Abstract
Primary antibody deficiencies (PADs) are the most common primary immunodeficiencies and are characterized by a defect in the production of normal amounts of antigen-specific antibodies. PADs represent a heterogeneous spectrum of conditions, ranging from often asymptomatic selective IgA and IgG subclass deficiencies to the severe congenital agammaglobulinemias, in which the antibody production of all immunoglobulin isotypes is severely decreased. Apart from recurrent respiratory tract infections, PADs are associated with a wide range of other clinical complications. This review will describe the pathophysiology, diagnosis, and treatment of the different PADs.
Keywords: Primary antibody deficiency, Child, Agammaglobulinemia, Common variable, Immunodeficiency, IgA deficiency, IgG2 deficiency, Specific anti-polysaccharide antibody deficiency
Introduction
Primary antibody deficiencies (PADs) are the most common primary immunodeficiencies [24]. The hallmark of PADs is a defect in the production of normal amounts of antigen-specific antibodies. These antibodies or immunoglobulins are indispensible for the adaptive immune response against a wide variety of pathogens. A defect in antibody production results in recurrent and/or severe infections. PADs represent a heterogeneous spectrum of conditions, ranging from often asymptomatic selective IgA and IgG subclass deficiencies to the severe congenital agammaglobulinemias, in which antibody production of all immunoglobulin isotypes is severely decreased. The majority of patients with symptomatic PADs present with recurrent ENT and airway infections and are difficult to discover among the many children presenting to pediatric practice. Therefore, a diagnostic strategy for immunodeficiency in children has been recently presented in this journal [16]. Apart from recurrent infections, there is a wide range of other clinical complications associated with primary antibody deficiency [13, 15, 43], affecting the child’s quality of life and life expectancy. After an introduction on normal B cell development, this review will describe the pathophysiology, diagnosis, and treatment of the different PADs.
Normal B cell differentiation and maturation
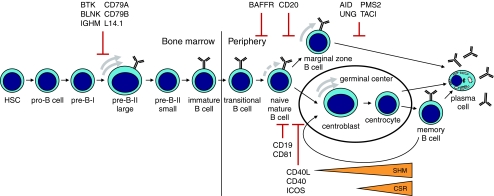
Plasma cells and memory B cells represent the end stages of B cell differentiation and maturation (Fig. 1). They are responsible for the continuous production of specific antibodies and long-lasting immunological memory, respectively [59]. Memory B cells are able to rapidly differentiate in new plasma cells in case of reinfections. B cells originate from lymphoid precursors in the bone marrow, where a large repertoire of B cells with different B cell receptors (BCRs) is formed, consisting of a signaling complex and a membrane bound IgM molecule. These BCRs are able to recognize a wide variety of antigens. After leaving the bone marrow, IgM positive B cells can be activated by antigen and enter a germinal center in lymphoid tissue. Here, the interaction of B and T cells, characteristic for the T cell-dependent antibody response, is contributing to the generation IgG, IgA, and IgE positive plasmacells and memory B cells by initiation of class switch recombination (CSR). Somatic hypermutation (SHM) of the variable region of Ig heavy and Ig light chains increases the affinity of the BCR for antigen (affinity maturation). The production of antibodies to polysaccharide antigens, present in the cell wall of encapsulated bacteria like pneumococci and meningococci, is supported by a second T cell-independent B cell response, which localizes in the marginal zone of the spleen. The T cell-independent B cell response is still immature in healthy children below the age of 2 years, hence their susceptibility to severe complications of pneumococcal and meningococcal infections.
Fig. 1.
Antigen(Ag)-independent B cell differentiation occurs in the bone marrow, whereas Ag-dependent B cell differentiation occurs in the periphery. After activation by Ag, B cells develop in a T cell-dependent way in the germinal center and in a T cell-independent way in the marginal zone of the spleen. Defects of survival, Ag stimulation, B–T interaction, TI response, CSR/SHM can result in PAD. Identified PAD genes affecting these processes are indicated
The spectrum of primary antibody deficiencies
Defects in all critical stages of B cell development have the potential to cause PAD (Fig. 1); however, the immunological and genetic defects of most patients with PAD are still unknown. Evaluating a child with a suspected PAD, the secondary causes for the antibody deficiency, such as nephrotic syndrome, protein-loosing enteropathy, use of certain drugs, and hematological malignancies have to be excluded. Depending on the nature of the B cell defect, PADs can be separated in different categories (Table 1), with their own clinical, immunological, and genetic characteristics. In this section, we will discuss the different PADs in more detail including specific diagnostic features and most important clinical complications (Table 2).
Table 1.
The heterogeneous spectrum of primary antibody deficiencies
| Disease | Circulating B cells | Serum Ig decrease |
|---|---|---|
| Congenitial agammaglobulinemias | ||
| X-linked | ||
| BTK deficiency | Severe decrease | All isotypes |
| Autosomal recessive | ||
| μ Heavy chain deficiency | Severe decrease | All isotypes |
| λ 5 deficiency | Severe decrease | All isotypes |
| Ig (or CD79) α deficiency | Severe decrease | All isotypes |
| Ig (or CD79) β deficiency | Severe decrease | All isotypes |
| BLNK | Severe decrease | All isotypes |
| Class switch recombination deficiency | ||
| X-linked | ||
| CD40 ligand deficiency | Normal | IgG, IgA |
| Autosomal recessive | ||
| CD40 deficiency | Normal | IgG, IgA |
| AID deficiency | Normal | IgG, IgA |
| UNG deficiency | Normal | IgG, IgA |
| Anhydrotic ectodermal dysplasia with | Normal | IgG and/or IgA and/or |
| Immunodeficiency (NEMO deficiency, syndromic) | Specific anti-polysaccharide | |
| PMS2 deficiency | Normal/decrease | IgG variable, IgA |
| Other PADs with known genetic defect | ||
| CD19 deficiency | Normal | IgG, IgA, and/or IgM |
| CD81 deficiency | Normal | IgG, IgA, and/or IgM |
| ICOS deficiency | Normal | IgG, IgA, and/or IgM |
| BAFF receptor deficiency | Decrease | Variable |
| TACI deficiency (increased disease susceptibility) | Normal | Variable |
| Idiopathic primary antibody deficiencies | ||
| Common variable immunodeficiency disordersb | Normal/decrease | IgG, IgA, and/or IgMc |
| Possible CVID/CVID-like disorders | Normal/decrease | IgG |
| Transient hypogammaglobulinemia of infancy | Normal | IgG, IgA, and/or IgM |
| Selective IgM deficiency | Normal | IgM |
| Selective IgA deficiencya | Normal | IgA |
| IgG2 deficiencya | Normal | IgG2 |
| Specific anti-polysaccaride antibody deficiencya | Normal | Specific anti-polysaccharide |
| Other PIDs associated with antibody deficiency | ||
| Severe combined immunodeficiency | Normal/decreased | All isotypes |
| DNA repair disorders | Normal/decrease | Variable |
| AD Hyper-IgE syndrome | Normal | Specific antibodies |
| Wiscott–Aldrich syndrome | Normal | IgM, specific anti-polysaccharide |
BTK Bruton’s tyrosine kinase, CVID common variable immunodeficiency, TACI transmembrane activator and CAML interactor, AID activation-induced cytidine deaminase, UNG uracil-n glycosylase, PMST2 postmeiotic segregation increased 2, ICOS inducible costimulator, BAFF B cell-activating factor, PID pelvic inflammatory disease
aOften combined in one patient
bCan be preceded by conditions marked with a
cAge >2–4 years and decreased response to vaccination
dT-cells show a severe decrease in most patients
Table 2.
Clinical complications of primary antibody deficiency
| Clinical complications | Predominantly associated with | Occurrencea |
|---|---|---|
| Recurrent URTI | All PAD | Very common |
| Recurrent ENT infections | All PAD | Very common |
| Recurrent severe pneumonia | Cong. agamma., CSR def., CVID | Very common |
| Bronchiectasis | Cong. agamma., CSR def., CVID | Common |
| Interstitial pulmonary abnormalities | CVID | Rare |
| Sepsis | Cong. agamma., CSR def., CVID | Rare |
| Bacterial osteomyelitis/arthritis | Cong. agamma., CSR def., CVID | Rare |
| Bacterial meningitis | Cong. agamma., CSR def., CVID | Rare |
| Chronic enteroviral meningoencephalitis | Cong.agamma., CD40L def | Rare |
| Recurrent giardiasis | All PAD | Common |
| Campylobacter gastroenteritis | All PAD | Rare |
| Nodular lymphoid hyperplasia | CVID | Rare |
| Inflammatory bowel disease | AID, CVID | Rare |
| Enteropathy (chronic diarrhoea of origin) | CVID, cong. agamma, sIgAD, AID | Common unknown |
| Autoimmune disease | CVID, sIgAD, AID | Common |
| Allergic disorders | CVID, sIgAD | Common |
| Granulomas (lung, bowel, other) | CVID | Rare |
| (Hepato)splenomegaly | CVID | Common |
| Lymphoid hyperplasia | CVID, AID | Common |
| Malignancies, Mainly lymphoma | CVID | Rare |
| Neutropenia | XLA, CD40L | Common |
URTI upper respiratory tract infection, BTK Bruton’s tyrosine kinase, AID activation-induced cytidine deaminase, PAD primary antibody deficiencies, ENT cong. agamma congenital agammaglobulinemias ears, nose, throat, XLA X-linked agammaglobulinemia, CVID common variable immunodeficiency, sIgAD selective IgA deficiency
aEstimated occurrency rate; rare (<10%), common (10–50%), very common (>50%)
Congenital agammaglobulinemias
The first report of a congenital agammaglobulinemia dates from 1952 [6], when Bruton described a boy with recurrent infections and a deficiency of gammaglobulins. Many years later, it appeared that boys with X-linked agammaglobulinemia (XLA) suffer from a defect in the gene for Bruton’s tyrosine kinase or BTK [62], which is crucial for (pre)B cell receptor signalling. BTK deficiency causes an early block in B cell development in the bone marrow, resulting in the (near) absence of B lymphocytes in peripheral blood and peripheral lymphoid tissues. As a result, antibody production of all immunoglobulin isotypes, including the response to vaccinations is severely impaired. XLA accounts for 85% of all cases of congenital agammaglobulinemia. Patients with autosomal recessive forms also have B cell defects affecting the pre-BCR or the downstream signalling cascade (Fig. 1, Table 1). The clinical problems of these patients are comparable to XLA, but the clinical phenotype tends to be more severe because of a more absolute block in early B cell development [23].
Over half the XLA patients present before 1 year of age and more than 90% are diagnosed at the age of 5 years [66]. Fewer than 10% of the patients have symptoms in the first 3 months because of protection by placentally transferred maternal antibodies. Recurrent ENT and airway infections are the most frequent presenting symptoms, but children may also present with severe bacterial infections in other organ systems [66]. Apart from a severe antibody deficiency, 11% of children with XLA suffer from concomitant neutropenia, which can be misdiagnosed as congenital neutropenia. Following the diagnostic strategy for children with recurrent ENT and airway infections, congenital agammaglobulinemias can be easily suspected by low immunoglobulin levels of IgG, IgA, and IgM [16]. Subsequent lymphocyte subset analysis will reveal that B cells in the peripheral blood are severely decreased. In case B cells are present, other PADs have to be considered, especially transient hypogammaglobulinemia of infancy and class switch recombination deficiencies (discussed below). When B cells are absent, a definite diagnosis can be made by genetic analysis of the candidate genes.
Treatment consists of immunoglobulin replacement therapy and antibiotic treatment of suspected bacterial infections. If neutropenia is present, it disappears on immunoglobulin replacement. Chronic lung disease is the most frequent long-term complication. XLA patients are susceptible to chronic enteroviral meningoencephalitis, which is an important cause of death [66].
Class switch recombination deficiencies
Class switch recombination deficiencies, formerly known as hyper IgM syndromes, are rare conditions characterized by decreased serum IgG and IgA levels, but normal or increased levels of IgM [28]. The disease-causing mechanism is either a disturbed co-stimulation of B cells and T cells in the germinal centre, affecting the initiation of CSR, or a deregulation of the class switch process itself (Fig. 1).
The prototype of a co-stimulation defect is X-linked CD40-ligand deficiency [1, 2, 18, 67]. CD40L deficiency not only causes a PAD, but also results in a profound T cell deficiency because the antibody deficiency is secondary to decreased CD40L expression on T cells. Therefore, CD40L deficiency is nowadays primarily classified as a T cell disorder [28]. Because of the T cell deficiency, an important difference with other PADs is the occurrence of opportunistic infections. Apart from a bacterial pneumonia, which occurs in 80% of the children, 41% suffer from Pneumocystis jiroveci pneumonia [67]. Severe combined immunodeficiency (SCID) is part of the differential diagnosis of CD40L deficiency (van der Burg M, Gennery A, the expanding spectrum of severe combined immunodeficiency, EJP in press), but in contrast to most cases of SCID, analysis of lymphocyte subpopulation in PAD patients will show normal T cell counts. Similar to XLA patients, neutropenia can be present and CD40L patients also share the increased susceptibility chronic enteroviral meningoencephalitis. Furthermore, CD40L patients are prone to a fatal sclerosing cholangitis secondary to a Cryptosporidium parvum infection. Initial treatment consists of immunoglobulin replacement and Pneumocystis jiroveci prophylaxis, but because of the high frequency of life-threatening complications before the age of 25 years [36], hematopoietic stem cell transplantation is now the treatment of choice [19].
A deregulation of the CSR process itself is caused by AID [45], UNG [27], NEMO [30], and PMS2 [40] deficiency. X-linked anhidrotic ectodermal dysplasia with immunodeficiency secondary to mutations in NEMO gives a much broader immunodeficiency in addition to the CSR defect and has been described in the EJP review on syndromic primary immunodeficiencies of Kersseboom et al. [34] in this journal. The immune defect in AID and UNG deficiency is limited to the B cell lineage. These patients usually present at an older age than patients with CD40L deficiency. Apart from recurrent infections, they often suffer from lymphoid hyperplasia, inflammatory bowel disease, and autoimmunity [27, 42].
Common variable immunodeficiency
Common variable immunodeficiency (CVID) is an idiopathic antibody deficiency with an estimated prevalence of 1:25,000. It is defined by serum IgG levels below 2 SD of normal controls in the presence of decreased IgA and/or IgM levels, recurrent infections, impaired response to immunization, exclusion of defined causes of hypogammaglobulinemia, and an age above 2 years (ESID-PAGID-criteria “probable CVID”, www.esid.org). A considerable group of patients suffer from a similar idiopathic hypogammaglobulinemia, but do not fulfil all the diagnostic criteria. These patients are usually diagnosed as “possible” CVID or as having a “CVID-like” disorder. The clinical characteristics are indistinguishable from CVID. Less than 10% of the CVID patients have a positive family history [13] and a genetic defect has only been identified in less than 10% of the patients who have been reported to the ESID primary immunodeficiency database with the clinical phenotype of CVID [24]. Reported defects involve B cell activation (CD19 [60] and CD81 deficiency [61]), co-stimulation (ICOS deficiency [25]) and B cell survival (BAFF-R deficiency [64]). Moreover, genetic defects have been identified that do not cause hypogammaglobulinemia per se, but increase disease susceptibility (TACI deficiency [8, 41, 44, 47, 48]). The heterogeneity of the immunological and clinical features of CVID hampers the discovery of underlying disease-causing mechanisms, clinically relevant prognostic factors, and genetic defects.
Most of the CVID patients present in young adulthood, but symptoms start in childhood in more than half of the cases [43]. Consequently, a diagnostic delay of more than 5 years is the rule [13, 54]. Sometimes CVID is preceded by IgA deficiency, IgG subclass deficiency, or a specific anti-polysaccharide antibody deficiency.
The presenting symptoms in CVID are diverse, but recurrent ENT and airway infections are present in more than 90% of the patients. Some patients present with autoimmune disease, most often autoimmune cytopenias (Table 2). Other clinical features are granulomatous inflammation of the lungs and gastrointestinal tract, chronic diarrhoea secondary to unexplained enteropathy, and haematological malignancies, which are important causes of death. CVID patients who suffer from at least one non-infectious complication have higher mortality than patients who only suffer from infectious complications [9].
Treatment consists of immunoglobulin replacement and antibiotic treatment of infections. Immunosuppressants are indicated in some cases with autoimmunity, but therapeutic guidelines on how to use these agents in CVID patients are lacking. A low number of switched memory B cells in the peripheral blood is associated with autoimmunity, granulomas, and respiratory infections [7, 57, 63, 65]. Also, children who are non-responders to pneumococcal polysaccharide vaccination more often suffer from respiratory infections and bronchiectasis [46]. Growth monitoring is important because one third of the patients develops a growth retardation [54], which is associated with recurrent infectious episodes, but can also develop irrespective of infectious complications secondary to disturbances of the growth hormone axis [17, 55]. A combination of short stature and antibody deficiency may also have its origin in a syndromic form of primary immunodeficieny [34].
Transient hypogammaglobulinemia of infancy
Transient hypogammaglobulinemia of infancy (THI) has to be considered in the differential diagnosis of every young child with hypogammaglobulinemia. It is best defined by low levels of IgG (<2 SD below the mean for age), with or without a decrease of IgA or IgM while other causes of the hypogammaglobulinemia have been excluded [52]. The pathophysiology is unknown. In some patients, THI might be a variation of the physiological hypogammaglobulinemia secondary to the disappearance of maternal IgG from the circulations that normally occurs between 3 and 6 month of age. Many cases probably go unnoticed because they are asymptomatic. Of the symptomatic cases, most children present with recurrent ENT and airway infections before the age of 1 year. Much rarer presenting symptoms are sepsis [51] or meningitis [26, 50]. Other symptoms include recurrent diarrhoea, severe varicella infection, or prolonged oral candidiasis [52]. In more than two thirds of the children, immunoglobulin levels normalize before the age of 2 years, but in some children, the condition can persist up to the age of 5 years [14]. A minority of children will appear to suffer from a long-lasting PAD. Children who do not recover from a suspected THI have more infectious complications, lower switched memory B cells, and lower levels of IgM compared to children with a self-limiting course [38]. Treatment consists of prophylactic antibiotics; immunoglobulin replacement is usually only given to patients with severe or frequent infections despite antibiotic prophylaxis.
Selective IgA, IgG2 subclass, and specific anti-polysaccharide antibody deficiency
These three PADs are the most prevalent in children and tend to appear in combination. As single conditions they are often asymptomatic, but a combination more often leads to a clinically significant immunodeficiency. This implies that if one of these conditions is diagnosed, it is useful to search for the others.
Selective IgA deficiency (sIgAD) is defined as a decrease of serum IgA <2 SD of age-matched controls. The prevalence of sIgAD in Europe varies between 1:163 and 1:875 [11, 33]. The incidence is much lower in Asian populations [32]. Although the cause of sIgAD is unknown, mutations in TACI increase disease susceptibility, similar to CVID [44].
Secretory IgA, secreted in the dimeric form, is the prominent immunoglobulin in luminal secretions of the respiratory and gastrointestinal tract and as such an important component of mucosal immunity. Secretory IgA cannot be measured in the serum; the serum level of monomeric IgA is rather an indirect reflection of the level of secretory IgA in the body. The diagnosis of sIgAD is usually made by analysis of immunoglobulin levels during the evaluation of recurrent respiratory infections. However, sIgAD is also often diagnosed “by accident” as part of a laboratory evaluation for celiac disease, allergy, or autoimmune disease. The clinical course is asymptomatic in many patients. If children have complaints, they usually suffer recurrent ENT and airway infections. A minority of patients develop recurrent lower respiratory tract infections and/or bronchiectasis. Patients with sIgAD are especially at risk of chronic diarrhoea and giardiasis because of their defect in mucosal immunity. Furthermore, sIgAD is associated with a higher prevalence of allergy/atopy and a range of autoimmune diseases, including autoimmune cytopenias [12, 20, 29].
The four IgG subclasses are defined by the structure of their constant regions. Of the IgG subclass deficiencies, at least IgG2 deficiency is clinically relevant. A decrease in IgG1 cannot be considered as an IgG subclass deficiency a decrease of IgG1 in it results in hypogammaglobulinemia. Most patients with IgG3 deficiency also suffer from a deficiency in another subclass, whereas IgG4 subclass deficiency is very common, but asymptomatic as a rule. In healthy children, IgG2 antibodies are low in the first years of life, after which they gradually increases with age. Antibodies against encapsulated bacteria are mainly of the IgG2 subclass, and IgG2 deficiency increased the susceptibility to these bacteria. Children often present with recurrent respiratory tract and ENT infections. Symptomatic children with IgG2 subclass deficiency should be tested for a concomitant specific anti-polysaccharide antibody deficiency (SPAD) if they are older than 2 years [20]. Children under the age of 10 years may recover spontaneously [52].
If patients continue to experience recurrent respiratory tract or ENT infections despite normal levels of IgG, IgA, and IgM, a SPAD has to be excluded. Although the pathophysiology is unknown, a deficiency of CD20 has recently been linked to SPAD in one patient [35]. The antibody response to polysaccharide antigens is impaired in healthy children under the age of 2 to 3 years, which contributes to their susceptibility to infections with encapsulated bacteria. However, some infants are able to produce normal responses to certain pneumococcal serotypes [3, 5]. After the age of 2 to 3 years, children should be able to mount a sufficient response to pneumococcal polysaccharides. An insufficient response after this age defines the presence of a SPAD. However, the interpretation of the pneumococcal serology for the diagnosis of SPAD is problematic. Jeurissen et al. [31] and Borgers et al. [4] published the best available serotype-specific cut off values for anti-pneumococcal antibodies in children and adults so far. Their fifth percentiles of normal controls of an ELISA for six different serotypes [31] and a commercially available bead assay for 14 serotypes [4] can be used for the diagnosis of SPAD. Pneumococcal antibodies are measured before and 2 to 4 weeks after pneumococcal vaccination. Healthy children should be able to respond to at least seven of the 14 serotypes, above a level of 0.23 to 1.66 mg l, depending on age and serotype (Table 3). Furthermore, an increase of two to fourfold of the pre-vaccination anti-pneumococcal antibody level is often used as additional criteria. If children received previous vaccinations with pneumococcal conjugate vaccines, the serotypes present these vaccines cannot be used for the interpretation of the anti-pneumococcal polysaccharide response (Table 3), so the introduction of pneumococcal conjugate vaccines in national vaccination programs limits the usage of these assays for the diagnosis of SPAD. The assays measuring the anti-pneumococcal IgA or IgM response might be able to overcome these limitations.
Table 3.
Cut off values of antipneumococcal antibodies for the diagnosis of SPAD as defined by Jeurissen et al. [31] and Borgers et al. [4] in relation to serotypes in pneumococcal conjugate vaccines
| Pneumococcal serotype | Serotypes of pneumococcal conjugate vaccine | Anti-pneumococcal antibodies by ELISA (mg/L)a | Anti-pneumococcal antibodies by multiplex bead assay (mg/L)a | ||||
|---|---|---|---|---|---|---|---|
| PCV-7 | PCV-10 | PCV-13 | Age | Age | Age | Age | |
| 3–15 years | 19–30 years | 3–30 years | 3–30 years | ||||
| 1 | x | x | 0.53 | ||||
| 3 | x | 0.99 | 0.58 | 0.67 | 0.64 | ||
| 4 | x | x | x | 0.73 | 0.37 | 0.45 | 0.49 |
| 6B | x | x | x | 0.80 | |||
| 7F | x | x | 1.45 | ||||
| 8 | 1.02 | ||||||
| 9N | 0.78 | 0.23 | 0.46 | 0.63 | |||
| 9V | x | x | x | 0.69 | |||
| 12F | 0.46 | ||||||
| 14 | x | x | x | 0.57 | |||
| 18C | x | x | x | 0.55 | 0.31 | 0.31 | 0.34 |
| 19A | x | 0.96 | |||||
| 19F | x | x | x | 1.66 | 0.91 | 1.04 | 0.74 |
| 23F | x | x | x | 0.24 | |||
Serotypes present in 23-valent pneumococcal polysaccharide (1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19F, 19A, 20, 22F, 23F, and 33F)
aFifth percentile of normal controls
x serotypes cannot be used for the interpretation of the pneumococcal polysaccharide response, if the child received one of the PCV, PCV pneumococcal conjugate vaccines, ELISA enzyme-linked immunosorbent assay
Treatment for IgAD, IgG2 deficiency, or SPAD consists of antibiotic prophylaxis in symptomatic cases, especially during autumn and winter. If children present with bronchiectasis or severe recurrent lower respiratory tract infections, immunoglobulin replacement therapy has to be considered, especially in case of a combination of sIgAD and/or IgG2 deficiency with a SPAD. Data for the efficacy of immunoglobulin replacement for this indication is lacking, but clinical studies are ongoing. If diagnosed at young age, children tend to recover from IgG2, sIgAD, and SPAD. For individuals diagnosed at an older age, these conditions are usually permanent. Follow-up with large intervals is necessary to check for the development of CVID [21].
Treatment and follow-up of PAD
Immunoglobulin replacement therapy
Before the introduction of immunoglobulin replacement therapy, the life expectancy of patients with a- or hypogammaglobulinemia was severely reduced due to chronic lung disease secondary to recurrent pneumonia and bronchiectasis. Immunoglobulin replacement prevents many, but not all, pulmonary complications. Over the past years, it has become obvious that individual dosing, rather than supplementation above a certain “through level” of IgG is necessary to prevent infections complications in PAD patients [37]. There is no difference in efficacy between subcutaneous substitution or IV treatment [10]. Home therapy is very well possible and increases the quality of life [22].
Antibiotic prophylaxis
The use of prophylactic antibiotics to prevent infections in PAD is widely practiced, but is not evidence based. There is an urgent need for controlled trials to address this issue because besides immunoglobulin replacement, it is one of the few therapeutic options in patients with PAD.
Follow-up and prognosis
The long-term follow-up of PAD patients on immunoglobulin replacement should focus on the early detection of chronic lung disease. Apart from regularly pulmonary function tests, it is advisable to perform at least one high resolution CT thorax in these patients to exclude bronchiectasis or other pulmonary abnormalities [53]. Follow-up with high resolution low-dose CT thorax is promising, but needs further evaluation [58]. An increased incidence of respiratory complications and early onset of disease have been associated with polymorphisms in mannose-binding lectin in CVID patients [39], so once a PAD has been diagnosed, it is useful to check MBL levels. Furthermore, attention should be paid to symptoms suggesting non-infectious complications such as autoimmunity, granulomas, splenomegaly, and enteropathy. A decrease of memory B cells in CVID patients is associated with a higher incidence of clinical complications [49, 56, 65, 68]. The use of age-related normal values is important for the interpretation of the results.
Conclusions, learning points
PADs represent a heterogeneous spectrum of conditions, ranging from often asymptomatic selective IgA and IgG subclass deficiencies to severe congenital agammaglobulinemias.
Patients with PAD most often present with recurrent or severe respiratory tract and ENT infections.
PADs have a range of non-infectious complications, such as autoimmunity and enteropathy, which might be the only presenting symptom.
Severe PADs can be easily detected by the determination of total IgG, IgA, and IgM levels.
Previous pneumococcal conjugate vaccinations limit the use of pneumococcal serology for the diagnosis of specific anti-polysaccharide antibody deficiency.
Intravenous or subcutaneous immunoglobulin replacement is the treatment of choice for PAD patients with reduced IgG levels.
Acknowledgments
Conflicts of interest
None to declare for all authors
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Allen RC, Armitage RJ, Conley ME, Rosenblatt H, Jenkins NA, Copeland NG, Bedell MA, Edelhoff S, Disteche CM, Simoneaux DK, et al. CD40 ligand gene defects responsible for X-linked hyper-IgM syndrome. Science. 1993;259:990–993. doi: 10.1126/science.7679801. [DOI] [PubMed] [Google Scholar]
- 2.Aruffo A, Farrington M, Hollenbaugh D, Li X, Milatovich A, Nonoyama S, Bajorath J, Grosmaire LS, Stenkamp R, Neubauer M, et al. The CD40 ligand, gp39, is defective in activated T cells from patients with X-linked hyper-IgM syndrome. Cell. 1993;72:291–300. doi: 10.1016/0092-8674(93)90668-G. [DOI] [PubMed] [Google Scholar]
- 3.Balloch A, Licciardi PV, Russell FM, Mulholland EK, Tang ML. Infants aged 12 months can mount adequate serotype-specific IgG responses to pneumococcal polysaccharide vaccine. J Allergy Clin Immunol. 2010;126:395–397. doi: 10.1016/j.jaci.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borgers H, Moens L, Picard C, Jeurissen A, Raes M, Sauer K, Proesmans M, De Boeck K, Casanova JL, Meyts I, Bossuyt X. Laboratory diagnosis of specific antibody deficiency to pneumococcal capsular polysaccharide antigens by multiplexed bead assay. Clin Immunol. 2009;134:198–205. doi: 10.1016/j.clim.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Bossuyt X, Borgers H, Moens L, Verbinnen B, Meyts I. Age- and serotype-dependent antibody response to pneumococcal polysaccharides. J Allergy Clin Immunol. 2011;127(4):1079–1080. doi: 10.1016/j.jaci.2010.12.1109. [DOI] [PubMed] [Google Scholar]
- 6.Bruton OC. Agammaglobulinemia. Pediatrics. 1952;9:722–728. [Google Scholar]
- 7.Carsetti R, Rosado MM, Donnanno S, Guazzi V, Soresina A, Meini A, Plebani A, Aiuti F, Quinti I. The loss of IgM memory B cells correlates with clinical disease in common variable immunodeficiency. J Allergy Clin Immunol. 2005;115:412–417. doi: 10.1016/j.jaci.2004.10.048. [DOI] [PubMed] [Google Scholar]
- 8.Castigli E, Wilson SA, Garibyan L, Rachid R, Bonilla F, Schneider L, Geha RS. TACI is mutant in common variable immunodeficiency and IgA deficiency. Nat Genet. 2005;37:829–834. doi: 10.1038/ng1601. [DOI] [PubMed] [Google Scholar]
- 9.Chapel H, Lucas M, Lee M, Bjorkander J, Webster D, Grimbacher B, Fieschi C, Thon V, Abedi MR, Hammarstrom L. Common variable immunodeficiency disorders: division into distinct clinical phenotypes. Blood. 2008;112:277–286. doi: 10.1182/blood-2007-11-124545. [DOI] [PubMed] [Google Scholar]
- 10.Chapel HM, Spickett GP, Ericson D, Engl W, Eibl MM, Bjorkander J. The comparison of the efficacy and safety of intravenous versus subcutaneous immunoglobulin replacement therapy. J Clin Immunol. 2000;20:94–100. doi: 10.1023/A:1006678312925. [DOI] [PubMed] [Google Scholar]
- 11.Crisanti MC, Wallace AF, Kapoor V, Vandermeers F, Dowling ML, Pereira LP, Coleman K, Campling BG, Fridlender ZG, Kao GD, Albelda SM. The HDAC inhibitor panobinostat (LBH589) inhibits mesothelioma and lung cancer cells in vitro and in vivo with particular efficacy for small cell lung cancer. Mol Cancer Ther. 2009;8:2221–2231. doi: 10.1158/1535-7163.MCT-09-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cunningham-Rundles C. Physiology of IgA and IgA deficiency. J Clin Immunol. 2001;21:303–309. doi: 10.1023/A:1012241117984. [DOI] [PubMed] [Google Scholar]
- 13.Cunningham-Rundles C, Bodian C. Common variable immunodeficiency: clinical and immunological features of 248 patients. Clin Immunol. 1999;92:34–48. doi: 10.1006/clim.1999.4725. [DOI] [PubMed] [Google Scholar]
- 14.Dalal I, Reid B, Nisbet-Brown E, Roifman CM. The outcome of patients with hypogammaglobulinemia in infancy and early childhood. J Pediatr. 1998;133:144–146. doi: 10.1016/S0022-3476(98)70195-7. [DOI] [PubMed] [Google Scholar]
- 15.Daniels JA, Lederman HM, Maitra A, Montgomery EA. Gastrointestinal tract pathology in patients with common variable immunodeficiency (CVID): a clinicopathologic study and review. Am J Surg Pathol. 2007;31:1800–1812. doi: 10.1097/PAS.0b013e3180cab60c. [DOI] [PubMed] [Google Scholar]
- 16.de Vries E, Driessen G. Educational paper: primary immunodeficiencies in children: a diagnostic challenge. Eur J Pediatr. 2011;170:169–177. doi: 10.1007/s00431-010-1358-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delvecchio M, De Bellis A, De Mattia D, Cavallo L, Martire B. Growth hormone deficiency and antipituitary antibodies in a patient with common variable immunodeficiency. J Endocrinol Invest. 2009;32:637–640. doi: 10.1007/BF03345733. [DOI] [PubMed] [Google Scholar]
- 18.DiSanto JP, Bonnefoy JY, Gauchat JF, Fischer A, de Saint BG. CD40 ligand mutations in x-linked immunodeficiency with hyper-IgM. Nature. 1993;361:541–543. doi: 10.1038/361541a0. [DOI] [PubMed] [Google Scholar]
- 19.Du L, Dunn-Walters DK, Chrzanowska KH, Stankovic T, Kotnis A, Li X, Lu J, Eggertsen G, Brittain C, Popov SW, Gennery AR, Taylor AM, Pan-Hammarstrom Q. A regulatory role for NBS1 in strand-specific mutagenesis during somatic hypermutation. PLoS ONE. 2008;3:e2482. doi: 10.1371/journal.pone.0002482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edwards E, Razvi S, Cunningham-Rundles C. IgA deficiency: clinical correlates and responses to pneumococcal vaccine. Clin Immunol. 2004;111:93–97. doi: 10.1016/j.clim.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 21.Espanol T, Catala M, Hernandez M, Caragol I, Bertran JM. Development of a common variable immunodeficiency in IgA-deficient patients. Clin Immunol Immunopathol. 1996;80:333–335. doi: 10.1006/clin.1996.0132. [DOI] [PubMed] [Google Scholar]
- 22.Fasth A, Nystrom J. Quality of life and health-care resource utilization among children with primary immunodeficiency receiving home treatment with subcutaneous human immunoglobulin. J Clin Immunol. 2008;28:370–378. doi: 10.1007/s10875-008-9180-9. [DOI] [PubMed] [Google Scholar]
- 23.Garcia Rodriguez MC, Lopez Granados E, Cambronero Martinez R, Ferreira Cerdan A, Fontan Casariego G. [Molecular diagnosis of primary immunodeficiencies] Diagnostico molecular de inmunodeficiencias primarias. Allergol Immunopathol (Madr) 2001;29:107–113. doi: 10.1016/s0301-0546(01)79028-3. [DOI] [PubMed] [Google Scholar]
- 24.Gathmann B, Grimbacher B, Beaute J, Dudoit Y, Mahlaoui N, Fischer A, Knerr V, Kindle G, Party ERW. The European internet-based patient and research database for primary immunodeficiencies: results 2006–2008. Clin Exp Immunol. 2009;157(Suppl 1):3–11. doi: 10.1111/j.1365-2249.2009.03954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grimbacher B, Hutloff A, Schlesier M, Glocker E, Warnatz K, Drager R, Eibel H, Fischer B, Schaffer AA, Mages HW, Kroczek RA, Peter HH. Homozygous loss of ICOS is associated with adult-onset common variable immunodeficiency. Nat Immunol. 2003;4:261–268. doi: 10.1038/ni902. [DOI] [PubMed] [Google Scholar]
- 26.Hsueh KC, Chiu HH, Lin HC, Hsu CH, Tsai FJ. Transient hypogammaglobulinemia of infancy presenting as Staphylococcus aureus sepsis with deep neck infection. J Microbiol Immunol Infect. 2005;38:141–144. [PubMed] [Google Scholar]
- 27.Imai K, Slupphaug G, Lee WI, Revy P, Nonoyama S, Catalan N, Yel L, Forveille M, Kavli B, Krokan HE, Ochs HD, Fischer A, Durandy A. Human uracil-DNA glycosylase deficiency associated with profoundly impaired immunoglobulin class-switch recombination. Nat Immunol. 2003;4:1023–1028. doi: 10.1038/ni974. [DOI] [PubMed] [Google Scholar]
- 28.International Union of Immunological Societies Expert Committee on Primary I. Notarangelo LD, Fischer A, Geha RS, Casanova JL, Chapel H, Conley ME, Cunningham-Rundles C, Etzioni A, Hammartrom L, Nonoyama S, Ochs HD, Puck J, Roifman C, Seger R, Wedgwood J. Primary immunodeficiencies: 2009 update. J Allergy Clin Immunol. 2009;124:1161–1178. doi: 10.1016/j.jaci.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jacob CM, Pastorino AC, Fahl K, Carneiro-Sampaio M, Monteiro RC. Autoimmunity in IgA deficiency: revisiting the role of IgA as a silent housekeeper. J Clin Immunol. 2008;28(Suppl 1):S56–S61. doi: 10.1007/s10875-007-9163-2. [DOI] [PubMed] [Google Scholar]
- 30.Jain A, Ma CA, Lopez-Granados E, Means G, Brady W, Orange JS, Liu S, Holland S, Derry JM. Specific NEMO mutations impair CD40-mediated c-Rel activation and B cell terminal differentiation. J Clin Invest. 2004;114:1593–1602. doi: 10.1172/JCI21345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jeurissen A, Moens L, Raes M, Wuyts G, Willebrords L, Sauer K, Proesmans M, Ceuppens JL, De Boeck K, Bossuyt X. Laboratory diagnosis of specific antibody deficiency to pneumococcal capsular polysaccharide antigens. Clin Chem. 2007;53:505–510. doi: 10.1373/clinchem.2006.080051. [DOI] [PubMed] [Google Scholar]
- 32.Kanoh T, Mizumoto T, Yasuda N, Koya M, Ohno Y, Uchino H, Yoshimura K, Ohkubo Y, Yamaguchi H. Selective IgA deficiency in Japanese blood donors: frequency and statistical analysis. Vox Sang. 1986;50:81–86. doi: 10.1111/j.1423-0410.1986.tb04851.x. [DOI] [PubMed] [Google Scholar]
- 33.Kerr SH, Valdiserri RO, Loft J, Bresolin L, Holtgrave D, Moore M, MacGowan R, Marder W, Rinaldi R. Primary care physicians and their HIV prevention practices. AIDS Patient Care STDs. 1996;10:227–235. doi: 10.1089/apc.1996.10.227. [DOI] [PubMed] [Google Scholar]
- 34.Kersseboom R, Brooks A, Weemaes C. Educational paper: syndromic forms of primary immunodeficiency. Eur J Pediatr. 2011;170:295–308. doi: 10.1007/s00431-011-1396-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuijpers TW, Bende RJ, Baars PA, Grummels A, Derks IA, Dolman KM, Beaumont T, Tedder TF, van Noesel CJ, Eldering E, van Lier RA. CD20 deficiency in humans results in impaired T cell-independent antibody responses. J Clin Invest. 2010;120:214–222. doi: 10.1172/JCI40231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levy J, Espanol-Boren T, Thomas C, Fischer A, Tovo P, Bordigoni P, Resnick I, Fasth A, Baer M, Gomez L, Sanders EA, Tabone MD, Plantaz D, Etzioni A, Monafo V, Abinun M, Hammarstrom L, Abrahamsen T, Jones A, Finn A, Klemola T, DeVries E, Sanal O, Peitsch MC, Notarangelo LD. Clinical spectrum of X-linked hyper-IgM syndrome. J Pediatr. 1997;131:47–54. doi: 10.1016/S0022-3476(97)70123-9. [DOI] [PubMed] [Google Scholar]
- 37.Lucas M, Lee M, Lortan J, Lopez-Granados E, Misbah S, Chapel H. Infection outcomes in patients with common variable immunodeficiency disorders: relationship to immunoglobulin therapy over 22 years. J Allergy Clin Immunol. 2010;125:1354–1360. doi: 10.1016/j.jaci.2010.02.040. [DOI] [PubMed] [Google Scholar]
- 38.Moschese V, Graziani S, Avanzini MA, Carsetti R, Marconi M, La Rocca M, Chini L, Pignata C, Soresina AR, Consolini R, Bossi G, Trizzino A, Martino S, Cardinale F, Bertolini P, Marseglia GL, Zecca M, Di Cesare S, Quinti I, Rondelli R, Pietrogrande MC, Rossi P, Plebani A. A prospective study on children with initial diagnosis of transient hypogammaglobulinemia of infancy: results from the Italian Primary Immunodeficiency Network. Int J Immunopathol Pharmacol. 2008;21:343–352. doi: 10.1177/039463200802100211. [DOI] [PubMed] [Google Scholar]
- 39.Mullighan CG, Marshall SE, Welsh KI. Mannose binding lectin polymorphisms are associated with early age of disease onset and autoimmunity in common variable immunodeficiency. Scand J Immunol. 2000;51:111–122. doi: 10.1046/j.1365-3083.2000.00697.x. [DOI] [PubMed] [Google Scholar]
- 40.Peron S, Metin A, Gardes P, Alyanakian MA, Sheridan E, Kratz CP, Fischer A, Durandy A. Human PMS2 deficiency is associated with impaired immunoglobulin class switch recombination. J Exp Med. 2008;205:2465–2472. doi: 10.1084/jem.20080789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poodt AE, Driessen GJ, de Klein A, van Dongen JJ, van der Burg M, de Vries E. TACI mutations and disease susceptibility in patients with common variable immunodeficiency. Clin Exp Immunol. 2009;156:35–39. doi: 10.1111/j.1365-2249.2008.03863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Quartier P, Bustamante J, Sanal O, Plebani A, Debre M, Deville A, Litzman J, Levy J, Fermand JP, Lane P, Horneff G, Aksu G, Yalcin I, Davies G, Tezcan I, Ersoy F, Catalan N, Imai K, Fischer A, Durandy A. Clinical, immunologic and genetic analysis of 29 patients with autosomal recessive hyper-IgM syndrome due to activation-induced cytidine deaminase deficiency. Clin Immunol. 2004;110:22–29. doi: 10.1016/j.clim.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 43.Quinti I, Soresina A, Spadaro G, Martino S, Donnanno S, Agostini C, Claudio P, Franco D, Maria Pesce A, Borghese F, Guerra A, Rondelli R, Plebani A, Italian Primary Immunodeficiency N. Long-term follow-up and outcome of a large cohort of patients with common variable immunodeficiency. J Clin Immunol. 2007;27:308–316. doi: 10.1007/s10875-007-9075-1. [DOI] [PubMed] [Google Scholar]
- 44.Rachid R, Castigli E, Geha RS, Bonilla FA. TACI mutation in common variable immunodeficiency and IgA deficiency. Curr Allergy Asthma Rep. 2006;6:357–362. doi: 10.1007/s11882-996-0004-9. [DOI] [PubMed] [Google Scholar]
- 45.Revy P, Muto T, Levy Y, Geissmann F, Plebani A, Sanal O, Catalan N, Forveille M, Dufourcq-Labelouse R, Gennery A, Tezcan I, Ersoy F, Kayserili H, Ugazio AG, Brousse N, Muramatsu M, Notarangelo LD, Kinoshita K, Honjo T, Fischer A, Durandy A. Activation-induced cytidine deaminase (AID) deficiency causes the autosomal recessive form of the Hyper-IgM syndrome (HIGM2) Cell. 2000;102:565–575. doi: 10.1016/S0092-8674(00)00079-9. [DOI] [PubMed] [Google Scholar]
- 46.Rezaei N, Aghamohammadi A, Read RC. Response to polysaccharide vaccination amongst pediatric patients with common variable immunodeficiency correlates with clinical disease. Iran J Allergy Asthma Immunol. 2008;7:231–234. [PubMed] [Google Scholar]
- 47.Salzer U, Bacchelli C, Buckridge S, Pan-Hammarstrom Q, Jennings S, Lougaris V, Bergbreiter A, et al. Relevance of biallelic versus monoallelic TNFRSF13B mutations in distinguishing disease-causing from risk-increasing TNFRSF13B variants in antibody deficiency syndromes. Blood. 2009;113:1967–1976. doi: 10.1182/blood-2008-02-141937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Salzer U, Chapel HM, Webster AD, Pan-Hammarstrom Q, Schmitt-Graeff A, Schlesier M, Peter HH, Rockstroh JK, Schneider P, Schaffer AA, Hammarstrom L, Grimbacher B. Mutations in TNFRSF13B encoding TACI are associated with common variable immunodeficiency in humans. Nat Genet. 2005;37:820–828. doi: 10.1038/ng1600. [DOI] [PubMed] [Google Scholar]
- 49.Sanchez-Ramon S, Radigan L, Yu JE, Bard S, Cunningham-Rundles C. Memory B cells in common variable immunodeficiency: clinical associations and sex differences. Clin Immunol. 2008;128:314–321. doi: 10.1016/j.clim.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schiffer JT, Sterling TR. Timing of antiretroviral therapy initiation in tuberculosis patients with AIDS: a decision analysis. J Acquir Immune Defic Syndr. 2007;44:229–234. doi: 10.1097/QAI.0b013e31802e2975. [DOI] [PubMed] [Google Scholar]
- 51.Simon JL, Bosch J, Puig A, Grau M. Two relapses of group B streptococcal sepsis and transient hypogammaglobulinemia. Pediatr Infect Dis J. 1989;8:729–730. doi: 10.1097/00006454-198910000-00018. [DOI] [PubMed] [Google Scholar]
- 52.Stiehm ER. The four most common pediatric immunodeficiencies. J Immunotoxicol. 2008;5:227–234. doi: 10.1080/15476910802129646. [DOI] [PubMed] [Google Scholar]
- 53.Thickett KM, Kumararatne DS, Banerjee AK, Dudley R, Stableforth DE. Common variable immune deficiency: respiratory manifestations, pulmonary function and high-resolution CT scan findings. QJM. 2002;95:655–662. doi: 10.1093/qjmed/95.10.655. [DOI] [PubMed] [Google Scholar]
- 54.Urschel S, Kayikci L, Wintergerst U, Notheis G, Jansson A, Belohradsky BH. Common variable immunodeficiency disorders in children: delayed diagnosis despite typical clinical presentation. J Pediatr. 2009;154:888–894. doi: 10.1016/j.jpeds.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 55.van Bilsen K, Driessen GJ, de Paus RA, van de Vosse E, van Lom K, van Zelm MC, Lam KH, Hartwig NG, Baarsma GS, van de Burg M, van Hagen PM. Low level IGF-1 and common variable immune deficiency: an unusual combination. Neth J Med. 2008;66:368–372. [PubMed] [Google Scholar]
- 56.van de Ven AA, van de Corput L, van Tilburg CM, Tesselaar K, van Gent R, Sanders EA, Boes M, Bloem AC, van Montfrans JM. Lymphocyte characteristics in children with common variable immunodeficiency. Clin Immunol. 2010;135:63–71. doi: 10.1016/j.clim.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 57.van de Ven AA, van de Corput L, van Tilburg CM, Tesselaar K, van Gent R, Sanders EA, Boes M, Bloem AC, van Montfrans JM. Lymphocyte characteristics in children with common variable immunodeficiency. Clin Immunol. 2010;135:63–71. doi: 10.1016/j.clim.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 58.van de Ven AA, van Montfrans JM, Terheggen-Lagro SW, Beek FJ, Hoytema van Konijnenburg DP, Kessels OA, de Jong PA. A CT scan score for the assessment of lung disease in children with common variable immunodeficiency disorders. Chest. 2010;138:371–379. doi: 10.1378/chest.09-2398. [DOI] [PubMed] [Google Scholar]
- 59.van der Burg M, van Zelm MC, van Dongen JJ. Molecular diagnostics of primary immunodeficiencies: benefits and future challenges. Adv Exp Med Biol. 2009;634:231–241. doi: 10.1007/978-0-387-79838-7_19. [DOI] [PubMed] [Google Scholar]
- 60.van Zelm MC, Reisli I, van der Burg M, Castano D, van Noesel CJ, van Tol MJ, Woellner C, Grimbacher B, Patino PJ, van Dongen JJ, Franco JL. An antibody-deficiency syndrome due to mutations in the CD19 gene. N Engl J Med. 2006;354:1901–1912. doi: 10.1056/NEJMoa051568. [DOI] [PubMed] [Google Scholar]
- 61.van Zelm MC, Smet J, Adams B, Mascart F, Schandene L, Janssen F, Ferster A, Kuo CC, Levy S, van Dongen JJ, van der Burg M. CD81 gene defect in humans disrupts CD19 complex formation and leads to antibody deficiency. J Clin Invest. 2010;120:1265–1274. doi: 10.1172/JCI39748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vetrie D, Vorechovsky I, Sideras P, Holland J, Davies A, Flinter F, Hammarstrom L, Kinnon C, Levinsky R, Bobrow M, et al. The gene involved in X-linked agammaglobulinaemia is a member of the src family of protein-tyrosine kinases. Nature. 1993;361:226–233. doi: 10.1038/361226a0. [DOI] [PubMed] [Google Scholar]
- 63.Vodjgani M, Aghamohammadi A, Samadi M, Moin M, Hadjati J, Mirahmadian M, Parvaneh N, Salavati A, Abdollahzade S, Rezaei N, Srrafnejad A. Analysis of class-switched memory B cells in patients with common variable immunodeficiency and its clinical implications. J Investig Allergol Clin Immunol. 2007;17:321–328. [PubMed] [Google Scholar]
- 64.Warnatz K, Salzer U, Rizzi M, Fischer B, Gutenberger S, Bohm J, Kienzler AK, Pan-Hammarstrom Q, Hammarstrom L, Rakhmanov M, Schlesier M, Grimbacher B, Peter HH, Eibel H. B-cell activating factor receptor deficiency is associated with an adult-onset antibody deficiency syndrome in humans. Proc Natl Acad Sci USA. 2009;106:13945–13950. doi: 10.1073/pnas.0903543106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wehr C, Kivioja T, Schmitt C, Ferry B, Witte T, Eren E, Vlkova M, et al. The EUROclass trial: defining subgroups in common variable immunodeficiency. Blood. 2008;111:77–85. doi: 10.1182/blood-2007-06-091744. [DOI] [PubMed] [Google Scholar]
- 66.Winkelstein JA, Marino MC, Lederman HM, Jones SM, Sullivan K, Burks AW, Conley ME, Cunningham-Rundles C, Ochs HD. X-linked agammaglobulinemia: report on a United States registry of 201 patients. Med Baltim. 2006;85:193–202. doi: 10.1097/01.md.0000229482.27398.ad. [DOI] [PubMed] [Google Scholar]
- 67.Winkelstein JA, Marino MC, Ochs H, Fuleihan R, Scholl PR, Geha R, Stiehm ER, Conley ME. The X-linked hyper-IgM syndrome: clinical and immunologic features of 79 patients. Med Baltim. 2003;82:373–384. doi: 10.1097/01.md.0000100046.06009.b0. [DOI] [PubMed] [Google Scholar]
- 68.Yong PL, Orange JS, Sullivan KE. Pediatric common variable immunodeficiency: immunologic and phenotypic associations with switched memory B cells Pediatr Allergy Immunol. 2010;21:852–858. doi: 10.1111/j.1399-3038.2010.01004.x. [DOI] [PubMed] [Google Scholar]