Non-technical summary
When under stress, the heart beat becomes stronger, in part due to enhanced fluxes of Ca2+ at the level of the cardiac cell. It is known that this effect is mediated by activation of β-receptors on the cardiac cell surface. This leads to modifications of intracellular proteins that in turn increase the flux of Ca2+ within the cell. In this study we show that activation of β-receptors increases the production of reactive oxygen species (ROS) in the heart cell. These ROS generate enhanced Ca2+ fluxes and more vigorous contraction. This finding shows a new cellular signalling route for regulating the power of the heart beat and might contribute to our understanding of diseases with defective cardiac contraction, such as heart failure.
Abstract
Abstract
The sympathetic adrenergic system plays a central role in stress signalling and stress is often associated with increased production of reactive oxygen species (ROS). Furthermore, the sympathetic adrenergic system is intimately involved in the regulation of cardiomyocyte Ca2+ handling and contractility. In this study we hypothesize that endogenously produced ROS contribute to the inotropic mechanism of β-adrenergic stimulation in mouse cardiomyocytes. Cytoplasmic Ca2+ transients, cell shortening and ROS production were measured in freshly isolated cardiomyocytes using confocal microscopy and fluorescent indicators. As a marker of oxidative stress, malondialdehyde (MDA) modification of proteins was detected with Western blotting. Isoproterenol (ISO), a β-adrenergic agonist, increased mitochondrial ROS production in cardiomyocytes in a concentration- and cAMP–protein kinase A-dependent but Ca2+-independent manner. Hearts perfused with ISO showed a twofold increase in MDA protein adducts relative to control. ISO increased Ca2+ transient amplitude, contraction and L-type Ca2+ current densities (measured with whole-cell patch-clamp) in cardiomyocytes and these increases were diminished by application of the general antioxidant N-acetylcysteine (NAC) or the mitochondria-targeted antioxidant SS31. In conclusion, increased mitochondrial ROS production plays an integral role in the acute inotropic response of cardiomyocytes to β-adrenergic stimulation. On the other hand, chronically sustained adrenergic stress is associated with the development of heart failure and cardiac arrhythmias and prolonged increases in ROS may contribute to these defects.
Introduction
The sympathetic adrenergic system plays a central role in our ability to rapidly respond to various types of threats. One important target of adrenergic stimulation is the heart, where activation of β-adrenergic receptors causes increases in heart rate (chronotropy), relaxation speed (lusitropy) and contractility (inotropy). β-Adrenergic-induced increases in relaxation speed and contractility are considered to be a consequence of cAMP-dependent protein kinase A (PKA)-mediated phosphorylation of proteins involved in cardiomyocyte Ca2+ handling (Bers, 2002). Two important Ca2+ handling proteins that are targeted by this phosphorylation are phospholamban and the sarcoplasmic reticulum (SR) Ca2+ release channel (the ryanodine receptor, RyR). Phosphorylation of phospholamban leads to increased activity of the SR Ca2+-ATPase (SERCA), which accelerates SR Ca2+ uptake and hence increases SR Ca2+ loading (MacLennan & Kranias, 2003). SR Ca2+ release can be enhanced by RyR phosphorylation that increases the Ca2+ channel open probability (Zalk et al. 2007). Moreover, Ca2+ entry into the cardiac cell is increased by PKA-mediated effects on the L-type Ca2+ channel and increased Ca2+ entry through this channel will further amplify the release of SR Ca2+ via the Ca2+-induced Ca2+ release mechanism (Bassani et al. 1995). The Cav1.2-subunit of the L-type Ca2+ channel can be phosphorylated on Ser1928 and this is widely considered to be mediated by PKA (Davare & Hell, 2003). However, it is uncertain whether this phosphorylation is the direct cause of enhanced L-type Ca2+ current (ICaL) following β-adrenergic stimulation because the response is still present in knock-in mice with targeted mutation of Ser1928 to alanine, which abolishes the PKA-induced phosphorylation (Lemke et al. 2008). Furthermore, a recent study identified Ser1700 as the primary regulatory site for the response to β-adrenergic stimulation (Fuller et al. 2010).
The faster and larger Ca2+ transients following β-adrenergic stimulation result in enhanced myofilament contractions. Adrenergic signalling in the heart is thus of critical importance for acute increases of cardiac output. However, chronically sustained adrenergic stress is associated with the development of heart failure and cardiac arrhythmias (Clark & Cleland, 2000; Marx et al. 2000; Curran et al. 2007).
Various pathological states have been coupled to increased cellular production of reactive oxygen species (ROS), which include: superoxide (O2·−), hydrogen peroxide (H2O2) and hydroxyl radical (OH·) (Houstis et al. 2006; Moylan & Reid, 2007). However, ROS are not only involved in pathological processes, but also participate in normal physiological signalling (Droge, 2002; Linnane & Eastwood, 2006). For instance, Ca2+ handling in cardiac cells has been shown to be altered by changes in ROS, where ROS activates the RyR and inhibits SR Ca2+ uptake (Zima & Blatter, 2006). Moreover, the cardiac ICaL has been shown to be redox sensitive (Sims & Harvey, 2004; Hool, 2008). In this context, we recently showed that the saturated fatty acid palmitate disturbs Ca2+ handling in healthy cardiomyocytes via a mechanism involving markedly increased ROS production (Fauconnier et al. 2007).
In the present study, we measured mitochondrial ROS production and cellular Ca2+ handling in isolated mouse cardiomyocytes. We tested the following hypotheses: (i) β-adrenergic stimulation increases mitochondrial ROS production in cardiomyocytes; (ii) this endogenous ROS production alters cellular Ca2+ handling such that it effectuates cardiac inotropy; (iii) the effect involves altered ICaL. Our results are in accordance with the hypotheses. Thus, application of the β-adrenergic agonist isoproterenol (ISO) increased mitochondrial ROS production in a cAMP–PKA-dependent manner and the stimulatory effect of ISO on cytoplasmic Ca2+ transients, cell shortening and ICaL was blunted by pre-exposure to antioxidants.
Methods
Ethical approval
All experiments complied with the Swedish Animal Welfare Act, the Swedish Animal Welfare ordinance, and applicable regulations and recommendations from Swedish authorities. The study was approved by the Stockholm North Ethical Committee on Animal Experiments. The experiments comply with the policies and regulations of The Journal of Physiology (Drummond, 2009).
Cell isolation and stimulation
We used ∼4-month-old C57BL6 mice; in total 41 were used. The mice were killed by rapid neck disarticulation and the heart was excised. Single cardiac cells were isolated from the ventricles following the protocols developed by the Alliance for Cellular Signalling (AfCS Procedure Protocol ID PP00000 125) (Sambrano et al. 2002). After being loaded with fluorescent indicators, cardiomyocytes were attached to the bottom of a stimulation chamber, superfused with standard Tyrode solution at room temperature (∼24°C) and stimulated at 1 Hz with 1 ms current pulses (Fauconnier et al. 2007). The signals of fluorescent indicators were measured with confocal microscopy using a Bio-Rad MRC 1024 unit attached to a Nikon Diaphot inverted microscope. Measurements were performed on the largest possible in-focus areas of individual cells.
Mitochondrial ROS production
Changes in mitochondrial O2·− production were monitored using MitoSOX Red (Invitrogen/Molecular Probes) and confocal microscopy (Fauconnier et al. 2007). Isolated cardiomyocytes were loaded with MitoSOX Red (5 μm) for 30 min at room temperature, followed by washout. MitoSOX Red was excited with light at 488 nm while measuring the emitted light collected through a 585 nm long-pass filter. Confocal images were obtained at the start, after 10 min of 1 Hz stimulation and after 10 min at 1 Hz stimulation in the presence of the following drugs: the β-adrenergic agonist isoproterenol (ISO, 1–100 nm; Sigma); the adenylyl cyclase activator forskolin (1 μm; Sigma), or the L-type Ca2+ channel agonist (–)-Bay K 8644 (1 μm; Sigma). Before application of ISO (100 nm), some cells were exposed to the PKA inhibitor H89 (5 μm; Calbiochem) or the cell-permeant myristoylated PKA inhibitor 14–22 amide (PKI, 5 μm; Calbiochem) (Xiao et al. 2006). In some experiments with ISO exposure, a cell-permeant form of the Ca2+ buffer BAPTA (BAPTA AM, 50 μm; Invitrogen/Molecular Probes) was added together with the fluorescent indicator. The signal from each cell was normalized to that immediately before application of a drug.
Changes in ROS induced by application of a high concentration of H2O2 (1 mm) were monitored with MitoSOX Red as described above but confocal images were obtained at 2 min intervals. Cardiomyocytes were stimulated at 1 Hz for 20 min before H2O2 application with the last 10 min in the absence or presence of the mitochondrial respiratory complex I inhibitor rotenone (2.4 μm; Sigma).
The fluorescence of endogenous flavin adenine dinucleotide (FAD)-linked proteins can alter with changes in mitochondrial energy metabolism and redox state in cardiomyocytes (Zima et al. 2003). The FAD-linked autofluorescence overlaps in wavelengths with the MitoSOX Red fluorescence and changes in autofluorescence might then interfere with the MitoSOX Red measurements. We therefore performed experiments with application of 100 nm ISO (same protocol as above) using cardiomyocytes not loaded with any fluorescent dye.
Mitochondrial membrane potential
Tetramethylrhodamine, ethyl ester, (TMRE; Invitrogen/Molecular Probes) was used to measure mitochondrial membrane potential (ΔΨm) (Duchen et al. 1998). Isolated cardiomyocytes were loaded with TMRE (10 nm) for 15 min at room temperature, followed by washout. Confocal images of TMRE fluorescence were obtained by excitation at 488 nm while measuring the emitted light at ≥585 nm. Images were taken every 5 min and fluorescence signals were normalized to the fluorescence measured in each cell at the start of the experiment, which was set to 100%. At the end of each experiment, cells were depolarized by exposure to the mitochondrial uncoupler carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone (FCCP; 10 μm).
Mitochondrial Ca2+ concentration
Rhod-2 (Invitrogen/Molecular Probes) was used to assess changes in the mitochondrial Ca2+ concentration. Isolated cells were loaded with rhod-2 (10 μm) for 30 min at 4°C, followed by 60 min washout at room temperature. Confocal images were obtained with excitation at 531 nm while measuring the emitted light at ≥585 nm.
Western blotting
Whole hearts were excised and immediately perfused in a Langendorff set-up with Tyrode solution in the presence or absence of ISO or H2O2. Hearts were then snap-frozen and analysed for malondialdehyde (MDA) protein adducts using Western blot and rabbit anti-MDA (Academy Bio-Medical, no. MD20A-R1a) and donkey anti-rabbit horseradish peroxidase (HRP) secondary antibody (Bio-Rad, no. 170-6515) as described previously (Aydin et al. 2009).
Cytosolic Ca2+ and cell shortening
The cytosolic Ca2+ concentration was measured with the fluorescent Ca2+ indicator fluo-3 and confocal microscopy (Fauconnier et al. 2007). Confocal images were obtained by line scanning along the long axis, with focus in the middle of the cell. Stored confocal images were analysed with ImageJ (National Institutes of Health; available at http://rsb.info.nih.gov/ij). To enable comparisons between cells, the amplitude of Ca2+ transients was assessed as the change in the fluo-3 fluorescence signal (ΔF) divided by the fluorescence immediately before a stimulation pulse given under control conditions (F0). The decay rate of Ca2+ transients was assessed by measuring the time constant of the exponential part of the decay phase, ignoring the initial phase that often diverges from a monoexponential function. In some experiments the edges of the fluorescence images were used to measure cell length in the rested and the maximally contracted state and shortening is expressed as a percentage of the resting cell length.
The relationship between the free cytosolic [Ca2+] ([Ca2+]i) and fluorescence intensity becomes steeper at high [Ca2+]i as fluo-3 approaches saturation. To assess the importance of this non-linearity of the fluo-3 signal, we used a pseudo-ratio to calculate [Ca2+]i (Santiago et al. 2010):
where KD is the dissociation constant of fluo-3, which was set to 1.1 μm, and [Ca2+]dia is the diastolic [Ca2+]i, which was set to 100 nm.
In some experiments the effect of Ca2+ transient facilitating agents (ISO, forskolin or (–)-Bay K 8644) were tested in the presence of the general antioxidant N-acetylcystein (NAC; 5 mm) or the mitochondria-targeted peptide antioxidant SS31 (200 nm) (Zhao et al. 2004; Szeto, 2006). Cardiomyocytes were then exposed to the antioxidant for 20 min before applying one of the Ca2+ transient facilitating agents.
L-type Ca2+ current (ICaL) measurements
Whole-cell patch-clamp experiments were performed on isolated cardiomyocytes at room temperature (∼24°C) using an Axopatch 200B amplifier (Axon Instruments/Molecular Devices, Sunnyvale, CA, USA). Currents, recorded with 2–3 MΩ patch pipettes, were normalized to cell membrane capacitance and expressed as current densities (pA pF−1). To record ICaL, the following pipette solution was used (mm): 120 CsCl, 6.8 MgCl2, 5 Na2ATP, 5 sodium creatine phosphate, 0.4 Na2GTP, 11 EGTA, 4.7 CaCl2 (120 nm free [Ca2+]), and 20 Hepes; pH was adjusted with CsOH to 7.2. The bath solution contained (mm): 135 TEA-Cl, 2 MgCl2, 10 glucose, 10 Hepes, 1.8 CaCl2; pH adjusted to 7.4 with TEAOH. The current–voltage relationship was obtained by giving test pulses varying from −80 mV to +60 mV from a holding potential of −80 mV. Electrophysiological data acquisition and analyses were performed using pCLAMP (v. 6, Axon Instruments/Molecular Devices).
Statistics
Student's t test for paired or unpaired data was used when two measurements were compared. One-way ANOVA was used for multiple comparisons vs. a control group. One-way repeated measures ANOVA was used for repeated measurements. The Holm–Sidak post hoc test was used with both ANOVAs. P < 0.05 was considered significant. Data are expressed as means ± SEM.
Results
β-Adrenergic stimulation increases mitochondrial ROS production in cardiomyocytes
The effect of the β-adrenergic agonist ISO on mitochondrial ROS production was studied in freshly isolated cardiomyocytes using the fluorescent indicator MitoSOX Red (Fauconnier et al. 2007; Martins et al. 2008). Cardiomyocytes were paced at 1 Hz for 10 min in standard Tyrode solution and then exposed to various drugs for 10 min. FAD-linked autofluorescence may interfere with the MitoSOX Red signal and control experiments were therefore performed on cells not loaded with any dye. The fluorescence of unloaded cells amounted to ∼20% of the fluorescence in cells loaded with MitoSOX Red and the signal was not affected by exposure to 100 nm ISO for 10 min (Δfluorescence 0 ± 3%; n = 9 cells). Thus, FAD-linked autofluorescence would not affect the MitoSOX Red measurements. Experiments with cells not exposed to any drug showed no significant (P > 0.05) increase in MitoSOX Red fluorescence (Fig. 1A). Application of ISO caused a concentration-dependent increase in the MitoSOX Red fluorescence, with ∼10% increase at 1 nm ISO (Fig. 2A) and a maximum increase of ∼25% at ≥10 nm ISO. Application of forskolin, which directly stimulates cAMP formation, resulted in an increase in the MitoSOX Red fluorescence similar to the maximum response with ISO. Moreover, ISO did not increase the MitoSOX Red fluorescence in cardiomyocytes pre-exposed to the PKA inhibitors H89 or PKI. These results indicate that the increased mitochondrial ROS production induced by β-adrenergic stimulation occurred via cAMP–PKA-dependent signalling.
Figure 1. β-Adrenergic stimulation of mouse cardiomyocytes results in increased mitochondrial ROS production.
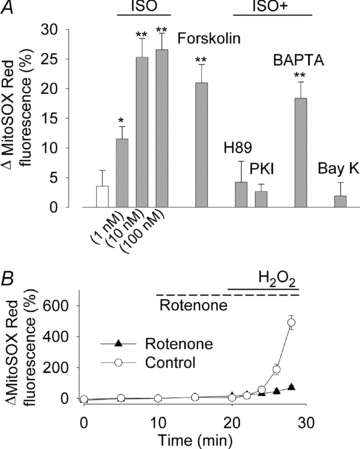
A, mean data (±SEM) of the relative increase in mitochondrial superoxide production measured with MitoSOX Red following 10 min of 1 Hz stimulation under control conditions (white bar) or exposure to: ISO (1–100 nm as indicated); forskolin (1 μm); ISO (100 nm) in cells pre-exposed to H89 (5 μm), PKI (5 μm) or BAPTA AM (50 μm); (–)-Bay K 8644 (1 μm). Data in each group were obtained from ≥8 cells from at least 2 mice. *P < 0.05 and **P < 0.001 vs. the control group. B, MitoSOX Red fluorescence in cardiomyocytes exposed to H2O2 (1 mm) in the presence or absence (Control) of the respiratory chain inhibitor rotenone (2.4 μm) (n≥ 13 cells).
Figure 2. β-Adrenergic stimulation increases mitochondrial ROS, whereas it does not affect mitochondrial membrane potential (ΔΨm) or mitochondrial [Ca2+].
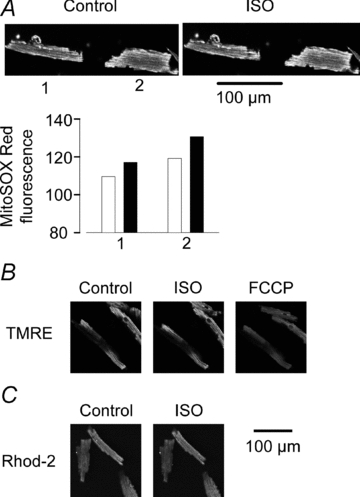
A, representative confocal images showing two MitoSOX Red-loaded cardiomyocytes under control conditions and after exposure to 1 nm ISO. Measurements show ∼10% increase in fluorescence in both cells after ISO exposure (black bars). B, confocal images showing three cardiomyocytes loaded with TMRE to measure ΔΨm. Images were obtained under control conditions, after exposure to ISO (100 nm), and after dissipating ΔΨm with FCCP (10 μm). There was no noticeable effect of ISO exposure, whereas the fluorescence decreased after application of FCCP. C, confocal images of three cardiomyocytes loaded with rhod-2 to measure mitochondrial [Ca2+] under control conditions and after 10 min exposure to ISO (100 nm), which had no noticeable effect on the fluorescence. Common scale bar for B and C.
Previous studies on intact and permeabilized cardiomyocytes have shown large increases in mitochondrial ROS production to be accompanied by depolarized ΔΨm (Fauconnier et al. 2007; Nagasaka et al. 2007). We used TMRE to assess whether exposure to ISO induces a change in ΔΨm and confocal images from one such experiment are shown in Fig. 2B. Exposure to 100 nm ISO for 10 min had no effect on the TMRE fluorescence (112 ± 5% of the pre-exposure control; n = 10 cells from 2 mice, P > 0.05), whereas the subsequent exposure to the mitochondrial uncoupler FCCP (10 μm) resulted in a marked depolarization of ΔΨm with the TMRE signal decreasing to 29 ± 10% of the control (P < 0.01).
The ISO-induced increase in mitochondrial ROS production may be a consequence of increases in SR Ca2+ release and/or the amplitude of cytosolic Ca2+ transients. Therefore experiments were performed where the Ca2+ transient amplitude was increased by application of the L-type Ca2+ channel agonist (–)-Bay K 8644, but this did not increase the MitoSOX Red fluorescence (Fig. 1A). Experiments were also performed on cardiomyocytes pre-loaded with the Ca2+ buffer BAPTA. Although Ca2+ transients were minimal (ΔF/F0= 0.25 ± 0.09, n = 5) and contractions undetectable in these BAPTA-loaded cells, ISO still induced an increase in the MitoSOX Red fluorescence (Fig. 1A). Thus, these experiments indicate that the increase in mitochondrial ROS production induced by β-adrenergic stimulation is not a consequence of increases in SR Ca2+ release or amplitude of cytosolic Ca2+ transients.
It has been suggested that there are Ca2+ microdomains through which Ca2+ may be directly channelled between spatially associated SR Ca2+ release sites and mitochondria (Maack & O'Rourke, 2007). These tentative Ca2+ microdomains might then allow an ISO-induced stimulation of SR Ca2+ release to increase the mitochondrial Ca2+ concentration, and hence affect mitochondrial ROS, even when the cytosolic Ca2+ is buffered by BAPTA. We therefore measured the mitochondrial Ca2+ concentration with rhod-2 before and after exposing BAPTA-loaded cardiomyocytes to ISO (100 nm) and confocal images from one such experiment are shown in Fig. 2C. The results showed no ISO-induced change in the rhod-2 fluorescence (105 ± 3% of the pre-exposure control; n = 11 cells from one mouse, P > 0.05).
Control experiments were performed to assess the extent of ROS increase induced by β-adrenergic stimulation. Cardiomyocytes paced at 1 Hz were then exposed to 1 mm H2O2, which would increase the mitochondrial [O2·−] by inducing product inhibition of the superoxide dismutase in the mitochondrial matrix (SOD2) and thereby inhibit conversion of O2·− to H2O2 (McAdam et al. 1977; Hearn et al. 1999; Aydin et al. 2009). Application of H2O2 resulted in ∼500% increase in the MitoSOX Red fluorescence and this increase depended on mitochondrial respiration, because it was markedly smaller when the mitochondrial respiratory complex I was inhibited by rotenone (Fig. 1B). Thus, the increase in MitoSOX Red fluorescence obtained with a high concentration of H2O2 was markedly larger than that observed with β-adrenergic stimulation.
β-Adrenergic stimulation increases oxidation-induced protein modifications
Increased ROS production is associated with decomposition of polyunsaturated fatty acids, which leads to the formation of reactive carbonyl species that can bind to protein (Aldini et al. 2007). To further address the question whether β-adrenergic stimulation increased ROS production, we used Western blotting and an antibody specific for protein binding of one such carbonyl species, malondialdehyde (MDA). Whole hearts were perfused in a Langendorff set-up for 30 min with Tyrode solution containing or lacking ISO (100 nm) and then frozen and analysed for MDA protein modifications. Hearts perfused with ISO showed a ∼twofold increase of MDA protein adducts relative to controls (Fig. 3A and B). The relative magnitude of this change was assessed by perfusing hearts with H2O2 (0.2 mm) and this resulted in a ∼sevenfold increase in MDA protein adducts (Fig. 3C and D), i.e. an increase much larger than that with ISO exposure. Thus, these results concur with the MitoSOX Red results presented above and imply that β-adrenergic stimulation triggers a robust, but not excessive, increase in mitochondrial ROS production.
Figure 3. β-Adrenergic stimulation of mouse hearts increases MDA protein adducts.
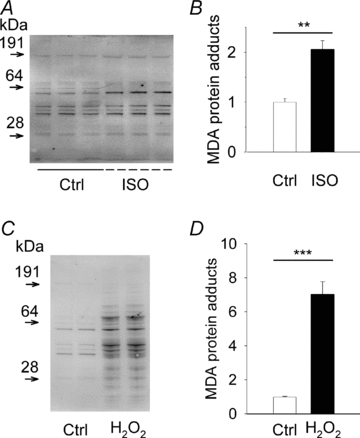
Western blots and mean (±SEM) densitometric quantifications of total MDA protein adducts in hearts perfused under control conditions (Ctrl) or in the presence of ISO (100 nm; A and B) or H2O2 (0.2 mm; C and D). Data normalized to the mean value in control, which was set to 1.0; n = 3–4 hearts in each group. **P < 0.01, ***P < 0.001.
Decreased inotropic response to β-adrenergic stimulation in cardiomyocytes in the presence of antioxidant
ROS and the redox state are known to alter SR Ca2+ cycling at multiple sites, including the release and uptake machineries (Zima & Blatter, 2006). Since β-adrenergic stimulation resulted in an increased ROS production in cardiomyocytes, we investigated whether ISO-induced changes in cardiomyocyte Ca2+ handling are affected by the general antioxidant NAC. Ca2+ transient properties and contractility (cell shortening) were not affected by application of NAC on its own (Fig. 4). Application of ISO (100 nm) induced larger and faster Ca2+ transients and increased the contractility (cell shortening). The ISO-induced increase of the Ca2+ transient amplitude and cell shortening was reduced, but not abolished, in the presence of NAC, whereas the rate of Ca2+ decline was unaffected (Fig. 4). The actual size of the ISO-induced increase in Ca2+ transient amplitude, as well as the inhibitory effect of NAC on this increase, is underestimated when expressed as ΔF/F0 because fluo-3 approaches saturation (cf. Fig. 4B and C). Thus, the ISO-induced increase in Ca2+ transient amplitude was ∼60% when expressed as ΔF/F0 and ∼150% when converted to [Ca2+]i. When forskolin was used to directly activate the cAMP–PKA pathway, NAC decreased the Ca2+ transient amplitude (from ΔF/F0 6.22 ± 0.23 to 5.46 ± 0.26 (representing ∼30% decrease in [Ca2+]i; P < 0.05) and cell shortening (from 16.0 ± 0.6 to 10.8 ± 1.1% of the cell length; P < 0.001), whereas it did not affect the time constant of Ca2+ decline (from 101 ± 8 to 95 ± 6 ms; P > 0.05) (data obtained from ≥12 cells from at least two mice). On the other hand, the response to application of the L-type Ca2+ channel agonist (–)-Bay K 8644 was not significantly different (P > 0.05) in the absence and presence of NAC: Ca2+ transient amplitude (ΔF/F0) 6.13 ± 0.33 vs. 6.31 ± 0.32; cell shortening 17.7 ± 1.9 vs. 17.2 ± 1.4% of the cell length; time constant of Ca2+ decline 180 ± 16 vs. 162 ± 15 ms (data obtained from ≥10 cells from at least two mice). To sum up, the increases in Ca2+ transient amplitude and cell shortening induced by β-adrenergic stimulation or by direct activation of the cAMP–PKA pathway are blunted by the general antioxidant NAC.
Figure 4. The β-adrenergic stimulatory effect on cardiomyocyte Ca2+ transient amplitude and cell shortening is blunted by the antioxidant NAC.
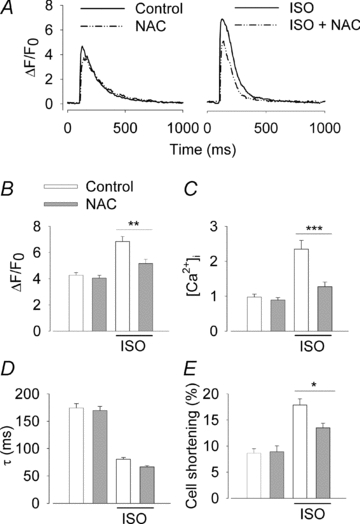
A, representative Ca2+ transients from cardiomyocytes in the presence of ISO (100 nm) and/or NAC (5 mm) as indicated. Average (±SEM; n≥ 19 cells from at least three mice) amplitude of Ca2+ transients expressed as ΔF/F0 (B) or translated into [Ca2+]i (C), Ca2+ transient decay time constant (D), and fractional cell shortening (E) without and with ISO as indicated and in the absence (white bars) or presence (grey bars) of NAC. *P < 0.05, **P < 0.01, ***P < 0.001.
In an additional series of experiments we tested the effect of SS31, a novel cell-permeant peptide antioxidant targeted to mitochondria (Zhao et al. 2004; Szeto, 2006), on the Ca2+ transient changes induced by β-adrenergic stimulation. Application of SS31 (200 nm) had no effect on Ca2+ transients under control conditions, whereas it decreased the Ca2+ transient amplitude in the presence of ISO (100 nm) by ∼30% (Fig. 5). SS31 had no effect on the time constant of Ca2+ decline either in the absence or presence of ISO. Thus, these results with a mitochondria-targeted antioxidant are very similar to those obtained with the general antioxidant NAC.
Figure 5. The β-adrenergic stimulatory effect on cardiomyocyte Ca2+ transient amplitude is blunted by the mitochondria-targeted antioxidant SS31.
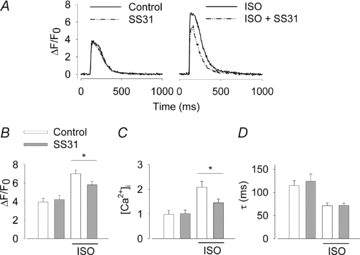
A, representative Ca2+ transients from cardiomyocytes in the presence of ISO (100 nm) and/or SS31 (200 nm) as indicated. Average (±SEM; n≥ 10 cells from at least two mice) amplitude of Ca2+ transients expressed as ΔF/F0 (B) or translated into [Ca2+]i (C), and Ca2+ transient decay time constant (D) without and with ISO as indicated and in the absence (white bars) or presence (grey bars) of SS31. *P < 0.05.
To directly test whether increased mitochondrial ROS production affects Ca2+ transients, cardiomycytes were exposed to the mitochondrial complex III inhibitor antimycin A, which is known to increase ROS production (Chen et al. 2003). Initial experiments were performed with application of 25 μm antimycin A but this resulted in irregular contractions and cells stopped contracting in response to electrical stimulation after ∼5 min exposure. We interpret this deleterious effect as a consequence of antimycin A-induced inhibition of mitochondrial respiration, because cardiomyocytes are heavily dependent on aerobic metabolism during repeated contractions. In subsequent experiments we therefore used a lower concentration of antimycin A (5 μm) and this concentration gave a 7.4 ± 1.9% increase in MitoSOX Red fluorescence (P < 0.01, n = 27 cells from two mice). The Ca2+ transient amplitude (ΔF/F0) increased from 4.06 ± 0.17 under control conditions to 4.63 ± 0.13 in the presence of antimycin A (P < 0.01; representing an increase in [Ca2+]i of ∼20%), whereas the time constant of Ca2+ decline was not affected (170 ± 8 vs. 168 ± 7 ms) (data obtained from >30 cells from two mice).
β-Adrenergic stimulation is known to increase ICaL in ventricular cardiomyocytes and this effect may not depend on PKA-induced phosphorylation of the channel (Lemke et al. 2008). Moreover, the cardiac ICaL has been shown to be redox sensitive (Sims & Harvey, 2004; Hool, 2008). We therefore tested whether the NAC-induced inhibition of ISO-mediated inotropy in cardiomyocytes involves a ROS-dependent effect on the L-type Ca2+ channels. Whole cell patch clamp of isolated cardiomyocytes was used to measure ICaL. Superfusing the cells with ISO (100 nm) caused a marked increase in ICaL and a leftward shift of the I–V curve (Fig. 6A). The ISO-induced stimulation of ICaL was almost fully abolished in the presence of NAC (Fig. 6B).
Figure 6. The β-adrenergic stimulation of cardiomyocyte L-type Ca2+ currents is inhibited by the antioxidant NAC.
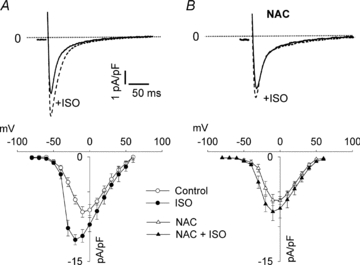
A, representative records of the L-type Ca2+ current (ICaL) in cardiomyocytes ±100 nm ISO (upper panel, voltage step from −80 mV holding potential to −10 mV) and mean data (±SEM) of the peak current density (lower panel) (n = 6 cells in each group). B, same as A but in the presence of 5 mm NAC (n = 7 cells in each group).
Discussion
The effects of β-adrenergic stimulation are generally mediated via cAMP and PKA. It was recently shown that cAMP can be generated within the mitochondria (Acin-Perez et al. 2009). Moreover, PKA, as well as PKA anchoring proteins, can be localized in the mitochondria (Papa et al. 2002). Prolonged (24 h) β-adrenergic stimulation has been shown to induce mitochondrial membrane depolarization and apoptosis in adult rat cardiomyocytes (Remondino et al. 2003; Menon et al. 2006). This apoptosis was inhibited by SOD/catalase mimetics and by overexpression of catalase, which indicates that the apoptotic signalling induced by β-adrenergic stimulation involves increased ROS production (Remondino et al. 2003). A recent study on permeabilized rat cardiomyocytes shows that addition of activated PKA results in a rapid and reversible increase in mitochondrial ROS production (Nagasaka et al. 2007). In accordance with these results, we now show that acute β-adrenergic stimulation with ISO induces a cAMP- and PKA-dependent increase in mitochondrial ROS production in intact ventricular mouse cardiomyocytes. In addition, we show that this increase in ROS plays a crucial role in the β-adrenergic inotropic effect, because the ISO-induced increases in Ca2+ transient amplitude is diminished in the presence of the general antioxidant NAC as well as the mitochondria-targeted antioxidant SS31.
The increase in mitochondrial ROS production induced by β-adrenergic stimulation might be a direct effect of activation of the cAMP–PKA signalling pathway. Alternatively, it can occur as a consequence of the increased Ca2+ transients in the presence of β-adrenergic stimulation. To study these possibilities we used forskolin to directly activate cAMP signalling, H89 or PKI to inhibit ISO-induced PKA signalling and (–)-Bay K 8644 or BAPTA to independently increase or decrease Ca2+ transients, respectively. The results show that mitochondrial ROS production was increased by application of forskolin and the ISO-induced increase was inhibited by H89 and PKI. On the other hand, buffering of Ca2+ transients with BAPTA had no significant impact on the ISO-induced increase in ROS production and increasing the amplitude of Ca2+ transients with (–)-Bay K 8644 did not increase ROS production. Moreover, the increases in Ca2+ transient amplitude and cell shortening induced by ISO and forskolin were diminished by NAC, whereas NAC had no effect on the (–)-Bay K 8644-induced increases. Taken together, these results demonstrate that the increased mitochondrial ROS production with β-adrenergic stimulation occurs as a direct consequence of cAMP–PKA-dependent signalling and not as an indirect effect of increased Ca2+ transient amplitudes. Our results also show that the increased mitochondrial ROS production induced by β-adrenergic stimulation is not dependent on mitochondrial Ca2+ accumulation or membrane depolarization (see Fig. 3). However, the direct mechanism(s) by which activation of the cAMP–PKA signalling pathway leads to increased mitochondrial ROS production remains to be elucidated. Furthermore, the effects of ISO on cardiomyocyte Ca2+ handling have recently been shown to also depend on nitric oxide (Collins & Rodrigo, 2010) and possible interactions between reactive nitrogen species and ROS requires further attention.
The ROS-mediated increase in Ca2+ transient amplitude could, in principle, be due to either increased Ca2+ entry over the plasma membrane or increased Ca2+ release from intracellular Ca2+ stores. Our data support a mechanism whereby ROS, induced by β-adrenergic stimulation, facilitate Ca2+ entry through L-type Ca2+ channels. An increase in L-type Ca2+ entry would not only in itself increase cytosolic Ca2+, but also enhance RyR Ca2+ release via increasing Ca2+-induced Ca2+ release (Bassani et al. 1995).
Our results show faster Ca2+ transient decays with both ISO and forskolin and this effect, which is considered to be due to phosphorylation of phospholamban resulting in faster SR Ca2+ uptake (MacLennan & Kranias, 2003), was not affected by NAC or SS31. This shows that not all effects of β-adrenergic stimulation are ROS dependent. There are two subtypes of β-adrenergic receptors (β1ARs and β2ARs). β1ARs are distributed across the entire surface of cardiomyocytes and activation of these is required for PKA-induced phosphorylation of phospholamban and acceleration of the decay of Ca2+ transients (Xiao, 2001; Nikolaev et al. 2010). β2ARs, on the other hand, are specifically localized to the transverse tubules and hence more prone to affect plasma membrane proteins, such as L-type Ca2+ channels (Xiao, 2001; Nikolaev et al. 2010). Thus, localized signalling induced by activation of β2ARs in the transverse tubules may involve increased ROS production, whereas this does not appear to be the case for the generalized signalling mediated via β1ARs.
In the present study we exposed cardiomyocytes to antimycin A (5 μm) to directly stimulated mitochondrial ROS production and this resulted in ∼7% increase in MitoSOX Red fluorescence and ∼20% increase in [Ca2+]i transient amplitude, which compares to increases of ∼25% and ∼150% after application of ISO. This indicates a dose-dependent ROS-induced stimulation of Ca2+ transients; unfortunately a higher concentration of antimycin A (25 μm) could not be used because of deleterious effects on cardiomyocyte function. On the other hand, we previously showed that application of palmitate resulted in a markedly increased ROS production in wild-type cardiomyocytes, which was accompanied by decreased Ca2+ transient amplitude and contractility, i.e. opposite to the present findings (Fauconnier et al. 2007). The opposite effect on Ca2+ transients can be explained by the fact that the ROS production was larger with palmitate than with ISO or antimycin A; the increase in MitoSOX Red fluorescence was ∼50% during 10 min exposure to palmitate compared to ∼25% with ISO and 7% with antimycin A. Moreover, application of palmitate caused a marked depolarization of ΔΨm (Fauconnier et al. 2007), whereas the present results show no effect of ISO on ΔΨm. On the other hand, a marked depolarization of ΔΨm was observed in rat cardiomyocytes after prolonged (24 h) exposure to ISO (Menon et al. 2006). Thus, these findings represent examples of the dual effects of ROS where an acute limited increase in ROS acts as a beneficial integral part in normal physiological signalling, whereas a large or prolonged increases can have adverse effects and contributes to pathological changes (Andrade et al. 2001; Goldstein et al. 2005; Rojas et al. 2006; Zima & Blatter, 2006).
Conclusions
Our results show that increased mitochondrial ROS production plays an integral role in the acute β-adrenergic-induced inotropic response of cardiomyocytes. This stimulatory effect is mediated via cAMP–PKA-dependent and Ca2+-independent signalling. On the other hand, prolonged increases in ROS can have deleterious effects and may contribute to the development of heart failure and cardiac arrhythmias associated with chronically sustained adrenergic stress.
Acknowledgments
The work is supported by the Swedish Heart Lung Foundation, the Swedish Research Council, the Swedish Diabetes Foundation, Lars Hierta Memorial Foundation, Sigurd and Elsa Golje Memorial Foundation, and Funds at the Karolinska Institute. SS31 was a gift from University of Kentucky Centre for Muscle Biology.
Glossary
Abbreviations
- ΔΨm
mitochondrial membrane potential
- ICaL
L-type Ca2+ current
- FCCP
carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone
- HRP
horseradish peroxidase
- ISO
isoproterenol
- MDA
malondialdehyde
- NAC
N-acetylcysteine
- PKA
protein kinase A
- PKI
PKA inhibitor 14–22 amide (myristoylated)
- ROS
reactive oxygen species
- RyR
ryanodine receptor
- SERCA
sarcoplasmic reticulum ATPase
- SOD
superoxide dismutase
- SR
sarcoplasmic reticulum
- TMRE
Tetramethylrhodamine ethyl ester
Author contributions
All experiments were performed at the Department of Physiology and Pharmacology, Karolinska Institutet. D.C.A., J.F. and H.W. were involved in the conception and design of the studies. All authors were involved in the collection, analysis and interpretation of the data. D.C.A. was mainly responsible for drafting the article and critical input was obtained from all other authors. All authors approved the final version of the manuscript.
References
- Acin-Perez R, Salazar E, Kamenetsky M, Buck J, Levin LR, Manfredi G. Cyclic AMP produced inside mitochondria regulates oxidative phosphorylation. Cell Metab. 2009;9:265–276. doi: 10.1016/j.cmet.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldini G, Dalle-Donne I, Facino RM, Milzani A, Carini M. Intervention strategies to inhibit protein carbonylation by lipoxidation-derived reactive carbonyls. Med Res Rev. 2007;27:817–868. doi: 10.1002/med.20073. [DOI] [PubMed] [Google Scholar]
- Andrade FH, Reid MB, Westerblad H. Contractile response of skeletal muscle to low peroxide concentrations: myofibrillar calcium sensitivity as a likely target for redox-modulation. FASEB J. 2001;15:309–311. doi: 10.1096/fj.00-0507fje. [DOI] [PubMed] [Google Scholar]
- Aydin J, Andersson DC, Hänninen SL, Wredenberg A, Tavi P, Park CB, Larsson NG, Bruton JD, Westerblad H. Increased mitochondrial Ca2+ and decreased sarcoplasmic reticulum Ca2+ in mitochondrial myopathy. Human Mol Genet. 2009;18:278–288. doi: 10.1093/hmg/ddn355. [DOI] [PubMed] [Google Scholar]
- Bassani JW, Yuan W, Bers DM. Fractional SR Ca release is regulated by trigger Ca and SR Ca content in cardiac myocytes. Am J Physiol Cell Physiol. 1995;268:C1313–C1319. doi: 10.1152/ajpcell.1995.268.5.C1313. [DOI] [PubMed] [Google Scholar]
- Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- Chen Q, Vazquez EJ, Moghaddas S, Hoppel CL, Lesnefsky EJ. Production of reactive oxygen species by mitochondria: central role of complex III. J Biol Chem. 2003;278:36027–36031. doi: 10.1074/jbc.M304854200. [DOI] [PubMed] [Google Scholar]
- Clark AL, Cleland JG. The control of adrenergic function in heart failure: therapeutic intervention. Heart Fail Rev. 2000;5:101–114. doi: 10.1023/A:1009854325711. [DOI] [PubMed] [Google Scholar]
- Collins HE, Rodrigo GC. Inotropic response of cardiac ventricular myocytes to β-adrenergic stimulation with isoproterenol exhibits diurnal variation: involvement of nitric oxide. Circ Res. 2010;106:1244–1252. doi: 10.1161/CIRCRESAHA.109.213942. [DOI] [PubMed] [Google Scholar]
- Curran J, Hinton MJ, Rios E, Bers DM, Shannon TR. β-Adrenergic enhancement of sarcoplasmic reticulum calcium leak in cardiac myocytes is mediated by calcium/calmodulin-dependent protein kinase. Circ Res. 2007;100:391–398. doi: 10.1161/01.RES.0000258172.74570.e6. [DOI] [PubMed] [Google Scholar]
- Davare MA, Hell JW. Increased phosphorylation of the neuronal L-type Ca2+ channel Cav1.2 during aging. Proc Natl Acad Sci U S A. 2003;100:16018–16023. doi: 10.1073/pnas.2236970100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchen MR, Leyssens A, Crompton M. Transient mitochondrial depolarizations reflect focal sarcoplasmic reticular calcium release in single rat cardiomyocytes. J Cell Biol. 1998;142:975–988. doi: 10.1083/jcb.142.4.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauconnier J, Andersson DC, Zhang SJ, Lanner JT, Wibom R, Katz A, Bruton JD, Westerblad H. Effects of palmitate on Ca2+ handling in adult control and ob/ob cardiomyocytes: impact of mitochondrial reactive oxygen species. Diabetes. 2007;56:1136–1142. doi: 10.2337/db06-0739. [DOI] [PubMed] [Google Scholar]
- Fuller MD, Emrick MA, Sadilek M, Scheuer T, Catterall WA. Molecular mechanism of calcium channel regulation in the fight-or-flight response. Sci Signal. 2010;3:ra70. doi: 10.1126/scisignal.2001152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein BJ, Mahadev K, Wu X. Redox paradox: insulin action is facilitated by insulin-stimulated reactive oxygen species with multiple potential signaling targets. Diabetes. 2005;54:311–321. doi: 10.2337/diabetes.54.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearn AS, Tu C, Nick HS, Silverman DN. Characterization of the product-inhibited complex in catalysis by human manganese superoxide dismutase. J Biol Chem. 1999;274:24457–24460. doi: 10.1074/jbc.274.35.24457. [DOI] [PubMed] [Google Scholar]
- Hool LC. Evidence for the regulation of L-type Ca2+ channels in the heart by reactive oxygen species: mechanism for mediating pathology. Clin Exp Pharmacol Physiol. 2008;35:229–234. doi: 10.1111/j.1440-1681.2007.04727.x. [DOI] [PubMed] [Google Scholar]
- Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440:944–948. doi: 10.1038/nature04634. [DOI] [PubMed] [Google Scholar]
- Lemke T, Welling A, Christel CJ, Blaich A, Bernhard D, Lenhardt P, Hofmann F, Moosmang S. Unchanged β-adrenergic stimulation of cardiac L-type calcium channels in Cav1.2 phosphorylation site S1928A mutant mice. J Biol Chem. 2008;283:34738–34744. doi: 10.1074/jbc.M804981200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnane AW, Eastwood H. Cellular redox regulation and prooxidant signaling systems: a new perspective on the free radical theory of aging. Ann N Y Acad Sci. 2006;1067:47–55. doi: 10.1196/annals.1354.008. [DOI] [PubMed] [Google Scholar]
- Maack C, O'Rourke B. Excitation-contraction coupling and mitochondrial energetics. Basic Res Cardiol. 2007;102:369–392. doi: 10.1007/s00395-007-0666-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLennan DH, Kranias EG. Phospholamban: a crucial regulator of cardiac contractility. Nat Rev Mol Cell Biol. 2003;4:566–577. doi: 10.1038/nrm1151. [DOI] [PubMed] [Google Scholar]
- Martins AS, Shkryl VM, Nowycky MC, Shirokova N. Reactive oxygen species contribute to Ca2+ signals produced by osmotic stress in mouse skeletal muscle fibres. J Physiol. 2008;586:197–210. doi: 10.1113/jphysiol.2007.146571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, Marks AR. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101:365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- McAdam ME, Levelle F, Fox RA, Fielden EM. A pulse-radiolysis study of the manganese-containing superoxide dismutase from Bacillus stearothermophilus. Biochem J. 1977;165:81–87. doi: 10.1042/bj1650081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon B, Singh M, Ross RS, Johnson JN, Singh K. β-Adrenergic receptor-stimulated apoptosis in adult cardiac myocytes involves MMP-2-mediated disruption of β1 integrin signaling and mitochondrial pathway. Am J Physiol Cell Physiol. 2006;290:C254–C261. doi: 10.1152/ajpcell.00235.2005. [DOI] [PubMed] [Google Scholar]
- Moylan JS, Reid MB. Oxidative stress, chronic disease, and muscle wasting. Muscle Nerve. 2007;35:411–429. doi: 10.1002/mus.20743. [DOI] [PubMed] [Google Scholar]
- Nagasaka S, Katoh H, Niu CF, Matsui S, Urushida T, Satoh H, Watanabe Y, Hayashi H. Protein kinase A catalytic subunit alters cardiac mitochondrial redox state and membrane potential via the formation of reactive oxygen species. Circ J. 2007;71:429–436. doi: 10.1253/circj.71.429. [DOI] [PubMed] [Google Scholar]
- Nikolaev VO, Moshkov A, Lyon AR, Miragoli M, Novak P, Paur H, Lohse MJ, Korchev YE, Harding SE, Gorelik J. β2-Adrenergic receptor redistribution in heart failure changes cAMP compartmentation. Science. 2010;327:1653–1657. doi: 10.1126/science.1185988. [DOI] [PubMed] [Google Scholar]
- Papa S, Scacco S, Sardanelli AM, Petruzzella V, Vergari R, Signorile A, Technikova-Dobrova Z. Complex I and the cAMP cascade in human physiopathology. Biosci Rep. 2002;22:3–16. doi: 10.1023/a:1016004921277. [DOI] [PubMed] [Google Scholar]
- Remondino A, Kwon SH, Communal C, Pimentel DR, Sawyer DB, Singh K, Colucci WS. β-Adrenergic receptor-stimulated apoptosis in cardiac myocytes is mediated by reactive oxygen species/c-Jun NH2-terminal kinase-dependent activation of the mitochondrial pathway. Circ Res. 2003;92:136–138. doi: 10.1161/01.res.0000054624.03539.b4. [DOI] [PubMed] [Google Scholar]
- Rojas A, Figueroa H, Morales MA, Re L. Facing up the ROS labyrinth – Where to go? Curr Vasc Pharmacol. 2006;4:277–289. doi: 10.2174/157016106777698441. [DOI] [PubMed] [Google Scholar]
- Sambrano GR, Fraser I, Han H, Ni Y, O'Connell T, Yan Z, Stull JT. Navigating the signalling network in mouse cardiac myocytes. Nature. 2002;420:712–714. doi: 10.1038/nature01306. [DOI] [PubMed] [Google Scholar]
- Santiago DJ, Curran JW, Bers DM, Lederer WJ, Stern MD, Rios E, Shannon TR. Ca sparks do not explain all ryanodine receptor-mediated SR Ca leak in mouse ventricular myocytes. Biophys J. 2010;98:2111–2120. doi: 10.1016/j.bpj.2010.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims C, Harvey RD. Redox modulation of basal and β-adrenergically stimulated cardiac L-type Ca2+ channel activity by phenylarsine oxide. Br J Pharmacol. 2004;142:797–807. doi: 10.1038/sj.bjp.0705845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeto HH. Mitochondria-targeted peptide antioxidants: novel neuroprotective agents. AAPS J. 2006;8:E521–E531. doi: 10.1208/aapsj080362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao B, Zhong G, Obayashi M, Yang D, Chen K, Walsh MP, Shimoni Y, Cheng H, Ter Keurs H, Chen SR. Ser-2030, but not Ser-2808, is the major phosphorylation site in cardiac ryanodine receptors responding to protein kinase A activation upon β-adrenergic stimulation in normal and failing hearts. Biochem J. 2006;396:7–16. doi: 10.1042/BJ20060116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao RP. β-Adrenergic signaling in the heart: dual coupling of the β2-adrenergic receptor to Gs and Gi proteins. Sci STKE. 2001;2001:re15. doi: 10.1126/stke.2001.104.re15. [DOI] [PubMed] [Google Scholar]
- Zalk R, Lehnart SE, Marks AR. Modulation of the ryanodine receptor and intracellular calcium. Annu Rev Biochem. 2007;76:367–385. doi: 10.1146/annurev.biochem.76.053105.094237. [DOI] [PubMed] [Google Scholar]
- Zhao K, Zhao GM, Wu D, Soong Y, Birk AV, Schiller PW, Szeto HH. Cell-permeable peptide antioxidants targeted to inner mitochondrial membrane inhibit mitochondrial swelling, oxidative cell death, and reperfusion injury. J Biol Chem. 2004;279:34 682–34 690. doi: 10.1074/jbc.M402999200. [DOI] [PubMed] [Google Scholar]
- Zima AV, Blatter LA. Redox regulation of cardiac calcium channels and transporters. Cardiovasc Res. 2006;71:310–321. doi: 10.1016/j.cardiores.2006.02.019. [DOI] [PubMed] [Google Scholar]
- Zima AV, Kockskamper J, Mejia-Alvarez R, Blatter LA. Pyruvate modulates cardiac sarcoplasmic reticulum Ca2+ release in rats via mitochondria-dependent and -independent mechanisms. J Physiol. 2003;550:765–783. doi: 10.1113/jphysiol.2003.040345. [DOI] [PMC free article] [PubMed] [Google Scholar]


