Abstract
As a result of injury caused by chronic gastroesophageal reflux, Barrett's esophagus with high-grade dysplasia and esophageal adenocarcinoma are rapidly increasing problems in the United States. The current standard of care involves esophagectomy, a procedure associated with a high morbidity, a negative impact on long term quality of life, and a mortality rate of 1–6 percent. An entirely endoscopic technique for circumferential, long segment en bloc removal of the mucosa and submucosa with subsequent placement of a biologic scaffold material that promotes a constructive remodeling response and minimizes stricture is described herein. The results of this approach are reported for five patients with 4–24-month follow-up. Restoration of normal mature, K4+/K14+, squamous epithelium, and return to a normal diet without significant dysphagia is reported for all patients. Two of five patients show a small focus of recurrent Barrett's esophagus at the gastroesophageal junction, but the entire length and circumference of the reconstituted esophageal mucosa remains free of disease. This experience provides evidence that a regenerative medicine approach may, for the first time, enable aggressive endoscopic resection of early stage neoplasia without the need for esophagectomy and its associated complications.
Introduction
The incidence of Barrett's esophagus and esophageal adenocarcinoma is dramatically increasing and esophageal cancer has become the world's sixth leading cause of cancer death.1,2 Esophageal resection has been challenged as a treatment for high-grade dysplasia (HGD) with intramucosal cancer because lymph node involvement is unlikely (<5%),3–5 and morbidity and mortality rates associated with esophagectomy are substantial.6–8 However, early stage lesions have the potential to become lethal and are only curable if completely removed.9 Surveillance and screening programs have increased the number of patients detected with early stage disease10,11 and interest in less invasive endoscopic treatments has grown in parallel with early diagnosis. Currently available endoscopic techniques have significant limitations. Photodynamic therapy has been abandoned by many centers due to photosensitivity-related side effects, recurrent disease, uncontrolled depth of ablation, and subsequent stricture formation.12,13 Early results of radiofrequency ablation in the treatment of dysplasia have been encouraging, but just as with photodynamic therapy there is no specimen available for histopathologic examination, which precludes accurate staging of the cancer. With these approaches, patients are committed to a lifetime of surveillance endoscopy and the need for subsequent interventions.13–15
The use of endoscopic resection (ER) has generated excellent results in the treatment of HGD and early adenocarcinoma with an extrapolated 5-year survival rate of 98% in highly selected patients,6,16 and permits the complete histopathologic examination of the resected lesions.17 However, disease recurrence secondary to synchronous or metachronous cancers is common and requires frequent surveillance endoscopies with the need for subsequent ER and combination therapy with radiofrequency ablation.18,19
Furthermore, current ER techniques are limited by a maximum nodule size of 20 mm, necessitating piecemeal resections of larger lesions with a compromised histologic assessment of lateral resection margins. Circumferential lesions require a repeated stepwise approach to prevent stricture formation with the need for multiple procedures and exhaustive follow-up.20,21 Recent studies have shown that successive ER can successfully eliminate neoplasm in ∼97% of patients. However, the lesions are generally limited to <5 cm, and up to 50% of patients develop a symptomatic stricture that requires endoscopic dilation.17,20,21 A strategy that would enable en bloc resection and histopathologic evaluation of a full circumference of mucosa and submucosa with minimal risk of stricture would represent a quantum leap forward in the treatment of Barrett's associated esophageal neoplasia. A preclinical study recently showed that endoscopic en bloc sleeve resection of the entire esophageal inner layers is possible in swine with reasonable operative time and no complications, but there is still a need for secondary treatment to promote constructive remodeling of the resected mucosa and submucosa without stricture formation.22
Biologic scaffold materials composed of xenogeneic extracellular matrix (ECM) have been investigated extensively in the context of regenerative medicine for their ability to modify the default tissue healing response in numerous anatomic sites.23–31 including the esophagus.7,32–34 In a preclinical model, critically sized, circumferential defects could be repaired with minimal stricture formation and near-normal restitution of the esophageal histomorphology if adjacent autologous muscle tissue was placed in direct apposition to the ECM scaffold at the time of surgery.33 A follow-up preclinical study of esophageal transection that was designed to reinforce the anastomosis of a “gastric pull-up” procedure showed restoration of a mature epithelium and regeneration of muscle tissue that bridged the gap between the native muscle tissue on either side of the surgical transection site.7 Most recently, the successful use of ECM has been extended to a preclinical model of aggressive ER such as that proposed for treatment of Barrett's disease.34 Based upon these preclinical findings, five patients with either Barrett's esophagus and multifocal HGD and/or mucosal adenocarcinoma were treated with long segment, circumferential sleeve resection of the mucosa and submucosa and placement of an ECM scaffold material over the site of the resected tissue.
Materials and Methods
Patient history
Five patients with advanced esophageal pathology and limited therapeutic options were selected for circumferential ER. All patients had a hiatal hernia and associated gastroesophageal reflux disease.
The first patient was a 65-year-old man who presented with heartburn and regurgitation and was found to have Barrett's esophagus and HGD. History was significant for back and leg pain and a myocardial infarction in the 1980s for which the patient was treated with coronary artery bypass surgery. Coronary artery stents were placed in 1990s for recurrent disease and he also underwent left carotid endarterectomy. The patient was a current cigarette smoker with a 100-pack-year history and had a history of alcohol abuse. Because of these comorbidities, he was not a candidate for esophagectomy. Upper endoscopy showed a 5-cm-long, circumferential region of nodular Barrett's mucosa with extensive multifocal HGD and features suspicious for adenocarcinoma. Endoscopic ultrasound (EUS), positron emission tomography/computed tomography (PET/CT) showed no evidence of lymph node involvement or distant disease.
The second patient was a 54-year-old man with a history of a 6-cm-long circumferential Barrett's esophagus that had been followed by surveillance endoscopy. He developed HGD and mucosal adenocarcinoma and was initially treated with ER and several rounds of radiofrequency ablation; despite these measures, he developed recurrence of HGD. This patient declined esophagectomy. EUS and PET/CT were negative for extra-esophageal disease.
The third patient was a 60-year-old man with a 15-cm-long nodular Barrett's esophagus with HGD and mucosal adenocarcinoma that was initially treated with photodynamic therapy, single-site ER, and radiofequency ablation on multiple occasions, and developed recurrent disease. He had a history of severe chronic obstructive pulmonary disease and pulmonary embolism, and was not a candidate for esophagectomy. EUS and PET/CT were negative for lymph node involvement or distant disease.
The fourth patient was a 63-year-old man who presented with heartburn since coronary bypass surgery 13 years previously. Surveillance endoscopy showed 9-cm-long segment Barrett's esophagus with HGD and mucosal adenocarcinoma. EUS showed no lymph node involvement. PET/CT showed no metastatic lesions.
The fifth patient was a 68-year-old man with a history of heartburn. Surveillance endoscopy showed a 6-cm-long segment circumferential Barrett's esophagus with HGD, which was treated by five sessions of radiofrequency ablation and ER. Histologic examination of biopsies from the last ER specimens showed intramucosal adenocarcinoma within a background of HGD. EUS and PET/CT were negative for lymph node involvement or metastatic disease.
An overview of the five case histories, including the length of resected mucosal and submucosal tissue for each patient, is presented in Table 1.
Table 1.
Case Summary for Each of the Four Patients
| Case no. | Age (years) | Sex | InitialaBarret lesion | Previous treatment | Lesion at procedure | Length of resection | Hospital stay (days) | Perioperative complication |
|---|---|---|---|---|---|---|---|---|
| 1 | 65 | M | C5M5 | Multiple ER | C2M2 with islands and nodules | 8 cm | 1 | None |
| 2 | 54 | M | C5M6 | Multiple ER/RFA | Two islands with nodularity at 35 cm, stricture at 37 cm | 8 cm | 3 | Left ear/thigh compression |
| 3 | 60 | M | C15M15 | Multiple ER/RFA/PDT | Several islands over 11 cm | 13 cm | 21 | 3-cm muscular tear |
| 4 | 63 | M | C9M9 | None | C9M9 | 10 cm | 4 | Small perforation |
| 5 | 68 | M | C6M6 | Multiple ER/RFA | C1M7 with multiple isolated islands | 8 cm | 9 | Stent migration (day 5) |
C&M Prague classification: C, circumferential extent of Barrett's segment; M, maximal proximal extent of Barrett's segment or tongue(s).
ER, endoscopic resection; RFA, radiofrequency ablation; PDT, photodynamic therapy.
Surgical procedure
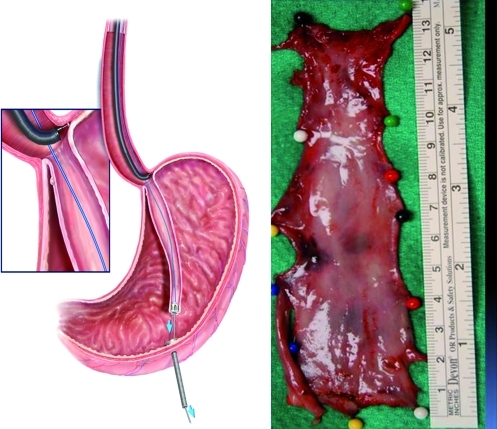
Endoscopic long segment, en bloc circumferential resection of the mucosa and submucosa was performed for the entire length of Barrett's esophagus similar to a procedure previously described.22 An endoscopic gastrostomy tube was placed at the beginning of the procedure. A dissection plane between the submucosa and muscularis externa was created by injection of sodium hyaluronate (HYALGAN®) mixed with indigo carmine and epinephrine into a site immediately distal to the gastroesophageal junction. This site represented the “goal” or terminus of resection. The normal esophageal mucosa 1 cm proximal to the squamocolumnar junction represented the “entry” or beginning of resection. Circumferential ER was then performed at both of these sites, thereby defining the proximal and distal resection margins. More sodium hyaluronate was injected and endoscopic submucosal dissection with a needle knife (Olympus America) was performed to create a 9 mm diameter submucosal tunnel along the entire length of the specimen. The bridging vessels between the submucosal layer and the muscular layer were cauterized using hemostat forceps. The creation of 2–3 submucosal tunnels was performed leaving the attachments between each tunnel for counter-traction. A proximal, circumferential cuff of mucosa and submucosa was then developed over a 2 cm length using this dissection technique. A small-diameter plastic cable was passed retrograde through the gastrostomy tube, retrieved endoscopically, and exited orally. A 9.5-mm olive-shaped cap was attached to the oral side of the plastic cable and this was subsequently secured to the tissue cuff using an endoscopic suture loop applicator (Olympus America). Drawing back on the plastic cable at the site of the gastrostomy facilitated inversion of a sleeve of attached mucosal and submucosal layers. Using this technique, the remaining attachments were stripped away from the muscularis externa, thereby freeing the entire sleeve of tissue. The specimen was then retrieved through the mouth (Fig. 1). The inversion approach was converted to overlapping ER if severe submucosal adhesions were encountered as a result of prior treatments such as ER and radiofrequency ablation.
FIG. 1.
Left panel shows the inversion technique. Drawing back on the plastic cable at the site of the gastrostomy facilitates inversion of a sleeve of attached mucosal and submucosal layers. Using this technique, the remaining attachments are stripped away from the muscularis externa, thereby freeing the entire sleeve of tissue. The sleeve is then retrieved and exited through the mouth. The right panel demonstrates a 13-cm-long sleeve of esophageal inner layers removed from patient no. 3. The tube shaped sleeve was split longitudinally for subsequent fixation and histopathologic processing. Color images available online at www.liebertonline.com/tea
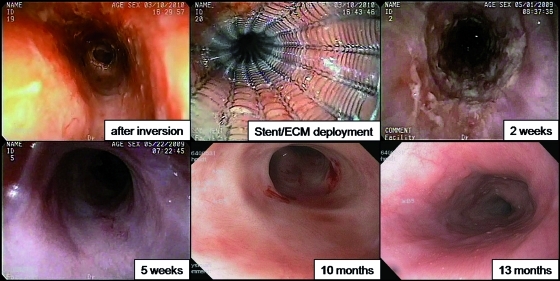
Following resection, the exposed muscular layer was covered with a biologic scaffold material composed of xenogeneic ECM derived from porcine small intestine (Surgisis; Cook). Using a “wall paper” technique, the ECM was secured into position with a radially expanding intralumenal stent (Ultraflex; Boston Scientific). A flat sheet of ECM was tubularized to a diameter of 3 cm and placed around the collapsed stent and insertion catheter. The stent was deployed under combined fluoroscopic and endoscopic guidance resulting in gentle compression of the ECM against the muscularis externa. The stent was removed between 9 and 18 days after ER leaving the now incorporated ECM in position. Endoscopic follow-up was performed every 2–3 weeks following removal of the stent (Fig. 2). A video of the procedure can be found in Supplementary Movie S1.
FIG. 2.
Representative endoscopic views of each stage in the procedure and follow-up. (Upper row) Left: 8-cm-long circumferential muscularis externa was exposed after inversion and resection of the entire sleeve of mucosal and submucosal layers. Middle: the stent was deployed resulting in the gentle compression of ECM against the entire exposed mucsularis externa. Right: 2-week follow-up, immediately after the stent removal. ECM could be seen firmly attached to the area of resection. (Bottom row) Left: 5-week follow-up, the ECM was no longer visible and the resected area was completely covered by squamous epithelium. Middle: 10-month follow-up. The entire esophageal mucosa appeared normal with soft, short segment circumferential strictures. Right: 13-month follow-up. The entire resected area was covered by normal esophageal epithelium without stricture formation. ECM, extracellular matrix. Color images available online at www.liebertonline.com/tea
Biopsy and histopathology
Esophageal mucosal biopsies were taken from patients 1–5 at 55, 27, 18, 13, and 16 weeks postoperatively, respectively. The biopsy specimens were placed directly into formalin, fixed for ∼24 h and subsequently embedded in paraffin. Serial 5-μm sections were cut for hematoxylin and eosin staining and immunolabeling. Hematoxylin and eosin staining was performed using the Leica Autostainer XL (Leica Microsystems). Immunolabeling for cytokeratin 14 (K14), a basal cell marker, was conducted with the primary antibody at 1:200 dilution (clone LL002; Novocastra). Labeling for cytokeratin 4 (K4), a suprabasal cell marker, was conducted with the primary antibody at 1:200 dilution (clone 6B10; Abcam).35 Mouse immunoglobulin G and tris buffered saline were used as controls. Antigen retrieval was conducted by incubating slides in 10 mM citrate buffer (pH 6) for 20 min at 95°C. Red and green fluorescent tags were attached to the K14 and K4 secondary antibodies, respectively.
Results
Patient 1
Multiple ER and endoscopic submucosal dissection were performed including an 8-cm circumferential segment of mucosa and submucosa, followed by ECM scaffold and stent deployment. The stent was carefully removed after 17 days and the biologic scaffold could be seen firmly attached to the area of resection. At 5 weeks, the scaffold was no longer visible and the operative field was completely covered by squamous epithelium. The patient was clinically asymptomatic and there were no signs of esophageal stricture. At 3 months, the entire esophageal mucosa appeared normal with a soft, short segment circumferential stricture identified at the gastroesophageal junction. The 9.8-mm endoscope was able to traverse this narrowing without difficulty. Dilation of the stricture to a diameter of 20 mm was easily achieved. A biopsy of the neoepithleum at 55 weeks post surgery showed normal squamous mucosa. Immunolabeling studies showed a normal distribution of K4+and K14+ keratinocytes (Fig. 3). Although a small focus of recurrent intestinal metaplasia was identified at the gastroesophageal junction during post-surgical surveillance at 4 weeks, neither recurrent dysplasia nor adenocarcinoma has been identified in any postsurgical endoscopic surveillance biopsies. At 24 months, the patient is disease free and consuming a normal diet without dysphagia.
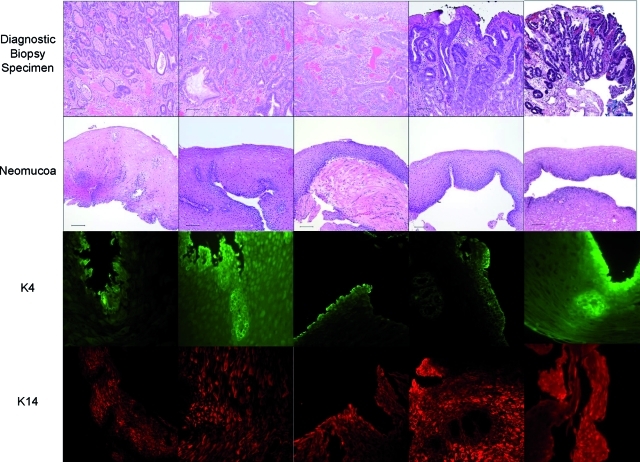
FIG. 3.
Diagnostic biopsy (top row), postoperative biopsy (second row), K4 immunolabeling (third row), and K14 immunolabeling images from each of the four patients. The number of weeks indicated at the bottom of the figure represents the time at which the postoperative biopsy was taken. The diagnostic biopsies all show adenocarcinoma. The postoperative biopsies show replacement of the ECM scaffold with mature, differentiated squamous epithelium. Scale bars represent 100 μm. Color images available online at www.liebertonline.com/tea
Patient 2
A preprocedure upper endoscopy showed two islands of Barrett's esophagus with nodularity and a nonobstructing circumferential stricture caused by prior endoscopic therapy. An endoscopic gastrostomy tube was placed and an 8 cm circumferential resection was performed using the inversion technique described previously. Submucosal scar tissue was present at the level of stricture. ECM and stent were deployed to cover the entire resected area. After 9 days, the stent was removed without difficulty and partial neo-epithelialization was observed. Mild dysphagia without weight loss was reported at 6 weeks and upper endoscopy revealed a <1 cm high-grade stricture at the interface between the proximal resection margin and untreated esophagus. The stricture was soft and easily dilated. During follow-up monitoring, dilation of this region was performed three subsequent times although no symptoms of dysphagia were reported. A biopsy of the neoepithelium at 27 weeks postsurgery showed normal squamous mucosa with normal distribution of K4+ and K14+ keratinocytes (Fig. 3). No Barrett's esophagus, dysplasia, or adenocarcinoma has been identified in any postsurgical endoscopic surveillance biopsies. At 11 months postprocedure, the patient remains disease free with no dysphagia or weight loss.
Patient 3
An endoscopic gastrostomy tube was placed and a 13-cm en-bloc circumferential resection was performed using the inversion technique described previously. A 3-cm tear of the muscular layer occurred during the inversion and stripping maneuver and this was likely secondary to dense scarring between submucosa and muscularis externa from prior endoscopic therapy. Two stents with their corresponding ECM sheets were placed in tandem to cover the resected area. A contrast study showed no leak as a result of the muscle tear. The patient required mechanical ventilation for 4 days because of postoperative respiratory failure due to exacerbation of chronic obstructive pulmonary disease and he was successfully extubated on day 5. At 4 weeks postprocedure, the stents were endoscopically removed under direct visualization without difficulty. There was a 2-cm length of muscularis externa located at the level of the gastroesophageal junction that had not been covered by ECM and a nonobstructing stricture was noted and dilated. Neo-epithelialization was present in the remaining length of the resection. At 6 weeks, mild dysphagia was reported and upper endoscopy demonstrated stricturing at the level of the gastroesophageal junction, which was not covered by ECM; this region required six subsequent dilations. A biopsy of the neoepithelium at 18 weeks postsurgery showed normal squamous mucosa including K4+ and K14+ keratinocytes (Fig. 3). Neither recurrent Barrett's dysplasia nor adenocarcinoma has been identified in postsurgical endoscopic surveillance biopsies. At 9 months the patient is disease free and tolerating a normal diet without dysphagia or weight loss.
Patient 4
An endoscopic gastrostomy tube was placed and a 10-cm en-bloc resection was performed. A small perforation was noted next to the gastroesophageal junction. Two stents with their corresponding ECM sheets were deployed to cover the entire resected area and the small perforation. A contrast study showed no leakage at the perforation site. After 18 days, the stents were endoscopically removed under direct visualization without difficulty. Neo-epithelialization was observed along the entirety of the resected area and the small perforation was healed. Endoscopic follow-up at 26 days showed a focal stricture that was dilated. The stricture was <1 cm in length and located at the interface between the proximal resection margin and untreated esophagus. This region required three subsequent dilations. A biopsy of the neoepithelium at 13 weeks postsurgery showed normal squamous mucosa with normal K4+ and K14+ labeled keratinocytes (Fig. 3). In postsurgical endoscopic surveillance biopsies at the gastroesophageal junction, there is a focus of recurrent Barrett's esophagus with dysplasia. There is no recurrent adenocarcinoma. At 8 months the patient is tolerating a normal diet without dysphagia or weight loss.
Patient 5
A preprocedure upper endoscopy showed multiple isolated islands of Barrett's esophagus without any obvious nodules and a nonobstructing, segmental stricture likely caused by prior endoscopic procedures. An endoscopic gastrostomy tube was not placed and an 8-cm en-bloc circumferential resection was performed using the inversion technique described previously. One stent with its corresponding ECM sheet was deployed to cover the entire resected area. On the postoperative day 5, the stent migrated into the stomach and was replaced with a new stent with ECM. On day 19, the stent was endoscopically removed under direct visualization without difficulty. There was a 1-cm length of muscularis externa located at the level of the gastroesophageal junction that had not been covered by ECM and a nonobstructing stricture was noted at that location and was dilated. Neo-epithelialization was present in the remaining length of the resection that had been covered by ECM. On day 21, the patient presented with dysphagia. Upper endoscopy showed a 2-cm length of stricture at the gastroesophageal junction due to postoperative edema and an impaction of esophageal mucosal debris. This stricture was dilated. On day 26, repeat upper endoscopy showed a 1-cm length of stricture at the gastroesophageal junction. This stricture required nine subsequent dilations and all stricture recurrences were at the only site that had not been originally covered by the ECM sheet. A biopsy of the neoepithelium at 16 weeks postsurgery showed normal squamous mucosa with normal distribution of K4+ and K14+ keratinocytes (Fig. 3). During follow-up endoscopy, neither recurrent Barrett's esophagus nor adenocarcinoma has been identified. At 4 months the patient is tolerating a normal diet without dysphagia or weight loss.
Discussion
This article describes both a new endoscopic technique for en bloc sleeve resection of the esophageal mucosa and submucosa and a regenerative medicine approach to restore the functional inner layers of the esophagus with minimal stricture. The outcome of the first five patients in which this combined approach was used is described with a postoperative follow-up of 4–24 months. The biopsy specimens obtained from these five patients showed nearly complete mature esophageal squamous epithelium in place of the ECM scaffold material as early as 4 months postsurgery.
Avoiding esophagectomy is highly dependent upon the knowledge that the tumor has not penetrated through the submucosal layer and that the lymph nodes are free of disease. Assurance that the cancer has remained superficial becomes challenging in the presence of multifocal HGD and current endoscopic techniques fail to fully address this problem by their destructive nature or the inability to obtain wide tissue margins. The en bloc circumferential inner layer resection described herein provides the necessary tissue to accurately assess the extent of pathology.
Stricture is a common result of esophageal mucosal resection that involves 50%–70% of the circumference.17,36,37 Circumferential, long segment inner layer resection as described in these patients would be expected to uniformly cause severe long segment stricture. Given the fact that only the portion of stripped esophagus that was not covered by ECM formed stricture and required dilation, the ECM placement clearly had a beneficial effect upon the host response such that intractable stricture was avoided and constructive remodeling was facilitated. These findings parallel those found in preclinical animal studies.32,33
The mechanism(s) by which a biologic scaffold composed of non-crosslinked ECM can alter the default esophageal response to injury are partially understood. Preclinical studies have shown that modulation of the innate immune response, specifically the macrophage phenotype, and the recruitment of endogenous stem and progenitor cells to the site of scaffold placement are important factors.38–40 Sequential biopsy specimens were not collected from these patients because avoidance of additional trauma to the mucosa, especially during the early remodeling phase, was more important than examining the microscopic appearance and risking stricture formation. However, studies of ECM scaffold remodeling in preclinical animal models of body wall replacement show that a robust mononuclear cell infiltrate occurs in the 4–28 day postsurgical period and that these cells represent a predominantly M2 macrophage phenotype.38,41 The M2 phenotype is associated with a constructive tissue rebuilding response rather than the typical proinflammatory response mediated by the classically activated M1 macrophage phenotype.42,43 The absence of an adverse immune response to xenogeneic scaffold materials (i.e., typically porcine origin) such as Surgisis has been previously reported44,45 and is thought to be a result of the high degree of homology among the matrix molecules across different species and the alternatively activated innate immune response.46,47
The surgically placed ECM scaffold could not be identified in any of the patients by 2 weeks following stent removal, a finding consistent with the reported rapid degradation of such scaffold materials in radioisotope studies conducted in preclinical animal models.48 Preclinical work also suggests that scaffold degradation results in the formation of cryptic peptides that are potent chemoattractants for endogenous stem and progenitor cells that may contribute to the constructive remodeling process.39,40,49 These mechanisms were not investigated in these first five patients but represent an important area of future work.
The five patients included in this report continue to experience reflux-induced esophageal injury as a result of hiatal hernia and a defective lower esophageal sphincter. Barrett's esophagus is considered a downstream complication of chronic reflux50 and it is therefore important to note that although this approach represents a potential treatment for Barrett's associated neoplasia, it does not correct a major contributing factor to recurrent pathology; that is, gastroesophageal reflux. It is likely because of this continued injury that some of the patients in this report have recurrent Barrett's esophagus at the level of the gastroesophageal junction, the region closest to the caustic effects of gastric fluid. It is reasonable that follow-up surgical correction of gastroesophageal reflux should be considered to prevent recurrent disease. In the present report, all patients had intramucosal adenocarcinoma in the background of HGD, which required resection of the neoplastic tissue rather than anti-reflux surgery. All five patients happen to be male, but this sex bias is simply a matter of chance and not deliberate patient selection. There is no preclinical data to suggest that an identical response would also be found in females. In fact, almost all preclinical studies were conducted in female animal models.
Conclusion
A minimally invasive endoscopic procedure for treatment of Barrett's esophagus with HGD and mucosal adenocarcinoma is possible when combined with the use of a biologic scaffold material to promote reconstruction of functional esophageal mucosa. Esophagectomy with its associated morbidity and mortality can be avoided in selected patients with HGD and mucosal adenocarcinoma.
Supplementary Material
Acknowledgment
We appreciate the assistance provided by Kristen Lippert for facilitating the immunolabeling studies.
Disclosure Statement
The authors disclose they have no competing financial interests.
References
- 1.Ries L.A. Wingo P.A. Miller D.S. Howe H.L. Weir H.K. Rosenberg H.M. Vernon S.W. Cronin K. Edwards B.K. The annual report to the nation on the status of cancer, 1973–1997, with a special section on colorectal cancer. Cancer. 2000;88:2398. doi: 10.1002/(sici)1097-0142(20000515)88:10<2398::aid-cncr26>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 2.Enzinger P.C. Mayer R.J. Medical progress—esophageal cancer. N Engl J Med. 2003;349:2241. doi: 10.1056/NEJMra035010. [DOI] [PubMed] [Google Scholar]
- 3.Oh D.S. Hagen J.A. Chandrasoma P.T. Dunst C.M. Demeester S.R. Alavi M. Bremner C.G. Lipham J. Rizzetto C. Cote R. Demeester T.R. Clinical biology and surgical therapy of intramucosal adenocarcinoma of the esophagus. J Am Coll Surg. 2006;203:152. doi: 10.1016/j.jamcollsurg.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 4.Rice T.W. Blackstone E.H. Adelstein D.J. Zuccaro G., Jr. Vargo J.J. Goldblum J.R. Murthy S.C. DeCamp M.M. Rybicki L.A. Role of clinically determined depth of tumor invasion in the treatment of esophageal carcinoma. J Thorac Cardiovasc Surg. 2003;125:1091. doi: 10.1067/mtc.2003.404. [DOI] [PubMed] [Google Scholar]
- 5.Rice T.W. Zuccaro G., Jr. Adelstein D.J. Rybicki L.A. Blackstone E.H. Goldblum J.R. Esophageal carcinoma: depth of tumor invasion is predictive of regional lymph node status. Ann Thorac Surg. 1998;65:787. doi: 10.1016/s0003-4975(97)01387-8. [DOI] [PubMed] [Google Scholar]
- 6.Ell C. May A. Pech O. Gossner L. Guenter E. Behrens A. Nachbar L. Huijsmans J. Vieth M. Stolte M. Curative endoscopic resection of early esophageal adenocarcinomas (Barrett's cancer) Gastrointest Endosc. 2007;65:3. doi: 10.1016/j.gie.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 7.Nieponice A. Gilbert T.W. Badylak S.F. Reinforcement of esophageal anastomoses with an extracellular matrix scaffold in a canine model. Ann Thorac Surg. 2006;82:2050. doi: 10.1016/j.athoracsur.2006.06.036. [DOI] [PubMed] [Google Scholar]
- 8.Schuchert M.J. Abbas G. Nason K.S. Pennathur A. Awais O. Santana M. Pereira R. Oostdyk A. Luketich J.D. Landreneau R.J. Impact of anastomotic leak on outcomes after transhiatal esophagectomy. Surgery. 2010;148:831. doi: 10.1016/j.surg.2010.07.034. [DOI] [PubMed] [Google Scholar]
- 9.Hameeteman W. Tytgat G.N. Houthoff H.J. van den Tweel J.G. Barrett's esophagus: development of dysplasia and adenocarcinoma. Gastroenterology. 1989;96:1249. doi: 10.1016/s0016-5085(89)80011-3. [DOI] [PubMed] [Google Scholar]
- 10.Fitzgerald R.C. Saeed I.T. Khoo D. Farthing M.J. Burnham W.R. Rigorous surveillance protocol increases detection of curable cancers associated with Barrett's esophagus. Dig Dis Sci. 2001;46:1892. doi: 10.1023/a:1010678913481. [DOI] [PubMed] [Google Scholar]
- 11.van Sandick J.W. van Lanschot J.J.B. Kuiken B.W. Tytgat G.N.J. Offerhaus G.J.A. Obertop H. Impact of endoscopic biopsy surveillance of Barrett's oesophagus on pathological stage and clinical outcome of Barrett's carcinoma. Gut. 1998;43:216. doi: 10.1136/gut.43.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Overholt B.F. Panjehpour M. Halberg D.L. Photodynamic therapy for Barrett's esophagus with dysplasia and/or early stage carcinoma: long-term results. Gastrointest Endosc. 2003;58:183. doi: 10.1067/mge.2003.327. [DOI] [PubMed] [Google Scholar]
- 13.Prasad G.A. Wang K.K. Buttar N.S. Wongkeesong L.M. Krishnadath K.K. Nichols F.C., 3rd Lutzke L.S. Borkenhagen L.S. Long-term survival following endoscopic and surgical treatment of high-grade dysplasia in Barrett's esophagus. Gastroenterology. 2007;132:1226. doi: 10.1053/j.gastro.2007.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaheen N.J. Sharma P. Overholt B.F. Wolfsen H.C. Sampliner R.E. Wang K.K. Galanko J.A. Bronner M.P. Goldblum J.R. Bennett A.E. Jobe B.A. Eisen G.M. Fennerty M.B. Hunter J.G. Fleischer D.E. Sharma V.K. Hawes R.H. Hoffman B.J. Rothstein R.I. Gordon S.R. Mashimo H. Chang K.J. Muthusamy V.R. Edmundowicz S.A. Spechler S.J. Siddiqui A.A. Souza R.F. Infantolino A. Falk G.W. Kimmey M.B. Madanick R.D. Chak A. Lightdale C.J. Radiofrequency ablation in Barrett's esophagus with dysplasia. N Engl J Med. 2009;360:2277. doi: 10.1056/NEJMoa0808145. [DOI] [PubMed] [Google Scholar]
- 15.Ganz R.A. Overholt B.F. Sharma V.K. Fleischer D.E. Shaheen N.J. Lightdale C.J. Freeman S.R. Pruitt R.E. Urayama S.M. Gress F. Pavey D.A. Branch M.S. Savides T.J. Chang K.J. Muthusamy V.R. Bohorfoush A.G. Pace S.C. DeMeester S.R. Eysselein V.E. Panjehpour M. Triadafilopoulos G. Circumferential ablation of Barrett's esophagus that contains high-grade dysplasia: a U.S. Multicenter Registry. Gastrointest Endosc. 2008;68:35. doi: 10.1016/j.gie.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 16.Adams D.S. Masi A. Levin M. H+ pump-dependent changes in membrane voltage are an early mechanism necessary and sufficient to induce Xenopus tail regeneration. Development. 2007;134:1323. doi: 10.1242/dev.02812. [DOI] [PubMed] [Google Scholar]
- 17.Namasivayam V. Wang K.K. Prasad G.A. Endoscopic mucosal resection in the management of esophageal neoplasia: current status and future directions. Clin Gastroenterol Hepatol. 2010;8:743. doi: 10.1016/j.cgh.2010.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giovannini M. Bories E. Pesenti C. Moutardier V. Monges G. Danisi C. Lelong B. Delpero J.R. Circumferential endoscopic mucosal resection in Barrett's esophagus with high-grade intraepithelial neoplasia or mucosal cancer. Preliminary results in 21 patients. Endoscopy. 2004;36:782. doi: 10.1055/s-2004-825813. [DOI] [PubMed] [Google Scholar]
- 19.Lopes C.V. Hela M. Pesenti C. Bories E. Caillol F. Monges G. Giovannini M. Circumferential endoscopic resection of Barrett's esophagus with high-grade dysplasia or early adenocarcinoma. Surg Endosc. 2007;21:820. doi: 10.1007/s00464-006-9187-3. [DOI] [PubMed] [Google Scholar]
- 20.Chennat J. Konda V.J. Ross A.S. de Tejada A.H. Noffsinger A. Hart J. Lin S. Ferguson M.K. Posner M.C. Waxman I. Complete Barrett's eradication endoscopic mucosal resection: an effective treatment modality for high-grade dysplasia and intramucosal carcinoma—an American single- center experience. Am J Gastroenterol. 2009;104:2684. doi: 10.1038/ajg.2009.465. [DOI] [PubMed] [Google Scholar]
- 21.Pouw R.E. Seewald S. Gondrie J.J. Deprez P.H. Piessevaux H. Pohl H. Rosch T. Soehendra N. Bergman J.J. Stepwise radical endoscopic resection for eradication of Barrett's oesophagus with early neoplasia in a cohort of 169 patients. Gut. 2010;59:1169. doi: 10.1136/gut.2010.210229. [DOI] [PubMed] [Google Scholar]
- 22.Witteman B.P. Foxwell T.J. Monsheimer S. Gelrud A. Eid G.M. Nieponice A. O'Rourke R.W. Hoppo T. Bouvy N.D. Badylak S.F. Jobe B.A. Transoral endoscopic inner layer esophagectomy: management of high-grade dysplasia and superficial cancer with organ preservation. J Gastrointest Surg. 2009;13:2104. doi: 10.1007/s11605-009-1053-x. [DOI] [PubMed] [Google Scholar]
- 23.Badylak S.F. Tullius R. Kokini K. Shelbourne K.D. Klootwyk T. Voytik S.L. Kraine M.R. Simmons C. The use of xenogeneic small intestinal submucosa as a biomaterial for Achilles tendon repair in a dog model. J Biomed Mater Res. 1995;29:977. doi: 10.1002/jbm.820290809. [DOI] [PubMed] [Google Scholar]
- 24.Dejardin L.M. Arnoczky S.P. Ewers B.J. Haut R.C. Clarke R.B. Tissue-engineered rotator cuff tendon using porcine small intestine submucosa. Histologic and mechanical evaluation in dogs. Am J Sports Med. 2001;29:175. doi: 10.1177/03635465010290021001. [DOI] [PubMed] [Google Scholar]
- 25.Gilbert T.W. Nieponice A. Spievack A.R. Holcomb J. Gilbert S. Badylak S.F. Repair of the thoracic wall with an extracellular matrix scaffold in a canine model. J Surg Res. 2008;147:61. doi: 10.1016/j.jss.2007.04.035. [DOI] [PubMed] [Google Scholar]
- 26.Knoll L.D. Use of small intestinal submucosa graft for the surgical management of Peyronie's disease. J Urol. 2007;178:2474. doi: 10.1016/j.juro.2007.08.044. [DOI] [PubMed] [Google Scholar]
- 27.Kochupura P.V. Azeloglu E.U. Kelly D.J. Doronin S.V. Badylak S.F. Krukenkamp I.B. Cohen I.S. Gaudette G.R. Tissue-engineered myocardial patch derived from extracellular matrix provides regional mechanical function. Circulation. 2005;112:I144. doi: 10.1161/CIRCULATIONAHA.104.524355. [DOI] [PubMed] [Google Scholar]
- 28.Kropp B.P. Eppley B.L. Prevel C.D. Rippy M.K. Harruff R.C. Badylak S.F. Adams M.C. Rink R.C. Keating M.A. Experimental assessment of small intestinal submucosa as a bladder wall substitute. Urology. 1995;46:396. doi: 10.1016/S0090-4295(99)80227-1. [DOI] [PubMed] [Google Scholar]
- 29.Mase V.J., Jr. Hsu J.R. Wolf S.E. Wenke J.C. Baer D.G. Owens J. Badylak S.F. Walters T.J. Clinical application of an acellular biologic scaffold for surgical repair of a large, traumatic quadriceps femoris muscle defect. Orthopedics. 2010;33:511. doi: 10.3928/01477447-20100526-24. [DOI] [PubMed] [Google Scholar]
- 30.Metcalf M.H. Savoie F.H. Kellum B. Surgical technique for xenograft (SIS) augmentation of rotator-cuff repairs. Oper Tech Orthop. 2002;12:204. [Google Scholar]
- 31.Ueno T. Pickett L.C. de la Fuente S.G. Lawson D.C. Pappas T.N. Clinical application of porcine small intestinal submucosa in the management of infected or potentially contaminated abdominal defects. J Gastrointest Surg. 2004;8:109. doi: 10.1016/j.gassur.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 32.Badylak S.F. Meurling S. Chen M. Spievack A. Simmons-Byrd A. Resorbable bioscaffold for esophageal repair in a dog model. J Pediatr Surg. 2000;35:1097. doi: 10.1053/jpsu.2000.7834. [DOI] [PubMed] [Google Scholar]
- 33.Badylak S.F. Vorp D.A. Spievack A.R. Simmons-Byrd A. Hanke J. Freytes D.O. Thapa A. Gilbert T.W. Nieponice A. Esophageal reconstruction with ECM and muscle tissue in a dog model. J Surg Res. 2005;128:87. doi: 10.1016/j.jss.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 34.Nieponice A. McGrath K. Qureshi I. Beckman E.J. Luketich J.D. Gilbert T.W. Badylak S.F. An extracellular matrix scaffold for esophageal stricture prevention after circumferential EMR. Gastrointest Endosc. 2008;69:289. doi: 10.1016/j.gie.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 35.Green N. Huang Q. Khan L. Battaglia G. Corfe B. MacNeil S. Bury J.P. The development and characterization of an organotypic tissue-engineered human esophageal mucosal model. Tissue Eng Part A. 2010;16:1053. doi: 10.1089/ten.TEA.2009.0217. [DOI] [PubMed] [Google Scholar]
- 36.Giovannini M. Bories E. Pesenti C. Moutardier V. Monges G. Danisi C. Lelong B. Delpero J.R. Circumferential endoscopic mucosal resection in Barrett's esophagus with high-grade intraepithelial neoplasia or mucosal cancer. Preliminary results in 21 patients. Endoscopy. 2004;36:782. doi: 10.1055/s-2004-825813. [DOI] [PubMed] [Google Scholar]
- 37.Soehendra N. Seewald S. Groth S. Omar S. Seitz U. Zhong Y. de Weerth A. Thonke F. Schroeder S. Use of modified multiband ligator facilitates circumferential EMR in Barrett's esophagus (with video) Gastrointest Endosc. 2006;63:847. doi: 10.1016/j.gie.2005.06.052. [DOI] [PubMed] [Google Scholar]
- 38.Badylak S.F. Valentin J.E. Ravindra A.K. McCabe G.P. Stewart-Akers A.M. Macrophage phenotype as a determinant of biologic scaffold remodeling. Tissue Eng Part A. 2008;14:1835. doi: 10.1089/ten.tea.2007.0264. [DOI] [PubMed] [Google Scholar]
- 39.Beattie A.J. Gilbert T.W. Guyot J.P. Yates A.J. Badylak S.F. Chemoattraction of progenitor cells by remodeling extracellular matrix scaffolds. Tissue Eng Part A. 2009;15:1119. doi: 10.1089/ten.tea.2008.0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reing J.E. Zhang L. Myers-Irvin J. Cordero K.E. Freytes D.O. Heber-Katz E. Bedelbaeva K. McIntosh D. Dewilde A. Braunhut S.J. Badylak S.F. Degradation products of extracellular matrix affect cell migration and proliferation. Tissue Eng Part A. 2009;15:605. doi: 10.1089/ten.tea.2007.0425. [DOI] [PubMed] [Google Scholar]
- 41.Brown B.N. Valentin J.E. Stewart-Akers A.M. McCabe G.P. Badylak S.F. Macrophage phenotype and remodeling outcomes in response to biologic scaffolds with and without a cellular component. Biomaterials. 2009;30:1482. doi: 10.1016/j.biomaterials.2008.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Balbani A.P. Duarte J.G. Montovani J.C. [Retrospective analysis of toxicity of eardrops, topical nasal and oropharyngeal medicines, documented in Sao Paulo, Brazil] Rev Assoc Med Bras. 2004;50:433. doi: 10.1590/s0104-42302004000400036. [DOI] [PubMed] [Google Scholar]
- 43.Kou P.M. Babensee J.E. Macrophage and dendritic cell phenotypic diversity in the context of biomaterials. J Biomed Mater Res A. 2011;96:239. doi: 10.1002/jbm.a.32971. [DOI] [PubMed] [Google Scholar]
- 44.Badylak S.F. Gilbert T.W. Immune response to biologic scaffold materials. Semin Immunol. 2008;20:109. doi: 10.1016/j.smim.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Allman A.J. McPherson T.B. Badylak S.F. Merrill L.C. Kallakury B. Sheehan C. Raeder R.H. Metzger D.W. Xenogeneic extracellular matrix grafts elicit a TH2-restricted immune response. Transplantation. 2001;71:1631. doi: 10.1097/00007890-200106150-00024. [DOI] [PubMed] [Google Scholar]
- 46.Constantinou C.D. Jimenez S.A. Structure of cDNAs encoding the triple-helical domain of murine alpha 2 (VI) collagen chain and comparison to human and chick homologues. Use of polymerase chain reaction and partially degenerate oligonucleotide for generation of novel cDNA clones. Matrix. 1991;11:1. doi: 10.1016/s0934-8832(11)80221-0. [DOI] [PubMed] [Google Scholar]
- 47.Exposito J.Y. D'Alessio M. Solursh M. Ramirez F. Sea urchin collagen evolutionarily homologous to vertebrate pro-alpha 2(I) collagen. J Biol Chem. 1992;267:15559. [PubMed] [Google Scholar]
- 48.Record R.D. Hillegonds D. Simmons C. Tullius R. Rickey F.A. Elmore D. Badylak S.F. In vivo degradation of 14C-labeled small intestinal submucosa (SIS) when used for urinary bladder repair. Biomaterials. 2001;22:2653. doi: 10.1016/s0142-9612(01)00007-2. [DOI] [PubMed] [Google Scholar]
- 49.Crisan M. Yap S. Casteilla L. Chen C.W. Corselli M. Park T.S. Andriolo G. Sun B. Zheng B. Zhang L. Norotte C. Teng P.N. Traas J. Schugar R. Deasy B.M. Badylak S. Buhring H.J. Giacobino J.P. Lazzari L. Huard J. Peault B. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 50.Holmes R.S. Vaughan T.L. Epidemiology and pathogenesis of esophageal cancer. Semin Radiat Oncol. 2007;17:2. doi: 10.1016/j.semradonc.2006.09.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.