Non-technical summary
The endothelin-3 (ET-3) gene is essential for the development of the enteric nervous system in the gastrointestinal tract of mammals, including humans and mice. Loss of the ET-3 gene leads to the formation of an aganglionic colorectum and impaired bowel function. Endogenous endothelin peptides and their receptors also play a major role in nociception in a variety of organs and species, including humans. However, whether nociception is altered in the aganglionic region of the colorectum is unknown. We show that in ET-3 deficient mice, there is a loss of nociception from the aganglionic rectum, but not other visceral organs. This loss of nociception is due to a reduction in spinal afferent innervation and a selective deficiency in specific classes of rectal afferent nerve fibres, which are necessary for detection of noxious stimuli from this region.
Abstract
Abstract
Endothelin peptides and their endogenous receptors play a major role in nociception in a variety of different organs. They also play an essential role in the development of the enteric nervous system. Mice with deletions of the endothelin-3 gene (lethal spotted mice, ls/ls) develop congenital aganglionosis. However, little is known about how nociception might be affected in the aganglionic rectum of mice deficient in endothelin-3. In this study we investigated changes in spinal afferent innervation and visceral pain transmission from the aganglionic rectum in ls/ls mice. Electromyogram recordings from anaesthetized ls/ls mice revealed a deficit in visceromotor responses arising from the aganglionic colorectum in response to noxious colorectal distension. Loss of visceromotor responses (VMRs) in ls/ls mice was selective, as no reduction in VMRs was detected after stimulation of the bladder or somatic organs. Calcitonin gene related peptide (CGRP) immunoreactivity, retrograde neuronal tracing and extracellular afferent recordings from the aganglionic rectum revealed decreased colorectal spinal innervation, combined with a reduction in mechanosensitivity of rectal afferents. The sensory defect in ls/ls mice is primarily associated with changes in low threshold wide dynamic range rectal afferents. In conclusion, disruption of endothelin 3 gene expression not only affects development and function of the enteric nervous system, but also specific classes of spinal rectal mechanoreceptors, which are required for visceral nociception from the colorectum.
Introduction
Hirschsprung's disease is a common congenital defect, affecting approximately 1 in 4000 newborn humans, in which the enteric nervous system fails to colonize the terminal bowel (Newgreen & Young, 2002; Young et al. 2002; Furness, 2006; Mundt & Bates, 2010). Typically, there is an absence of both enteric neurons and ganglia (aganglionosis) from a variable length of the colorectum, which can be classified as short or long segment aganglionosis. Long segment aganglionosis is associated with failure to pass meconium, obstruction, stasis and megacolon, which necessitates surgical removal of the affected bowel. Many patients with short segment aganglionosis require surgery and removal of the aganglionic segment, but in some cases these patients can live into adulthood, or live their entire life without having the aganglionic segment removed (Fishbein et al. 1986).
The genetic basis of Hirschsprung's disease has been extensively investigated and mutations of at least nine genes contribute in a complex fashion (Tam & Garcia-Barcelo, 2009), making it an ideal model for genetic disease with complex patterns of inheritance (Amiel et al. 2008). Mutations in the gene coding for endothelin-3 (ET-3) or its cognate receptor (Ednrb) each account for approximately 5% of human cases of Hirschsprung's disease, indicating that the endothelin-3 signalling pathway is required for normal development of the enteric nervous system. Mice with deletions of ET-3 (lethal spotted mouse, ls/ls) or Ednrb (piebald lethal mouse, sl/sl) show comparable defects in the enteric nervous system in the distal bowel (Baynash et al. 1994; Hosoda et al. 1994)
In addition to their role in development of the enteric nervous system, there is substantial evidence that endogenous endothelins and their receptors participate in a wide range of nociceptive behaviours (Baamonde et al. 2004; Khodorova et al. 2009; Stösser et al. 2010). Endothelin receptors are found extensively on small diameter sensory neurons, often corresponding to c-fibre afferents, which carry pain impulses. Exogenous endothelin elicits pain when injected into many different parts of the body in several species (Katugampola et al. 2000; Hans et al. 2007; Claudino et al. 2010; Liang et al. 2010). Furthermore, tumour-derived endothelins are involved in the activation and sensitization of nociceptors in a variety of experimental models of pain and hypersensitivity arising from cancerous tissues (Hamamoto et al. 2008; Khodorova et al. 2009).
Despite the absence of enteric ganglia in the aganglionic rectum of mice lacking endothelin-3, extrinsic nerve fibres remain abundant in the aganglionic segment (Larsson, 1994). Interestingly, we recently identified the existence of low threshold stretch sensitive rectal afferents innervating the aganglionic rectum of mutant piebald-lethal (sl/sl) and lethal-spotted (ls/ls) mice (Spencer et al. 2008a,b;). However, preliminary in vivo studies from the aganglionic region of mice with deficiencies in endothelin pathways suggested that noxious distension of this region fails to activate normal pain responses (Spencer, 2005). Therefore, mutant mice with colorectal aganglionosis might provide an ideal model to test whether colorectal aganglionosis is associated with changes in sensory nerve function and visceral pain pathways arising from this region. Comparison of the properties of different classes of afferents between these mutant mice and controls could provide important information about the afferent basis of visceral pain from the colorectum (Blackshaw et al. 2010).
Here, we demonstrate that ET-3 deficient mice not only develop colorectal aganglionosis, but also selectively lack mechanonociception from the aganglionic region. We identified that this is due to both a reduction in the density of sensory innervation and impaired mechanosensitivity predominantly in low-threshold stretch-sensitive afferents.
Methods
Homozygous lethal spotted (ls/ls) mice, deficient in endogenous endothelin-3, were obtained in-house by intercrossing homozygous lethal spotted mice, as previously described (Spencer et al. 2008a). All progeny were therefore homozygous and readily identified by their black and white spotting coat colour (Baynash et al. 1994). Lethal spotted mice were studied between 4 and 6 months of age and age-matched C57BL/6 mice were used as experimental controls throughout all experiments.
Protocol for in vivo visceromotor responses
Mice were anaesthetized with pentobarbital sodium (200–300 μl of 6 mg ml−1); depth of anaesthesia was assessed by lack of response to hindlimb or tail pinch. A collapsible balloon was inserted 4–6 mm into the colorectum of mice and pressure was continuously monitored by a Gould pressure transducer, connected to a Powerlab recording system using Chart software (v. 5.3, ADInstruments, Sydney, Australia). When fully inflated, the balloon measured 8 mm in length and 7 mm in width. Thus the maximum length of colorectum distended by the balloon never exceeded the length of the aganglionic region in ls/ls mice. Electromyographic (EMG) electrodes were placed into the left external oblique muscle and a reference electrode placed in the quadriceps muscle of the opposing leg. EMG recordings were acquired at 20 kHz and recorded on a PC running LabChart 6 Pro software, high pass filtered (100 Hz) and analysed using Spike Histogram software (ADInstruments). Intraluminal pressure was increased by two protocols: the first by raising a column of water in 20 mmHg increments, from 0 mmHg to 100–120 mmHg, with each step maintained for 15–20 s, and the second by an instantaneous increase in pressure from 0 mmHg to 120 mmHg maintained for 10–20 s. Manual balloon inflations were also made up to 300 mmHg. Following EMG experiments mice were humanely killed at the end of each experiment, by an overdose of pentobarbital sodium, followed by cervical dislocation.
Extracellular afferent nerve recordings
Adult mice (N = 18 C57BL/6 and N = 23 ls/ls) were killed by inhalation of isoflurane (overdose) followed by cervical dislocation, in accordance with a permit from the Animal Welfare Committee at Flinders University. A midline incision was made along the abdominal cavity and the entire colon with attached pelvic ganglia and pelvic nerves was removed and placed in a Petri dish containing cold Krebs solution (mm: NaCl, 118; KCl, 4.75; NaH2PO4, 1.0; NaHCO3, 25; MgCl2, 1.2; CaCl2, 2.5; glucose, 11; bubbled with 95% O2–5% CO2). Full thickness, small (12 mm length × 8–9 mm width) flat sheet preparations were studied with the mucosa uppermost. The same length segment of colorectum (12 mm total length measured from the anus) was removed from ls/ls and C57BL/6 controls. ‘Close to target’ extracellular recordings (Zagorodnyuk & Brookes, 2000; Zagorodnyuk et al. 2005) were made from sensory axons in fine rectal nerve trunks (98 nerve trunks from 41 mice) entering the colorectum. The majority of the preparations were stretched by attaching a 12 mm array of hooks to an isotonic transducer (Harvard Bioscience 52-9511, S. Natick, MA, USA) and increasing the counterweight (0.5–40 g), while recording changes in circumference. In some preparations, the hooks were connected to an isometric force transducer (DSC no. 46-1001-01, Kistler-Morse, Redmond, WA, USA), mounted on a ‘tissue stretcher’ (Brookes et al. 1999). Preparations were stretched by the microprocessor-controlled tissue stretcher at 1000 μm s−1 up to 4–5 mm (which correspond to 50–60% of resting length) and held for 10 s at 3–4 min intervals while recording intramural tension. Mean firing rate of afferent units was calculated during 10 s stretches.
Fine rectal nerve trunks, originating from the pelvic ganglia, were dissected free and pulled into a small chamber (∼1 ml volume) separated by a coverslip and silicone grease barrier (Ajax Chemicals, Ajax, Auburn, Australia). The small chamber was filled with paraffin oil and differential extracellular recordings were made via platinum electrodes from the nerve trunks. Signals were amplified (DAM 80, WPI, Sarasota, FL, USA) and recorded at 20 kHz with a MacLab 8sp (ADInstruments) attached to an Apple iMac G5 computer using LabChart 6 Pro software (ADInstruments). Single units were discriminated by amplitude and duration using Spike Histogram software.
To distinguish the major classes of rectal mechanoreceptors, the following strategy was used. First, the preparation was stretched isotonically and stretch-sensitive single units (if any) were identified. Then, the mucosa was stroked with light von Frey hairs (10–100 mg) to determine receptive field (hotspots) of ‘muscular-mucosal’ and ‘mucosal’ mechano-sensitive units. The receptive field was then marked with carbon particles attached to the von Frey hair (Zagorodnyuk & Brookes, 2000). For afferents insensitive to mucosal stroking, the receptive field was located by compressing the tissue (from the mucosal surface) with calibrated (200–1000 mg) von Frey hairs. ‘Hotspots’ identified in this way were marked with carbon particles attached to the von Frey hair. For stimulus–response curves, five probes with each von Frey hair were delivered and the three largest responses were averaged. Hot Krebs solution (45–46°C) was applied directly from a pipette onto the marked receptive field onto mucosa. In most cases, at the end of experiments capsaicin (10 μm for 10 s) was pressure ejected from a pipette onto the receptive field (mucosa was removed from hotspot area). To compare the effects of capsaicin and hot Krebs solutions, the firing rate of afferent units was averaged for a 5 s period around the peak response (mean maximum firing). All studies on afferents were carried out in the presence of nicardipine (3 μm) to minimize mechanical interference from active contractions evoked by stretch or von Frey hair probing and stroking.
Drugs
Capsaicin was obtained from Sigma-Aldrich Corp. (St Louis, MO, USA) and 1,1′-didodecyl-3,3,3,3′-tetramethylindocarbocyanine perchlorate (DiI, C12) from Molecular Probes (Eugene, OR, USA).
DiI retrograde tracing
Mice of either sex were aneasthetized with pentobarbital sodium (200–300 μl of 6 mg ml−1); depth of anaesthesia was monitored by lack of response to a hindlimb or tail pinch. When anaesthetized, sterile Hamilton syringe (5 μl maximum volume; Hamilton Company, Reno, Nevada) was used to penetrate the mucosal surface of the terminal rectum (1–4 mm from the anus). No surgery was required for injections of DiI into the terminal rectum. A single site injection of DiI (200–400 nl volume) was made into the smooth muscle layers of colorectum and 6 days later mice were killed. The distal bowel was then removed and inspected for leakage of DiI: preparations showing leakage onto the surface of the colorectum were discarded. Dorsal root ganglia (DRGs) from bilateral spinal levels (T13 to S5, inclusive) were then removed. DRGs were fixed in paraformadehyde (4%) for 2 days before cryoprotection in sucrose for 2 days. Dorsal root ganglia were cryostat-sectioned at −19°C and 12 μm sections made throughout all ganglia. Two sections (sections 5 and 9) from each DRG were retained and mounted under a coverslip. Fluorescence images of all DRG sections were photographed within 2 days to avoid spread of DiI from filled nerve cell bodies. Because DRG neurons are occasionally autofluorescent, DiI-labelled cell bodies were discriminated by their having fluorescence intensities three times above the background, which was defined by the mean fluorescence of afferent cell bodies from animals of similar age that had not been injected with DiI (Robinson et al. 2004).
Immunohistochemistry
Preparations of bladder and colorectum were fixed overnight in modified Zamboni's fixative (15% saturated picric acid, 2% formaldehyde in 0.1 m phosphate buffer, pH 7.0), cleared in dimethylsulfoxide (3 × 10 min washes) then rinsed in 0.1 m phosphate buffered saline (PBS, 0.15 m NaCl, pH 7.2). Preparations were obtained from control C57BL/6 and ls/ls mice and stained overnight for calcitonin gene related peptide (CGRP; IHC 6006, Peninsula Laboratories, IgG raised in rabbit), at a concentration of 1:1600. Donkey anti-rabbit CY3 was used as a secondary antibody (catalogue: 711-165-152, Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA) at a concentration of 1:200. We confirmed that CGRP immunoreactivity labelled extrinsic afferents by showing that after 5 days in organ culture, CGRP immunoreactive fibres degenerated and were not detected.
Hematoxylin and eosin (H & E stain) was used to determine if the circular and longitudinal muscles, the mucosa and muscularis mucosa were of different thicknesses in control and ls/ls mice. Prior to staining, control and ls/ls preparations were all pinned under the same level of circumferential stretch, then fixed in 4% paraformaldehyde at room temperature for 48 h. Regions of colorectum stained were taken at distances of 5 mm, 10 mm, 15 mm and 20 mm from the anal sphincter. Usually, the region taken 20 mm from the anal sphincter was ganglionated with myenteric plexus, while the other regions taken were aganglionic.
Data analysis
Mean firing rate of afferent units was calculated during 10 s stretches. Results are expressed as means ± standard error of the mean, with n referring to the number of units and N to the number of animals on which observations were made. Statistical analysis was performed by Student's two-tailed t test for paired or unpaired data or by repeated measures analysis of variance (ANOVA, one-way or two-way) using Prism v.5 software (GraphPad Software, Inc., San Diego, CA, USA). Differences were considered significant if P < 0.05.
Results
Visceromotor responses from colorectum
Intraluminal distension (maintained for 15–20 s with increments of 20 mmHg), applied to the colorectum of anaesthetized wild-type mice, consistently evoked electromyographic visceromotor responses (VMRs) in the left external oblique muscle of control mice. The VMRs had a threshold of approximately 20 mmHg and increased linearly with pressure up to 100 mmHg (see Fig. 1A and C) (N = 8), consistent with previous reports (Sipe et al. 2008).
Figure 1. Loss of visceromotor responses to rectal distension in ls/ls mice.
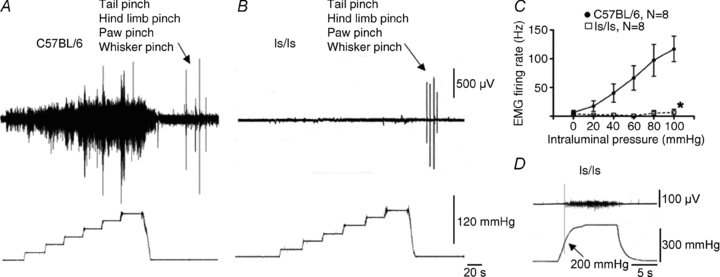
A, electromyographic recording of visceromotor responses to incremental rectal distension in a control C57BL/6 mouse (up to 120 mmHg). B, rectal distension up to 120 mmHg did not evoke a measurable visceromotor response in ls/ls mouse, although somatic stimuli (tail pinch, hindlimb pinch, paw pinch and whisker pinch) were effective. C, quantification of visceromotor responses to rectal distension in control and ls/ls mice. D, distension pressures over 200 mmHg evoked small visceromotor responses in some, but not all ls/ls mice.
When the same incremental distensions were applied to the aganglionic colorectum of ls/ls mice, no detectable visceromotor responses were elicited (Fig. 1B and C) (N = 8). We then applied an instantaneous step change from 0 mmHg to 120 mmHg, and this again failed to elicit visceromotor responses in 7 of 8 ls/ls mice. One mouse showed small VMRs at 120 mmHg. Intrarectal pressures up to 180 mmHg were then tested in five ls/ls mice, but still no visceromotor responses were detected (N = 5). Above 200 mmHg, the intraluminal balloon sometimes ruptured the rectum. In 3 of 5 ls/ls mice, intraluminal pressures above 200 mmHg evoked small visceromotor responses (Fig. 1D).
Responses to other noxious stimuli
We investigated whether the impairment in nociception in ls/ls mice was specific for stimulation of the aganglionic rectum, or if nociception from other visceral organs was also impaired. In wild-type mice, graded intraluminal distension (20–100 mmHg) applied to the bladder (N = 6), reliably elicted VMRs (Fig. 2A and C). When the same distension stimuli were applied to ls/ls mice, similar VMRs were recorded (Fig. 2B and C) (N = 6), which were not significantly different from control (N = 6, F = 0.03, P = 0.86, two-way ANOVA). We also tested somatic stimuli by applying a calibrated pinch to the tail or hindlimb for 5 s, similar to that described previously (Shafton et al. 2006). Overall, there was no difference in the electromyographic responses from left external oblique muscle in C57BL/6 (N = 14) and ls/ls mice (Fig. 3A, B) (N = 16, NS, one-way ANOVA). These results indicate that the ls/ls mice did not have a generalized deficit in either somatic or visceral pain mechanisms, but had selective suppression of responses to colorectal distension.
Figure 2. ls/ls mice show normal visceromotor responses to bladder distension.
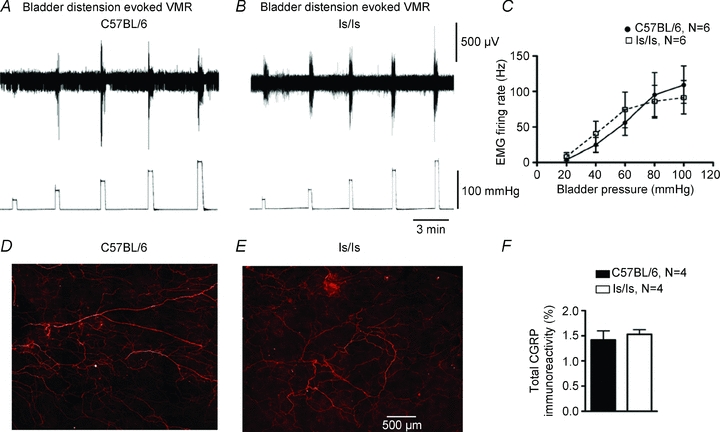
A, visceromotor responses in control mouse to graded bladder distensions (20–100 mmHg). B, similar responses were seen in ls/ls mice. C, averaged visceromotor responses did not differ significantly in controls and ls/ls mice. D and E, similar CGRP immunoreactivity from spinal afferents innervating the control and ls/ls mouse bladder detrusor muscle. F, no statistical difference was found in total density of CGRP immunoreactivity between the bladders of control and ls/ls mice when measured as a percentage of field of view.
Figure 3. Changes in visceromotor response evoked by focal electrical stimulation of the exposed colorectum in ls/ls mice.
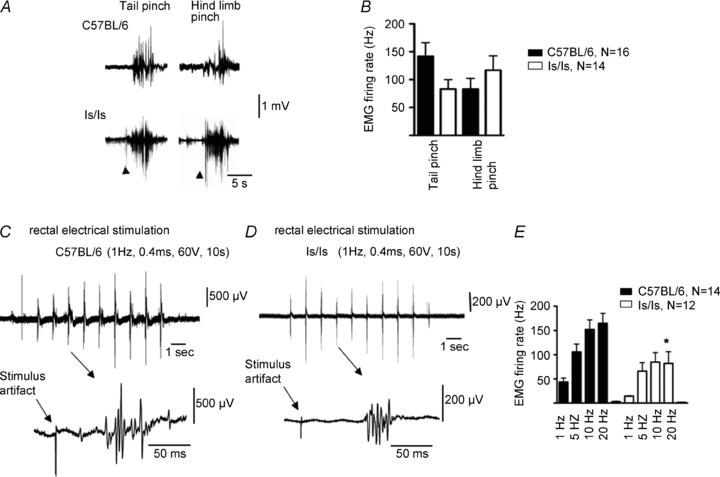
A, electromyographic responses evoked by calibrated tail pinch and hindlimb pinch did not differ between C57BL/6 and ls/ls mice. B, averaged responses to tail or hindlimb pinch for C57BL/6 and ls/ls mice showed no significant differences. C, focal electrical stimulation (1 Hz, 0.4 ms, 60 V, 10 s) of the surface of the exposed control rectum elicited brief visceromotor responses with latencies of ∼50 ms. D, VMRs elicited from the aganglionic colorectum were of reduced intensity. E, the average number per second of multiunit action potentials were significantly reduced in ls/ls mice.
Electrical stimulation of colorectal nerve pathways
We also tested whether there was a loss of a functional pain pathway from the aganglionic colorectum in ls/ls mice, without relying on mechanical stimuli. To test this, electrical nerve stimulation was applied directly to the surface of gut wall, in the same region of aganglionic rectum subjected to colorectal distension. Nerve selective stimuli (1–20 Hz, 0.4 ms, 60 V, 10 s) applied to the exposed rectum consistently evoked VMRs in both wild-type (N = 14) and ls/ls mice (N = 12) (Fig. 3C and D). However, responses in mutant mice were significantly (F = 13.5, P < 0.001, two-way ANOVA) smaller (by 50% at 20 Hz) than controls (Fig. 3E).
CGRP immunoreactivity and DiI retrograde labelling
The selective loss of VMRs to rectal distension and reduced responses to electrical stimulation raised the possibility that the innervation of the distal bowel by spinal afferent neurons might be abnormal. The retrograde neuronal tracer, DiI, was injected into the wall of the colorectum of mice in the region distended by the intraluminal balloon (200–400 nl single injections) a distance of 1–4 mm from the anus. In control mice (N = 4), the greatest number of neurons was located in dorsal root ganglia of L5 and L6, with a small proportion of neurons labelled in S1 and S2 (see Fig. 4A). In ls/ls mice (N = 7), significantly fewer neurons (60–80% loss) were labelled in S1 and S2 than in wild-type controls (N = 7, F = 11.5, P < 0.001 two-way ANOVA). Consistent with this, in ls/ls mice, the aganglionic rectum had a significant reduction (by 60%, P < 0.0001, N = 4) in CGRP immunoreactive nerve fibres, compared with wild-type mice (N = 4) (Fig. 4B, C and D). In contrast, there was no significant difference in axonal CGRP immunoreactivity between the bladders from either wild-type and ls/ls mice (N = 4) (Fig. 2D–F).
Figure 4. DiI retrograde tracing and CGRP immunoreactivity reveals differences in spinal innervation from the aganglionic rectum of ls/ls mice.
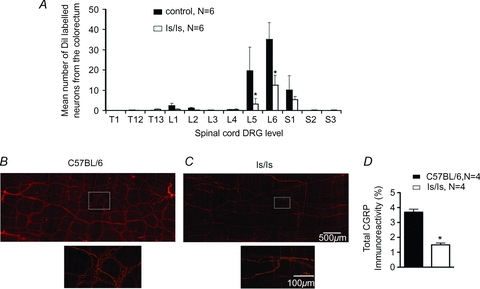
A, retrograde neuronal tracing from the rectum, 2–4 mm from the anal sphincter revealed DiI labelled sensory nerve cell bodies in dorsal root ganglia, with the peak distribution at L5 and L6 in both C57BL/6 and ls/ls mice. There were significantly less DRG labelled neurons in ls/ls mice. B and C, CGRP immunoreactivity in control mouse colorectum (B) and in aganglionic ls/ls colorectum (C). Dense immunoreactivity is present in the myenteric plexus of control mice, but it is significantly reduced in the aganglionic colorectum of ls/ls mice. D, CGRP immunoreactivity was significantly reduced in ls/ls mouse rectum, when measured as a percentage of field of view.
Extracellular recordings from different classes of rectal afferent nerve in wild-type and endothelin-3 deficient (ls/ls) mice
Recently, we identified that lesions applied to the rectal nerves abolished the visceral pain response underlying the VMRs to colorectal distension, but lesions to the lumbar colonic or hypogastric nerves did not reduce VMRs (Brookes & Spencer, 2008). Therefore, extracellular recordings were made from extrinsic sensory axons in rectal nerves, close to the gut wall, in vitro from both ls/ls and wild-type mice. In most cases, one to three units were reliably discriminated from medium to large diameter nerve trunks innervating colorectum. First, we analysed total stretch-sensitive firing using multi-unit recordings. While stretch-evoked responses could be evoked from both control and ls/ls mice, there was a threefold reduction (F = 34.7, P < 0.0001, two-way ANOVA) of multi-unit stretch-induced firing in ls/ls mice compared with controls (Fig. 5F). We tested whether this was due to a reduction in the number of distension-sensitive units per nerve trunk and/or to reduced stretch-sensitivity of specific classes of distension-sensitive afferents. Three major classes of stretch-sensitive rectal afferents were readily distinguished in single unit recordings from rectal nerve trunks in control C57BL/6 and ls/ls mice. Muscular afferents and muscular-mucosal afferents both had low thresholds to distension whereas serosal afferents had significantly higher thresholds (Fig. 7D). The electrophysiological parameters (spike amplitude and duration) of muscular and muscular-mucosal afferents were similar while serosal afferents had significantly lower spike amplitude and longer spike duration (Fig. 5E).
Figure 5. Characteristics of functional classes of distension-sensitive rectal afferents in wild-type and ls/ls mice.
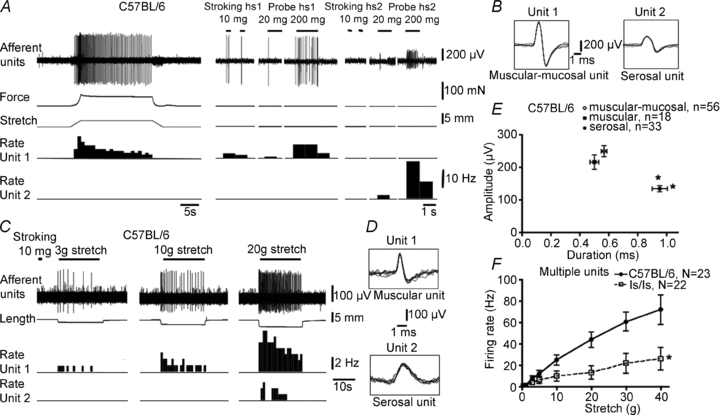
A, wild-type mouse: unit 1 is a muscular-mucosal afferent (sensitive to stroking and probing with light von Frey hairs); unit 2 is a serosal afferent (only activated by 200–500 mg von Frey hair probing. B, superimposed action potentials (×7) of units 1 and 2 confirm single unit recordings. C, recording from a different nerve reveals a muscular afferent (activated by small distensions) and a serosal afferent, with a typically higher distension threshold. D, superimposed action potentials confirm single unit recordings and show characteristic shapes of action potentials. E, plot of action potential amplitude and half-duration for each class (muscular, muscular-mucosal and serosal) show consistent differences in profiles. Typically, serosal afferents had a characteristic smaller amplitude and longer duration than muscular-mucosal afferents. F, comparison of total stretch-evoked firing from all functional classes of afferents (multi-unit recording from rectal nerves) for control C57BL/6 and ls/ls mice. Total stretch sensitivity of afferents was significantly reduced in ls/ls mice compared with controls.
Figure 7. Properties of high threshold serosal rectal afferents in C57BL/6 and ls/ls mice.
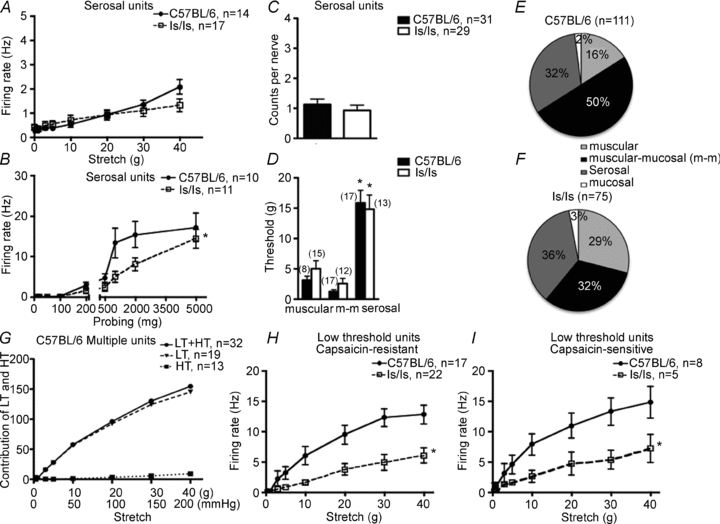
A, the stretch sensitivity of high threshold rectal afferents did not differ between control and ls/ls mice (note the low rate of firing by comparison with Fig. 6E and G), although the sensitivity of serosal afferents to von Frey hair probing was significantly reduced in ls/ls mice at high intensity stimuli (B). C, numbers of serosal afferents in each rectal nerve did not differ significantly between ls/ls and control mice. D, comparison of thresholds to circumferential distension between 3 classes of afferents in both wild-type and ls/ls mice show that serosal afferents had significantly higher thresholds than either muscular or muscular-mucosal afferents. E and F, the relative proportions of each class of afferents recorded from rectal nerves in control and ls/ls mice differed, reflecting the reduced numbers of low threshold wide-dynamic range fibres. G shows relative contribution of low threshold (LT) and high threshold (HT) afferents on the y-axis in the total mean firing frequency (in Hz) in response to stretch. High threshold afferents contribute little to the total rectal nerve firing even at maximum stretch. H and I show that responses to stretch of low threshold capsaicin-sensitive and capsaicin-resistant rectal afferents were both significantly reduced in ls/ls mice.
Muscular afferents
Muscular afferents were silent in in vitro preparations of colorectum from both wild-type (n = 16) and ls/ls mice (n = 34) when the gut was minimally distended. In both control and ls/ls mice, muscular afferents had low thresholds to stretch (3.1 ± 0.7 g, n = 8, N = 8 and 5.0 ± 1.3 g, n = 15, N = 11, respectively, NS, t test) (Figs 5C and 7D). Despite this, stretch-induced firing of muscular afferents in ls/ls mice was approximately half that of control mice (F = 29.1, P < 0.0001, two-way ANOVA, Fig. 6G). In addition, the threshold to probing with a series of von Frey hairs was significantly higher in mutant mice than controls (67 ± 10 mg, n = 10, N = 7 and 259 ± 89 mg, n = 11, N = 8, P < 0.05, t test). Firing rates evoked by probing receptive fields with von Frey hairs were also significantly reduced (F = 6.5, P < 0.01, two-way ANOVA) in ls/ls mice compared with wild-type (Fig. 6H).
Figure 6. Differences in firing of low threshold wide dynamic range distension-sensitive rectal afferents in control and ls/ls mice.
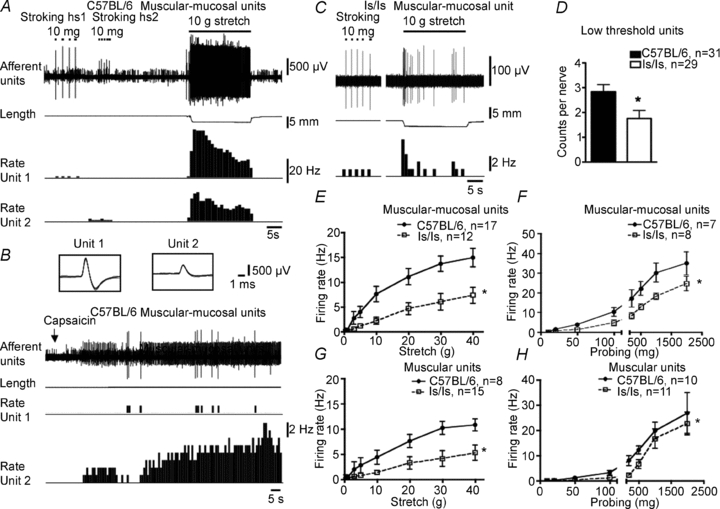
A, two typical muscular-mucosal afferents from a control mouse, responded to both von Frey hair stroking and stretch. B, both units responded to capsaicin (10 μm) (superimposed action potentials shown in inset). C, a muscular-mucosal unit in an ls/ls mouse showed normal responses to mucosal stroking but diminished response to distension by a 10 g load, compare to A. D, the total count of low threshold mechanoreceptors (a mixture of muscular and muscular mucosal afferents) in recorded rectal nerve trunks showed a significant reduction in ls/ls mice compared to control animals. E and F, the stretch and probing sensitivity of muscular-mucosal afferents was significantly reduced compared to controls. G and H, muscular afferents of ls/ls mice showed a similar reduction in stretch and probe-induced firing compared to controls. Note that stretch-induced responses in both muscular and muscular-mucosal afferents did not saturate across the entire range of loads applied. For this reason, these two classes were both considered to belong to low threshold wide dynamic range mechanoreceptors.
Muscular-mucosal afferent mechanoreceptors
These mechanoreceptors showed similar activity to muscular afferents but could be distinguished by their sensitivity to light stroking and probing of the surface of the mucosa. They were typically silent in undistended segments of gut, but could be activated both by distension and by stroking the overlying mucosa with light von Frey hairs (10 mg) in control (n = 51, N = 15) and mutant mice (n = 30, N = 22) (Figs 5A and 6A and C). The distension threshold was similar in control and mutant mice: (1.2 ± 0.03 g, n = 17, N = 11 and 2.6 ± 0.8 g respectively, n = 12, N = 7, NS, t test) (Fig. 7D). However, the number of action potentials evoked by distension was approximately half that recorded from controls (F = 45, P < 0.0001, two-way ANOVA) (Fig. 6A, C and E). Responses to probing of their receptive fields with von Frey hairs were also significantly reduced (F = 25.3, P < 0.0001, two-way ANOVA) (Fig. 6F): at 1000 mg probing, 30 ± 4.8 Hz, n = 7, N = 7) versus 18 ± 1.5 Hz, n = 8, N = 7, respectively, P < 0.01, two-way ANOVA, Bonferroni's post hoc test).
Low threshold stretch-sensitive afferents
Both muscular and muscular-mucosal afferents responded to low levels of circumferential stretch, with their responses increasing across a wide range of distensions, without saturating. We therefore grouped them as low threshold, wide dynamic range fibres. The number of such low threshold wide dynamic range afferents in each recorded nerve trunk was significantly lower (by 36%, P < 0.05, t test) in ls/ls mice compared to wild-type controls: 1.8 ± 0.3 (29 nerves, N = 15) compared with 2.8 ± 0.3 (31 nerves, N = 15), respectively (Fig. 6D). We tested these afferents with both capsaicin (10 μm applied locally) and hot Krebs solution (45–46°C) which activate axons expressing the TRPV1 channel. In 24% of low threshold single units (21 out of 86 units, N = 12) capsaicin (10 μm) activated low threshold wide dynamic range afferents, evoking maximal firing of 7.7 ± 1.5 Hz (n = 14, N = 11). Most of the capsaicin-sensitive fibres were also activated by hot Krebs solution (10 out 12 units, N = 9) with mean maximal firing of 6.8 ± 1.9 Hz (n = 11, N = 8). Overall, ls/ls mice had approximately half as many capsaicin-sensitive, low threshold, stretch-sensitive units as control animals: 17% of ls/ls mice (6 out of 35 units) and 29% of controls (15 out of 51 units) (P < 0.001, chi square test). It is noteworthy that stretch-induced firing of both capsaicin-sensitive and capsaicin-resistant low threshold stretch-sensitive afferents in mutant mice was approximately a half that in wild-type mice (Fig. 7H and I)
High threshold serosal mechanoreceptors
High threshold serosal afferents were recorded from both wild-type and ls/ls mice. They did not respond to mucosal stroking with light von Frey hairs (10 mg) and had high thresholds to both distension and to probing of their receptive fields with von Frey hairs. The distension threshold and the threshold for von Frey hair probing of serosal afferents were significantly higher than those for low threshold, wide dynamic range afferents (P < 0.05, one way ANOVA, Bonferroni's post hoc tests) (Fig. 7B and D).
The majority of serosal afferents (75%, 27 out of 36, N = 18) fired spontaneously at low frequencies when the preparation was not distended, often in a bursting pattern, with mean frequency of 0.3 ± 0.1 Hz (n = 14, N = 7) and 0.4 ± 0.1 Hz (n = 17, N = 7, P > 0.05, t test unpaired) in wild-type and ls/ls mice, respectively. Stretch-induced firing of serosal afferents did not significantly differ (F = 0.42, P = 0.52, two-way ANOVA,) between control and mutant mice: 30 g stretch evoking a change in firing of 1.4 ± 0.2 Hz (n = 14, N = 7) in control versus 1.1 ± 0.3 Hz in mutant mice (n = 17, N = 7, P > 0.05, two-way ANOVA, Bonferroni's post hoc test) (Fig. 7A). The distension thresholds for activation of serosal mechanoreceptors were similar in control and mutant mice: (15.9 ± 2.1 g, n = 17, N = 10 and 14.9 ± 2.3 g, n = 13, N = 7, P > 0.05, t test) (Fig. 7D). The number of serosal afferents that could be discriminated in each nerve trunk did not significantly differ between control and ls/ls mice: 1.1 ± 0.2 (31 nerves, N = 15) and 0.9 ± 0.2 (27 nerves, N = 15, P > 0.05, t test) (Fig. 7C). However, the threshold to von Frey hair probing was significantly higher in ls/ls mice than in controls: in control 210 ± 35 mg (n = 10, N = 8) and in ls/ls mice 480 ± 98 mg (n = 10, N = 9, P < 0.05, t test) and firing rate evoked by probing was smaller in ls/ls mice than in controls (P < 0.01, two-way ANOVA, Bonferroni's post hoc test) (Fig. 7B). In both control and mutant mice, the majority of these afferents (87%, 19 out of 22 units, N = 15) were activated by both capsaicin (10 μm) and by hot Krebs solution (45–46°C), with mean maximum firing rates of 7.0 ± 1.2 Hz (n = 20, N = 15) and 5.3 ± 0.7 Hz (n = 21, N = 15, not significantly different, P > 0.05, unpaired t test), respectively.
Population distribution of afferent classes and their contribution to afferent signalling
We were interested in whether the ratio of different classes of rectal afferent differed between control and ls/ls mice. Overall, there were proportionally fewer muscular-mucosal afferents and more muscular afferents in ls/ls mice (Fig. 7E and F). Hematoxylin and eosin (H & E) stain was used to identify if anatomical differences exist between the colorectum of control and ls/ls mice. Compared to control animals, there was no significant difference in the thickness of circular or longitudinal muscle, mucosa or muscularis-mucosa of ls/ls mice, when aganglionic regions of colorectum were studied 5 mm, 10 mm and 15 mm from the anal sphincter (N = 4, P > 0.05, t test). The major difference was that there were no myenteric ganglia in the terminal colorectum from ls/ls mice lacking ET-3 expression (Baynash et al. 1994).
It was of particular interest to compare the relative contribution of low threshold stretch-sensitive afferents versus high threshold afferents to total afferent signalling evoked by colorectal stretch. When the total stretch-induced afferent firing was summed and weighted according to the proportion of low and high threshold afferents, it was found that even at maximal stretch (range of 20–40 g), 95% of the total afferent firing was attributed to low threshold afferents with only 5% of action potentials contributed by high threshold afferents (Fig. 7G).
Compliance of the colorectum
Changes in nociception to distension of the aganglionic rectum could be caused by changes in wall compliance of the aganglionic musculature. Indeed, wall tension of the colorectum influences the mechanosensitivity of extrinsic afferent fibres (Zagorodnyuk et al. 2005). To test this, we examined the effects of both imposed length and imposed load applied to equally sized segments of colorectum in vitro. First, we stretched control and mutant colorectum by imposed gradual changes in circumference (at 100 μm s−1 up to 4–5 mm), using a microprocessor controlled tissue stretcher (Brookes et al. 1999), while simultaneously recording developed passive force of the preparations. We also imposed changes in circumferential load (0.5–40 g), while simultaneously recording changes in length. No difference in compliance was detected between control and ls/ls mice with either methodology (Fig. 8). In the former experiments, where length was the independent variable, stretching preparations by 50% of resting circumference evoked 126 ± 31 mN (N = 5) in control mice and 144 ± 39 mN (N = 5, F = 0.23, P = 0.63, NS, two-way ANOVA) in ls/ls mice. In the latter experiments, when tension was the independent variable, stretch by 40 g induced 5.8 ± 0.2 mm (N = 12) lengthening in control mice and 5.9 ± 0.2 mm (N = 13, NS, two-way ANOVA, Bonferroni's post hoc test) in mutant mice (Fig. 8).
Figure 8. Compliance of the aganglionic ls/ls colorectum did not differ from that of the control C57BL/6 bowel.
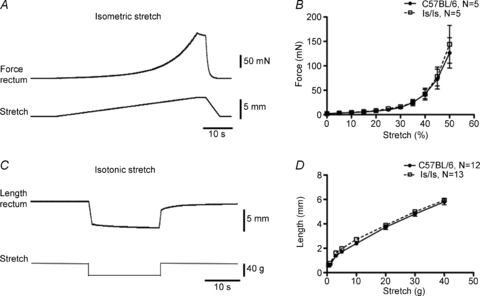
A, typical example of force generated during ramp distension by imposed changes in length (100 μm s−1, 4 mm) with averaged traces shown in B. C, typical example of lengthening of control colorectum evoked by a 40 g load (distension by imposed load) with averaged data in D not showing any difference between control and ls/ls mice. Changes in compliance are unlikely to explain different stimulus–response functions of mechanoreceptors between control and mutant mice.
Discussion
In a number of different mammals, mutation of the ET-3 gene leads to colorectal aganglionosis, or Hirschsprung's disease in humans (Amiel et al. 2008; Tam & Garcia-Barcelo, 2009). The current study has identified that in addition to colorectal aganglionosis, mice deficient in ET-3 also have a selective deficit in nociception from the aganglionic colorectum, which has not been previously described. The loss of nociception from this region of the gastrointestinal tract was not due to an absence of sensory nerve endings, nor to changes in wall compliance. Rather, it was associated with a reduction in the density of low threshold, wide dynamic range rectal afferents and significant functional changes in their mechanosensory properties.
Sensory pathway involved in detection of noxious rectal distension
One striking finding of the current study was the absence of behavioural visceromotor responses to rectal distension in ls/ls mice. Only at the highest pressures tested, often close to the point where the gut ruptured, was any electromyographic activity recorded from the abdominal muscles in ls/ls mice. In contrast, robust VMRs could be evoked in wild-type mice with stimuli over 20 mmHg. This is unlikely to reflect a generalized deficit in central sensory or motor pathways, since responses to both bladder distension (a visceral stimulus) and both tail and hindlimb compression (a somatic stimulus) were normal in ls/ls mice. Thus, the deficit is likely to lie specifically in the sensory pathways to the distal bowel which shows the characteristic aganglionosis in ls/ls mice.
Retrograde tracing studies demonstrated that the total number of spinal afferent neurons projecting to the aganglionic region of colorectum (where distension stimuli were applied to evoke visceromotor responses) was reduced by about 60–80%. This almost certainly contributes to the reduction of visceromotor responses but is unlikely to explain its abolition. In the present study, electrical stimulation applied to the surface of the aganglionic colorectum still evoked visceromotor responses in ls/ls mice, although these were significantly smaller than those in wild-type mice. These data suggest that afferent fibres capable of evoking VMRs were still present in the aganglionic rectum in ls/ls mice, albeit in decreased numbers, but their mechanosensitivity was significantly suppressed.
The identity of the nociceptive neurons from the distal bowel has been a subject of speculation (Brierley et al. 2009; Hughes et al. 2009a; Song et al. 2009; Feng et al. 2010; Blackshaw et al. 2010). Visceral afferents retrogradely labelled in this study from the colorectum (1–4 mm from the anal sphincter) were primarily located in lumbosacral region of the spinal cord, predominantly L5 and L6. These findings differ somewhat from previous reports which showed that spinal afferents innervating the descending colon were largely located in thoracolumbar segments (Robinson et al. 2004; Brierley et al. 2005; Tan et al. 2008). In these previous studies, dye was injected into the descending colon, accessed via laparotomy, rostral to the pelvic brim, which must have been at least 15–20 mm from the anus. In a study by Christianson et al. (2006) injections of cholera toxin B were also made into the descending colon and DRG neurons were labelled primarily in the lumbosacral region, which is more consistent with our findings. In our retrograde tracing studies, DiI was injected 1–4 mm from the anus, without laparotomy, and as far as we are aware, no studies have previously injected neuronal tracers into this terminal region of rectum. In a recent study, we identified that lesions applied to the rectal nerves, but not lumbar colonic or hypogastric nerves, abolished the visceromotor responses following noxious rectal distension applied to the terminal 15 mm of colorectum (Brookes & Spencer, 2008). Taken together, our data strongly suggest that sacral afferents, travelling via pelvic and rectal nerves to the distal bowel, are the primary pathway involved in detecting noxious distension of the colorectum.
Functional classes of afferents involved in detection of noxious distension
The question then arises as to which class(es) of sacral afferents mediated mechano-nociception from the terminal region of bowel. Two hypotheses can account for pain perception from the viscera. The ‘intensity coding hypothesis’ suggests that low threshold wide dynamic range afferents, with thresholds in the innocuous range, are primarily responsible for activation of central pain circuits, as they have an ability to encode into the noxious range. On the other hand, the ‘specificity hypothesis’ suggests that high threshold afferents mediate pain perception, as they respond only to noxious intensities of stimulation (Cervero & Janig, 1992; Fioramonti & Gebhart, 2007). Splanchnic (thoracolumbar) and pelvic (sacral) sensory pathways to the colon contain different complements of sensory neurons. In mouse, low threshold mechanoreceptors account for approximately 67% of all sacral afferents, but only 14% of splanchnic afferents (Brierley et al. 2004). The remaining 33% of sacral mechanoreceptors are high threshold serosal receptors. The present study allows us to address which classes of sacral afferents are responsible for transmitting nociceptive information from the distal bowel.
The sensory pathways encoding noxious distension from the rectum should meet three criteria. As pointed out above, they most likely project in the sacral rectal/pelvic nerve pathway. This rules out a major contribution by ‘mesenteric’ afferents (sensory nerves with endings on mesenteric blood vessels; Song et al. 2009) since these are not present in the sacral pathway (Brierley et al. 2004). Secondly, the afferents responsible for nociception are likely to show diminished responses to distension and may also be reduced in number in ls/ls mice. Third, the afferents in question should encode distension in a graded fashion across the noxious range and have a threshold for activation comparable to the visceromotor responses.
Four classes of pelvic/sacral afferents have been identified in the mouse colorectum: muscular, muscular-mucosal, serosal and mucosal afferents (Brierley et al. 2004). Mucosal afferents are not sensitive to distension and hence cannot encode noxious distension. Of the other three classes, muscular and muscular-mucosal afferents responded to distension with low thresholds and wide dynamic ranges which did not saturate across the entire range of stretch up to the point where the gut tissue tore. Serosal afferents had significantly higher thresholds. Visceromotor responses to rectal distension were consistently activated at distension pressures in the range of 20–40 mmHg in the present study on anaesthetized mice, or 10–20 mmHg in conscious mice (Sipe et al. 2008). In our study, serosal mechanoreceptors had thresholds of 10–20 g circumferential load, which corresponded (using the Young–Laplace law) to intraluminal pressures of 50–100 mmHg. In contrast, low threshold wide dynamic range fibres had thresholds below 3 g (∼15 mmHg), favouring the latter as carrying the signals for visceromotor responses. In addition, low threshold, wide dynamic range fibres were reduced in number in rectal nerve trunks in ls/ls mice, consistent with the sensory deficit in these mutants. The significant loss in low threshold mechanosensory rectal afferents from the aganglionic colorectum is consistent with a population of low threshold rectal mechanoreceptors normally located in myenteric ganglia (Spencer et al. 2008a). In contrast the number of serosal mechanoreceptors per nerve trunk was not significantly affected by the lethal spotting mutation. In addition, stretch-dependent firing of low threshold wide-dynamic range afferents was significantly reduced in ls/ls mice compared to controls, whereas stretch dependent firing of serosal mechanoreceptors was unaffected by the mutation. Thus all these data strongly suggest that low threshold stretch-sensitive fibres make a major contribution to VMRs evoked by colorectal distension.
Our observation that VMRs could be elicited by colorectal distension with stimuli as low as 10–20 mmHg is noteworthy, as these levels of distension are generally not thought to be noxious. Despite the extensive literature of using VMRs as a surrogate marker for visceral pain, our finding suggests that VMRs elicted by rectal distension may include a significant non-noxious component. Since we found that VMRs were absent at low and high levels of rectal distension in ls/ls mice, this observation is consistent with a view that low threshold wide dynamic range rectal stretch-sensitive afferents encode stimuli from the innocuous to noxious range, likely mediating both physiological reflexes (such as defaecatory motor reflexes) and pain responses (such as VMRs) (Cervero 1994; Yamanouchi et al. 2002; Lynn et al. 2005; Fioramonti & Gebhart, 2007).
In this study, all afferent recordings in vitro were made in the presence of nicardipine. This was because spinal afferents in the rectum potently respond to contraction of the gut wall (Lynn et al. 2005; Spencer et al. 2008b), which can confound the firing evoked by rectal distension and probing with a von Frey hair, since both stimuli can induce rectal contraction. To prevent this, nicardipine was used for in vitro afferent recordings. Indeed, it is known that blockade of L-type Ca2+ channels substantially reduces the release of 5-hydroxytryptamine from enterochromaffin (EC) cells (Kojima et al. 2000). Therefore, it is possible that this alters the sensitivity of muscular-mucosal afferents to mucosal stimulation. The observation that rectal afferents potently responded to rectal distension and mucosal stroking in the presence of nicardipine shows that release of 5-HT from EC cells is not required for activation of rectal afferents by distension or mucosal stimulation.
Role of high threshold mechanoreceptors
Serosal receptors are likely to play a much smaller role in distension-evoked visceromotor responses from the distal bowel. Even at maximum stretch (20–40 g, which corresponds to 100–200 mmHg using the Young–Laplace law) serosal units contributed only 5% of total stretch-induced firing from colorectum. It should be noted, however, that the current study was carried out in naive, non-inflamed bowel. Inflammation reduces the threshold and increases the amplitude of visceromotor responses to bowel distension (Shinoda et al. 2009). Correspondingly it also increases the sensitivity of spinal mechanoreceptors, in particular, serosal and mesenteric afferents (Hughes et al. 2009a,b;) with a modest sensitization of mucosal afferents. It can be speculated that low threshold wide dynamic range mechanoreceptors mediate most of the sensory traffic evoked by noxious distension in naive gut, but high threshold serosal afferents are sensitized by inflammation and play a much larger role in the inflamed or post-inflammatory bowel.
Extrinsic innervation of the aganglionic rectum
In human colon, there are conflicting results with regards to the extrinsic innervation of the aganglionic rectum. Some suggest an absence of cholinergic fibres (Sun et al. 1987), or an increase in the number of cholinergic nerve fibres (Watanabe et al. 1998). In our results on mouse rectum, there was clearly a functional reduction in sensory innervation of the aganglionic rectum, which was consistent with the significant reduction in CGRP positive afferent fibres in the aganglionic rectum. Consistent with our results in mouse, in human patients with Hirschsprung's disease, a reduction in the number of CGRP positive fibres has also been reported in the aganglionic rectum (Larsson, 1994; Watanabe et al. 1999).
Endothelins as potential trophic factors for development and survival of low threshold stretch-sensitive afferents
Lethal spotted (ls/ls) mice fail to produce endothelin-3 (Baynash et al. 1994). In contrast, piebald lethal mice (sl/sl) lack a major receptor for endothelin 3 (the endothelin receptor B, encoded by the ednrb gene) and show a comparable aganglionosis (Hosoda et al. 1994). Our present findings on ls/ls mice and previous on sl/sl mice (Spencer, 2005) showing an abolition of visceromotor responses to colorectal distension suggest that the lack of this signalling pathway causes a deficit in sensory innervation of the distal bowel. Endogenous endothelin signalling plays an important role in nociceptive pathways in both visceral and somatic organs (Baamonde et al. 2004; Khodorova et al. 2009; Stösser et al. 2010). Direct injections of endothelins into mammals leads to pain sensations (Katugampola et al. 2000; Hans et al. 2007). Furthermore, selective endothelin antagonists reduce pain sensations induced by inflammation, such as 2,4,6-trinitrobenzene sulphonic acid (TNBS)-induced colitis (Claudino et al. 2010). Exactly how disruption of endothelin-3 signalling causes changes in afferent density and mechanosensitivity remains unclear. Recent studies have shown that family members of glial cell line-derived neurotrophic factor (GDNF), which are known to be essential for migration of the enteric neural crest during development of the enteric nervous system (Heanue & Pachnis, 2007), also play a major role in visceral hypersensitivity and sensitization of muscular and muscular-mucosal colorectal afferents (Tanaka et al. 2011). It is exciting to speculate that endothelin-3 may play a trophic role in the development and/or survival of low threshold stretch-sensitive afferents involved in rectal nociception. It is known that endothelin-3 signalling mutations produce about 5% of cases of Hirschsprung's disease, while Ret mutations account for about 40–45% of cases (Heanue & Pachnis, 2007). As both pathways have similar consequences for enteric ganglia, the possibility of GDNF signalling pathway mutations affecting sensory nerve function is particularly interesting.
Conclusion
In summary, the findings of the current study show that in mutant ls/ls mice, which lack endogenous endothelin-3, there is a selective loss of nociception from the aganglionic colorectum, following noxious levels of rectal distension. This was not associated with a loss of sensory nerves from the rectum, but rather with a reduction in sensory innervation and impairment in mechanosensation primarily of low threshold wide dynamic range rectal muscular and muscular-mucosal afferents.
Acknowledgments
We wish to acknowledge the financial support provided by the National Health and Medical Research Council (NH&MRC) of Australia with grant nos 535033 and 535034.
Glossary
Abbreviations
- CGRP
calcitonin gene related peptide
- DRG
dorsal root ganglion
- ET-3
endothelin-3
- VMR
visceromotor response
Author contributions
Experiments were designed by N.J.S. and V.P.Z. and were performed by V.P.Z., N.J.S., M.K. and S.N. at Flinders University of South Australia. B.C. and H.P. contributed to immunohistochemical experiments. The paper was written by N.J.S. and V.P.Z. S.J.B. contributed to design and interpretation of data as well as to revising the article. All authors approved the final version.
References
- Amiel J, Sproat-Emison E, Garcia-Barcelo M, Lantieri F, Burzynski G, Borrego S, Pelet A, Arnold S, Miao X, Griseri P, Brooks AS, Antinolo G, de Pontual L, Clement-Ziza M, Munnich A, Kashuk C, West K, Wong KK, Lyonnet S, Chakravarti A, Tam PK, Ceccherini I, Hofstra RM, Fernandez RC. Hirschsprung disease, associated syndromes and genetics: a review. J Med Genet. 2008;45:1–14. doi: 10.1136/jmg.2007.053959. [DOI] [PubMed] [Google Scholar]
- Baamonde A, Lastra A, Villazon M, Bordallo J, Hidalgo A, Menendez L. Involvement of endogenous endothelins in thermal and mechanical inflammatory hyperalgesia in mice. Naunyn-Schmiedebergs Arch Pharmacol. 2004;369:245–251. doi: 10.1007/s00210-003-0841-1. [DOI] [PubMed] [Google Scholar]
- Baynash AG, Hosoda K, Giaid A, Richardson JA, Emoto N, Hammer RE, Yanagisawa M. Interaction of endothelin-3 with endothelin-B receptor is essential for development of epidermal melanocytes and enteric neurons. Cell. 1994;79:1277–1285. doi: 10.1016/0092-8674(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Blackshaw LA, Brierley SM, Hughes PA. TRP channels: new targets for visceral pain. Recent advances in basic science. Gut. 2010;59:126–135. doi: 10.1136/gut.2009.179523. [DOI] [PubMed] [Google Scholar]
- Brierley SM, Hughes PA, Page AJ, Kwan KY, Martin CM, O'Donnell TA, Cooper NJ, Harrington AM, Adam B, Liebregts T, Holtmann G, Corey DP, Rychkov GY, Blackshaw LA. The ion channel TRPA1 is required for normal mechanosensation and is modulated by algesic stimuli. Gastroenterology. 2009;137:2084–2095. doi: 10.1053/j.gastro.2009.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brierley SM, Jones RC, 3rd, Gebhart GF, Blackshaw LA. Splanchnic and pelvic mechanosensory afferents signal different qualities of colonic stimuli in mice. Gastroenterology. 2004;127:166–178. doi: 10.1053/j.gastro.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Brierley SM, Carter R, Jones W, 3rd, Xu L, Robinson DR, Hicks GA, Gebhart GF, Blackshaw LA. Differential chemosensory function and expression of splanchnic and pelvic colonic afferents in mice. J Physiol. 2005;567:267–281. doi: 10.1113/jphysiol.2005.089714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes SJ, Chen BN, Costa M, Humphreys CM. Initiation of peristalsis by circumferential stretch of flat sheets of guinea-pig ileum. J Physiol. 1999;516:525–538. doi: 10.1111/j.1469-7793.1999.0525v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes SJ, Spencer NJ. Identification of the pain pathway activated by rectal distension in mice. Proc Aust Neurosci Soc. 2008;18:130. [Google Scholar]
- Cervero F, Janig W. Visceral nocieptors: a new world order ? Trends Neurosci. 1992;15:374–378. doi: 10.1016/0166-2236(92)90182-8. [DOI] [PubMed] [Google Scholar]
- Cervero F. Sensory innervation of the viscera: peripheral basis of visceral pain. Physiol Rev. 1994;74:95–138. doi: 10.1152/physrev.1994.74.1.95. [DOI] [PubMed] [Google Scholar]
- Christianson JA, Traub RJ, Davis BM. Differences in spinal distribution and neurochemical phenotype of colonic afferents in mouse and rat. J Comp Neurol. 2006;494:246–259. doi: 10.1002/cne.20816. [DOI] [PubMed] [Google Scholar]
- Claudino RF, Marcon R, Bento AF, Chichorro JG, Rae GA. Endothelins implicated in referred mechanical hyperalgesia associated with colitis induced by TNBS in mice. Can J Physiol Pharmacol. 2010;88:661–667. doi: 10.1139/Y10-043. [DOI] [PubMed] [Google Scholar]
- Feng B, Brumovsky PR, Gebhart GF. Differential roles of stretch-sensitive pelvic nerve afferents innervating mouse distal colon and rectum. Am J Physiol Gastrointest Liver Physiol. 2010;298:G402–409. doi: 10.1152/ajpgi.00487.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishbein RH, Handelsman JC, Schuster MM. Surgical treatment of Hirschsprung's disease in adults. Surg Gynecol Obstet. 1986;163:458–464. [PubMed] [Google Scholar]
- Fioramonti J, Gebhart GF. In vivo and transgenic animal models used to study visceral hypersensitivity. Neurogastroenterol Motil. 2007;19:20–28. doi: 10.1111/j.1365-2982.2006.00872.x. [DOI] [PubMed] [Google Scholar]
- Furness JB. The Enteric Nervous System. Oxford, UK: Blackwell Publishing; 2006. [Google Scholar]
- Hans G, Deseure K, Robert D, De Hert S. Neurosensory changes in a human model of endothelin-1 induced pain: a behavioral study. Neurosci Lett. 2007;418:117–121. doi: 10.1016/j.neulet.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Hamamoto DT, Khasabova SG, Cain DM, Simone DA. Tumor-evoked sensitization of C nociceptors: a role for endothelin. J Neurophysiol. 2008;100:2300–2311. doi: 10.1152/jn.01337.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heanue TA, Pachnis V. Enteric nervous system development and Hirschsprung's disease: advances in genetic and stem cell studies. Nat Rev Neurosci. 2007;8:466–479. doi: 10.1038/nrn2137. [DOI] [PubMed] [Google Scholar]
- Hosoda K, Hammer RE, Richardson JA, Baynash AG, Cheung JC, Giaid A, Yanagisawa M. Targeted and natural (piebald-lethal) mutations of endothelin-B receptor gene produce megacolon associated with spotted coat color in mice. Cell. 1994;79:1267–1276. doi: 10.1016/0092-8674(94)90017-5. [DOI] [PubMed] [Google Scholar]
- Hughes PA, Brierley SM, Blackshaw LA. Post-inflammatory modification of colonic afferent mechanosensitivity. Clin Exp Pharmacol Physiol. 2009a;36:1034–1040. doi: 10.1111/j.1440-1681.2009.05248.x. [DOI] [PubMed] [Google Scholar]
- Hughes PA, Brierley SM, Martin CM, Brookes SJ, Linden DR, Blackshaw LA. Post-inflammatory colonic afferent sensitisation: different subtypes, different pathways and different time courses. Gut. 2009b;58:1333–1341. doi: 10.1136/gut.2008.170811. [DOI] [PubMed] [Google Scholar]
- Katugampola R, Church MK, Clough GF. The neurogenic vasodilator response to endothelin-1: a study in human skin in vivo. Exp Physiol. 2000;85:839–846. [PubMed] [Google Scholar]
- Khodorova A, Montmayeur JP, Strichartz G. Endothelin receptors and pain. J Pain. 2009;10:4–28. doi: 10.1016/j.jpain.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima S, Ikeda M, Kamikawa Y. Investigations in the 5-hydroxytryptophan-evoked luminal 5-hydroxytryptamine release from the guinea-pig colon. Jpn J Pharmacol. 2000;84:174–178. doi: 10.1254/jjp.84.174. [DOI] [PubMed] [Google Scholar]
- Larsson LT. Hirschsprung's disease – immunohistochemical findings. Histol Histopathol. 1994;9:615–629. [PubMed] [Google Scholar]
- Liang J, Bi H, Ji W. Involvement of TRPA1 in ET-1-induced pain-like behavior in mice. Neuroreport. 2010;21:201–205. doi: 10.1097/WNR.0b013e328335b3c5. [DOI] [PubMed] [Google Scholar]
- Lynn P, Zagorodnyuk VP, Hennig G, Costa M, Brookes SJ. Mechanical activation of rectal intraganglionic laminar endings in the guinea-pig distal gut. J Physiol. 2005;564:589–601. doi: 10.1113/jphysiol.2004.080879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundt E, Bates MD. Genetics of Hirschsprung disease and anorectal malformations. Semin Pediatr Surg. 2010;19:107–117. doi: 10.1053/j.sempedsurg.2009.11.015. [DOI] [PubMed] [Google Scholar]
- Newgreen D, Young HM. Enteric nervous system: development and developmental disturbances – part 1. Pediatr Dev Pathol. 2002;5:224–247. doi: 10.1007/s10024-001-0142-y. [DOI] [PubMed] [Google Scholar]
- Robinson DR, McNaughton PA, Evans ML, Hicks GA. Characterization of the primary spinal afferent innervation of the mouse colon using retrograde labelling. Neurogastroenterol Motil. 2004;16:113–124. doi: 10.1046/j.1365-2982.2003.00456.x. [DOI] [PubMed] [Google Scholar]
- Shafton AD, Furness JB, Ferens D, Bogeski G, Koh SL, Lean NP, Kitchener PD. The visceromotor responses to colorectal distension and skin pinch are inhibited by simultaneous jejunal distension. Pain. 2006;123:127–136. doi: 10.1016/j.pain.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Shinoda M, Feng B, Gebhart GF. Peripheral and central P2X3 receptor contributions to colon mechanosensitivity and hypersensitivity in the mouse. Gastroenterology. 2009;137:2096–2104. doi: 10.1053/j.gastro.2009.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipe WE, Brierley SM, Martin CM, Phillis BD, Cruz FB, Grady EF, Liedtke W, Cohen DM, Vanner S, Blackshaw LA, Bunnett NW. Transient receptor potential vanilloid 4 mediates protease activated receptor 2-induced sensitization of colonic afferent nerves and visceral hyperalgesia. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1288–1298. doi: 10.1152/ajpgi.00002.2008. [DOI] [PubMed] [Google Scholar]
- Song X, Chen BN, Zagorodnyuk VP, Lynn PA, Blackshaw LA, Grundy D, Brunsden AM, Costa M, Brookes SJ. Identification of medium/high-threshold extrinsic mechanosensitive afferent nerves to the gastrointestinal tract. Gastroenterology. 2009;137:274–284. doi: 10.1053/j.gastro.2009.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer NJ. Impaired extrinsic afferent reflex responses in aganglionic piebald-lethal mouse colon in vivo. Neurogastroenterol Motil. 2005;17:71. [Google Scholar]
- Spencer NJ, Kerrin A, Singer CA, Hennig GW, Gerthoffer WT, McDonnell O. Identification of capsaicin-sensitive rectal mechanoreceptors activated by rectal distension in mice. Neuroscience. 2008a;153:518–534. doi: 10.1016/j.neuroscience.2008.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer NJ, Kerrin A, Zagorodnyuk VP, Hennig GW, Muto M, Brookes SJ, McDonnell O. Identification of functional intramuscular rectal mechanoreceptors in aganglionic rectal smooth muscle from piebald lethal mice. Am J Physiol Gastrointest Liver Physiol. 2008b;294:G855–867. doi: 10.1152/ajpgi.00502.2007. [DOI] [PubMed] [Google Scholar]
- Stösser S, Agarwal N, Tappe-Theodor A, Yanagisawa M, Kuner R. Dissecting the functional significance of endothelin A receptors in peripheral nociceptors in vivo via conditional gene deletion. Pain. 2010;148:206–214. doi: 10.1016/j.pain.2009.09.024. [DOI] [PubMed] [Google Scholar]
- Sun CC, Caniano DA, Hill JL. Intestinal aganglionosis: a histologic and acetylcholinesterase histochemical study. Pediatr Pathol. 1987;7:421–435. doi: 10.3109/15513818709161404. [DOI] [PubMed] [Google Scholar]
- Tan LL, Bornstein JC, Anderson CR. Distinct chemical classes of medium-sized transient receptor potential channel vaniloid 1-immunoreactive dorsal root ganglia neurons innervate the adult mouse jejunum and colon. Neuroscience. 2008;156:334–343. doi: 10.1016/j.neuroscience.2008.06.071. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Shinoda M, Feng B, Albers KM, Gebhart GF. Modulation of visceral hypersensitivity by glial cell line-derived neurotrophic factor (GDNF) family receptor alpha-3 in colorectal afferents. Am J Physiol Gastrointest Liver Physiol. 2011 doi: 10.1152/ajpgi.00456.2010. (in press, doi: 10.1152/ajpgi.00456.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam PK, Garcia-Barcelo M. Genetic basis of Hirschsprung's disease. Pediatric Surgery Intern. 2009;25:543–558. doi: 10.1007/s00383-009-2402-2. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Ito T, Ando H, Seo T, Kaneko K, Harada T, Lino S. Morphological investigation of the enteric nervous system in Hirschsprung's disease and hypoganglionosis using whole-mount colon preparation. J Pediatr Surg. 1999;34:445–449. doi: 10.1016/s0022-3468(99)90496-7. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Ito T, Harada T. Extrinsic nerve strands in the aganglionic segment of Hirschsprung's disease. J Pediatr Surg. 1998;8:1233–1237. doi: 10.1016/s0022-3468(98)90157-9. [DOI] [PubMed] [Google Scholar]
- Yamanouchi M, Shimatani H, Kadowaki M, Yoneda S, Nakagawa T, Fujii H, Takaki M. Integrative control of rectoanal reflex in guinea-pigs through lumbar colonic nerves. Am J Physiol Gastrointest Liver Physiol. 2002;283:G148–156. doi: 10.1152/ajpgi.00497.2001. [DOI] [PubMed] [Google Scholar]
- Young HM, Jones BR, McKeown SJ. The projections of early enteric neurons are influenced by the direction of neural crest cell migration. J Neurosci. 2002;22:6005–6018. doi: 10.1523/JNEUROSCI.22-14-06005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagorodnyuk VP, Brookes SJ. Transduction sites of vagal mechanoreceptors in the guinea pig esophagus. J Neurosci. 2000;20:6249–6255. doi: 10.1523/JNEUROSCI.20-16-06249.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagorodnyuk VP, Lynn P, Costa M, Brookes SJ. Mechanisms of mechanotransduction by specialized low-threshold mechanoreceptors in the guinea pig rectum. Am J Physiol Gastrointest Liver Physiol. 2005;289:G397–406. doi: 10.1152/ajpgi.00557.2004. [DOI] [PubMed] [Google Scholar]


