Abstract
This review aims to highlight the current and significant work in the use of adipose-derived stem cells (ASC) in functional bone tissue engineering framed through the bone mechanobiology perspective. Over a century of work on the principles of bone mechanosensitivity is now being applied to our understanding of bone development. We are just beginning to harness that potential using stem cells in bone tissue engineering. ASC are the primary focus of this review due to their abundance and relative ease of accessibility for autologous procedures. This article outlines the current knowledge base in bone mechanobiology to investigate how the knowledge from this area has been applied to the various stem cell-based approaches to engineering bone tissue constructs. Specific emphasis is placed on the use of human ASC for this application.
Introduction
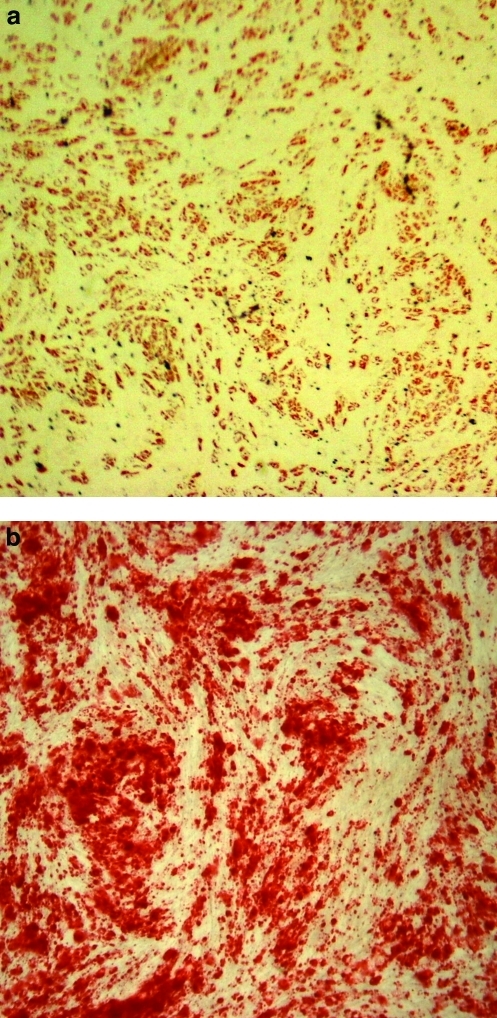
Adipose-derived stem cells (ASC) have become an attractive multipotent cell population for use in tissue replacement therapies. They are a rapidly emerging alternative to the traditional bone marrow-derived mesenchymal stem cells (MSC), though the two cell types have many phenotypic similarities. As an abundant and autologous cell source, use of ASC in tissue-engineered constructs minimizes immunogenicity concerns associated with allograft-based methods. ASC are relatively easy to maintain in culture as they readily self-renew and have the ability to commit to a range of lineages including adipogenic (Fig. 1a), osteogenic (Fig. 1b), chondrogenic, myogenic, neuronal,1,2 cardiomyogenic,3 and endothelial.4 Due to their vast clinical potential in treating critical defect injuries, ASC have gained popularity in cartilage and bone tissue engineering constructs.5
FIG. 1.
Adipogenic and osteogenic differentiation of ASC. (a) Oil Red O staining of ASC cultured in adipogenic media for 14 days; presence of cherry red oil droplets indicates adipogenic differentiation. (b) Alizarin Red staining of ASC cultured in osteogenic media for 14 days; presence of dark red calcium deposits indicates osteogenic differentiation. ASC, adipose-derived stem cell. Color images available online at www.liebertonline.com/teb
It has long been established that bone responds to changes in its mechanical environment. Documented observations date back to the development of Wolff's Law, in the late 19th century, which described loading induced architectural adaptations in bone, remodeling its structure through a feedback system.6 In later years, these ideas were expanded further by Harold M. Frost, who proposed that a minimum effective strain, or “set point,” determined the remodeling process; when strains in the bone exceed the set point, mechanically controlled remodeling acts to increase bone mass and the reverse occurs with strains below the set point.7
Much of the contemporary evidence of bone mechanosensitivity has derived from a multitude of disuse osteoporosis studies8 and microgravity experiments,9 as well as exercise and loading studies.10,11 This body of work provided substantial evidence that increased loading conditions induced bone formation, and reduced loading conditions induced osteoporotic phenotypes, leading to exploration of these patterns in in vitro experimental models. Corresponding work demonstrated that bone cells in culture exhibit mechanosensitivity, and upregulate genes associated with bone formation, in response to mechanical strain and fluid shear, as previously reviewed by Ehrlich and Lanyon.12 Given the wealth of in vivo and in vitro evidence, mechanical forces are considered increasingly crucial for success of current bone tissue engineering methods,13 and are of particular interest in the context of directing ASC osteogenic differentiation.
In 2001 Zuk et al. were the first to establish ASC as a multipotent stem cell population, with the ability to assume osteogenic as well as chondrogenic, adipogenic, and neurogenic phenotypes, through chemically induced differentiation.1,14 Zuk et al. found that when ASC were cultured in osteogenic differentiation media for 2–6 weeks, osteogenic specification was detected by increases in alkaline phosphatase activity, calcium accretion, and upregulation of bone specific gene markers.1 In general, chemical induction of lineage specification has been the most prevalent method used to direct stem cells for tissue engineering applications. However, it is now understood that functional tissue engineering of load bearing tissues likely requires additional physical stimuli (mechanical or electrical) concurrently with chemical stimuli.15–23
A quickly emerging scheme in stem cell differentiation for tissue engineering applications involves simulating a physiologically relevant growth environment for the generated tissue construct. A large part of this effort includes emulating the in vivo mechanical environment experienced by the cells in an in vitro culture. Two major approaches have been used to modulate the mechanical environment of cells and/or tissue-engineered constructs in culture: (1) bioreactors applying “active” mechanical signals such as fluid shear, electrical stimulation, tensile, or compressive strain13,15,24–26 and (2) somewhat “passive” signaling applied through modulation of substrate biochemical composition and stiffness.27–30 A number of custom-designed31–34 and commercially available bioreactor systems15,21,22 are used to apply the signaling modalities discussed in this article, though the versatility of each system is often constrained by design criteria. Bioreactor development remains an active area of research, as bioreactors have become increasingly relevant to overcoming common challenges in tissue engineering. They are also emerging as tools to seed cells throughout three-dimensional (3D) scaffold materials,31,35 as well as devices to validate mechanical and electrochemical properties of a construct.36 As bone functions largely as structural support for the body, construct mechanical integrity is tantamount to applying physiological loading regimes in an in vitro culture environment to generate the construct. Both substrate properties and bioreactors have proven to be an integral part of mechanical approaches to directing ASC lineage specification toward an osteogenic phenotype.21,30,34,37
To create an ASC-derived functional tissue-engineered bone construct for regenerative medicine applications, the construct must carry out the necessary biochemical processes characteristic of healthy bone. To that end, it must emulate the morphology and mechanical behavior of native bone tissue. We refer to this tissue-level construct design approach as a “Top-Down” approach to creating ASC-derived tissue-engineered bone constructs. In contrast, we refer to cell-level approaches as “Bottom-Up.” On a cellular level, the construct should contain cells of an osteogenic phenotype, which respond appropriately to physiologically relevant biochemical and mechanical stimuli. The successful generation of these functional constructs relies on the intersection of Top-Down and Bottom-Up approaches, thoroughly elucidating how mechanical signals affect ASC fate and behavior, in the context of synthesized tissue-level replacements.
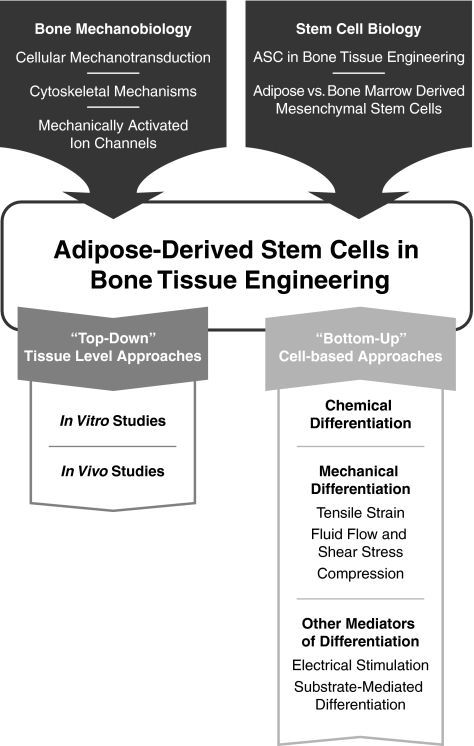
This review aims to summarize the current knowledge of mechanotransduction in ASC lineage specification and how this information has been used in bone tissue engineering with ASC. We will begin by briefly reviewing relevant current knowledge of the mechanical environment and mechanotransduction processes in bone. The knowledge in this area has built the foundation for understanding appropriate physical stimuli and growth environments for the creation of stem cell-derived bone tissue engineering constructs. Framed through the context of bone mechanobiology, we will discuss current mechanobiological approaches applied in ASC osteogenic differentiation and methods in bone tissue engineering. Additionally, we will discuss some of the major studies involving bone marrow-derived MSC as a basis for comparison with ASC, though we will not cover MSC in depth. For a comprehensive review of mechanical control of MSC differentiation to osteochondral tissues, we refer the reader to a recent review by Knothe Tate et al.29 We will conclude with a general summary of the field and comments on its future directions. A schematic illustrating the progression of the field and the topics covered in the article is provided in Figure 2.
FIG. 2.
General schematic illustrating the flow of information provided in this review describing the development and progression of the field. The field of stem-cell-derived bone tissue engineering emerged as a marriage of approaches from bone mechanobiology and stem cell biology. The combination of these two fields has now developed into two different approaches to creating a bone tissue construct: “Top-Down” approaches utilizing the more traditional cell and scaffold tissue level approach meant for immediate translation from bench-to-bedside, and “Bottom-Up” cell-level approaches to characterize the cell population behavior for construct component-level optimization.
Mechanosensitivity of Bone
To determine appropriate mechanical loads for functional bone tissue engineering using ASC, we must first understand the typical mechanical environment experienced by bone cells in vivo. From the cellular perspective in vivo, active mechanical loading of bone translates into either strain through small deformation of the calcified matrix, or fluid shear stress produced by interstitial fluid movement in the osteocyte canaliculi.33 When bone is loaded, both bending and compressive forces create degrees of strain on the bone surface38 and concurrently, on the osteocytes and bone-lining cells.5 The tensile strain imposed on the osteocytes and bone lining cells leads to a change in cytoskeletal conformation. This change is associated with induced activation of stretch-activated ion channels,38,39 voltage sensitive channels via an influx of calcium and shift in membrane potential,38,40 and stretch-activated cation channels.38
Additionally, the resulting compressive strain from external loading causes an increase in interstitial fluid pressure, forcing the fluid to flow from regions of high pressure to regions of lower pressure33,41 within the bone matrix. However, because the matrix is so stiff, the deformation as a result of physiological loads is very small (on the order of 0.2%).42,43 This leads to the canalicular fluid flow hypothesis, which proposes that these small strains impose a local force that initiates fluid flow between thin layers of non-mineralized matrix surrounding the osteocytes' bodies and processes, thus creating a shear stress (8–30 dyn/cm2) at the osteocyte cell membrane.44,45
This section provides a brief overview citing some of the major studies in bone mechanotransduction. All current work in ASC osteogenesis builds off of the basis of bone's chemical, electrical, and mechanical environments, and we will limit the discussion to the relevant mechanisms currently applied in directing ASC differentiation. For thorough reviews on the current state of bone mechanobiology and its implications in tissue engineering applications, we refer the reader to reviews by Riddle and Donahue46 and Allori et al.47
Cellular mechanotransduction in bone
Osteocytes and bone-lining cells are presently thought to be the primary mechanosensory cells responsible for interpreting mechanical forces in bone tissue and translating them to osteoblasts and osteoclasts for bone remodeling.33,45,48,49 Multiple investigators report evidence supporting the key mechanosensory role of osteocytes in bone formation as detected by changes in matrix protein expression, and production of nitric oxide (NO) and prostaglandin E2 (PGE2), a potent stimulator for bone formation.50,51 Osteocytes have been found to be more mechanically sensitive to pulsatile fluid flow (PFF) than osteoblasts and periosteal fibroblasts, as only osteocytes increase production of PGE2 in response to such mechanical stimulation.51 These findings have been further validated by increased NO production in osteocytes in response to PFF, with increases not exhibited by periosteal fibroblasts.45,51 More recent studies using microarray analysis have also identified the mechanosensitivity of osteoblasts to PFF. Thi et al. identified the upregulation of vascular endothelial growth factor and other associated genes in MC3T3E1 cells, an osteoblastic cell line, in response to PFF.52 That work suggested that PFF stimulates signaling pathways crucial to the bone healing and remodeling processes, and identifying these markers of osteogenic healing is of particular relevance in ASC osteogenic lineage transition.
Osteoblasts, perhaps the most relevant cell type to the ASC-derived osteogenic phenotype, have also demonstrated sensitivity to oscillatory fluid flow (OFF), believed by some to be a more physiologically relevant mode of mechanical stimulation in bone.52,53 OFF has been shown to affect osteoblasts: calcium mobilization, mitogen-activated protein kinase activity, and expression of osteopontin (OPN), a bone-specific matrix protein.53 Increases in these metabolic bone markers have been reported to occur in minutes to hours after continuous exposure to OFF.53 Subsequent studies have reported differences in osteoblast behavior in response to continuous OFF and rest-inserted OFF, manifested in changes in intracellular calcium and OPN expression.54 Qin et al. illustrated an analogous mechanosensitivity in vivo through oscillating the intramedullary pressure in the marrow cavity of functionally isolated ulnae in adult turkeys. The adaptive response of the bone was observed after 4 weeks of disuse. The disuse ulna exposed to 10 minutes of OFF per day exhibited increased bone formation on both the endosteal and periosteal surfaces as compared to the control, an ulna not exposed to fluid shear.55 Additional work has suggested osteoblast and osteocyte behavioral response to fluid flow is further modulated by the surface micro-architecture of the cell substrate.56 Generally, in both in vitro and in vivo studies, bone cells have shown sensitivity to dynamic fluid shear, which has led directly to its exploration as a mechanical stimulation modality in ASC, as discussed in section Fluid flow and shear stress.34
Uniaxial tensile strain has been used as another mode of mechanical stimulation to successfully induce bone regeneration in vivo via distraction osteogenesis.57–62 Buchman et al. developed a rat model establishing specific parameters, including critical bone defect size as greater than 3 mm to sufficiently study the mechanisms of distraction osteogenesis and provide a quantitative distinction from conventional bone fracture healing.59 Following this work, in an in vivo study utilizing rat models, Loboa et al.63 reported that gradual distraction of the hemi-mandible (0.25 mm every 12 h) over 8 days, followed by 28 days of rest resulted in periosteal bone formation by postoperative day 7 and a full bridge of new bone spanning the width of the distraction gap by postoperative day 41.57 Our empirical and computational investigations of the regions with the highest rate of new bone formation indicated that tensile strains in the range of 10%–12.5% appeared to optimally induce the highest rate of bone regeneration in the distraction callus.57,63 A similar study by Meyer et al. reported that distraction osteogenesis of the mandible under physiological magnitudes (2000 microstrain) resulted in woven bone formation and some lamellar ossification after 14 days. Over the same time period, a magnitude of 20,000 microstrains resulted in thin trabecular bone formation over the entire gap, and active osteoblasts could be seen on a layer of primary bone.62 These in vivo strain-stimulated bone formation studies have provided the parameter basis adapted to in vitro cyclic strain systems to stimulate osteogenesis in ASC.21 Taken together, these studies provide convincing evidence that osteoblasts and osteocytes are highly mechanosensitive cells capable of differentially sensing mechanical deformation. Harnessing these sensing mechanisms is likely significant for ASC osteogenic lineage specification.
Cytoskeletal mechanisms of mechanotransduction in bone
There are many possible mechanisms by which bone cells interpret external mechanical loads and transmit them via biochemical signals. One such method involves the extracellular matrix-integrin-cytoskeleton network.38,50,64,65 Transmission of mechanical stimulation across the cell surface is modulated by transmembrane receptors (i.e., integrins, cell adhesion molecules, and cadherins) that connect the cytoskeleton to an external substrate.66 This connection provides a molecular pathway for mechanical signals to be passed across the cell surface, allowing focal adhesion molecules to act as mechanoreceptors.
The transmission of mechanical signaling via the connection between integrins and the cytoskeleton has been linked to intracellular pathways that dictate cell viability,67 proliferation,68–70 morphology,67,70 and differentiation.65,68,69,71,72 Tong et al. reported evidence of mechanically transduced signaling as mediated by integrins and focal adhesion kinase in critical defect healing during distraction osteogenesis. Further, they suggested that the mechanical signals were specifically inducing bone formation as detected by bone sialoprotein mRNA expression patterns.65 Mechanistic studies report that PFF results in fluid shear stress-induced reorganization of actin, concurrent with actin-dependent increases in cyclooxygenase-2 (COX-2), c-Fos expression, and PGE2 release, important markers of mechanically induced bone formation.50,73 Together, these results demonstrate the critical role that actin stress fibers and their anchorage to the substrate via focal adhesions have on the mechanotransduction of external mechanical loads and subsequent bone formation.
Similarly, cell–cell interactions and connections have been implicated as a mechanism in transmitting intercellular mechanical signals in bone cells. This cell–cell signaling is associated with the initiation of bone formation and has been suggested to occur via a network of gap junctions and cadherins connecting osteocytes to osteoblasts and osteoclasts.49,74–76 Osteocytes, exposed to a fluid shear stress of 4.4 dyn/cm2 in an osteocyte–osteoblast co-culture system, mediate the upregulated alkaline phosphatase activity response in osteoblasts, as evidenced by Taylor et al. in 2007.76 Although not immediately relevant to the current state of ASC osteogenic work, this study brings up an important point relating the dynamic process of mechanical signaling in bone. The intercellular transmission of mechanical signals among different osteogenic cell types will be directly relevant to the functionality of an ASC-derived bone construct in the future.
More recently, primary cilia have been implicated in the mechanosensitivity and transduction of mechanical signals in bone cells.32,77 The primary cilium, present on most mammalian cell types, was previously believed to be a vestigial organelle. It was later characterized as a mechanosensing organelle in kidney epithelial cells with its dysfunctionality linked to development of polycystic kidney diseases.78 Similarly, its role as a mechanosensor on osteoblasts has been characterized through physical abrogation of the primary cilia as well as siRNA knockdown of ciliary proteins. Malone et al. illustrated significant reduction in gene expression of PGE2 and OPN in primary cilia-free MC3T3E1 cells, as compared to MC3T3E1 cells with intact primary cilia. The reduction in expression was consistent for both siRNA protein knockdown and physical removal of the cilia.32 The role of primary cilia in bone mechanotransduction is under active investigation, as more knowledge is needed to truly understand its role in bone mechanotransduction and its mechanism of action. However, this emerging evidence along with preliminary work in our group hints that it may be a potential mechanistic mediator of osteogenic differentiation in adult stem cells.79
Mechanisms of mechanically activated ion channels
Although they are not extensively studied in mechanically mediated ASC osteogenesis, mechanically sensitive channels such as stretch-activated ion channels,80–83 L-type voltage-sensitive calcium channels,80,84 and potassium-selective channels also play a role in mechanotransduction signaling. Work by Rawlinson et al. suggests the importance of stretch-sensitive channels in mechanically transduced signals. They demonstrated that tensile strain in a rat ulna resulted in activation of stretch/shear-sensitive nonselective cation channels and L-type voltage-dependent calcium channels, involved in osteogenic potential and metabolic activity.80 Li et al. further demonstrated that blocking L-type voltage-sensitive calcium channels in vivo significantly reduced the mechanical loading-induced increase in mineralizing surface, mineral apposition rate, and bone formation rate.84 It is apparent that these calcium channels are significantly involved in bone adaptation and mechanical response in vivo, and elucidating their role in osteogenesis will play a future role in validating the functional ASC osteogenic phenotype.
For a comprehensive review of mechanotransduction and the effects of biomechanical stimulation in bone, please refer to reviews by Riddle and Donahue46 and Allori et al.47 For the remainder of the discussion, we will primarily focus on the current understanding of ASC and how they have been incorporated into the field of bone tissue engineering.
ASC in Bone Tissue Engineering
Adult stem cells such as ASC and MSC demonstrate vast potential in regenerative medicine applications. Traditional methods of treating degenerative skeletal diseases and wounds include use of allografts, autografts, or artificial implants; however, for some types of injuries these treatments are not an option.85 These techniques often present complications such as donor site morbidity, low tissue availability, immunogenicity, or loosening of the implant.30,86–89 The use of autologous ASC for tissue replacement treatments minimizes immunogenic response, and yields a more abundant cell source than bone marrow-derived MSC.
As previously mentioned, the general top-down approach to creating a generic tissue-engineered construct has two primary components: (1) tissue-specific cells and (2) a biocompatible, mechanically appropriate scaffold on which cells can adhere to produce extracellular matrix and encourage regeneration at the defect site.21,87,90,91 A tissue-engineered construct using the general cell-seeded scaffold of the top-down tissue-level approach has been utilized by researchers and physicians for tissue replacement therapies in: (1) tendon92–94; (2) cartilage95–99; and (3) bone100–105 repairs. With increasing use of ASC and other types of stem cells as the primary cell source in an implanted tissue-engineered construct, a second cell-based bottom-up approach has emerged. The bottom-up approach has begun to elucidate the cellular behavior and function within the construct, providing knowledge on how to optimize the scaffold environment. To discuss the ASC approaches currently used in the creation of living bone tissue equivalents, it is important to briefly delineate the current knowledge on the differences between MSC and ASC.
Adipose-derived versus bone marrow-derived MSC
As much of the ASC tissue engineering work to date has arisen from foundational MSC studies, we would be remiss to exclude MSC from the discussion. The majority of stem cell-based tissue replacement efforts to date have typically used bone marrow-derived MSC.18,92,95,100–103,106,107 However, the limited supply of these cells constrains the feasibility of using them in large commercial applications. This constraint has led to the study of stem cells derived from adipose tissue. In contrast to bone marrow, adipose tissue is an abundant and more readily available source of cells.108 In a study performed by De Ugarte et al., ASC showed a similar capacity for adherent cell yield per gram tissue, cell expansion, growth kinetics, and differentiation as that of MSC.109 It should be noted that investigators have reported scalability issues with large volume bone marrow aspirates as peripheral blood contamination reduces MSC cell yields.110 Scalability is not an issue with large ASC isolates and comparisons of cell yield per gram of tissue correspond to optimized volumes for MSC isolation.109 Studies involving ASC, including investigations from our group, have demonstrated their MSC-like multipotency by inducing these cells down osteogenic, myogenic, adipogenic, and chondrogenic lineages.1,109,111–115 Moreover, with few exceptions the surface marker expression profile of ASC seems to generally align with MSC.14,116 However, the use of ASC as a substitute for MSC in certain applications has stimulated controversy due to inconsistent reports of ASC differentiation potential.
While some investigators have reported that there are no differences between the potential for MSC and ASC to differentiate into multiple lineages,109,114,117 others report that ASC are inferior to MSC with respect to their ability to differentiate down particular pathways.1,118–122 De Ugarte et al. examined the multilineage potential of bone marrow-derived MSC to ASC and found that under chemically induced differentiation, there was no difference between the two cell types in their ability to undergo osteogenic and adipogenic differentiation, express neuron-like morphology, or express discrepancies in growth kinetics.109 Likewise, Hattori et al. reported that both MSC and ASC cultured in osteogenic medium expressed similar quantities of calcium phosphate deposition and osteocalcin secretion.
However, studies by Im et al. and Mehlhorn et al. argue that these two cell types do not have the same potential to differentiate down osteogenic or chondrogenic lineages. Im et al. found that the level of mineralization and alkaline phosphatase activity in MSC was significantly greater than that in ASC after 2 and 3 weeks of differentiation, and likewise reported consistent data with specific markers for chondrogenesis.120 The results of a study by Mehlhorn et al. focusing on chondrogenesis agreed with the Im et al. study. MSC cultured in TGF-β1-supplemented medium showed an increase expression of chondrogenic gene markers collagen type II, type X, cartilage oligomeric matrix protein, and aggrecan at least three times higher than expression levels in ASC.118
While some comparisons among different studies have led to a suspicion that ASC may exhibit reduced stem cell potency as compared to MSC, it is important to note that the consensus data are generally inconclusive. Other distinctions between ASC and MSC such as their expression of different surface markers,1,109 requirements of additional medium supplements to differentiate down specific lineages,123–125 and upregulation in different genes during differentiation122 all suggest that ASC are, in fact, not less potent but simply behave differently than MSC. Recently, an in-depth comparison between the gene expression profiles of ASC and MSC demonstrated distinct and unique differences inherent to the specific cell populations.126 Despite emerging evidence supporting inherent differences between ASC and MSC, further characterization of the cell populations remains a critical step in their effective use in tissue engineering applications.
ASC isolation
ASC can be derived from fat pads removed from almost any site of the body, though studies suggest variation in ASC potency as dependent on their derived body location and among donors.127 Generally, ASC are isolated from adipose tissue using a collagenase (most commonly Type I) tissue digest and a series of centrifugation steps to separate the pelleted stromal cell fraction from the red blood cells, adipocyte and adipogenic progenitor fraction.14,128 Final culture selection procedures range from culturing cells that adhere to the culture surface to rigorous cell sorting techniques and clonal culture.1,128–130 General ASC cell surface marker profiles have been characterized, though specific marker discrepancies regarding the surface expression of Stro-1, CD34, and VCAM (CD106) have been reported among ASC population studies from different groups.14,116,129 In spite of these discrepancies, for the most part adherent cells isolated from adipose tissue generally have a defined surface marker expression profile, and Katz et al. suggest that differences are likely related to variations in isolation procedure, propagation time in culture, and exposure to tissue culture plastic. The most widely utilized method to isolate ASC is simply to propagate the adherent cell fraction from adipose tissue without surface marker selection. Consistent reports of the surface expression profiles emerging from different research groups have validated this method.14,116,129 Taken together, this evidence suggests that exposure and adherence to tissue culture plastic may play an important role in defining the ASC immunophenotype. Nonetheless, the surface profile is not necessarily an indicator of ASC potency. Population heterogeneity and donor-to-donor variation still remain challenges that need to be further investigated when manipulating ASC.
Top-Down ASC Living Tissue Equivalent Approaches
We refer to the top-down approach as a primarily tissue level approach, which has rapidly advanced the development of bone tissue constructs, generating potentially implantable living tissue equivalents. The top-down approach has provided the quickest path to usable implants, translating bench-top work by researchers to bedside application by clinicians. However, their success has largely been validated through characterizing tissue-level morphology with some limited evaluation of in vivo functionality. To date, the validation has focused primarily on the performance of the entire construct emulating basic tissue level organization, exhibiting a simplified version of native tissue morphology, with limited understanding of cellular activity and phenotype. In vitro and in vivo top-down approaches to creating living bone tissue equivalents have been largely similar for ASC and MSC and below is a brief summary highlighting some key ASC studies.
In vitro studies
Clinically, autologous bone grafts can provide a treatment method for critical defect repair, but the quantity of donor tissue is limited and there is potential for donor-site morbidity. Tissue engineering using ASC combined with a biocompatible scaffold is emerging as a novel approach for bone tissue replacements in repair of critical defect injuries.22,31,131–134 Work by Hattori et al. demonstrated the osteogenic potential of ASC cultured on β-tricalcium phosphate (TCP) scaffolds with osteogenic media in vitro. Osteocalcin secretion and histology demonstrated an acquired osteogenic phenotype within these constructs.135 Similarly, our group has recently shown that ASC-seeded composite TCP/poly (L-lactic acid) (PLA) scaffolds enhance cell-mediated mineralization and alkaline phosphatase activity in osteogenic media, as compared to the same growth conditions on a purely PLA scaffold.131 This suggests that biochemical composition of the scaffold can play a significant role in directing ASC differentiation and enhancing functionality of the tissue engineered construct.
More recently, decellularized bone scaffolds derived from native bone have shown significant promise as a viable and instructive scaffold material for ASC due to their biochemical and mechanical properties. Fröhlich et al. have presented an approach using ASC seeded on a decellularized bone matrix and reported cell survival, mineral deposition, and expression of bone-specific markers (collagen, bone sialoprotein, and OPN) in histological sections, up to 5 weeks in culture.31 That comparative study showed ASC-seeded bone matrices supported osteogenic differentiation of ASC under static and perfusion culture. Perfusion culture improved cellular distribution throughout the scaffold, thus enhancing the potential three-dimensionality of the construct, a consistent challenge in creating tissue constructs.31
A wide variety of scaffold materials, derived from both natural and synthetic sources, have been used as a platform for ASC growth and induction toward an osteogenic phenotype in two-dimensional and 3D culture. We and others have shown that ASC acquire an osteogenic phenotype in vitro when grown on collagen scaffolds in 3D,37,136 decellularized bone scaffolds,31 PLA scaffolds,132 β-TCP,131,137 bioceramics,138 and composite scaffolds containing the aforementioned materials,131,134,135,139,140 among others. In general, most top-down in vitro approaches apply similar methodologies with ASC and simply vary the scaffold type, but all focus on tissue level resolution and function. The cell-level effects of scaffold variation will be further discussed in the section exploring bottom-up approaches.
In vivo studies
In 2005, a study by Cowan et al. reported successful calvarial critical defect healing by 12 weeks in mice with implantation of ASC-seeded, apatite-coated poly(lactic-co-glycolic acid) (PLGA) scaffolds.141 The cell-seeded scaffolds were biochemically stimulated with bone morphogenetic protein-2 (BMP-2) and retinoic acid for 4 weeks ex vivo before implantation.141 Although the mechanism was not understood and the differentiation process not optimized, this was one of the initial studies reporting the potential use of ASC in healing a critical bone defect. In 2006, Conejero et al. reported successful repair of surgically created palatal bone defects in rats using osteogenically differentiated ASC on a PLA scaffold. After 12 weeks, the osteogenically differentiated ASC produced osseous regeneration of bone, calcium accretion, and positive staining for osteocalcin, a bone matrix protein, at the defect site. Defect sites implanted with PLA alone, PLA seeded with undifferentiated ASC, or left implant free exhibited only fibrous tissue production with little evidence of bone formation.142 Similarly, Yoon et al. implanted osteogenically differentiated ASC-seeded scaffolds into critical-sized rat calvarial defects and observed robust bone regeneration after 12 weeks.143
These studies suggest that the osteogenic phenotype of pre-differentiated ASC is functionally maintained in vivo and that they can operate in a regenerative capacity at a bone defect site. Work by Jeon et al. further evaluated the ability of ASC to differentiate in vivo through BMP-2 stimulation, without the need for an in vitro pre-differentiation step.144 It is important to highlight that this study directly evaluated in vivo differentiation capacity only in the sub-cutaneous space, and did not evaluate its performance within a critical bone defect. ASC seeded on PLGA/hydroxyapatite scaffolds loaded with BMP-2 and implanted subcutaneously into athymic mice generated bone formation and calcification after 8 weeks. The phenotype of the explanted cell population was confirmed through upregulation in bone genetic markers after 8 weeks.144
Further, a comparative study explored the osteogenic potential of BMP-4 retrovirally transduced ASC and MSC and their capacity to ossify a calvarial defect. The transduced ASC and MSC embedded in fibrin gel both formed bone when implanted in the calvarial defect with no significant differences between the groups, though the ASC deposited a higher amount of calcified matrix.145 Similarly, gene therapy approaches using ASC transduced to express BMP-7 derived from rats146 and humans147 have shown evidence for enhanced bone formation in vitro and in vivo. The BMP-7 was encased in a collagen I gel and implanted subcutaneously into rats146 or SCID mice,147 respectively, and caused an increase in mineralization, alkaline phosphatase activity, and osteocalcin expression.146,147 Taken together, these transduction results are consistent with an in vitro study by Dragoo et al. showing increased frequency of a BMP-2-transduced ASC-derived osteoblastic phenotype comparable to exogenous BMP-2 stimulation.148 Another transduction study inducing overexpression of osterix, an important transcription factor in bone development, also produced similar results and differentiation of ASC into an osteoblastic phenotype.149 Gene therapy techniques show promise as an in vivo single-step alternative to chemically induced differentiation before and/or during implantation, minimizing the complexity of the construct components. This body of work suggests feasible techniques for in vivo differentiation of ASC, potentially simplifying therapeutic procedures.
ASC Differentiation: Bottom-Up Cell-Based Approaches
With the emergence of ASC and other stem cell types as a cell source in tissue constructs, a more cell-based, mechanistic, bottom-up approach has led to extensive study on the mechanisms of differentiation. This more basic science approach focuses largely on characterizing cell behavior, phenotype, and the cell-level activity as it contributes to the potential function of a tissue-engineered construct. Such cell-level understanding is becoming particularly important following the increasing popularity of stem cells as cell sources in tissue engineering (Fig. 2). With the growing evidence supporting that chemical, mechanical, and electrical environments all significantly affect ASC differentiation and behavior, the bottom-up approach has generated more rigorous validation methods to understand cell activity. This allows researchers to best optimize tissue-construct design, leading to in-depth study of the differentiation process. Table 1 highlights the major studies applying bottom-up approaches modifying the physical environment, for using ASC in bone tissue engineering.
Table 1.
Major Studies Utilizing Bottom-Up Physical Approaches in the Generationof Adipose Stem Cell-Derived Osteogenic Tissue Constructs
| Author | External mechanical load | Medium | Substrate | Evidence of differentiation | Conclusions |
|---|---|---|---|---|---|
| Wall et al.113 | Cyclic tensile strain | ODM | 3D culture in Collagen I in Tissue Train strain culture system. (Flexcell Int.) | Alizarin Red Staining Opn protein expression | Palladin expression is upregulated with chemically induced osteogenesis and tensile strain. |
| Hanson et al.21 | Cyclic tensile strain | ODM | 2D culture on Col I-coated Bioflex membraneTM (Flexcell Int.) | Quantified calcium accretion | Continuous and rest-inserted cyclic tensile strain enhances osteogenic differentiation. |
| Huang et al.167 | Cyclic tensile strain | ODM | 2D culture on Col I-coated Bioflex membraneTM (Flexcell Int.) | Quantified calcium deposition Alizarin Red Staining | Proliferative capacity of ASC is reduced with age, but restored with tensile strain stimulation. Osteogenic potential is unchanged with age. |
| Knippenberg et al.34 | PFF | ODM | Polylysine-coated glass | NO production cox-2 gene expression | PFF upregulates NO production and cox-2 gene expression in osteogenically differentiated ASC. |
| Tjabringa et al.171 | PFF | ODM | Polylysine-coated glass | NO production, cox-2, runx2 and poly-amine associated SSAT gene expression | PFF upregulates SSAT gene expression. Polyamines mediate mechanically induced upregulation of NO activity and cox-2 gene expression. |
| Fröhlich et al.31 | Continuous flow | ODM | 3D culture in decellularized bone matrix scaffold | Histological and Immunostaining detection of Col I, BSP, and Opn SEM detection of mineralization | Perfusion culture of ASC on 3D deceullarized bone scaffolds improves cellular distribution and enhances mass transport through the scaffold. |
| ODM in perfusion culture system enhances expression of bone specific markers throughout the construct. | |||||
| Hammerick et al.25 | Electrical stimulation (DC field) | ODM | Tissue culture plastic | Gene expression of ALP, Opn, Col I, Runx2 and Osc Atomic force microscopy ALP activity Cytosolic calcium Immunostaining | ALP, Opn, Col I, and Runx2 gene expression is upregulated with DC field exposure. DC field exposure increases cytoskeletal tension Increase in cytoskeletal tension is not necessary for DC field induced differentiation. |
| McCullen et al.26 | Electrical stimulation (AC field) | ODM | Glass slides | Intracellular calcium Mineralized calcium accretion | AC field exposure induces intracellular calcium signaling and increases mineralized calcium accretion. |
ODM, osteogenic differentiation medium; 3D, three-dimensional; 2D, two-dimensional; ASC, adipose-derived stem cell; OPN, osteopontin; PFF, pulsatile fluid flow; NO, nitric oxide; cox-2, cyclo-oxygenase-2; SSAT, spermidine/spermine N (1)-acetyltransferase; BSP, bone sialoprotein; Runx2, runt-related transcription factor-2; SEM, scanning electron microscope; ALP, alkaline phosphatase; OSC, osteocalcin; DC, direct current; AC, alternating current.
Differentiation via chemical stimulation
As stem cell-based tissue engineering technology continues to progress, it is imperative to establish techniques yielding a well characterized and consistent cell population following the differentiation process. The most prevalent bottom-up approach to creating tissue engineering therapeutics operates in the realm of in vitro expansion of the stem cells and subsequent induction of differentiation before implantation into the defect or disease site. ASC can be differentiated by chemical stimulation using media supplements and growth factors to induce lineage specification. Typically, osteogenesis can be obtained by treating ASC with dexamethasone or 1,25-dihydroxyvitamin D3, ascorbic acid, and β-glycerolphosphate in the standard cell growth/expansion media.1,14,115,150 Additional components and growth factors such as BMP-2 and retinoic acid,141 tumor necrosis factor-α,151 growth and differentiation factor-5,152 and histone deacetylase inhibitor valproic acid153 among others have also been studied as osteogenic enhancers. Much of the differentiation media formulation has been based on previous work with pre-osteoblasts and MSC osteogenic differentiation media.154–156
To evaluate the level of osteogenic differentiation response, differentiated cells are characterized using histological stains, protein and gene markers, and morphological properties specific to the osteogenic lineage. Typically, the most straightforward test for differentiation is the Alizarin Red stain for calcium deposits (Fig. 1b), indicating the presence of an osteogenic phenotype.1,115,122,157 Osteogenesis can also be quantified nonspecifically by measuring calcium content using a colorimetric assay or specifically by the upregulation of osteogenic gene and protein markers such as BMP-2, collagen I, alkaline phosphatase, osteocalcin, OPN (SPP1), bone sialoprotein (IBSP), and runt-related transcription factor-2 (Runx2 also known as CBFα1).1,28,31,113,115,156,157 Chemically induced differentiation still remains the gold standard to produce an osteogenic phenotype from ASC, though it has become quite clear that chemical signals are certainly not the only mediators in that process.
Differentiation via mechanical stimulation
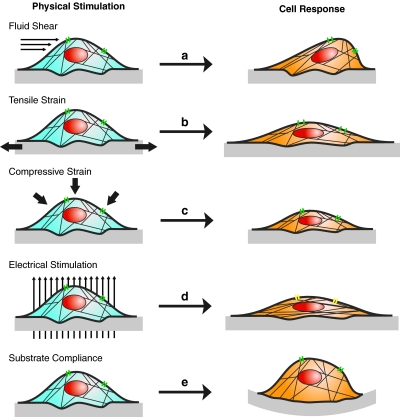
While chemical differentiation methods are generally effective at inducing osteogenesis and production of bone ECM products, studies including mechanical stimulation15,21,158–161 are proving to be more appropriate for creation of functional tissue-engineered constructs. Further knowledge on the native mechanical environments cells experience in vivo has suggested an entirely different mode of differentiation signaling from the physical environment (Fig. 3).
FIG. 3.
Schematic of ASC responses to physical stimulation in 2D culture. Each type of stimulation results in physical changes in cell morphology, alignment, conformation of actin cytoskeleton, and ion channel activity. (a) Fluid shear deforms the apical surface of the cell when sensed by cytoskeletal proteins and activation of stretch-activated ion channels. (b) Tensile strain elongates the basal surface of the cell through stretching of the substrate. Cytoskeletal proteins sense the cellular deformation via connections to integrin–substrate adhesions. Strain applied to the basal surface also induces activation of stretch-activated ion channels. (c) Compressive strain applies pressure to the apical surface of the cell leading to compaction of the cytoplasm and cytoskeleton. (d) Electrical stimulation results in cellular and cytoskeletal alignment perpendicular to the direction of the electric field. Ion channel activity changes, though the mechanism is not clear. (e) Substrate compliance alters the cell's ability to form focal adhesions, limiting cell spreading and causing integrin-cytoskeleton mediated changes in cell behavior. 2D, two-dimensional. Color images available online at www.liebertonline.com/teb
The role of mechanical stimulation in ASC differentiation is following in the stride of MSC, though the mechanotransduction mechanisms in both cell types remain an active area of investigation. However, the basis for mechanically directed differentiation is increasingly supported through the evidence of behavioral changes in bone tissue and cells (osteoblasts, osteoclasts, and osteocytes) in response to mechanical signals such as fluid flow and tensile strain in committed cell types, as discussed previously.
Tensile strain
Early work by Thomas and El Haj in 1996 and Yoshikawa et al. in 1997 demonstrated some of the first evidence of in vitro mechanosensitivity in MSC, implicating the role of tensile strain for MSC osteogenic specification.162,163 Further, stemming from in vivo work with distraction osteogenesis,57,59 cyclic tensile strain has been shown to establish successfully enhanced osteogenic induction of bone marrow-derived MSC in vitro.15,39,162–166 Extending the optimal in vivo distraction osteogenesis parameters to an in vitro model, Sumanasinghe et al. found that even in the absence of osteogenic differentiation medium (i.e., cells maintained in complete growth medium) 10% cyclic tensile strain, applied at a frequency of 1 Hz, for 4 h/day, resulted in an upregulation of BMP-2 in MSC seeded in a 3D collagen I matrix after 1 week, a significant fourfold increase over unstrained samples.15 Likewise, Ignatius et al. reported that cyclic tensile strain (1% at 1 Hz for 1800 cycles/day) applied to osteoblastic precursor cells for 3 weeks resulted in slight increases of histone H4, alkaline phosphatase, CBFα1 (runx2), and OPN compared to unstrained controls.19 Lower frequencies of strain (2.5% at 0.17 Hz) have also been shown to enhance osteogenesis in MSC and reduce their proliferation rate, hinting at the relationship between mechanically signaled proliferation and differentiation.20 Additionally, that mechanistic study demonstrated the critical role of stretch activated cation channels and kinases such as ERK, p38, and PI3K in mediating the mechanically transduced differentiation signals.20
Subsequent work with ASC has similarly demonstrated mechanosensitivity during osteogenic differentiation, though the specific mechanisms of the process are less clear. We have shown that ASC exhibit enhanced osteogenic differentiation when exposed to both continuous (10% strain, 1 Hz) and rest inserted strain (10% strain, 1 Hz, 10 rest between each cycle).21 That particular study specifically compared ASC from two different donors: one line with high mineralization potential in response to chemical stimulation with osteogenic supplements and the other with low mineralization potential. Both modalities of tensile strain enhanced cell-mediated calcium accretion in both ASC lines; however, the ASC with a predisposition to greater calcium accretion expressed a relatively higher osteogenic response to tensile strain, suggesting increased mechanical sensitivity in this line.21 Differences in the observed osteogenic response support the idea that all ASC do not always behave the same way. Just as human ASC from different donors vary in their chemical differentiation potential, so do they in their mechanosensitivity and differentiation potential. Much investigation is still needed to understand the underlying mechanisms of these predispositions and how they are related.
Our group has published a mechanistic study with the goal of elucidating proteins and mechanisms associated with mechanically induced osteogenic differentiation of ASC. We have described the upregulation of palladin expression, an actin-associated cytoskeletal protein, during chemically induced osteogenesis and under cyclic tensile strain.37 That study identified a mechanosensitive protein associated with both osteogenesis and signaling transduction of tensile strain, though it is unclear whether this protein is crucial to mechanically enhanced osteogenesis.
Very recent work with mouse-derived ASC has shown that tensile strain can mechanically mediate age-related variations in ASC proliferation and differentiation potential, altering their cell fate in a magnitude and frequency-dependent fashion.167 However, that study reported significant age and strain-related differentiation effects only in ASC adipogenesis and no significant age and strain related differences in osteogenesis.167 Characteristics such as magnitude,15 number of cycles,168 and frequency168 of strain have been shown to be important variables for optimal tissue regeneration. It is apparent that mechanical signals differentially affect cell behavior more widely than initially hypothesized. Elucidating this process will allow researchers to better harness these characteristics for optimal ASC differentiation.
Fluid flow and shear stress
As stated previously, interstitial fluid flow is believed to impose a physical signal on osteocytes altering their proliferation and metabolic activity.33,50,55,72,73 Empirical and computational studies on the effects of fluid flow signaling osteogenic proliferation and differentiation in MSC16,17,169,170,172 have opened the door for similar studies in ASC.31,34,35,171 Fluid perfusion has also been used as a culture tool to increase dimensionality and cellular distribution throughout a scaffold material, enhancing nutrient transport, to create a more functional construct.35,172 Direct osteogenic signaling via fluid shear in ASC has been primarily PFF, though constant flow perfusion culture regimes have been specifically used to promote 3D cell seeding.31,173
Knippenberg et al. harnessed this principle in an attempt to enhance ASC osteogenesis. ASC differentiation was chemically initialized with 1,25-dihydroxyvitamin D3 and subsequently the cells were cultured under PFF. They reported significant differences in phenotypic behavior between ASC-derived osteogenic cells cultured under PFF and those in static culture. Osteogenic ASC cultured under PFF showed increases in production of NO and upregulation of cox-2 gene expression. This suggests functional validation and enhancement of the osteogenic phenotype through application of physiologically relevant mechanical stimulation, and further demonstrates the innate mechanosensitivity of ASC.34 A subsequent study from the same group identified the role of polyamines in the process of fluid shear-enhanced differentiation in ASC.171 They reported that PFF also led to increased gene expression of spermidine/spermine N (1)-acetyltransferase (SSAT), an enzyme associated with polyamine activity, suggesting that PFF affected polyamine levels. Furthermore, the authors showed that the addition of polyamine spermine inhibited mechanically induced NO-production and cox-2 gene expression.171 These data imply that polyamines play a role in modulating ASC response to mechanical stimulation, which may have a profound effect on ASC approaches to bone tissue engineering and potential therapeutic uses for polyamines in skeletal disorders.
Additionally, studies by Grayson et al. and Li et al. have utilized a perfusion culture system with steady fluid flow to distribute MSC throughout 3D scaffold materials.35,172 Grayson et al. reported substantial cellular penetration and distribution throughout a decellularized bone matrix scaffold and enhanced expression of specific bone matrix markers, concluding flow rate alone can directly control the quality of an MSC-derived bone construct.172 Likewise Li et al. achieved cell survival and shear stress-level dependent osteogenic differentiation and matrix mineralization throughout a TCP scaffold in perfusion culture.35
Following the Grayson et al. article, the same group applied their perfusion culture system to ASC seeded on decellularized bone scaffolds for a comparative investigation, using the same parameters as their MSC study and other static culture methods (study also discussed in “In vitro studies”).31 Similar to the MSC study, ASC also achieved improved 3D cellular distribution and expression of bone specific markers, validating the perfusion culture method for creating a 3D ASC-derived bone construct.31 In general, a variety of fluid flow modalities are proving to provide directive mechanical cues for ASC differentiation as well as cell-seeding methods and improved nutrient delivery within a 3D construct.
Unconfined and confined compression
Cyclic compression has been shown to stimulate osteoblast differentiation and bone formation in vitro and in vivo.36,174,175 Although there has been little work to date on the effect of compression on ASC osteogenic differentiation, it has been implicated in MSC osteogenesis and thus likely is another important mediator of ASC lineage specification. A majority of the work in MSC suggests that cyclic compressive loading leads to a chondrogenic phenotype,176,177 though there is some evidence it may also enhance osteogenic differentiation.174,175 However, it is unclear whether it is the compressive force initiating the mechanically transduced signal, or rather the tensile force acting along the unconfined axis, orthogonally to the direction of compression.
Other environmental mediators of differentiation
Electrical stimulation
Electrical stimulation has been used clinically as a therapeutic procedure to stimulate bone growth and enhance healing of nonunion bone fractures, though some argue the evidenced benefits of the procedure.178 Regardless, the effect of various modalities of electrical stimulation on osteoblast,179 MSC,180–182 and ASC25,26 osteogenic differentiation remains an active area of research. Tsai et al. demonstrated enhanced early osteogenic induction in MSC via application of low-frequency (7.5 Hz) pulsed electromagnetic fields as determined by an increase in alkaline phosphatase activity and upregulation of Runx2 and ALP gene expression.180 Hammerick et al. used pulsed direct current (DC) at a higher frequency (50 Hz) applied to ASC and likewise observed enhanced osteogenic differentiation via upregulation of Opn, Col I, and Runx2 gene expression as well as an increase in cytoskeletal tension, as measured by atomic force microscopy.25 Interestingly, this study reported an important mechanistic finding: the addition of an inhibitor disrupting the tensional action of the cytoskeleton did not yield an apparent decrease in osteogenesis, suggesting that electric field effects are not mediated by mechanical changes in cytoskeletal tension, but rather through another pathway.25 Similarly, active research in our group has observed enhanced osteogenesis and intracellular calcium activity in ASC when exposed to low frequency alternating current (AC) fields via ASC growth and stimulation on interdigitated electrodes.26 Further understanding of this process is needed to fully harness the potential of using electrical stimulation as a tool in creating tissue-engineered bone constructs.
Substrate-mediated differentiation
Another development in directing stem cell differentiation has come out of investigating cell–substrate interactions. Substrate biochemical composition, stiffness, and surface morphology can greatly affect ASC and MSC adhesion, proliferation, migration, and differentiation.28,131–134,183–189 Engler et al. demonstrated distinct morphological, gene, and protein differences among MSC cultured on collagen-coated polyacrylamide gels with elastic moduli of 0.1–1 kPa, 8–17 kPa, and 25–40 kPa. These values were predicted to emulate the tissue compliance of brain, muscle, and collagenous bone, respectively.28 They described synergistic lineage specification effects when combining chemical differentiation media with the appropriate substrate stiffness for the target phenotype. Following that study, work by Rowlands et al. described the interplay between substrate stiffness and surface ligand properties, through testing various ECM coatings. This group reported greatest osteogenic differentiation of MSC on the stiffest (80 kPa) collagen I-coated substrate through expression of Runx2 as compared to other ECM coatings. Interestingly, MSC showed peak expression of myogenic marker MyoD1 at varying moduli depending on type of ECM coating.188
In addition to these mechanistic studies, others have explored the use of bioinstructive materials to direct functional differentiation through inducing characteristic metabolic activities. A study by Au et al. used a well-defined protein peptide for cellular adhesion (GRGDSPY) and a peptide derived from bone sialoprotein (FHRRIKA) to observe integrin-mediated processes in MSC osteogenic differentiation and alkaline phosphatase activity.183 Although much of the work on substrate-mediated differentiation has characterized MSC behavior, our group has shown similar findings of substrate-mediated differentiation with ASC. Studies in our lab have shown that incorporating TCP into electrospun PLA scaffolds enhances ASC endogenous alkaline phosphatase activity and cell-mediated calcium accretion.131 These results were consistent with a similar study conducted by Haimi et al. confirming that PLA/TCP composite scaffolds significantly enhance osteogenic differentiation of ASC.140 These studies suggest that the biochemical composition of the substrate can provide important environmental cues to direct lineage specification of ASC and MSC, particularly for osteogenesis. Although there are fewer studies on substrate-controlled differentiation of ASC, there is substantial evidence that ASC may have similar mechanosensing properties to MSC.
Conclusions
From the development of Wolff's law in the late 19th century to the principles of mechanobiology applied to stem cell osteogenic differentiation, the field has made huge strides toward engineering bone tissue replacements. Mechanical stimulation is now a well-established inducer of osteogenesis in native bone and its role in ASC and MSC osteogenic differentiation is relatively undeniable. Creating a viable tissue-engineered bone construct derived from ASC is truly a multifaceted process necessitating a multidisciplinary approach to optimize the culture environment, as discussed in various sections of this review. Although ASC are a relatively abundant cell source, they are not as well characterized as their MSC counterparts. Further understanding of both of these cell populations will improve our understanding of their multipotency and their limitations for use in bone tissue engineering. Additionally, development of consensus in ASC isolation procedures, though not emphasized in this article, is a necessary step for an accurate characterization definition of the ASC population, as some studies have a more stringent and specific selection process for their ASC population than others.
Most importantly, the crux of this review aimed to explore the current knowledge base in ASC mechanobiology as framed through established principles in bone, though a thorough mechanistic understanding of mechanotransduction processes in these cells remains elusive. It is quite clear that mechanical forces, particularly tensile strain and fluid shear among others, are crucial signals in ASC osteogenic differentiation. Emerging evidence of hypothesized mediators such as the cytoskeletal proteins, primary cilia, and ion channels shows great promise in elucidating the mechanisms behind mechanotransduction in these cells. As we expand our understanding of the ASC mechanosensing process, we can better optimize the chemical, mechanical, and electrical culture environment, as well as the properties of the culture substrate, to direct ASC toward the specified osteogenic phenotype. ASC continue to show great promise as a cell source for autologous bone replacement and regeneration procedures, and as each facet of bone tissue engineering advances in conjunction with further understanding of the ASC populations, they will very likely be cell source candidates in these future therapeutic constructs.
Acknowledgments
This work was supported by NIH/NCRR 10KR51023(EGL), NIH/NIBIB R03Eb008790(EGL) and the NSF Graduate Research Fellowship Program (JCB). We thank B. Hartsfield for his artwork design guidance & E.D. Jerkins for her critical comments on this review.
Disclosure Statement
No competing financial interests exist.
References
- 1.Zuk P. Zhu M. Ashjian P. De Ugarte D. Huang J. Mizuno H., et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gimble J. Guilak F. Adipose-derived adult stem cells: isolation, characterization, and differentiation potential. Cytotherapy. 2003;5:362. doi: 10.1080/14653240310003026. [DOI] [PubMed] [Google Scholar]
- 3.Strem B. Zhu M. Alfonso Z. Daniels E. Schreiber R. Begyui R., et al. Expression of cardiomyocytic markers on adipose tissue-derived cells in a murine model of acute myocardial injury. Cytotherapy. 2005;7:282. doi: 10.1080/14653240510027226. [DOI] [PubMed] [Google Scholar]
- 4.Dimuzio P. Tulenko T. Tissue engineering applications to vascular bypass graft development: The use of adipose-derived stem cells. J Vasc Surg. 2007;45:A99. doi: 10.1016/j.jvs.2007.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rada T. Reis R. Gomes M. Adipose tissue-derived stem cells and their application in bone and cartilage tissue engineering. Tissue Eng Part B Rev. 2009;15:113. doi: 10.1089/ten.teb.2008.0423. [DOI] [PubMed] [Google Scholar]
- 6.Frost H.M. Skeletal structural adaptations to mechanical usage (SATMU): 1. Redefining Wolff's law: the bone modeling problem. Anat Rec. 1990;226:403. doi: 10.1002/ar.1092260402. [DOI] [PubMed] [Google Scholar]
- 7.Frost H.M. Bone “mass” and the “mechanostat”: a proposal. Anat Rec. 1987;219:1. doi: 10.1002/ar.1092190104. [DOI] [PubMed] [Google Scholar]
- 8.Schneider V. McDonald J. Skeletal calcium homeostasis and countermeasures to prevent disuse osteoporosis. Calcif Tissue Int. 1984;36:151. doi: 10.1007/BF02406149. [DOI] [PubMed] [Google Scholar]
- 9.Morey E. Baylink D. Inhibition of bone formation during space flight. Science. 1978;201:1138. doi: 10.1126/science.150643. [DOI] [PubMed] [Google Scholar]
- 10.Robling A. Hinant F. Burr D. Turner C. Improved bone structure and strength after long-term mechanical loading is greatest if loading is separated into short bouts. J Bone Miner Res. 2002;17:1545. doi: 10.1359/jbmr.2002.17.8.1545. [DOI] [PubMed] [Google Scholar]
- 11.Greene D.A. Naughton G.A. Adaptive skeletal responses to mechanical loading during adolescence. Sports Med. 2006;36:723. doi: 10.2165/00007256-200636090-00001. [DOI] [PubMed] [Google Scholar]
- 12.Ehrlich P. Lanyon L. Mechanical strain and bone cell function: a review. Osteoporos Int. 2002;13:688. doi: 10.1007/s001980200095. [DOI] [PubMed] [Google Scholar]
- 13.van Griensven M. Diederichs S. Roeker S. Boehm S. Mechanical strain using 2D and 3D bioreactors induces osteogenesis: implications for bone tissue engineering. Adv Biochem Eng Biotechnol. 2008;112:95. doi: 10.1007/978-3-540-69357-4_5. [DOI] [PubMed] [Google Scholar]
- 14.Zuk P. Zhu M. Mizuno H. Huang J. Futrell J. Katz A., et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 15.Sumanasinghe R.D. Bernacki S.H. Loboa E.G. Osteogenic differentiation of human mesenchymal stem cells in collagen matrices: effect of uniaxial cyclic tensile strain on bone morphogenetic protein (BMP-2) mRNA expression. Tissue Eng. 2006;12:3459. doi: 10.1089/ten.2006.12.3459. [DOI] [PubMed] [Google Scholar]
- 16.Arnsdorf E.J. Tummala P. Kwon R.Y. Jacobs C.R. Mechanically induced osteogenic differentiation - the role of RhoA, ROCKII and cytoskeletal dynamics. J Cell Sci. 2009;122:546. doi: 10.1242/jcs.036293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arnsdorf E.J. Tummala P. Jacobs C.R. Non-canonical Wnt signaling and N-cadherin related beta-catenin signaling play a role in mechanically induced osteogenic cell fate. PLoS ONE. 200;4:e5388. doi: 10.1371/journal.pone.0005388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee W. Maul T. Vorp D. Rubin J. Marra K. Effects of uniaxial cyclic strain on adipose-derived stem cell morphology, proliferation, and differentiation. Biomech Model Mechanobiol. 2007;6:265. doi: 10.1007/s10237-006-0053-y. [DOI] [PubMed] [Google Scholar]
- 19.Ignatius A. Blessing H. Liedert A. Schmidt C. Neidlinger-Wilke C. Kaspar D., et al. Tissue engineering of bone: effects of mechanical strain on osteoblastic cells in type I collagen matrices. Biomaterials. 2005;26:311. doi: 10.1016/j.biomaterials.2004.02.045. [DOI] [PubMed] [Google Scholar]
- 20.Kearney E.M. Farrell E. Prendergast P.J. Campbell V.A. Tensile strain as a regulator of mesenchymal stem cell osteogenesis. Ann Biomed Eng. 2010;38:1767. doi: 10.1007/s10439-010-9979-4. [DOI] [PubMed] [Google Scholar]
- 21.Hanson A. Marvel S. Bernacki S.H. Banes A. Aalst J. Loboa E.G. Osteogenic effects of rest inserted and continuous cyclic tensile strain on hASC lines with disparate osteodifferentiation capabilities. Ann Biomed Eng. 2009;37:955. doi: 10.1007/s10439-009-9648-7. [DOI] [PubMed] [Google Scholar]
- 22.Sumanasinghe R. Osborne J. Loboa E. Mesenchymal stem cell-seeded collagen matrices for bone repair: effects of cyclic tensile strain, cell density, and media conditions on matrix contraction in vitro. J Biomed Mater Res. 2009;88:778. doi: 10.1002/jbm.a.31913. [DOI] [PubMed] [Google Scholar]
- 23.Ozcivici E. Luu Y.K. Adler B. Qin Y-X. Rubin J. Judex S., et al. Mechanical signals as anabolic agents in bone. Nat Rev Rheumatol. 2010;6:50. doi: 10.1038/nrrheum.2009.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Altman G. Lu H. Horan R. Calabro T. Ryder D. Kaplan D., et al. Advanced bioreactor with controlled application of multi-dimensional strain for tissue engineering. J Biomech Eng. 2002;124:742. doi: 10.1115/1.1519280. [DOI] [PubMed] [Google Scholar]
- 25.Hammerick K.E. James A.W. Huang Z. Prinz F.B. Longaker M.T. Pulsed direct current electric fields enhance osteogenesis in adipose-derived stromal cells. Tissue Eng Part A. 2010;16:917. doi: 10.1089/ten.tea.2009.0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCullen S. McQuilling J. Grossfeld R. Lubischer J. Clarke L. Loboa E. Application of low frequency AC electric fields via interdigitated electrodes: effects on cellular viability, cytoplasmic calcium, and osteogenic differentiation of human adipose-derived stem cells. Tissue Eng Part C Methods. 2010;16:1377. doi: 10.1089/ten.tec.2009.0751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Discher D.E. Janmey P. Wang Y.L. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 28.Engler A.J. Sen S. Sweeney H.L. Discher D.E. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 29.Knothe Tate M. Falls T. McBride S. Atit R. Knothe U. Mechanical modulation of osteochondroprogenitor cell fate. Int J Biochem Cell Biol. 2008;40:2720. doi: 10.1016/j.biocel.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCullen S.D. Haslauer C.M. Loboa E.G. Musculoskeletal mechanobiology: Interpretation by external force and engineered substratum. J Biomech. 2010;43:119. doi: 10.1016/j.jbiomech.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 31.Fröhlich M. Grayson W.L. Marolt D. Gimble J.M. Kregar-Velikonja N. Vunjak-Novakovic G. Bone grafts engineered from human adipose-derived stem cells in perfusion bioreactor culture. Tissue Eng Part A. 2010;16:179. doi: 10.1089/ten.tea.2009.0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malone A.M. Anderson C.T. Tummala P. Kwon R.Y. Johnston T.R. Stearns T., et al. Primary cilia mediate mechanosensing in bone cells by a calcium-independent mechanism. Proc Natl Acad Sci U S A. 2007;104:13325. doi: 10.1073/pnas.0700636104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klein-Nulend J. Semeins C. Burger E. Plas A.V.d. Ajubi N. Nijweide P. Response of isolated osteocytes to mechanical loading in vitro. In: Odgaard A., editor; Weinans H., editor. Bone Structure and Remodeling. Singapore: World Scientific Publishing Co. Pvt. Ltd.; 1994. pp. 37–49. [Google Scholar]
- 34.Knippenberg M. Helder M. Zandieh Doulabi B. Semeins C. Wuisman P. Klein-Nulend J. Adipose tissue-derived mesenchymal stem cells acquire bone cell-like responsiveness to fluid shear stress on osteogenic stimulation. Tissue Eng. 2005;11:1780. doi: 10.1089/ten.2005.11.1780. [DOI] [PubMed] [Google Scholar]
- 35.Li D. Tang T. Lu J. Dai K. Effects of flow shear stress and mass transport on the construction of a large-scale tissue-engineered bone in a perfusion bioreactor. Tissue Eng Part A. 2009;15:2773. doi: 10.1089/ten.TEA.2008.0540. [DOI] [PubMed] [Google Scholar]
- 36.David V. Guignandon A. Martin A. Malaval L. Lafage-Proust M.-H. Rattner A., et al. Ex vivo bone formation in bovine trabecular bone cultured in a dynamic 3D bioreactor is enhanced by compressive mechanical strain. Tissue Eng Part A. 2008;14:117. doi: 10.1089/ten.a.2007.0051. [DOI] [PubMed] [Google Scholar]
- 37.Wall M. Rachlin A. Otey C. Loboa E. Human adipose-derived adult stem cells upregulate palladin during osteogenesis and in response to cyclic tensile strain. Am J Physiol Cell Physiol. 2007;293:C1532. doi: 10.1152/ajpcell.00065.2007. [DOI] [PubMed] [Google Scholar]
- 38.Duncan R.L. Turner C.H. Mechanotransduction and the functional response of bone to mechanical strain. Calcif Tissue Int. 1995;57:344. doi: 10.1007/BF00302070. [DOI] [PubMed] [Google Scholar]
- 39.Carter D.R. Beaupre G.S. Giori N.J. Helms J.A. Mechanobiology of skeletal regeneration. Clin Orthop Relat Res. 1998;355(Suppl):S41. doi: 10.1097/00003086-199810001-00006. [DOI] [PubMed] [Google Scholar]
- 40.Rubin J. Rubin C. Jacobs C.R. Molecular pathways mediating mechanical signaling in bone. Gene. 2006;367:1. doi: 10.1016/j.gene.2005.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Knothe Tate M.L. “Whither flows the fluid in bone?” An osteocyte's perspective. J Biomech. 2003;36:1409. doi: 10.1016/s0021-9290(03)00123-4. [DOI] [PubMed] [Google Scholar]
- 42.Rubin C.T. Lanyon L.E. Regulation of bone formation by applied dynamic loads. J Bone Joint Surg Am. 1984;66:397. [PubMed] [Google Scholar]
- 43.Cowin S.C. Moss-Salentijn L. Moss M.L. Candidates for the mechanosensory system in bone. J Biomech Eng. 1991;113:191. doi: 10.1115/1.2891234. [DOI] [PubMed] [Google Scholar]
- 44.Weinbaum S. Cowin S.C. Zeng Y. A model for the excitation of osteocytes by mechanical loading-induced bone fluid shear stresses. J Biomech. 1994;27:339. doi: 10.1016/0021-9290(94)90010-8. [DOI] [PubMed] [Google Scholar]
- 45.Burger E.H. Klein-Nulend J. Mechanotransduction in bone—role of the lacuno-canalicular network. Faseb J. 1999;13(Suppl):S101. [PubMed] [Google Scholar]
- 46.Riddle R. Donahue H. From streaming-potentials to shear stress: 25 years of bone cell mechanotransduction. J Orthop Res. 2009;27:143. doi: 10.1002/jor.20723. [DOI] [PubMed] [Google Scholar]
- 47.Allori A. Sailon A. Pan J. Warren S. Biological basis of bone formation, remodeling, and repair—part III: biomechanical forces. Tissue Eng Part B: Rev. 2008;14:285. doi: 10.1089/ten.teb.2008.0084. [DOI] [PubMed] [Google Scholar]
- 48.Burger E.H. Klein-Nulend J. Responses of bone cells to biomechanical forces in vitro. Adv Dental Res. 1999;13:93. doi: 10.1177/08959374990130012201. [DOI] [PubMed] [Google Scholar]
- 49.Sikavitsas V.I. Temenoff J.S. Mikos A.G. Biomaterials and bone mechanotransduction. Biomaterials. 2001;22:2581. doi: 10.1016/s0142-9612(01)00002-3. [DOI] [PubMed] [Google Scholar]
- 50.Ajubi N.E. Klein-Nulend J. Nijweide P.J. Vrijheid-Lammers T. Alblas M.J. Burger E.H. Pulsating fluid flow increases prostaglandin production by cultured chicken osteocytes—a cytoskeleton-dependent process. Biochem Biophys Res Commun. 1996;225:62. doi: 10.1006/bbrc.1996.1131. [DOI] [PubMed] [Google Scholar]
- 51.Klein-Nulend J. Semeins C.M. Ajubi N.E. Nijweide P.J. Burger E.H. Pulsating fluid flow increases nitric oxide (NO) synthesis by osteocytes but not periosteal fibroblasts—correlation with prostaglandin upregulation. Biochem Biophys Res Commun. 1995;217:640. doi: 10.1006/bbrc.1995.2822. [DOI] [PubMed] [Google Scholar]
- 52.Thi M. Iacobas D. Iacobas S. Spray D. Fluid shear stress upregulates vascular endothelial growth factor gene expression in osteoblasts. Ann N Y Acad Sci. 2007;1117:73. doi: 10.1196/annals.1402.020. [DOI] [PubMed] [Google Scholar]
- 53.You J. Reilly G.C. Zhen X. Yellowley C.E. Chen Q. Donahue H.J., et al. Osteopontin gene regulation by oscillatory fluid flow via intracellular calcium mobilization and activation of mitogen-activated protein kinase in MC3T3-E1 osteoblasts. J Biol Chem. 2001;276:13365. doi: 10.1074/jbc.M009846200. [DOI] [PubMed] [Google Scholar]
- 54.Batra N. Li Y. Yellowley C. You L. Malone A. Kim C., et al. Effects of short-term recovery periods on fluid-induced signaling in osteoblastic cells. J Biomech. 2005;38:1909. doi: 10.1016/j.jbiomech.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 55.Qin Y. Kaplan T. Saldanha A. Rubin C. Fluid pressure gradients, arising from oscillations in intramedullary pressure, is correlated with the formation of bone and inhibition of intracortical porosity. J Biomech. 2003;36:1427. doi: 10.1016/s0021-9290(03)00127-1. [DOI] [PubMed] [Google Scholar]
- 56.Schwartz Z. Denison T. Bannister S. Cochran D. Liu Y. Lohmann C., et al. Osteoblast response to fluid induced shear depends on substrate microarchitecture and varies with time. J Biomed Mater Res. 2007;83A:20. doi: 10.1002/jbm.a.31185. [DOI] [PubMed] [Google Scholar]
- 57.Loboa E.G. Fang T.D. Warren S.M. Lindsey D.P. Fong K.D. Longaker M.T., et al. Mechanobiology of mandibular distraction osteogenesis: experimental analyses with a rat model. Bone. 2004;34:336. doi: 10.1016/j.bone.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 58.Richards M. Goulet J.A. Weiss J.A. Waanders N.A. Schaffler M.B. Goldstein S.A. Bone regeneration and fracture healing. Experience with distraction osteogenesis model. Clin Orthop Relat Res. 1998;355(Suppl):S191. [PubMed] [Google Scholar]
- 59.Buchman S.R. Ignelzi M.A. Radu C. Wilensky J. Rosenthal A.H. Tong L., et al. Unique rodent model of distraction osteogenesis of the mandible. Ann Plast Surg. 2002;49:511. doi: 10.1097/00000637-200211000-00012. [DOI] [PubMed] [Google Scholar]
- 60.Waanders N.A. Richards M. Steen H. Kuhn J.L. Goldstein S.A. Goulet J.A. Evaluation of the mechanical environment during distraction osteogenesis. Clin Orthop Relat Res. 1998;349:225. doi: 10.1097/00003086-199804000-00028. [DOI] [PubMed] [Google Scholar]
- 61.Smith-Adaline E.A. Volkman S.K. Ignelzi M.A., Jr. Slade J. Platte S. Goldstein S.A. Mechanical environment alters tissue formation patterns during fracture repair. J Orthop Res. 2004;22:1079. doi: 10.1016/j.orthres.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 62.Meyer U. Wiesmann H.P. Kruse-Losler B. Handschel J. Stratmann U. Joos U. Strain-related bone remodeling in distraction osteogenesis of the mandible. Plast Reconstr Surg. 1999;103:800. doi: 10.1097/00006534-199903000-00005. [DOI] [PubMed] [Google Scholar]
- 63.Loboa E. Fang T. Parker D. Warren S. Fong K. Longaker M., et al. Mechanobiology of mandibular distraction osteogenesis: finite element analyses with a rat model. J Orthop Res. 2005;23:663. doi: 10.1016/j.orthres.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 64.Plotkin L.I. Mathov I. Aguirre J.I. Parfitt A.M. Manolagas S.C. Bellido T. Mechanical stimulation prevents osteocyte apoptosis: requirement of integrins, Src kinases, and ERKs. Am J Physiol Cell Physiol. 2005;289:C633. doi: 10.1152/ajpcell.00278.2004. [DOI] [PubMed] [Google Scholar]
- 65.Tong L. Buchman S.R. Ignelzi M.A. Rhee S. Goldstein S.A. Focal adhesion kinase expression during mandibular distraction osteogenesis: evidence for mechanotransduction. Plast Reconstr Surg. 2003;111:211. doi: 10.1097/01.PRS.0000033180.01581.9A. [DOI] [PubMed] [Google Scholar]
- 66.Ingber D.E. Tensegrity: the architectural basis of cellular mechanotransduction. Annu Rev Physiol. 1997;59:575. doi: 10.1146/annurev.physiol.59.1.575. [DOI] [PubMed] [Google Scholar]
- 67.Ingber D.E. Folkman J. Mechanochemical switching between growth and differentiation during fibroblast growth factor-stimulated angiogenesis in vitro: role of extracellular matrix. J Cell Biol. 1989;109:317. doi: 10.1083/jcb.109.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rana B. Mischoulon D. Xie Y. Bucher N.L. Farmer S.R. Cell-extracellular matrix interactions can regulate the switch between growth and differentiation in rat hepatocytes: reciprocal expression of C/EBP alpha and immediate-early growth response transcription factors. Mol Cell Biol. 1994;14:5858. doi: 10.1128/mcb.14.9.5858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mooney D. Hansen L. Vacanti J. Langer R. Farmer S. Ingber D. Switching from differentiation to growth in hepatocytes: control by extracellular matrix. J Cell Physiol. 1992;151:497. doi: 10.1002/jcp.1041510308. [DOI] [PubMed] [Google Scholar]
- 70.Hansen L.K. Mooney D.J. Vacanti J.P. Ingber D.E. Integrin binding and cell spreading on extracellular matrix act at different points in the cell cycle to promote hepatocyte growth. Mol Biol Cell. 1994;5:967. doi: 10.1091/mbc.5.9.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McGarry J.G. Klein-Nulend J. Prendergast P.J. The effect of cytoskeletal disruption on pulsatile fluid flow-induced nitric oxide and prostaglandin E2 release in osteocytes and osteoblasts. Biochem Biophys Res Commun. 2005;330:341. doi: 10.1016/j.bbrc.2005.02.175. [DOI] [PubMed] [Google Scholar]
- 72.Mullender M. El Haj A.J. Yang Y. van Duin M.A. Burger E.H. Klein-Nulend J. Mechanotransduction of bone cells in vitro: mechanobiology of bone tissue. Med Biol Eng Comput. 2004;42:14. doi: 10.1007/BF02351006. [DOI] [PubMed] [Google Scholar]
- 73.Pavalko F. Chen N. Turner C. Burr D. Atkinson S. Hsieh Y., et al. Fluid shear-induced mechanical signaling in MC3T3-E1 osteoblasts requires cytoskeleton-integrin interactions. Am J Physiol Cell Physiol. 1998;275:1591. [PubMed] [Google Scholar]
- 74.Liedert A. Kaspar D. Blakytny R. Claes L. Ignatius A. Signal transduction pathways involved in mechanotransduction in bone cells. Biochem Biophys Res Commun. 2006;349:1. doi: 10.1016/j.bbrc.2006.07.214. [DOI] [PubMed] [Google Scholar]
- 75.Jiang J.X. Siller-Jackson A.J. Burra S. Roles of gap junctions and hemichannels in bone cell functions and in signal transmission of mechanical stress. Front Biosci. 2007;12:1450. doi: 10.2741/2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Taylor A.F. Saunders M.M. Shingle D.L. Cimbala J.M. Zhou Z. Donahue H.J. Mechanically stimulated osteocytes regulate osteoblastic activity via gap junctions. Am J Physiol Cell Physiol. 2007;292:C545. doi: 10.1152/ajpcell.00611.2005. [DOI] [PubMed] [Google Scholar]
- 77.Whitfield J. The solitary (primary) cilium–A mechanosensory toggle switch in bone and cartilage cells. Cell Signal. 2008;20:1019. doi: 10.1016/j.cellsig.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 78.Zhou J. Polycystins and primary cilia: primers for cell cycle progression. Ann Rev Physiol. 2009;71:83. doi: 10.1146/annurev.physiol.70.113006.100621. [DOI] [PubMed] [Google Scholar]
- 79.Tummala P. Arnsdorf E.J. Jacobs C.R. The role of primary cilia in mesenchymal stem cell differentiation: a pivotal switch in guiding lineage commitment. Cell Mol Bioeng. 2010;3:207. doi: 10.1007/s12195-010-0127-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rawlinson S.C. Pitsillides A.A. Lanyon L.E. Involvement of different ion channels in osteoblasts' and osteocytes' early responses to mechanical strain. Bone. 1996;19:609. doi: 10.1016/s8756-3282(96)00260-8. [DOI] [PubMed] [Google Scholar]
- 81.Charras G.T. Williams B.A. Sims S.M. Horton M.A. Estimating the sensitivity of mechanosensitive ion channels to membrane strain and tension. Biophys J. 2004;87:2870. doi: 10.1529/biophysj.104.040436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Davidson R.M. Membrane stretch activates a high-conductance K+ channel in G292 osteoblastic-like cells. J Membr Biol. 1993;131:81. doi: 10.1007/BF02258536. [DOI] [PubMed] [Google Scholar]
- 83.Davidson R.M. Tatakis D.W. Auerbach A.L. Multiple forms of mechanosensitive ion channels in osteoblast-like cells. Pflugers Arch. 1990;416:646. doi: 10.1007/BF00370609. [DOI] [PubMed] [Google Scholar]
- 84.Li J. Duncan R.L. Burr D.B. Turner C.H. L-type calcium channels mediate mechanically induced bone formation in vivo. J Bone Miner Res. 2002;17:1795. doi: 10.1359/jbmr.2002.17.10.1795. [DOI] [PubMed] [Google Scholar]
- 85.Khan Y. Yaszemski M.J. Mikos A.G. Laurencin C.T. Tissue engineering of bone: material and matrix considerations. J Bone Joint Surg. 2008;90(Suppl 1):36. doi: 10.2106/JBJS.G.01260. [DOI] [PubMed] [Google Scholar]
- 86.Younger E.M. Chapman M.W. Morbidity at bone graft donor sites. J Orthop Trauma. 1989;3:192. doi: 10.1097/00005131-198909000-00002. [DOI] [PubMed] [Google Scholar]
- 87.Ringe J. Kaps C. Burmester G.R. Sittinger M. Stem cells for regenerative medicine: advances in the engineering of tissues and organs. Naturwissenschaften. 2002;89:338. doi: 10.1007/s00114-002-0344-9. [DOI] [PubMed] [Google Scholar]
- 88.Moore W.R. Graves S.E. Bain G.I. Synthetic bone graft substitutes. ANZ J Surg. 2001;71:354. [PubMed] [Google Scholar]
- 89.Hui J.H. Ouyang H.W. Hutmacher D.W. Goh J.C. Lee E.H. Mesenchymal stem cells in musculoskeletal tissue engineering: a review of recent advances in National University of Singapore. Ann Acad Med Singapore. 2005;34:206. [PubMed] [Google Scholar]
- 90.Bonassar L.J. Vacanti C.A. Tissue engineering: the first decade and beyond. J Cell Biochem Suppl. 1998;30–31:297. [PubMed] [Google Scholar]
- 91.Vacanti C.A. Vacanti J.P. The science of tissue engineering. Orthop Clin North Am. 2000;31:351. doi: 10.1016/s0030-5898(05)70155-3. [DOI] [PubMed] [Google Scholar]
- 92.Young R.G. Butler D.L. Weber W. Caplan A.I. Gordon S.L. Fink D.J. Use of mesenchymal stem cells in a collagen matrix for Achilles tendon repair. J Orthop Res. 1998;16:406. doi: 10.1002/jor.1100160403. [DOI] [PubMed] [Google Scholar]
- 93.Juncosa-Melvin N. Shearn J.T. Boivin G.P. Gooch C. Galloway M.T. West J.R., et al. Effects of mechanical stimulation on the biomechanics and histology of stem cell-collagen sponge constructs for rabbit patellar tendon repair. Tissue Eng. 2006;12:2291. doi: 10.1089/ten.2006.12.2291. [DOI] [PubMed] [Google Scholar]
- 94.Awad H.A. Boivin G.P. Dressler M.R. Smith F.N. Young R.G. Butler D.L. Repair of patellar tendon injuries using a cell-collagen composite. J Orthop Res. 2003;21:420. doi: 10.1016/S0736-0266(02)00163-8. [DOI] [PubMed] [Google Scholar]
- 95.Wakitani S. Goto T. Pineda S.J. Young R.G. Mansour J.M. Caplan A.I., et al. Mesenchymal cell-based repair of large, full-thickness defects of articular cartilage. J Bone Joint Surg Am. 1994;76:579. doi: 10.2106/00004623-199404000-00013. [DOI] [PubMed] [Google Scholar]
- 96.Liu Y. Shu X.Z. Prestwich G.D. Osteochondral defect repair with autologous bone marrow-derived mesenchymal stem cells in an injectable, in situ, cross-linked synthetic extracellular matrix. Tissue Eng. 2006;12:3405. doi: 10.1089/ten.2006.12.3405. [DOI] [PubMed] [Google Scholar]
- 97.Yan H. Yu C. Repair of full-thickness cartilage defects with cells of different origin in a rabbit model. Arthroscopy. 2007;23:178. doi: 10.1016/j.arthro.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 98.Kuroda R. Ishida K. Matsumoto T. Akisue T. Fujioka H. Mizuno K., et al. Treatment of a full-thickness articular cartilage defect in the femoral condyle of an athlete with autologous bone-marrow stromal cells. Osteoarthritis Cartilage. 2007;15:226. doi: 10.1016/j.joca.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 99.Wakitani S. Imoto K. Yamamoto T. Saito M. Murata N. Yoneda M. Human autologous culture expanded bone marrow mesenchymal cell transplantation for repair of cartilage defects in osteoarthritic knees. Osteoarthritis Cartilage. 2002;10:199. doi: 10.1053/joca.2001.0504. [DOI] [PubMed] [Google Scholar]
- 100.Endres M. Hutmacher D.W. Salgado A.J. Kaps C. Ringe J. Reis R.L., et al. Osteogenic induction of human bone marrow-derived mesenchymal progenitor cells in novel synthetic polymer-hydrogel matrices. Tissue Eng. 2003;9:689. doi: 10.1089/107632703768247386. [DOI] [PubMed] [Google Scholar]
- 101.Bruder S.P. Kraus K.H. Goldberg V.M. Kadiyala S. The effect of implants loaded with autologous mesenchymal stem cells on the healing of canine segmental bone defects. J Bone Joint Surg Am. 1998;80:985. doi: 10.2106/00004623-199807000-00007. [DOI] [PubMed] [Google Scholar]
- 102.Arinzeh T.L. Peter S.J. Archambault M.P. van den Bos C. Gordon S. Kraus K. et al. Allogeneic mesenchymal stem cells regenerate bone in a critical-sized canine segmental defect. J Bone Joint Surg Am. 2003;85A:1927. doi: 10.2106/00004623-200310000-00010. [DOI] [PubMed] [Google Scholar]
- 103.Schantz J.T. Hutmacher D.W. Lam C.X. Brinkmann M. Wong K.M. Lim T.C. et al. Repair of calvarial defects with customised tissue-engineered bone grafts II. Evaluation of cellular efficiency and efficacy in vivo. Tissue Eng. 2003;9(Suppl 1):S127. doi: 10.1089/10763270360697030. [DOI] [PubMed] [Google Scholar]
- 104.Quarto R. Mastrogiacomo M. Cancedda R. Kutepov S.M. Mukhachev V. Lavroukov A., et al. Repair of large bone defects with the use of autologous bone marrow stromal cells. N Engl J Med. 2001;344:385. doi: 10.1056/NEJM200102013440516. [DOI] [PubMed] [Google Scholar]
- 105.Kon E. Muraglia A. Corsi A. Bianco P. Marcacci M. Martin I., et al. Autologous bone marrow stromal cells loaded onto porous hydroxyapatite ceramic accelerate bone repair in critical-size defects of sheep long bones. J Biomed Mater Res. 2000;49:328. doi: 10.1002/(sici)1097-4636(20000305)49:3<328::aid-jbm5>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 106.Park J.S. Chu J.S. Cheng C. Chen F. Chen D. Li S. Differential effects of equiaxial and uniaxial strain on mesenchymal stem cells. Biotechnol Bioeng. 2004;88:359. doi: 10.1002/bit.20250. [DOI] [PubMed] [Google Scholar]
- 107.Endres M. Hutmacher D.W. Salgado A.J. Kaps C. Ringe J. Reis R.L. Sittinger M. Brandwood A. Schantz J.T. Osteogenic induction of human bone marrow-derived mesenchymal progenitor cells in novel synthetic polymer-hydrogel matrices. Tissue Eng. 2003;9:689. doi: 10.1089/107632703768247386. [DOI] [PubMed] [Google Scholar]
- 108.Aust L. Devlin B. Foster S.J. Halvorsen Y.D. Hicok K. du Laney T., et al. Yield of human adipose-derived adult stem cells from liposuction aspirates. Cytotherapy. 2004;6:7. doi: 10.1080/14653240310004539. [DOI] [PubMed] [Google Scholar]
- 109.De Ugarte D.A. Morizono K. Elbarbary A. Alfonso Z. Zuk P.A. Zhu M., et al. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs. 2003;174:101. doi: 10.1159/000071150. [DOI] [PubMed] [Google Scholar]
- 110.Batinić D. Marusić M. Pavletić Z. Bogdanić V. Uzarević B. Nemet D., et al. Relationship between differing volumes of bone marrow aspirates and their cellular composition. Bone Marrow Transplant. 1990;6:103. [PubMed] [Google Scholar]
- 111.Guilak F. Lott K.E. Awad H.A. Cao Q. Hicok K.C. Fermor B., et al. Clonal analysis of the differentiation potential of human adipose-derived adult stem cells. J Cell Physiol. 2006;206:229. doi: 10.1002/jcp.20463. [DOI] [PubMed] [Google Scholar]
- 112.Weinzierl K. Hemprich A. Frerich B. Bone engineering with adipose tissue derived stromal cells. J Craniomaxillofac Surg. 2006;34:466. doi: 10.1016/j.jcms.2006.07.860. [DOI] [PubMed] [Google Scholar]
- 113.Halvorsen Y.D. Franklin D. Bond A.L. Hitt D.C. Auchter C. Boskey A.L., et al. Extracellular matrix mineralization and osteoblast gene expression by human adipose tissue-derived stromal cells. Tissue Eng. 2001;7:729. doi: 10.1089/107632701753337681. [DOI] [PubMed] [Google Scholar]
- 114.Hattori H. Sato M. Masuoka K. Ishihara M. Kikuchi T. Matsui T., et al. Osteogenic potential of human adipose tissue-derived stromal cells as an alternative stem cell source. Cells Tissues Organs. 2004;178:2. doi: 10.1159/000081088. [DOI] [PubMed] [Google Scholar]
- 115.Wall M. Bernacki S.H. Loboa E.G. Effects of serial passaging on the adipogenic and osteogenic differentiation potential of adipose-derived human mesenchymal stem cells. Tissue Eng. 2007;13:1291. doi: 10.1089/ten.2006.0275. [DOI] [PubMed] [Google Scholar]
- 116.Gronthos S. Franklin D.M. Leddy H.A. Robey P.G. Storms R.W. Gimble J.M. Surface protein characterization of human adipose tissue-derived stromal cells. J Cell Physiol. 2001;189:54. doi: 10.1002/jcp.1138. [DOI] [PubMed] [Google Scholar]
- 117.Mauney J.R. Nguyen T. Gillen K. Kirker-Head C. Gimble J.M. Kaplan D.L. Engineering adipose-like tissue in vitro and in vivo utilizing human bone marrow and adipose-derived mesenchymal stem cells with silk fibroin 3D scaffolds. Biomaterials. 2007;28:5280. doi: 10.1016/j.biomaterials.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mehlhorn A.T. Niemeyer P. Kaiser S. Finkenzeller G. Stark G.B. Sudkamp N.P., et al. Differential expression pattern of extracellular matrix molecules during chondrogenesis of mesenchymal stem cells from bone marrow and adipose tissue. Tissue Eng. 2006;12:2853. doi: 10.1089/ten.2006.12.2853. [DOI] [PubMed] [Google Scholar]
- 119.Vidal M.A. Kilroy G.E. Lopez M.J. Johnson J.R. Moore R.M. Gimble J.M. Characterization of equine adipose tissue-derived stromal cells: adipogenic and osteogenic capacity and comparison with bone marrow-derived mesenchymal stromal cells. Vet Surg. 2007;36:613. doi: 10.1111/j.1532-950X.2007.00313.x. [DOI] [PubMed] [Google Scholar]
- 120.Im G.I. Shin Y.W. Lee K.B. Do adipose tissue-derived mesenchymal stem cells have the same osteogenic and chondrogenic potential as bone marrow-derived cells? Osteoarthritis Cartilage. 2005;13:845. doi: 10.1016/j.joca.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 121.Afizah H. Yang Z. Hui J.H. Ouyang H.W. Lee E.H. A comparison between the chondrogenic potential of human bone marrow stem cells (BMSC) and adipose-derived stem cells (ADSCs) taken from the same donors. Tissue Eng. 2007;13:659. doi: 10.1089/ten.2006.0118. [DOI] [PubMed] [Google Scholar]
- 122.Liu T.M. Martina M. Hutmacher D.W. Hui J.H. Lee E.H. Lim B. Identification of common pathways mediating differentiation of bone marrow- and adipose tissue-derived human mesenchymal stem cells into three mesenchymal lineages. Stem Cells. 2007;25:750. doi: 10.1634/stemcells.2006-0394. [DOI] [PubMed] [Google Scholar]
- 123.Estes B.T. Wu A.W. Guilak F. Potent induction of chondrocytic differentiation of human adipose-derived adult stem cells by bone morphogenetic protein 6. Arthritis Rheum. 2006;54:1222. doi: 10.1002/art.21779. [DOI] [PubMed] [Google Scholar]
- 124.Estes B.T. Wu A.W. Storms R.W. Guilak F. Extended passaging, but not aldehyde dehydrogenase activity, increases the chondrogenic potential of human adipose-derived adult stem cells. J Cell Physiol. 2006;209:987. doi: 10.1002/jcp.20808. [DOI] [PubMed] [Google Scholar]
- 125.Puetzer J.L. Petitte J.N. Loboa E.G. Comparative review of growth factors for induction of three-dimensional in vitro chondrogenesis in human mesenchymal stem cells isolated from bone marrow and adipose tissue. Tissue Eng Part B Rev. 2010;16:435. doi: 10.1089/ten.TEB.2009.0705. [DOI] [PubMed] [Google Scholar]
- 126.Jansen B.J. Gilissen C. Roelofs H. Schaap-Oziemlak A. Veltman J. Raymakers R.A., et al. Functional differences between mesenchymal stem cell populations are reflected by their transcriptome. Stem Cells Dev. 2010;19:481. doi: 10.1089/scd.2009.0288. [DOI] [PubMed] [Google Scholar]
- 127.Prunet-Marcassus B. Cousin B. Caton D. André M. Pénicaud L. Casteilla L. From heterogeneity to plasticity in adipose tissues: site-specific differences. Exp Cell Res. 2006;312:727. doi: 10.1016/j.yexcr.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 128.Bernacki S. Wall M. Loboa E. Isolation of human mesenchymal stem cells from bone and adipose tissue. Methods Cell Biol. 2008;86:257. doi: 10.1016/S0091-679X(08)00011-3. [DOI] [PubMed] [Google Scholar]
- 129.Katz A.J. Tholpady A. Tholpady S.S. Shang H. Ogle R.C. Cell surface and transcriptional characterization of human adipose-derived adherent stromal (hADAS) cells. Stem Cells. 2005;23:412. doi: 10.1634/stemcells.2004-0021. [DOI] [PubMed] [Google Scholar]
- 130.Rada T. Reis R.L. Gomes M.E. Distinct stem cells subpopulations isolated from human adipose tissue exhibit different chondrogenic and osteogenic differentiation potential. Stem Cell Rev. 2011;7:64. doi: 10.1007/s12015-010-9147-0. [DOI] [PubMed] [Google Scholar]
- 131.McCullen S. Zhu Y. Bernacki S. Narayan R. Pourdeyhimi B. Gorga R., et al. Electrospun composite poly(L-lactic acid)/tricalcium phosphate scaffolds induce proliferation and osteogenic differentiation of human adipose-derived stem cells. Biomed Mater. 2009;4:035002. doi: 10.1088/1748-6041/4/3/035002. [DOI] [PubMed] [Google Scholar]
- 132.Hanson A. Wall M. Pourdeyhimi B. Loboa E. Effects of oxygen plasma treatment on adipose-derived human mesenchymal stem cell adherence to poly (L-lactic acid) scaffolds. J Biomater Sci Poly Ed. 2007;18:1387. doi: 10.1163/156856207782246812. [DOI] [PubMed] [Google Scholar]
- 133.Hyde G. McCullen S. Jeon S. Stewart S. Jeon H. Loboa E., et al. Atomic layer deposition and biocompatibility of titanium nitride nano-coatings on cellulose fiber substrates. Biomed Mater. 2009;4:025001. doi: 10.1088/1748-6041/4/2/025001. [DOI] [PubMed] [Google Scholar]
- 134.McCullen S. Stevens D. Roberts W. Clarke L. Bernacki S. Gorga R., et al. Characterization of electrospun nanocomposite scaffolds and biocompatibility with adipose-derived human mesenchymal stem cells. Int J Nanomed. 2007;2:253. [PMC free article] [PubMed] [Google Scholar]
- 135.Hattori H. Masuoka K. Sato M. Ishihara M. Asazuma T. Takase B., et al. Bone formation using human adipose tissue-derived stromal cells and a biodegradable scaffold. J Biomed Mater Res Part B Appl Biomater. 2006;76:230. doi: 10.1002/jbm.b.30357. [DOI] [PubMed] [Google Scholar]
- 136.Kakudo N. Shimotsuma A. Miyake S. Kushida S. Kusumoto K. Bone tissue engineering using human adipose-derived stem cells and honeycomb collagen scaffold. J Biomed Mater Res Part A. 2008;84:191. doi: 10.1002/jbm.a.31311. [DOI] [PubMed] [Google Scholar]
- 137.Marino G. Rosso F. Cafiero G. Tortora C. Moraci M. Barbarisi M., et al. Beta-tricalcium phosphate 3D scaffold promote alone osteogenic differentiation of human adipose stem cells: in vitro study. J Mater Sci Mater Med. 2010;21:353. doi: 10.1007/s10856-009-3840-z. [DOI] [PubMed] [Google Scholar]
- 138.Liu Q. Cen L. Yin S. Chen L. Liu G. Chang J., et al. A comparative study of proliferation and osteogenic differentiation of adipose-derived stem cells on akermanite and beta-TCP ceramics. Biomaterials. 2008;29:4792. doi: 10.1016/j.biomaterials.2008.08.039. [DOI] [PubMed] [Google Scholar]
- 139.Hao W. Pang L. Jiang M. Lv R. Xiong Z. Hu Y.-Y. Skeletal repair in rabbits using a novel biomimetic composite based on adipose-derived stem cells encapsulated in collagen I gel with PLGA-β-TCP scaffold. J Orthop Res. 2010;28:252. doi: 10.1002/jor.20969. [DOI] [PubMed] [Google Scholar]
- 140.Haimi S. Suuriniemi N. Haaparanta A.M. Ellä V. Lindroos B. Huhtala H., et al. Growth and osteogenic differentiation of adipose stem cells on PLA/bioactive glass and PLA/beta-TCP scaffolds. Tissue Eng Part A. 2009;15:1473. doi: 10.1089/ten.tea.2008.0241. [DOI] [PubMed] [Google Scholar]
- 141.Cowan C.M. Aalami O.O. Shi Y.-Y. Chou Y.-F. Mari C. Thomas R., et al. Bone morphogenetic protein 2 and retinoic acid accelerate in vivo bone formation, osteoclast recruitment, and bone turnover. Tissue Eng. 2005;11:645. doi: 10.1089/ten.2005.11.645. [DOI] [PubMed] [Google Scholar]
- 142.Conejero J. Lee J. Parrett B. Terry M. Wear-Maggitti K. Grant R., et al. Repair of palatal bone defects using osteogenically differentiated fat-derived stem cells. Plast Reconstr Surg. 2006;117:857. doi: 10.1097/01.prs.0000204566.13979.c1. [DOI] [PubMed] [Google Scholar]
- 143.Yoon E. Dhar S. Chun D. Gharibjanian N. Evans G. In vivo osteogenic potential of human adipose-derived stem cells/poly lactide-co-glycolic acid constructs for bone regeneration in a rat critical-sized calvarial defect model. Tissue Eng. 2007;13:619. doi: 10.1089/ten.2006.0102. [DOI] [PubMed] [Google Scholar]
- 144.Jeon O. Rhie J. Kwon I. Kim J. Kim B. Lee S. In vivo bone formation following transplantation of human adipose–derived stromal cells that are not differentiated osteogenically. Tissue Eng Part A. 2008;14:1285. doi: 10.1089/ten.tea.2007.0253. [DOI] [PubMed] [Google Scholar]
- 145.Lin L. Shen Q. Wei X. Hou Y. Xue T. Fu X., et al. Comparison of osteogenic potentials of BMP4 transduced stem cells from autologous bone marrow and fat tissue in a rabbit model of calvarial defects. Calcif Tissue Int. 2009;85:55. doi: 10.1007/s00223-009-9250-x. [DOI] [PubMed] [Google Scholar]
- 146.Yang M. Ma Q.J. Dang G.T. Ma K.t. Chen P. Zhou C.Y. In vitro and in vivo induction of bone formation based on ex vivo gene therapy using rat adipose-derived adult stem cells expressing BMP-7. Cytotherapy. 2005;7:273. doi: 10.1080/14653240510027244. [DOI] [PubMed] [Google Scholar]
- 147.Kang Y. Liao W.-M. Yuan Z.-H. Sheng P.-Y. Zhang L.-J. Yuan X.-W., et al. In vitro and in vivo induction of bone formation based on adeno-associated virus-mediated BMP-7 gene therapy using human adipose-derived mesenchymal stem cells. Acta Pharmacol Sin. 2007;28:839. doi: 10.1111/j.1745-7254.2007.00583.x. [DOI] [PubMed] [Google Scholar]
- 148.Dragoo J.L. Choi J.Y. Lieberman J.R. Huang J. Zuk P.A. Zhang J., et al. Bone induction by BMP-2 transduced stem cells derived from human fat. J Orthop Res. 2003;21:622. doi: 10.1016/S0736-0266(02)00238-3. [DOI] [PubMed] [Google Scholar]
- 149.Wu L. Wu Y. Lin Y. Jing W. Nie X. Qiao J., et al. Osteogenic differentiation of adipose derived stem cells promoted by overexpression of osterix. Mol Cell Biochem. 2007;301:83. doi: 10.1007/s11010-006-9399-9. [DOI] [PubMed] [Google Scholar]
- 150.Gupta A. Leong D.T. Bai H.F. Singh S.B. Lim T.-C. Hutmacher D.W. Osteo-maturation of adipose-derived stem cells required the combined action of vitamin D3, beta-glycerophosphate, and ascorbic acid. Biochem Biophys Res Commun. 2007;362:17. doi: 10.1016/j.bbrc.2007.07.112. [DOI] [PubMed] [Google Scholar]
- 151.Cho H.H. Shin K.K. Kim Y.J. Song J.S. Kim J.M. Bae Y.C., et al. NF-kappaB activation stimulates osteogenic differentiation of mesenchymal stem cells derived from human adipose tissue by increasing TAZ expression. J Cell Physiol. 2010;223:168. doi: 10.1002/jcp.22024. [DOI] [PubMed] [Google Scholar]
- 152.Zeng Q. Li X. Beck G. Balian G. Shen F.H. Growth and differentiation factor-5 (GDF-5) stimulates osteogenic differentiation and increases vascular endothelial growth factor (VEGF) levels in fat-derived stromal cells in vitro. Bone. 2007;40:374. doi: 10.1016/j.bone.2006.09.022. [DOI] [PubMed] [Google Scholar]
- 153.Cho H.H. Park H.T. Kim Y.J. Bae Y.C. Suh K.T. Jung J.S. Induction of osteogenic differentiation of human mesenchymal stem cells by histone deacetylase inhibitors. J Cell Biochem. 2005;96:533. doi: 10.1002/jcb.20544. [DOI] [PubMed] [Google Scholar]
- 154.Jørgensen N.R. Henriksen Z. Sørensen O.H. Civitelli R. Dexamethasone, BMP-2, and 1,25-dihydroxyvitamin D enhance a more differentiated osteoblast phenotype: validation of an in vitro model for human bone marrow-derived primary osteoblasts. Steroids. 2004;69:219. doi: 10.1016/j.steroids.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 155.Karageorgiou V. Meinel L. Hofmann S. Malhotra A. Volloch V. Kaplan D. Bone morphogenetic protein-2 decorated silk fibroin films induce osteogenic differentiation of human bone marrow stromal cells. J Biomed Mater Res Part A. 2004;71:528. doi: 10.1002/jbm.a.30186. [DOI] [PubMed] [Google Scholar]
- 156.Jaiswal N. Haynesworth S.E. Caplan A.I. Bruder S.P. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J Cell Biochem. 1997;64:295. [PubMed] [Google Scholar]
- 157.Pittenger M.F. Mackay A.M. Beck S.C. Jaiswal R.K. Douglas R. Mosca J.D., et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 158.Raab-Cullen D.M. Akhter M.P. Kimmel D.B. Recker R.R. Bone response to alternate-day mechanical loading of the rat tibia. J Bone Miner Res. 1994;9:203. doi: 10.1002/jbmr.5650090209. [DOI] [PubMed] [Google Scholar]
- 159.Zhao F. Chella R. Ma T. Effects of shear stress on 3-D human mesenchymal stem cell construct development in a perfusion bioreactor system: Experiments and hydrodynamic modeling. Biotechnol Bioeng. 2007;96:584. doi: 10.1002/bit.21184. [DOI] [PubMed] [Google Scholar]
- 160.Cartmell S.H. Porter B.D. Garcia A.J. Guldberg R.E. Effects of medium perfusion rate on cell-seeded three-dimensional bone constructs in vitro. Tissue Eng. 2003;9:1197. doi: 10.1089/10763270360728107. [DOI] [PubMed] [Google Scholar]
- 161.Goldstein A.S. Juarez T.M. Helmke C.D. Gustin M.C. Mikos A.G. Effect of convection on osteoblastic cell growth and function in biodegradable polymer foam scaffolds. Biomaterials. 2001;22:1279. doi: 10.1016/s0142-9612(00)00280-5. [DOI] [PubMed] [Google Scholar]
- 162.Thomas G.P. el Haj A.J. Bone marrow stromal cells are load responsive in vitro. Calcif Tissue Int. 1996;58:101. doi: 10.1007/BF02529731. [DOI] [PubMed] [Google Scholar]
- 163.Yoshikawa T. Peel S.A. Gladstone J.R. Davies J.E. Biochemical analysis of the response in rat bone marrow cell cultures to mechanical stimulation. Biomed Mater Eng. 1997;7:369. [PubMed] [Google Scholar]
- 164.Mauney J. Sjostorm S. Blumberg J. Horan R. O'leary J. Vunjak-Novakovic G., et al. Mechanical stimulation promotes osteogenic differentiation of human bone marrow stromal cells on 3-D partially demineralized bone scaffolds in vitro. Calcif Tissue Int. 2004;74:458. doi: 10.1007/s00223-003-0104-7. [DOI] [PubMed] [Google Scholar]
- 165.Ward D.F., Jr. Salasznyk R.M. Klees R.F. Backiel J. Agius P. Bennett K., et al. Mechanical strain enhances extracellular matrix-induced gene focusing and promotes osteogenic differentiation of human mesenchymal stem cells through an extracellular-related kinase-dependent pathway. Stem Cells Dev. 2007;16:467. doi: 10.1089/scd.2007.0034. [DOI] [PubMed] [Google Scholar]
- 166.Simmons C. Matlis S. Thornton A. Chen S. Wang C. Mooney D. Cyclic strain enhances matrix mineralization by adult human mesenchymal stem cells via the extracellular signal-regulated kinase (ERK1/2) signaling pathway. J Biomech. 2003;36:1087. doi: 10.1016/s0021-9290(03)00110-6. [DOI] [PubMed] [Google Scholar]
- 167.Huang S.-C. Wu T.-C. Yu H.-C. Chen M.-R. Liu C.-M. Chiang W.-S., et al. Mechanical strain modulates age-related changes in the proliferation and differentiation of mouse adipose-derived stromal cells. BMC Cell Biol. 2010;11:18. doi: 10.1186/1471-2121-11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kaspar D. Seidl W. Neidlinger-Wilke C. Beck A. Claes L. Ignatius A. Proliferation of human-derived osteoblast-like cells depends on the cycle number and frequency of uniaxial strain. J Biomech. 2002;35:873. doi: 10.1016/s0021-9290(02)00058-1. [DOI] [PubMed] [Google Scholar]
- 169.Gurkan U.A. Akkus O. The mechanical environment of bone marrow: a review. Ann Biomed Eng. 2008;36:1978. doi: 10.1007/s10439-008-9577-x. [DOI] [PubMed] [Google Scholar]
- 170.Loboa E.G. Wren T.A.L. Beaupré G.S. Carter D.R. Mechanobiology of soft skeletal tissue differentiation—a computational approach of a fiber-reinforced poroelastic model based on homogeneous and isotropic simplifications. Biomech Model Mechanobiol. 2003;2:83. doi: 10.1007/s10237-003-0030-7. [DOI] [PubMed] [Google Scholar]
- 171.Tjabringa G. Vezeridis P. Zandieh-Doulabi B. Helder M. Wuisman P. Klein-Nulend J. Polyamines modulate nitric oxide production and Cox-2 gene expression in response to mechanical loading in human adipose tissue-derived mesenchymal stem cells. Stem Cells. 2006;24:2262. doi: 10.1634/stemcells.2005-0625. [DOI] [PubMed] [Google Scholar]
- 172.Grayson W.L. Bhumiratana S. Cannizzaro C. Chao P.-H.G. Lennon D.P. Caplan A.I., et al. Effects of initial seeding density and fluid perfusion rate on formation of tissue-engineered bone. Tissue Eng Part A. 2008;14:1809. doi: 10.1089/ten.tea.2007.0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Grayson W.L. Martens T.P. Eng G.M. Radisic M. Vunjak-Novakovic G. Biomimetic approach to tissue engineering. Semin Cell Dev Biol. 2009;20:665. doi: 10.1016/j.semcdb.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sim W.Y. Park S.W. Park S.H. Min B.H. Park S.R. Yang S.S. A pneumatic micro cell chip for the differentiation of human mesenchymal stem cells under mechanical stimulation. Lab Chip. 2007;7:1775. doi: 10.1039/b712361m. [DOI] [PubMed] [Google Scholar]
- 175.Duty A.O. Oest M.E. Guldberg R.E. Cyclic mechanical compression increases mineralization of cell-seeded polymer scaffolds in vivo. J Biomech Eng. 2007;129:531. doi: 10.1115/1.2746375. [DOI] [PubMed] [Google Scholar]
- 176.Pelaez D. Charles Huang C.-Y. Cheung H.S. Cyclic compression maintains viability and induces chondrogenesis of human mesenchymal stem cells in fibrin gel scaffolds. Stem Cells Dev. 2009;18:93. doi: 10.1089/scd.2008.0030. [DOI] [PubMed] [Google Scholar]
- 177.Kisiday J.D. Frisbie D.D. McIlwraith C.W. Grodzinsky A.J. Dynamic compression stimulates proteoglycan synthesis by mesenchymal stem cells in the absence of chondrogenic cytokines. Tissue Eng Part A. 2009;15:2817. doi: 10.1089/ten.tea.2008.0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Goldstein C. Sprague S. Petrisor B.A. Electrical stimulation for fracture healing: current evidence. J Orthop Trauma. 2010;24(Suppl 1):S62. doi: 10.1097/BOT.0b013e3181cdde1b. [DOI] [PubMed] [Google Scholar]
- 179.Tsai M.-T. Chang W.H.-S. Chang K. Hou R.-J. Wu T.-W. Pulsed electromagnetic fields affect osteoblast proliferation and differentiation in bone tissue engineering. Bioelectromagnetics. 2007;28:519. doi: 10.1002/bem.20336. [DOI] [PubMed] [Google Scholar]
- 180.Tsai M.-T. Li W.-J. Tuan R.S. Chang W.H. Modulation of osteogenesis in human mesenchymal stem cells by specific pulsed electromagnetic field stimulation. J Orthop Res. 2009;27:1169. doi: 10.1002/jor.20862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kim I.S. Song J.K. Song Y.M. Cho T.H. Lee T.H. Lim S.S., et al. Novel effect of biphasic electric current on in vitro osteogenesis and cytokine production in human mesenchymal stromal cells. Tissue Eng Part A. 2009;15:2411. doi: 10.1089/ten.tea.2008.0554. [DOI] [PubMed] [Google Scholar]
- 182.Aaron R.K. Ciombor D.M. Acceleration of experimental endochondral ossification by biophysical stimulation of the progenitor cell pool. J Orthop Res. 1996;14:582. doi: 10.1002/jor.1100140412. [DOI] [PubMed] [Google Scholar]
- 183.Au A. Boehm C. Mayes A. Muschler G. Griffith L. Formation of osteogenic colonies on well-defined adhesion peptides by freshly isolated human marrow cells. Biomaterials. 2007;28:1847. doi: 10.1016/j.biomaterials.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Chaubey A. Ross K. Leadbetter R. Burg K. Surface patterning: tool to modulate stem cell differentiation in an adipose system. J Biomed Mater Res. 2007;84B:70. doi: 10.1002/jbm.b.30846. [DOI] [PubMed] [Google Scholar]
- 185.Kim T. Seong J. Ouyang M. Sun J. Lu S. Hong J., et al. Substrate rigidity regulates Ca 2+oscillation via RhoA pathway in stem cells. J Cell Physiol. 2009;218:285. doi: 10.1002/jcp.21598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Martino M.M. Mochizuki M. Rothenfluh D.A. Rempel S.A. Hubbell J.A. Barker T.H. Controlling integrin specificity and stem cell differentiation in 2D and 3D environments through regulation of fibronectin domain stability. Biomaterials. 2008;30:1089. doi: 10.1016/j.biomaterials.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Neal R. McClugage S., III. Link M. Sefcik L. Ogle R. Botchwey E. Laminin nanofiber meshes that mimic morphological properties and bioactivity of basement membranes. Tissue Eng Part C: Methods. 2008;15:11. doi: 10.1089/ten.tec.2007.0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Rowlands A. George P. Cooper-White J. Directing osteogenic and myogenic differentiation of MSC: interplay of stiffness and adhesive ligand presentation. Am J Physiol Cell Physiol. 2008;295:C1037. doi: 10.1152/ajpcell.67.2008. [DOI] [PubMed] [Google Scholar]
- 189.Reed C. Han L. Andrady A. Caballero M. Jack M. Collins J., et al. Composite tissue engineering on polycaprolactone nanofiber scaffolds. Ann Plast Surg. 2009;62:505. doi: 10.1097/SAP.0b013e31818e48bf. [DOI] [PubMed] [Google Scholar]