 30 s. In this way, circular PEG discs with diameters of 4 mm were created so that one side was smooth due to its surface contact with the glass slide and the other side had perpendicularly oriented channels due to its contact with the mesh. The PEG discs were immersed in 100% ethanol, washed in PBS, and soaked in culture medium to remove residual PEG monomer followed by Matrigel coating (5 μL/disc).
Cell seeding
D4T EC were from an embryoid body–derived mouse EC line.12 They were propagated in culture medium consisting of Iscove's modified Dulbecco's media (Gibco, Burlington, Canada) with 5% fetal bovine serum (FBS; Gibco), 100 units/mL penicillin, and 100 μg/mL streptomycin (Gibco/Invitrogen, Burlington, Canada). FB and CM were primary cells isolated from the neonatal rat heart ventricles by serial collagenase digestion, as described by Radisic et al.13 and according to the protocol approved by the Committee on Animal Care at University of Toronto. FB and CM were cultured in high-glucose Dulbecco's modified Eagle's medium (Gibco) with 4.5 g/L glucose, 4 mM L-glutamine, 10% FBS, 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES Buffer, Gibco), 100 units/mL penicillin, and 100 μg/mL streptomycin.
Enriched CM were obtained by preplating neonatal rat heart cells for 1 h in T75 flasks. Cardiac FB preferentially attach to the bottom of the flasks, while CM preferentially remain in suspension. Therefore, other cell types such as FB were present in the “enriched CM” group, rather than having 100% pure CM. Nonmyocytes were precultured on PEG discs for 2 days prior to cardiomyocyte seeding (Fig. 1C). For experimental groups with enriched CM, 80,000 enriched CM were seeded onto the discs. For preculture with FB, 26,666 FB were seeded and cultured for 2 days, followed by seeding of 80,000 enriched CM. For preculture with EC, 93,333 EC were seeded and cultured for 2 days, followed by 80,000 enriched CM. For preculture with FB and EC, 26,666 FB and 93,333 EC were simultaneously seeded and cultured for 2 days, followed by seeding of 80,000 enriched CM. These cell ratios were motivated by the study of Levenberg et al.14 The desired cell number was applied in 5 μL of culture medium/PEG disc placed in 96-well plates.
Electrical stimulation
Electrical stimulation was started 2 days after cardiomyocyte seeding (Fig. 1C). PEG discs were placed inside the holes of a rubber gasket to prevent discs from floating in the culture medium away from the stimulation field (Supplementary Fig. 1B). Two parallel carbon electrodes (1/4 in diameter; Ladd Research Industries, Williston, VT) were placed 1 cm apart in a glass chamber (60 mm diameter) and connected through platinum wires to a stimulator (Grass S88X) as described previously.6 Fresh culture medium (12 mL) with 10 μM ascorbic acid (Sigma A4544) was added to the chamber, and the cells were cultured for 5 days with culture medium exchange once per 24 h. Monophasic group was field stimulated using monophasic square pulses at 5 V/cm, 1 Hz, 2 ms. Biphasic group was stimulated using biphasic square pulses at 2.5 V/cm, 1 Hz, 1 ms (per phase). Therefore, the total amplitude and duration of one complete biphasic pulse cycle were 5 V/cm and 2 ms, respectively, which were identical to the monophasic pulses.
Methods of analysis
Functionality
Functionality was determined at the end of cultivation by three parameters: excitation threshold (ET), maximum capture rate (MCR), and success rate as described previously.15 ET is the minimum voltage at which the engineered cardiac organoids cultured on the PEG discs are observed to beat synchronously under electrical field stimulation at 1 Hz using 2 ms square monophasic pulses. MCR is defined as the maximum pacing frequency for synchronous contractions at 200% of ET. MCR was measured by increasing the stimulation frequency until the contractions of the organoids become asynchronous or ceased completely. Success rate was defined as the number of PEG discs containing cardiac organoids capable of synchronous contractions in response to electrical field stimulation, out of the total number of discs in an experimental group.
Live/dead staining
Live/dead staining with Carboxy Fluoroscein Succinimidyl Ester (staining live cells green) and propidium iodide (staining dead cells red) was performed according to the manufacturer's protocol (Molecular Probes, Burlington, Canada) and imaged using a fluorescence microscope (Leica DMIRE2, Wetzlar, Germany). Manual counts were used to obtain percentage viability from live/dead images. Cell density was calculated by dividing the total number of cells by the total area of the live/dead image taken (1 × 10−2 cm2).
Immunohistochemistry
PEG discs were fixed in 4% paraformaldehyde overnight at 4°C, embedded in TissueTek optimal cutting temperature medium (O.C.T.; Sakura, Torrance, CA), and snap frozen in liquid nitrogen for 30 s. Face sections of the discs (10 μm thick) were obtained using a cryostat (Leica CM3050S) operating at an ambient and specimen temperature of −24°C. The cells were double-stained for vimentin (Vim; to identify nonmyocytes, specifically FB and EC) and troponin-I (TnI; to identify CM), and also stained for Connexin-43 (Cx-43; to identify gap junctions) and cluster of differentiation 31 (CD31; to identify EC) according to our previously described protocols11,16 using the following antibodies (all at 1:50): anti-vimentin (Cy3 conjugated; Sigma C9080) and anti-cardiac TnI (Chemicon AB1627, Temecula, CA), or anti-Cx-43 (Chemicon AB1728), or anti-CD31 (BD 557355). Manual counts were used to determine percentages of Vim+ and TnI+ cells. Double staining for TnI/Vim was validated in monolayers of heart cells by observing cell type–specific structural elements (i.e., sarcomeres or intermediate filaments) in addition to the cell color (i.e., green or red) (Supplementary Fig. 2).
XTT assay
The number of cells on the PEG discs in the CM control, CM monophasic, and CM biphasic groups was determined at the end of cultivation by performing XTT ((sodium 3′-[1-(phenylaminocarbonyl)-3,4-tetrazolium]-bis (4-methoxy-6-nitro) benzene sulfonic acid hydrate) assay using cell proliferation kit II (Roche Diagnostics, Indianapolis, IN) according to the manufacturer's instructions. XTT assay works by the principle that live cells cleave the yellow tetrazolium salt XTT to form an orange formazan dye. Calibration curves were created by seeding known number of cells on PEG discs and performing XTT assay on these standards.
Image analysis
The levels of Cx-43 were assessed in immunostained images by manual counting as follows: A region of 100 by 100 pixel (11 μm by 11 μm) was randomly placed in an immunostained image such that 1–4 cells were contained in the box. The number of Cx-43–positive dots and the number of nuclei were counted for each box. For each immunostained image, counting was performed in 3–5 such regions. For each experimental group, n = 4 independent images were evaluated except for the CM nonstimulated group where n = 8 independent images were evaluated. The number of Cx-43 positive dots was divided by the number of nuclei in each region to get a measure of Cx-43 levels per cell (Tables 1 and 2).
Table 1.
Levels of Connexin-43 in Organoids Based on Enriched Cardiomyocytes as Assessed by Manual Counts of Immunostained Sections
Indicates significantly smaller than CM biphasic as assessed by one-way ANOVA in conjunction with Dunn's test. p < 0.05 considered significant. CM, cardiomyocytes.
Table 2.
Levels of Connexin-43 in Organoids in Preculture Groups as Assessed by Manual Counts of Immunostained Sections
| |
Number of Cx-43 dots/cell |
|
|---|---|---|
| Group | Nonstimulated | Biphasic |
| Pre FB | 1 ± 1 | 1 ± 1 |
| Pre EC | 2 ± 1a | 4 ± 2 |
| Pre FB and EC | 3 ± 2a | 5 ± 4 |
Indicates significantly smaller than CM biphasic as assessed by t-test. Within each stimulation regime, CM, Pre FB, Pre EC, Pre FB, and EC groups were compared by one-way ANOVA in conjunction with Dunn's test. Bracket indicates significant difference. For both nonstimulated and biphasic, the presence of Connexin-43 in CM groups (Table 1) was significantly higher than in the Pre FB and Pre EC groups. p < 0.05 considered significant. FB, fibroblast; EC, endothelial cell.
Quantitative polymerase chain reaction
RNA from CM control, CM monophasic, and CM biphasic groups (n = 3/group) was extracted using Trizol (Invitrogen, Burlington, Canada), and 0.5 μg was used to synthesize cDNA with SuperScript™ III (Invitrogen) according to manufacturers' protocols. Quantitative polymerase chain reaction (qPCR) was performed using SYBR Green (Applied Biosystems, Foster City, CA) according to the product insert. Relative concentrations of each sample were determined using an ABI Prism 7900HT Fast Real-Time PCR System with SDS version 2.3 software. Agarose gels (1%) were run for 30 min at 100 V with 100 base pair ladder (Invitrogen) as a standard. A standard curve for each gene was created using a dilution series (undiluted, 1:2, 1:4, 1:8, and 1:16) of the cDNA from a flask of cultured CM. In brief, for each reaction, the cycle number (Ci), which yielded the inflection point on the curve of fluorescence versus cycle number, was recorded. The dilution series was used to plot Ci values vs. log of dilution factors (undiluted sample having a value of 100; no units). Straight line was fitted to this data. All subsequent readings of (Ci) from unknown samples were then plotted, and the relative concentration of each unknown was calculated using the linear fit described above. To normalize, the relative concentration for each gene of each sample was divided by its corresponding relative against GAPDH and β-actin. Analysis of the reaction was completed using an ABI Prism 7900HT with SDS version 2.0 software. The primers were tested in n = 3 independent qPCR runs with the standard curve samples as well as the unknown samples. For each gene, a minimum of three independent samples of our experimental groups (CM nonstimulated, CM monophasic, and CM biphasic) were tested at each plate run. Three technical replicates were used. In these multiple repetitions, the average slope of Cx-43 standard curve was −3.35 ± 0.10, of GAPDH was −3.49 ± 0.04, and of β-actin was −3.37 ± 0.07. Statistical analysis indicated that there were no significant differences between the slopes of the standard curves for these genes (p = 0.244, one-way ANOVA). In addition, these average slopes corresponded to PCR efficiencies of 99%, 93%, and 98% for Cx-43, GAPDH, and β-actin, respectively. The range of Ci values for the unknown samples were: 25.75–36.96 for Cx-43, 26.07–32.27 for GAPDH, and 26.27–35.96 for β-actin.
The following primers were used:
GAPDH: Forward (5′–3′), GTATGTCGTGGAGTCTACTG; Reverse (5′–3′), GGGAGTTGTCATATTTCTCGT β-Actin: Forward (5′–3′), TAAAGACCTCTATGCCAACAC; Reverse (5′–3′), GATAGAGCCACCAATCCAC
Cx-43: Forward (5′–3′), GTTCTATGTGATGAGGAAGG; Reverse (5′–3′), ACTTCTTGATTTCAATCTGC
Statistical analysis
One-way ANOVA using α = 0.05, in conjunction with post hoc Tukey's test, was performed to compare the enriched CM groups: nonstimulated, monophasic stimulated, and biphasic stimulated (SigmaStat 3.0). For the second study, one-way ANOVA was used to compare the results within the biphasic stimulated groups or within the nonstimulated groups of enriched CM, preculture with FB, preculture with EC, and preculture with FB and EC. Furthermore, t-tests were performed to compare the biphasic stimulated groups to their corresponding nonstimulated controls.
Results
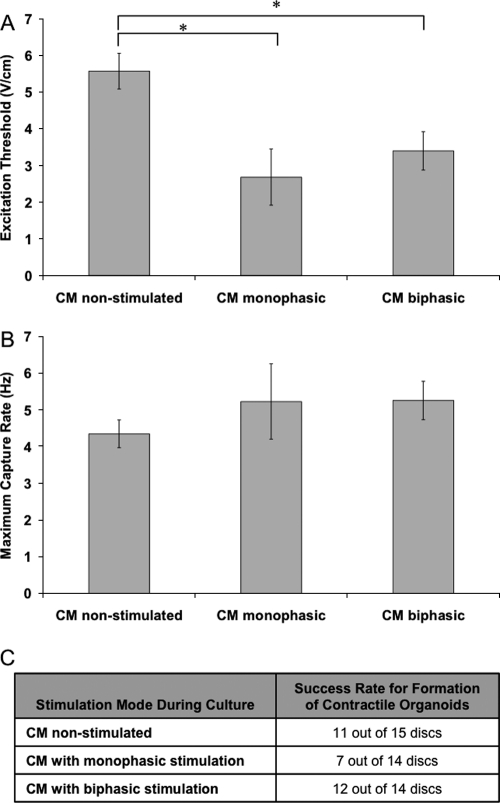
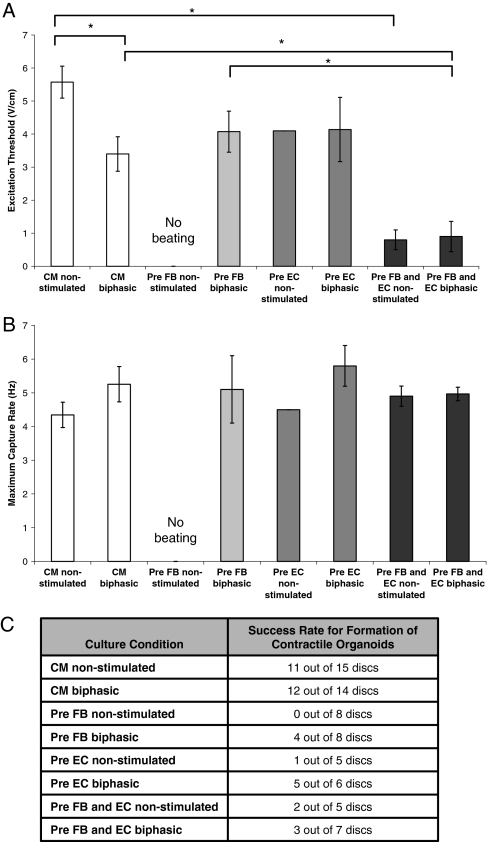
In the first study, enriched CM were cultured with monophasic or biphasic pulses of identical amplitude and duration (Fig. 1A). MCRs were comparable for all groups (p = 0.530), while the ETs were significantly lower for both monophasic and biphasic field stimulated CM when compared to the nonstimulated controls (p = 0.007; p = 0.019) (Fig. 2A, B). Although the average values of ET were not significantly different between the monophasic and biphasic groups (p = 0.683), a significantly higher success rate for formation of cardiac organoids (Fig. 2C) was observed for the biphasic group.
FIG. 2.
Functional properties for cardiac organoids based on enriched cardiomyocytes at different stimulation modes: (1) no stimulation, (2) monophasic square pulses at 5 V/cm, 1 Hz, and duration of 2 ms, and (3) biphasic square pulses at 2.5 V/cm, 1 Hz, and duration of 1 ms. (A) Excitation threshold. (B) Maximum capture rate. (C) Success rate. Asterisks (*) denote statistically significant difference (p < 0.05; one-way ANOVA with post hoc Tukey's test).
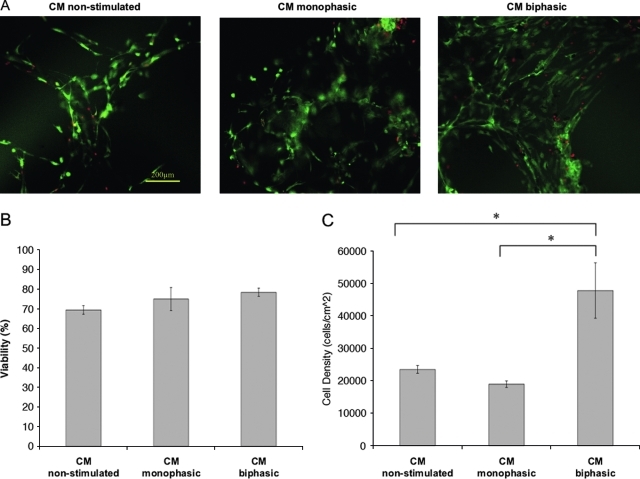
Live/dead images (Fig. 3) indicated that most cells were alive in the three groups, with no significant differences in viability (p = 0.148), but with the highest cell density in the biphasic group (p = 0.009). The nonstimulated group had sparse distribution of cells throughout the PEG microchannels, as compared to the monophasic and biphasic groups that exhibited denser three-dimensional structures. The results of the XTT assay were consistent with the image analysis, illustrating the highest cell density in the CM biphasic group (Fig. 3D).
FIG. 3.
Viability and cell density of cardiac organoids based on enriched cardiomyocytes cultivated at different stimulation modes. (A) Live/dead images of enriched cardiomyocytes (live cells appear green, and dead cells appear red). (B) Viability of engineered organoids in percentage of live cells compared to the total number of cells. (C) Cell density in number of cells per square centimeter of the image taken. Asterisks (*) denote statistically significant difference (p < 0.05; one-way ANOVA with post hoc Tukey's test). (D) Cell number per disc as determined by XTT assay. Color images available online at www.liebertonline.com/tea
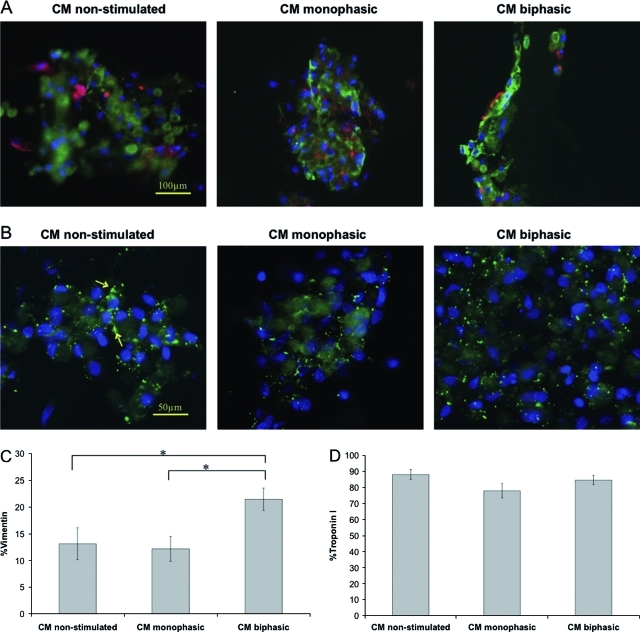
The enriched CM population used for cell seeding consisted mostly of cardiomyocyte and a small percentage of nonmyocytes (Vim+) that remained in the cell suspension after preplating.2,11 At the end of cultivation, there was no significant difference amongst the average percentages of TnI+ cells (Fig. 4D) for the three experimental groups (p = 0.228), and the presence of elongated TnI+ cells was clearly documented in the biphasic group (Fig. 4A). The biphasic group had significantly higher percentage of Vim+ cells (Fig. 4C), as compared to the nonstimulated group (p = 0.015) and the monophasic group (p = 0.009). Punctate distribution of Cx-43, a gap junctional protein, was documented in all three groups (Fig. 4B) consistent with the ability of the organoids to respond to electrical field stimulation (Fig. 2).
FIG. 4.
Immunostaining of cardiac organoids based on enriched cardiomyocytes cultivated at different stimulation modes. (A) Vimentin staining shows fibroblasts in red. Troponin-I staining shows cardiomyocytes in green. (B) Connexin-43 staining shows gap junctions in green (arrows). Cell nuclei are stained blue with 4′, 6-diamidino-2-phenylindole (DAPI). (C) Percentage of vimentin-positive cells. (D) Percentage of troponin-I–positive cells. Asterisks (*) denote statistically significant difference (p < 0.05; one-way ANOVA with post hoc Tukey's test). Color images available online at www.liebertonline.com/tea
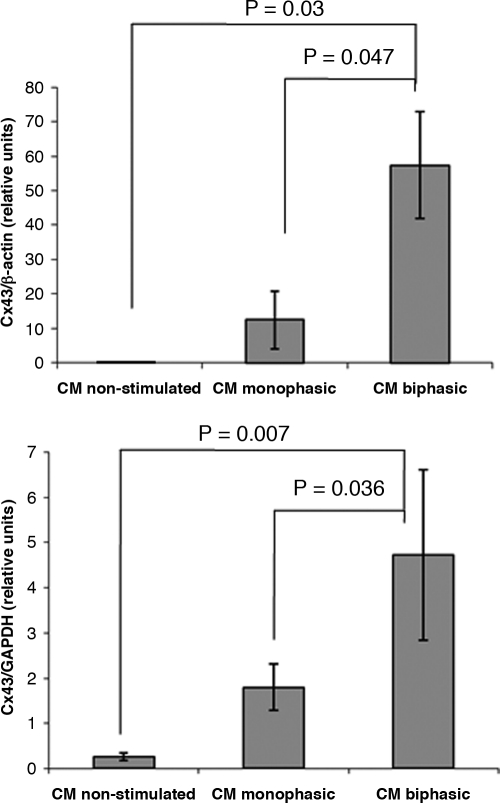
The results of qPCR analysis indicated that CM biphasic group had significantly higher expression levels of Cx-43 compared to the CM monophasic group (Fig. 5). Both stimulated groups had higher expression levels of Cx-43 compared to the nonstimulated controls (Fig. 5).
FIG. 5.
Expression levels of Connexin-43 in organoids based on enriched cardiomyocytes. Quantitative polymerase chain reaction (qPCR) analysis of Connexin-43 expression.
Subsequently, we introduced FB and EC into the cardiac organoids and stimulated them using biphasic pulses, as determined most beneficial in the first study. Enriched CM were seeded onto the preformed networks of FB alone, or EC alone, or the mixture of the two cell types (FB and EC), resulting in total of eight study groups (Fig. 1B).
The most remarkable difference in the functional properties was observed in the preculture with FB. None of the samples in the nonstimulated group were beating (Fig. 6C). In contrast, half of the samples for the biphasic stimulated group were beating with reasonable ET and MCR. For preculture with EC, the biphasic stimulated group had a much higher success rate, 5/6, compared to 1/5 for its nonstimulated control. For preculture with FB and EC, the biphasic and nonstimulated groups had comparable functional properties in terms of ET and MCR values (p = 0.884; p = 0.859). Interestingly, the groups of preculture with FB and EC had significantly lower ET compared to their respective groups of enriched CM (p = 0.002 for nonstimulated groups; p = 0.039 for biphasic stimulated groups). However, preculture with FB and EC and preculture with FB showed lower success rates of forming beating organoids than enriched CM groups, suggesting the need to further optimize the stimulation conditions.
FIG. 6.
Functional properties for cardiac organoids based on (1) enriched cardiomyocytes, (2) enriched cardiomyocytes cultivated on precultured networks of FB, (3) enriched cardiomyocytes cultivated on precultured networks of EC, and (4) enriched cardiomyocytes cultivated on precultured networks of FB and EC. Stimulated groups used biphasic square pulses at 2.5 V/cm, 1 Hz, and duration of 1 ms. (A) Excitation threshold. (B) Maximum capture rate. (C) Success rate. Asterisks (*) denote statistically significant difference (p < 0.05; one-way ANOVA with post hoc Tukey's test).
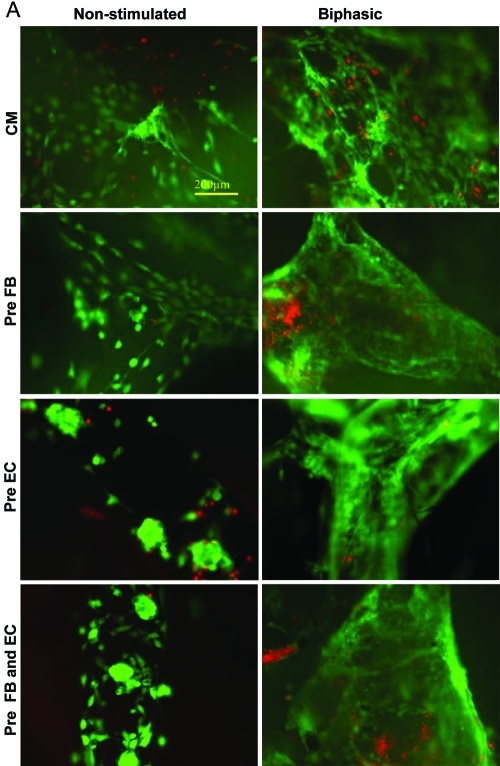
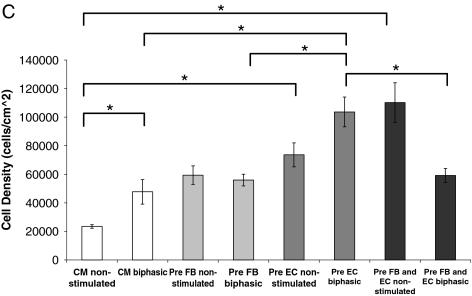
All biphasic stimulated groups showed three-dimensional networks of live cells (Fig. 7A live/dead staining), as compared to a sparser and more two-dimensional structure in the corresponding nonstimulated groups. For the preculture with EC (p = 0.003) and preculture with FB and EC (p = 0.042), viability was significantly higher in biphasic compared to the respective nonstimulated groups (Fig. 7B). Within the nonstimulated groups, preculture with FB resulted in the organoids with the highest viability compared to the other cellular compositions. Within the biphasic groups, no significant differences were observed for different cellular compositions. In terms of cell density, the nonstimulated groups of preculture with EC and preculture with FB and EC had a significantly higher cell density than the nonstimulated CM group (p < 0.001; p < 0.001), as expected by the experimental design. Within the biphasic stimulated groups, pre-EC group had the highest cell density.
FIG. 7.
Viability and cell density of cardiac organoids. (A) Live/dead images of organoids (live cells appear green, and dead cells appear red). (B) Viability of engineered organoids in percentage of live cells compared to the total number of cells. (C) Cell density in number of cells per square centimeter of the image taken. Asterisks (*) denote statistically significant difference. Color images available online at www.liebertonline.com/tea
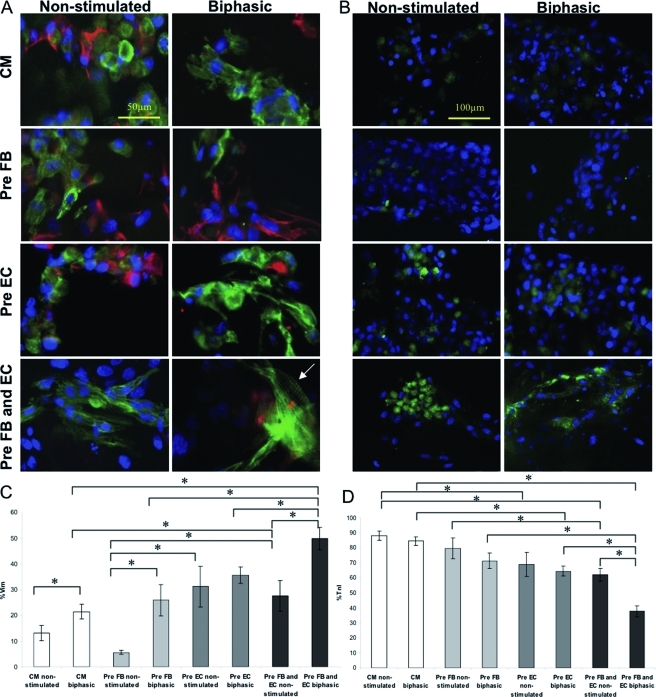
Double staining for vimentin and cardiac TnI (Fig. 8A) showed that all biphasic stimulated groups had enhanced cellular elongation, as compared to the rounder cells in the nonstimulated groups. Striations were observed for the biphasic group of preculture with FB and EC (Fig. 8A, arrow). With the exception of preculture with EC, all biphasic stimulated groups had significantly higher percentages of Vim+ cells (Fig. 8C) than their corresponding nonstimulated groups. For nonstimulated groups, both preculture with EC and preculture with FB and EC had significantly higher percentage of Vim+ cells compared to preculture with FB and enriched CM. For stimulated groups, preculture with FB and EC showed the highest percentage of Vim+ cells (p < 0.001).
FIG. 8.
Immunostaining of cardiac organoids. (A) Images showing double staining for vimentin and troponin-I. Vimentin staining shows fibroblasts in red. Troponin-I staining shows cardiomyocytes in green. (B) CD31 staining shows endothelial cells in green. DAPI stains all cell nuclei blue. (C) Percentage of vimentin-positive cells. (D) Percentage of troponin-I–positive cells. (E) Percentage of CD31+ cells. Asterisks (*) denote statistically significant difference. Color images available online at www.liebertonline.com/tea
The percentage of TnI+ cells was lower in the preculture groups compared to the enriched CM group, as expected in this experimental design (i.e., lower percentages of CM were originally seeded in the preculture groups). The biphasic groups had similar percentages of TnI+ cells as their corresponding nonstimulated groups, except for the preculture with FB and EC, where stimulated group had lower percentage of TnI+ cells (p < 0.001). Enriched CM group and preculture with FB group had significantly higher percentages of TnI+ cells (Fig. 8D), compared to preculture with FB and EC group under both nonstimulated and biphasic stimulated conditions (p < 0.001).
As expected, the nonstimulated and biphasic stimulated groups of both preculture with EC and preculture with FB and EC had significantly higher percentages of CD31+ cells (Fig. 8B, E), which are EC, in comparison with enriched CM. This was probably due to the initial cell seeding ratios (i.e., the only EC found in the CM and Pre FB groups is a small percentage that comes from the native heart isolate).
The presence of Cx-43 was decreased in the preculture groups as compared to the enriched CM groups (Fig. 9). However, it increased in the field-stimulated samples for all preculture groups. Manual counts indicated that the presence of Cx-43 increased significantly with the application of biphasic electrical field stimulation (Table 1) in organoids based on enriched CM. Application of biphasic stimulation also significantly elevated the presence of Cx-43 dots in the Pre FB and EC and Pre EC groups (Table 2). Pre FB and Pre EC groups exhibited significantly smaller presence of Cx-43 in comparison to the respective CM groups (Tables 1 and 2). In contrast, Pre FB and EC groups exhibited slightly but not significantly smaller presence of Cx-43 compared to their corresponding (nonstimulated or biphasic) CM groups (Tables 1 and 2).
FIG. 9.
Connexin-43 staining of cardiac organoids. Connexin-43 staining shows gap junctions in green. DAPI stains cell nuclei blue. Color images available online at www.liebertonline.com/tea
Discussion
Recent studies6 reported that the use of monophasic electrical field stimulation improved cardiomyocyte alignment and coupling, differentiation levels, functional properties, and cell organization in engineered cardiac tissue.6 However, studies were not conducted to compare the use of different pulse forms, as well as to look at the combined effect of nonmyocyte coculturing and electrical field stimulation on engineered heart tissues. This is partly due to large amounts of scaffolds and cells required for such complex studies in the standard mesoscale tissue engineering systems.
Here, we cultivated cardiac organoids in microchannels fabricated on PEG discs (Supplementary Fig. 1).11 PEG microstructures have been used previously for encapsulating living cells.17,18 We made the microchannels supportive of cardiac organoid assembly by Matrigel coating. Since cells cannot attach to PEG, Matrigel coating was required to make the channels supportive of cell attachment and self-assembly. The advantage of microfabrication is that small volumes of reagents are needed and the size of microchannels (100–200 μm in width and 4 mm in length) can be chosen to mimic the dimensions of cardiac myofibers.
The experimental timeline for the present study (Fig. 1C) was chosen based on our previous studies. Previously, we observed that cultivation of enriched CM on preformed networks of nonmyocytes improved electrical excitability of the resulting tissue compared to simultaneous culture at identical cell ratios.3,11,16 Thus, in the current study we precultured nonmyocytes (FB alone, or EC alone, or a mixture of FB and EC) for 2 days in the PEG microchannels to allow them to connect and start secreting soluble factors and ECM components, followed by the addition of enriched CM and subsequent electrical field stimulation. In the previous studies, we also found that there exists an optimal time point for initiation of electrical field stimulation corresponding to ∼3 days after cardiomyocyte seeding.6 Therefore, electrical field stimulation was initiated 2 days after CM seeding in the current study.
In this study, monophasic and biphasic square pulses of identical total amplitude and total pulse duration were compared. The stimuli were supra-threshold (total amplitude 5 V/cm as in6) with pulse duration (2 ms) chosen to be comparable to that of 1-week-old rats.19 Ascorbic acid (10 μM) was added as a reducing agent to prevent the ionic and free radical buildup as reported by Sathaye et al.20
Although electrical stimulation did not significantly improve MCR (Fig. 2B), both monophasic and biphasic field stimulation improved ET (Fig. 2A) compared to no stimulation. For organoids based on enriched CM, the nonstimulated group had the highest ET (indicating that high voltage must be applied before synchronous contractions are first observed) and lowest MCR (indicating that the maximum beating frequency of the tissue was low). This is consistent with past studies,6 which showed improved electrical excitability using monophasic electrical field stimulation, as compared to no stimulation. Importantly, however, biphasic pulses excelled in the success rate of forming contractile organoids (Fig. 2C) compared to monophasic pulses (7/14 for monophasic and 12/14 for biphasic). In our previous studies,6 we used collagen scaffolds that were capable of freely contracting in response to electrical field stimulation while suspended in the culture medium achieving 100% success rate with monophasic stimulation. In the current systems, the organoids are grown within the PEG microchannels, with few anchor points to the underlying PEG/Matrigel substrate.11 As such, the organoids were not freely suspended. This difference in geometry may have translated into a difference in the success rate for contractile organoids formation.
The higher success rate for biphasic stimulated group could not be explained by the viability of cells, since all three stimulation modes yielded similar percentage of live cells (Fig. 3B). Rather, biphasic stimulated group showed higher cell density (Fig. 3C, D) in the organoids and increased Cx-43 presence (Fig. 4B), which presumably allowed for the improved propagation of electrical signals. The data from the XTT assay (Fig. 3D) and image analysis (Fig. 3C) are consistent, indicating more cells (Fig. 3D) and higher cell density (Fig. 3C) in the CM biphasic group compared to the CM monophasic and CM nonstimulated groups. Cells occupy mostly the channels on PEG discs, which are 100–200 μm in diameter, and on average there are six channels on each disc.11 Image analysis was performed on the cells in the channels, and due to the nature of regular fluorescence microscopy, the images were confined to a single plane. The XTT assay results accounted for the summation of cell numbers in the planes along the depth of the channels, whereas image analysis showed only the cell density at a particular plane. Moreover, if any cells were present on the PEG surface between the channels, XTT assay would also account for these cells, whereas image analysis would not. That is why it is not possible to directly convert from cell density to cell number by simply multiplying cell density by the area of a 4 mm PEG disc.
Quantitative PCR (Fig. 5) and manual counts (Tables 1 and 2) of immunostained images consistently showed higher levels of Cx-43 in the CM biphasic group compared to the CM monophasic and nonstimulated controls. Although Cx-43–positive dots were clearly identified in the CM nonstimulated immunostains (Fig. 4), the expression levels in the nonstimulated group were very low (i.e., values close to 0 in Fig. 5).
Although increase in Cx-43 expression contributed to the increase in excitability of cardiac organoids (e.g., for CM nonstimulated and biphasic), this relationship was not linear, consistent with the presence of other factors that also influence organoid excitability. For example, our manual counts indicate that the Cx-43 presence was slightly but not significantly lower in the Pre FB and EC groups in comparison to the CM groups (Tables 1 and 2), yet the Pre FB and EC groups had a significantly lower ET than the CM groups. This was offset by a higher success rate in the CM groups in comparison to the Pre FB and EC groups.
The cell number at the end of cultivation was lower in all enriched cardiomyocyte groups (Fig. 3D) compared to the cell number seeded onto the discs, consistent with the inability of these cells to proliferate significantly and viability of the initial cell population lower than 100% (70–80%). With biphasic stimulation, the percentage of Vim+ cells increased from ∼13% to ∼21% (Fig. 4C), perhaps optimizing the ratio between FB and CM in culture. The percentage of TnI+ cells remained comparable in the three groups and similar to the percentage in the original cell suspension used for seeding (81 ± 14% by fluorescence activated cell sorting11).
One possible explanation for this phenomenon is that biphasic stimulation may have promoted the secretion of growth factors, causing the proliferation of FB. A control experiment was performed where FB alone were seeded in PEG microchannels and subjected to biphasic electrical field stimulation (Vim+; Supplementary Fig. 3D). There was no significant difference in the viability (p = 0.105) with or without stimulation (Supplementary Fig. 3A–C). The cell density was slightly, but not significantly (p = 0.123), higher in the biphasic stimulated group of FB. Bundles of elongated FB could be observed under biphasic stimulation (Supplementary Fig. 3A).
Similarly, in all preculture groups biphasic electrical field stimulation improved functional and structural properties (Figs. 6 and 7A), as compared to the nonstimulated controls. With the aid of biphasic electrical field stimulation, the success rate for preculture with FB was increased from 0/8 to 4/8 (Fig. 6C), with ET and MCR values similar to the organoids based on enriched CM with biphasic stimulation. Interestingly, although preculture with FB and EC had lower percentage of CM (62% for nonstimulated and 38% for biphasic stimulated), it exhibited a significantly lower ET and significantly higher MCR than the enriched CM group with a higher percentage of CM (88% for nonstimulated and 85% for biphasic stimulated). The cellular composition of this group (Fig. 8, preculture with FB and EC biphasic) was comparable to that reported for the native myocardium,21 with elongated striated CM. Yet, only 50% of the Pre FB and EC organoids contracted in comparison to the 70–80% in the CM groups, indicating that at the current cell ratios Pre FB and EC organoids were not clearly superior in functional properties to the CM organoids. This indicates the need to further optimize the seeding ratios of the three cell types as well as the stimulation amplitude and duration in order to increase the success rate for contractile organoid formation to 100% in the Pre FB and EC group.
Viability of the cells in the organoids could not be directly related to the functional properties. For example, the nonstimulated group of preculture with FB had significantly higher viability compared to the nonstimulated group of enriched CM (Fig. 7B), yet contractile organoids were not formed in the preculture with FB (Fig. 6). Instead, functional properties of the organoids improved with the increase in the presence of Cx-43 (Table 2).
The overall functional properties were improved by the use of all three cell types in comparison to preculture with FB alone or preculture with EC alone, consistent with our previous work.11 A possible explanation for the observed phenomena is that the presence of both the ECM components from FB22 and pro-survival factors from EC4 prior to the seeding of CM may be important for functional assembly of cardiac organoids. Moreover, the presence of FB and EC was shown by Levenberg et al.14 to result in VEGF secretion in an engineered muscle. Upregulation of VEGF may have resulted in the upregulation of Cx-43 expression by the CM in our organoids, as reported by Pimentel et al.23
Within the nonstimulated groups, overall cell viability in the organoids was not increased by the preculture of EC alone or the preculture of FB and EC (Fig. 7B), which appeared to be contradictory to the reported observation that nonmyocytes improved the viability of CM.4 A possible explanation is that the viability of CM was indeed improved, but the total viability was decreased by the death of EC. A control experiment was performed where EC alone were seeded into PEG microchannels (CD31+, Supplementary Fig. 3D). Although biphasic stimulation significantly improved the viability of EC (p = 0.003), the viability of nonstimulated EC alone (53.94 ±3.94%) was lower than that of FB alone (80.14 ± 3.65%) (Supplementary Fig. 3B) and of enriched CM (69.42 ± 2.20) (Fig. 3B).
In addition, the contractile apparatus of CM in the current study was not as well developed as the one we observed in our previous studies with collagen scaffolds, presumably due to the differences in the underlying substrate (collagen/Matrigel vs. PEG/Matrigel). However, we were still able to observe the differences amongst experimental groups, with the best-developed sarcomeres in the stimulated Pre FB and EC group (Supplementary Fig. 4).
The use of biphasic pulses can maintain biocompatibility due to the lowered cumulative energy requirement, as well as its charge-balanced property, thus avoiding damage to the tissues and electrodes.24 Although the amplitude of each phase of the biphasic pulse was only half of that of the monophasic pulse, biphasic stimulation was more effective in improving electrical excitability of cardiac organoids compared to the monophasic stimulation. Our data are consistent with that of Jones et al.,25 who reported decreased ETs for spheroids based on enriched CM cultivated for 24 h. However, total pulse duration of the biphasic pulse in Jones et al.25 was twice that of the monophasic pulse (i.e., monophasic pulse duration equaled just one of the two phases of the biphasic pulse). Modeling studies of Tung and Borderies8 confirm that under those conditions biphasic stimulation will be more effective than the monophasic stimulation over the entire range of pulse durations. When the total duration of monophasic and biphasic pulses is set to be equal, the study by Tung and Borderies8 predicts that biphasic pulses will have more efficacy in inducing actions potentials at higher total pulse durations (≥ 2.5 ms). The main explanation for this phenomena is that the two phases of the biphasic pulse act synergistically during field stimulation, with the first phase acting as a conditioning sub-threshold prepulse and the second phase as the excitatory pulse.8,25
Conclusions
Biphasic electrical field stimulation during cultivation resulted in the improved functional and structural properties of cardiac organoids based on enriched CM, in comparison to nonstimulated controls and stimulation using monophasic square pulses. Biphasic stimulation was also effective at improving electrical excitability of cardiac organoids based on mixed cell populations (FB, EC, and CM). The functionality of the engineered organoids was not correlated to the cell viability. Instead, the improved functional properties for the biphasic stimulated groups could be explained by the three-dimensional organization of cells, increased cell elongation, higher cell density, the presence of cross-striations, and increased expression of Cx-43.
Supplementary Material
Acknowledgments
This study was supported by NSERC Discovery Grant, NIH Grant R01HL076485 Ontario Graduate Scholarship (to R.K.I.), and NSERC Undergraduate Summer Research Award (to L.L.C.). The authors thank Melissa Brown for immunostaining in Supplementary Figure 2.
Disclosure Statement
No competing financial interests exist.
References
- 1.Zimmermann W.H. Eschenhagen T. Cardiac tissue engineering for replacement therapy. Heart Fail Rev. 2003;8:259. doi: 10.1023/a:1024725818835. [DOI] [PubMed] [Google Scholar]
- 2.Naito H. Melnychenko I. Didie M. Schneiderbanger K. Schubert P. Rosenkranz S. Eschenhagen T. Zimmermann W.H. Optimizing engineered heart tissue for therapeutic applications as surrogate heart muscle. Circulation. 2006;114:I72. doi: 10.1161/CIRCULATIONAHA.105.001560. [DOI] [PubMed] [Google Scholar]
- 3.Radisic M. Park H. Martens T.P. Salazar-Lazaro J.E. Geng W. Wang Y. Langer R. Freed L.E. Vunjak-Novakovic G. Pre-treatment of synthetic elastomeric scaffolds by cardiac fibroblasts improves engineered heart tissue. J Biomed Mater Res A. 2008;86:713. doi: 10.1002/jbm.a.31578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Narmoneva D.A. Vukmirovic R. Davis M.E. Kamm R.D. Lee R.T. Endothelial cells promote cardiac myocyte survival and spatial reorganization: implications for cardiac regeneration. Circulation. 2004;110:962. doi: 10.1161/01.CIR.0000140667.37070.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tokudome T. Horio T. Fukunaga M. Okumura H. Hino J. Mori K. Yoshihara F. Suga S. Kawano Y. Kohno M. Kangawa K. Ventricular nonmyocytes inhibit doxorubicin-induced myocyte apoptosis: involvement of endogenous endothelin-1 as a paracrine factor. Endocrinology. 2004;145:2458. doi: 10.1210/en.2003-1322. [DOI] [PubMed] [Google Scholar]
- 6.Radisic M. Park H. Shing H. Consi T. Schoen F.J. Langer R. Freed L.E. Vunjak-Novakovic G. Functional assembly of engineered myocardium by electrical stimulation of cardiac myocytes cultured on scaffolds. Proc Natl Acad Sci USA. 2004;101:18129. doi: 10.1073/pnas.0407817101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kavanagh K.M. Duff H.J. Clark R. Robinson K.V. Giles W.R. Wyse D.G. Monophasic versus biphasic cardiac stimulation: mechanism of decreased energy requirements. Pacing Clin Electrophysiol. 1990;13:1268. doi: 10.1111/j.1540-8159.1990.tb02026.x. [DOI] [PubMed] [Google Scholar]
- 8.Tung L. Borderies J.R. Analysis of electric field stimulation of single cardiac muscle cells. Biophys J. 1992;63:371. doi: 10.1016/S0006-3495(92)81632-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flaker G.C. Schuder J.C. McDaniel W.C. Stoeckle H. Dbeis M. Superiority of biphasic shocks in the defibrillation of dogs by epicardial patches and catheter electrodes. Am Heart J. 1989;118:288. doi: 10.1016/0002-8703(89)90187-7. [DOI] [PubMed] [Google Scholar]
- 10.Wharton J.M. Richard V.J. Murry C.E. Dixon E.G. Reimer K.A. Meador J. Smith W.M. Ideker R.E. Electrophysiological effects of monophasic and biphasic stimuli in normal and infarcted dogs. Pacing Clin Electrophysiol. 1990;13:1158. doi: 10.1111/j.1540-8159.1990.tb02174.x. [DOI] [PubMed] [Google Scholar]
- 11.Iyer R.K. Chiu L.L. Radisic M. Microfabricated poly(ethylene glycol) templates enable rapid screening of tri-culture conditions for cardiac tissue engineering. J Biomed Mater Res A. 2008 Apr 28; doi: 10.1002/jbm.a.32014. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 12.Choi K. Kennedy M. Kazarov A. Papadimitriou J.C. Keller G. A common precursor for hematopoietic and endothelial cells. Development. 1998;125:725. doi: 10.1242/dev.125.4.725. [DOI] [PubMed] [Google Scholar]
- 13.Radisic M. Euloth M. Yang L. Langer R. Freed L.E. Vunjak-Novakovic G. High-density seeding of myocyte cells for cardiac tissue engineering. Biotechnol Bioeng. 2003;82:403. doi: 10.1002/bit.10594. [DOI] [PubMed] [Google Scholar]
- 14.Levenberg S. Rouwkema J. Macdonald M. Garfein E.S. Kohane D.S. Darland D.C. Marini R. van Blitterswijk C.A. Mulligan R.C. D’Amore P.A. Langer R. Engineering vascularized skeletal muscle tissue. Nat Biotechnol. 2005;23:879. doi: 10.1038/nbt1109. [DOI] [PubMed] [Google Scholar]
- 15.Radisic M. Yang L. Boublik J. Cohen R.J. Langer R. Freed L.E. Vunjak-Novakovic G. Medium perfusion enables engineering of compact and contractile cardiac tissue. Am J Physiol Heart Circ Physiol. 2004;286:H507. doi: 10.1152/ajpheart.00171.2003. [DOI] [PubMed] [Google Scholar]
- 16.Radisic M. Park H. Chen F. Salazar-Lazzaro J.E. Wang Y. Dennis R. Langer R. Freed L.E. Vunjak-Novakovic G. Biomimetic approach to cardiac tissue engineering: oxygen carriers and channeled scaffolds. Tissue Eng. 2006;12:2077. doi: 10.1089/ten.2006.12.2077. [DOI] [PubMed] [Google Scholar]
- 17.Koh W.G. Revzin A. Pishko M.V. Poly(ethylene glycol) hydrogel microstructures encapsulating living cells. Langmuir. 2002;18:2459. doi: 10.1021/la0115740. [DOI] [PubMed] [Google Scholar]
- 18.Khademhosseini A. Yeh J. Jon S. Eng G. Suh K.Y. Burdick J.A. Langer R. Molded polyethylene glycol microstructures for capturing cells within microfluidic channels. Lab Chip. 2004;4:425. doi: 10.1039/b404842c. [DOI] [PubMed] [Google Scholar]
- 19.Gomes P.A. de Galvao K.M. Mateus E.F. Excitability of isolated hearts from rats during postnatal development. J Cardiovasc Electrophysiol. 2002;13:355. doi: 10.1046/j.1540-8167.2002.00355.x. [DOI] [PubMed] [Google Scholar]
- 20.Sathaye A. Bursac N. Sheehy S. Tung L. Electrical pacing counteracts intrinsic shortening of action potential duration of neonatal rat ventricular cells in culture. J Mol Cell Cardiol. 2006;41:633. doi: 10.1016/j.yjmcc.2006.06.076. [DOI] [PubMed] [Google Scholar]
- 21.Nag A.C. Study of non-muscle cells of the adult mammalian heart: a fine structural analysis and distribution. Cytobios. 1980;28:41. [PubMed] [Google Scholar]
- 22.Sussman M.A. McCulloch A. Borg T.K. Dance band on the Titanic: biomechanical signaling in cardiac hypertrophy. Circ Res. 2002;91:888. doi: 10.1161/01.res.0000041680.43270.f8. [DOI] [PubMed] [Google Scholar]
- 23.Pimentel R.C. Yamada K.A. Kleber A.G. Saffitz J.E. Autocrine regulation of myocyte Cx43 expression by VEGF. Circ Res. 2002;90:671. doi: 10.1161/01.res.0000014823.75393.4d. [DOI] [PubMed] [Google Scholar]
- 24.Rubinstein J.T. Miller C.A. Mino H. Abbas P.J. Analysis of monophasic and biphasic electrical stimulation of nerve. IEEE Trans Biomed Eng. 2001;48:1065. doi: 10.1109/10.951508. [DOI] [PubMed] [Google Scholar]
- 25.Jones J.L. Jones R.E. Balasky G. Improved cardiac cell excitation with symmetrical biphasic defibrillator waveforms. Am J Physiol. 1987;253:H1418. doi: 10.1152/ajpheart.1987.253.6.H1418. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.