Abstract
Autologous adult cardiomyocytes are not utilized for heart repair strategies because of their rapid apoptosis after implantation. We examined whether induction of heme oxygenase-1 (HO-1), a mediator of preconditioning, could enhance early postimplant myocyte survival. Three-dimensional 5×5 mm patches of full-thickness adult murine atrial wall, including cardiomyocytes, capillary networks, and extracellular matrix, were cultured with or without HO-1 inducer cobalt protoporphyrin (CoPP), or the HO-1 inhibitor, tin protoporphyrin (SnPP), or both. Patches were then implanted subcutaneously. Freshly procured atrial wall patches implanted without preculturing served as additional controls. By 14 days postimplant, graft cardiomyocyte content was significantly greater in CoPP-treated patches than in either control group (p<0.02). Adult cardiomyocytes did not contract in culture or immediately after implantation. However, by 14 days postimplant, spontaneous contraction had recovered in 47% of CoPP-treated patches, but in only 6% of precultured patches without CoPP, 0% of SnPP-treated patches, and 0% of uncultured patches (p<0.03). CoPP-treated adult cardiomyocyte patches were also observed to remodel spontaneously into endothelial-lined chambers that pumped nonclotting blood. These findings demonstrate that adult cardiomyocytes have more plasticity and capacity for functional recovery than previously recognized and could have application as an autologous cardiomyocyte source for tissue engineering.
Introduction
As the only expendable part of the human heart, the right or left atrial appendages could serve as a readily available source of autologous adult cardiomyocytes for cardiac tissue engineering. To date, though, the use of adult cardiomyocytes has been limited by their rapid apoptosis in the face of ischemia. Absent blood flow, the viability of adult cardiomyocytes drops precipitously within hours.1 However, a minimum of several days is required to establish connections between the host vasculature and implanted cells or tissues, even with angiogenic growth factor supplementation. The necessity for cellular grafts to endure this period of ischemia after implantation, a limitation only magnified in a hypoperfused implant site such as an infarct bed, has effectively precluded the use of adult cardiomyocytes in cardiac repair strategies.
At the same time, it is well known that, in animal models, pretreating intact hearts with repeated short cycles of ischemia and reperfusion, known as preconditioning, can seemingly “tolerize” cardiomyocytes to ischemic injury and reduce myocyte loss during a subsequent myocardial infarction.2 Although standard preconditioning regimens provide relative protection for cardiac muscle during ischemic events that occur within the following hours,2 delayed, or late preconditioning, operating through different mediators, can protect cardiac muscle through ischemic events occurring over the ensuing 3–4 days.3 This longer time frame might be sufficient to protect newly implanted cardiomyocytes over the several-day “at risk” period while links with host vascular networks are being developed. In these experiments, we explored whether preconditioning could be adapted to three-dimensional tissue grafts destined for in vivo implantation. Specifically, we examined whether induction of a mediator of late preconditioning, heme oxygenase-1 (HO-1), could improve the postimplant survival and contractile recovery of fragile adult cardiomyocytes in full-thickness heart wall grafts.
Of the mediators of late preconditioning, HO-1 is an especially attractive target for interventional therapy.4 In mammals, HO-1 is the rate-limiting step in the degradation of heme into carbon monoxide, iron, and biliverdin, which, in turn, is rapidly converted to bilirubin. Carbon monoxide activates cell survival pathways, such as MAPK and Akt,5–12 and has downstream effects that preserve mitochondrial homeostasis.13,14 Biliverdin and its endproduct, bilirubin, are both potent antioxidants with demonstrated cell-protective effects in ischemia–reperfusion injury.15–18 Importantly, besides these key roles in cell survival, HO-1 has additional, complementary effects that are pro-angiogenic,19–21 anti-inflammatory,8,9,18,22–24 and anti-fibrotic,12,23,25–27 all of which would be highly desirable in implanted engineered tissues.
Materials and Methods
Animals
Adult C57BL/6 male mice (Charles River Laboratories, Wilmington, MA), 8–12 weeks of age, 20–25 g in weight, were used as donors and recipients under protocols approved by the Institutional Animal Care and Use Committee, in compliance with National Institutes of Health Guidelines (NIH Publication No. 85-23, revised 1996). To model autologous adult cardiac cell transplantation as a potential future clinical application, atrial tissues were procured from adult donor mice and implanted into age- and gender-matched isogenic recipient mice.
Atrial tissue procurement
For atrial tissue procurement, donor mice were euthanized (intraperitoneal sodium pentobarbital, 250 mg) and systemically heparinized (intravenous sodium heparin, 100 units). Following thoracotomy, the pocket-like left atrial appendage was rapidly excised in its entirety, rinsed in cold heparinized saline, then maintained at 4°C in cardioplegia solution (St. Thomas' solution with 30 mM/L 2,3-butanedione 2-monoxime [Sigma–Aldrich, St. Louis, MO]) for 30 min until either transfer into culture or immediate implantation.
HO-1 induction in atrial tissues in organ culture
Procured whole atrial appendages were placed into organ culture as full-thickness tissues with 5 mL of Dulbecco's modified Eagle's medium containing 5% fetal bovine serum, 4 mM l-glutamine, 100 μg/mL penicillin, and 100 IU/mL streptomycin and cultured with gentle agitation for 3 days at 37°C in 5% CO2. In the atrial tissues designated for in vivo implantation, the media in the different treatment arms were randomized to contain: (1) 25 μM cobalt protoporphyrin (CoPP; Sigma, St. Louis, MO), a transcriptional activator of HO-128,29; (2) 25 μM tin protoporphyrin (SnPP; Frontier Scientific, Logan, UT), a specific inhibitor of HO-1 activity; (3) a combination of 25 μM CoPP+25 μM SnPP; or (4) no additives. CoPP and/or SnPP were delivered only once at the initiation of the 3-day organ culture. No treatments were given to recipient animals.
Assessments in cultured tissue: HO-1, cardiac troponin T, and cell viability
HO-1 and troponin T expression in organ-cultured atrial tissue were assessed by western blotting using antibodies to HO-1 (Enzo Biochem, New York, NY) and cardiac troponin T (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), respectively. Results were confirmed by immunohistochemistry using the same antibodies in 4% paraformaldehyde-fixed, paraffin-embedded specimens. Cyclic guanosine monophosphate (cGMP) was measured in triplicate samples of frozen atrial tissue as an index of HO-1 enzyme activity5–8,30 using a commercially available enzyme immunoassay (GE Healthcare Life Sciences, Piscataway, NJ). Cell viability in cultured and freshly procured atrial tissues was determined by MTS tetrazolium assay (Promega, Madison, WI) according to standard instructions. Absorbance (abs) at 490 nm was measured by spectrophotometry and normalized per milligram of wet tissue weight.
Cardiac patch implantation
Just before implantation, the donor atrial appendages were converted into rectangular, flattened, three-dimensional patches by delicate surgical division of muscular pectinate trabeculae that bridge across the pocket-like appendage lumen. The patches thus consisted of full-thickness myocardial wall including both endocardium and epicardium, with final graft size measuring approximately 5×5 mm in area. Given the inherent ridges of muscular bundles in the atrial appendage wall, tissue thickness across each graft varied from 50 to 300 μm. Isogenic adult recipient C57/Bl6 mice were anesthetized with an intraperitoneal injection of 1.25% tribromoethanol solution (0.02 mL/g body weight). Dissected, flattened patches were stretched slightly (∼10%) and implanted subcutaneously onto the anterior chest wall, tacking the margins of the patch to the underlying pectoralis muscle fascia with three or four 8-0 Prolene sutures. Care was taken to situate the grafts well lateral of the midline skin incision so that skin wound healing was not in proximity to the implant site. A subcutaneous vessel consistently coursing under the skin of the anterolateral chest wall was ligated in the axilla so that there was no ready source of vasculature for the graft, ensuring that implant beds were uniformly ischemic across all groups.
Postimplant assessment of graft contraction in vivo
At 14 days postimplant, recipient mice were re-anesthetized and the skin overlying the implant site was opened to expose the grafts in their subcutaneous position on the pectoralis fascia. Graft contractions, both spontaneous and in response to mechanical stretch, were recorded according to a semi-quantitative scale: 2+: spontaneous, ongoing graft beating without requiring a stimulus; 1+: unsustained, transient graft contraction occurring only in response to mechanical stretch stimulus; 0: no spontaneous and no mechanically inducible contractions.
Assessment of myocyte survival in implanted atrial grafts
Following assessments of contractility, recipient mice were euthanized with pentobarbitol as mentioned above. Patch grafts were excised to include a surrounding rim of host tissue, fixed in 4% paraformaldehyde, paraffin-embedded, and then sectioned coronally at 5 μm intervals, sampling across the entirety of each graft. Atrial cardiomyocytes surviving at 14 days postimplant were identified on immunohistochemistry using an antibody to atrial natriuretic peptide (ANP) (rabbit polyclonal, gift from Jolanta Gutkowska, Ph.D., Montreal, Canada), constitutively expressed by atrial cardiomyocytes, visualized with a biotinylated goat anti-rabbit secondary antibody and the biotin–avidin-peroxidase DAB Substrate Kit (both Vector Laboratories, Burlingame, CA). The mean cardiomyocyte-containing content in each graft was quantified by computerized image analysis as the percentage of the total graft area immunopositive for ANP, averaged over three to four sections per graft, encompassing the entire specimen. Staining of serial sections with antibodies to sarcomeric myosin heavy chain (mAb MF-20; Developmental Studies Hyridoma Bank, Iowa City, IA),31 and murine cardiac α-like myosin heavy chain (mAb BA-G5; Abcam, Inc., Cambridge, MA)32 was used to confirm that the ANP-immunopositive cells accurately represented the entire cardiomyocyte population. The subcutaneous location of the implants allowed for clear discrimination of graft cardiomyocytes, since, unlike implants onto the heart, the muscle underlying the graft was skeletal instead of cardiac muscle.
Statistical methods
Data are expressed as mean values±the standard deviation of the mean. Continuous variables were compared among multiple groups using analysis of variance, while two-tailed Student's t tests were used for comparisons between two groups. Categorical variables were compared using Chi square tests; ordinal variables such as graft contractility were compared using nonparametric Kruskal–Wallis tests. p-values ≤0.05 were considered significant.
Results
HO-1 expression and activity in three-dimensional atrial tissue following CoPP induction
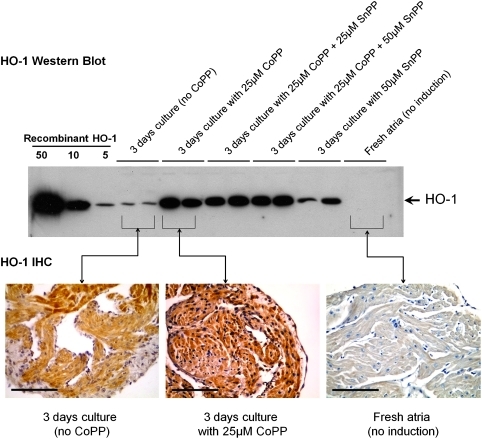
Adding CoPP to the culture medium increased HO-1 expression in organ-cultured full-thickness adult atrial appendage tissues. Following a single dose of 25 μM CoPP on day 0, HO-1 protein levels increased daily, peaking at 72 h, as assessed by both western blotting and immunohistochemistry (Fig. 1). A 25 μM CoPP dose produced higher levels of HO-1 than 5 μM CoPP (data not shown). Atrial tissues cultured with combinations of 25 μM CoPP plus either 25 or 50 μM of SnPP evidenced similar elevations of HO-1 protein on western blots as those cultured with 25 μM CoPP alone, confirming that the inhibitory effects of SnPP are on HO-1 enzyme activity rather than on transcription, consistent with previous reports.30,33 Atrial tissue samples cultured without CoPP were found to progressively develop low, but detectable, levels of endogenous HO-1 expression over 3 days in culture (Fig. 1), likely a response to relative hypoxia of three-dimensional tissues in static culture. No HO-1 expression was seen in atrial tissue tested immediately after procurement from donor animals without the intervening culture.
FIG. 1.
Western blot and IHC of atrial appendage wall after 3 days of in vitro organ culture. Adding CoPP to the culture medium on day 0 induces HO-1 protein production. Inhibitor SnPP does not inhibit HO-1 protein production (but instead sterically inhibits HO-1 enzymatic activity). Culturing full-thickness atrial wall in the absence of CoPP results in low levels of endogenous HO-1 production, whereas no HO-1 is seen in freshly procured atrial wall that has not been cultured. On IHC, CoPP treatment results in widespread HO-1 expression (brown DAB staining) in cardiomyocytes, endothelial cells, endocardium, and epicardium. Atrial walls cultured without CoPP develop low levels (lighter staining) of HO-1 in cardiomyocytes, with patchy areas of darker staining in central cardiomyocytes. No HO-1 staining is seen in freshly procured, uncultured atrial tissue. Magnification bars=100 μm. IHC, immunohistochemistry; CoPP, cobalt protoporphyrin; SnPP, tin protoporphyrin; HO-1, heme oxygenase-1.
Changes in cGMP production in response to CoPP were assessed as an indicator of HO-1 activity. After 3 days in organ culture, levels of cGMP in CoPP-treated atrial tissues (1660±254 fmol/mg protein) were greater than in tissues cultured without CoPP (1093±273 fmol/mg protein; p<0.03). In the cultured atrial tissues, adding 25 μM SnPP to CoPP-dosed culture media prevented the CoPP-associated increase in cGMP production (1150±307 fmol/mg protein; p<0.04 vs. CoPP-treated). Levels of cGMP in CoPP-treated tissues were also significantly higher than in freshly excised control atrial tissues, that is, neither cultured nor porphyrin-treated (369±155 fmol/mg; p<0.0003). To rule out any secondary effects of nitric oxide on cGMP production, experiments were repeated with the addition of the nitric oxide synthase inhibitor N(G)-nitro-l-arginine methyl ester (L-NAME) to the culture media which did not alter the results.
Cell viability in tissues prior to in vivo implantation was similar between groups. After 3 days in culture, total cell viability as measured by MTS activity in cultured left atrial tissues treated with 0, 5, or 25 μM CoPP (0.69±0.14, 0.75±0.14, 0.78±0.15 abs/mg [n=4/group], respectively) was not different from freshly procured left and right normal atrial appendages (0.63±0.09 abs/mg, p=0.43).
Graft cardiomyocyte content at 14 days following implantation
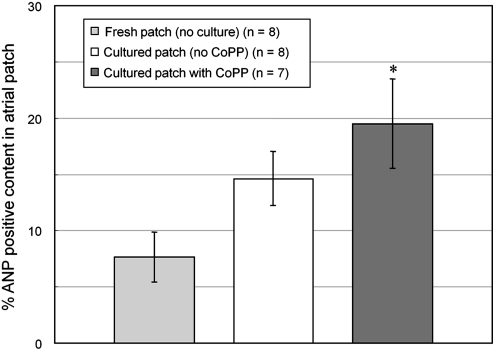
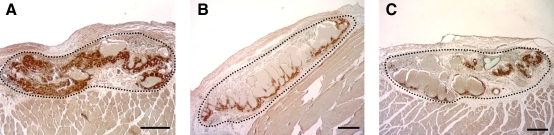
By 14 days postimplant, the mean size of implanted grafts, including myocyte, vascular and interstitial spaces (2.55±0.85×105 pixels, n=23) was somewhat smaller than the mean size of freshly excised atrial appendage tissues prior to implant ((3.5±1.31)×105 pixels, n=10), but not significantly so (p=0.06). Importantly, there were no significant differences in mean graft sizes among the different treatment groups at 14 days (p=0.19). However, the mean percentage of the total graft volume occupied by surviving cardiomyocytes in grafts precultured with 25 μM CoPP (19.5%±4.0%, n=7) was significantly higher than in either of the two control groups: that is, grafts precultured without CoPP (14.6%±2.4%, n=8); or those implanted immediately after procurement, without either preculturing or CoPP treatment (7.7%±2.2%, n=8, p<0.001, analysis of variance) (Figs. 2 and 3). In both the treatment and control groups, the myocyte-containing areas immunopositive for ANP corresponded to the areas staining positive for cardiomyocytes using sarcomeric myosin and cardiac-specific myosin heavy chain antibodies (Fig. 4).
FIG. 2.
Cardiomyocyte volume in atrial grafts at 14 days after in vivo implantation in different pretreatment groups. *p<0.001 versus the two control groups, analysis of variance. ANP, atrial natriuretic peptide.
FIG. 3.
Examples of subcutaneous atrial grafts in vivo at 14 days postimplant, with IHC for ANP (brown DAB staining) to demonstrate cardiomyocyte content. Black dotted lines indicate graft margins, but are not those actually used to calculate graft volumes. (A) Graft pretreated with CoPP prior to implantation; (B) graft precultured prior to implantation without CoPP; (C) graft implanted with neither preculture nor CoPP treatment. Magnification bars=200 μM. Color images available online at www.liebertonline.com/tea
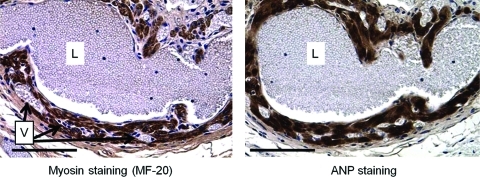
FIG. 4.
Chamber formation seen in cross-sections of an atrial graft at 14 days after subcutaneous implantation. Atrial cardiomyocytes are stained by antibodies to both sarcomeric myosin (mAb MF-20) and ANP. Endothelial-lined lumens (L) are filled with red blood cells without clot formation. Numerous smaller vessels, also filled with red blood cells, are seen in the chamber walls (V). Magnification bars=100 μm. Color images available online at www.liebertonline.com/tea
Graft contraction following in vivo implantation
Spontaneous contraction was not seen in any of the grafts examined at 3 or 7 days after implantation in any of the groups. However, by 14 days following in vivo implantation, almost half (7/15 or 47%) of the grafts pretreated with CoPP were seen to be spontaneously and synchronously contracting (Table 1 and Supplementary Video S1; Supplementary Data are available online at www.liebertonline.com/tea). This finding was significantly different from implanted control grafts that had been precultured without CoPP, and from the freshly procured control grafts implanted without preculture, in which 1/16 and 0/16 grafts contracted spontaneously, respectively (p<0.03). Also, no spontaneous contraction was seen in grafts precultured with the HO-1 activity inhibitor SnPP, whether SnPP was delivered to culture media alone (0/7 grafts contracting) or added in combination with CoPP (0/7 grafts contracting). Total graft size was similar among grafts with different grades of contraction (p=0.33). But, as expected, the grafts that evidenced contraction were those in which the surviving cardiomyocyte contents were highest. Contraction rates of spontaneously beating patches were 100–120 beats/min. Of note, neither arrhythmias nor atrial fibrillation were observed.
Table 1.
Percentage of Beating Grafts at 2 Weeks After In Vivo Implantation
| |
Beating grade at 2 weeks after implant |
||
|---|---|---|---|
| Atrial patch treatment groups | 0+ | 1+ | 2+ |
| Precultured with CoPP (high level HO-1 induction) | 27% (4/15) | 27% (4/15) | 47% (7/15)a |
| Precultured without CoPP (low level HO-1 induction) | 44% (7/16) | 50% (8/16) | 6% (1/16)a |
| Implanted without culture or CoPP (no HO-1 induction at implantation) | 56% (9/16) | 44% (7/16) | 0% (0/16)a |
| Precultured with CoPP+SnPP | 86% (6/7) | 14% (1/7) | 0% (0/7) |
| Precultured with SnPP alone | 86% (6/7) | 14% (1/7) | 0% (0/7) |
p<0.03, Differences between treatment groups, Kruskal–Wallis.
Beating grades are compared between groups under different treatment conditions.
HO-1, heme oxygenase-1; CoPP, cobalt protoporphyrin, an inducer of HO-1; SnPP, tin protoporphyrin, a specific inhibitor of HO-1 activity.
In the two control groups, that is, grafts precultured without CoPP and grafts implanted immediately after atrial tissue procurement without preculturing, approximately half of the grafts exhibited nonsustained contraction in response to mechanical stimulation, although spontaneous contraction was rarely seen. In the precultured patches, even this lesser degree of stimulus-dependent responsiveness was abrogated by the addition of SnPP to the culture medium (Table 1).
Chamber formation within grafts after implantation
Although atrial tissues had been dissected into flattened patches prior to implant, by 14 days postimplantation, histologic cross-sections revealed that vascular chambers, lined with CD31+ endothelial cells and filled with blood, had formed within all of the grafts (Figs. 3 and 4) except those treated with the HO-1 inhibitor, SnPP. Of note, no clots were seen within the chambers. Where myocytes were preserved, up to several layers of myocytes surrounded the chambers (Fig. 4) and formed trabeculae crossing the chambers (Fig. 5). On videography, in some grafts, blood could be seen flowing into the graft from small, feeding host arterioles, passing through the chamber(s), and being pumped out by the contracting graft into small host vessels exiting the graft on the far side. Of interest, virtually all grafts were filled with blood, and pink or dark red in color, when viewed at explant, even those that were not contracting, showing that communication to the host vasculature had been re-established over the 14 days following implantation, irrespective of treatment protocol or the extent of myocyte loss. However, only those grafts containing surviving myocytes had regained contractility.
FIG. 5.
Troponin T staining at 7 days after in vivo implantation. On IHC, troponin T staining is seen in some CoPP-treated patches at 7 days (A) and early sarcomeric structures are occasionally seen (B), whereas no troponin T is seen at 7 days in patches without CoPP pretreatment (C).
Vascular chambers were seen in only 36% (5/14) of the grafts pretreated with the HO-1 inhibitor, SnPP, whether given alone or in combination with CoPP. In comparison, 100% of grafts in all other groups (CoPP-pretreated grafts, those cultured without CoPP, and grafts implanted without culture or CoPP) had evident vascular chambers (p<0.001). The SnPP-treated grafts were, by and large, reduced to dense fibrotic tissue. A blood supply was still seen in SnPP-pretreated grafts at 14 days, but these were small vessels within the scar, consistent with granulation tissue formation.
Troponin T reappearance
Cardiac troponin T, essential for sarcomeric contraction, was lost rapidly from atrial tissue during organ culture. As assessed by both western blotting and histology, the troponin T content of atrial tissues dropped to 50% or less of the original levels during the first 3 days in culture medium. If, instead of implanting atrial tissues after 3 days of culture, they were maintained longer in culture, troponin T was completely lost after 7 days.
In the implanted atrial grafts, troponin T staining was utilized to gauge the time to recovery for graft sarcomeres. At 3 days following in vivo implantation, minimal to no troponin T was seen on immunohistochemistry in any of the treatment groups. Sarcomeric structures were also absent. However, by day 7 after implantation, although no patches were yet beating, in some CoPP-treated patches, occasional troponin T staining was now observed, as well as some sarcomeric structures (Fig. 5). Troponin T staining was not seen by day 7 in patches without CoPP treatment.
Discussion
Whether atrial appendage tissue might eventually be used as a patch-like construct, potentially augmented with stem cells, or dissociated into injectable cell clusters, it represents a source of a surprisingly large mass of autologous cardiomyocytes, coronary endothelial cells, and normal cardiac extracellular matrix for use in cardiac repair strategies. Atrial appendage tissue is commonly excised and discarded during routine cardiac surgery, causing no impingement on the atrial chamber itself. It can also be procured without open surgery by minimally invasive devices in current clinical use, making application to autologous cell transplantation quite feasible. Appendage wall is highly infolded and trabeculated,34 such that simple division of the pectinate muscles bridging the appendage lumen can produce a sheet of myocardium sufficient in area to cover one-third of the ventricle. This myocyte population might in itself be sufficient for infarct repair purposes, even if engrafted adult cardiomyocytes have little proliferation. The unique properties of atrial myocytes, such as remodeling capacity,35 atrial natriuretic hormone secretion,36–38 and the potential to form gap junctions with ventricular myocytes,39 might be harnessed to advantage in infarct repair. Recently, atrial wall has been reported to harbor a repository of putative cardiac stem cells,40–44 even in human hearts.40,45 Further, the new methods being developed for dedifferentiation of somatic cells46–49 might provide a future means by which autologous adult atrial myocytes could augment their proliferative potential or be transitioned toward ventricular phenotypes, perhaps with less extensive cell reprogramming than that entailed for induced pluripotent cells.
However, to date, the main limitation to the use of adult cardiomyocytes in repair strategies has been their historically poor viability after transplantation. Reinecke et al. found that no adult cardiomyocytes survived the first 24 h after intramyocardial injection, whether injections were made into infarcts or even into normally vascularized hearts without infarction.1 Sakai et al.50 injected adult atrial myocytes into cryoinjured hearts, but found few surviving cells at 5 weeks. Nonetheless, atrial cell injections improved left ventricular function, most likely through paracrine effects. The findings here now show that HO-1 induction can significantly increase the viability of implanted adult cardiomyocytes, bringing engrafted areas and cell survival, although still modest, up to the general ranges reported for neonatal51 and embryonic-derived cardiomyocytes,52,53 as well as mesenchymal stem cells,54 recognizing that the different models, species, and assessment modalities preclude exact comparison.
In these experiments, adult cardiomyocytes were implanted in a three-dimensional patch, effectively a “co-culture” with inherent endothelial cells, fibroblasts, and cardiac extracellular matrix. Whether maintaining the normal cardiac niche environment might have facilitated myocyte survival was not examined. However, for both neonatal55,56 and embryonic cardiomyocytes,57,58 culturing myocytes in conjunction with other cell types has been previously shown to enhance cell survival. Also, cardiomyocyte hypertrophy was not investigated as a potential variable affecting myocyte volumes, but this was not suggested by the histology, and the fact that HO-1 inhibits cardiac hypertrophic pathways59 makes this unlikely.
Importantly, HO-1 was found not only to enhance cardiomyocyte viability, but also to improve the contractile recovery of adult cardiomyocytes. Adding the HO-1 inhibitor, SnPP, precluded contractile recovery in both the CoPP-treated grafts and even in the grafts from the culture-only control group which had low levels of endogenously generated HO-1 at implant. An interesting incidental observation was that preservation of troponin T did not appear to be important for contractile recovery. In fact, the atrial grafts implanted immediately after procurement, which had the highest troponin T content at the time of implantation, had the lowest graft recovery rates by 14 days postimplant. Instead, initial loss and later reorganization or resynthesis of structural proteins seemed to characterize adult cardiomyocyte recovery potential.
The observation that flat three-dimensional patches of adult myocardium could remodel spontaneously into chambered structures with lumens was surprising and most likely represents a response to the loss of central cells where patch thickness exceeded diffusion capacities. The potential for spontaneous structural reorganization of atrial cells was reported in 1986 by Jockusch et al.,35 but in neonatal myocardium where greater plasticity is expected. In that study, subcutaneously implanted minced neonatal atrial tissue spontaneously reformed into “organ-like” chambered structures with blood-filled lumens by 5–11 weeks postimplant. Similar to the current HO-1-induced adult cardiomyocyte grafts, myofibrillar structures were absent in the first week, but then reformed by 14 days when spontaneous beating resumed. Also noteworthy in that study was the fact that, at least in neonatal heart tissue, cell survival, contractile recovery, and the capacity to remodel into large morphological structures were greater for atrial than for ventricular myocardium.
A notable feature of the full-thickness atrial wall grafts used in these experiments is that they contain both a preformed capillary network and a layer of CD31+ endocardium which, like endothelium, has anticoagulant properties.60 As seen in skin grafts,61,62 and as implicated in some engineered tissues,58,63 the presence of pre-existing endothelial cells and networks may have facilitated host vascular connections by inosculation. That HO-1 may play a role in this process is supported by the fact that chamber formation was significantly reduced when patches were pretreated with an HO-1 inhibitor. HO-1 is known to protect endothelial cells from apoptosis.10,20,21,64–66 and to upregulate both vascular endothelial growth factor and stromal cell derived factor-1 (SDF-1).19–21 Induction of HO-1 expression in patches prior to implantation may have further promoted or accelerated host vascularization. If so, then rapidity of vascularization might be partly responsible for myocyte survival in grafts with HO-1 expression. For translation to myocardial infarct beds, where the infarct bed would likely be more ischemic than the subcutaneous site used in this model, the addition of a large vessel inflow source, such as an omental flap, may be advantageous, especially if a thicker patch, intrinsic patch growth, or additional layers67 are to be supported.
Although preconditioning has well-documented benefits for myocardial preservation in experimental myocardial infarction, it has not been broadly applied therapeutically in clinical medicine because of the unpredictable nature of cardiac events. Autologous cellular transplantation, however, offers one such opportunity. For these experiments, instead of using HO-1 gene therapy,17,23,25,26,68 HO-1 was induced pharmacologically by topical application of CoPP as a rapid and less cumbersome technique that could appropriately be applied to cell or tissue transplantation. In humans, the wall of the atrial appendage is thinner than the wall of the atrium,69 and only slightly thicker than in rodents, so it should be amenable to HO-1 induction by topical CoPP either delivered in culture media or incorporated into a gel. (The use of topical gels for local plasmid DNA delivery to atrial wall has been reported.70) Although such localized delivery to tissues or cells destined for implantation would likely be preferable, alternatively, CoPP could be given systemically prior to planned tissue procurement for an autologous transplant in the clinical setting. CoPP, as well as other HO-1 inducers such as hemin,15,24 and carbon monoxide-releasing molecules,71,72 have been used parenterally in experimental ischemia/reperfusion injury and organ allograft transplantation models.73–75 Among HO-1 inducers, CoPP has been proposed as perhaps the safest for systemic delivery since it is neither a pro-oxidant nor an HO-1 substrate.29
In summary, these results show that upregulation of HO-1 can improve the survival and contractile recovery of adult cardiomyocytes implanted in vivo as three-dimensional tissues. This represents one of the first demonstrations that adult myocardium, unlike neonatal or embryonic heart tissue or progenitor cell transplants,42 can survive in vivo implantation without requiring immediate vascularization by direct vessel anastomosis (as, e.g., in heart transplant operations). Additionally, the remodeling observed in these grafts suggests that adult cardiomyoctyes may have more plasticity than previously recognized. Taken together, these findings suggest that autologous adult cardiomyocytes might be added to the armamentarium of cell sources adaptable for future use in cardiac tissue engineering. Further, similar preconditioning strategies might be adapted to enhance the survival of other cell types in three-dimensional tissues destined for transplantation.
Supplementary Material
Acknowledgments
This work was funded by the National Institutes of Health grants HL086709, HL64387, and EB002386, and by The Hope Heart Institute's Lester A. Sauvage Fellowship to S.K. The authors wish to thank Dr. Virginia M. Green for her help in editing and preparation of the manuscript.
Disclosure Statement
There are no competing financial interests, commercial associations, or conflicts of interest for any of the authors.
References
- 1.Reinecke H. Zhang M. Bartosek T. Murry C.E. Survival, integration, and differentiation of cardiomyocyte grafts: a study in normal and injured rat hearts. Circulation. 1999;100:193. doi: 10.1161/01.cir.100.2.193. [DOI] [PubMed] [Google Scholar]
- 2.Murry C.E. Jennings R.B. Reimer K.A. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 3.Bolli R. The late phase of preconditioning. Circ Res. 2000;87:972. doi: 10.1161/01.res.87.11.972. [DOI] [PubMed] [Google Scholar]
- 4.Dawn B. Bolli R. HO-1 induction by HIF-1: a new mechanism for delayed cardioprotection? Am J Physiol Heart Circ Physiol. 2005;289:H522. doi: 10.1152/ajpheart.00274.2005. [DOI] [PubMed] [Google Scholar]
- 5.Wu L. Wang R. Carbon monoxide: endogenous production, physiological functions, and pharmacological applications. Pharmacol Rev. 2005;57:585. doi: 10.1124/pr.57.4.3. [DOI] [PubMed] [Google Scholar]
- 6.Hausenloy D.J. Yellon D.M. Survival kinases in ischemic preconditioning and postconditioning. Cardiovasc Res. 2006;70:240. doi: 10.1016/j.cardiores.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 7.Burley D.S. Ferdinandy P. Baxter G.F. Cyclic GMP and protein kinase-G in myocardial ischaemia–reperfusion: opportunities and obstacles for survival signaling. Br J Pharmacol. 2007;152:855. doi: 10.1038/sj.bjp.0707409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bilban M. Haschemi A. Wegiel B. Chin B.Y. Wagner O. Otterbein L.E. Heme oxygenase and carbon monoxide initiate homeostatic signaling. J Mol Med. 2008;86:267. doi: 10.1007/s00109-007-0276-0. [DOI] [PubMed] [Google Scholar]
- 9.Fujimoto H. Ohno M. Ayabe S. Kobayashi H. Ishizaka N. Kimura H. Yoshida K. Nagai R. Carbon monoxide protects against cardiac ischemia–reperfusion injury in vivo via MAPK and Akt–eNOS pathways. Arterioscler Thromb Vasc Biol. 2004;24:1848. doi: 10.1161/01.ATV.0000142364.85911.0e. [DOI] [PubMed] [Google Scholar]
- 10.Zhang X. Shan P. Alam J. Fu X.Y. Lee P.J. Carbon monoxide differentially modulates STAT1 and STAT3 and inhibits apoptosis via a phosphatidylinositol 3-kinase/Akt and p38 kinase-dependent STAT3 pathway during anoxia-reoxygenation injury. J Biol Chem. 2005;280:8714. doi: 10.1074/jbc.M408092200. [DOI] [PubMed] [Google Scholar]
- 11.Gozzelino R. Jeney V. Soares M.P. Mechanisms of cell protection by heme oxygenase-1. Annu Rev Pharmacol Toxicol. 2010;50:323. doi: 10.1146/annurev.pharmtox.010909.105600. [DOI] [PubMed] [Google Scholar]
- 12.Wang G. Hamid T. Keith R.J. Zhou G. Partridge C.R. Xiang X. Kingery J.R. Lewis R.K. Li Q. Rokosh D.G. Ford R. Spinale F.G. Riggs D.W. Srivastava S. Bhatnagar A. Bolli R. Prabhu S.D. Cardioprotective and antiapoptotic effects of heme oxygenase-1 in the failing heart. Circulation. 2010;121:1912. doi: 10.1161/CIRCULATIONAHA.109.905471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suliman H.B. Carraway M.S. Tatro L.G. Piantadosi C.A. A new activating role for CO in cardiac mitochondrial biogenesis. J Cell Sci. 2007;120:299. doi: 10.1242/jcs.03318. [DOI] [PubMed] [Google Scholar]
- 14.Suliman H.B. Carraway M.S. Ali A.S. Reynolds C.M. Welty-Wolf K.E. Piantadosi C.A. The CO/HO system reverses inhibition of mitochondrial biogenesis and prevents murine doxorubicin cardiomyopathy. J Clin Invest. 2007;117:3730. doi: 10.1172/JCI32967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clark J.E. Foresti R. Sarathchandra P. Kaur H. Green C.J. Motterlini R. Heme oxygenase-1-derived bilirubin ameliorates postischemic myocardial dysfunction. Am J Physiol Heart Circ Physiol. 2000;278:H643. doi: 10.1152/ajpheart.2000.278.2.H643. [DOI] [PubMed] [Google Scholar]
- 16.Baranano D.E. Rao M. Ferris C.D. Snyder S.H. Biliverdin reductase: a major physiologic cytoprotectant. Proc Natl Acad Sci U S A. 2002;99:16093. doi: 10.1073/pnas.252626999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pachori A.S. Smith A. McDonald P. Zhang L. Dzau V.J. Melo L.G. Heme-oxygenase-1-induced protection against hypoxia/reoxygenation is dependent on biliverdin reductase and its interaction with PI3K/Akt pathway. J Mol Cell Cardiol. 2007;43:580. doi: 10.1016/j.yjmcc.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ollinger R. Wang H. Yamashita K. Wegiel B. Thomas M. Margreiter R. Bach F.H. Therapeutic applications of bilirubin and biliverdin in transplantation. Antioxid Redox Signal. 2007;9:2175. doi: 10.1089/ars.2007.1807. [DOI] [PubMed] [Google Scholar]
- 19.Bussolati B. Ahmed A. Pemberton H. Landis R.C. Di Carlo F. Haskard D.O. Mason J.C. Bifunctional role for VEGF-induced heme oxygenase-1 in vivo: induction of angiogenesis and inhibition of leukocytic infiltration. Blood. 2004;103:761. doi: 10.1182/blood-2003-06-1974. [DOI] [PubMed] [Google Scholar]
- 20.Dulak J. Deshane J. Jozkowicz A. Agarwal A. Heme oxygenase-1 and carbon monoxide in vascular pathobiology: focus on angiogenesis. Circulation. 2008;117:231. doi: 10.1161/CIRCULATIONAHA.107.698316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lakkisto P. Kyto V. Forsten H. Siren J.M. Segersvard H. Voipio-Pulkki L.M. Laine M. Pulkki K. Tikkanen I. Heme oxygenase-1 and carbon monoxide promote neovascularization after myocardial infarction by modulating the expression of HIF-1α, SDF-1α and VEGF-B. Eur J Pharmacol. 2010;635:156. doi: 10.1016/j.ejphar.2010.02.050. [DOI] [PubMed] [Google Scholar]
- 22.Ockaili R. Natarajan R. Salloum F. Fisher B.J. Jones D. Fowler A.A., 3rd Kukreja R.C. HIF-1 activation attenuates postischemic myocardial injury: role for heme oxygenase-1 in modulating microvascular chemokine generation. Am J Physiol Heart Circ Physiol. 2005;289:H542. doi: 10.1152/ajpheart.00089.2005. [DOI] [PubMed] [Google Scholar]
- 23.Tang Y.L. Qian K. Zhang Y.C. Shen L. Phillips M.I. A vigilant, hypoxia-regulated heme oxygenase-1 gene vector in the heart limits cardiac injury after ischemia–reperfusion in vivo. J Cardiovasc Pharmacol Ther. 2005;10:251. doi: 10.1177/107424840501000405. [DOI] [PubMed] [Google Scholar]
- 24.Hangaishi M. Ishizaka N. Aizawa T. Kurihara Y. Taguchi J. Nagai R. Kimura S. Ohno M. Induction of heme oxygenase-1 can act protectively against cardiac ischemia/reperfusion in vivo. Biochem Biophys Res Commun. 2000;279:582. doi: 10.1006/bbrc.2000.3973. [DOI] [PubMed] [Google Scholar]
- 25.Liu X. Pachori A.S. Ward C.A. Davis J.P. Gnecchi M. Kong D. Zhang L. Murduck J. Yet S.F. Perrella M.A. Pratt R.E. Dzau V.J. Melo L.G. Heme oxygenase-1 (HO-1) inhibits postmyocardial infarct remodeling and restores ventricular function. FASEB J. 2006;20:207. doi: 10.1096/fj.05-4435com. [DOI] [PubMed] [Google Scholar]
- 26.Liu X. Simpson J.A. Brunt K.R. Ward C.A. Hall S.R. Kinobe R.T. Barrette V. Tse M.Y. Pang S.C. Pachori A.S. Dzau V.J. Ogunyankin K.O. Melo L.G. Preemptive heme oxygenase-1 gene delivery reveals reduced mortality and preservation of left ventricular function 1 yr after acute myocardial infarction. Am J Physiol Heart Circ Physiol. 2007;293:H48. doi: 10.1152/ajpheart.00741.2006. [DOI] [PubMed] [Google Scholar]
- 27.Jadhav A. Torlakovic E. Ndisang J. Interaction among heme oxygenase, nuclear factor-κB, and transcription activating factors in cardiac hypertrophy in hypertension. Hypertension. 2008;52:910. doi: 10.1161/HYPERTENSIONAHA.108.114801. [DOI] [PubMed] [Google Scholar]
- 28.Kappas A. Drummond G.S. Control of heme metabolism with synthetic metalloporphyrins. J Clin Invest. 1986;77:335. doi: 10.1172/JCI112309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shan Y. Lambrecht R.W. Donohue S.E. Bonkovsky H.L. Role of Bach1 and Nrf2 in up-regulation of the heme oxygenase-1 gene by cobalt protoporphyrin. FASEB J. 2006;20:2651. doi: 10.1096/fj.06-6346fje. [DOI] [PubMed] [Google Scholar]
- 30.Morita T. Perrella M.A. Lee M.E. Kourembanas S. Smooth muscle cell-derived carbon monoxide is a regulator of vascular cGMP. Proc Natl Acad Sci U S A. 1995;92:1475. doi: 10.1073/pnas.92.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bader D. Masaki T. Fischman D.A. Immunochemical analysis of myosin heavy chain during avian myogenesis in vivo and in vitro. J Cell Biol. 1982;95:763. doi: 10.1083/jcb.95.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones W.K. Grupp I.L. Doetschman T. Grupp G. Osinska H. Hewett T.E. Boivin G. Gulick J. Ng W.A. Robbins J. Ablation of the murine alpha myosin heavy chain gene leads to dosage effects and functional deficits in the heart. J Clin Invest. 1996;98:1906. doi: 10.1172/JCI118992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoshinaga T. Sassa S. Kappas A. Purification and properties of bovine spleen heme oxygenase. Amino acid composition and sites of action of inhibitors of heme oxidation. J Biol Chem. 1982;257:7778. [PubMed] [Google Scholar]
- 34.Veinot J.P. Harrity P.J. Gentile F. Khandheria B.K. Bailey K.R. Eickholt J.T. Seward J.B. Tajik A.J. Edwards W.D. Anatomy of the normal left atrial appendage: a quantitative study of age-related changes in 500 autopsy hearts: implications for echocardiographic examination. Circulation. 1997;96:3112. doi: 10.1161/01.cir.96.9.3112. [DOI] [PubMed] [Google Scholar]
- 35.Jockusch H. Fuchtbauer E.M. Fuchtbauer A. Leger J.J. Leger J. Maldonado C.A. Forssmann W.G. Long-term expression of isomyosins and myoendocrine functions in ectopic grafts of atrial tissue. Proc Natl Acad Sci U S A. 1986;83:7325. doi: 10.1073/pnas.83.19.7325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Okawa H. Horimoto H. Mieno S. Nomura Y. Yoshida M. Shinjiro S. Preischemic infusion of alpha-human atrial natriuretic peptide elicits myoprotective effects against ischemia reperfusion in isolated rat hearts. Mol Cell Biochem. 2003;248:171. doi: 10.1023/a:1024148621505. [DOI] [PubMed] [Google Scholar]
- 37.Kasama S. Toyama T. Hatori T. Sumino H. Kumakura H. Takayama Y. Ichikawa S. Suzuki T. Kurabayashi M. Effects of intravenous atrial natriuretic peptide on cardiac sympathetic nerve activity and left ventricular remodeling in patients with first anterior acute myocardial infarction. J Am Coll Cardiol. 2007;49:667. doi: 10.1016/j.jacc.2006.09.048. [DOI] [PubMed] [Google Scholar]
- 38.Li P. Wang D. Lucas J. Oparil S. Xing D. Cao X. Novak L. Renfrow M.B. Chen Y.F. Atrial natriuretic peptide inhibits transforming growth factor β-induced Smad signaling and myofibroblast transformation in mouse cardiac fibroblasts. Circ Res. 2008;102:185. doi: 10.1161/CIRCRESAHA.107.157677. [DOI] [PubMed] [Google Scholar]
- 39.Eppenberger H.M. Zuppinger C. In vitro reestablishment of cell-cell contacts in adult rat cardiomyocytes. Functional role of transmembrane components in the formation of new intercalated disk-like cell contacts. FASEB J. 1999;13(Suppl):S83. doi: 10.1096/fasebj.13.9001.s83. [DOI] [PubMed] [Google Scholar]
- 40.Laugwitz K.L. Moretti A. Lam J. Gruber P. Chen Y. Woodard S. Lin L.Z. Cai C.L. Lu M.M. Reth M. Platoshyn O. Yuan J.X. Evans S. Chien K.R. Postnatal isl1+cardioblasts enter fully differentiated cardiomyocyte lineages. Nature. 2005;433:647. doi: 10.1038/nature03215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hsieh P.C. Segers V.F. Davis M.E. MacGillivray C. Gannon J. Molkentin J.D. Robbins J. Lee R.T. Evidence from a genetic fate-mapping study that stem cells refresh adult mammalian cardiomyocytes after injury. Nat Med. 2007;13:970. doi: 10.1038/nm1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith R.R. Barile L. Cho H.C. Leppo M.K. Hare J.M. Messina E. Giacomello A. Abraham M.R. Marban E. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- 43.Bearzi C. Rota M. Hosoda T. Tillmanns J. Nascimbene A. De Angelis A. Yasuzawa-Amano S. Trofimova I. Siggins R.W. Lecapitaine N. Cascapera S. Beltrami A.P. D'Alessandro D.A. Zias E. Quaini F. Urbanek K. Michler R.E. Bolli R. Kajstura J. Leri A. Anversa P. Human cardiac stem cells. Proc Natl Acad Sci U S A. 2007;104:14068. doi: 10.1073/pnas.0706760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Urbanek K. Cesselli D. Rota M. Nascimbene A. De Angelis A. Hosoda T. Bearzi C. Boni A. Bolli R. Kajstura J. Anversa P. Leri A. Stem cell niches in the adult mouse heart. Proc Natl Acad Sci U S A. 2006;103:9226. doi: 10.1073/pnas.0600635103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hosoda T. D'Amario D. Cabral-Da-Silva M.C. Zheng H. Padin-Iruegas M.E. Ogorek B. Ferreira-Martins J. Yasuzawa-Amano S. Amano K. Ide-Iwata N. Cheng W. Rota M. Urbanek K. Kajstura J. Anversa P. Leri A. Clonality of mouse and human cardiomyogenesis in vivo. Proc Natl Acad Sci U S A. 2009;106:17169. doi: 10.1073/pnas.0903089106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Okita K. Ichisaka T. Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 47.Yu J. Vodyanik M.A. Smuga-Otto K. Antosiewicz-Bourget J. Frane J.L. Tian S. Nie J. Jonsdottir G.A. Ruotti V. Stewart R. Slukvin II. Thomson J.A. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 48.Nakagawa M. Koyanagi M. Tanabe K. Takahashi K. Ichisaka T. Aoi T. Okita K. Mochiduki Y. Takizawa N. Yamanaka S. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. 2008;26:101. doi: 10.1038/nbt1374. [DOI] [PubMed] [Google Scholar]
- 49.Yi B.A. Wernet O. Chien K.R. Pregenerative medicine: developmental paradigms in the biology of cardiovascular regeneration. J Clin Invest. 2010;120:20. doi: 10.1172/JCI40820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sakai T. Li R.K. Weisel R.D. Mickle D.A. Kim E.J. Tomita S. Jia Z.Q. Yau T.M. Autologous heart cell transplantation improves cardiac function after myocardial injury. Ann Thorac Surg. 1999;68:2074. doi: 10.1016/s0003-4975(99)01148-0. [DOI] [PubMed] [Google Scholar]
- 51.Zhang M. Methot D. Poppa V. Fujio Y. Walsh K. Murry C.E. Cardiomyocyte grafting for cardiac repair: graft cell death and anti-death strategies. J Mol Cell Cardiol. 2001;33:907. doi: 10.1006/jmcc.2001.1367. [DOI] [PubMed] [Google Scholar]
- 52.Laflamme M.A. Gold J. Xu C. Hassanipour M. Rosler E. Police S. Muskheli V. Murry C.E. Formation of human myocardium in the rat heart from human embryonic stem cells. Am J Pathol. 2005;167:663. doi: 10.1016/S0002-9440(10)62041-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Laflamme M.A. Chen K.Y. Naumova A.V. Muskheli V. Fugate J.A. Dupras S.K. Reinecke H. Xu C. Hassanipour M. Police S. O'Sullivan C. Collins L. Chen Y. Minami E. Gill E.A. Ueno S. Yuan C. Gold J. Murry C.E. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25:1015. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 54.Tang Y.L. Tang Y. Zhang Y.C. Qian K. Shen L. Phillips M.I. Improved graft mesenchymal stem cell survival in ischemic heart with a hypoxia-regulated heme oxygenase-1 vector. J Am Coll Cardiol. 2005;46:1339. doi: 10.1016/j.jacc.2005.05.079. [DOI] [PubMed] [Google Scholar]
- 55.Narmoneva D.A. Vukmirovic R. Davis M.E. Kamm R.D. Lee R.T. Endothelial cells promote cardiac myocyte survival and spatial reorganization: implications for cardiac regeneration. Circulation. 2004;110:962. doi: 10.1161/01.CIR.0000140667.37070.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Naito H. Melnychenko I. Didie M. Schneiderbanger K. Schubert P. Rosenkranz S. Eschenhagen T. Zimmermann W.H. Optimizing engineered heart tissue for therapeutic applications as surrogate heart muscle. Circulation. 2006;114:I72. doi: 10.1161/CIRCULATIONAHA.105.001560. [DOI] [PubMed] [Google Scholar]
- 57.Caspi O. Lesman A. Basevitch Y. Gepstein A. Arbel G. Habib I.H. Gepstein L. Levenberg S. Tissue engineering of vascularized cardiac muscle from human embryonic stem cells. Circ Res. 2007;100:263. doi: 10.1161/01.RES.0000257776.05673.ff. [DOI] [PubMed] [Google Scholar]
- 58.Stevens K.R. Kreutziger K.L. Dupras S.K. Korte F.S. Regnier M. Muskheli V. Nourse M.B. Bendixen K. Reinecke H. Murry C.E. Physiological function and transplantation of scaffold-free and vascularized human cardiac muscle tissue. Proc Natl Acad Sci U S A. 2009;106:16568. doi: 10.1073/pnas.0908381106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tongers J. Fiedler B. König D. Kempf T. Klein G. Heineke J. Kraft T. Gambaryan S. Lohmann S.M. Drexler H. Wollert K.C. Heme oxygenase-1 inhibition of MAP kinases, calcineurin/NFAT signaling, and hypertrophy in cardiac myocytes. Cardiovasc Res. 2004;63:545. doi: 10.1016/j.cardiores.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 60.Yamashita T. Sekiguchi A. Iwasaki Y.K. Sagara K. Hatano S. Iinuma H. Aizawa T. Fu L.T. Thrombomodulin and tissue factor pathway inhibitor in endocardium of rapidly paced rat atria. Circulation. 2003;108:2450. doi: 10.1161/01.CIR.0000102969.09658.F2. [DOI] [PubMed] [Google Scholar]
- 61.Hickey M.J. Wilson Y. Hurley J.V. Morrison W.A. Mode of vascularization of control and basic fibroblast growth factor-stimulated prefabricated skin flaps. Plast Reconstr Surg. 1998;101:1296. [PubMed] [Google Scholar]
- 62.Gibot L. Galbraith T. Huot J. Auger F.A. A preexisting microvascular network benefits in vivo revascularization of a microvascularized tissue-engineered skin substitute. Tissue Eng Part A. 2010;16:3199. doi: 10.1089/ten.tea.2010.0189. [DOI] [PubMed] [Google Scholar]
- 63.Lesman A. Habib M. Caspi O. Gepstein A. Arbel G. Levenberg S. Gepstein L. Transplantation of a tissue-engineered human vascularized cardiac muscle. Tissue Eng Part A. 2010;16:115. doi: 10.1089/ten.TEA.2009.0130. [DOI] [PubMed] [Google Scholar]
- 64.Balla G. Jacob H.S. Balla J. Rosenberg M. Nath K. Apple F. Eaton J.W. Vercellotti G.M. Ferritin: a cytoprotective antioxidant strategem of endothelium. J Biol Chem. 1992;267:18148. [PubMed] [Google Scholar]
- 65.Brouard S. Otterbein L.E. Anrather J. Tobiasch E. Bach F.H. Choi A.M. Soares M.P. Carbon monoxide generated by heme oxygenase 1 suppresses endothelial cell apoptosis. J Exp Med. 2000;192:1015. doi: 10.1084/jem.192.7.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dulak J. Jozkowicz A. Foresti R. Kasza A. Frick M. Huk I. Green C.J. Pachinger O. Weidinger F. Motterlini R. Heme oxygenase activity modulates vascular endothelial growth factor synthesis in vascular smooth muscle cells. Antioxid Redox Signal. 2002;4:229. doi: 10.1089/152308602753666280. [DOI] [PubMed] [Google Scholar]
- 67.Shimizu T. Sekine H. Yang J. Isoi Y. Yamato M. Kikuchi A. Kobayashi E. Okano T. Polysurgery of cell sheet grafts overcomes diffusion limits to produce thick, vascularized myocardial tissues. FASEB J. 2006;20:708. doi: 10.1096/fj.05-4715fje. [DOI] [PubMed] [Google Scholar]
- 68.Melo L.G. Agrawal R. Zhang L. Rezvani M. Mangi A.A. Ehsan A. Griese D.P. Dell'Acqua G. Mann M.J. Oyama J. Yet S.F. Layne M.D. Perrella M.A. Dzau V.J. Gene therapy strategy for long-term myocardial protection using adeno-associated virus-mediated delivery of heme oxygenase gene. Circulation. 2002;105:602. doi: 10.1161/hc0502.103363. [DOI] [PubMed] [Google Scholar]
- 69.Figueiredo A.M. Poggetti R.S. Quintavalle F.G. Fontes B. Dalva M. Younes R.N. Jatene F.B. Birolini D. Isolated right atrial appendage (RAA) rupture in blunt trauma—a case report and an anatomic study comparing RAA and right atrium (RA) wall thickness. World J Emerg Surg. 2007;2:5. doi: 10.1186/1749-7922-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kikuchi K. McDonald A.D. Sasano T. Donahue J.K. Targeted modification of atrial electrophysiology by homogeneous transmural atrial gene transfer. Circulation. 2005;111:264. doi: 10.1161/01.CIR.0000153338.47507.83. [DOI] [PubMed] [Google Scholar]
- 71.Clark J.E. Naughton P. Shurey S. Green C.J. Johnson T.R. Mann B.E. Foresti R. Motterlini R. Cardioprotective actions by a water-soluble carbon monoxide-releasing molecule. Circ Res. 2003;93:e2. doi: 10.1161/01.RES.0000084381.86567.08. [DOI] [PubMed] [Google Scholar]
- 72.Stein A.B. Guo Y. Tan W. Wu W.J. Zhu X. Li Q. Luo C. Dawn B. Johnson T.R. Motterlini R. Bolli R. Administration of a CO-releasing molecule induces late preconditioning against myocardial infarction. J Mol Cell Cardiol. 2005;38:127. doi: 10.1016/j.yjmcc.2004.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kotsch K. Francuski M. Pascher A. Klemz R. Seifert M. Mittler J. Schumacher G. Buelow R. Volk H.D. Tullius S.G. Neuhaus P. Pratschke J. Improved long-term graft survival after HO-1 induction in brain-dead donors. Am J Transplant. 2006;6:477. doi: 10.1111/j.1600-6143.2005.01208.x. [DOI] [PubMed] [Google Scholar]
- 74.Katori M. Buelow R. Ke B. Ma J. Coito A.J. Iyer S. Southard D. Busuttil R.W. Kupiec-Weglinski J.W. Heme oxygenase-1 overexpression protects rat hearts from cold ischemia/reperfusion injury via an antiapoptotic pathway. Transplantation. 2002;73:287. doi: 10.1097/00007890-200201270-00023. [DOI] [PubMed] [Google Scholar]
- 75.Akamatsu Y. Haga M. Tyagi S. Yamashita K. Graca-Souza A.V. Ollinger R. Czismadia E. May G.A. Ifedigbo E. Otterbein L.E. Bach F.H. Soares M.P. Heme oxygenase-1-derived carbon monoxide protects hearts from transplant associated ischemia reperfusion injury. FASEB J. 2004;18:771. doi: 10.1096/fj.03-0921fje. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.