Abstract
Positive social interactions are essential for emotional well-being and proper behavioral development of young individuals. Here, we studied the neural underpinnings of social reward by investigating the involvement of opioid neurotransmission in the nucleus accumbens (NAc) in social play behavior, a highly rewarding social interaction in adolescent rats. Intra-NAc infusion of morphine (0.05–0.1 μg) increased pinning and pouncing, characteristic elements of social play behavior in rats, and blockade of NAc opioid receptors with naloxone (0.5 μg) prevented the play-enhancing effects of systemic morphine (1 mg/kg, s.c.) administration. Thus, stimulation of opioid receptors in the NAc was necessary and sufficient for morphine to increase social play. Intra-NAc treatment with the selective μ-opioid receptor agonist [d-Ala2,N-MePhe4,Gly5-ol]enkephalin (DAMGO) (0.1–10 ng) and the μ-opioid receptor antagonist Cys-Tyr-d-Trp-Arg-Thr-Pen-Thr-NH2 (CTAP) (0.3–3 μg) increased and decreased social play, respectively. The δ-opioid receptor agonist DPDPE ([d-Pen2,d-Pen5]-enkephalin) (0.3–3 μg) had no effects, whereas the κ-opioid receptor agonist U69593 (N-methyl-2-phenyl-N-[(5R,7S,8S)-7-(pyrrolidin-1-yl)-1-oxaspiro[4.5]dec-8-yl]acetamide) (0.01–1 μg) decreased social play. Intra-NAc treatment with β-endorphin (0.01–1 μg) increased social play, but met-enkephalin (0.1–5 μg) and the enkephalinase inhibitor thiorphan (0.1–1 μg) were ineffective. DAMGO (0.1–10 ng) increased social play after infusion into both the shell and core subregions of the NAc. Last, intra-NAc infusion of CTAP (3 μg) prevented the development of social play-induced conditioned place preference. These findings identify NAc μ-opioid receptor stimulation as an important neural mechanism for the attribution of positive value to social interactions in adolescent rats. Altered NAc μ-opioid receptor function may underlie social impairments in psychiatric disorders such as autism, schizophrenia, or personality disorders.
Introduction
The experience of positive emotions during interactions with others is an important feature of social relationships. Social reward and attachment are crucial for emotional well-being, and impairments in this domain are an important element of psychiatric disorders, such as autism, schizophrenia, and personality disorders (American Psychiatric Association, 2000). However, the neural mechanisms that mediate the rewarding properties of social interactions are incompletely understood.
To investigate the neural substrates of social reward, we focused on social play behavior in adolescent rats. Social play is the most characteristic and rewarding component of the social repertoire of young mammals, and it serves the development of physical, cognitive, and social capacities (Panksepp et al., 1984; Spinka et al., 2001; Pellis and Pellis, 2009; Trezza et al., 2010). For example, deprivation of social play causes impairments in the ability of rats to deal with challenging social situations (Van den Berg et al., 1999a; Von Frijtag et al., 2002). Comparable with other natural and drug rewards, social play is an incentive for maze learning, lever pressing, and place conditioning in rats and primates, providing empirical support for the notion that social play is rewarding (Falk, 1958; Mason et al., 1963; Humphreys and Einon, 1981; Normansell and Panksepp, 1990; Calcagnetti and Schechter, 1992; Crowder and Hutto, 1992; Ikemoto and Panksepp, 1992; Van den Berg et al., 1999b; Douglas et al., 2004; Thiel et al., 2008, 2009; Trezza et al., 2009; Vanderschuren, 2010). Furthermore, the neurotransmitter systems that mediate the motivational and hedonic properties of food and drug rewards, such as endogenous opioids, endocannabinoids, and dopamine, also modulate social play (Panksepp et al., 1987; Vanderschuren et al., 1997; Siviy, 1998; Trezza et al., 2010). Systemic treatment with opioid receptor agonists, such as morphine, enhances social play in adolescent rats and primates through stimulation of μ-opioid receptors, whereas treatment with opioid receptor antagonists suppresses it (Beatty and Costello, 1982; Panksepp et al., 1985; Siegel et al., 1985; Siegel and Jensen, 1986; Vanderschuren et al., 1995a,b, 1997; Guard et al., 2002; Trezza and Vanderschuren, 2008a,b). Interestingly, a study using a play-rewarded spatial discrimination task (Normansell and Panksepp, 1990) indicated that opioids do not modulate social play through changes in the motivation for play, but perhaps through changes in its positive subjective properties.
The positive subjective properties of natural and drug rewards are mediated by corticolimbic circuits, comprising the dopaminergic, GABAergic, and glutamatergic interconnections between the nucleus accumbens (NAc), ventral tegmental area, frontal cortex, and amygdala (Cardinal et al., 2002; Ikemoto and Wise, 2004; Voorn et al., 2004; Everitt and Robbins, 2005; Berridge and Kringelbach, 2008; Haber and Knutson, 2010). Within this circuit, we hypothesized that opioids exert their stimulating effects on social play in the NAc, for two reasons. First, opioid neurotransmission in the NAc mediates hedonic properties of natural and drug rewards (Kelley, 2004; Berridge and Kringelbach, 2008; Le Merrer et al., 2009). Second, social play is associated with enhanced endogenous opioid activity in the NAc (Vanderschuren et al., 1995c).
Materials and Methods
Subjects
Male Wistar rats (Charles River) arrived in our animal facility at 21 d of age and were housed in groups of four in 40 × 26 × 20 [length (l) × width (w) × height (h)] Macrolon cages under controlled conditions (i.e., temperature, 20–21°C; 60–65% relative humidity; and 12 h light/dark cycle with lights on at 7:00 A.M.). Food and water were available ad libitum.
All animals used were experimentally naive. All experiments were approved by the Animal Ethics Committee of Utrecht University and were conducted in agreement with Dutch laws (Wet op de Dierproeven, 1996) and European regulations (Guideline 86/609/EEC).
Surgery
At 28 d of age, rats were anesthetized with 0.08 ml/100 g (subcutaneous) Hypnorm (fentanylcitrate, 0.315 mg/ml, and fluanison, 10 mg/ml; Janssen) and positioned into a stereotaxic frame (David Kopf Instruments). Guide cannulae, consisting of 24 gauge thin-walled stainless-steel tubing (Cooper's Needleworks), were implanted bilaterally, aimed 1.0 mm above the NAc [border between the shell and core subregions; coordinates: anteroposterior (AP), +1.5 mm from bregma; mediolateral (ML), ±1.9 mm from the midline; dorsoventral (DV), −7.0 mm from skull surface]. Other groups of rats were implanted with bilateral guide cannulae (24 gauge) aimed 1.0 mm above the dorsal striatum (coordinates: AP, +1.5 mm; ML, ±1.9 mm; DV, −5.0 mm), NAc core (coordinates: AP, +1.5 mm; ML, ±1.9 mm; DV, −6.5 mm), and NAc shell (coordinates: AP, +1.8 mm; ML, ±2.8 mm; DV, −7.5 mm, 10° angle). Coordinates for each brain region were determined by pilot placement experiments in 28-d-old rats using the atlas of Paxinos and Watson (2007). Cannulae were secured with stainless-steel screws and dental acrylic; 29 gauge wire stylets (Cooper's Needleworks) were inserted into the guide cannulae to maintain patency. After surgery, rats were individually housed and allowed to recover for 4 d; on the fifth day, they were rehoused in groups of four with their original cage mates. Behavioral testing began 1 week after surgery.
Behavioral testing
Social play behavior.
All the experiments were performed in a sound-attenuated chamber under dim light conditions. The testing arena consisted of a Plexiglas cage measuring 40 × 40 × 60 cm (l × w × h), with ∼2 cm of wood shavings covering the floor. The behaviors of the animals were recorded using a camera with zoom lens, videotape recorder, and television monitor.
One week after surgery, at 35 d of age, the rats were habituated to the experimental procedures on 2 consecutive days. On the first habituation day, rats were individually placed into the test cage for 10 min. On the second habituation day, the animals were socially isolated for 2 h before testing. Pairs of rats were then infused simultaneously with vehicle solutions and placed into the test cage for 15 min, to habituate them to the infusion procedures and determine baseline levels of social play behavior. The animals in a pair did not differ by >10 g in body weight and had no previous common social experience. On the test day, the animals were socially isolated for 2 h before testing. Pairs of rats were then infused simultaneously with either vehicle or drug solutions and placed into the test cage for 15 min.
Behavior was assessed per pair of animals using the Observer 3.0 software (Noldus Information Technology). The following behavioral elements were scored per 15 min (Panksepp et al., 1984; Vanderschuren et al., 1997; Pellis and Pellis, 2009; Trezza et al., 2010): (1) frequency of pinning: one animal lying with its dorsal surface on the floor with the other animal standing over it; this is the most characteristic posture in social play in rats, which occurs when one animal is solicited to play by its test partner and rotates to its dorsal surface to prolong the playful interaction; (2) frequency of pouncing: this is an index of play solicitation (i.e., one animal is soliciting the other to play, by attempting to nose or rub the nape of the neck of the test partner); (3) time spent in social exploration: sniffing any part of the body of the test partner, including the anogenital area.
Locomotor activity.
At 28 d of age, rats were implanted with bilateral guide cannulae aimed 1.0 mm above the NAc and allowed to recover, as described above.
To assess whether opioid effects on social play were secondary to changes in locomotor activity, adolescent rats were tested for horizontal locomotor activity in plastic cages (l × w × h, 50 × 33 × 40 cm) using a videotracking system (EthoVision; Noldus Information Technology), which determined the position of the animal five times per second. Rats were infused with a dose of [d-Ala2,N-MePhe4,Gly5-ol]enkephalin (DAMGO) that maximally increased social play (10 ng/0.3 μl) and then individually transferred from the home cage to the test cage, where locomotor activity was monitored for 60 min.
Social play-induced conditioned place preference.
At 28 d of age, rats were implanted with bilateral guide cannulae aimed 1.0 mm above the NAc and allowed to recover as described above.
The place conditioning setup (TSE System) comprised eight boxes, each consisting of three compartments with removable Plexiglas lids: two equally sized large conditioning compartments (l × w × h, 30 × 25 × 30 cm) separated by a smaller, neutral compartment (l × w × h, 10 × 25 × 30 cm). The two conditioning compartments had different visual and tactile cues: one had black-and-white striped walls and a floor with wide metal mesh, and the other had black walls and a floor with fine metal mesh. The compartment with black walls had a white light (2 W) mounted on the Plexiglas lid, to achieve a comparable light intensity in both conditioning compartments. The middle compartment had white walls, a smooth floor, and a white light (2 W) on the lid. During conditioning, closed dividers between the compartments were used to confine the animals to the conditioning compartment. During habituation and testing, the closed dividers were replaced by dividers that contained an arched gateway (w × h, 9 × 11.5 cm), allowing the rats access to all compartments. During habituation and testing, the position of the animal in the apparatus was monitored by an array of infrared photobeam sensors located 2.5 cm above the floor. A computer recorded the time (in seconds) that the animals spent in each compartment.
Conditioning and testing were performed as described in our previous studies (Trezza et al., 2009). Briefly, 1 week after surgery, each rat was placed into the apparatus and allowed to move freely around the three compartments for 15 min (day 1). The time spent in each compartment was recorded and served to determine baseline side preference for each subject. Rats were socially isolated after this baseline preference session and assigned to a compartment in which they would be allowed social interaction during conditioning, so that the baseline preference in each test group for the (to be) social-paired and (to be) non-social-paired compartments approximated 50% [counterbalanced place conditioning design (Tzschentke, 2007; Veeneman et al., 2011)]. Rats were conditioned during the following 8 d, with two conditioning sessions per day [30 min in one compartment with an initially unfamiliar partner (social session) and 30 min alone in the other compartment (nonsocial session)]. Before every social session, the rats received an infusion with the selective μ-opioid receptor antagonist Cys-Tyr-d-Trp-Arg-Thr-Pen-Thr-NH2 (CTAP) (3 μg/0.3 μl) or vehicle. On day 10, the rats were placed in the middle compartment and allowed to freely move throughout the apparatus for 15 min. Time spent in each compartment was recorded.
Drugs and infusion procedures
The opioid receptor agonist morphine (OPG; 0.05–0.1 μg/0.3 μl), the opioid receptor antagonist naloxone (Tocris Bioscience; 0.5 μg/0.3 μl), the selective μ-opioid receptor agonist DAMGO (Sigma-Aldrich; 0.1–1-10 ng/0.3 μl), the selective δ-opioid receptor agonist [d-Pen2,d-Pen5]-enkephalin (DPDPE) (Sigma-Aldrich; 0.3–1-3 μg/0.3 μl), the selective κ-opioid receptor agonist N-methyl-2-phenyl-N-[(5R,7S,8S)-7-(pyrrolidin-1-yl)-1-oxaspiro[4.5]dec-8-yl]acetamide (U69593) (Sigma-Aldrich; 0.01–0.1–1 μg/0.3 μl), the selective μ-opioid receptor antagonist CTAP (Sigma-Aldrich; 0.3–3 μg/0.3 μl), β-endorphin (Bachem Biosciences; 0.01–0.1–1 μg/0.3 μl), and met-enkephalin (Bachem Biosciences; 0.1–1-5 μg/0.3 μl) were dissolved in saline; the enkephalinase inhibitor thiorphan (Sigma-Aldrich; 0.1–1 μg/0.3 μl) was dissolved in 5% Tween 80/5% polyethylene glycol/saline. All drugs were freshly prepared before each experiment. Drug doses were chosen on the basis of literature data (Cunningham and Kelley, 1992; Meyer et al., 1994; Zhang and Kelley, 1997; Simmons and Self, 2009). Bilateral infusions of drugs or an equivalent volume of the corresponding vehicle were made by using 30 gauge injection needles (Bilaney) connected to 10 μl Hamilton microsyringes by polyethylene (PE-20) tubing. The injection needles protruded 1.0 mm beyond the cannula tips, and a 0.3 μl injection volume per hemisphere was infused over 60 s simultaneously to both sides of the brain using a syringe pump (model 975A; Harvard Apparatus). The injection needles were retained within the cannulae for 60 s after drug infusion to maximize diffusion and to prevent backflow of drug along the cannula track. After all infusions, stylets were replaced, and the animals were left in a holding cage for 5 min before testing.
In the experiment shown in Figure 1, e and f, animals were subcutaneously treated with morphine (OPG; 1 mg/kg) 1 h before intra-NAc infusion of naloxone (Tocris Bioscience; 0.5 μg/0.3 μl).
Figure 1.
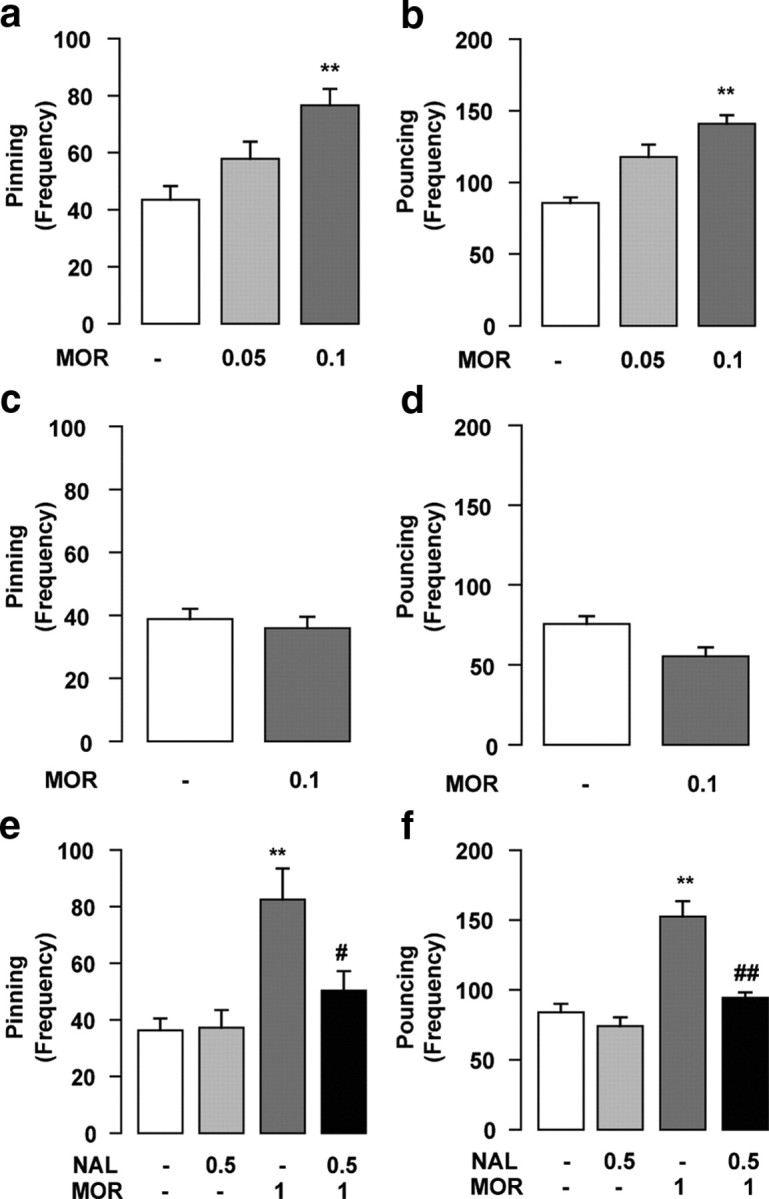
Opioid receptors in the NAc are necessary and sufficient for morphine to increase social play behavior. Intra-NAc infusion of morphine (MOR) (0.05–0.1 μg/0.3 μl) increased pinning (a) and pouncing (b). The effects of morphine on social play were site specific, since infusion of morphine (0.1 μg/0.3 μl) into the dorsal striatum had no effect on pinning (c) or pouncing (d). Intra-NAc infusion of the opioid receptor antagonist naloxone (NAL) (0.5 μg/0.3 μl) completely blocked the increase in pinning (e) and pouncing (f) induced by systemic administration of morphine (1 mg/kg, s.c.). Data represent mean ± SEM frequency of pinning and pouncing. **p < 0.01 versus vehicle/vehicle; #p < 0.05, ##p < 0.01 versus vehicle/morphine (Tukey's post hoc test; n = 6–14 per treatment group).
Histological confirmation of injection sites
Injection sites were verified according to the procedure described by Mahler et al. (2007) and Simmons and Self (2009). After testing, animals were killed by carbon dioxide inhalation and microinjected with 0.3 μl of black ink over 60 s through the guide cannulae. Animals were immediately decapitated, and their brains were removed. Slices (20 μm thick) were collected throughout the forebrain and analyzed under a dissecting microscope for the location of the infusion sites according to the atlas of Paxinos and Watson (2007). Only pairs in which both animals had bilateral needle tracks terminating into the target area and no damage to the target tissues were included in the final analysis.
Statistical analysis
Pinning and pouncing frequencies and time spent in social exploration are expressed as mean ± SEM. To assess the effects of single treatments on social play behavior, data were analyzed using either one-way ANOVA followed by Tukey's post hoc test, or Student's t test. To assess the effects of combined treatments on social play behavior, data were analyzed using two-way ANOVA, followed by Tukey's post hoc test.
Horizontal locomotor activity was expressed as mean ± SEM traveled distance (in centimeters/15 min). The effects of drug treatment on locomotor activity were analyzed with a one-way repeated-measures ANOVA.
Social play-induced conditioned place preference (CPP) data were expressed as mean ± SEM time spent in the social-paired and non-social-paired compartments on the test day, and analyzed by a paired Student t test.
Results
Opioid receptors in the NAc are necessary and sufficient for morphine to increase social play behavior
First, we tested the effects of intra-NAc infusion of morphine (0.05–0.1 μg/0.3 μl; n = 6–14 per treatment group) on social play behavior in adolescent rats. Morphine, infused into the NAc at the dose of 0.1 μg/0.3 μl, increased pinning (F(2,36) = 10.8; p < 0.001) (Fig. 1a) and pouncing (F(2,36) = 32.47; p < 0.001) (Fig. 1b), with no effect on social exploration (F(2,36) = 1.24; NS) (Table 1). The increase in social play induced by intra-NAc morphine infusion was mediated within the NAc rather than by diffusion to adjacent brain areas, since infusion of morphine into the overlying dorsal striatum had no effects on either pinning (t = 0.58; NS) (Fig. 1c) or pouncing (t = 2.11; NS) (Fig. 1d) (n = 6 per treatment group).
Table 1.
Intra-NAc infusion of morphine (0.05–0.1 μg/0.3 μl), DAMGO (0.1–10 ng/0.3 μl), CTAP (0.3–3 μg/0.3 μl), and β-endorphin (0.01–1 μg/0.3 μl) had no effect on social exploration
| Social exploration (s/15 min) | ||
|---|---|---|
| Vehicle | 44 ± 6 | F = 1.24, NS |
| Morphine (0.05 μg/0.3 μl) | 42 ± 7 | |
| Morphine (0.1 μg/0.3 μl) | 55 ± 5 | |
| Vehicle | 34 ± 4 | F = 1.3, NS |
| DAMGO (0.1 ng/0.3 μl) | 28 ± 4 | |
| DAMGO (1 ng/0.3 μl) | 29 ± 5 | |
| DAMGO (10 ng/0.3 μl) | 40 ± 5 | |
| Vehicle | 39 ± 6 | F = 1.24, NS |
| CTAP (0.3 μg/0.3 μl) | 45 ± 10 | |
| CTAP (3 μg/0.3 μl) | 40 ± 5 | |
| Vehicle | 51 ± 8 | F = 0.5, NS |
| β-Endorphin (0.01 μg/0.3 μl) | 42 ± 4 | |
| β-Endorphin (0.1 μg/0.3 μl) | 48 ± 4 | |
| β-Endorphin (1 μg/0.3 μl) | 52 ± 8 |
Data represent mean ± SEM time spent in social exploration. n = 6–14 per treatment group.
Intra-NAc infusion of a dose of the opioid receptor antagonist naloxone (0.5 μg/0.3 μl) that did not affect social play by itself, completely blocked the effects of systemic morphine treatment (1 mg/kg, s.c.) on social play [pinning: F(naloxone)(1,30) = 4.43, NS; F(morphine)(1,30) = 15.88, p < 0.001; F(naloxone × morphine)(1,30) = 4.98, p < 0.05 (Fig. 1e); pouncing: F(naloxone)(1,30) = 23.64, p < 0.001; F(morphine)(1,30) = 39.86, p < 0.001; F(naloxone × morphine)(1,30) = 11.82, p < 0.001 (Fig. 1f); n = 7–10 per treatment group]. Post hoc analysis showed that morphine increased social play in rats that received intra-NAc vehicle, but not in animals that received intra-NAc naloxone. These results show that stimulation of opioid receptors within the NAc is necessary and sufficient for morphine to increase social play behavior.
Opioid effects on social play are mediated through μ-opioid receptors in the NAc
Morphine and naloxone are moderately selective for μ-opioid receptors, but they have considerable affinity for δ- and κ-opioid receptors as well (Goldstein and Naidu, 1989; Mansour et al., 1995). High densities of μ-, δ-, and κ-opioid receptors are found in the NAc (Mansour et al., 1988; Le Merrer et al., 2009). Therefore, we next determined the contribution of NAc μ-, δ-, and κ-opioid receptors in social play, by testing the effects of intra-NAc infusion of selective agonists for μ-, δ-, and κ-opioid receptors.
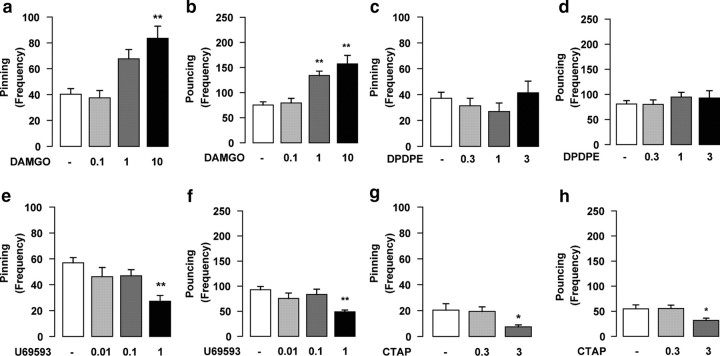
Intra-NAc infusion of the μ-opioid receptor agonist DAMGO (0.1–10 ng/0.3 μl; n = 8–11 per treatment group) increased pinning (F(3,37) = 8.57; p < 0.001) (Fig. 2a) and pouncing (F(3,37) = 11.22; p < 0.001) (Fig. 2b), without affecting social exploration (F(3,37) = 1.3; NS) (Table 1). Intra-NAc infusion of the δ-opioid receptor agonist DPDPE (0.3–3 μg/0.3 μl; n = 8–10 per treatment group) had no effects on social play [pinning: F(3,35) = 0.92, NS (Fig. 2c); pouncing: F(3,35) = 0.56, NS (Fig. 2d)], whereas the κ-opioid receptor agonist U69593 (0.01–1 μg/0.3 μl; n = 6–9 per treatment group) decreased social play behavior [pinning: F(3,25) = 6.68, p < 0.01 (Fig. 2e); pouncing: F(3,25) = 5.81, p < 0.01 (Fig. 2f)]. These data indicate that the stimulatory effects of opioid agonists on social play are exerted through μ-opioid receptors in the NAc.
Figure 2.
Opioid effects on social play are mediated through μ-opioid receptors in the NAc. Intra-NAc infusion of the selective μ-opioid receptor agonist DAMGO (0.1–10 ng/0.3 μl) increased pinning (a) and pouncing (b); the δ-opioid receptor agonist DPDPE (0.3–3 μg/0.3 μl) had no effect (c, d), whereas the κ-opioid receptor agonist U69593 (0.01–1 μg/0.3 μl) decreased pinning (e) and pouncing (f). Intra-NAc infusion of the selective μ-opioid receptor antagonist CTAP (0.3–3 μg/0.3 μl) reduced both pinning (g) and pouncing (h). Data represent mean ± SEM frequency of pinning and pouncing. *p < 0.05, **p < 0.01 versus vehicle (Tukey's post hoc test; n = 7–8 per treatment group).
To determine whether endogenous opioids acting on μ-opioid receptors mediate social play under physiological conditions, we investigated the effects of intra-NAc infusion of the selective μ-opioid receptor antagonist CTAP on social play. Intra-NAc infusion of CTAP (0.3–3 μg/0.3 μl; n = 6–7 per treatment group) reduced both pinning (F(2,19) = 4.37; p < 0.05) (Fig. 2g) and pouncing (F(2,19) = 4.57; p < 0.05) (Fig. 2h), without affecting social exploration (F(2,19) = 1.24; NS) (Table 1). This shows that that stimulation of μ-opioid receptors positively modulates social play under physiological conditions.
β-Endorphin, but not enkephalins, positively modulates social play behavior in the NAc
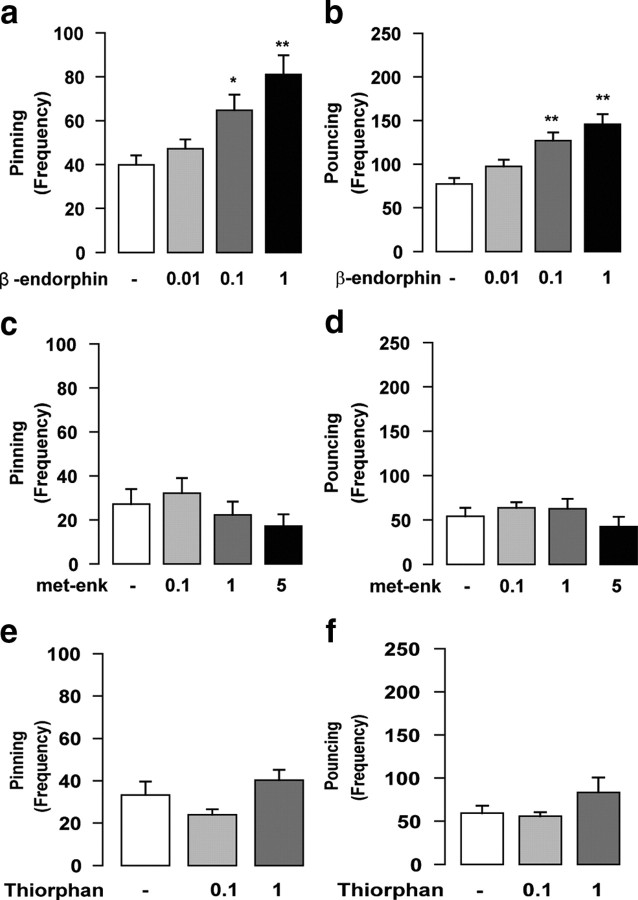
Having shown that stimulation of μ-opioid receptors in the NAc mediates social play behavior, we sought to identify the endogenous opioid ligand involved. We found that intra-NAc infusion of β-endorphin (0.01–1 μg/0.3 μl; n = 10 per treatment group) increased both pinning (F(3,39) = 8.25; p < 0.001) (Fig. 3a) and pouncing (F(3,39) = 11.35; p < 0.001) (Fig. 3b), with no changes in social exploration (F(3,39) = 0.5; NS) (Table 1). In contrast, intra-NAc infusion of met-enkephalin (0.1–5 μg/0.3 μl; n = 6 per treatment group) had no effect on social play [pinning: F(3,19) = 1.00, NS (Fig. 3c); pouncing: F(3,19) = 0.98, NS (Fig. 3d)]. It has been suggested that exogenously administered enkephalins are degraded too rapidly to produce effects in behavioral tests (Simmons and Self, 2009). Therefore, we also tested the effects of intra-NAc infusion of the enkephalinase inhibitor thiorphan, which prolongs and enhances the effects of locally released enkephalins. Thiorphan (0.1–1 μg/0.3 μl; n = 6 per treatment group) did not affect social play [pinning: F(2,11) = 2.78, NS (Fig. 3e); pouncing: F(2,11) = 1.70, NS (Fig. 3f)].
Figure 3.
In the NAc, β-endorphin but not enkephalins positively modulates social play behavior. Intra-NAc infusion of the endogenous opioid peptide β-endorphin (0.01–1 μg/0.3 μl) increased pinning (a) and pouncing (b), whereas both met-enkephalin (met-enk) (0.1–5 μg/0.3 μl) (c, d) and the enkephalinase inhibitor thiorphan (0.1–1 μg/0.3 μl) (e, f) had no effect. Data represent mean ± SEM frequency of pinning and pouncing. *p < 0.05, **p < 0.01 versus vehicle (Tukey's post hoc test; n = 6–8 per treatment group).
Collectively, these results suggest that under physiological circumstances, β-endorphin acts on μ-opioid receptors in the NAc to positively modulate social play behavior.
μ-Opioid receptors in both the NAc core and shell positively modulate social play behavior
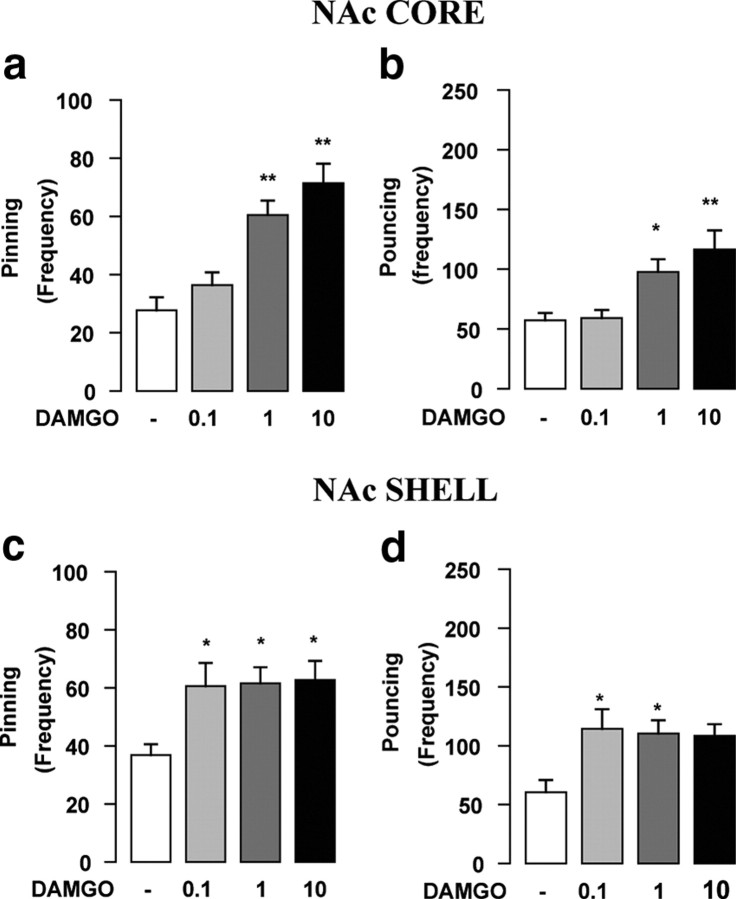
The NAc can be divided into two main subregions (i.e., core and shell) that differ in their anatomical architecture and functional characteristics (Zahm and Brog, 1992; Cardinal et al., 2002; Kelley, 2004; Voorn et al., 2004). In the previous experiments, it was not possible to differentiate between these two subregions, because cannula placements were located at the border between the core and the shell. To test whether the core and shell subregions of the NAc are differentially involved in opioid modulation of social reward, we implanted additional groups of rats with bilateral guide cannulae targeted at either the NAc core or shell, and injected the μ-opioid receptor agonist DAMGO (0.1–10 ng/0.3 μl) into these NAc subregions. DAMGO increased social play when injected both into the NAc core [pinning: F(3,23) = 15.16, p < 0.001 (Fig. 4a); pouncing: F(3,23) = 7.71, p = 0.001 (Fig. 4b); n = 6–7 per treatment group] and shell [pinning: F(3,38) = 3.91, p < 0.05 (Fig. 4c); pouncing: F(3,38) = 3.98, p < 0.05 (Fig. 4d); n = 9–11 per treatment group]. The lowest dose of DAMGO significantly increased social play only after infusion into the NAc shell, but the maximum effect of DAMGO was comparable in both regions. These data show that the entire NAc is involved in the opioid modulation of social reward, the shell being somewhat more sensitive.
Figure 4.
μ-Opioid receptors in both the NAc core and shell positively modulate social play behavior. The selective μ-opioid receptor agonist DAMGO (0.1–10 ng/0.3 μl) increased pinning (a, c) and pouncing (b, d) when infused both into the NAc core (a, b) and shell (c, d). Data represent mean ± SEM frequency of pinning and pouncing. *p < 0.05, *p < 0.01 versus vehicle (Tukey's post hoc test; n = 6–11 per treatment group).
Opioid effects on social play are not secondary to changes in locomotor activity
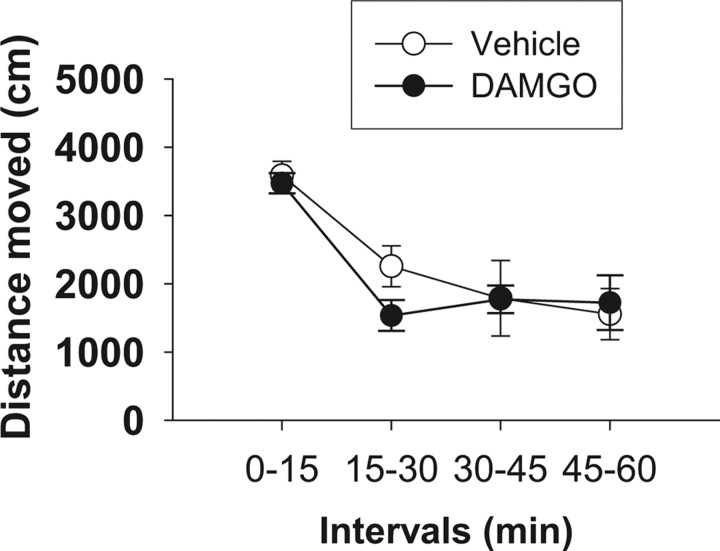
Intra-NAc infusion of a dose of DAMGO that maximally increased social play behavior (10 ng/0.3 μl) did not affect locomotor activity in adolescent rats (F(treatment)(1,14) = 0.21, NS; F(intervals)(1,14) = 30.84, p < 0.001; F(treatment × interval)(1,14) = 1.45, NS) (Fig. 5) (n = 6–7 per treatment group) at any of the time points tested, indicating that the effects of DAMGO on social play were not secondary to changes in locomotor activity.
Figure 5.
Opioid effects on social play are not secondary to changes in locomotor activity. Intra-NAc infusion of a dose of DAMGO that maximally increased social play behavior (10 ng/0.3 μl) did not affect locomotor activity in adolescent rats. Data represent mean ± SEM traveled distance (in centimeters/15 min intervals; n = 6–7 per treatment group).
μ-Opioid receptors in the NAc mediate social play-induced CPP
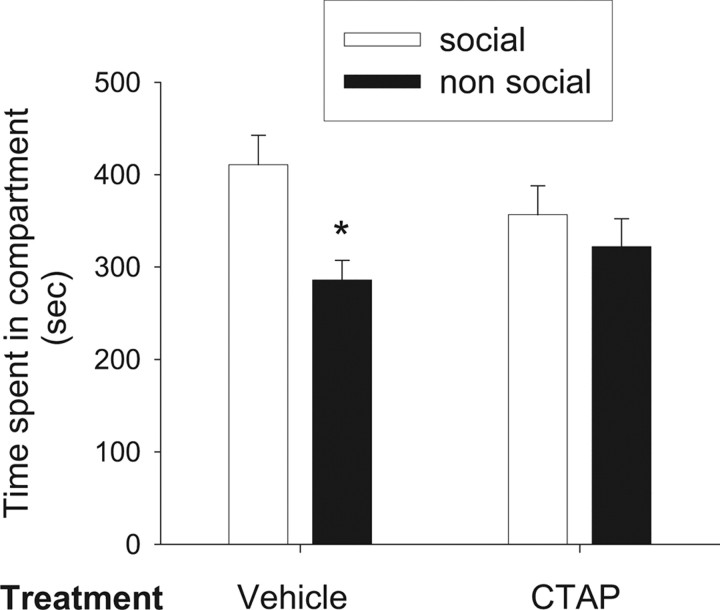
Social play behavior is known to induce CPP (Calcagnetti and Schechter, 1992; Crowder and Hutto, 1992; Van den Berg et al., 1999b; Douglas et al., 2004; Thiel et al., 2008, 2009; Trezza et al., 2009). Intra-NAc infusion of CTAP prevented the development of social play-induced CPP in adolescent rats. Animals infused with vehicle (n = 7) showed a significant preference for the compartment previously paired with social experience (t = 2.43; df = 6; p < 0.05), whereas animals infused with CTAP (3 μg/0.3 μl; n = 6) before conditioning spent an equal amount of time in the social- and non-social-paired compartments on the test day (t = 0.58; df = 5; NS) (Fig. 6). These results indicate that μ-opioid receptors in the NAc mediate the rewarding properties of social interaction in adolescent rats. For histological assessment of representative experiments, see Figure 7.
Figure 6.
μ-Opioid receptors in the NAc mediate social play-induced CPP. Intra-NAc infusion of CTAP prevented the development of social play-induced CPP in adolescent rats: animals infused with vehicle showed a significant preference for the compartment previously paired with social experience, whereas animals infused with CTAP (3 μg/0.3 μl) spent an equal amount of time in both the social- and non-social-paired compartments on the test day. Data represent mean ± SEM time spent in the social-paired (social, white bar) and non-social-paired (nonsocial, black bar) compartments on the test day. *p < 0.05 for difference in time spent in the social- and non-social-paired compartments (paired Student's t test; n = 6–7 per treatment group).
Figure 7.
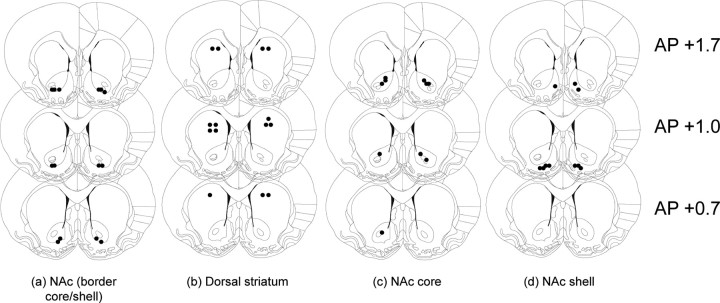
Diagrams of rat brain sections showing representative microinjection sites (filled circles) at the border between core and shell subregions of the NAc (a), dorsal striatum (b), NAc core (c), and NAc shell (d). Distances are in millimeters from bregma (adapted from Paxinos and Watson, 2007). Only data from pairs in which both animals had bilateral needle tracks terminating into the target area and no damage to the target tissues were included in the final analyses.
Discussion
It has been hypothesized that the endogenous opioid system is a critical component of the neural circuit that mediates the positive affective states associated with social interactions (Panksepp et al., 1980; Depue and Morrone-Strupinsky, 2005). Opioids mediate infant attachment behavior and the rewarding properties of mother-related stimuli, peer–peer interactions in young individuals, and adult sexual and social behaviors (Panksepp et al., 1980, 1987; van Furth et al., 1995; Moles et al., 2004; Depue and Morrone-Strupinsky, 2005; Trezza et al., 2010; Vanderschuren, 2010). The present study identifies the NAc as an important site of action for opioids to modulate social reward in adolescent rats.
NAc opioid receptor-mediated effects on social play are behaviorally and anatomically specific
Previous studies using systemic drug injections have shown that opioid effects on social play are behaviorally specific (i.e., not secondary to changes in locomotion or social interest) (Vanderschuren et al., 1995a; Trezza and Vanderschuren, 2008a,b). Consistent with this, we found that infusion of morphine, DAMGO, and β-endorphin into the NAc enhanced social play but not social exploratory behavior, indicating that NAc opioid neurotransmission specifically modulates playful aspects of social interaction in adolescent rats, rather than social behavior in general. Furthermore, intra-NAc infusion of a dose of DAMGO that maximally increased social play did not alter locomotor activity. The importance of the NAc for the opioid modulation of social play was further supported by our finding that blockade of NAc opioid receptors by naloxone antagonized the play-enhancing effects of systemic morphine administration. Together, these data demonstrate that stimulation of NAc opioid receptors is necessary and sufficient for morphine to increase social play.
The NAc comprises two main subregions, core and shell, that have distinct anatomical and functional properties (Zahm and Brog, 1992; Cardinal et al., 2002; Kelley, 2004; Voorn et al., 2004). Differences between the NAc core and shell in mediating the rewarding properties of food and drugs have been reported (Ikemoto and Wise, 2004; Kelley, 2004; Everitt and Robbins, 2005; Berridge and Kringelbach, 2008) whereby some studies show that μ-opioid neurotransmission enhances the hedonic impact of palatable food selectively in the NAc shell (Peciña and Berridge 2000) and others that the rewarding properties of food are enhanced by activation of μ-opioid receptors in the entire NAc (Zhang and Kelley, 2000). We found that the μ-opioid receptor agonist DAMGO significantly increased social play when injected into both the NAc core and shell. These results show that the NAc is a relatively homogeneous structure with respect to opioid modulation of social reward. The maximal effect of DAMGO was comparable in both regions, but the lowest dose of DAMGO increased social play after infusion into the shell but not the core. Thus, the NAc shell might be a somewhat more sensitive site for opioid modulation of social play. However, our finding that morphine did not increase social play after infusion into a striatal region dorsal to the NAc indicates that the augmenting effects of μ-opioid receptor stimulation on social play behavior are restricted to the NAc.
NAc opioid receptor-mediated effects on social play occur through μ-opioid receptors and involve β-endorphin
The NAc expresses high levels of μ-, δ-, and κ-opioid receptors (Mansour et al., 1988; Le Merrer et al., 2009) and μ-opioid receptors in the NAc are thought to mediate the hedonic properties of biologically relevant stimuli (van Ree et al., 1999; Kelley, 2004; Berridge and Kringelbach, 2008; Le Merrer et al., 2009). Systemic treatment with selective μ- and κ-opioid receptor agonists has previously been shown to increase and decrease social play, respectively, whereas δ-opioid receptor stimulation had no effects (Vanderschuren et al., 1995b). Consistent with this, we found that intra-NAc infusion of the selective μ-opioid receptor agonist DAMGO increased social play, the δ-opioid receptor DPDPE had no effect, whereas the κ-opioid receptor agonist U69593 decreased social play. It was also previously shown that systemic treatment with the selective μ-opioid receptor antagonist β-funaltrexamine decreased social play, whereas systemic administration of the δ-opioid receptor antagonist naltrindole or the κ-opioid receptor antagonist nor-binaltorphimine was ineffective (Vanderschuren et al., 1995b). This suggests that, under physiological conditions, μ- but not κ-opioid receptors are involved in the regulation of social play, and that the reduction in social play induced by systemic (Vanderschuren et al., 1995b) and intra-NAc administration of κ-opioid receptor agonists is related to the well known dysphoric effects of κ-opioid receptor agonists (Pfeiffer et al., 1986; van Ree et al., 1999; Le Merrer et al., 2009). Indeed, intra-NAc infusion of the selective μ-opioid receptor antagonist CTAP dose-dependently decreased social play behavior, without affecting social exploration. These results are consistent with previous findings of increased NAc opioid activity during social play (Vanderschuren et al., 1995c).
Met-enkephalin and β-endorphin are endogenous opioids with a high affinity for μ-opioid receptors (Mansour et al., 1995), and there are high densities of enkephalin- and β-endorphin-containing fibers in the NAc (Mansour et al., 1988; Olive et al., 2001; Roth-Deri et al., 2003; Le Merrer et al., 2009). Previous studies have shown that β-endorphin activity in the NAc may contribute to the positive reinforcing and motivational properties of ethanol, amphetamine, and cocaine (Olive et al., 2001; Roth-Deri et al., 2003; Simmons and Self, 2009). We found that intra-NAc infusion of the endogenous opioid peptide β-endorphin increased social play behavior but that infusion of met-enkephalin or the enkephalinase inhibitor thiorphan did not alter social play. Together with previous findings (Vanderschuren et al., 1995b,c), these results suggest that, during social play, β-endorphin is released in the NAc, where it binds to μ-opioid receptors to positively modulate this behavior.
NAc μ-opioid receptors mediate the rewarding properties of social play
The rewarding properties of social play behavior in rats have been demonstrated using T-maze discrimination tasks (Humphreys and Einon, 1981; Normansell and Panksepp, 1990; Ikemoto and Panksepp, 1992) and place conditioning paradigms (Calcagnetti and Schechter, 1992; Crowder and Hutto, 1992; Van den Berg et al., 1999b; Douglas et al., 2004; Thiel et al., 2008, 2009; Trezza et al., 2009). A previous study has shown that neither morphine nor naloxone affected the acquisition of social play-rewarded spatial discrimination but that morphine increased and naloxone decreased the amount of social play performed in the goal box of the T-maze (Normansell and Panksepp, 1990). This indicates that opioids do not modulate the motivational properties of play but may rather mediate its rewarding aspects. Indeed, our observation that intra-NAc infusion of the selective μ-opioid receptor antagonist CTAP prevented the development of social play-induced CPP provides evidence for the notion that μ-opioid receptor stimulation in the NAc mediates the rewarding properties of social interactions in adolescent rats.
Concluding remarks
Collectively, our results show that opioid neurotransmission in the NAc, a brain region widely implicated in reward and motivation (Cardinal et al., 2002; Ikemoto and Wise, 2004; Kelley, 2004; Everitt and Robbins, 2005; Berridge and Kringelbach, 2008; Haber and Knutson, 2010), mediates the positive subjective properties of social play behavior in adolescent rats. These findings are consistent with a larger body of literature implicating μ-opioid receptor stimulation in the positive properties of other social behaviors, such as the perception of mother-related stimuli and attachment behavior in infant chicks, mice, rats, and primates (Panksepp et al., 1978; Kalin et al., 1988; Carden et al., 1991; Moles et al., 2004; Barr et al., 2008), as well as in the regulation of sexual behavior (van Furth et al., 1995; Tian et al., 1997; Balfour et al., 2004; Coolen et al., 2004). In addition, enhanced nucleus accumbens activity has been found during positive social interactions in humans, such as simulated social interaction with friends in young adults (Güroğlu et al., 2008), during social cooperation (Rilling et al., 2002), and during eye contact with attractive faces (Kampe et al., 2001).
Dysfunction of NAc μ-opioid receptor activity may be an important etiological factor in psychiatric diseases characterized by social indifference and by the inability to form normal social bonds, such as autism, schizophrenia, and personality disorders. In support of this notion, aberrant μ-opioid receptor function in brain regions involved in emotion and stress processing, decision making, pain, and neuroendocrine control has recently been shown in patients with borderline personality disorder (Prossin et al., 2010). Thus, additional research into the role of the corticolimbic μ-opioid system in normal and abnormal social behavior may provide essential information to understand and treat social dysfunctions.
Footnotes
This work was supported by National Institute on Drug Abuse Grant R01 DA022628-01 (L.J.M.J.V.). This research was performed within the framework of Project T5-107 of the Dutch Top Institute Pharma.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. Ed 4. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- Balfour ME, Yu L, Coolen LM. Sexual behavior and sex-associated environmental cues activate the mesolimbic system in male rats. Neuropsychopharmacology. 2004;29:718–730. doi: 10.1038/sj.npp.1300350. [DOI] [PubMed] [Google Scholar]
- Barr CS, Schwandt ML, Lindell SG, Higley JD, Maestripieri D, Goldman D, Suomi SJ, Heilig M. Variation at the mu-opioid receptor gene (OPRM1) influences attachment behavior in infant primates. Proc Natl Acad Sci U S A. 2008;105:5277–5281. doi: 10.1073/pnas.0710225105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty WW, Costello KB. Naloxone and play fighting in juvenile rats. Pharmacol Biochem Behav. 1982;17:905–907. doi: 10.1016/0091-3057(82)90470-1. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Kringelbach ML. Affective neuroscience of pleasure: reward in humans and animals. Psychopharmacology (Berl) 2008;199:457–480. doi: 10.1007/s00213-008-1099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcagnetti DJ, Schechter MD. Place conditioning reveals the rewarding aspect of social interaction in juvenile rats. Physiol Behav. 1992;51:667–672. doi: 10.1016/0031-9384(92)90101-7. [DOI] [PubMed] [Google Scholar]
- Carden SE, Barr GA, Hofer MA. Differential effects of specific opioid receptor agonists on rat pup isolation calls. Brain Res Dev Brain Res. 1991;62:17–22. doi: 10.1016/0165-3806(91)90185-l. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002;26:321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Coolen LM, Fitzgerald ME, Yu L, Lehman MN. Activation of mu opioid receptors in the medial preoptic area following copulation in male rats. Neuroscience. 2004;124:11–21. doi: 10.1016/j.neuroscience.2003.10.045. [DOI] [PubMed] [Google Scholar]
- Crowder WF, Hutto CW., Jr Operant place conditioning measures examined using two nondrug reinforcers. Pharmacol Biochem Behav. 1992;41:817–824. doi: 10.1016/0091-3057(92)90233-6. [DOI] [PubMed] [Google Scholar]
- Cunningham ST, Kelley AE. Opiate infusion into nucleus accumbens: contrasting effects on motor activity and responding for conditioned reward. Brain Res. 1992;588:104–114. doi: 10.1016/0006-8993(92)91349-j. [DOI] [PubMed] [Google Scholar]
- Depue RA, Morrone-Strupinsky JV. A neurobehavioral model of affiliative bonding: implications for conceptualizing a human trait of affiliation. Behav Brain Sci. 2005;28:313–350. doi: 10.1017/S0140525X05000063. discussion 350–395. [DOI] [PubMed] [Google Scholar]
- Douglas LA, Varlinskaya EI, Spear LP. Rewarding properties of social interactions in adolescent and adult male and female rats: impact of social versus isolate housing of subjects and partners. Dev Psychobiol. 2004;45:153–162. doi: 10.1002/dev.20025. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Falk JL. The grooming behavior of the chimpanzee as a reinforcer. J Exp Anal Behav. 1958;1:83–85. doi: 10.1901/jeab.1958.1-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein A, Naidu A. Multiple opioid receptors: ligand selectivity profiles and binding site signatures. Mol Pharmacol. 1989;36:265–272. [PubMed] [Google Scholar]
- Guard HJ, Newman JD, Roberts RL. Morphine administration selectively facilitates social play in common marmosets. Dev Psychobiol. 2002;41:37–49. doi: 10.1002/dev.10043. [DOI] [PubMed] [Google Scholar]
- Güroğlu B, Haselager GJ, van Lieshout CF, Takashima A, Rijpkema M, Fernández G. Why are friends special? Implementing a social interaction simulation task to probe the neural correlates of friendship. Neuroimage. 2008;39:903–910. doi: 10.1016/j.neuroimage.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys AP, Einon DF. Play as a reinforcer for maze-learning in juvenile rats. Anim Behav. 1981;29:259–270. [Google Scholar]
- Ikemoto S, Panksepp J. The effects of early social isolation on the motivation for social play in juvenile rats. Dev Psychobiol. 1992;25:261–274. doi: 10.1002/dev.420250404. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Wise RA. Mapping of chemical trigger zones for reward. Neuropharmacology. 2004;47(Suppl 1):190–201. doi: 10.1016/j.neuropharm.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Barksdale CM. Opiate modulation of separation-induced distress in non-human primates. Brain Res. 1988;440:285–292. doi: 10.1016/0006-8993(88)90997-3. [DOI] [PubMed] [Google Scholar]
- Kampe KK, Frith CD, Dolan RJ, Frith U. Reward value of attractiveness and gaze. Nature. 2001;413:589. doi: 10.1038/35098149. [DOI] [PubMed] [Google Scholar]
- Kelley AE. Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning. Neurosci Biobehav Rev. 2004;27:765–776. doi: 10.1016/j.neubiorev.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Le Merrer J, Becker JA, Befort K, Kieffer BL. Reward processing by the opioid system in the brain. Physiol Rev. 2009;89:1379–1412. doi: 10.1152/physrev.00005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Smith KS, Berridge KC. Endocannabinoid hedonic hotspot for sensory pleasure: anandamide in nucleus accumbens shell enhances “liking” of a sweet reward. Neuropsychopharmacology. 2007;32:2267–2278. doi: 10.1038/sj.npp.1301376. [DOI] [PubMed] [Google Scholar]
- Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ. Anatomy of CNS opioid receptors. Trends Neurosci. 1988;11:308–314. doi: 10.1016/0166-2236(88)90093-8. [DOI] [PubMed] [Google Scholar]
- Mansour A, Hoversten MT, Taylor LP, Watson SJ, Akil H. The cloned mu, delta and kappa receptors and their endogenous ligands: evidence for two opioid peptide recognition cores. Brain Res. 1995;700:89–98. doi: 10.1016/0006-8993(95)00928-j. [DOI] [PubMed] [Google Scholar]
- Mason WM, Saxon SV, Sharpe LG. Preferential responses of young chimpanzees to food and social rewards. Psychol Rec. 1963;13:341–345. [Google Scholar]
- Meyer ME, McLaurin BI, Allen M, Meyer ME. Biphasic effects of intraaccumbens mu-opioid peptide agonist DAMGO on locomotor activities. Pharmacol Biochem Behav. 1994;47:827–831. doi: 10.1016/0091-3057(94)90283-6. [DOI] [PubMed] [Google Scholar]
- Moles A, Kieffer BL, D'Amato FR. Deficit in attachment behavior in mice lacking the mu-opioid receptor gene. Science. 2004;304:1983–1986. doi: 10.1126/science.1095943. [DOI] [PubMed] [Google Scholar]
- Normansell L, Panksepp J. Effects of morphine and naloxone on play-rewarded spatial discrimination in juvenile rats. Dev Psychobiol. 1990;23:75–83. doi: 10.1002/dev.420230108. [DOI] [PubMed] [Google Scholar]
- Olive MF, Koenig HN, Nannini MA, Hodge CW. Stimulation of endorphin neurotransmission in the nucleus accumbens by ethanol, cocaine, and amphetamine. J Neurosci. 2001;21:RC184. doi: 10.1523/JNEUROSCI.21-23-j0002.2001. (1–5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp J, Vilberg T, Bean NJ, Coy DH, Kastin AJ. Reduction of distress vocalization in chicks by opiate-like peptides. Brain Res Bull. 1978;3:663–667. doi: 10.1016/0361-9230(78)90014-x. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Herman BH, Vilberg T, Bishop P, DeEskinazi FG. Endogenous opioids and social behavior. Neurosci Biobehav Rev. 1980;4:473–487. doi: 10.1016/0149-7634(80)90036-6. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Siviy S, Normansell L. The psychobiology of play: theoretical and methodological perspectives. Neurosci Biobehav Rev. 1984;8:465–492. doi: 10.1016/0149-7634(84)90005-8. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Jalowiec J, DeEskinazi FG, Bishop P. Opiates and play dominance in juvenile rats. Behav Neurosci. 1985;99:441–453. doi: 10.1037//0735-7044.99.3.441. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Normansell L, Cox JF, Crepeau LJ, Sacks DS. Psychopharmacology of social play. In: Olivier B, Mos J, Brain BF, editors. Ethopharmacology of agonistic behaviour in animals and humans. Dordrecht, The Netherlands: Martinus Nijhoff; 1987. pp. 132–144. [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates, Ed 6. San Diego: Elsevier Academic; 2007. [Google Scholar]
- Peciña S, Berridge KC. Opioid site in nucleus accumbens shell mediates eating and hedonic “liking” for food: map based on microinjection Fos plumes. Brain Res. 2000;863:71–86. doi: 10.1016/s0006-8993(00)02102-8. [DOI] [PubMed] [Google Scholar]
- Pellis SM, Pellis V. The playful brain: venturing to the limits of neuroscience. Oxford, UK: Oneworld Publications; 2009. [Google Scholar]
- Pfeiffer A, Brantl V, Herz A, Emrich HM. Psychotomimesis mediated by kappa opiate receptors. Science. 1986;233:774–776. doi: 10.1126/science.3016896. [DOI] [PubMed] [Google Scholar]
- Prossin AR, Love TM, Koeppe RA, Zubieta JK, Silk KR. Dysregulation of regional endogenous opioid function in borderline personality disorder. Am J Psychiatry. 2010;167:925–933. doi: 10.1176/appi.ajp.2010.09091348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling J, Gutman D, Zeh T, Pagnoni G, Berns G, Kilts C. A neural basis for social cooperation. Neuron. 2002;35:395–405. doi: 10.1016/s0896-6273(02)00755-9. [DOI] [PubMed] [Google Scholar]
- Roth-Deri I, Zangen A, Aleli M, Goelman RG, Pelled G, Nakash R, Gispan-Herman I, Green T, Shaham Y, Yadid G. Effect of experimenter-delivered and self-administered cocaine on extracellular beta-endorphin levels in the nucleus accumbens. J Neurochem. 2003;84:930–938. doi: 10.1046/j.1471-4159.2003.01584.x. [DOI] [PubMed] [Google Scholar]
- Siegel MA, Jensen RA. The effects of naloxone and cage size on social play and activity in isolated young rats. Behav Neural Biol. 1986;45:155–168. doi: 10.1016/s0163-1047(86)90739-9. [DOI] [PubMed] [Google Scholar]
- Siegel MA, Jensen RA, Panksepp J. The prolonged effects of naloxone on play behavior and feeding in the rat. Behav Neural Biol. 1985;44:509–514. doi: 10.1016/s0163-1047(85)91024-6. [DOI] [PubMed] [Google Scholar]
- Simmons D, Self DW. Role of mu- and delta-opioid receptors in the nucleus accumbens in cocaine-seeking behavior. Neuropsychopharmacology. 2009;34:1946–1957. doi: 10.1038/npp.2009.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siviy SM. Neurobiological substrates of play behavior: glimpses into the structure and function of mammalian playfulness. In: Bekoff M, Byers JA, editors. Animal play: evolutionary, comparative, and ecological perspectives. Cambridge, UK: Cambridge UP; 1998. pp. 221–242. [Google Scholar]
- Spinka M, Newberry RC, Bekoff M. Mammalian play: training for the unexpected. Q Rev Biol. 2001;76:141–168. doi: 10.1086/393866. [DOI] [PubMed] [Google Scholar]
- Thiel KJ, Okun AC, Neisewander JL. Social reward-conditioned place preference: a model revealing an interaction between cocaine and social context rewards in rats. Drug Alcohol Depend. 2008;96:202–212. doi: 10.1016/j.drugalcdep.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel KJ, Sanabria F, Neisewander JL. Synergistic interaction between nicotine and social rewards in adolescent male rats. Psychopharmacology (Berl) 2009;204:391–402. doi: 10.1007/s00213-009-1470-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian M, Broxmeyer HE, Fan Y, Lai Z, Zhang S, Aronica S, Cooper S, Bigsby RM, Steinmetz R, Engle SJ, Mestek A, Pollock JD, Lehman MN, Jansen HT, Ying M, Stambrook PJ, Tischfield JA, Yu L. Altered hematopoiesis, behavior, and sexual function in mu opioid receptor-deficient mice. J Exp Med. 1997;185:1517–1522. doi: 10.1084/jem.185.8.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trezza V, Vanderschuren LJMJ. Bidirectional cannabinoid modulation of social behavior in adolescent rats. Psychopharmacology (Berl) 2008a;197:217–227. doi: 10.1007/s00213-007-1025-3. [DOI] [PubMed] [Google Scholar]
- Trezza V, Vanderschuren LJMJ. Cannabinoid and opioid modulation of social play behavior in adolescent rats: differential behavioral mechanisms. Eur Neuropsychopharmacol. 2008b;18:519–530. doi: 10.1016/j.euroneuro.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trezza V, Damsteegt R, Vanderschuren LJMJ. Conditioned place preference induced by social play behavior: parametrics, extinction, reinstatement and disruption by methylphenidate. Eur Neuropsychopharmacol. 2009;19:659–669. doi: 10.1016/j.euroneuro.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trezza V, Baarendse PJJ, Vanderschuren LJMJ. The pleasures of play: pharmacological insights into social reward mechanisms. Trends Pharmacol Sci. 2010;31:463–469. doi: 10.1016/j.tips.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol. 2007;12:227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- Van den Berg CL, Hol T, Van Ree JM, Spruijt BM, Everts H, Koolhaas JM. Play is indispensable for an adequate development of coping with social challenges in the rat. Dev Psychobiol. 1999a;34:129–138. [PubMed] [Google Scholar]
- Van den Berg CL, Pijlman FT, Koning HA, Diergaarde L, Van Ree JM, Spruijt BM. Isolation changes the incentive value of sucrose and social behaviour in juvenile and adult rats. Behav Brain Res. 1999b;106:133–142. doi: 10.1016/s0166-4328(99)00099-6. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJMJ. How the brain makes play fun. Am J Play. 2010;2:315–337. [Google Scholar]
- Vanderschuren LJMJ, Niesink RJM, Spruijt BM, Van Ree JM. Effects of morphine on different aspects of social play in juvenile rats. Psychopharmacology (Berl) 1995a;117:225–231. doi: 10.1007/BF02245191. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJMJ, Niesink RJM, Spruijt BM, Van Ree JM. Mu- and kappa-opioid receptor-mediated opioid effects on social play in juvenile rats. Eur J Pharmacol. 1995b;276:257–266. doi: 10.1016/0014-2999(95)00040-r. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJMJ, Stein EA, Wiegant VM, Van Ree JM. Social play alters regional brain opioid receptor binding in juvenile rats. Brain Res. 1995c;680:148–156. doi: 10.1016/0006-8993(95)00256-p. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJMJ, Niesink RJM, Van Ree JM. The neurobiology of social play behavior in rats. Neurosci Biobehav Rev. 1997;21:309–326. doi: 10.1016/s0149-7634(96)00020-6. [DOI] [PubMed] [Google Scholar]
- van Furth WR, Wolterink G, van Ree JM. Regulation of masculine sexual behavior: involvement of brain opioids and dopamine. Brain Res Brain Res Rev. 1995;21:162–184. doi: 10.1016/0165-0173(96)82985-7. [DOI] [PubMed] [Google Scholar]
- van Ree JM, Gerrits MAFM, Vanderschuren LJMJ. Opioids, reward and addiction: an encounter of biology, psychology, and medicine. Pharmacol Rev. 1999;51:341–396. [PubMed] [Google Scholar]
- Veeneman MMJ, Boleij H, Broekhoven MH, Snoeren EMS, Guitart Masip M, Cousijn J, Vanderschuren LJMJ. Dissociable roles of mGlu5 and dopamine receptors in the rewarding and sensitizing properties of morphine and cocaine. Psychopharmacology (Berl) 2011;214:863–876. doi: 10.1007/s00213-010-2095-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Frijtag JC, Schot M, van den Bos R, Spruijt BM. Individual housing during the play period results in changed responses to and consequences of a psychosocial stress situation in rats. Dev Psychobiol. 2002;41:58–69. doi: 10.1002/dev.10057. [DOI] [PubMed] [Google Scholar]
- Voorn P, Vanderschuren LJMJ, Groenewegen HJ, Robbins TW, Pennartz CMA. Putting a spin on the dorsal-ventral divide of the striatum. Trends Neurosci. 2004;27:468–474. doi: 10.1016/j.tins.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Zahm DS, Brog JS. On the significance of subterritories in the “accumbens” part of the rat ventral striatum. Neuroscience. 1992;50:751–767. doi: 10.1016/0306-4522(92)90202-d. [DOI] [PubMed] [Google Scholar]
- Zhang M, Kelley AE. Opiate agonists microinjected into the nucleus accumbens enhance sucrose drinking in rats. Psychopharmacology (Berl) 1997;132:350–360. doi: 10.1007/s002130050355. [DOI] [PubMed] [Google Scholar]
- Zhang M, Kelley AE. Enhanced intake of high-fat food following striatal mu-opioid stimulation: microinjection mapping and fos expression. Neuroscience. 2000;99:267–277. doi: 10.1016/s0306-4522(00)00198-6. [DOI] [PubMed] [Google Scholar]