Abstract
The mechanisms by which exposure to arsenic induces its myriad pathological effects are undoubtedly complex, while individual susceptibility to their type and severity is likely to be strongly influenced by genetic factors. Human metabolism of arsenic into methylated derivatives, once presumed to result in detoxification, may actually produce species with significantly greater pathological potential. We introduce a transgenic Drosophila model of arsenic methylation, allowing its consequences to be studied in a higher eukaryote exhibiting conservation of many genes and pathways with those of human cells while providing an important opportunity to uncover mechanistic details via the sophisticated genetic analysis for which the system is particularly well suited. The gene for the human enzyme, arsenic (+3 oxidation state) methyltransferase, was introduced into nonmethylating Drosophila under inducible control. Transgenic flies were characterized for enzyme inducibility, production of methylated arsenic species, and the dose-dependent consequences for chromosomal integrity and organismal longevity. Upon enzyme induction, transgenic flies processed arsenite into mono and dimethylated derivatives identical to those found in human urine. When induced flies were exposed to 9 ppm arsenite, chromosomal stability was clearly reduced, whereas at much higher doses, adult life span was significantly increased, a seemingly paradoxical pair of outcomes. Measurement of arsenic body burden in the presence or absence of methylation suggested that enhanced clearance of methylated species might explain this greater longevity under acutely toxic conditions. Our study clearly demonstrates both the hazards and the benefits of arsenic methylation in vivo and suggests a resolution based on evolutionary grounds.
Keywords: arsenic, AS3MT, transgenic model, Drosophila, methylated arsenical, LOH assay
The consumption of inorganic arsenic (iAs) in the drinking water of tens of millions in Bangladesh has been characterized as “the largest mass poisoning of a population in history” (Smith et al., 2000). Millions in other parts of the world are similarly at risk (Ng et al., 2003). This is because persistent ingestion of iAs (typically in the range 200–800 ppb) can lead to development of a wide range of pathologies, among which disfiguring skin cancers and life-threatening internal organ cancers are particularly noteworthy (Tapio and Grosche, 2006). Although the causative mechanism behind any one of these chronic toxicities is unknown, it has been suggested that iAs-linked induction of reactive oxygen species is responsible for damage to DNA bases (Wang et al., 2001), thereby leading to disruption of genetic information flow and/or key signaling cascade pathways (Kumagai and Sumi, 2007). Several other nonmutually exclusive possibilities also exist, including arsenic-dependent protein modification (Kitchin and Wallace, 2008) that might disrupt essential pathways, such as DNA damage repair (Schwerdtle et al., 2003) or epigenetic modification (Reichard and Puga, 2010), or affect expression of key genes regulating cell cycle progression, apoptosis, or stem cell maintenance and proliferation (Tokar et al., 2010). More generally, pathway interference by arsenicals leads to cytotoxicity followed by regenerative hyperplasia in a rodent model (Cohen et al., 2007), thus providing an alternative, nonoxidative damage-based explanation for its carcinogenic effects. The issue remains generally unresolved at this time.
There is renewed focus on such mechanistic questions because methylated arsenicals (MAs), rather than being merely metabolic detoxification products found in urine as monomethylated arsenicals (MMA) and dimethylated arsenicals (DMA), might be among the primary agents of chronic toxicity and/or carcinogenesis. In humans, a major pathway for arsenic metabolism involves the enzyme arsenic (+3 oxidation state) methyltransferase (AS3MT), which is able to methylate the reduced (+3 oxidation state) form of iAs to produce, in apparently sequential fashion, monomethylarsonous acid [MMA(III)] followed by dimethylarsinous acid [DMA(III)] (Lin et al., 2002), both of which can be oxidized to form derivatives of the +5 arsenic oxidation state [MMA(V) and DMA(V)] that are commonly found in urine of exposed individuals (Vahter, 2002). Several studies have reported that some of these MAs [particularly the trivalent MMA(III) species] have significantly greater toxicity than iAs in a range of assays, ranging from in vitro studies of DNA damage, chromosomal damage, cell viability, and cell transformation (Bredfeldt et al., 2006; Kligerman et al., 2003; Mass et al., 2001; Petrick et al., 2000) to in vivo studies of overt toxicity in rodents (Petrick et al., 2001). Furthermore, improved analyses have convincingly demonstrated the presence of such trivalent species in human urine (Le et al., 2000). Based on this altered paradigm, the issue of relative susceptibility to chronic iAs toxicity takes on a central role because interindividual differences in capacity to metabolize iAs could be of great importance in the progression to disease. In keeping with this, epidemiological studies inferring genetic variability in the metabolic processing of arsenic is associated with differential outcome and response to arsenic exposure have recently appeared (Steinmaus et al., 2010; Valenzuela et al., 2009).
This appreciation of the importance of genetic factors in the host response to iAs suggests that studying toxic mechanisms from a genetic perspective in an amenable model system would be very valuable. We have previously studied aspects of acute iAs toxicity using Drosophila where, by utilizing the natural genetic variation inherent in geographically dispersed populations (thus injecting no bias into the experiment), we identified the glutathione biosynthetic pathway as an essential component in the natural defense response to arsenic (Muñiz Ortiz et al., 2009). From the point of view of experimental genetics, Drosophila has proven to be an extremely useful higher eukaryote. The high conservation of genes and pathways in growth, development, and cellular physiology with those in mammalian systems has led to its use as a disease model in numerous fields, particularly for neurodegenerative conditions and cancer (Lu and Vogel, 2009; Vidal and Cagan, 2006). In the context of iAs metabolism, it was previously found that Drosophila neither methylates arsenite nor experiences genotoxicity upon exposure (Rizki et al., 2006). However, feeding DMA(V) to larvae led to observable chromosomal damage, suggesting that Drosophila does respond to MAs in ways similar to those reported in human studies (Ghosh et al., 2007). Because our basic local alignment search tool (BLAST) analysis suggested that Drosophila lacks an AS3MT homolog, introduction of the human gene (hAS3MT) into Drosophila would allow studies designed to mimic the chemical transformation of iAs in vivo, to be performed with the added attraction of studying the physiological responses in a system where sophisticated genetic analysis can be readily applied. We describe the initial characterization of such a transgenic model together with studies that suggest the benefits and disadvantages of iAs methylation in vivo reflect an adaptive evolutionary response. In the future, this transgenic model should allow us to more easily dissect the mechanisms behind the increased genotoxicity (and perhaps carcinogenicity) of MAs using the battery of genetic approaches for which Drosophila is particularly advantageous.
MATERIALS AND METHODS
Drosophila lines and manipulations.
Lines were maintained on standard cornmeal medium at room temperature. When tested for iAs responsiveness in various assays, flies were cultured on Drosophila 4-24 Instant Medium (Carolina Biological, Burlington, NC), prepared by hydration with either deionized water or sodium m-arsenite (iAsIII, 97%, lot no. 76H0240; Sigma-Aldrich, St Louis, MO) solution at the indicated concentration. Food preparation was carefully standardized to allow arsenic concentration in ppm to be calculated: for each 1 g of 4-24 medium, 5 ml of arsenite solution at a given concentration was added. The GAL4-expressing lines w1118; da-GAL4 and y1 w*; Act5C-GAL4 were obtained from the Drosophila Stock Center at Indiana University (Bloomington, IN). The w; latsx1/TM6B, Tb line was kindly provided by Dr Tian Xu (Xu et al., 1995). We created the UAS-hAS3MT; latsx1/TM3, Sb line following standard procedures using a variety of balancer lines.
Production of hAS3MT transgenic Drosophila.
The hAS3MT sequence was isolated by PCR using Phusion Polymerase (NEB, Ipswich, MA) from a human kidney complementary DNA library kindly provided by Dr William Miller (University of Cincinnati) using the forward primer 5′-CACCATGGCTGCACTTCGTGACGCTG and the reverse primer 5′-GCAGCTTTTCTTTGTGCCACAGCAG. The underlined sequence in the forward primer allowed for unidirectional cloning into the pENTR/D-TOPO vector according to manufacturer’s directions (Invitrogen, Carlsbad, CA), and identity to the standard wild-type hAS3MT gene (accession no. NM_020682) was verified by DNA sequencing (Genewiz, Inc., South Plainfield, NJ). To create suitable constructs for P-element–mediated transformation into flies, we used Drosophila-specific Gateway transformation vectors, obtained from the Drosophila Genomics Resource Center (Indiana University). The pTHW (1099) vector used consists of a GAL4-inducible UAS sequence driving expression of an N-terminal HA-tagged protein-encoding gene (in this case, hAS3MT), together with P-element sequences allowing for genomic insertion of the vector, and a w+ marker to identify positive transformants by their red eye color (http://www.ciwemb.edu/labs/murphy/Gateway%20vectors.html). Cloning of the hAS3MT gene into pTHW followed standard Gateway recombination procedures (Invitrogen). All constructs were confirmed via restriction endonuclease analysis, and verified Gateway recombinants were provided to Rainbow Transgenic Flies, Inc. (Newbury Park, CA) for P-element transformation into Drosophila. Eclosing adults from injected w1118 embryos were individually collected and crossed to w1118 males or females for detection of transgenic progeny via production of red eye color, followed by standard crossing procedures to identify the chromosomal location of the UAS-hAS3MT insert.
Molecular characterization of transgenic lines.
Analysis of hAS3MT messenger RNA (mRNA) transcription was performed by reverse transcriptase PCR (RT-PCR), basically as previously described (Muñiz Ortiz et al., 2009). For hAS3MT protein analysis, three adult flies were sonicated in SDS gel-loading buffer (200 μl), boiled for 5 min, and centrifuged at 15,800 × g for 3 min. Homogenates (20 μl) were separated by SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose membrane following standard procedures. Immunoblotting was performed with an HA antibody (provided by Dr William Miller) at 1:1000 dilution, hAS3MT antibody (provided by Dr Richard Weinshilboum, Mayo Clinic, MN) at 1:1000 dilution, and with anti-actin antibody as a loading control at 1:10,000 dilution. Horseradish peroxidase–conjugated anti-rabbit (HA and hAS3MT) or anti-mouse (actin) secondary antibody was used at a 1:1000 dilution and signal detected using SuperSignal West Pico Chemiluminescent Substrate (Pierce, Rockford, IL), followed by blue-sensitive x-ray film exposure.
Arsenic speciation and quantification analysis.
Arsenic speciation via ion-pairing reversed-phase liquid chromatography linked to an inductively coupled plasma mass spectrometer (ICPMS) was performed following methods previously described (Afton et al., 2008) with minimal adjustments. Similarly, quantification analysis of arsenic in whole adult flies was performed by standard ICPMS procedures. Both analyses were conducted at the University of Cincinnati/Agilent Technologies Metallomics Center of the Americas. A full description of the methodology is provided in the Supplementary data.
For speciation, embryos from relevant crosses were collected as described (Muñiz Ortiz et al., 2009) and allowed to develop in iAsIII-free or 0.1mM iAsIII-supplemented medium (6.25 ppm As). Sample preparation for analysis was adapted from a previous report (Rizki et al., 2006). Briefly, 30 third-instar larvae were collected and homogenized in 2 ml homogenizing buffer (250mM sucrose, 1mM MgCl2, and 10mM Tris-HCl [pH 7.4]) using a glass homogenizer with Teflon pestle. Homogenates were centrifuged at 105,000 × g for 90 min, transferred to a 1.5-ml microcentrifuge tube, and stored at −80°C until used in the analysis. For quantification analysis, flies were reared and adults subjected to 1mM iAsIII-supplemented food (62.5 ppm As), as described below (adult longevity assay). After 4 days of continuous feeding, flies were transferred to vials containing only 0.1% glucose-infiltrated filter paper for a further 24 h in order to clear the digestive tract of extraneous iAsIII-containing food prior to analysis. Data were subjected to paired t-test analysis, with significance level of α = 0.05.
Developmental genotoxicity (loss of heterozygosity) assay.
Flies of the genotypes w; UAS-hAS3MT; latsx1/TM3, Sb (transgenic) or w; latsx1/TM6B, Tb (nontransgenic control) were mated to flies of the w1118; da-GAL4 line and then females allowed to lay embryos on iAsIII-free or 0.15mM iAsIII-supplemented (9.4 ppm) medium. Eclosing (hatching) adults derived from development of these embryos were aged for 5 days, sorted into appropriate genotypes, and then observed under a stereomicroscope at ×40 magnification and scored for detectable tissue overgrowth. We analyzed and compared the data by dividing the number of tumors observed by the number of progeny flies derived from each treatment and genotype. Note that for each individual cross, lats-containing progeny could be directly scored and compared with their non-lats siblings. Data were analyzed by Fisher's exact test or Pearson's chi-square test for large sample sizes and p values computed for critical significance level α = 0.05.
Adult longevity assay.
Flies were reared and appropriate crosses performed in bottles containing standard cornmeal-molasses fly medium. Between 100 and 200 F1 males per experiment were collected within 24 h of eclosion and transferred into vials (25 per vial) containing 1mM iAsIII-supplemented (62.5 ppm) instant medium. Survivors were counted each day and transferred every 5 days into new vials containing fresh iAsIII-supplemented medium until all flies had perished. At least two replicate experiments were performed at room temperature (21°C–23°C). Survival data were subjected to log-rank (Mantel-Cox) test for significance with α = 0.05.
RESULTS
Transgenic Drosophila Lines Inducible for Human AS3MT
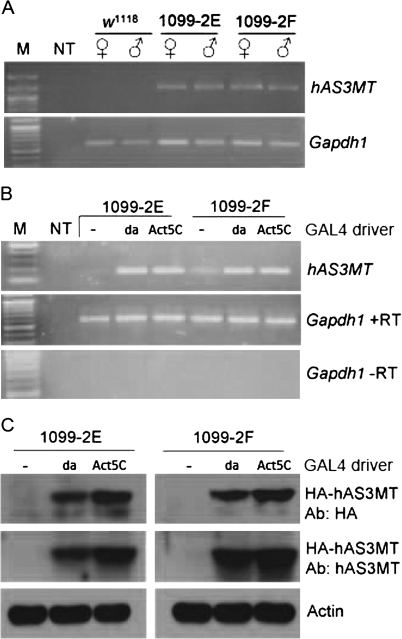
In designing an approach to produce Drosophila lines that could express the human AS3MT enzyme, we took advantage of the yeast GAL4-UAS transcriptional system that has been widely deployed in transgenic fly studies (Brand and Perrimon, 1993). In this scheme, flies that express the yeast GAL4 transcriptional activator protein under control of any chosen promoter/enhancer combination (driver) are mated with a second (responder) line containing a gene of interest (in our case hAS3MT) under control of the GAL4-responsive UAS promoter sequence. The progeny will express the transgene according to wherever the GAL4 protein is expressed. It is thus possible to control expression of such transgenes according to whatever enhancer-driven program is selected in the GAL4 driver. We tested two selected lines, shown by PCR to be transgenic for hAS3MT (Fig. 1A), for inducibility of hAS3MT mRNA by crossing each to either of two driver lines (da-GAL4 or Act5C-GAL4) that express the GAL4 protein in a fairly ubiquitous pattern. Both transgenic lines were strongly responsive to transcriptional induction by GAL4 (Fig. 1B) as monitored by RT-PCR. In addition, translation of the induced mRNA into an appropriately sized protein was detected via Western blotting performed with both an anti-HA antibody (constructs were designed with an N-terminal HA fusion tag) as well as with a bona fide anti-human AS3MT antibody (Fig. 1C). Of particular note is the lack of cross-reacting protein in uninduced (or nontransgenic) flies, confirming the GAL4-specific control imposed on the transgene.
FIG. 1.
Molecular characterization of hAS3MT transgene insertion and expression. The designations 1099-2E and 1099-2F are two independent pTHW transgenic lines. (A) Agarose gel analysis of PCR products derived from hAS3MT insertion into w1118 genome. Gapdh1 was used as a loading control. NT, no template. (B) Agarose gel analysis of RT-PCR for hAS3MT mRNA. Transgenic lines were crossed into either a non-GAL4 (w1118) or a GAL4-expressing (da-GAL4 or Act5C-GAL4) background. Gapdh1 was used as a loading control. (C) Western analyses for the expression of the AS3MT enzyme. Actin was used as a loading control. Ab, antibody used; these were active against either the HA tag or the human AS3MT protein.
Human AS3MT has Enzymatic Function in Drosophila
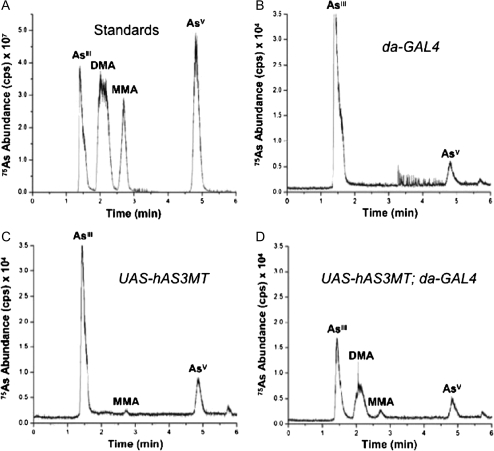
To determine if the expressed hAS3MT displayed arsenic-methylating enzymatic activity in flies, we collected 0.1mM iAsIII-exposed third-instar larvae (6.25 ppm) derived from either of two independent matings of the 1099-2E transgenic line, i.e., to the non–GAL4-containing w1118 line (uninduced control) or to the GAL4-expressing w1118; da-GAL4 line. In addition, to eliminate the possibility that GAL4 expression by itself could lead to production of MAs, nontransgenic w1118 flies were mated to the da-GAL4 line. Homogenates of the different larvae were analyzed for the presence of MAs using a high-performance liquid chromatography (HPLC)-linked inductively coupled mass spectrometry approach (Afton et al., 2008). Using arsenic standards, separation of iAsIII from iAsV, as well as the distinction of MMA and DMA species from their inorganic precursors, was readily accomplished (Fig. 2A). With larval-derived samples, neither the presence of GAL4 alone (Fig. 2B) nor the uninduced hAS3MT transgene (Fig. 2C) led to production of MAs. Importantly, only GAL4 induction of hAS3MT enzyme allowed high levels of DMA and somewhat lower levels of MMA species to be produced (Fig. 2D). As expected, third-instar larvae from all such crosses not exposed to iAsIII produced no trace of MAs (data not shown). It should be noted that the current methodology did not allow for determination of the +3 or +5 oxidation state of As in the MMA and DMA species, nor was the presence of trimethyl arsine oxide ascertained. These issues will be addressed in the future.
FIG. 2.
Functional enzymatic analysis of hAS3MT activity via HPLC/ICPMS. (A) Chromatogram of homogenizing buffer spiked with arsenic standards. (B) Chromatogram of larval extracts derived from a w1118 cross to da-GAL4 and exposed to 0.1mM iAsIII (6.25 ppm). (C) Chromatogram of larval extracts derived from a 1099-2E hAS3MT cross to w1118 exposed to 0.1mM iAsIII. (D) Chromatogram of larval extracts from a 1099-2E hAS3MT cross to da-GAL4 and exposed to 0.1mM iAsIII. Each peak in (B–D) represents the abundance of a specific arsenic species in larval homogenates.
Synthesis of MAs In Vivo Promotes Chromosomal Instability
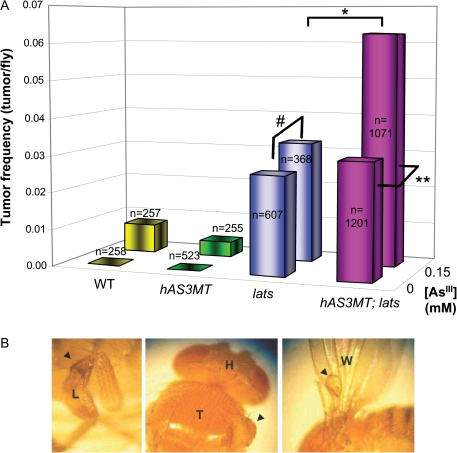
To determine if the induction of MAs in Drosophila had effects on chromosome structure and/or stability, we employed a loss of heterozygosity (LOH) assay that can be readily scored in flies exposed to iAsIII and that have been induced (or not) for the expression of hAS3MT. For this purpose, we introduced a mutated copy of the third-chromosomal lats gene into the tested flies. The lats gene (also known as wts in Drosophila) encodes a serine/threonine kinase tumor suppressor (Justice et al., 1995; Xu et al., 1995) involved in the Hippo signaling pathway that controls organ size by regulating cell growth, proliferation, and apoptosis (Pan, 2007). When the remaining wild-type copy of lats is mutated or, more likely, lost by mitotic recombination, chromosome breakage/deletion, etc., as a result of exposure to a genotoxin or clastogen, the ensuing LOH is readily observed as large, tumorigenic outgrowths of epithelial cell–based structures (derived from the larval imaginal disks) on the adult fly (Eeken et al., 2002; Xu et al., 1995). We screened several hundred flies of various genotypes, either induced for hAS3MT or not and exposed to iAsIII (or not). The data obtained (Fig. 3A) clearly show that significantly enhanced rates of LOH in lats heterozygotes (i.e., above the spontaneous background) were only seen when both the hAS3MT gene was induced and the flies were exposed to iAsIII (9.4 ppm) during development. In other words, significant enhancement of chromosomal instability is specific to the situation where MAs are synthesized in vivo and is not seen when iAsIII is encountered but cannot be metabolized. This strongly suggests that it is the MAs, and not iAs, that are the genotoxic (or clastogenic) species in vivo. Examples of tumor outgrowths in various parts of the body, derived from LOH of lats, are shown in Figure 3B.
FIG. 3.
Tumor induction via LOH of lats tumor suppressor reflects hAS3MT enzyme expression in the presence of iAsIII. (A) Four different genotypes (WT represents a common w1118 background) were tested for epithelial cell–derived tumor formation as a function of iAsIII dose (either 0 or 9.4 ppm). p values (α = 0.05) were as follows: *0.033; **0.0004; #0.56. All other comparisons (genotype or iAsIII dependent) were not significant. (B) Examples of tumor outgrowths in a variety of Drosophila tissues after LOH of the lats gene; leg (L), thorax (T) and wing (W). [Head (H)].
The Life span of iAsIII-Exposed Drosophila Is Extended by Human AS3MT Expression
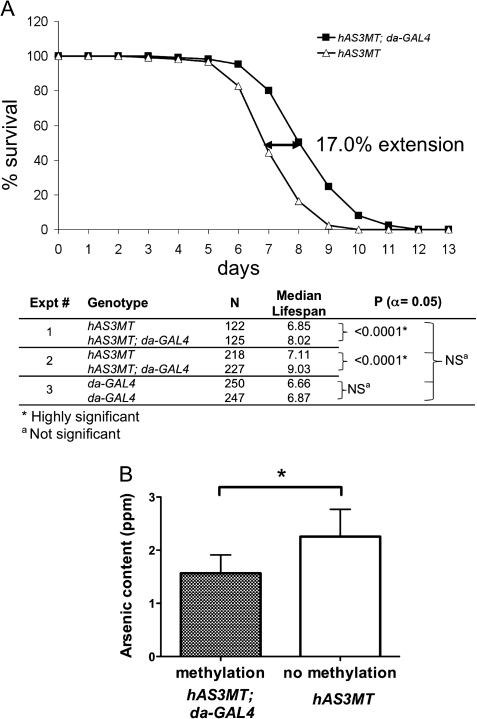
Because chromosomal instability as seen here was produced at nonlife-threatening concentrations of iAsIII (though such instability would likely have long-term detrimental consequences, such as disease initiation and/or progression, e.g., cancer, in an equivalent vertebrate situation), we were interested to know if expression of the human AS3MT gene had any effect on a measure of “acute” arsenic toxicity, e.g., early death after ingestion of high concentrations. To determine this, we tested the ability of freshly hatched adults of various genotypes to survive exposure to a much higher concentration (1mM) of iAsIII-containing food (62.5 ppm As), after having completed their growth and developmental phases (larval and pupal stages) in the absence of iAsIII. Most interestingly, flies that were induced to express the hAS3MT enzyme (hAS3MT; da-GAL4) lived for a significantly longer period (median lifetime) as compared with uninduced transgenic flies (hAS3MT) treated identically (Fig. 4A, upper). In the particular experiment shown, the median lifetime was extended 17% as compared with uninduced hAS3MT flies. Replicate experiments on these two genotypes (Fig. 4A, lower, experiments 1 and 2) found the extension of life span to be extremely significant (p < 0.0001) in each case, whereas biological replicates conducted on the nontransgenic da-GAL4 strain (Fig. 4A, lower, experiment 3) showed no significant life span difference compared with the uninduced hAS3MT strain. Interestingly, no differences in developmental viability were found when these same three genotypes were exposed to a much lower concentration (0.1mM, 6.25 ppm) of iAsIII in the food (Supplementary fig. S1). Thus, the data strongly suggest that, under conditions of life-threatening, high-concentration arsenic exposure, the expression of the human AS3MT enzyme is actually beneficial for organismal viability.
FIG. 4.
Upon high-dose iAsIII administration, adult life span is increased and arsenic body burden is decreased by human AS3MT enzyme expression. Flies of the appropriate genotype were derived from identical crosses to those described in Figure 2. (A) (Upper) Representative survival curve of adult hAS3MT transgenic flies exposed to 1mM iAsIII-supplemented food (62.5 ppm) and either induced (hAS3MT; da-GAL4) or not induced (hAS3MT) for hAS3MT expression. (Lower) Chart of survival parameters derived from two independent experiments using these genotypes (experiment numbers 1 and 2), as well as two independent tests of the control, nontransgenic da-GAL4 strain (experiment no. 3). (B) Arsenic was quantified by ICPMS after 4 days continuous feeding as in (A) followed by 24 h on 0.1% glucose. Groups of 25 flies were frozen and tested for As content; the measurement was repeated on six independent samples for each genotype. *p (α = 0.05) = 0.002.
Lower Arsenic Body Burden Correlates with Expression of Human AS3MT
We decided to examine the body burden of arsenic in these experiments to determine whether expression of hAS3MT (or lack thereof) might lead to differential arsenic accumulation, consistent with differential life span. Adult flies were fed as above (62.5 ppm) but after 4 days were transferred for a further 24 h to vials containing only 0.1% glucose. This allowed the digestive tract to be fully cleared of iAsIII-containing food prior to subjection to whole-body elemental analysis. In six independent experiments, transgenic flies induced for hAS3MT expression showed a consistent and significant reduction in total body arsenic as compared with uninduced transgenics fed identically (Fig. 4B). This strongly suggests that MAs produced in the induced flies were accumulating to a lower degree than iAsIII in the uninduced flies, perhaps because of enhanced cellular export of the chemically transformed species.
DISCUSSION
The work described here introduces a new transgenic model in which to study the effects of iAs in vivo. Although it might be argued that the field of arsenic research has more than enough different experimental systems and approaches and that greater integration among existing systems is needed, we believe that the Drosophila model has some significant advantages for experimentation and more than enough homology to vertebrate cellular and molecular pathways to encourage its consideration when it comes to exploring iAs action in vivo. This is not to say that Drosophila is a perfect toxicological model because it is clear that xenobiotic metabolism, transport, and toxicokinetics are often likely to differ from that in vertebrates. However, this is an important consideration in mammalian models too and particularly so in the case of iAs toxicity where rodent models also suffer in some of these respects in comparison to the human situation. Our goal in exploiting this fly model is to aid in the elucidation of potential molecular mechanisms of iAs toxicity through manipulating the genetic background (via the agency of the system's facile classical and “reverse” genetic manipulations) and observing the consequences on various phenotypic endpoints that resemble those seen in vertebrates; these can then be tested for relevance in suitable vertebrate-based assays. Because the methylation of iAs has become an important focus of recent investigations, particularly with respect to the outcomes of chronic exposure to iAs through drinking water, the lack of an equivalent methylation system in Drosophila, far from being a drawback, actually allows numerous genetic approaches aimed at exploring mechanistic features of iAs toxicity to be envisaged. We have explored this idea by creating transgenic Drosophila that express, under inducible control, a common allele of the human AS3MT gene and have shown that the two most common MAs species found in human urine, i.e., MMA and DMA, are now produced in induced flies. Neither species is detected when the corresponding uninduced transgenic flies are fed iAs, suggesting that there is tight regulation in the system and that unique biological effects uncovered in the induced transgenic flies will be specifically attributable to the presence of MAs.
With the system thus established and characterized and using two very different phenotypic endpoints, we have gone on to show that methylated metabolites of iAs can have strongly differential dose-dependent effects. Thus, methylation can ameliorate the overtly toxic life-threatening effects of high-dose iAsIII administration by extending life span, an effect presumably mediated by enhanced efflux of the synthesized MAs relative to iAs. Certainly, our demonstration of a lower As body burden in transgenic flies induced to methylate compared with those not induced is strongly consistent with this interpretation as being causative here. However, this same methylation of iAs significantly enhances its potential to act as a lower dose-based agent of chronic molecular damage (in this case chromosome instability), an outcome that undoubtedly contributes to pathological consequences for an organism chronically exposed to iAs over the long term. It is important to note that the doses we have used in these experiments (6–9 ppm As) are in precisely the same range as those employed in a recently reported “whole-life” exposure model of carcinogenesis developed in the mouse (Tokar et al., 2011). Our combined observations therefore neatly integrate, in one model system, a good deal of older literature that proposed methylation of iAs was a detoxification pathway (Buchet et al., 1981; Crecelius, 1977) with newer data, which have suggested that the methylated species (in particular the +3 oxidized form) are, albeit indirectly, more damaging to DNA (Mass et al., 2001) and (perhaps related to this) more active in causing cell transformation (Bredfeldt et al., 2006) and more highly associated with disease susceptibility in human populations (Steinmaus et al., 2010; Valenzuela et al., 2009). It is important to emphasize that the magnitude of the phenotypic effects described in this report is only altered when the hAS3MT transgene is “induced” in the presence of food-borne arsenite, strongly consistent with the notion that it is the transformation of iAs to the methylated species that is solely responsible for the altered phenotypic outcomes, i.e., decreased chromosome stability under one set of dose conditions and increased life span under a different (significantly higher) set of dose conditions.
Based on the results reported here, as well as previous data on the role of MAs, we are drawn to the conclusion that such observations may best be viewed as representing two sides of a single evolutionary coin. It is reasonable to hypothesize that the various xenobiotic defense mechanisms in animals have evolved to protect the life and (most importantly from the evolutionary perspective) the reproductive capacity of the afflicted animal. Thus, it is well known that acutely toxic doses of iAs uncouple mitochondrial oxidative phosphorylation (Ter Welle and Slater, 1964), which can rapidly lead to death. A metabolic processing system (like methylation) that leads to efficient cellular efflux of As in such a situation has the potential to preserve life and, most significantly, increase the chances of reproductive success, at least in the short term, and is therefore of distinct advantage when subjected to the forces of natural selection. There are numerous studies showing that ATP-binding cassette-type membrane transporters (e.g., multidrug resistance proteins [MRPs]) play an important role in exporting As from cells (see Thomas, 2007), and recent data are consistent with upregulation of MRP2 protein correlating with enhanced DMA transport from hepatocytes (Drobna et al., 2010). In other studies, methylation-competent cells accumulated less arsenic than their nonmethylating counterparts (Dopp et al., 2010; Drobna et al., 2005). Perhaps most important in this context are observations on an AS3MT knockout mouse, where loss of methylation ability leads to much slower clearance of As from tissues (Drobna et al., 2009) and consequently to an associated high accumulation of iAs (Hughes et al., 2010) leading to rapid systemic toxicity compared with the wild-type mouse (Yokohira et al., 2010). Interestingly, these latter studies were conducted using arsenic concentrations in the range 50–150 ppm, very similar to that (62.5 ppm) at which we tested hAS3MT-expressing and nonexpressing Drosophila for relative viability. Thus, we see strong parallels between this work and our own studies. The fact that the same MAs species produced by normal metabolism can be detrimental to cellular macromolecules (as shown here and by others previously) will likely have no short-term consequences in terms of pathology or impaired reproductive capacity. Of course, over the long term, such exposure to MAs (as will occur subsequent to ingestion of iAs-contaminated drinking water) will cause a slow accumulation of macromolecular damage that will ultimately lead to pathological outcomes. However, such a chronically exposed animal is expected to be well beyond its prime reproductive age by the time this happens, and so the metabolic capacity to produce MAs species is most unlikely to be eliminated through the agency of natural selection. Given these considerations, it makes sense that relatively long-lived animals (such as mammals) have evolved (and retained) a system for methylating iAs that is almost certainly linked to facilitated export. Although some mammals (e.g., certain nonhuman primates) do not exhibit significant iAs methylation (due to a mutation in the relevant AS3MT gene), we are not aware of studies on such animals that test their susceptibility to pathological outcomes after long-term exposure to iAs-contaminated drinking water, such as is encountered by many human populations. Such studies are certainly approachable with the knockout mouse. In our own model, an obviously much shorter lived animal, we will pursue such questions by developing appropriate dose-related phenotypic signatures at the cellular, subcellular, and molecular levels that are related to the presence or absence of MAs and that can be translated into an appropriate mammalian context.
The model of iAs methylation in Drosophila developed here, in addition to revealing possible advantages and disadvantages of this metabolic process, should facilitate the pursuit of more mechanistic questions related to chronic iAs toxicity. Thus, there has been much interest in the role that polymorphic variants of the human AS3MT enzyme (in particular the Met287Thr variant) may play in individual susceptibility to the effects of long-term As ingestion (Hernandez et al., 2008; Lindberg et al., 2007; Valenzuela et al., 2009). We have recently created transgenic fly lines expressing this particular variant under inducible control and plan to pursue a range of experiments under different dose regimes to determine if the Met287Thr variant can differentially affect phenotypic outcomes as compared with the “normal” human allele. Moreover, the GAL4-UAS inducible system we have built into our model offers many opportunities to vary the timing, the tissue specificity, and the degree of hAS3MT expression, all of which could be particularly informative in terms of differential toxic effects. Most importantly, we plan to exploit some of the highly refined genetic approaches available in Drosophila (e.g., transcriptome-wide RNAi) that, in combination with our transgenic model, should lend significant insight into the molecular pathways intersected by iAs and MAs in eliciting their highly dose-dependent range of differential phenotypic effects.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
FUNDING
National Institute of Environmental Health Sciences at the National Institutes of Health (R21-ES017235 to I.L.C. and P30-ES006096).
Supplementary Material
Acknowledgments
Dr Yinhuai Chen and Esteban Santana are gratefully thanked for their help in complementary DNA amplification.
References
- Afton S, Kubachka K, Catron B, Caruso JA. Simultaneous characterization of selenium and arsenic analytes via ion-pairing reversed phase chromatography with inductively coupled plasma and electrospray ionization ion trap mass spectrometry for detection applications to river water, plant extract and urine matrices. J. Chromatogr. A. 2008;1208:156–163. doi: 10.1016/j.chroma.2008.08.077. [DOI] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Bredfeldt TG, Jagadish B, Eblin KE, Mash EA, Gandolfi AJ. Monomethylarsonous acid induces transformation of human bladder cells. Toxicol. Appl. Pharmacol. 2006;216:69–79. doi: 10.1016/j.taap.2006.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchet JP, Lauwerys R, Roels H. Comparison of the urinary excretion of arsenic metabolites after a single oral dose of sodium arsenite, monomethylarsonate, or dimethylarsinate in man. Int. Arch. Occup. Environ. Health. 1981;48:71–79. doi: 10.1007/BF00405933. [DOI] [PubMed] [Google Scholar]
- Cohen SM, Ohnishi T, Arnold LL, Le XC. Arsenic-induced bladder cancer in an animal model. Toxicol. Appl. Pharmacol. 2007;222:258–263. doi: 10.1016/j.taap.2006.10.010. [DOI] [PubMed] [Google Scholar]
- Crecelius EA. Changes in the chemical speciation of arsenic following ingestion by man. Environ. Health Perspect. 1977;19:147–150. doi: 10.1289/ehp.7719147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dopp E, von Recklinghausen U, Diaz-Bone R, Hirner AV, Rettenmeier AW. Cellular uptake, subcellular distribution and toxicity of arsenic compounds in methylating and non-methylating cells. Environ. Res. 2010;110:435–442. doi: 10.1016/j.envres.2009.08.012. [DOI] [PubMed] [Google Scholar]
- Drobna Z, Naranmandura H, Kubachka KM, Edwards BC, Herbin-Davis K, Styblo M, Le XC, Creed JT, Maeda N, Hughes MF, et al. Disruption of the arsenic (+3 oxidation state) methyltransferase gene in the mouse alters the phenotype for methylation of arsenic and affects distribution and retention of orally administered arsenate. Chem. Res. Toxicol. 2009;22:1713–1720. doi: 10.1021/tx900179r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drobna Z, Walton FS, Paul DS, Xing W, Thomas DJ, Styblo M. Metabolism of arsenic in human liver: the role of membrane transporters. Arch. Toxicol. 2010;84:3–16. doi: 10.1007/s00204-009-0499-7. [DOI] [PubMed] [Google Scholar]
- Drobna Z, Waters SB, Devesa V, Harmon AW, Thomas DJ, Styblo M. Metabolism and toxicity of arsenic in human urothelial cells expressing rat arsenic (+3 oxidation state)-methyltransferase. Toxicol. Appl. Pharmacol. 2005;207:147–159. doi: 10.1016/j.taap.2004.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eeken JC, Klink I, van Veen BL, Pastink A, Ferro W. Induction of epithelial tumors in Drosophila melanogaster heterozygous for the tumor suppressor gene wts. Environ. Mol. Mutagen. 2002;40:277–282. doi: 10.1002/em.10119. [DOI] [PubMed] [Google Scholar]
- Ghosh P, Banerjee M, De Chaudhuri S, Das JK, Sarma N, Basu A, Giri AK. Increased chromosome aberration frequencies in the Bowen's patients compared to non-cancerous skin lesions individuals exposed to arsenic. Mutat. Res. 2007;632:104–110. doi: 10.1016/j.mrgentox.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Hernandez A, Xamena N, Sekaran C, Tokunaga H, Sampayo-Reyes A, Quinteros D, Creus A, Marcos R. High arsenic metabolic efficiency in AS3MT287Thr allele carriers. Pharmacogenet. Genomics. 2008;18:349–355. doi: 10.1097/FPC.0b013e3282f7f46b. [DOI] [PubMed] [Google Scholar]
- Hughes MF, Edwards BC, Herbin-Davis KM, Saunders J, Styblo M, Thomas DJ. Arsenic (+3 oxidation state) methyltransferase genotype affects steady-state distribution and clearance of arsenic in arsenate-treated mice. Toxicol. Appl. Pharmacol. 2010;249:217–223. doi: 10.1016/j.taap.2010.09.017. [DOI] [PubMed] [Google Scholar]
- Justice RW, Zilian O, Woods DF, Noll M, Bryant PJ. The Drosophila tumor suppressor gene warts encodes a homolog of human myotonic dystrophy kinase and is required for the control of cell shape and proliferation. Genes Dev. 1995;9:534–546. doi: 10.1101/gad.9.5.534. [DOI] [PubMed] [Google Scholar]
- Kitchin KT, Wallace K. The role of protein binding of trivalent arsenicals in arsenic carcinogenesis and toxicity. J. Inorg. Biochem. 2008;102:532–539. doi: 10.1016/j.jinorgbio.2007.10.021. [DOI] [PubMed] [Google Scholar]
- Kligerman AD, Doerr CL, Tennant AH, Harrington-Brock K, Allen JW, Winkfield E, Poorman-Allen P, Kundu B, Funasaka K, Roop BC, Mass MJ, et al. Methylated trivalent arsenicals as candidate ultimate genotoxic forms of arsenic: induction of chromosomal mutations but not gene mutations. Environ. Mol. Mutagen. 2003;42:192–205. doi: 10.1002/em.10192. [DOI] [PubMed] [Google Scholar]
- Kumagai Y, Sumi D. Arsenic: signal transduction, transcription factor, and biotransformation involved in cellular response and toxicity. Annu. Rev. Pharmacol. Toxicol. 2007;47:243–262. doi: 10.1146/annurev.pharmtox.47.120505.105144. [DOI] [PubMed] [Google Scholar]
- Le XC, Ma M, Cullen WR, Aposhian HV, Lu X, Zheng B. Determination of monomethylarsonous acid, a key arsenic methylation intermediate, in human urine. Environ. Health Perspect. 2000;108:1015–1018. doi: 10.1289/ehp.001081015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Shi Q, Nix FB, Styblo M, Beck MA, Herbin-Davis KM, Hall LL, Simeonsson JB, Thomas DJ. A novel S-adenosyl-L-methionine:arsenic(III) methyltransferase from rat liver cytosol. J. Biol. Chem. 2002;277:10795–10803. doi: 10.1074/jbc.M110246200. [DOI] [PubMed] [Google Scholar]
- Lindberg AL, Kumar R, Goessler W, Thirumaran R, Gurzau E, Koppova K, Rudnai P, Leonardi G, Fletcher T, Vahter M. Metabolism of low-dose inorganic arsenic in a central European population: influence of sex and genetic polymorphisms. Environ. Health Perspect. 2007;115:1081–1086. doi: 10.1289/ehp.10026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B, Vogel H. Drosophila models of neurodegenerative diseases. Annu. Rev. Pathol. 2009;4:315–342. doi: 10.1146/annurev.pathol.3.121806.151529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mass MJ, Tennant A, Roop BC, Cullen WR, Styblo M, Thomas DJ, Kligerman AD. Methylated trivalent arsenic species are genotoxic. Chem. Res. Toxicol. 2001;14:355–361. doi: 10.1021/tx000251l. [DOI] [PubMed] [Google Scholar]
- Muñiz Ortiz JG, Opoka R, Kane D, Cartwright IL. Investigating arsenic susceptibility from a genetic perspective in Drosophila reveals a key role for glutathione synthetase. Toxicol. Sci. 2009;107:416–426. doi: 10.1093/toxsci/kfn192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng JC, Wang J, Shraim A. A global health problem caused by arsenic from natural sources. Chemosphere. 2003;52:1353–1359. doi: 10.1016/S0045-6535(03)00470-3. [DOI] [PubMed] [Google Scholar]
- Pan D. Hippo signaling in organ size control. Genes Dev. 2007;21:886–897. doi: 10.1101/gad.1536007. [DOI] [PubMed] [Google Scholar]
- Petrick JS, Ayala-Fierro F, Cullen WR, Carter DE, Vasken Aposhian H. Monomethylarsonous acid (MMA(III)) is more toxic than arsenite in Chang human hepatocytes. Toxicol. Appl. Pharmacol. 2000;163:203–207. doi: 10.1006/taap.1999.8872. [DOI] [PubMed] [Google Scholar]
- Petrick JS, Jagadish B, Mash EA, Aposhian HV. Monomethylarsonous acid (MMA(III)) and arsenite: LD(50) in hamsters and in vitro inhibition of pyruvate dehydrogenase. Chem. Res. Toxicol. 2001;14:651–656. doi: 10.1021/tx000264z. [DOI] [PubMed] [Google Scholar]
- Reichard JF, Puga A. Effects of arsenic exposure on DNA methylation and epigenetic gene regulation. Epigenomics. 2010;2:87–104. doi: 10.2217/epi.09.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizki M, Kossatz E, Velazquez A, Creus A, Farina M, Fortaner S, Sabbioni E, Marcos R. Metabolism of arsenic in Drosophila melanogaster and the genotoxicity of dimethylarsinic acid in the Drosophila wing spot test. Environ. Mol. Mutagen. 2006;47:162–168. doi: 10.1002/em.20178. [DOI] [PubMed] [Google Scholar]
- Schwerdtle T, Walter I, Hartwig A. Arsenite and its biomethylated metabolites interfere with the formation and repair of stable BPDE-induced DNA adducts in human cells and impair XPAzf and Fpg. DNA Repair (Amst.) 2003;2:1449–1463. doi: 10.1016/j.dnarep.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Smith AH, Lingas EO, Rahman M. Contamination of drinking-water by arsenic in Bangladesh: a public health emergency. Bull. World Health Organ. 2000;78:1093–1103. [PMC free article] [PubMed] [Google Scholar]
- Steinmaus C, Yuan Y, Kalman D, Rey OA, Skibola CF, Dauphine D, Basu A, Porter KE, Hubbard A, Bates MN, et al. Individual differences in arsenic metabolism and lung cancer in a case-control study in Cordoba, Argentina. Toxicol. Appl. Pharmacol. 2010;247:138–145. doi: 10.1016/j.taap.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapio S, Grosche B. Arsenic in the aetiology of cancer. Mutat. Res. 2006;612:215–246. doi: 10.1016/j.mrrev.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Ter Welle HF, Slater EC. Uncoupling of respiratory-chain phosphorylation by arsenate and evidence for the existence of a stable X-P intermediate of oxidative phosphorylation. Biochim. Biophys. Acta. 1964;89:385–388. doi: 10.1016/0926-6569(64)90238-x. [DOI] [PubMed] [Google Scholar]
- Thomas DJ. Molecular processes in cellular arsenic metabolism. Toxicol. Appl. Pharmacol. 2007;222:365–373. doi: 10.1016/j.taap.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Tokar EJ, Diwan BA, Waalkes MP. Arsenic exposure transforms human epithelial stem/progenitor cells into a cancer stem-like phenotype. Environ. Health Perspect. 2010;118:108–115. doi: 10.1289/ehp.0901059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokar EJ, Diwan BA, Ward JM, Delker DA, Waalkes MP. Carcinogenic effects of “whole-life” exposure to inorganic arsenic in CD1 mice. Toxicol. Sci. 2011;119:73–83. doi: 10.1093/toxsci/kfq315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahter M. Mechanisms of arsenic biotransformation. Toxicology. 2002;181–182:211–217. doi: 10.1016/s0300-483x(02)00285-8. [DOI] [PubMed] [Google Scholar]
- Valenzuela OL, Drobna Z, Hernandez-Castellanos E, Sanchez-Pena LC, Garcia-Vargas GG, Borja-Aburto VH, Styblo M, Del Razo LM. Association of AS3MT polymorphisms and the risk of premalignant arsenic skin lesions. Toxicol. Appl. Pharmacol. 2009;239:200–207. doi: 10.1016/j.taap.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal M, Cagan RL. Drosophila models for cancer research. Curr. Opin. Genet. Dev. 2006;16:10–16. doi: 10.1016/j.gde.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Wang TS, Hsu TY, Chung CH, Wang AS, Bau DT, Jan KY. Arsenite induces oxidative DNA adducts and DNA-protein cross-links in mammalian cells. Free Radic. Biol. Med. 2001;31:321–330. doi: 10.1016/s0891-5849(01)00581-0. [DOI] [PubMed] [Google Scholar]
- Xu T, Wang W, Zhang S, Stewart RA, Yu W. Identifying tumor suppressors in genetic mosaics: the Drosophila lats gene encodes a putative protein kinase. Development. 1995;121:1053–1063. doi: 10.1242/dev.121.4.1053. [DOI] [PubMed] [Google Scholar]
- Yokohira M, Arnold LL, Pennington KL, Suzuki S, Kakiuchi-Kiyota S, Herbin-Davis K, Thomas DJ, Cohen SM. Severe systemic toxicity and urinary bladder cytotoxicity and regenerative hyperplasia induced by arsenite in arsenic (+3 oxidation state) methyltransferase knockout mice. A preliminary report. Toxicol. Appl. Pharmacol. 2010;246:1–7. doi: 10.1016/j.taap.2010.04.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.