Abstract
Prenatal alcohol exposure has numerous effects on the developing brain, including damage to selective brain structure. We review structural magnetic resonance imaging (MRI) studies of brain abnormalities in subjects prenatally exposed to alcohol. The most common findings include reduced brain volume and malformations of the corpus callosum. Advanced methods have been able to detect shape, thickness and displacement changes throughout multiple brain regions. The teratogenic effects of alcohol appear to be widespread, affecting almost the entire brain. The only region that appears to be relatively spared is the occipital lobe. More recent studies have linked cognition to the underlying brain structure in alcohol-exposed subjects, and several report patterns in the severity of brain damage as it relates to facial dysmorphology or to extent of alcohol exposure. Future studies exploring relationships between brain structure, cognitive measures, dysmorphology, age, and other variables will be valuable for further comprehending the vast effects of prenatal alcohol exposure and for evaluating possible interventions.
Keywords: Magnetic resonance imaging, Fetal alcohol syndrome, Fetal alcohol spectrum disorder, Brain, Dysmorphology
Introduction
Prenatal alcohol exposure affects fetal development and can lead to a variety of growth, facial, and neurological abnormalities, including structural brain damage. The earliest reports of structural brain damage in children with prenatal alcohol exposure were autopsy studies (Clarren et al. 1978; K. L. Jones and Smith 1973; Peiffer et al. 1979). These were, by nature, limited to a few subjects with severe cases of prenatal alcohol exposure, since only the most affected children will die during infancy. Nonetheless, the studies were valuable in reporting some of the more severe types of brain damage associated with prenatal alcohol exposure. Autopsies demonstrated cases of agenesis or malformation of the corpus callosum, ventriculomegaly, a small cerebellum and a variety of other abnormalities due to neuronal and glial migration errors (Clarren et al. 1978; Coulter et al. 1993; K. L. Jones and Smith 1973; Kinney et al. 1980; Peiffer et al. 1979; Ronen and Andrews 1991; Wisniewski et al. 1983). Although there were few consistent abnormalities observed across the autopsy studies, the vast majority reported microcephaly (small head) or microencephaly (small brain) (Roebuck et al. 1998).
More recently, magnetic resonance imaging (MRI) has transformed the study of brain development and brain abnormalities by allowing for in vivo measurements of the teratogenic effects of alcohol. This allows for much larger studies that may include less severely affected individuals, thus providing more information about the brain damage associated with prenatal alcohol exposure across the spectrum. Here, we review MRI studies of structural brain damage in alcohol-exposed subjects that have been published over the last 20 years. These studies range from case reports to sophisticated automated analyses of large subject groups, and collectively demonstrate widespread effects of prenatal alcohol exposure. In this review, we summarize these structural brain MRI studies, discuss emerging consistencies, and highlight controversies yet to be resolved.
There is a broad range of symptoms that may result from maternal alcohol consumption during pregnancy, and thus a variety of diagnoses have emerged (see Riley, this issue, for a discussion of diagnoses related to prenatal alcohol exposure). Briefly, diagnostic terms used in the papers reviewed here include fetal alcohol syndrome (FAS, the most severe end of the spectrum referring to a specific set of growth, facial and cognitive/behavioral deficits), partial FAS (pFAS, used to refer to subjects who have most, but not all of the characteristics of FAS), fetal alcohol effects (FAE, referring to symptoms less severe than those of FAS), alcohol-related neurobehavioral disorder (ARND, typically no facial dysmorphology), and prenatal exposure to alcohol (PEA; not a diagnostic term, but generally used to refer to subjects with alcohol exposure but without the facial features of FAS). Recently, diagnoses of static encephalopathy: alcohol-exposed (SE:AE, a set of growth, brain and possibly facial abnormalities that is not quite as severe as FAS) and neurobehavioral disorder: alcohol-exposed (NBD:AE, growth and brain abnormalities, but no facial dysmorphology) have seen increased usage, since they are a function of a diagnosis with the 4-digit code (Astley 2004). Finally, the term fetal alcohol spectrum disorder (FASD) is often used as an umbrella term that encompasses all of the various diagnoses related to prenatal alcohol exposure.
One crucial area of research investigates relationships between brain damage in FASD and other factors, including cognition, behavior, facial dysmorphology, and the amount and timing of alcohol exposure. Understanding the interplay between cognitive and behavioral deficits and the brain and face abnormalities associated with prenatal alcohol exposure will provide a more complete picture of the teratogenic effects of alcohol and may allow for earlier identification and more effective interventions to help individuals deal with the associated disorders. Numerous brain abnormalities correlate with cognitive and/or behavioral measures in subjects with prenatal alcohol exposure (Autti-Ramo et al. 2002; Bjorkquist et al. 2010; Bookstein et al. 2002b; Coles et al. 2011; Cortese et al. 2006; O’Hare et al. 2005; Roebuck et al. 2002; Roussotte et al. 2011; Sowell et al. 2001a; 2008; Willoughby et al. 2008), and recent studies show links between brain structure and facial dysmorphology (Astley et al. 2009; Roussotte et al. 2011). Therefore, in addition to structural findings, we review the relationship between brain structure and cognition, behavior, age, and facial dysmorphology in subjects with prenatal alcohol exposure; such research may be useful not only for understanding these disorders more fully, but may also provide clues as to the timing and nature of alcohol-induced brain damage during fetal development.
Mechanism of Alcohol Toxicity
Animal studies have provided clues as to the mechanisms of alcohol teratogenesis on the brain, although there remain many questions about the specific relationships between timing and amount of alcohol exposure and the observed brain defects. Mouse models suggest that the facial abnormalities associated with in utero alcohol exposure (e.g., short palpebral fissures, a smooth philtrum, and a thin upper lip) occur due to alcohol exposure at gestation day 7 in mice, which is equivalent to the third and fourth weeks of human gestation (Sulik 2005; Sulik et al. 1986). Both human and animal studies suggest that genetics play a significant role in the severity of alcohol toxicity (Chasnoff 1985; Chernoff 1980; Christoffel and Salafsky 1975; Gilliam et al. 1987; Streissguth and Dehaene 1993); other factors such as nutrition (S. I. Miller et al. 1983) and exposure to other drugs are also likely to contribute (Rivkin et al. 2008).
Alcohol is a central nervous system depressant, and when ingested by a pregnant woman, it can affect the developing fetus through a number of mechanisms. Alcohol can cross the placenta and directly affect fetal brain development by disrupting neuronal proliferation and migration (M. W. Miller 1986) or by causing cell death (Bonthius and West 1990; Ikonomidou et al. 2001; Marcussen et al. 1994). Furthermore, alcohol increases fetal glutamate levels (Karl et al. 1995; Thomas et al. 1997) and reduces glutamate N-methyl-D-aspartate receptors (Hoffman et al. 1989; Hughes et al. 1998), which may cause abnormal neuronal and glial migration. An important indirect mechanism of alcohol damage is alcohol-induced hypoxia in the fetus. Alcohol causes decreased umbilical artery blood flow (P. J. Jones et al. 1981; A. B. Mukherjee and Hodgen 1982), which can lead to growth retardation (Abel 1984, 1985). Alcohol also decreases protein synthesis and alters hormone levels, which can lead to additional growth retardation (Kennedy 1984; Pennington et al. 1983). Other mechanisms include increased oxidative stress on the embryo (Ornoy 2007; Pollard 2007) and disruption of growth factor signaling (Feng et al. 2005; M. W. Miller 2003).
Magnetic Resonance Imaging
Magnetic resonance imaging (MRI) is a safe, non-invasive technique that can be used to study brain structure and function. MRI uses a strong magnet and radio waves to measure signals from protons (water) within the brain. The signal measured is dependent on tissue properties, including density, local environment, blood oxygenation, water movement, and relaxation properties (T1, T2). With many different contrast mechanisms, numerous types of imaging are possible, including functional MRI (fMRI), diffusion tensor imaging (DTI), and structural imaging. DTI and fMRI are reviewed in separate chapters within this issue (see Wozniak and Muetzel, and Coles and Li, this issue); here we focus on structural MR imaging of the brain.
Structural MRI provides high-resolution images of the brain based on contrast from tissue density and relaxation properties (longitudinal relaxation—T1, and transverse relaxation—T2). Images obtained can then be analyzed qualitatively or quantitatively. Qualitative analysis can evaluate for gross structural abnormalities such as the presence of lesions, malformation of structures, ventriculomegaly, or other major anomalies. More subtle changes can often be picked up by a trained radiologist, but are still limited to qualitative observations within an individual. Quantitative analysis is able to detect more subtle abnormalities on either a group or individual level, and may be based on area, volume, thickness, and displacement of both the brain as a whole and of its individual lobes and components. Because the brain is made up primarily of white matter, gray matter and cerebrospinal fluid, the volumes of these are often considered separately, as are the volumes of each different brain lobe (frontal, parietal, occipital, temporal). In addition, many volume measurements are made on subcortical structures such as the corpus callosum, basal ganglia, and diencephalon.
MRI Studies of Prenatal Alcohol Exposure
In our literature search, we found 33 studies using structural MRI to examine subjects with prenatal alcohol exposure, beginning in 1992. These studies range in subject sample size from 1 to 117 alcohol-exposed subjects, and use a variety of analysis methods (basic methodological details and findings for each study are listed in Tables 1 and 2). There is some subject overlap among these studies; specifically, some subjects are shared in the early studies (Mattson et al. 1992, 1994; Riley et al. 1995; Roebuck et al. 2002; Sowell et al. 1996), between case reports (Johnson et al. 1996; Swayze et al. 1997), and within three other groups conducting studies in the last decade at the University of Washington (Bookstein et al. 2001; 2002a,b, 2006), University of California (Archibald et al. 2001; O’Hare et al. 2005; Sowell et al. 2008, 2001a,b, 2002a,b), and University of Alberta (Lebel et al. 2008; Nardelli et al. 2011). This overlap can be advantageous in that it provides the opportunity to study many brain structures and regions within the same individuals, highlighting the widespread nature of brain abnormalities associated with prenatal alcohol exposure, but it is also a limitation because of the smaller overall sample that has been investigated. Future studies in large, independent samples will help determine the consistency of findings discussed here.
Table 1.
Structural MRI studies of subjects with prenatal alcohol exposure and their findings in the total brain, cerebellum, and corpus callosum. All structural MRI studies of prenatal alcohol exposure are included in this table, regardless of whether they found significant results in the global brain, cerebellum, or corpus callosum. FAS=fetal alcohol syndrome, FAE=fetal alcohol effects, FASD=fetal alcohol spectrum disorder, pFAS=partial fetal alcohol syndrome, HC=healthy controls, CSF=cerebrospinal fluid, WM=white matter, GM=gray matter
| First author, year | Number of subjects | Age (years) | Global changes | Cerebellum | Corpus callosum |
|---|---|---|---|---|---|
| Mattson et al. 1992 | 2 FAS, 9 HC | 13–14 | ↓ Brain volume ↑ CSF | ↓ Volume | Agenesis in 1 subject |
| Mattson et al. 1994 | 2 FAE, 20 HC | 16 | ↓ Brain volume | ↓ Volume | |
| Riley et al. 1995 | 13 exposed (11 FAS), 12 HC | 8–19 | ↓ Volume in 4/5 subregions ↓ Overall area | ||
| Mattson et al. 1996 | 6 FAS, 7 HC | 8–19 | ↓ Brain volume | ↓ Volume | |
| Sowell et al. 1996 | 9 exposed (6 FAS), 24 HC | 8–24 | ↓ Anterior volume | ||
| Johnson et al. 1996 | 9 exposed | 4–20 | Micrencephaly in 7/9 subjetcs | Agenesis in 2, partial agenesis in 1 subject | |
| Swayze et al. 1997 | 10 FAS, 119 HC | 4–29 | ↓ Size in 7 subjects | Agenesis in 3 subjects | |
| Riikonen et al. 1999 | 11 FAS | 3–13 | Atrophy in 3 subjects | ||
| Clark et al. 2000 | 19 FAS | 16–30 | Thin in 1 subject | ||
| Archibald et al. 2001 | 26 exposed (14 FAS), 41 control | 8–24 | ↓ Total, WM and GM volume | ↓ Volume | |
| Sowell et al. 2001a a (Neuroreport) | 21 exposed (14 FAS), 21 controls | 8–24 | |||
| Bookstein et al. 2001 | 60 exposed (30 FAS), 30 HC | 18–37 | Higher shape variability | ||
| Sowell et al. 2001b (Neurology) | 20 exposed (13 FAS), 21 HC | 8–24 | ↓ Total, WM, GM, CSF volume | ↓ Area (esp. splenium) Shape and displacement changes | |
| Sowell et al. 2002a a (Cereb Cortex) | 21 exposed (14 FAS), 21 controls | 8–24 | |||
| Sowell et al. 2002b a (Neuroimage) | 21 exposed (14 FAS), 83 controls | 7–24 | |||
| Bookstein et al. 2002a (Anat Rec) | 117 exposed (60 FAS), 60 HC | 14–37 | Higher shape variability | ||
| Bookstein et al. 2002b (Neuroimage) | 30 exposed (15 FAS), 15 HC | 5–15 | Higher shape variability | ||
| Roebuck et al. 2002 a | 10 exposed, 6 HC | 18+ | |||
| Autti-Ramo et al. 2002 | 17 exposed (5 FAS) | 12–14 | ↓ Volume | Malformation/hypoplasia in 11/17 subjects | Thin in 1 subject, Hypoplastic in 1 sub ↓ Area, length, and splenium width |
| O’Hare et al. 2005 | 21 exposed (14 FAS), 21 controls | 8–24 | ↓ Anterior vermis Displacement of anterior vermis | ||
| Riikonen et al. 2005 | 12 exposed (10 FAS), 10 non-exposed with other diagnoses | 5–16 | ↓ Volume | ||
| Cortese et al. 2006 | 8 exposed (6 FAS), 4 HC | 9–12 | ↓ Volume | ||
| Bookstein et al. 2006 | 60 exposed (30 FAS), 30 HC | 14–37 | ↓ Volume | ||
| Sowell et al. 2008 a | 21 exposed (14 FAS), 21 controls | 8–24 | |||
| Willoughby et al. 2008 | 19 exposed (3 FAS), 18 HC | 9–15 | ↓ Volume | ||
| Lebel et al. 2008 | 24 exposed (2 FAS), 95 HC | 5–13 | ↓ Total, WM, GM volume | ||
| Li et al. 2008 a | 7 exposed, 7 HC | Young adults | |||
| Astley et al. 2009 | 61 FASD (20 FAS/pFAS), 20 HC | 8–16 | ↓ Volume across groups | ↓ Midsagittal area of vermis in FAS/pFAS | ↓ Midsagittal area and all subregions ↓ Mean length |
| Bjorkquist et al. 2010 | 21 exposed (10 FAS), 10 HC | 8–16 | ↓ WM, GM | ||
| Reinhardt et al. 2010 a | 1 FAS | 16 | |||
| Coles et al. 2010 | 66 exposed (30 dysmorphic), 26 HC | Young adults | ↓ Volume | ||
| Nardelli et al. 2011 | 28 exposed, 56 HC | 6–17 | ↓ Volume, WM, cortical GM, deep GM | ||
| Roussotte et al. 2011 | 56 exposed, 43 HC | 8–16 | ↓ Volume, WM, cortical GM |
aThese studies did not explicitly measure brain volume, the cerebellum or the corpus callosum. The demographics of these studies are included here for completeness; their findings are described in Table 2.
Table 2.
Structural MRI studies of subjects with prenatal alcohol exposure and their findings in frontal, parietal, temporal, occipital, and limbic/deep gray matter regions. Studies without significant findings in these regions are excluded from this table
| First author, year | Frontal | Parietal | Temporal | Occipital | Limbic/Deep gray matter |
|---|---|---|---|---|---|
| Mattson et al. 1992 | ↓ Caudate, thalamus | ||||
| Mattson et al. 1994 | ↓ Basal ganglia | ||||
| Riley et al. 1995 | |||||
| Mattson et al. 1996 | ↓ Basal ganglia, caudate, lenticular nucleus, diencephalon | ||||
| Riikonen et al. 1999 | Cortical atrophy in 1 subject | ↓WM in 1 subject | |||
| Archibald et al. 2001 | ↓ Total, cortical and WM volume | ↓ Caudate, hippocampus | |||
| Sowell et al. 2001b (Neuroreport) | ↑GM density | ↑GM density | |||
| Sowell et al. 2002a (Cereb Cortex) | ↓ Distance from center | ↓ Distance from center | ↓ Distance from center | ||
| ↓ Total, WM volume | ↓ Total, WM, GM volume | ↓ Total, WM volume | |||
| ↑ GM density | ↑ GM density | ||||
| Sowell et al. 2002b (Neuroimage) | Inferior region shifted backward | Superior region shifted back | |||
| ↓ Asymmetry in inferior region | |||||
| Autti-Ramo et al. 2002 | ↓ Mesencephalon area, width | ||||
| Small hippocampus in 3 (left) and 1 (right) subjects | |||||
| Riikonen et al. 2005 | ↓ Caudate | ||||
| Sowell et al. 2008 | ↑ Cortical thickness | ↑ Cortical thickness | ↑ Cortical thickness | ||
| Willoughby et al. 2008 | ↓ L hippocampus | ||||
| Li et al. 2008 | ↓ Volume in occipital/temporal region | ↓Volume in occipital/temporal region | |||
| Astley et al. 2009 | ↓ Volume across groups | ↓ Caudate, putamen, hippocampus | |||
| ↓WM and GM | |||||
| Bjorkquist et al. 2010 | ↓ Cingulate WM and GM | ||||
| Reinhardt et al. 2010 | Polymicrogyria in superior frontal gyrus | ||||
| Coles et al. 2010 | ↓ Caudal mid frontal, med orb frontal, sup frontal, rostral med frontal, pars triangularis, pars orbitalis | ↓ Fusiform gyrus | ↓ Hippocampus, parahippocampal gyrus, | ||
| Nardelli et al. 2011 | ↓ Caudate, putamen, thalamus, amygdala, hippocampus, globus pallidus | ||||
| Roussotte et al. 2011 | ↓ Caudate, putamen, thalamus, ventral diencephalon |
Most studies described in this review used T1-weighted MRI protocols to obtain structural brain images, although some used T2-weighted images (Autti-Ramo et al. 2002; Mattson et al. 1996; Riikonen et al. 2005). All studies were conducted on magnets operating at 1.5 or 3 T field strength, with the sole exception of a qualitative study conducted on a much weaker 0.15 T MRI scanner (Clark et al. 2000). Analysis methods ranged from visual inspection and qualitative descriptions to manual segmentations and automated analyses of area, volume, thickness, and other parameters.
Studies of prenatal alcohol exposure are challenging for several reasons. It is often difficult to obtain accurate and precise information about timing and amount of prenatal alcohol exposure, consequently making correlations between brain abnormalities and extent of exposure challenging, and impossible for some studies. Furthermore, children, adolescents, and adults with prenatal exposure to alcohol were often exposed to other substances in utero, including tobacco, cocaine, methamphetamines, marijuana, and opiates (Shor et al. 2009), all of which are substances known to affect brain structure (Paus et al. 2008; Sowell et al. 2010). In addition to other drug exposures, subjects with FASD are often likely to have co-morbid conditions, including attention deficit hyperactivity disorder (ADHD), mood disorders, and oppositional defiant disorder (Burd et al. 2003; O’Connor and Paley 2009), and imaging studies have shown brain abnormalities in these populations as well (Toga et al. 2006). Therefore, as they may complicate interpretation of findings, it is important to consider other possible substance exposures and co-morbid diagnoses in any study of prenatal alcohol exposure.
Despite these challenges, many informative neuroimaging studies have been conducted over the past two decades examining brain structure in children, youth and adults with prenatal alcohol exposure. These studies reveal widespread brain abnormalities, and some consistent patterns of abnormalities are beginning to emerge, while many questions remain to be answered. Below, we describe salient findings from structural imaging studies to date.
Global Brain Differences in Subjects with Prenatal Alcohol Exposure
Similar to early autopsy studies, MRI studies almost uniformly find small brain volumes in FASD, including total brain volume (Archibald et al. 2001; Astley et al. 2009; Coles et al. 2011; Johnson et al. 1996; Lebel et al. 2008; Nardelli et al. 2011; Roussotte et al. 2011; Sowell et al. 2001a; Swayze et al. 1997; Willoughby et al. 2008), cerebral volume (Archibald et al. 2001; Mattson et al. 1992, 1994, 1996), and cerebellar volume (Archibald et al. 2001; Astley et al. 2009; Mattson et al. 1992, 1994, 1996; O’Hare et al. 2005; Riikonen et al. 1999; Sowell et al. 1996). The volumes of white and gray matter have also been consistently reported as smaller in individuals with prenatal alcohol exposure compared to controls (Archibald et al. 2001; Bjorkquist et al. 2010; Lebel et al. 2008; Mattson et al. 1992, 1994; Nardelli et al. 2011; Roussotte et al. 2011; Sowell et al. 2001a), although these differences were no longer significant once total brain volume was taken into account in most of the studies that measured this. Notable exceptions are studies which found cerebral white matter (Archibald et al. 2001), total gray matter (Nardelli et al. 2011), or cortical gray matter (Roussotte et al. 2011) to be proportionally smaller in alcohol-exposed subjects, even beyond total brain volume. Fewer studies have measured cerebrospinal fluid (CSF) volume directly; one observed no change in total CSF volume between alcohol-exposed and controls (Archibald et al. 2001), another saw somewhat less CSF in FASD, which was not significant after taking into account total brain volume (Sowell et al. 2001a), and another saw a proportional increase of fluid volume from controls to FAS subjects (Mattson et al. 1992).
Although findings of smaller brains in subjects with prenatal alcohol exposure are nearly universal, most studies find group effects, where not every subject with alcohol exposure has a smaller brain than every control subject. A smaller brain is also not unique to prenatal alcohol exposure and is observed in many other disorders including ADHD (Castellanos and Acosta 2004) and child-onset schizophrenia (Arango et al. 2008). Thus, investigation of more subtle abnormalities is necessary to further understand the effects of prenatal alcohol exposure and the complex relationships between behavior, cognition, and brain structure, and may ultimately provide better diagnostic tools.
Regional Abnormalities
As the largest white matter tract in the human brain and one that is crucial for interhemispheric communication, the corpus callosum is also one of the most frequently examined brain structures. Imaging studies support early postmortem findings of callosal abnormalities (Clarren et al. 1978; K. L. Jones and Smith 1973; Peiffer et al. 1979), including cases of complete agenesis (Astley et al. 2009; Johnson et al. 1996; Mattson et al. 1992; Riley et al. 1995; Swayze et al. 1997), partial agenesis (Autti-Ramo et al. 2002; Johnson et al. 1996), and callosal thinning (Autti-Ramo et al. 2002; Clark et al. 2000). Figure 1 shows several examples of severe callosal abnormalities from imaging studies that are apparent upon visual inspection; such malformations are rare, but less severe forms of callosal abnormalities are common in subjects with prenatal alcohol exposure. Case reports have also described agenesis of the anterior and hippocampal commissures, other midline brain structures (Johnson et al. 1996; Swayze et al. 1997). Quantitative studies demonstrate significant displacement of the corpus callosum in alcohol-exposed subjects compared to healthy controls (see Fig. 2) (Sowell et al. 2001a), as well as smaller callosal volume (Riley et al. 1995), area (Astley et al. 2009; Autti-Ramo et al. 2002; Riley et al. 1995; Sowell et al. 2001a), and length (Astley et al. 2009; Autti-Ramo et al. 2002). Furthermore, the corpus callosum has much higher shape variability in alcohol-exposed subjects than in controls (Bookstein et al. 2001, 2002a,b). Abnormalities have been reported across the entire corpus callosum, but it seems that the splenium may be the most affected region, both in terms of frequency and severity of abnormalities (Autti-Ramo et al. 2002; Riley et al. 1995; Sowell et al. 2001a).
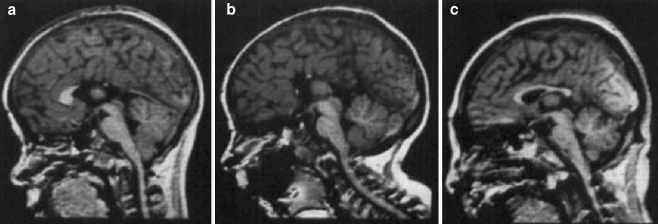
Fig. 1.
Examples of corpus callosum abnormalities in alcohol-exposed subjects, including (a, b) partial agenesis and (c) hypoplasia. Reproduced from Johnson et al., 1997 with permission. These severe malformations are observed in rare cases of subjects with very heavy prenatal alcohol exposure; however, more subtle callosal abnormalities have been observed in most studies of prenatal alcohol exposure
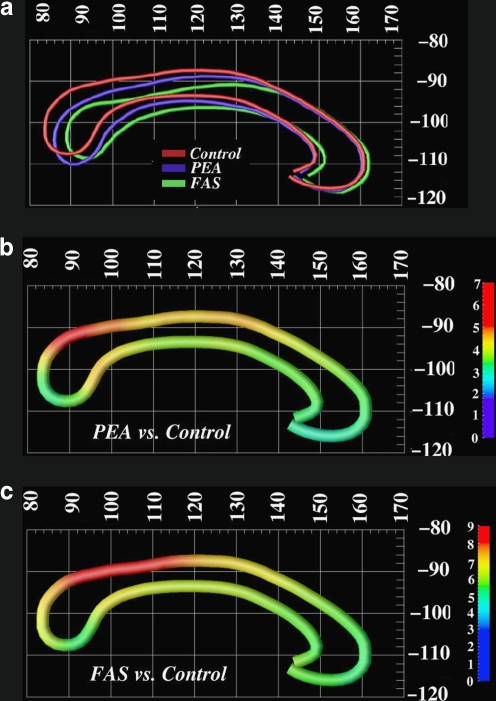
Fig. 2.
Sowell et al. (2001a), demonstrated changes in corpus callosum displacement. Here, the top row (a) shows callosal shape averages in native space in controls (red), subjects with fetal alcohol syndrome (FAS; green), and subjects with prenatal exposure to alcohol (PEA; blue). The middle row (b) shows displacement differences in millimeters between subjects with PEA and controls; the bottom row (c) shows the differences between FAS subjects and controls. Note that the abnormalities relative to controls that were observed in subjects with FAS and PEA are similar, although they are slightly more extensive in the FAS cohort. The callosal displacement here was significantly related to performance on the California Verbal Learning Test for children (CVLT-C). Figure used with permission
Deep gray matter structures also demonstrate substantial effects of prenatal alcohol exposure, particularly in the caudate nucleus and hippocampus. Some studies report significantly smaller hippocampi after accounting for total brain volume (Archibald et al. 2001; Nardelli et al. 2011; Willoughby et al. 2008), and other studies report significant differences only before correction (Astley et al. 2009; Coles et al. 2011; Riikonen et al. 2005; Roussotte et al. 2011). An additional qualitative report described smaller hippocampi on the left in three individuals (of 17 total) and on the right in one (Autti-Ramo et al. 2002). Significantly smaller volumes are also consistently reported in the basal ganglia (a group of nuclei consisting primarily of the globus pallidus and striatum) (Mattson et al. 1994, 1996). The caudate nucleus is smaller in alcohol-exposed subjects, even more so than total brain volume in some studies (Archibald et al. 2001; Astley et al. 2009; Mattson et al. 1992, 1996; Nardelli et al. 2011), and in proportion to total brain volume in other reports (Cortese et al. 2006; Riikonen et al. 2005; Roussotte et al. 2011). Other studies show reduced volumes in the globus pallidus on its own (Nardelli et al. 2011; Roussotte et al. 2011), and in the putamen (Astley et al. 2009; Nardelli et al. 2011; Riikonen et al. 2005; Roussotte et al. 2011); differences in the lenticular nucleus on its own (which comprises the putamen and globus pallidus) were significant in one study (Mattson et al. 1996), but not in another (Archibald et al. 2001).
Other deep gray matter structures display more mixed results, including the diencephalon, with either no change (Archibald et al. 2001; Mattson et al. 1994), significantly smaller volume (Mattson et al. 1996), or significantly lesser area and width (Autti-Ramo et al. 2002); the thalamus (part of the diencephalon), which has no significant changes beyond total brain volume (Archibald et al. 2001; Roussotte et al. 2011), or is significantly smaller (Mattson et al. 1992; Nardelli et al. 2011); and the amygdala, with no change beyond that of total brain volume (Archibald et al. 2001; Riikonen et al. 2005; Roussotte et al. 2011), or significant size differences (Nardelli et al. 2011). The nucleus accumbens, only measured in one study, had no significant volume changes (Archibald et al. 2001).
Like cerebral volume, cerebellar volume is consistently smaller in subjects prenatally exposed to alcohol. Cerebellar atrophy was reported in 3 of 11 children in one study (Riikonen et al. 1999), while malformation or hypoplasia was reported in 11 of 17 subjects in another (Autti-Ramo et al. 2002). Many studies report smaller cerebella across a goup of alcohol-exposed subjects compared to controls (Archibald et al. 2001; Astley et al. 2009; Bookstein et al. 2006; Mattson et al. 1992, 1994, 1996), and two studies localized these changes to the anterior vermis (O’Hare et al. 2005; Sowell et al. 1996). One report also noted a displacement of the cerebellar vermis in alcohol-exposed subjects relative to controls (O’Hare et al. 2005). Of the studies measuring cerebellar size in proportion to total brain volume, two report no changes beyond those of the total brain (Archibald et al. 2001; Astley et al. 2009), while one reports significantly smaller anterior cerebella even after accounting for total brain volume (O’Hare et al. 2005).
The brain frontal lobes have less white matter (Astley et al. 2009; Sowell et al. 2002a), gray matter (Astley et al. 2009) and total lobe volume (Astley et al. 2009; Sowell et al. 2002a) in subjects with prenatal alcohol exposure compared to controls, as well as smaller volumes of frontal lobe sub-regions (Coles et al. 2011). One study found a frontal volume difference in alcohol-exposed subjects that was no longer significant after controlling for total brain volume (Archibald et al. 2001). The cortex is thicker in frontal regions in alcohol-exposed subjects compared to controls (Sowell et al. 2008), as shown in Fig. 3, and brain surface extent is smaller in anterior and orbital frontal regions (Sowell et al. 2002a). Further, case studies report frontal cortical atrophy (Riikonen et al. 1999), and polymicrogyria in the superior frontal gyrus (Reinhardt et al. 2010). Notably, one study found a progressive decrease in frontal lobe volume across diagnostic groups, with the FAS/pFAS group demonstrating significant differences from the static encephalopathy group, suggesting that smaller volume may be related to the facial dysmorphology associated with prenatal alcohol exposure (Astley et al. 2009).
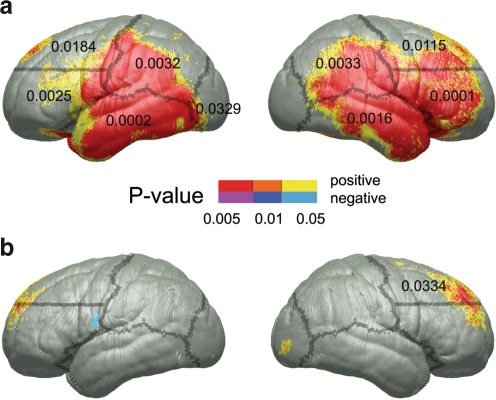
Fig. 3.
Sowell et al. (2008) demonstrated thicker cortices in alcohol-exposed subjects compared to healthy controls. The top row (a) shows the location of cortical thickness differences between exposed subjects and controls and the significance of those changes. They also observed correlations between the cortical thickness and performance on the CVLT-C, a test of verbal learning. The significant correlations were located in the right frontal lobe in exposed subjects (b). Figure used with permission
Like the frontal lobes, many abnormalities have been noted in the parietal and temporal lobes of subjects with prenatal alcohol exposure. These include significantly less white matter, gray matter, and total lobe volume (Archibald et al. 2001; Li et al. 2008; Sowell et al. 2002a), higher gray matter density (Sowell et al. 2001b, 2002a), and thicker cortices (Sowell et al. 2008), as shown in Fig. 3. Additional findings include a smaller fusiform gyrus (Coles et al. 2011), narrower temporal and parietal lobes (Sowell et al. 2002a), displacement of the inferior parietal and temporal regions (Sowell et al. 2002b), and reduced temporal asymmetry in alcohol-exposed subjects compared to controls (Sowell et al. 2002b).
One study specifically measured the cingulate area in subjects with prenatal alcohol exposure, and observed significantly less white and gray matter in alcohol-exposed subjects than in controls, with the white matter changes remaining significant even after accounting for total brain white matter (Bjorkquist et al. 2010). White matter volume differences were especially prominent in the right hemisphere. The parahippocampal gyrus has also been observed to be significantly smaller in subjects with prenatal alcohol exposure compared to controls (Coles et al. 2011).
In contrast to other brain regions, the occipital lobes seem to be relatively spared in subjects with prenatal exposure to alcohol. Most studies which measure the occipital lobe report no significant findings there for volume (Archibald et al. 2001; Sowell et al. 2002a), shape and displacement (Sowell et al. 2002a,b), or cortical thickness (Sowell et al. 2008). One study reported smaller volume in the occipital-temporal region in a group of seven young adults with prenatal exposure to alcohol compared to controls (Li et al. 2008), and one qualitative study reported less occipital white matter in one subject (Riikonen et al. 1999).
Structural abnormalities in subjects with prenatal alcohol exposure are widespread and have been reported in almost every brain structure and region that has been measured. While the occipital lobe appears to be one of the only regions that remain relatively spared, some of the most significantly affected seem to be the corpus callosum and the frontal lobes. The corpus callosum is one of several midline face and brain structures affected by prenatal alcohol exposure; other affected midline structures include the anterior and hippocampal commissures, lip, philtrum and palate (Johnson et al. 1996; Swayze et al. 1997). It is unclear why certain brain regions are more sensitive than others to the teratogenic effects of alcohol, but the timing of their development within the brain (Rakic and Yakovlev 1968; Yakovlev and Lecours 1967) and the differential susceptibility of neurons to alcohol-induced toxicity during the synaptogenesis period in the third trimester (Ikonomidou et al. 2000, 2001) are likely important factors. Furthermore, although consistent abnormalities in diverse subject groups with alcohol exposure at different times and extents suggest a vulnerability of brain structures throughout the prenatal period, the amount and timing of alcohol exposure are likely to play a role in the observed effects. Therefore, obtaining accurate histories and examining relationships between alcohol exposure and brain structure are crucial for further understanding this relationship.
Studying the relationships between structural brain abnormalities and cognition may also be helpful to understand development in subjects with prenatal alcohol exposure as well as to investigate possible diagnostic tools, interventions and treatments. The frontal lobes are particularly interesting in this regard, since they are responsible for executive function, and executive function deficits are common in individuals with prenatal exposure to alcohol (Jacobson and Jacobson 2002; Kodituwakku 2009). The consistently smaller frontal lobe volume, as well as the additional cortical thickness, surface extent and shape abnormalities may be linked to executive function or other cognitive and/or behavioral difficulties observed in FASD. The next section discusses studies examining such correlations in the frontal lobe and beyond.
Brain-Behavior Relationships
Subjects with FASD are delayed in a number of cognitive domains (Jacobson and Jacobson 2002; R. A. Mukherjee et al. 2006), and generally have lower IQ and other cognitive scores than controls (Mattson et al. 1997). Most studies report IQ for alcohol-exposed subjects, and in those studies that do report IQ, the alcohol-exposed group always has lower IQ than either standard scores (mean IQ score in the general population is 100) or the control group (Archibald et al. 2001; Astley et al. 2009; Autti-Ramo et al. 2002; Bjorkquist et al. 2010, 2001, 2002a,b; Clark et al. 2000; Coles et al. 2011; Cortese et al. 2006; Li et al. 2008; Mattson et al. 1992, 1994, 1996; O’Hare et al. 2005; Reinhardt et al. 2010; Riikonen et al. 1999, 2005; Riley et al. 1995; Roebuck et al. 2002; Roussotte et al. 2011; Sowell et al. 2001a,b, 2002a,b; Swayze et al. 1997; Willoughby et al. 2008). Linking cognitive deficits such as lower IQ in subjects with prenatal alcohol exposure to the underlying brain structure is important because it helps to understand the complex nature of the associated disorder and tease out the relationships between brain abnormalities and the observed behavior. Further, finding relationships between brain abnormalities and cognitive functions helps validate the clinical significance of sometimes-subtle abnormalities that can only be observed in relatively large groups of subjects. Recently, many studies have begun to investigate these links using various types of neuropsychological testing and quantitative measures obtained from structural MRI. The methods and findings of these studies are briefly outlined in Table 3 and summarized below.
Table 3.
Studies examining relationships between brain measures on structural MRI and cognitive/behavioral measures in subjects with prenatal alcohol exposure
| First author, year | Subjects | Cognitive/Behavior Tests | Results |
|---|---|---|---|
| Sowell et al. 2001a (Neurology) | 18 exposed (only 15 received ROCF), 18 HC | California verbal learning test for children (CVLT-C) | Posterior-anterior displacement of the corpus callosum is related to CVLT across entire group |
| Rey Osterrieth Complex Figure (ROCF) | Relationship significant in exposed group alone (more anterior=worse performance), but not controls | ||
| Bookstein et al. 2002b (Neuroimage) | 30 exposed (15 FAS), 15 HC | 25 different tests, see (Bookstein et al. 2002b) | Callosal thickening associated with executive function deficits |
| Callosal thinning associated with motor deficits | |||
| Roebuck et al. 2002 | 10 exposed, 6 HC | Finger localization task | Positive correlations between posterior callosal area and performance on 2-finger localization task in entire group and within only exposed |
| Positive area-FSIQ correlations across both groups | |||
| Anterior area positively correlated with 1-finger localization in exposed group | |||
| Autti-Ramo et al. 2002 | 17 exposed | Wechsler Intelligence Scale for Children (WISC-III) | No significant relationships found |
| Neuropsychological assessment (NEPSY) | |||
| O’Hare et al. 2005 | 17 exposed (only 14 received ROCF), 18 HC | CVLT-C | CVLT negatively correlated with anterior vermis morphology in exposed group (worse performance=greater dysmorphology) |
| ROCF | |||
| Sowell et al. 2008 | 18 exposed (only 15 received ROCF), 18 HC | CVLT-C | Controls have negative CVLT-thickness correlations in left occipital region |
| ROCF | FASD have positive correlations in right dorsal frontal region | ||
| Controls have negative ROCF-thickness correlations in bilateral parietal, left occipital, right dorsal and ventral frontal, and right temporal; none in exposed | |||
| Willoughby et al. 2008 | 19 exposed, 18 HC | Wechsler Abbreviated Scale of Intelligence (WASI), Children’s Memory Scale (CMS), CLVT-C, ROCF, Everyday Memory Questionnaire (EMQ) | In exposed, left hippocampal volume correlates positively with CVLT-C long-delay cued recall |
| Bjorkquist et al. 2010 | 21 exposed, 10 HC | WISC-III | Positive correlation between FD and posterior cingulate gray matter volume in exposed group |
| Freedom from Distractibility (FD) Index | |||
| Coles et al. 2010 | 66 exposed (30 dysmorphic), 26 HC | Verbal Selective Reminding Memory Test (VSRT) | Right hippocampus is a significant predictor of verbal and nonverbal recall |
| Frontal regions and left fusiform predict verbal recall | |||
| Nonverbal Selective Reminding Memory Test (NVSRT) | Right entorhinal cortex predicts nonverbal recall | ||
| Nardelli et al. 2011 | 17 FASD, 32 controls | Working Memory Test Battery for Children, Digit and Block Recall, Woodcock-Johnson III, Woodcock Reading Mastery Test, Comprehensive Receptive and Expressive Vocabulary Test, NEPSY | No correlations between standard scores and deep gray matter volumes |
| Roussotte et al. 2011 | 55 FASD | FSIQ | Positive correlation between FSIQ and right & left putamen volume |
In the general population, brain volume is weakly (correlation coefficients of ~0.2–0.4), but significantly correlated with intelligence (Lange et al. 2010; Witelson et al. 2006). However, this relationship is complex and does not hold for all populations or intelligence domains; for example, there are gender differences in the strength of correlations, and performance IQ tends to be significantly correlated with brain volume, while verbal IQ does not (Lange et al. 2010; Witelson et al. 2006). In the imaging studies of FASD that we found, a similar relationship was not tested or reported within alcohol-exposed subjects, but is likely to exist. However, relationships between more subtle brain abnormalities and cognitive abilities have been identified in several MRI studies of FASD.
With still relatively few studies examining brain-behavior relationships to date, it is difficult to make definitive conclusions. Nonetheless, of the 11 studies identified (Table 3), three focused on the corpus callosum, reporting correlations between callosal displacement and verbal learning (Sowell et al. 2001b), callosal thickness and executive function and motor abilities (Bookstein et al. 2002b), and callosal area and a finger localization task (Roebuck et al. 2002). Two studies have shown relationships between verbal abilities and hippocampal volume, one study highlighting the left hippocampus (Willoughby et al. 2008), and the other finding relationships between verbal recall and right hippocampal volume (Coles et al. 2011).
The California Verbal Learning Test (CVLT) has been used in many prenatal alcohol exposure studies (some with overlapping subjects). In addition to the relationships between verbal learning and callosal displacement and hippocampal volume noted above, verbal learning has been shown to correlate with cerebellar vermis morphology (O’Hare et al. 2005) and cortical thickness in the dorsal frontal region (Sowell et al. 2008). An independent study showed correlations between a different verbal task, the Verbal Selective Reminding Memory Test (VSRT), and both right hippocampal volume, and that of several frontal regions (Coles et al. 2011).
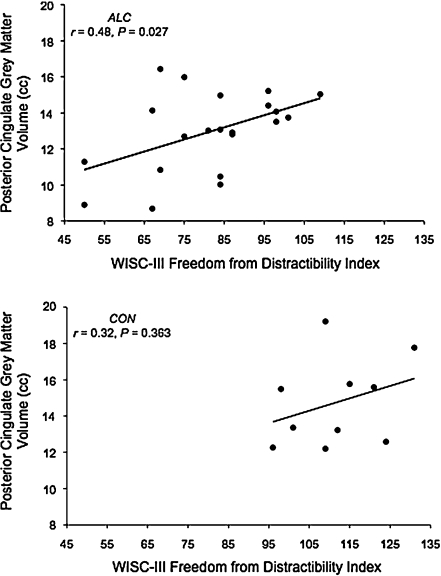
Other brain-behavior findings include a relationship between the freedom from distractibility (FD) index and cingulate gray matter volume (Bjorkquist et al. 2010) (see Fig. 4), and correlations between full scale IQ (FSIQ) and volume of the right and left putamen (Roussotte et al. 2011). On a nonverbal memory test, correlations were observed between test performance and volume of the right entorhinal cortex and hippocampus (Coles et al. 2011).
Fig. 4.
Bjorkquist et al. (2010) found a significant correlation between posterior cingulate gray matter volume and the freedom from distractibility index, indicating that smaller volumes were associated with more distractibility. Figure used with permission
In most studies mentioned above, the correlations were in the expected direction, with more severe brain damage being related to worse performance (i.e., smaller area=worse performance) (Bjorkquist et al. 2010; Coles et al. 2011; O’Hare et al. 2005; Roebuck et al. 2002; Roussotte et al. 2011; Sowell et al. 2001a; Willoughby et al. 2008). However, a few notable exceptions exist. Bookstein et al. (2002b) observed that callosal thickening was associated with deficits of executive function, while callosal thinning was related to poor motor skills, suggesting that variation in either direction from controls was associated with worse performance on specific tasks (Bookstein et al. 2002b). Sowell et al. (2008) observed positive correlations between verbal learning and thickness in alcohol-exposed subjects (thicker=better performance), which were in the opposite direction to correlations found in controls (Sowell et al. 2008). Coles et al. (2011) found mixed results between frontal lobe volumes and memory scores, with some positive and some negative correlations (Coles et al. 2011).
It should be noted, however, that in all the studies mentioned above that had significant findings, there were also brain regions or cognitive measures for which no significant relationships were found. Furthermore, two studies tested for relationships between brain structure and cognitive measures, but found no significant relationships at all (Autti-Ramo et al. 2002; Nardelli et al. 2011). Notably, one study found extensive regions of correlation between cortical thickness and performance on the Rey Osterrieth Complex Figure (ROCF) test in controls where thicker cortices were associated with worse performance, but no significant relationships in subjects with prenatal alcohol exposure (Sowell et al. 2008).
The brain-behavior links observed so far are varied and occur in widespread brain regions. Given different measurement and analysis techniques, as well as the diverse subject samples, it is difficult to draw general conclusions from the limited data available so far. With cross-sectional data alone, it is impossible to tell whether the structural abnormalities are a cause or result of the observed cognitive and behavioral deficits, or whether the two simply vary together due to a different factor. Another limitation of cross-sectional studies is that they provide only a single time point glimpse into complex brain-behavior relationships. Cognitive function, behavior and brain abnormalities may all change over time, particularly in relation to one another, and thus much more complex longitudinal studies are necessary in order to fully understand the relationships between structure and function. Nonetheless, these cross-sectional studies linking structure and cognition lay the foundation for future studies to examine longitudinal changes within subjects, and to test the efficacy of interventions within this population.
Brain Abnormalities and Age
The studies described here span a wide age range, from age 4 years to adulthood, with the majority focused on adolescents. Comparing across studies, the results in children, adolescents, and adults seem relatively consistent, although with so many different analysis methods, it is difficult to judge. The only group to use similar methods to study both adolescents and adults separately was Bookstein et al., who observe similar results in the corpus callosum in both groups (Bookstein et al. 2001, 2002a,b), specifically that those with prenatal alcohol exposure had higher shape variably in the corpus callosum than healthy controls.
Many studies show age-related changes in structural brain parameters in typically developing children, adolescents and young adults. These include changes of gray and white matter volume (Giedd et al. 1999; Good et al. 2001), gray matter density (Sowell et al. 1999, 2003), white matter density (Paus et al. 1999), cortical thickness (Lerch et al. 2006; Sowell et al. 2004), and the volumes of individual subcortical structures (Walhovd et al. 2005). To date, most studies of subjects with prenatal exposure to alcohol have age ranges too narrow or sample sizes too small to investigate age-related changes with sufficient statistical power and the majority of studies correct for age-effects to eliminate confounding effects from the results. Nonetheless, two groups measured age effects directly in subjects with FASD. One study observed significant hippocampal volume increases with age in the controls, but not in the subjects with FASD (Willoughby et al. 2008). Another reported significant increases in total brain, white matter, and globus pallidus volumes with age in both control and FASD groups, with the FASD subjects having consistently smaller volumes than controls across the age range (Nardelli et al. 2011). The same study found age-related changes in the thalamus (increases with age) and cortical gray matter volume (decreases with age) in control subjects, but not in those with FASD (Nardelli et al. 2011). Figure 5 shows volume-age plots for three brain structures in FASD and controls. The exploration of age-related brain changes in children and adolescents with prenatal alcohol exposure may be an interesting area for future research given that their trajectories may differ significantly from those of typically developing healthy controls. Larger sample sizes and/or longitudinal data will greatly benefit the study of development trajectories in children with prenatal alcohol exposure.
Fig. 5.
Nardelli et al. (2011) observed significantly smaller volume of six deep gray matter structures in alcohol-exposed subjects compared to controls. They also observed significant age-related changes within the globus pallidus (dark blue) for both groups, and within the thalamus (green) for controls (both shown above). The other four structures—the caudate (light blue), putamen, hippocampus, and amygdala (not shown) had no significant age-related changes. Across the age range, the mean volume of exposed subjects remained significantly below that of controls, suggesting consistency across the age range
Links to Alcohol Exposure and Facial Abnormalities
It is logical that the extent of brain damage caused by prenatal alcohol exposure is related to the timing and amount of exposure, although these links are difficult to investigate in humans. Studies of mice suggest that the facial dysmorphology associated with prenatal alcohol exposure is the result of alcohol exposure at gestation day 7, which is roughly equivalent to the third and fourth weeks of human gestation (Sulik 2005; Sulik et al. 1986). One of the challenges with studying FASD is obtaining accurate information about timing and amount of alcohol exposure, due to unreliable reports from the mother or relatives, or the unavailability of the biological mother (e.g., if the child is in foster or adoptive care). Furthermore, additional diagnoses and exposures confound results, and there is great inter-subject variation. Nonetheless, some studies have attempted to investigate this question (Table 4). One study found correlations between the size of various brain regions and the average number of drinking days per week (correlated with midsagittal area of the brain, absolute and relative hippocampal volume, and length of the corpus callosum), the maximum number of drinks per occasion (frontal lobe volume, relative caudate volume, and absolute and relative hippocampal volume), and the trimester of exposure (significant linear decrease in mean relative volume of the frontal lobe) (Astley et al. 2009). Another study found a significant negative correlation between amount of alcohol consumed around the time of conception and caudate nucleus volume, although this may reflect a global effect, as control subjects were included in the correlation (Cortese et al. 2006). Correlations have also been reported between the number of drinks per week in the first trimester and intracranial volume (Roussotte et al. 2011).
Table 4.
Correlations between brain abnormalities and measures of alcohol exposure or facial dysmorphology
| First author, year | Subjects | Findings |
|---|---|---|
| Astley et al. 2009 | 30 FAS/pFAS, 30 SE:AE | # Drinking days/week—midsagittal area, hippocampal volume, length of corpus callosum |
| Max # drinks/occasion—frontal lobe volume, caudate volume, hippocampal volume | ||
| Cortese et al. 2006 | 10 exposed (7 FAS), 4 HC | Amount of alcohol consumed at conception—caudate volume |
| Roussotte et al. 2011 | 17 exposed | # drinks/week in 1st trimester—intracranial volume |
| Facial Dysmorphology | ||
| Astley et al. 2009 | 30 FAS/pFAS, 30 SE:AE | FAS/pFAS group had smaller area/volume than SE:AE group for: midsagittal brain and cerebellar area, frontal lobe total and gray matter volume, putamen volume |
| Roussotte et al. 2011 | 52 exposed | Palpebral fissure length—ventral diencephalon volume |
| Lipometer—thalamus and left pallidum volume | ||
Another interesting question to investigate is whether brain structure correlates with facial dysmorphology. This approach reduces the need for detailed drinking histories, yet still provides valuable information about relationships between various aspects of the disorders associated with prenatal alcohol exposure. Furthermore, it seems logical that more severe brain damage would be related to more severe facial abnormalities, as both are likely related to the amount and timing of alcohol exposure. Several studies separate alcohol-exposed subjects into groups with and without facial dysmorphology (typically, facial dysmorphology is associated with a diagnosis of FAS or pFAS). Most studies then compare both groups separately to controls, and observe fewer significant differences (or less extensive regions of difference) between exposed subjects without dysmorphology and controls than between exposed subjects with dysmorphology and controls (Archibald et al. 2001; Bookstein et al. 2006; Coles et al. 2011; Cortese et al. 2006; Riley et al. 1995; Sowell et al. 2001a,b, 2008). Figure 2 shows an example of similar callosal abnormalities in FAS and PEA groups compared to controls, where the differences are slightly more extensive in the FAS group (Sowell et al. 2001a). However, several groups report no differences between results of dysmorphic versus controls or nondysmorphic versus controls comparisons (Autti-Ramo et al. 2002; Bjorkquist et al. 2010; Bookstein et al. 2001; 2002a,b).
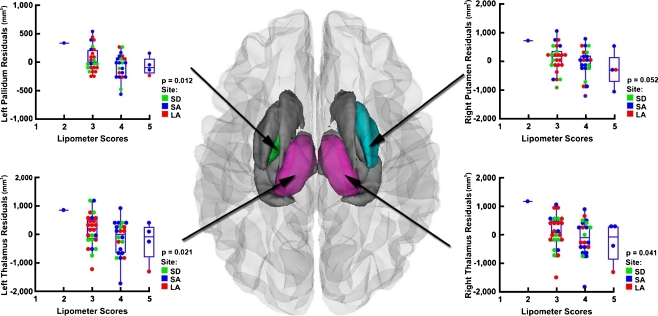
One study specifically tested for differences between a group of 30 subjects with FAS/pFAS and a group of 30 with SE:AE. They observed significant differences between these two groups in the total midsagittal brain and cerebellar areas, frontal lobe total and gray matter volumes, and putamen volume, suggesting a link between facial dysmorphology and brain abnormalities (Astley et al. 2009). Another recent study examined correlations between facial dysmorphology and brain volumes directly, using measures of palpebral fissure length and philtrum appearance (lipometer score). They observed significant correlations between palpebral fissure length and volume of the ventral diencephalon, as well as between lipometer scores and thalamus and left pallidum volumes; in all cases smaller volumes were associated with more severe dysmorphology (see Fig. 6) (Roussotte et al. 2011). Finally, it is important to note that findings of structural brain damage are robust in populations of exposed subjects both with and without dysmorphology, and that lack of facial features associated with FAS does not imply lack of cognitive, behavioral or neurological abnormalities.
Fig. 6.
Roussotte et al. (2011) showed correlations between lipometer score (a measure of philtrum dysmorphology) and volume of deep gray matter structures within subjects with prenatal alcohol exposure, demonstrating significantly smaller left pallidum and bilateral thalamus volumes (and a trend in right putamen) in FASD subjects with more severe facial abnormalities. Figure used with permission
Conclusions and Future Directions
Over the last 20 years, many studies have examined the effects of prenatal exposure to alcohol on brain structure in children, youth, and adults. Despite the diverse methods and sample populations, some consistent patterns of abnormalities have emerged. First, one of the most common findings in studies of subjects with prenatal alcohol exposure is reduced brain volume. Total brain volume reductions are almost always reported (Archibald et al. 2001; Astley et al. 2009; Autti-Ramo et al. 2002; Coles et al. 2011; Cortese et al. 2006; Johnson et al. 1996; Lebel et al. 2008; Mattson et al. 1992, 1994, 1996; Nardelli et al. 2011; Riikonen et al. 2005; Roussotte et al. 2011; Sowell et al. 2001a; Swayze et al. 1997; Willoughby et al. 2008), and because of this, total brain volume is often used as a covariate when testing the volumes of other brain structures, in order to determine whether they are reduced in proportion to total brain volume or beyond it. White matter and gray matter volumes are also typically smaller, although white matter seems to be affected slightly more than gray matter in terms of proportional reductions (Archibald et al. 2001; Bjorkquist et al. 2010; Lebel et al. 2008).
In terms of more specific changes, the corpus callosum, the largest white matter tract in the human brain, seems to be quite vulnerable to alcohol, with abnormalities ranging from agenesis and malformations (Astley et al. 2009; Autti-Ramo et al. 2002; Clark et al. 2000; Johnson et al. 1996; Mattson et al. 1992; Riley et al. 1995; Swayze et al. 1997) to shape variability (Bookstein et al. 2001; 2002a,b; Sowell et al. 2001a), displacement (Sowell et al. 2001a), and decreased area, width and/or length (Astley et al. 2009; Autti-Ramo et al. 2002; Sowell et al. 2001a). The deep gray matter structures have been assessed in a handful of studies, and are consistently reported to have reduced volume (Archibald et al. 2001; Astley et al. 2009; Autti-Ramo et al. 2002; Coles et al. 2011; Cortese et al. 2006; Mattson et al. 1992, 1994, 1996; Nardelli et al. 2011; Riikonen et al. 2005; Roussotte et al. 2011; Willoughby et al. 2008), suggesting that they also may be quite vulnerable to the teratogenic effects of alcohol. Most often, the caudate and hippocampus are reported to have reduced volume; however, many of the other structures have not been studied as extensively and thus it remains to be seen what the effects are on them.
The frontal, parietal and temporal lobes have been reported as abnormal in terms of a variety of measures, including volume (Archibald et al. 2001; Astley et al. 2009; Li et al. 2008), gray matter density (Sowell et al. 2001b, 2002a), shape (Sowell et al. 2002a,b), and cortical thickness (Sowell et al. 2008). Only the occipital lobes seem to be relatively spared by prenatal alcohol exposure, and were only reported to have reduced volume (in the occipital-temporal region) in one study (Li et al. 2008).
Thus, there seems to be a consensus that brain volume, and the volumes of many structures are reduced in subjects with prenatal alcohol exposure. More subtle abnormalities, such as shape variability, displacement, density, and cortical thickness have been reported as well, but were not examined by many studies. Thus, future research focusing on more subtle abnormalities may be beneficial for determining the precise effects of alcohol and perhaps providing more specific information about the potential causes of smaller volumes in FASD. Furthermore, examining the relationships between structural brain abnormalities and other parameters will provide valuable information. For example, an exploration of age-related changes within alcohol-exposed subjects may help determine whether developmental trajectories are similar in alcohol-exposed subjects and controls, or whether the two groups diverge at some point. Correlations with cognitive measures may be useful for studying interventions and possible treatments for these subjects, and research identifying links between the timing and amount of alcohol exposure and the observed structural brain abnormalities would greatly clarify how and when the damage occurs in utero. These types of studies generally require large sample sizes, with a large dynamic range on the variable of interest (e.g., spanning a wide age range or having diverse cognitive abilities) in order to detect differences.
Clearly, the teratogenic effects of alcohol on the brain are widespread and diverse. They range from global brain-volume reductions to specific shape abnormalities of individual structures, and affect not only subjects with the most severe diagnoses, but also subjects fewer cognitive/behavioral symptoms and those with no facial abnormalities. Future lines of research exploring subtle abnormalities and the relationship between structural brain abnormalities and other parameters will provide valuable insight into the causes and consequences of the brain damage caused by alcohol, and may ultimately help develop more effective ways of assessing and treating conditions associated with prenatal alcohol exposure.
Acknowledgments
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Abel EL. Prenatal effects of alcohol. Drug and Alcohol Dependence. 1984;14(1):1–10. doi: 10.1016/0376-8716(84)90012-7. [DOI] [PubMed] [Google Scholar]
- Abel EL. Prenatal effects of alcohol on growth: a brief overview. Federation Proceedings. 1985;44(7):2318–2322. [PubMed] [Google Scholar]
- Arango C, Moreno C, Martinez S, Parellada M, Desco M, Moreno D, et al. Longitudinal brain changes in early-onset psychosis. Schizophrenia Bulletin. 2008;34(2):341–353. doi: 10.1093/schbul/sbm157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archibald SL, Fennema-Notestine C, Gamst A, Riley EP, Mattson SN, Jernigan TL. Brain dysmorphology in individuals with severe prenatal alcohol exposure. Developmental Medicine and Child Neurology. 2001;43(3):148–154. [PubMed] [Google Scholar]
- Astley SJ. Diagnositc guide for fetal alcohol spectrum disorders: the 4-digit diagnostic code. Seattle: Fetal Alcohol Syndrome Diagnostic and Prevention Network, University of Washington; 2004. [Google Scholar]
- Astley SJ, Aylward EH, Olson HC, Kerns K, Brooks A, Coggins TE, et al. Magnetic resonance imaging outcomes from a comprehensive magnetic resonance study of children with fetal alcohol spectrum disorders. Alcoholism, Clinical and Experimental Research. 2009;33(10):1671–1689. doi: 10.1111/j.1530-0277.2009.01004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autti-Ramo I, Autti T, Korkman M, Kettunen S, Salonen O, Valanne L. MRI findings in children with school problems who had been exposed prenatally to alcohol. Developmental Medicine and Child Neurology. 2002;44(2):98–106. doi: 10.1017/S0012162201001748. [DOI] [PubMed] [Google Scholar]
- Bjorkquist OA, Fryer SL, Reiss AL, Mattson SN, Riley EP. Cingulate gyrus morphology in children and adolescents with fetal alcohol spectrum disorders. Psychiatry Research. 2010;181(2):101–107. doi: 10.1016/j.pscychresns.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonthius DJ, West JR. Alcohol-induced neuronal loss in developing rats: increased brain damage with binge exposure. Alcoholism, Clinical and Experimental Research. 1990;14(1):107–118. doi: 10.1111/j.1530-0277.1990.tb00455.x. [DOI] [PubMed] [Google Scholar]
- Bookstein FL, Sampson PD, Streissguth AP, Connor PD. Geometric morphometrics of corpus callosum and subcortical structures in the fetal-alcohol-affected brain. Teratology. 2001;64(1):4–32. doi: 10.1002/tera.1044. [DOI] [PubMed] [Google Scholar]
- Bookstein FL, Sampson PD, Connor PD, Streissguth AP. Midline corpus callosum is a neuroanatomical focus of fetal alcohol damage. The Anatomical Record. 2002a;269(3):162–174. doi: 10.1002/ar.10110. [DOI] [PubMed] [Google Scholar]
- Bookstein FL, Streissguth AP, Sampson PD, Connor PD, Barr HM. Corpus callosum shape and neuropsychological deficits in adult males with heavy fetal alcohol exposure. Neuroimage. 2002b;15(1):233–251. doi: 10.1006/nimg.2001.0977. [DOI] [PubMed] [Google Scholar]
- Bookstein FL, Streissguth AP, Connor PD, Sampson PD. Damage to the human cerebellum from prenatal alcohol exposure: the anatomy of a simple biometrical explanation. Anatomical Record. Part B: New Anatomist. 2006;289(5):195–209. doi: 10.1002/ar.b.20114. [DOI] [PubMed] [Google Scholar]
- Burd L, Klug MG, Martsolf JT, Kerbeshian J. Fetal alcohol syndrome: neuropsychiatric phenomics. Neurotoxicology and Teratology. 2003;25(6):697–705. doi: 10.1016/j.ntt.2003.07.014. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Acosta MT. The neuroanatomy of attention deficit/hyperactivity disorder. Revista de Neurologia. 2004;38(Suppl 1):S131–S136. [PubMed] [Google Scholar]
- Chasnoff IJ. Fetal alcohol syndrome in twin pregnancy. Acta Geneticae Medicae et Gemellologiae (Roma) 1985;34(3–4):229–232. doi: 10.1017/s0001566000004797. [DOI] [PubMed] [Google Scholar]
- Chernoff GF. The fetal alcohol syndrome in mice: maternal variables. Teratology. 1980;22(1):71–75. doi: 10.1002/tera.1420220110. [DOI] [PubMed] [Google Scholar]
- Christoffel KK, Salafsky I. Fetal alcohol syndrome in dizygotic twins. Jornal de Pediatria. 1975;87(6 Pt 1):963–967. doi: 10.1016/s0022-3476(75)80919-x. [DOI] [PubMed] [Google Scholar]
- Clark CM, Li D, Conry J, Conry R, Loock C. Structural and functional brain integrity of fetal alcohol syndrome in nonretarded cases. Pediatrics. 2000;105(5):1096–1099. doi: 10.1542/peds.105.5.1096. [DOI] [PubMed] [Google Scholar]
- Clarren SK, Alvord EC, Jr, Sumi SM, Streissguth AP, Smith DW. Brain malformations related to prenatal exposure to ethanol. Jornal de Pediatria. 1978;92(1):64–67. doi: 10.1016/s0022-3476(78)80072-9. [DOI] [PubMed] [Google Scholar]
- Coles, C. D., Goldstein, F. C., Lynch, M. E., Chen, X., Kable, J. A., Johnson, K. C., et al. (2011). Memory and brain volume in adults prenatally exposed to alcohol. Brain & Cognition, 75(1), 67–77. [DOI] [PMC free article] [PubMed]
- Cortese BM, Moore GJ, Bailey BA, Jacobson SW, Delaney-Black V, Hannigan JH. Magnetic resonance and spectroscopic imaging in prenatal alcohol-exposed children: preliminary findings in the caudate nucleus. Neurotoxicology and Teratology. 2006;28(5):597–606. doi: 10.1016/j.ntt.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Coulter CL, Leech RW, Schaefer GB, Scheithauer BW, Brumback RA. Midline cerebral dysgenesis, dysfunction of the hypothalamic-pituitary axis, and fetal alcohol effects. Archives of Neurology. 1993;50(7):771–775. doi: 10.1001/archneur.1993.00540070083022. [DOI] [PubMed] [Google Scholar]
- Feng MJ, Yan SE, Yan QS. Effects of prenatal alcohol exposure on brain-derived neurotrophic factor and its receptor tyrosine kinase B in offspring. Brain Research. 2005;1042(2):125–132. doi: 10.1016/j.brainres.2005.02.017. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nature Neuroscience. 1999;2(10):861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Gilliam DM, Stilman A, Dudek BC, Riley EP. Fetal alcohol effects in long- and short-sleep mice: activity, passive avoidance, and in utero ethanol levels. Neurotoxicology and Teratology. 1987;9(5):349–357. doi: 10.1016/0892-0362(87)90030-4. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14(1 Pt 1):21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Hoffman PL, Rabe CS, Moses F, Tabakoff B. N-methyl-D-aspartate receptors and ethanol: inhibition of calcium flux and cyclic GMP production. Journal of Neurochemistry. 1989;52(6):1937–1940. doi: 10.1111/j.1471-4159.1989.tb07280.x. [DOI] [PubMed] [Google Scholar]
- Hughes PD, Kim YN, Randall PK, Leslie SW. Effect of prenatal ethanol exposure on the developmental profile of the NMDA receptor subunits in rat forebrain and hippocampus. Alcoholism, Clinical and Experimental Research. 1998;22(6):1255–1261. doi: 10.1111/j.1530-0277.1998.tb03906.x. [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, Bittigau P, Ishimaru MJ, Wozniak DF, Koch C, Genz K, et al. Ethanol-induced apoptotic neurodegeneration and fetal alcohol syndrome. Science. 2000;287(5455):1056–1060. doi: 10.1126/science.287.5455.1056. [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, Bittigau P, Koch C, Genz K, Hoerster F, Felderhoff-Mueser U, et al. Neurotransmitters and apoptosis in the developing brain. Biochemical Pharmacology. 2001;62(4):401–405. doi: 10.1016/S0006-2952(01)00696-7. [DOI] [PubMed] [Google Scholar]
- Jacobson JL, Jacobson SW. Effects of prenatal alcohol exposure on child development. Alcohol Research & Health. 2002;26(4):282–286. [PMC free article] [PubMed] [Google Scholar]
- Johnson VP, Swayze VW, II, Sato Y, Andreasen NC. Fetal alcohol syndrome: craniofacial and central nervous system manifestations. American Journal of Medical Genetics. 1996;61(4):329–339. doi: 10.1002/(SICI)1096-8628(19960202)61:4<329::AID-AJMG6>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Jones KL, Smith DW. Recognition of the fetal alcohol syndrome in early infancy. Lancet. 1973;2(7836):999–1001. doi: 10.1016/S0140-6736(73)91092-1. [DOI] [PubMed] [Google Scholar]
- Jones PJ, Leichter J, Lee M. Placental blood flow in rats fed alcohol before and during gestation. Life Sciences. 1981;29(11):1153–1159. doi: 10.1016/0024-3205(81)90204-6. [DOI] [PubMed] [Google Scholar]
- Karl PI, Kwun R, Slonim A, Fisher SE. Ethanol elevates fetal serum glutamate levels in the rat. Alcoholism, Clinical and Experimental Research. 1995;19(1):177–181. doi: 10.1111/j.1530-0277.1995.tb01488.x. [DOI] [PubMed] [Google Scholar]
- Kennedy LA. Changes in the term mouse placenta associated with maternal alcohol consumption and fetal growth deficits. American Journal of Obstetrics and Gynecology. 1984;149(5):518–522. doi: 10.1016/0002-9378(84)90028-0. [DOI] [PubMed] [Google Scholar]
- Kinney H, Faix R, Brazy J. The fetal alcohol syndrome and neuroblastoma. Pediatrics. 1980;66(1):130–132. [PubMed] [Google Scholar]
- Kodituwakku PW. Neurocognitive profile in children with fetal alcohol spectrum disorders. Developmental Disabilities Research Reviews. 2009;15(3):218–224. doi: 10.1002/ddrr.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange N, Froimowitz MP, Bigler ED, Lainhart JE. Associations between IQ, total and regional brain volumes, and demography in a large normative sample of healthy children and adolescents. Developmental Neuropsychology. 2010;35(3):296–317. doi: 10.1080/87565641003696833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Rasmussen C, Wyper K, Walker L, Andrew G, Yager J, et al. Brain diffusion abnormalities in children with fetal alcohol spectrum disorder. Alcoholism, Clinical and Experimental Research. 2008;32(10):1732–1740. doi: 10.1111/j.1530-0277.2008.00750.x. [DOI] [PubMed] [Google Scholar]
- Lerch JP, Worsley K, Shaw WP, Greenstein DK, Lenroot RK, Giedd J, et al. Mapping anatomical correlations across cerebral cortex (MACACC) using cortical thickness from MRI. Neuroimage. 2006;31(3):993–1003. doi: 10.1016/j.neuroimage.2006.01.042. [DOI] [PubMed] [Google Scholar]
- Li Z, Ma X, Peltier S, Hu X, Coles CD, Lynch ME. Occipital-temporal reduction and sustained visual attention deficit in prenatal alcohol exposed adults. Brain Imaging Behavior. 2008;2(1):39–48. doi: 10.1007/s11682-007-9013-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcussen BL, Goodlett CR, Mahoney JC, West JR. Developing rat Purkinje cells are more vulnerable to alcohol-induced depletion during differentiation than during neurogenesis. Alcohol. 1994;11(2):147–156. doi: 10.1016/0741-8329(94)90056-6. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Jernigan TL, Ehlers CL, Delis DC, Jones KL, et al. Fetal alcohol syndrome: a case report of neuropsychological, MRI and EEG assessment of two children. Alcoholism, Clinical and Experimental Research. 1992;16(5):1001–1003. doi: 10.1111/j.1530-0277.1992.tb01909.x. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Jernigan TL, Garcia A, Kaneko WM, Ehlers CL, et al. A decrease in the size of the basal ganglia following prenatal alcohol exposure: a preliminary report. Neurotoxicology and Teratology. 1994;16(3):283–289. doi: 10.1016/0892-0362(94)90050-7. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Sowell ER, Jernigan TL, Sobel DF, Jones KL. A decrease in the size of the basal ganglia in children with fetal alcohol syndrome. Alcoholism, Clinical and Experimental Research. 1996;20(6):1088–1093. doi: 10.1111/j.1530-0277.1996.tb01951.x. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Gramling L, Delis DC, Jones KL. Heavy prenatal alcohol exposure with or without physical features of fetal alcohol syndrome leads to IQ deficits. Jornal de Pediatria. 1997;131(5):718–721. doi: 10.1016/s0022-3476(97)70099-4. [DOI] [PubMed] [Google Scholar]
- Miller MW. Effects of alcohol on the generation and migration of cerebral cortical neurons. Science. 1986;233(4770):1308–1311. doi: 10.1126/science.3749878. [DOI] [PubMed] [Google Scholar]
- Miller MW. Expression of transforming growth factor-beta in developing rat cerebral cortex: effects of prenatal exposure to ethanol. The Journal of Comparative Neurology. 2003;460(3):410–424. doi: 10.1002/cne.10658. [DOI] [PubMed] [Google Scholar]
- Miller SI, Del Villano BC, Flynn A, Krumhansl M. Interaction of alcohol and zinc in fetal dysmorphogenesis. Pharmacology, Biochemistry and Behavior. 1983;18(Suppl 1):311–315. doi: 10.1016/0091-3057(83)90192-2. [DOI] [PubMed] [Google Scholar]
- Mukherjee AB, Hodgen GD. Maternal ethanol exposure induces transient impairment of umbilical circulation and fetal hypoxia in monkeys. Science. 1982;218(4573):700–702. doi: 10.1126/science.6890235. [DOI] [PubMed] [Google Scholar]
- Mukherjee RA, Hollins S, Turk J. Fetal alcohol spectrum disorder: an overview. Journal of the Royal Society of Medicine. 2006;99(6):298–302. doi: 10.1258/jrsm.99.6.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardelli, A., Lebel, C., Rasmussen, C., Andrew, G., & Beaulieu, C. (2011). Extensive deep gray matter volume reductions in children and adolescents with fetal alcohol spectrum disorder. Alcohol Clin Exp Res, in press. [DOI] [PubMed]
- O’Connor MJ, Paley B. Psychiatric conditions associated with prenatal alcohol exposure. Developmental Disabilities Research Reviews. 2009;15(3):225–234. doi: 10.1002/ddrr.74. [DOI] [PubMed] [Google Scholar]
- O’Hare ED, Kan E, Yoshii J, Mattson SN, Riley EP, Thompson PM, et al. Mapping cerebellar vermal morphology and cognitive correlates in prenatal alcohol exposure. NeuroReport. 2005;16(12):1285–1290. doi: 10.1097/01.wnr.0000176515.11723.a2. [DOI] [PubMed] [Google Scholar]
- Ornoy A. Embryonic oxidative stress as a mechanism of teratogenesis with special emphasis on diabetic embryopathy. Reproductive Toxicology. 2007;24(1):31–41. doi: 10.1016/j.reprotox.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Paus T, Zijdenbos A, Worsley K, Collins DL, Blumenthal J, Giedd JN, et al. Structural maturation of neural pathways in children and adolescents: in vivo study. Science. 1999;283(5409):1908–1911. doi: 10.1126/science.283.5409.1908. [DOI] [PubMed] [Google Scholar]
- Paus T, Nawazkhan I, Leonard G, Perron M, Pike GB, Pitiot A, et al. Corpus callosum in adolescent offspring exposed prenatally to maternal cigarette smoking. Neuroimage. 2008;40(2):435–441. doi: 10.1016/j.neuroimage.2007.10.066. [DOI] [PubMed] [Google Scholar]
- Peiffer J, Majewski F, Fischbach H, Bierich JR, Volk B. Alcohol embryo- and fetopathy. Neuropathology of 3 children and 3 fetuses. Journal of the Neurological Sciences. 1979;41(2):125–137. doi: 10.1016/0022-510X(79)90033-9. [DOI] [PubMed] [Google Scholar]
- Pennington SN, Boyd JW, Kalmus GW, Wilson RW. The molecular mechanism of fetal alcohol syndrome (FAS). I. Ethanol-induced growth suppression. Neurobehavioral Toxicology and Teratology. 1983;5(2):259–262. [PubMed] [Google Scholar]
- Pollard I. Neuropharmacology of drugs and alcohol in mother and fetus. Seminars in Fetal & Neonatal Medicine. 2007;12(2):106–113. doi: 10.1016/j.siny.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Rakic P, Yakovlev PI. Development of the corpus callosum and cavum septi in man. The Journal of Comparative Neurology. 1968;132(1):45–72. doi: 10.1002/cne.901320103. [DOI] [PubMed] [Google Scholar]
- Reinhardt K, Mohr A, Gartner J, Spohr HL, Brockmann K. Polymicrogyria in fetal alcohol syndrome. Birth Defects Research. Part A: Clinical and Molecular Teratology. 2010;88(2):128–131. doi: 10.1002/bdra.20629. [DOI] [PubMed] [Google Scholar]
- Riikonen RS, Salonen I, Partanen K, Verho S. Brain perfusion SPECT and MRI in foetal alcohol syndrome. Developmental Medicine and Child Neurology. 1999;41(10):652–659. doi: 10.1017/S0012162299001358. [DOI] [PubMed] [Google Scholar]
- Riikonen RS, Nokelainen P, Valkonen K, Kolehmainen AI, Kumpulainen KI, Kononen M, et al. Deep serotonergic and dopaminergic structures in fetal alcoholic syndrome: a study with nor-beta-CIT-single-photon emission computed tomography and magnetic resonance imaging volumetry. Biological Psychiatry. 2005;57(12):1565–1572. doi: 10.1016/j.biopsych.2005.01.029. [DOI] [PubMed] [Google Scholar]
- Riley EP, Mattson SN, Sowell ER, Jernigan TL, Sobel DF, Jones KL. Abnormalities of the corpus callosum in children prenatally exposed to alcohol. Alcoholism, Clinical and Experimental Research. 1995;19(5):1198–1202. doi: 10.1111/j.1530-0277.1995.tb01600.x. [DOI] [PubMed] [Google Scholar]
- Rivkin MJ, Davis PE, Lemaster JL, Cabral HJ, Warfield SK, Mulkern RV, et al. Volumetric MRI study of brain in children with intrauterine exposure to cocaine, alcohol, tobacco, and marijuana. Pediatrics. 2008;121(4):741–750. doi: 10.1542/peds.2007-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roebuck TM, Mattson SN, Riley EP. A review of the neuroanatomical findings in children with fetal alcohol syndrome or prenatal exposure to alcohol. Alcoholism, Clinical and Experimental Research. 1998;22(2):339–344. doi: 10.1111/j.1530-0277.1998.tb03658.x. [DOI] [PubMed] [Google Scholar]
- Roebuck TM, Mattson SN, Riley EP. Interhemispheric transfer in children with heavy prenatal alcohol exposure. Alcoholism, Clinical and Experimental Research. 2002;26(12):1863–1871. doi: 10.1111/j.1530-0277.2002.tb02494.x. [DOI] [PubMed] [Google Scholar]
- Ronen GM, Andrews WL. Holoprosencephaly as a possible embryonic alcohol effect. American Journal of Medical Genetics. 1991;40(2):151–154. doi: 10.1002/ajmg.1320400206. [DOI] [PubMed] [Google Scholar]
- Roussotte, F. F., Sulik, K., Mattson, S. N., Riley, E. P., Jones, K. L., Adnams, C., et al. (2011). Regional brain volume reductions relate to facial dysmorphology and neurocognitive function in fetal alcohol spectrum disorders. Human Brain Mapping, in press. [DOI] [PMC free article] [PubMed]
- Shor, S., Nulman, I., Kulaga, V., & Koren, G. (2009). Heavy in utero ethanol exposure is associated with the use of other drugs of abuse in a high-risk population. Alcohol. [DOI] [PubMed]
- Sowell ER, Jernigan TL, Mattson SN, Riley EP, Sobel DF, Jones KL. Abnormal development of the cerebellar vermis in children prenatally exposed to alcohol: size reduction in lobules I-V. Alcoholism, Clinical and Experimental Research. 1996;20(1):31–34. doi: 10.1111/j.1530-0277.1996.tb01039.x. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nature Neuroscience. 1999;2(10):859–861. doi: 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Mattson SN, Thompson PM, Jernigan TL, Riley EP, Toga AW. Mapping callosal morphology and cognitive correlates: effects of heavy prenatal alcohol exposure. Neurology. 2001a;57(2):235–244. doi: 10.1212/wnl.57.2.235. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Mattson SN, Tessner KD, Jernigan TL, Riley EP, et al. Voxel-based morphometric analyses of the brain in children and adolescents prenatally exposed to alcohol. NeuroReport. 2001b;12(3):515–523. doi: 10.1097/00001756-200103050-00018. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Mattson SN, Tessner KD, Jernigan TL, Riley EP, et al. Regional brain shape abnormalities persist into adolescence after heavy prenatal alcohol exposure. Cerebral Cortex. 2002a;12(8):856–865. doi: 10.1093/cercor/12.8.856. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Peterson BS, Mattson SN, Welcome SE, Henkenius AL, et al. Mapping cortical gray matter asymmetry patterns in adolescents with heavy prenatal alcohol exposure. Neuroimage. 2002b;17(4):1807–1819. doi: 10.1006/nimg.2002.1328. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nature Neuroscience. 2003;6(3):309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Toga AW. Mapping changes in the human cortex throughout the span of life. The Neuroscientist. 2004;10(4):372–392. doi: 10.1177/1073858404263960. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Mattson SN, Kan E, Thompson PM, Riley EP, Toga AW. Abnormal cortical thickness and brain behavior correlation patterns in individuals with heavy prenatal alcohol exposure. Cerebral Cortex. 2008;18(1):136–144. doi: 10.1093/cercor/bhm039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Leow AD, Bookheimer SY, Smith LM, O’Connor MJ, Kan E, et al. Differentiating prenatal exposure to methamphetamine and alcohol versus alcohol and not methamphetamine using tensor-based brain morphometry and discriminant analysis. The Journal of Neuroscience. 2010;30(11):3876–3885. doi: 10.1523/JNEUROSCI.4967-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streissguth AP, Dehaene P. Fetal alcohol syndrome in twins of alcoholic mothers: concordance of diagnosis and IQ. American Journal of Medical Genetics. 1993;47(6):857–861. doi: 10.1002/ajmg.1320470612. [DOI] [PubMed] [Google Scholar]
- Sulik KK. Genesis of alcohol-induced craniofacial dysmorphism. Experimental Biology and Medicine (Maywood) 2005;230(6):366–375. doi: 10.1177/15353702-0323006-04. [DOI] [PubMed] [Google Scholar]
- Sulik KK, Johnston MC, Daft PA, Russell WE, Dehart DB. Fetal alcohol syndrome and DiGeorge anomaly: critical ethanol exposure periods for craniofacial malformations as illustrated in an animal model. American Journal of Medical Genetics. Supplement. 1986;2:97–112. doi: 10.1002/ajmg.1320250614. [DOI] [PubMed] [Google Scholar]
- Swayze VW, 2nd, Johnson VP, Hanson JW, Piven J, Sato Y, Giedd JN, et al. Magnetic resonance imaging of brain anomalies in fetal alcohol syndrome. Pediatrics. 1997;99(2):232–240. doi: 10.1542/peds.99.2.232. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Weinert SP, Sharif S, Riley EP. MK-801 administration during ethanol withdrawal in neonatal rat pups attenuates ethanol-induced behavioral deficits. Alcoholism, Clinical and Experimental Research. 1997;21(7):1218–1225. doi: 10.1111/j.1530-0277.1997.tb04441.x. [DOI] [PubMed] [Google Scholar]
- Toga AW, Thompson PM, Sowell ER. Mapping brain maturation. Trends in Neurosciences. 2006;29(3):148–159. doi: 10.1016/j.tins.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM, Reinvang I, Lundervold A, Dale AM, Eilertsen DE, et al. Effects of age on volumes of cortex, white matter and subcortical structures. Neurobiology of Aging. 2005;26(9):1261–1270. doi: 10.1016/j.neurobiolaging.2005.05.020. [DOI] [PubMed] [Google Scholar]
- Willoughby KA, Sheard ED, Nash K, Rovet J. Effects of prenatal alcohol exposure on hippocampal volume, verbal learning, and verbal and spatial recall in late childhood. Journal of the International Neuropsychological Society. 2008;14(6):1022–1033. doi: 10.1017/S1355617708081368. [DOI] [PubMed] [Google Scholar]
- Wisniewski K, Dambska M, Sher JH, Qazi Q. A clinical neuropathological study of the fetal alcohol syndrome. Neuropediatrics. 1983;14(4):197–201. doi: 10.1055/s-2008-1059578. [DOI] [PubMed] [Google Scholar]
- Witelson SF, Beresh H, Kigar DL. Intelligence and brain size in 100 postmortem brains: sex, lateralization and age factors. Brain. 2006;129(Pt 2):386–398. doi: 10.1093/brain/awh696. [DOI] [PubMed] [Google Scholar]
- Yakovlev PI, Lecours A-R. The myelogenetic cycles of regional maturation of the brain. In: Minkowski A, editor. Regional development of the brain early in life. Boston: Blackwell Scientific Publications Inc; 1967. pp. 3–70. [Google Scholar]