Background
It is well established that exercise training increases skeletal muscle mitochondrial content, and this adaptive response is linked with increased exercise tolerance and improved health. However, the mechanisms responsible for exercise-induced mitochondrial biogenesis are only beginning to be elucidated. Mitochondrial adaptations to exercise training in skeletal muscle are likely to be attributable to repeated transient increases in mRNA expression following each exercise session (see Perry et al. (2010) and references within). Over time, these successive ‘pulses’ of increased mRNA expression eventually lead to an accumulation of new muscle proteins, which constitute training adaptation and improve metabolic capacity. Data obtained from muscle-like cell lines and transgenic rodent models suggest a key role for transcriptional regulatory proteins in mediating the increase in mitochondrial content and the identification of those proteins responsible has become vital to advancing the field. Chief among these regulatory proteins is the transcriptional co-activator peroxisome proliferator-activated receptor γ co-activator (PGC)-1α, which has often been described as a ‘master regulator of mitochondrial biogenesis’. Overexpression of PGC-1α in skeletal muscle leads to many of the same adaptations as exercise training, such as an increase in mitochondrial content, improved fatigue resistance, and increased expression of proteins involved in lipid metabolism (e.g. Calvo et al. 2008). Therefore, it is generally accepted that transcriptional regulatory proteins, such as PGC-1α, are involved in mediating the adaptive increase in skeletal muscle mitochondrial content following exercise training. However, equally important to identifying those proteins responsible for regulating post-exercise elevations in mRNA expression is the characterization of the temporal relationship between and among transcriptional regulatory proteins and mitochondrial genes. Currently, there is a paucity of research examining the time course of the increase in transcriptional regulators in association with markers of mitochondrial content, especially in humans. A recent study published in The Journal of Physiology (Perry et al. 2010) comprehensively describes the time course for the increase in transcription factors and co-activators implicated in mitochondrial biogenesis along with markers of mitochondrial content, in human skeletal muscle, to shed light on this issue.
Data of interest
To directly examine the hypothesis that cumulative increases in mRNA expression precede adaptive increases in protein and enzyme activity in human skeletal muscle in response to training, Perry and associates (2010) used a unique experimental design in which sequential muscle biopsies were obtained from nine healthy men over the course of seven sessions of high-intensity interval training (HIT) performed over a 2 week period. Each training session consisted of ten 4 min cycling intervals at an intensity eliciting ∼90%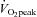 , based on previous work from these authors that showed this short-term protocol was a potent stimulus to induce muscle mitochondrial and exercise performance adaptations (see Perry et al. (2010) and references within). Using this design, Perry and colleagues (2010) were able to assess the time course of the adaptive changes in mRNA and protein of various transcriptional regulatory proteins as well as mitochondrial enzymes. Furthermore, six different transcriptional regulators and several markers of mitochondrial content were assessed, thus making the paper rich with data.
, based on previous work from these authors that showed this short-term protocol was a potent stimulus to induce muscle mitochondrial and exercise performance adaptations (see Perry et al. (2010) and references within). Using this design, Perry and colleagues (2010) were able to assess the time course of the adaptive changes in mRNA and protein of various transcriptional regulatory proteins as well as mitochondrial enzymes. Furthermore, six different transcriptional regulators and several markers of mitochondrial content were assessed, thus making the paper rich with data.
Consistent with the theory that transcriptional regulation is a key aspect of adaptations to training, the results from Perry et al. (2010) showed that an increase in mRNA preceded an increase in protein for any given gene. Furthermore, the increase in protein content of transcriptional regulators tended to precede the increase in mitochondrial enzymes that were measured. Notably, the PGC-1α data lend important insight into the mechanisms of mitochondrial adaptation to exercise training. PGC-1α mRNA was increased ∼10-fold 4 h following the first training session, yet total PGC-1α protein was unchanged at this time point. Because PGC-1α is known to co-activate its own promoter, the increase in PGC-1α mRNA suggests that existing PGC-1α protein was activated by acute exercise. This is consistent with recent studies demonstrating that subcellular redistribution and/or post-translational modifications activate existing PGC-1α protein in response to acute endurance exercise (e.g. Little et al. 2010). The large increase in PGC-1α mRNA measured after 4 h of recovery from the first training session was followed by an increase in total PGC-1α protein at 24 h. This increase in total PGC-1α protein preceded increases in citrate synthase (CS) and β-hydroxyacyl-CoA dehydrogenase (β-HAD) enzyme activity, which were not apparent until 4–24 h following the third training session. Significantly, the authors also observed that with each successive training session, the acute response of PGC-1α, CS, and β-HAD mRNA was attenuated. These observations led to the attractive hypothesis that reduced transcriptional responses with subsequent training bouts may play a role in the oft-observed ‘plateau effect’ that occurs over time with training. This plateau effect was in opposition to previous findings by Pilegaard and colleagues (2003) who showed a greater mRNA response to acute exercise in trained human muscle. These results also appear at odds with data from Wilkinson et al. (2008) who demonstrated that trained muscle appears to become more efficient at directing protein synthesis towards its primary adaptive need (i.e. hypertrophic or metabolic) over time. These contrasting findings may, as the authors point out, be due to the unique stimulus presented by HIT. Alternatively, it presents the possibility that as training progresses, the primary mechanism behind the adaptive response may shift from transcriptional regulation to that of, for example, post-translational modification of existing proteins. Further research into these mechanisms will surely provide even greater insight.
The authors also measured the content of mitochondrial DNA (mtDNA), which is regarded by many as a more robust indicator of mitochondrial content. The increase in mtDNA followed a similar pattern as CS and β-HAD, being significantly elevated at 24 h following the third training session, preceded by the increase in total PGC-1α protein. Collectively, these findings support the notion that an increase in PGC-1α protein may be important for inducing or sustaining an increase in skeletal muscle mitochondrial content following exercise training in humans. Interestingly, total protein content of mitochondrial transcription factor A (Tfam), which is required for mtDNA transcription and replication, did not increase following training. This suggests that basal amounts of Tfam are sufficient to support an increase in mtDNA. Alternatively, the location or activation of existing Tfam within the muscle cell or mitochondria may play a role in regulating mitochondrial biogenesis.
In additional to transcriptional regulators, the authors also measured proteins involved in mitochondrial fission and fusion. The fission proteins dynamin-related protein-1 (Drp1) and fission protein-1 (Fis1) were increased following three sessions of HIT and the fusion protein mitofusin 1 (Mfn1) was increased following seven sessions, but the fusion protein Mfn2 was unaltered at all time points. Fusion and fission of mitochondria in skeletal muscle are now well-established and disordered mitochondrial dynamics have been implicated in several chronic disease states. Currently, little is known regarding the effects of exercise on mitochondrial fusion and fission and the findings provide evidence that exercise may influence mitochondrial dynamics. However, it is difficult to discern whether increases in fusion and fission proteins measured by Western blotting were primarily the result of an increase in total mitochondrial content or were indicative of increased fusion and fission events. Further research is clearly needed to clarify the importance of mitochondrial fusion and fission in the adaptive response to exercise.
Significance
In summary, the results of Perry et al. (2010) provide important mechanistic insight into the time course of the mitochondrial adaptive response to exercise in human skeletal muscle. The findings provide experimental support for the notion that an increase in transcriptional regulatory proteins, in particular PGC-1α, precedes an increase in markers of mitochondrial content throughout the course of training. Future research on the regulation of mtDNA transcription and replication, as well as mitochondrial fusion and fission, should help to improve our expanding understanding of how skeletal muscle mitochondrial biogenesis is controlled in response to exercise.
Acknowledgments
The authors would like to thank Dr Martin Gibala for his critical review of the manuscript prior to submission.
References
- Calvo JA, Daniels TG, Wang X, Paul A, Lin J, Spiegelman BM, Stevenson SC, Rangwala SM. Muscle-specific expression of PPARγ coactivator-1α improves exercise performance and increases peak oxygen uptake. J Appl Physiol. 2008;104:1304–1312. doi: 10.1152/japplphysiol.01231.2007. [DOI] [PubMed] [Google Scholar]
- Little JP, Safdar A, Cermak N, Tarnopolsky MA, Gibala MJ. Acute endurance exercise increases the nuclear abundance of PGC-1α in trained human skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2010;298:R912–R917. doi: 10.1152/ajpregu.00409.2009. [DOI] [PubMed] [Google Scholar]
- Perry CG, Lally J, Holloway GP, Heigenhauser GJ, Bonen A, Spriet LL. Repeated transient mRNA bursts precede increases in transcriptional and mitochondrial proteins during training in human skeletal muscle. J Physiol. 2010;588:4795–4810. doi: 10.1113/jphysiol.2010.199448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilegaard H, Saltin B, Neufer PD. Exercise induces transient transcriptional activation of the PGC-1α gene in human skeletal muscle. J Physiol. 2003;546:851–858. doi: 10.1113/jphysiol.2002.034850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson SB, Phillips SM, Atherton PJ, Patel R, Yarasheski KE, Tarnopolsky MA, Rennie MJ. Differential effects of resistance and endurance exercise in the fed state on signalling molecule phosphorylation and protein synthesis in human muscle. J Physiol. 2008;586:3701–3717. doi: 10.1113/jphysiol.2008.153916. [DOI] [PMC free article] [PubMed] [Google Scholar]


