Abstract
Background
Interferon (IFN) alpha is one of the central agents in immunotherapy for renal cell carcinoma (RCC). It acts by binding to the IFN-alpha receptor (IFNAR). We previously reported that increased tumor expression of IFNAR2 mRNA was associated with the metastatic potential and progression of RCC, as well as with a poor response of metastatic RCC to IFN-alpha therapy. This study investigated the influence of serum IFNAR2 in RCC patients.
Methods
We measured serum IFNAR2 mRNA levels and quantified IFNAR mRNA expression in paired tumor and non-tumor tissues from the surgical specimens of 66 consecutive RCC patients by the real-time reverse transcription polymerase chain reaction (RT-PCR). We also measured phosphorylated Akt (Ser-473) and phosphorylated-S6 ribosomal protein (Ser-235/236) proteins levels in paired tumor and non-tumor tissues of patients with metastatic RCC by Western blotting.
Results
The serum level of IFNAR2 mRNA was not associated with its tumor tissue level. Serum IFNAR2 mRNA was positively correlated with tumor size (P < 0.05), but not with tumor grade, pT stage, metastasis, microscopic vascular invasion, or serum C-reactive protein. Serum levels of IFNAR2 mRNA were significantly higher in patients with a good response to IFN-alpha ± sorafenib than in those with a poor response (P < 0.0001). Tumor tissue IFNAR2 mRNA levels and phosphorylated-S6 ribosomal protein (Ser-235/236) levels were associated with metastatic potential (P < 0.001 and P < 0.01, respectively), and patients with a low IFNAR2 mRNA level and low phosphorylated Akt (Ser-473) protein level in the primary tumor showed a good response to IFN-α ± sorafenib (IFN-α ± Sor: CR-PR) (P < 0.01 and P < 0.05, respectively). Kaplan–Meier survival analysis showed that a higher serum IFNAR2 mRNA level was associated with longer overall survival of treated patients (P < 0.05), while a higher tumor tissue IFNAR2 mRNA level was related to shorter overall survival (P < 0.01).
Conclusions
Our findings suggest that a high serum level of IFNAR2 mRNA may be a useful marker for predicting the response of metastatic RCC to IFN-alpha ± sorafenib therapy.
Keywords: Interferon alpha, Receptor, mTOR, Sorafenib, Renal cell carcinoma, Metastasis
Introduction
Localized renal cell carcinoma (RCC) is generally considered to be a surgical disease. However, almost 30% of the patients who present with limited disease and undergo surgery develop metastasis during the next 3 years [1]. The incidence of RCC is steadily increasing, and it accounts for 2–3% of all adult malignancies. Around 40% of RCC patients die of metastasis, because of the high frequency of metastasis at diagnosis and relapse following nephrectomy [2]. Patients with distant metastases have a very poor prognosis, and their 5-year survival rate is less than 10% [2]. Clear cell RCC is considered to be an immunogenic tumor [3], with immunocytokine therapy being the mainstay of treatment [4], while it is notoriously resistant to chemotherapy and radiotherapy [2]. Targeting novel pathways associated with the evolution of malignancy may lead to an improved outcome for patients with RCC. Novel treatment options include immunotherapy [5], monoclonal antibodies [6], anti-angiogenesis therapy [7], and inhibitors of mammalian target of rapamycin (mTOR) [8–10].
Japanese patients who receive immunocytokine therapy including interferon alpha (IFN-α) show a better response and survival compared with American or European patients [11]. With the advent of molecular-targeting therapy, the use of sorafenib and sunitinib for RCC has been covered by the Japanese national health insurance system since 2008. Recently, Motzer et al. [12] identified five prognostic factors for RCC, which are known as the Memorial Slone-Kettering Cancer Center (MSKCC) classification (Karnofsky performance status, time from diagnosis of RCC to treatment or recurrence, serum lactate dehydrogenase, corrected serum calcium, and hemoglobin). The MSKCC classification is correlated with the overall survival of patients with metastatic RCC receiving IFN-α as initial systemic therapy. It has the potential to be employed for predicting the efficacy of immunocytokine therapy, and it may also be useful for predicting the response to molecular-targeting therapy. Recently, a number of molecular markers have been investigated in RCC patients to assess both their predictive value and their potential as therapeutic targets. Identification of new targets may lead to an improvement in the outcome of RCC, but no biomarkers have been established so far.
Individual variations of mRNA expression can have an important influence [13]. We previously reported that increased expression of IFNAR2 mRNA in tumor tissue is associated with the metastatic potential and progression of RCC and with a poor response to IFN-α therapy [14]. Our previous study also showed that a 102-KDa IFNAR2c protein, a functional domain of IFNAR2, is more highly expressed in the primary tumors compared with the non-tumor tissues of patients with metastatic RCC, but not localized RCC, indicating that the main component of IFNAR2 is the long form of IFNAR2c and that this protein may be important in the progression of RCC [14]. However, the influence of circulating (serum) IFNAR2 mRNA in patients with RCC has not been elucidated. In the present study, we examined serum levels of IFNAR2 mRNA, as well as the IFNAR2 mRNA levels in corresponding tumor tissue and non-tumor tissue samples, by the real-time reverse transcription polymerase chain reaction (RT-PCR). The relations among serum and tumor tissue levels of IFNAR2 mRNA and various clinicopathologic features of RCC patients were also examined. Furthermore, we investigated whether IFNAR2 mRNA could be used to predict the response of metastatic RCC to IFN-α ± sorafenib therapy. It was hoped that the information obtained might contribute to elucidation of the role of IFNAR2 in RCC.
Materials and methods
Patients, blood samples, and tissue specimens
We studied 66 consecutive Japanese patients (39 men and 27 women) aged 32–82 years (mean age: 62.9 years), who were newly diagnosed with clear cell RCC from 2008 to 2010. All patients routinely underwent imaging with CT and/or MRI for preoperative staging prior to radical nephrectomy. The postoperative follow-up period ranged from 2 to 35 months (median: 19 months). Surgery was done before the patients received any other therapy.
A peripheral whole blood sample (10 ml) was collected from each patient and was diluted in PBS with 2 mM EDTA. Then, peripheral blood mononuclear cells (PBMC) were separated using Ficol-Hypaque medium (Biocoll, Berlin, Germany) and gradient centrifugation and stored at −80°C for future use. Total RNA was isolated with an RNeasy kit (Qiagen, Hamburg, Germany), including a DNA digestion step using the RNase-free DNase set.
In every patient, three different tumor tissue specimens and various parts of the non-neoplastic kidney were harvested for this study and stored at −80°C, as described previously [13–15]. The tumor grade and clinical stage were determined according to the Fuhrman grading system and the TNM classification, respectively [16, 17]. This study was conducted in accordance with the Helsinki Declaration, and approval of the Dokkyo Medical University Hospital institutional review board was obtained. In addition, each patient signed a consent form that was approved by the Committee on Human Rights in Research of our institution.
Postoperative immunotherapy with IFN-α was given to 26 patients with extra-renal involvement. These patients received 3, 5, or 6 million units of natural human IFN-α intravenously or intramuscularly two or three times a week until tumor progression occurred. If the tumor was refractory to IFN-α monotherapy (progressive disease; PD), concomitant treatment with IFN-α and sorafenib (400 mg/day) was performed [18].
The doses of IFN-α and sorafenib were decreased if grade 3/4 toxicity occurred. Peripheral whole blood samples (10 ml) were obtained every 2 or 3 months from each metastatic RCC patient on treatment with IFN-α ± sorafenib. Tumor response was assessed according to RECIST criteria [19].
Real-time RT-PCR
Total RNA was purified from all 66 sets of serum, tumor tissue samples, and non-tumor tissue samples with an RNA preparation kit (“High Pure RNA Kit”; Roche Diagnostic Ltd., Germany) and was used as the template for cDNA synthesis. A 100-μl reaction mixture containing 1 mg of random hexamers and 100 units of MMLV reverse transcriptase was incubated at 25°C for 10 min, at 42°C for 30 min, and then at 99°C for 5 min. The IFNAR gene expression profile was analyzed with an ABI PRISM 7700 Sequence Detection System (Applied Biosystems, Foster City, CA) using the SYBR Green method. The following primers were employed to amplify the β-actin gene after confirming their specificity: sense; 5′-CTGGCATCGTGATGGACTCCGG-3′; anti-sense, 5′-GTGGATGCCACAGGACTCCATG-3′. The primers used for IFNAR1 and IFNAR2 have been previously reported [14]. Real-time RT-PCR was performed in a 25-μl reaction mixture containing 20 ng of sample cDNA, 100 nM sense primer, 100 nM anti-sense primer, and 12.5 μl of SYBR Green PCR Master Mix (Applied Biosystems), with 45 cycles of 95°C for 15 s and 60°C for 1 min. A standard curve for each mRNA was generated using fivefold dilutions of a control RNA sample (25×, 5×, 1×, 0.2×, and 0.04×). The expression of each IFNAR mRNA was calculated as a ratio to that of β-actin in the serum, tumor tissue, and corresponding normal tissue samples to determine the relative level of expression [13, 14]. Individual variations of mRNA expression considered to be important [13]. To investigate the influence of such individual variations in the expression of IFNAR1 mRNA and IFNAR2 mRNA in the blood and tissues, we compared the expression of these mRNAs in serum and paired samples of tumor and non-tumor tissues. For quantification of mRNA expression, the relative amounts of IFNAR1 and IFNAR2 mRNAs in the tumors were calculated as a ratio of the optical density of the bands for the tumor specimens to the density of the bands for the corresponding normal tissue specimens (T/N ratio) by densitometric analysis, as described previously [13, 14]. The mean value for three tissue samples was used, as described previously [13, 14]. We also measured the serum C-reactive protein (CRP) level (normal < 0.3 mg/dl) in each patient.
Western blotting
We could only perform Western blotting for 15 M1 tumors and four M0 tumors. Samples of tumor tissue and normal tissue were carefully dissected free of stromal tissue. Western blotting for phosphorylated Akt (Ser-473), Akt, and phosphorylated S6 ribosomal protein (Ser-235/236) was carried out as described previously [15, 20, 21]. Briefly, 50 μg of cytosolic protein was separated by SDS–PAGE on 12.5% gel and electrotransferred to a polyvinylidene difluoride membrane (Immobilon-P membrane; Millipore, Bedford, MA). After the membrane was blocked, the bound proteins were probed with an anti-rabbit monoclonal antibody for phosphorylated Akt (Ser-473) and an anti-rabbit monoclonal antibody for Akt (Cell Signaling Technology, Inc; PhosphoPlus Akt (Ser-473) Antibody Kit; # 9270, Danvers, MA), as well as an anti-rabbit monoclonal antibody for phosphorylated S6 ribosomal protein (Ser-235/236) (2F9) (Cell Signaling Technology, Inc; # 4856) and a primary antibody for β-actin (Santa Cruz Biotechnology, Santa Cruz, CA). Hela cells were used as the positive control. Next, the membranes were washed and incubated with horseradish peroxidase-conjugated secondary antibodies. Bands of antibody-bound proteins were visualized by chemiluminescence; the blotted membrane was scanned for densitometry with a PDI imaging scanner (Agfa Japan, Tokyo), and the data were analyzed with NIH Image software. Expression of phosphorylated Akt (Ser-473), Akt, and phosphorylated S6 ribosomal protein (Ser-235/236) was calculated relative to that of β-actin in the tumor tissue specimens and corresponding normal tissue specimens. For quantification of these proteins, the relative amount of phosphorylated Akt (Ser-473), Akt, or phosphorylated S6 ribosomal protein (Ser-235/236) in tumor tissue was expressed as a ratio of the optical density of the band for the tumor tissue specimen to that for the corresponding normal tissue specimen (set at 1.0) by densitometric analysis, as described previously [15, 20, 21]. The mean values for specimens of tumor and non-tumor tissue were calculated from three experiments [15, 21].
Statistical analysis
Real-time RT-PCR and Western blotting data were analyzed by the Mann–Whitney U-test for comparisons between two groups (pT stage, metastasis, microscopic pathological vascular invasion, and serum CRP), while the Kruskal–Wallis test was used to compare three groups (histological grade and treatment effect) [13–15]. Spearman’s rank correlation coefficient analysis was performed to determine the relations between IFNAR2 mRNA expression and the serum CRP level or tumor size. The Kaplan–Meier method was used to estimate survival, and differences of survival were assessed by the log-rank test. In all analyses, a P value of less than 0.05 was considered significant. Data were analyzed with commercially available software.
Results
Serum IFNAR mRNA and characteristics of RCC
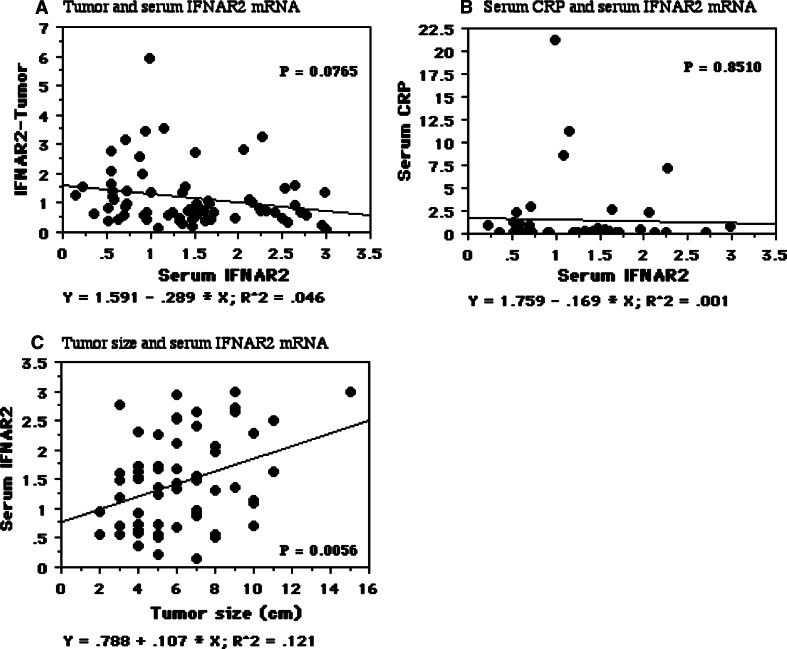
Although the preoperative serum IFNAR2 mRNA level was not related to the IFNAR2 mRNA level in tumor tissues (Fig. 1a) or the preoperative serum CRP level (Fig. 1b), there was a weak positive correlation between serum IFNAR2 and tumor size (r 2 = 0.121, P = 0.0056, Fig. 1c).
Fig. 1.
Spearman rank correlation coefficient relationship between mRNA expression levels of serum IFNAR2 mRNA and other factors. X axis is an independent variable. Y axis is a dependent variable. Serum IFNAR2 mRNA levels were associated with tumor size (c), but not with the IFNAR2 mRNAs in tumors (a) and serum CRP levels (b)
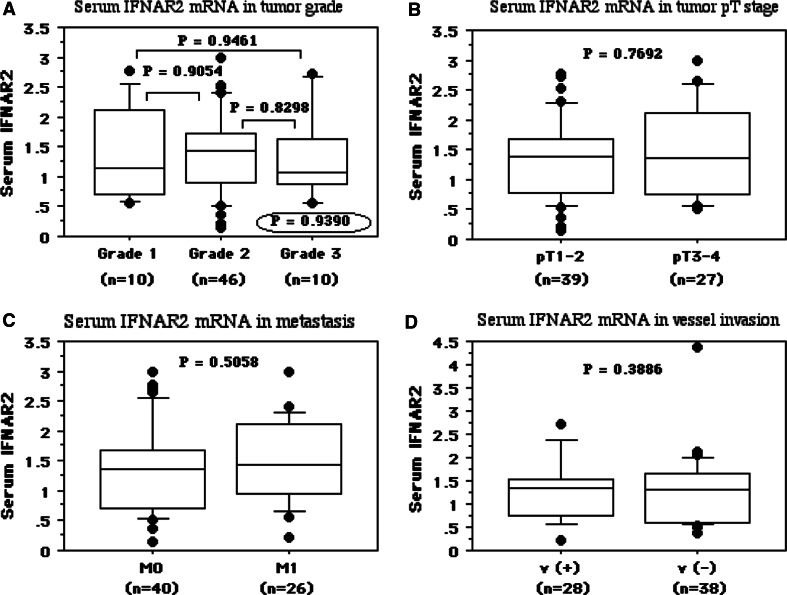
The preoperative serum IFNAR2 mRNA level was not associated with the histological grade of RCC (mean ± S.D., grade 1, 1.38 ± 0.80; grade 2, 1.41 ± 0.71; grade 3, 1.36 ± 0.78, P = 0.9390, Fig. 2a), the pT stage (pT1–2, 1.35 ± 0.67; pT3–4, 1.47 ± 0.80, P = 0.7692, Fig. 2b), tumor metastasis (M0, 1.37 ± 0.74; M1, 1.49 ± 0.71, P = 0.5058, Fig. 2c), or microscopic vascular invasion (v(−), 1.36 ± 0.69; v(+), 1.55 ± 0.80, P = 0.3886, Fig. 2d).
Fig. 2.
Serum levels for IFNAR2 mRNAs were not associated with tumor grade (a), pT stage (b), metastasis (c), and pathological microscopic vessel invasion (d). The median value is the central line, the box is the interquartile range, the bars are the full range, and the points are the outliers. Bold circled P values were obtained by comparing the three groups with the Kruskal–Wallis test
The preoperative serum level of IFNAR1 mRNA was not associated with the IFNAR1 mRNA level in tumor tissues, the preoperative serum CRP level, tumor size, histological grade, pT stage, tumor metastasis, or microscopic vascular invasion (data not shown).
A higher preoperative serum CRP level was associated with local invasion (pT1–2, 0.24 ± 0.30; pT3–4, 3.50 ± 5.48, P < 0.0001), metastatic disease (M0, 0.39 ± 0.63; M1, 4.11 ± 6.09, P = 0.0002, and microscopic vascular invasion (v(−), 0.49 ± 0.73; v(+), 4.11 ± 6.41, P = 0.0217), but not with the histological grade of RCC (grade 1, 1.18 ± 3.03; grade 2, 0.83 ± 1.47; grade 3, 4.49 ± 7.74, P = 0.0771) (data not shown).
Tumor expression of IFNAR mRNA and characteristics of RCC
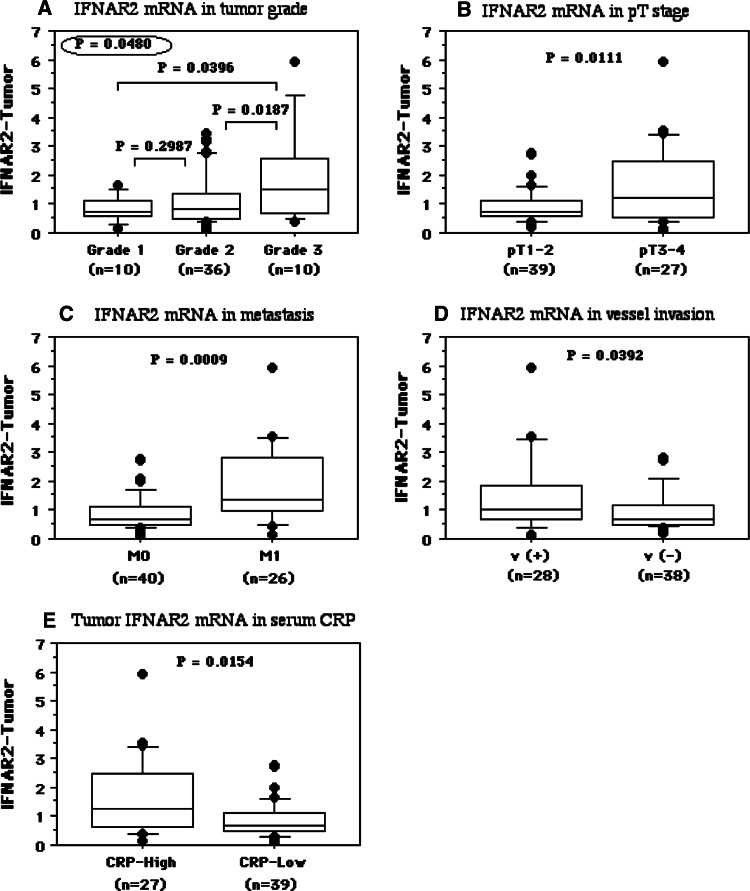
The IFNAR2 mRNA level in tumor tissue specimens was correlated with the histological grade of RCC (grade 1, 0.82 ± 0.46; grade 2, 1.12 ± 0.86; grade 3, 1.97 ± 1.61, P = 0.0480, Fig. 3a), pT stage (pT1–2, 0.94 ± 0.59; pT3–4, 1.58 ± 1.37, P = 0.0111, Fig. 3b), tumor metastasis (M0, 0.89 ± 0.61; M1, 1.83 ± 1.39, P = 0.0009, Fig. 3c), microscopic vascular invasion (v(−), 0.97 ± 0.70; v(+), 1.54 ± 1.39, P = 0.0392, Fig. 3d), and serum CRP (normal, <0.30 mg/dl; high CRP, 1.63 ± 1.35; low CRP, 0.88 ± 0.61; P = 0.0154, Fig. 3e). In contrast, there was no relationship between IFNAR1 mRNA expression and the histological grade (grade 1, 1.07 ± 0.79; grade 2, 1.75 ± 3.79; grade 3, 0.90 ± 0.67, P = 0.7472), stage (pT1–2, 0.87 ± 0.58; pT3–4, 2.30 ± 4.61, P = 0.2439), tumor metastasis (M0, 0.74 ± 0.51; M1, 3.07 ± 5.18, P = 0.0735), microscopic vascular invasion (v(-), 1.59 ± 3.91; v(+), 1.54 ± 1.08, P = 0.9736), or serum CRP (normal, < 0.30 mg/dl; high CRP, 2.50 ± 4.77; low CRP, 0.91 ± 0.71; P = 0.2646).
Fig. 3.
The IFNAR2 mRNA levels in tumors were associated with tumor grade (a), pT stage (b), metastasis (c), pathological microscopic vessel invasion (d), and serum CRP levels (e). The median value is the central line, the box is the interquartile range, the bars are the full range, and the points are the outliers. Bold circled P values were obtained by comparing the three groups with the Kruskal–Wallis test
Tumor expression of phosphorylated Akt (Ser-473), Akt, and phosphorylated S6 ribosomal protein (Ser-235/236) and characteristics of RCC
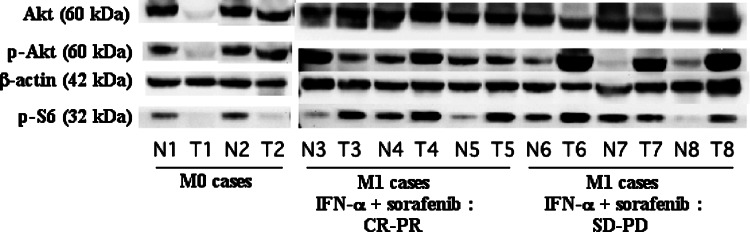
We could only perform Western blotting for 15 M1 tumors and four M0 tumors. The levels of phosphorylated Akt (Ser-473) and phosphorylated S6 ribosomal protein (Ser-235/236) were significantly higher in M1 tumors than in M0 tumors (3.93 ± 3.13, vs. 0.89 ± 0.40, P = 0.0257 and 3.82 ± 2.28 vs. 0.96 ± 0.84, P = 0.0079, respectively, Fig. 4). In contrast, there was no difference of Akt expression between M1 tumors and M0 tumors (1.13 ± 0.26, vs. 1.48 ± 1.87, P = 0.3662).
Fig. 4.
Expression of phosphorylated Akt (Ser-473) (60 kDa), Akt (60 kDa), phosphorylated S6 ribosomal protein (Ser-235/236) (32 kDa), and beta actin (42 kDa) proteins in the primary tumor tissues with metastatic lesions (M1) and without (M0) using Western blotting. N non-tumor tissue, T primary tumor tissue with metastatic lesions. Each number corresponds to a case number
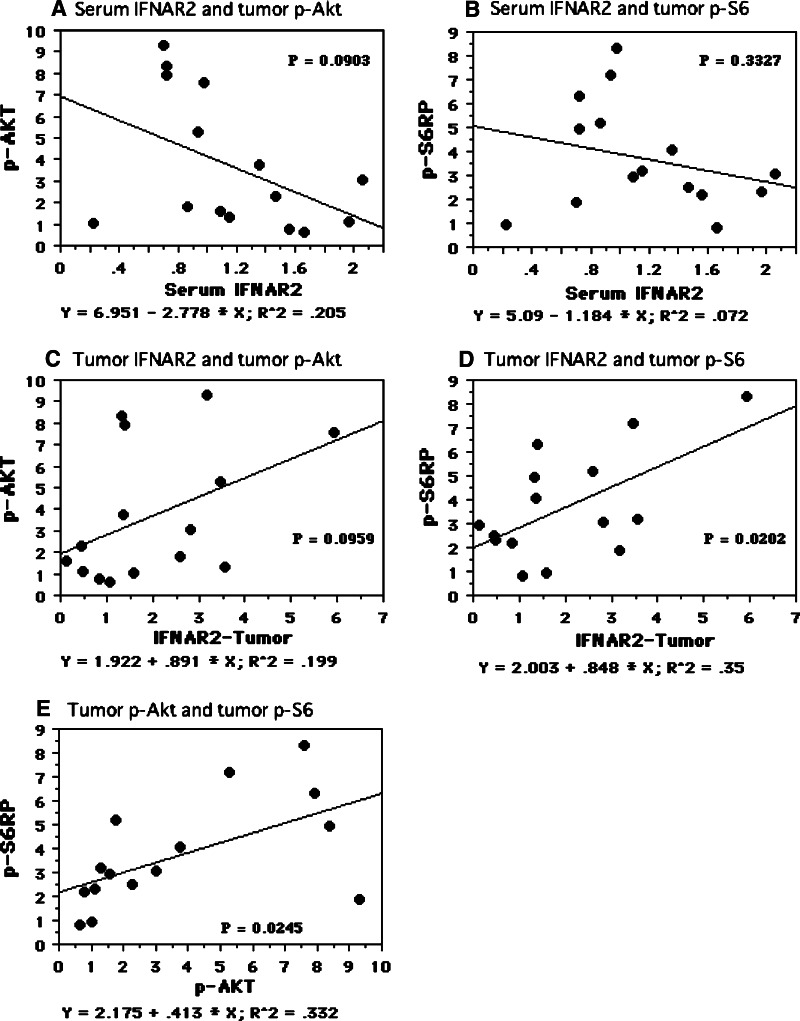
Preoperative serum level of IFNAR2 mRNA showed a weak negative correlation with tumor tissue levels of phosphorylated Akt (Ser-473) (r 2 = 0.205, P = 0.0903, Fig. 5a), but not with tumor tissue levels of AKT (r 2 = 0.017, P = 0.6587) or phosphorylated S6 ribosomal protein (Ser-235/236) (r 2 = 0.072, P = 0.3327, Fig. 5b). On the other hand, tumor tissue levels of IFNAR2 mRNA were positively associated with the levels of phosphorylated S6 ribosomal protein (Ser-235/236) (r 2 = 0.35, P = 0.0202, Fig. 5d) and also showed a weak positive correlation with phosphorylated Akt (Ser-473) (r 2 = 0.199, P = 0.0959, Fig. 5c), but not with Akt (r 2 = 0.001, P = 0.9154). Although there was a positive correlation between tumor tissue levels of phosphorylated Akt (Ser-473) and phosphorylated S6 ribosomal protein (Ser-235/236) (r 2 = 0.332, P = 0.0245, Fig. 5e), there was no relationship between either of these two phosphorylated proteins and Akt (data not shown).
Fig. 5.
Spearman rank correlation coefficient relationship. X axis is an independent variable. Y axis is a dependent variable. Serum IFNAR2 mRNA levels inversely correlated with tumor phosphorylated Akt (Ser-473) (a), but not tumor phosphorylated S6 ribosomal protein (Ser-235/236) (b). Tumor IFNAR2 mRNA levels were associated with tumor phosphorylated S6 ribosomal protein (Ser-235/236) (c), and weak positive correlation with tumor phosphorylated Akt (Ser-473) (d). Tumor phosphorylated Akt (Ser-473) and phosphorylated S6 ribosomal protein (Ser-235/236) were related each other (e)
Relationship between IFNAR2 mRNA, phosphorylated Akt (Ser-473), Akt, or phosphorylated S6 ribosomal protein (Ser-235/236) and the response to therapy
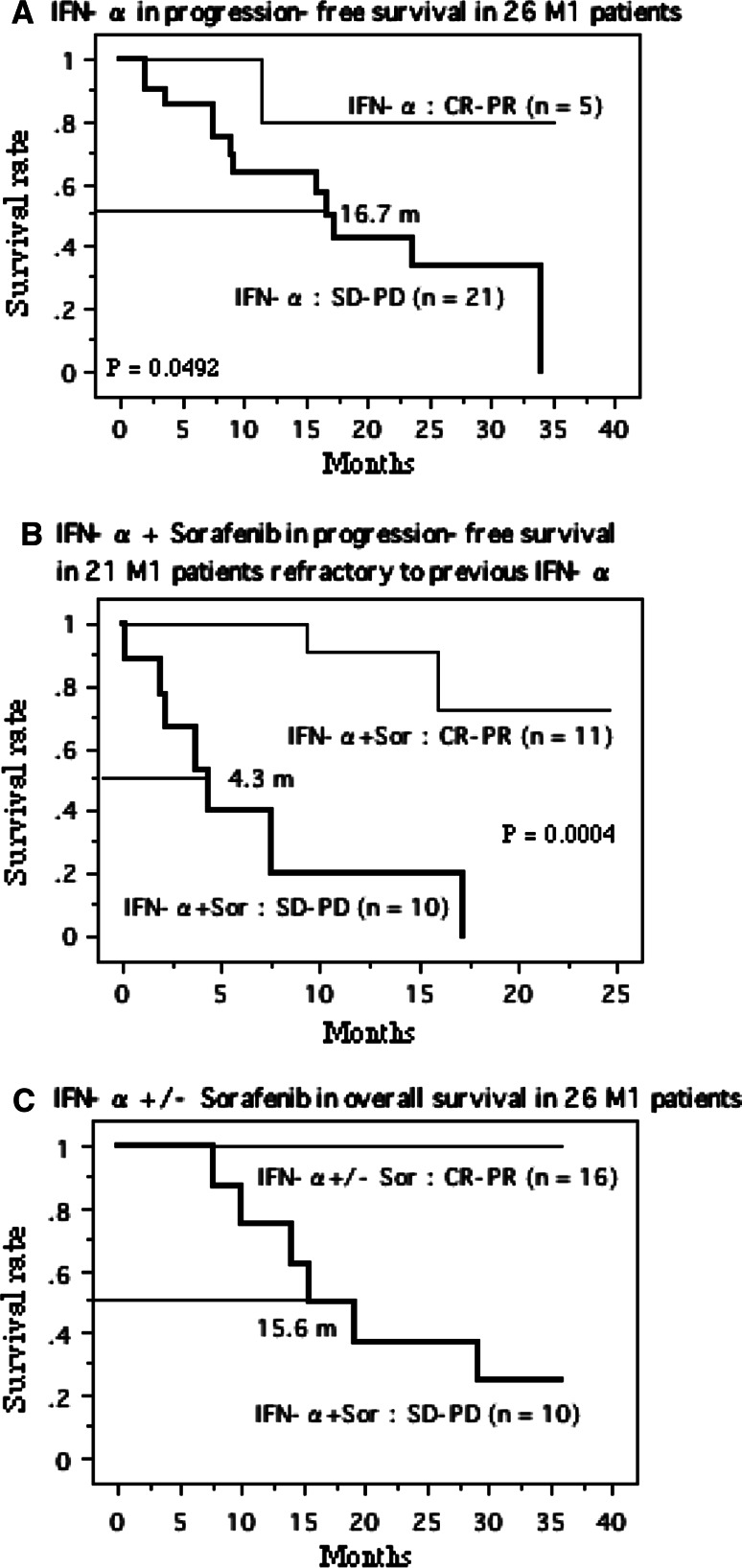
Kaplan–Meier survival plots for twenty-six metastatic RCCs treated with IFN-α monotherapy showed that the patients with a good response to this agent had better progression-free survival (Fig. 6a). Among the 21 patients refractory to IFN-α, IFN-α + Sor: CR-PR group had longer progression-free survival than IFN-α + Sor: SD-PD group (Fig. 6b). Thus, the patients with a good response to IFN-α with/without sorafenib had favorable overall survival (Fig. 6c).
Fig. 6.
Survival curve in the patients with metastatic lesions (M1) based on the treatment effects. a Progression-free survival curve in M1 patients treated with IFN-α alone. One patient of IFN-α: CR-PR group progressed gradually into progressive disease (PD) after partial response (PR), but continued IFN-α monotherapy. Median survival time in IFN-α: SD-PD group is 16.7 months. Survival time of IFN-α: CR-PR group does not reach. b Progression-free survival curve in M1 patients after combination therapy with IFN-α plus sorafenib. Median survival time in IFN-α + Sor: SD-PD group is 4.3 months. Survival time of IFN-α + Sor: CR-PR group does not reach. c Overall survival curve in M1 patients treated with IFN-α with/without sorafenib. Median survival time in IFN-α + Sor: SD-PD group is 15.6 months. Survival time of IFN-α ± Sor: CR-PR group does not reach. P value was analyzed by log-rank test
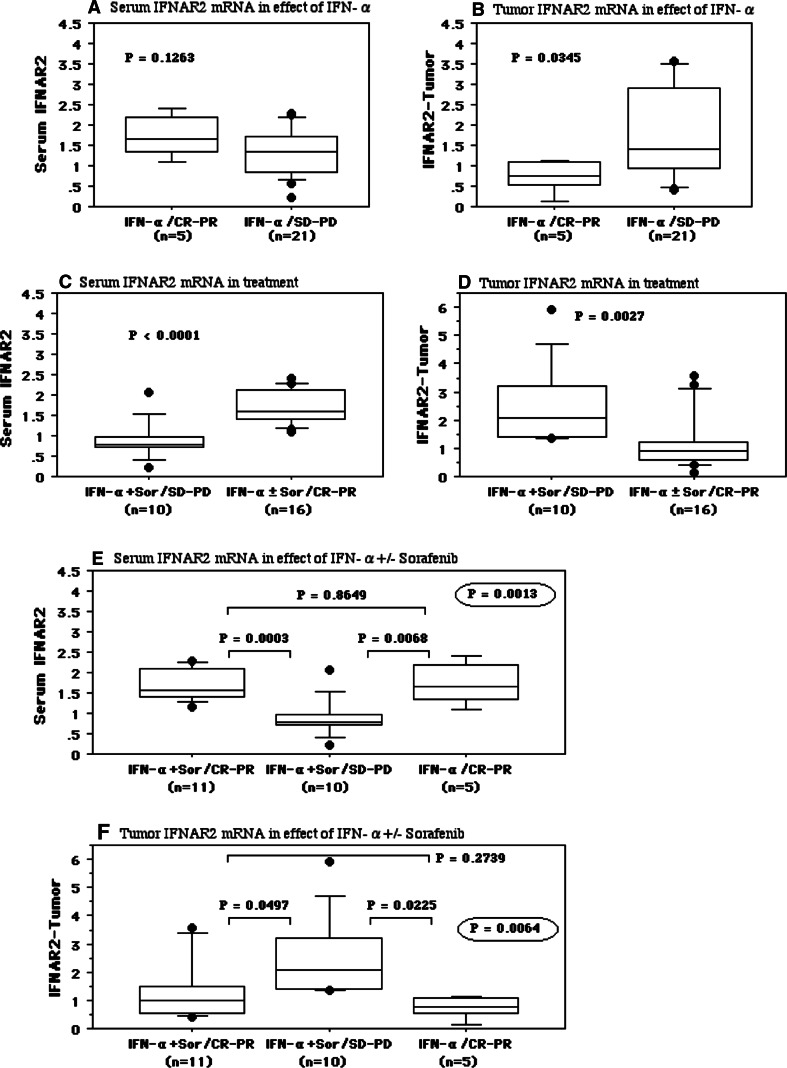
Twenty-six patients with metastatic disease received IFN-α as first-line adjuvant therapy. If these patients showed a poor response to IFN-α monotherapy, they received concomitant treatment with IFN-α and sorafenib (Sor) as second-line therapy. Five of the 26 patients showed a complete or partial response to IFN-α alone (IFN-α: CR-PR), while the other 21 patients received concomitant IFN-α + sorafenib as second-line therapy. The five patients with a good response to IFN-α alone (IFN-α: CR-PR) were low risk according to the MSKCC criteria. There was no difference of the preoperative serum IFNAR2 level between the patients with a good response to IFN-α (IFN-α: CR-PR, 1.74 ± 0.53) and those with a poor response, including stable disease or progressive disease (IFN-α: SD-PD; 1.31 ± 0.59, P = 0.1263, Fig. 7a). Lower tumor expression of IFNAR2 mRNA was related to a good response (IFN-α: CR-PR, 0.76 ± 0.40; IFN-α: SD-PD, 1.89 ± 1.38, P = 0.0345, Fig. 7b).
Fig. 7.
The relationship between treatment effect and mRNA levels of serum and tumor IFNAR2. The serum and tumor IFNAR2 mRNAs levels in the IFN-α monotherapy (a, b), and IFN-α ± sorafenib therapy (c–f). The median value is the central line, the box is the interquartile range, the bars are the full range, and the points are the outliers. Bold circled P values were obtained by comparing the three groups with the Kruskal–Wallis test
Among the 21 patients treated with IFN-α + sorafenib, eleven patients showed a good response (IFN-α + Sor: CR-PR), while the other 10 patients had stable disease or progressive disease (IFN-α + Sor: SD-PD). The background characteristics and the outcomes of the patients receiving concomitant therapy with IFN-α + sorafenib are summarized in Table 1. A higher preoperative serum IFNAR2 level was correlated with a good outcome (IFN-α + Sor: CR-PR, 1.70 ± 0.39; IFN-α: CR-PR, 1.74 ± 0.53; IFN-α + Sor: SD-PD, 0.88 ± 0.47, P = 0.0013, Fig. 7e). In order to better assess the effect of combination therapy, we combined the IFN-α : CR-PR group and the IFN-α + Sor: CR-PR group into a single good response group (IFN-α ± Sor: CR-PR). The preoperative serum IFNAR2 level was significantly higher in this good response group than in the group with a poor response to IFN-α + sorafenib (INF-α ± Sor: CR-PR, 1.71 ± 0.42; IFN-α + Sor: SD-PD, 0.88 ± 0.47, P < 0.0001) (Fig. 7c). On the other hand, low tumor expression of IFNAR2 mRNA was related to a good response (IFN-α + Sor: CR-PR, 1.34 ± 1.09; IFN-α: CR-PR, 0.76 ± 0.40; IFN-α + Sor: SD-PD, 2.51 ± 1.46, P = 0.0064, Fig. 7f, IFN-α ± Sor: CR-PR, 1.16 ± 0.95; IFN-α + Sor: SD-PD, 2.51 ± 1.46, P = 0.0027, Fig. 7d).
Table 1.
Background of 26 metastatic RCCs treated with IFN-α ± sorafenib (400 mg/day)
| Option | IFN-α alone | IFN-α + sorafenib | ||
|---|---|---|---|---|
| CR-PR (n = 5) | CR-PR (n = 11) | SD-PD (n = 10) | ||
| Sex (male/female) | ||||
| 19/7 | ||||
| Years (median) | ||||
| 39–78 (63) | ||||
| ECOG PS* (0/1/2) | ||||
| 17/7/2 | 5/0/0 | 8/3/0 | 4/4/2 | |
| MSKCC* (Fav/Int/Poor) | ||||
| 11/12/3 | 4/1/0 | 5/6/0 | 2/5/3 | |
| Duration of IFN-α* (mean:months) | 3–29 (14.7) | |||
| Duration of pre-IFN-α* (mean:months) | 1–27 (7.8) | |||
| Duration of IFN-α + sorafenib (mean:months) | 1–23 (9.7) | |||
| Metastatic lesions* (numbers) | ||||
| PUL | ||||
| 25 | 5 | 17 | 3 | |
| PLE | ||||
| 2 | 0 | 1 | 1 | |
| HEP | ||||
| 4 | 0 | 1 | 3 | |
| OSS | ||||
| 5 | Radiation for bone lesions | 0 | 2 | 3 |
| LYM | ||||
| 8 | 0 | 4 | 4 | |
| Others | ||||
| 2 | 0 | 0 | 2 | |
| AE*: Grade 1/2 (numbers) | ||||
| HT | 0 | 14 | ||
| Fatigue | 3 | 9 | ||
| Alopecia | 0 | 13 | ||
| Fever | 4 | 8 | ||
| HFS | 0 | 6 | ||
| Thrombocytopenia | 2 | 4 | ||
| Elevation of amylase and/or lipase | 0 | 5 | ||
| Others | 2 | 9 | ||
| AE*: Grade 3/4 (numbers) | ||||
| HT | 0 | 1 | ||
| Fatigue | 1 | 1 | ||
| Alopecia | 0 | 2 | ||
| Fever | 0 | 3 | ||
| Thrombocytopenia | 0 | 2 | ||
| Elevation of ALT and AST | 0 | 2 | ||
| Erythema multiforme | 0 | 1 | ||
ECOG PS* eastern cooperative oncology group (ECOG) performance status, MSKCC* Memorial Sloen-Kettering Cancer Center, Fav favorable risk, Int intermediated risk, Duration of IFN-α* duration of IFN-α monotherapy, Duration of pre-IFN-α* duration of IFN-α monotherapy prior to IFN-α plus sorafenib, Metastatic lesions* PUL Lung, PLE Pleura, HEP Liver, OSS Bone, LYM lymph node, AE* adverse events, HT hypertension, HFS hand-foot syndrome
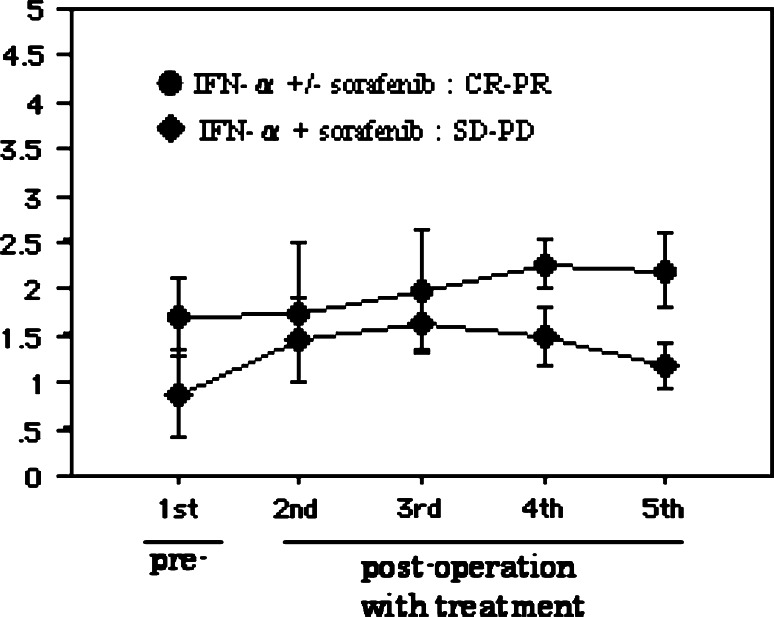
Interestingly, chronological analysis of serum IFNAR2 mRNA levels showed that these levels remained relatively high in the IFN-α ± Sor: CR-PR group and remained lower in the IFN-α + Sor: SD-PD group (Fig. 8).
Fig. 8.
Chronological analysis of serum IFNAR2 mRNA throughout IFN-α ± sorafenib treatment; IFN-α ± Sor: CR-PR group (n = 12), IFN-α + Sor: SD-PD group (n = 7)
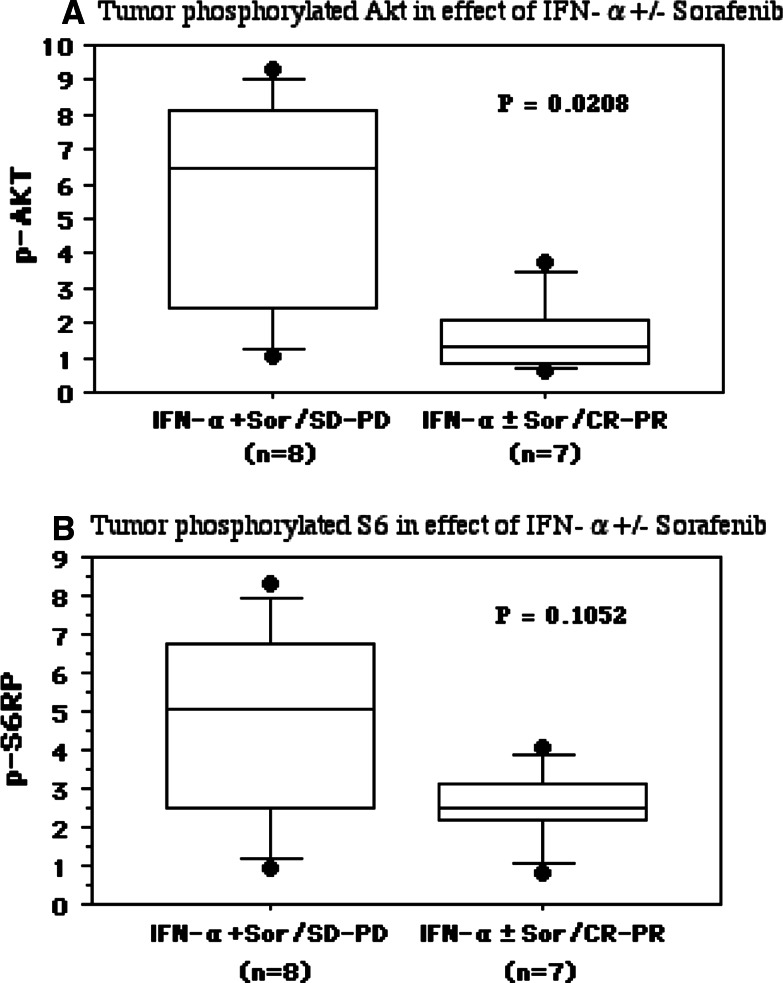
The IFN-α + Sor: CR-PR group had lower phosphorylated Akt (Ser-473) levels than the IFN-α + Sor: SD-PD group (1.65 ± 1.08 vs. 5.54 ± 3.22, P = 0.0208, Fig. 9a). Lower expression of phosphorylated S6 ribosomal protein (Ser-235/236) tended to be associated with a better response (IFN-α ± Sor: CR-PR, 2.50 ± 1.09; IFN-α + Sor: SD-PD, 4.72 ± 2.59, P = 0.1052, Fig. 9b). On the other hand, expression of Akt was similar in both the IFN-α + Sor: CR-PR group and the IFN-α + Sor: SD-PD group (1.11 ± 0.19 vs. 1.16 ± 0.34, P = 0.9078).
Fig. 9.
The relationship between treatment effect and protein expression levels of Akt, phosphorylated Akt (Ser-473) (a) and phosphorylated S6 ribosomal protein (Ser-235/236) (b). The relative expression levels of targeted proteins in the primary tumor to those in corresponding non-tumor portion, which was set to 1.0. Hela cell was used as the positive control. The median value is the central line, the box is the interquartile range, the bars are the full range, and the points are the outliers
Unlike IFNAR2 mRNA, serum and tissue levels of IFNAR1 mRNA were unrelated to the response to treatment (data not shown).
Prognostic value of IFNAR2 mRNA, phosphorylated Akt (Ser-473), and phosphorylated S6 ribosomal protein (Ser-235/236)
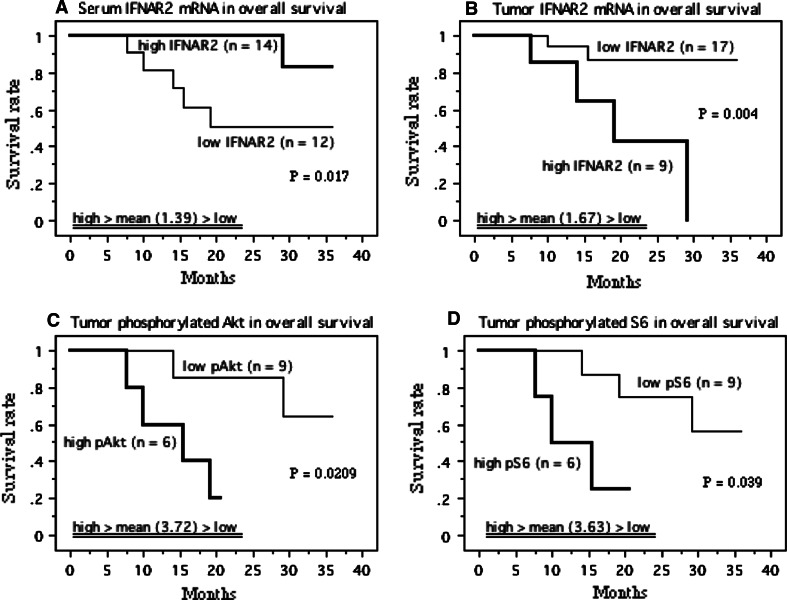
The mean serum level of IFNAR2 mRNA and mean tumor tissue levels of IFNAR2 mRNA, phosphorylated Akt (Ser-473), and phosphorylated S6 ribosomal protein (Ser-235/236) in M1 patients treated by IFN-α ± sorafenib were 1.39 (±0.60), 1.67 (±1.33), 3.72 (±3.12), and 3.63 (±2.24), respectively. Patients were divided into two groups based on these mean values (i.e., a high group and a low group), as described previously [13–15, 21]. Kaplan–Meier survival plots for patients with low versus high levels of these possible prognostic factors showed that a lower serum level of IFNAR2 mRNA (P < 0.05), a higher tumor tissue levels of IFNAR2 mRNA (P < 0.01), phosphorylated Akt (Ser-473) (P < 0.05), and phosphorylated S6 ribosomal protein (Ser-235/236) (P < 0.05) were correlated with shorter overall survival (OS) (Fig. 10a–d).
Fig. 10.
Survival curve in the patients with metastatic lesions (M1) based on the mean values of mRNA levels of serum and tumor lFNAR2 s, and of protein levels of tumor phosphorylated Akt (Ser-473) and phosphorylated S6 ribosomal protein (Ser-235/236), the cases were divided into two groups at this levels—high and low expression. Progression-free survival curve based on serum (a) and tumor IFNAR2 mRNA levels (b) in M1 patients treated with IFN-α ± sorafenib therapy. Progression-free survival curve based on tumor proteins for phosphorylated Akt (Ser-473) (c) and phosphorylated S6 ribosomal protein (Ser-235/236) (d) in M1 patients treated with IFN-α + sorafenib therapy. Overall survival curve based on serum (e) and tumor IFNAR2 mRNA levels (f) in M1 patients treated with IFN-α ± sorafenib therapy. Overall survival curve based on tumor proteins for phosphorylated Akt (Ser-473) (g) and phosphorylated S6 ribosomal protein (Ser-235/236) (h) in M1 patients treated with IFN-α + sorafenib therapy. P value was analyzed by log-rank test
Discussion
There were five main findings of this study. First, the preoperative serum level of IFNAR2 mRNA was correlated with tumor size. Second, the tumor tissue levels of IFNAR2 mRNA and phosphorylated S6 ribosomal protein (Ser-235/236) were positively correlated with each other, and both were related to tumor metastatic potential. Third, while the patients with a high preoperative serum level of IFNAR2 mRNA showed a good response to IFN-α ± sorafenib (IFN-α ± Sor; CR-PR) and a longer overall survival, the patients with a low serum level and a high tumor tissue level of IFNAR2 mRNA level showed a poor response (SD-PD) and a shorter overall survival. Fourth, the patients with a high serum level of IFNAR2 mRNA throughout treatment showed a good response to IFN-α ± Sor. Fifth, a high level of phosphorylated Akt (Ser-473), but not a high level of phospho-S6 ribosomal protein (Ser-235/236), in the primary tumor was related to a poor response to IFN-α ± sorafenib. These findings suggest that the serum level of IFNAR2 mRNA might be useful to predict the efficacy of IFN-α ± sorafenib therapy, while the tumor tissue level of IFNAR2 mRNA could be associated with metastatic potential and tumor resistance.
Combination therapy with IFN-α and sorafenib
While the efficacy of combination therapy with IFN-α and sorafenib needs clarification, the adverse effects of this regimen are related to the doses of each agent and are not additive [22–25]. We previously reported a good response to combination therapy with IFN-α and half the usual dose of sorafenib (400 mg/day rather than 800 mg/day) in patients with IFN-α-resistant RCC, along with tolerable adverse events [18].
It has also been reported that combined treatment with IFN-α + sorafenib suppresses proliferation and vascular endothelial growth factor (VEGF) production by several RCC cell lines more strongly than either agent alone [26, 27]. RCC is considered to be an immunogenic tumor [3], since cytotoxic T lymphocytes recognize and selectively kill autologous RCC cells, while tumor-specific T cells can be detected in the blood of RCC patients [3]. Sorafenib is a multikinase inhibitor targeting VEGF receptors 1–3, PDGFβ receptor, and Raf kinase, and it has both direct antitumor activity and antiangiogenic activity [28]. In a randomized phase III trial comparing sorafenib with placebo as second-line therapy for RCC, the response rate to sorafenib was 10%, and the stable disease rate was 74%, with the median progression-free survival time being 5.5 months in the sorafenib group versus 2.8 months in the placebo group [29]. In addition to its immunomodulatory effects, IFN-α also has direct antitumor activity as well as antiangiogenic activity, including inhibition of VEGF [30]. Antitumor immunity is usually suppressed in tumor-bearing mice because of the influence of regulatory T cells and suppressive cytokines, such as TGF-β and IL-10 [31]. Takeuchi et al. recently reported that the synergistic effect of sorafenib and IFN-α on RCC both in vitro and in tumor-bearing mice was related to a combination of antitumor, antiangiogenic, and immunologic responses [27]. In their study, sorafenib had no effect on the levels of natural killer (NK) cells, T cells, and regulatory T cells in the spleens of tumor-bearing BALB/c mice, irrespective of the use of IFN-α, while IFN-α showed weaker direct antitumor activity than sorafenib but stimulated CTL, NK cells, and tumor-infiltrating lymphocytes (which sorafenib did not), so that a synergistic antiproliferative effect of these two agents was demonstrated in vitro [27].
Although an additive effect of IFN-α to sorafenib therapy in patients with metastatic RCC was recently reported [24], the clinical efficacy of this combination remains to be confirmed. Jonasch et al. reported that the outcome after addition of IFN-α to sorafenib was comparable to that of sorafenib monotherapy, when patients were randomized to treatment with either sorafenib (400 mg twice daily) or the combination of sorafenib (400 mg twice daily) plus IFN-α (0.5 MU twice daily) [25]. However, their IFN-α dose of 1 million units daily was probably too low to assess its additive effect because the average dose is 3–9 million units daily. So far, the published studies on combination therapy with IFN-α and sorafenib have employed concomitant therapy with both agents. In contrast, our treatment strategy was to add sorafenib (400 mg/day) to IFN-α in RCC patients whose tumors were refractory to IFN-α alone, i.e., first-line IFN-α monotherapy and second-line combination therapy with IFN-α plus sorafenib [18]. In the present study, 26 patients had metastatic disease (M1) at diagnosis. Among them, five patients showed a good response to IFN-α alone (IFN-α: CR-PR), 11 patients showed a good response to IFN-α + sorafenib (IFN-α + Sor: CR-PR, Table 1), and the remaining 10 patients had stable disease or showed a poor response to IFN-α + sorafenib (IFN-α + Sor: SD-PD, Table 1). Although half-dose sorafenib (400 mg/day) caused grade 1/2 toxicity, grade 3/4 toxicity was rare in the present study and such toxicity resolved when patients suspended sorafenib therapy. The response rate to the combination of IFN-α plus half-dose sorafenib was 52.4% (11/21 patients resistant to previous IFN-α monotherapy achieved CR or PR), indicating that sorafenib may be synergistic with IFN-α, leading to an increase of antitumor activity. Furthermore, the patients with good response to this combination therapy had favorable prognosis (Fig. 6). Thus, this combination seems to be tolerable and could be a useful treatment option for advanced RCC resistant to IFN-α monotherapy [18].
Role of the IFNAR2 and mTOR pathways in progression of RCC
The preoperative serum level of IFNAR2 mRNA was not correlated with the effect of IFN-α monotherapy, but a lower tumor tissue level of IFNAR2 mRNA was related to a better response to IFN-α. The present finding that a higher tumor tissue level of IFNAR2 mRNA was associated with a poor response to IFN-α is consistent with our previous results [14]. In contrast, regarding the relationship between IFNAR2 mRNA levels and the effect of combination therapy with IFN-α plus sorafenib, the preoperative serum level of IFNAR2 mRNA was higher in the IFN-α + Sor: CR-PR group and the IFN-α: CR-PR group than in the IFN-α + Sor: SD-PD group (Fig. 6e), while the tumor tissue level of IFNAR2 mRNA was lower in the IFN-α + Sor: CR-PR group and the IFN-α: CR-PR group than in the IFN-α + Sor: SD-PD group (Fig. 6f). These findings suggested that a different molecular mechanism might be involved in IFN-α ± Sor: CR-PR group and IFN-α + Sor: SD-PD group.
IFNs are pleiotropic cytokines that regulate antiviral, antitumor, apoptotic, antiangiogenic, and cellular immune responses via activation of multiple downstream signaling cascades, including the Janus tyrosine kinase (Jak)-signal transducer and activator of transcription (STAT) pathway, the p38 mitogen-activated protein kinase (MAPK) pathway, and the mTOR pathway [32, 33]. The Jak-STAT and p38-MAPK signaling pathways have been shown to be responsible for transcription of the genes encoding proteins related to the antiviral and/or antiproliferative effects of IFNs. On the other hand, it has been reported that activation of the mTOR pathway by IFNs has an important regulatory role in mRNA translation and induction of the interferon response [34, 35]. IFN-α-induced tumor cell apoptosis is also mediated via the mTOR pathway in a nucleus-independent manner [36, 37]. Moreover, the Jak-STAT and mTOR pathways act separately from each other after activation by IFN-α [37]. Thus, it is likely that mTOR signaling selectively mediates apoptosis and survival.
The rapamycin-sensitive mTOR-raptor (regulatory-associated protein of mTOR) complex controls cell growth by regulating protein synthesis, so mTOR-raptor signaling is a potential antitumor target, and mTOR inhibitors are currently under investigation for the treatment of various human cancers. On the other hand, mTOR also interacts with rictor (rapamycin-insensitive companion of mTOR), and recent findings have suggested that the rapamycin-insensitive effect of mTOR on cell survival is overactive in many cancers. Thus, mTOR has dual rapamycin-sensitive (mTOR-raptor complex: mTORC1) and insensitive (mTOR-rictor complex: mTORC2) functions, indicating that treatment with rapamycin will not completely inhibit mTOR activity [38, 39]. Phosphatidylinositol 3`kinase (PI3 K), serine/threonine kinase Akt, and the mTOR pathway are all overactive in human cancers. mTORC1 lies downstream of PI3 K and is part of a pathway that is frequently activated in human cancers, so mTORC1 represents a pivotal target for anticancer therapy. The best-characterized pathways regulated by mTORC1 are phosphorylation and activation of ribosomal S6 kinase-1 (S6K1) and phosphorylation and inactivation of 4E-BP1, the suppressor of mRNA cap-binding protein eIF4E, leading to effects on cell growth and metabolism by acting as a restriction point in cells subjected to stresses [40, 41] such as hypoxia [42–44].
Phosphorylation at two sites is required for full activation of Akt, since it is phosphorylated by PI3 K-dependent kinase-1 (PDK1) at a threonine residue in the catalytic domain (Thr 308) and by PI3 K-dependent kinase-2 (PDK2) at a serine residue (Ser 473) in the carboxy-terminal hydrophobic motif [45]. It has been reported that mTORC2 regulates the actin cytoskeleton and also possesses PDK2 activity that phosphorylates Ser-473 in the carboxy-terminal of Akt, making it essential for Akt activity [46]. Importantly, activation of Akt may lead to cell survival when mTORC1 is inhibited or could potentially increase VEGF production because PI3 K/Akt signaling induces tumor angiogenesis by regulating VEGF via both HIF-1α-dependent and -independent mechanisms [47]. It has been reported that hypoxia-inducible factor (HIF) 1α expression is dependent on both raptor and rictor, whereas HIF2α expression only depends on rictor and HIF2α is more important in RCC [48]. These findings suggest that phosphorylation of Ser 473 in AKT is a key molecular step in the progression of RCC and could be a target for treating these tumors [25]. In agreement with this, our current study showed that the tumor tissue levels of IFNAR2 mRNA and phosphorylated S6 ribosomal protein (Ser-235/236) were positively correlated and that both were related to tumor metastatic potential, while a high phosphorylated Akt (Ser-473) level in the primary tumor was associated with a poor response to IFN-α ± sorafenib therapy (stable or progressive disease).
Current efforts to achieve the clinical development of mTOR inhibitors are based on the role of mTOR signaling in promoting the proliferation and survival of tumor cells. It has been reported that treatment with mTOR inhibitors can improve the outcome of patients with metastatic RCC [8–10]. On the other hand, the mTOR pathway is also important for IFN-dependent translational responses, and IFN-α is widely used to treat advanced RCC. Although we could not exclude a possible detrimental effect of IFN-α treatment in the patients with metastatic RCC and higher IFNAR2 mRNA levels in their tumors, our findings suggested that there may be different molecular mechanisms of cancer progression in the patients with a good or poor response to IFN-α ± sorafenib. It is possible that IFNAR2 signaling has different biological effects from normal when upregulated in RCC.
Jonasch et al. recently reported that an increase of phosphorylated Akt (Ser-473) was associated with worse survival by microarray analysis of paraffin-embedded specimens [25]. In the present study, the tumors with higher phosphorylated Akt (Ser-473) levels, but not higher phospho-S6 ribosomal protein (Ser-235/236) levels, were resistant to IFN-α ± sorafenib therapy. In addition, the tumor tissue levels of IFNAR2 mRNA and phosphorylated S6 ribosomal protein (Ser-235/236) were positively correlated, and both were related to metastatic potential. Tumor levels of IFNAR2 mRNA also had a weak positive correlation with those of phosphorylated Akt (Ser-473). Moreover, the patients whose tumors had higher levels of phosphorylated Akt (Ser-473), phosphorylated S6 ribosomal protein (Ser-235/236), and IFNAR2 mRNA showed shorter overall survival. Taken together, it is possible that phosphorylation of Ser 473 on Akt is a key molecular step in the progression of RCC and a potential therapeutic target, so that the tumor level of phosphorylated Akt (Ser-473) may be useful for predicting the response to treatment. At present, it remains to be elucidated why upregulation of IFNAR2 expression is linked to the progression of RCC and to a poor response to treatment, and it is unclear how IFNAR2 interacts with mTORC1 and mTORC2, but our findings suggested that the IFNAR2-mTORC1 pathway via phosphorylated S6 ribosomal protein (Ser-235/236) may act locally within tumors to promote proliferation and metastasis by modifying mRNA translation, while the IFNAR2-mTORC2 pathway via phosphorylated Akt (Ser-473) may be associated with tumor resistance. So, these interactions should be elucidated in the future. As Lekmine et al. have indicated, therefore, caution should be exercised when designing clinical trials that combine an mTOR inhibitor and IFN-α due to possible antagonism of antitumor activity [34]. In fact, Huges et al. reported that patients treated with temsirolimus alone had better overall survival than those given IFN-α alone, while patients treated with temsirolimus plus IFN-α did not [49]. In the future, the downstream targets of IFNAR2 should be identified, and the expression or activity of one or two such targets should be studied in cell lines or tissue samples. A better understanding of the IFNAR2 pathway may help to elucidate its role in cancer.
Although none of our patients were treated by sorafenib alone, it would be interesting to assess the expression of not only IFNAR2, phosphorylated Akt (Ser-473), and phosphorylated S6 ribosomal protein (Ser-235/236), but also VEGF receptor and Raf, in tumor cells and the effects of sorafenib, sunitinib, or mTOR inhibitors. Such information could lead to elucidation of the role of the IFNAR2-mTOR pathway in the progression of RCC and the selection of patients who will benefit from treatment with IFN-α, sorafenib, sunitinib, or mTOR inhibitors.
Role of serum IFNAR2 in progression of RCC
The serum CRP level is associated with the stage and outcome of RCC [50, 51]. Elevation of CRP is primarily determined by an increase of circulating IL-6 [52], and the IL-6 level is correlated with the serum CRP level as well as with tumor histological grade and tumor metastasis [53]. We previously reported that increased serum levels of CRP and IL-6 were associated with local tumor invasion and metastasis [54]. In the present study, a higher preoperative serum CRP level was associated with local invasion and metastasis of RCC, but not with the response to treatment (data not shown). These findings suggest that the serum CRP level is associated with tumor aggressiveness, so that elevation of CRP might reflect the poorer general condition of the patient rather than the response to therapy. On the other hand, IFN-α has immunomodulatory effects and direct antitumor activity as well as antiangiogenic activity, including inhibition of VEGF [30]. Therefore, it is unclear exactly what serum IFNAR2 reflects, but it is likely to be associated with the overall immune status. Accordingly, we expected to find a relation between the preoperative serum levels of IFNAR2 mRNA and CRP, but no relation was observed.
Although we could not examine how IFN-α binds to IFNAR2 on peripheral blood cells and then exhibits antitumor and antiangiogenic activity, our findings showed that the preoperative serum level of IFNAR2 mRNA was positively correlated with tumor size and was higher in patients with metastatic RCC who showed a good response to IFN-α ± sorafenib therapy than in those with a poor response. Because obtaining blood samples from patients is easier than harvesting tissue samples, chronological analysis of serum IFNAR2 mRNA levels is preferable for evaluation of the role of IFNAR2. While the effect of IFN-α therapy on the serum level of IFNAR2 mRNA is still unclear, our chronological evaluation of IFNAR2 mRNA throughout treatment showed that its serum level remained higher in the IFN-α ± Sor: CR-PR group than in the IFN-α + Sor: SD-PD group. Taken together, these observations suggest that an increased serum level of IFNAR2 mRNA may represent a systemic immunologic and antitumor response to the tumor burden in RCC patients, as well as showing antimicrobial activity if infection occurs.
Regarding the effect of genetic polymorphism on the response of metastatic RCC to IFN-α, it has been reported that STAT3 polymorphism is a useful diagnostic marker for predicting the response to IFN-α therapy in these patients [55]. An efficient marker of the response to IFN-α is needed to establish individualized optimal treatment strategies, especially when newer therapies are used as first-line treatment for metastatic RCC. Our study showed that patients with higher serum levels of IFNAR2 mRNA may be more likely to respond to IFN-α ± sorafenib therapy and to show a longer overall survival, while patients with higher tumor tissue levels of IFNAR2 mRNA may show poor response and unfavorable overall survival. Although we need a surgical specimen to examine tumor tissue levels of IFNAR2 mRNA and protein, the serum level of IFNAR2 mRNA can be conveniently measured, so it may be more useful for predicting the response to IFN-α ± sorafenib therapy and as a prognostic indicator.
Conclusion
This study showed that an elevated serum level of IFNAR2 mRNA could predict the response of metastatic RCC to IFN-α ± sorafenib and may be associated with favorable prognosis.
Acknowledgments
The authors are special grateful to Hitomi Yamazaki for her excellent technique and to Dr Kazumoto Kimura for his constructive suggestions regarding statistical analysis in this study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
The authors Nobutaka Furuya and Takao Kamai are equal contributors to this work.
References
- 1.Athar U, Gentile TC. Treatment options for metastatic renal cell carcinoma: a review. Can J Urol. 2008;15:523–532. [PubMed] [Google Scholar]
- 2.Motzer RJ, Bander NH, Nanus DM. Renal-cell carcinoma. N Engl J Med. 1996;335:865–875. doi: 10.1056/NEJM199609193351207. [DOI] [PubMed] [Google Scholar]
- 3.Jantzer P, Schendel DJ. Human renal carcinoma antigen-specific CTLs, antigen-driven selection and long-term persistence in vivo. Cancer Res. 1998;58:3078–3086. [PubMed] [Google Scholar]
- 4.Coppin C, Le L, Porzsolt F, et al. Targeted therapy for advanced renal cell carcinoma. Cochrane Database Syst Rev. 2008;CD006017(2):1–38. doi: 10.1002/14651858.CD006017.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coppin C. Immunotherapy for renal cell cancer in the era of targetd therapy. Expert Rev Anticancer Ther. 2008;8:907–919. doi: 10.1586/14737140.8.6.907. [DOI] [PubMed] [Google Scholar]
- 6.Dalle S, Thieblemont C, Thomas L, et al. Monoclonal antibodies in clinical oncology. Anticancer Agents Med Chem. 2008;8:523–532. doi: 10.2174/187152008784533071. [DOI] [PubMed] [Google Scholar]
- 7.Sciarra A, Gentile V, Salciccia S. New anti-angiogenic targeted therapy in advanced renal cell carcinoma (RCC): current status and future prospects. Rev Recent Clin Trials. 2008;3:97–103. doi: 10.2174/157488708784223808. [DOI] [PubMed] [Google Scholar]
- 8.Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449–456. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 9.Oudard S, Medioni J, Aylllon J, et al. Everolimus (RAD001): an mTOR inhibitor for the treatment of metastatic renal cell carcinoma. Expert Rev Anticancer Ther. 2009;9:705–717. doi: 10.1586/era.09.27. [DOI] [PubMed] [Google Scholar]
- 10.Kapoor A, Figlin RA. Targeted inhibition of mammalian target of rapamycin for the treatment of advanced renal cell carcinoma. Cancer. 2009;115:3618–3630. doi: 10.1002/cncr.24409. [DOI] [PubMed] [Google Scholar]
- 11.Naito S, Yamamoto N, Takayama T, et al. Prognosis of Japanese metastatic renal cell carcinoma patients in the cytokine era: a cooperative group report of 1463 patients. Eur Urol. 2010;57:317–326. doi: 10.1016/j.eururo.2008.12.026. [DOI] [PubMed] [Google Scholar]
- 12.Motzer RJ, Bacik J, Murphy BA, et al. Interferon-alpha as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol. 2002;20:289–296. doi: 10.1200/JCO.20.1.289. [DOI] [PubMed] [Google Scholar]
- 13.Suwa H, Ohshio G, Imamura T, et al. Overexpression of the rhoC gene correlates with progression of ductal adenocarcinoma of the pancreas. Br J Cancer. 1998;77:147–152. doi: 10.1038/bjc.1998.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamai T, Yanai Y, Arai K, et al. Increased interferon alpha receptor 2 mRNA levels is associated with renal cell carcinoma metastasis. BMC Cancer. 2007;7:1–11. doi: 10.1186/1471-2407-7-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamai T, Tsujii T, Arai K, et al. Significant association of Rho/ROCK pathway with invasion and metastasis of bladder cancer. Clin Cancer Res. 2003;9:2632–2641. [PubMed] [Google Scholar]
- 16.Fuhrman SA, Lasky LC, Lmas C. Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol. 1982;6:655–663. doi: 10.1097/00000478-198210000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Sobin H, Wittekind CH (eds) (2002) International union against cancer. UICC. In: TNM classification of malignant tumors, 6th edn. Wiley, New York
- 18.Furuya N, Kamai T, Tokui N, et al. Interferon alpha and half-dose sorafenib is an effective treatment modality for interferon alpha-resistant metastatic renal cell carcinoma: a case report. Hinyokika Kiyo. 2009;55:323–326. [PubMed] [Google Scholar]
- 19.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 20.Fritz G, Just I, Kaina B. Rho GTPase over-expressed in human tumors. Int J Cancer. 1999;81:682–687. doi: 10.1002/(SICI)1097-0215(19990531)81:5<682::AID-IJC2>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 21.Abe H, Kamai T, Shirataki H, et al. High expression of Ran GTPase is associated with local invasion and metastasis of human clear cell renal cell carcinoma. Int J Cancer. 2008;122:2391–2397. doi: 10.1002/ijc.23400. [DOI] [PubMed] [Google Scholar]
- 22.Escudier B, Lassau N, Angevin E, et al. Phase I trial of sorafenib in combination with IFN alpha-2a in patients with unresectable and/or metastatic renal cell carcinoma or malignant melanoma. Clin Cancer Res. 2007;13:1801–1809. doi: 10.1158/1078-0432.CCR-06-1432. [DOI] [PubMed] [Google Scholar]
- 23.Ryan CW, Goldman BH, Lara PN, Jr, et al. Sorafenib with interferon alfa-2b as first-line treatment of advanced renal carcinoma: a phase II study of the Southwest Oncology Group. J Clin Oncol. 2007;25:3296–3301. doi: 10.1200/JCO.2007.11.1047. [DOI] [PubMed] [Google Scholar]
- 24.Gollob JA, Rathmell WK, Richmond TM, et al. Phase II trial of sorafenib plus interferon alfa-2b as first- or second-line therapy in patients with metastatic renal cell cancer. J Clin Oncol. 2007;25:3288–3295. doi: 10.1200/JCO.2007.10.8613. [DOI] [PubMed] [Google Scholar]
- 25.Jonasch E, Corn P, Pagloaro LC, et al. Upfront, randomized, phase 2 trial of sorafenib versus sorafenob and low-dose interferon alpha in patients with advanced renal cell carcinoma. Clinical and biomarker analysis. Cancer. 2010;116:57–65. doi: 10.1002/cncr.24685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tochizawa S, Masumori N, Yanai Y, et al. Antitumor effects of a combination of interferon-alpha and sorafenib on human renal cell carcinoma cell lines. Biomed Res. 2008;29:271–278. doi: 10.2220/biomedres.29.271. [DOI] [PubMed] [Google Scholar]
- 27.Takeuchi A, Eto M, Tatsugami K, et al. Mechanism of synergistic antitumor effect of sorafenib and interferon-alpha in the treatment of renal cell carcinoma. J Urol. 2010;184:2549–2556. doi: 10.1016/j.juro.2010.07.033. [DOI] [PubMed] [Google Scholar]
- 28.Wilhelm SM, Carter C, Tang L, et al. BAY 43–9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–7109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 29.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 30.von Marschall Z, Scholz A, Cramer T, et al. Effects of interferon alpha on vascular endothelial growth factor gene transcription and tumor angiogenesis. J Natl Cancer Inst. 2003;95:437–478. doi: 10.1093/jnci/95.6.437. [DOI] [PubMed] [Google Scholar]
- 31.Frumento G, Piazza T, Di Carlo E, et al. Targeting tumor-related immunosuppression for cancer immunotherapy. Endocr Metab Immune Disord Drug Targets. 2006;6:233–237. doi: 10.2174/187153006778250019. [DOI] [PubMed] [Google Scholar]
- 32.Platanias LC. Mechanisms of type-I- and type II-interferon-mediated signaling. Nat Rev Immunol. 2005;5:375–386. doi: 10.1038/nri1604. [DOI] [PubMed] [Google Scholar]
- 33.De Weerd NA, Samarajiwa SA, Hertzog PJ. Type-I interferon receptors: biochemistry and biological functions. J Biol Chem. 2007;282:20053–20057. doi: 10.1074/jbc.R700006200. [DOI] [PubMed] [Google Scholar]
- 34.Lekmine F, Uddin S, Sassano A, et al. Activation of the p70 S6 kinase and phosphorylation of the 4E-BP1 repressor of mRNA translation by Type I interferons. J Biol Chem. 2003;278:27772–27780. doi: 10.1074/jbc.M301364200. [DOI] [PubMed] [Google Scholar]
- 35.Kaur S, Lal L, Sassano A, et al. Regulatory effects of mammalian target of rapamycin-activated pathways in Type I and II interferon signalling. J Biol Chem. 2007;282:1757–1768. doi: 10.1074/jbc.M607365200. [DOI] [PubMed] [Google Scholar]
- 36.Thyrell L, Hjortsberg L, Arulampalam V, et al. Interferon α-induced apoptosis in tumor cells is mediated through the phosphoinositide 3-kinase/mammalian target of rapamycin signaling pathway. J Biol Chem. 2004;279:24152–24162. doi: 10.1074/jbc.M312219200. [DOI] [PubMed] [Google Scholar]
- 37.Pnaretakis T, Hjortsberg L, Tamm KJ, et al. Interferon α induces nucleus-independent apoptosis by activating extracellular signal-regulated kinase 1/2 and phosphatidylinositol 3-kinase and mammalian target of rapamycin. Mol Biol Cell. 2008;19:41–50. doi: 10.1091/mbc.E07-04-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petroulakis E, Mamane Y, Le Bacquer O. mTOR signaling: implications for cancer and anticancer therapy. Brit J Cancer. 2006;94:195–199. doi: 10.1038/sj.bjc.6602902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guertin DA, Sabatini DM. An expanding role for mTOR in cancer. Trends Mol Med. 2005;11:353–361. doi: 10.1016/j.molmed.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 40.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 41.Bjornsti MA, Houghton PJ. The mTOR pathway: a target for cancer therapy. Nat Rev Cancer. 2004;4:335–348. doi: 10.1038/nrc1362. [DOI] [PubMed] [Google Scholar]
- 42.Arsham AM, Howell JJ, Simon MC. A novel hypoxia-inducible factor-independent hypoxic response regulating mammalian target of rapamycin and its targets. J Biol Chem. 2003;278:29655–29660. doi: 10.1074/jbc.M212770200. [DOI] [PubMed] [Google Scholar]
- 43.Brugarolas J, Lei K, Hurley RL, et al. Regulation of mTOR function in response to hypoxia by REDD1 an the TSC1/TSC2 tumor suppressor complex. Genes Dev. 2004;18:2893–2904. doi: 10.1101/gad.1256804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deyonug MP, Horak P, Sofer A, et al. Hypoxia regulates TSC1/2 mTOR signaling and tumor suppression through REDD1-mediated 14 3 3 shuttling. Genes Dev. 2008;22:239–251. doi: 10.1101/gad.1617608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hay N. The Akt-mTOR tango and its relevance to cancer. Cancer Cell. 2005;8:179–183. doi: 10.1016/j.ccr.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 46.Sarbassov DD, Guertin DA ALISM, et al. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 47.Arsham AM, Plas DR, Thompson CB, et al. Akt and hypoxia-inducible factor-1 independently enhance tumor growth and angiogenesis. Cancer Res. 2004;64:3500–3507. doi: 10.1158/0008-5472.CAN-03-2239. [DOI] [PubMed] [Google Scholar]
- 48.Toschi A, Lee E, Gadir N, et al. Differential dependence of hypoxia-inducible factors 1α and 2α on mTORC1 and mTORC2. J Biol Chem. 2010;283:34495–34499. doi: 10.1074/jbc.C800170200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2008;356:2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 50.Saito K, Tatokoro M, Fujii Y, et al. Impact of C-Reactive protein kinetics on survival of patients with metastatic renal cell carcinoma. Eur Urol. 2008;55:1143–1153. doi: 10.1016/j.eururo.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 51.Iimura Y, Saito K, Fujii Y, et al. Development and external validation of a new outcome prediction model for patients with clear cell renal cell carcinoma treated with nephrectomy based on preoperative serum C-reactive protein and TNM classification: the TNM-C Score. J Urol. 2009;181:1004–1012. doi: 10.1016/j.juro.2008.10.156. [DOI] [PubMed] [Google Scholar]
- 52.Gabay C, Kushner I. Acute phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 53.Blay JY, Negrier S, Combaret V, et al. Serum level of Il-6 as a prognostic factor in metastatic renal cell carcinoma. Cancer Res. 1997;52:3317–3322. [PubMed] [Google Scholar]
- 54.Kamai T, Tomosugi N, Abe H et al (2009) Increased serum hepcidin-25 level and increased tumor expression of hepcidin mRNA are associated with metastasis of renal cell carcinoma. BMC Cancer 9:1–9. doi:10.1186/1471-2407-9-270 [DOI] [PMC free article] [PubMed]
- 55.Ito N, Eto M, Nakamura E, et al. STAT3 polymorphism predicts interferon-alpha response in patients with metastatic renal cell carcinoma. J Clin Oncol. 2007;25:2785–2791. doi: 10.1200/JCO.2006.09.8897. [DOI] [PubMed] [Google Scholar]