Abstract
The growth and the metabolic activity of Shewanella putrfaciens, Brochothrix thermosphacta, and Pseudomonas sp., when cultured individually or in all possible combinations in gel cassettes system supplemented with 0.1% glucose at 5°C, were investigated. The overall outcome was that the coexistence of the above-mentioned microorganisms affected not only each growth rate but also their type of metabolic end products compared to the control cultures. These effects were varied and depended on the selection of the combination of the tested bacteria. For example, the growth of Pseudomonas sp. strains cocultured with either B. thermosphacta or S. putrefaciens strains resulted in different effects: a promoting one for the first and an inhibitory one for the second. Moreover, the production of formic acid and two unidentified organic acids (peaks a and b) was characteristic in all cases in which S. putrefaciens was cultured.
There is no doubt that microbiological activity is by far the most important factor influencing spoilage in raw muscle food and vegetables (23, 28, 31). Recent studies in food microbiology established that spoilage is caused only by a small fraction of the initial microbial association, the so-called specific spoilage organisms (21). This concept has contributed significantly to our understanding, in particular of muscle foods spoilage (23, 28). Various factors, either intrinsic (pH, aw, nutrients, and oxidation or reduction) or extrinsic (modified atmosphere packaging or temperature), affect the initial and the final microbial association of a given food ecosystem (21). For example, Shewanella putrefaciens and Photobacterium phosphoreum are considered to be the specific spoilage organisms of aerobically stored and modified-atmosphere-packaged fish, respectively (8). On the basis of numerous data reported in the literature, spoilage of fresh fish can be considered as a result of the activity of more than one specific spoilage organism (8, 11, 23, 24, 32). Indeed, both Pseudomonas sp. and S. putrefaciens are considered to be the main spoilage bacteria in fish from the Mediterranean Sea that are stored in ice. On the other hand, B. thermosphacta is a minor contributor to spoilage (8, 23, 32), whereas both B. thermosphacta and lactic acid bacteria are the dominant bacteria in the microbial association during the storage of these fish under vacuum and modified atmosphere packaging (11, 12, 24). Thus, the qualitative and quantitative composition of the microbial flora at the end of storage of fish will eventually characterize the type of spoilage (21). Although there is a wide knowledge for the detection of microorganisms that cause spoilage, researchers in the food industry are searching for techniques to replace the time-consuming and retrospective traditional microbiological methods and to detect rapidly the spoilage.The correlation between microbial growth and the development of chemical changes during spoilage has been continuously recognized as a means of revealing specific substrates and/or end products that may be useful for assessing food quality (6, 15, 21, 28). However, the selection of the microbial association and the subsequent chemical changes during food spoilage depends not only on the imposed environmental conditions, as is well known, but also on microbial interaction (17, 28, 30, 34). This concept has only been partly exploited in fish microbiology. For example, although considerable data concerning the correlation between H2S-producing bacteria (S. putrefaciens) and freshness have been collected, Pseudomonas spp. have not received the appropriate attention with regard to the effect of microbial interaction on spoilage. This may be important in understanding spoilage, since it has been found that there is an interaction between the above-mentioned bacteria. Indeed, Pseudomonas sp. can inhibit the growth of S. putrefaciens due to the ability of the former to produce siderophores, and this interaction can be the major factor governing the development of spoilage flora (20). Furthermore, the competition for other nutrients (e.g., glucose), metabiosis (production of a favorable environment), and cell-to-cell communication could also affect the physiological attributes of the organisms under the imposed ecological determinants (10, 13, 25). Indeed, Koutsoumanis and Nychas (23) reported that the chemical changes occurring in naturally contaminated fish significantly differed from those on sterile fish tissue when it was individually inoculated with the specific spoilage organisms. Therefore, studies in coculture model systems could be helpful in simplifying the natural food ecosystem and help both to elucidate the mechanisms whereby the development of potential specific spoilage organisms is affected by possible interactive behaviors and to identify the responsible metabolite that may be further used as a unique chemical spoilage index.
Since the food matrix affects the growth rate and biochemical behavior of microorganisms (36), a gel cassette system was used instead of broth to simulate structured (food) systems. Such a system allowed us to quantify more accurately the growth and metabolic activity (36) of the specific spoilage bacteria examined in the present study, which were grown in every possible combination.
MATERIALS AND METHODS
Bacterial strains.
Strains of Pseudomonas sp., S. putrefaciens, and B. thermosphacta were originally isolated from gilt head fish (Sparus aurata). Colonies were isolated from Pseudomonas agar supplemented with cetrimide-fucidin-cephaloridine (SR 103E [Oxoid, Basingstoke, United Kingdom]), iron agar, and streptomycin sulfate-thallous acetate-actidione agar (CM 559; Oxoid) and were initially characterized by using the following criteria: Gram staining, cell morphology, flagellar arrangement, oxidase reaction, catalase formation, aerobic and anaerobic breakdown of glucose, ammonia production from arginine, acid production of maltose, decarboxylation of ornithine, lipolytic activity, production of fluorescent pigments, and growth at different temperatures, as described by Tryfinopoulou et al. (32, 33).
Preparation of gel cassettes.
The cassette comprised an acetate frame that was 2 mm thick and had outer measurements of 130 by 145 mm and a window within the frame that measured 100 by 100 mm, sealed within a sleeve of polyvinyl chloride (PVC) packaging film that was 15 μm thick (5). Cassettes were formed by enveloping the frame within a sleeve of PVC that was heat sealed on three sides as described by Brocklehurst et al. (4). The cassettes were sterilized by autoclaving at 121°C for 15 min, and the PVC on either side of the sterile cassette was made taut in a stream of hot air.
Preparation of inocula and inoculation.
The growth of Pseudomonas sp., B. thermosphacta, and S. putrefaciens strains were monitored individually or in all of their possible combinations in the gel cassette containing nutrient broth supplemented with 0.1% (wt/vol) glucose (4). This cassette was prepared as follows. Portions of a sterile solution of 20% (wt/vol) gelatin (Merck, Darmstadt, Germany; pH 6.55 ± 0.02) were mixed with equal portions of filter (0.22-μm pore size; Millipore, Vienna, Austria)-sterilized double-strength nutrient broth (pH 6.55 ± 0.02) supplemented with 0.1% (wt/vol) glucose. The isolates were subcultured twice in 100 ml of nutrient broth (catalog no. 1.05443.0500; Merck) and then incubated at 25°C for 18 h. Cells were harvested and washed with sterile quarter-strength Ringer's solution. Aliquots of inoculum, serially diluted in quarter-strength Ringer solution (NaCl, 2.15 g liter−1; KCl, 0.075 g liter−1; CaCl, 0.12 g liter−1; NaHSO4, 0.5 g liter−1 [Lab M, Bury, United Kingdom]), were mixed with the gelatin culture medium at 30°C and loaded into the gel cassette (3, 4; T. F. Brocklehurst, A. R. Mackie, D. C. Steer, and D. R. Wilson, international patent application WO 95/00661) in order to obtain a final cell (colony) concentration of ca. 103 CFU ml−1. This procedure was performed three times, and duplicate samples for each treatment were obtained.
Microbiological analysis.
Samples (10 g) from gel cassette system were weighted aseptically, added to sterile quarter-strength Ringer's solution (90 ml), and homogenized in a stomacher (Lab Blender 400; Seward Medical, London, United Kingdom) for 60 s at room temperature. Decimal dilutions in quarter-strength Ringer's solution were prepared, and duplicate 1- or 0.1-ml samples of appropriate dilutions poured or spread on plate count agar (catalog no. 1.05463.0500; Merck) for determination of the total viable count, incubated at 25°C for 72 h. B. thermosphacta was placed on streptomycin sulfate-thallous acetate-actidione medium supplemented with streptomycin sulfate, thallous acetate, and cycloheximide (Actidione) as described by Gardner (18); this medium was made from basic ingredients in the laboratory and incubated at 25°C for 72 h. S. putrefaciens was placed on iron agar (CM867; Oxoid), overlaid with the same medium, and incubated at 25°C for 96 h under anaerobic conditions. Pseudomonas spp. were placed on cetrimide-fucidin-cephaloridine agar (CM559 with selective supplement SR 103E; Oxoid) and incubated at 25°C for 48 h.
Growth monitoring.
The growth data from plate counts were transformed to log10 values. The Baranyi model (1) was fitted to the logarithm of the viable cell concentration. For curve fitting, the in-house program DMFit (Institute of Food Research, Reading, United Kingdom) was used (kindly provided by J. Baranyi).
pH measurement.
The pH of the sample homogenates was measured with a combination glass electrode with a pH meter (catalog no. RL 150; Russell, Fife, Scotland).
Determination of glucose.
The concentrations of glucose in supernatants were assayed by using the GOD-PERID kit (Boehringer Mannheim GmBH).
HPLC determination of certain organic acids.
The profiles of the organic acids (treated with trifluoacetic acid) of the samples were analyzed by high-pressure liquid chromatography (HPLC; Spectra Physics P2000 two-pump system with a UV/VIS detector using low-inertia scanning technology [similar to a photodiode array] and software from Photodiode, San Jose, Calif.), using a Rheodyne 7125 injector and a 300-by-7.8-mm Aminex HPX-87H (5 μm; pore size; Bio-Rad Laboratories, Richmond, Calif.). The compounds were separated isocratically with 4.5 mM H2SO4 in distilled water (flow rate, 0.7 ml/min). The peak width was 12; the peak threshold was 600 and 0.034 absorbance units full scale (AUFS). The whole spectrum (190 to 330 nm) of the chromatograms was analyzed (14). The solvents were HPLC grade and, for the identification of peaks, solutions of reference substances (citric, lactic, acetic, tartaric, malic, succinic, formic, and propionic acids) were analyzed by using the same program; their retention times (RT) and spectra were then compared. The precision of the results (peak area) was always better than ±5%.
RESULTS
Microbiological changes.
Growth of Pseudomonas sp., B. thermosphacta, and S. putrefaciens strains were monitored individually (control cultures) and in all possible combinations (Pseudomonas sp. and B. thermosphacta [MPB], Pseudomonas sp. and S. putrefaciens [MPS], B. thermosphacta and S. putrefaciens [MBS], and Pseudomonas sp., B. thermosphacta, and S. putrefaciens [MPBS]) in gel cassette systems at 5°C. Maximum specific growth rates, lag-phase durations, and final counts of the bacteria are presented in Table 1.
TABLE 1.
Maximum specific growth rate, final population, and lag period of control and mixed cultures in gel cassettes stored at 5°Ca
| Cassette(s) | Mean rate (h−1) ± SD | Final count (mean log10 CFU/g) ± SD | Mean lag time (h) ± SD |
|---|---|---|---|
| CP (Pseudomonas sp.) | 0.057 ± 0.005 | 8.544 ± 0.103 | 0 |
| CB (B. thermosphacta) | 0.036 ± 0.002 | 8.360 ± 0.122 | 0 |
| CS (S. putrefaciens) | 0.053 ± 0.003 | 8.682 ± 0.089 | 8.384 ± 4.372 |
| MPB | |||
| Pseudomonas sp. | 0.060 ± 0.005 | 8.319 ± 0.103 | 0 |
| B. thermosphacta | 0.048 ± 0.003 | 8.211 ± 0.113 | 0 |
| MPS | |||
| Pseudomonas sp. | 0.055 ± 0.004 | 8.415 ± 0.086 | 0 |
| S. putrefaciens | 0.037 ± 0.004 | 8.294 ± 0.183 | 0 |
| MBS | |||
| B. thermosphacta | 0.042 ± 0.004 | 8.146 ± 0.146 | 0 |
| S. putrefaciens | 0.047 ± 0.005 | 7.977 ± 0.186 | 0 |
| MPBS | |||
| Pseudomonas sp. | 0.058 ± 0.006 | 8.072 ± 0.140 | 0 |
| B. thermosphacta | 0.039 ± 0.002 | 8.360 ± 0.122 | 0 |
| S. putrefaciens | 0.045 ± 0.005 | 7.587 ± 0.147 | 0 |
CP, control Pseudomonas sp.; CB, control B. thermosphacta; CS, control S. putrefaciens; MPB, Pseudomonas sp. and B. thermosphacta; MPS, Pseudomonas sp. and S. putrefaciens; MBS, B. thermosphacta and S. putrefaciens; MPBS, Pseudomonas sp., B. thermosphacta, and S. putrefaciens.
The growth rate of Pseudomonas sp. strains in control cultures was 0.057 h−1. The presence of B. thermosphacta, S. putrefaciens, or both did not significantly affect the growth of Pseudomonas sp. (Table 1). B. thermosphacta presented a greater growth rate (P < 0.05) in the mixed cultures MPB and MBS than in the other cultures. The only bacterium that presented a lag period was S. putrefaciens, principally in the control culture. The most apparent decrease in growth rate of S. putrefaciens was observed in the mixed cultures of this bacterium with Pseudomonas sp. (Table 1). In the latter mixed culture, the final population of S. putrefaciens was reduced by ∼10-fold compared to its control culture. In the remaining cases, the type of the culture had no effect on the final population of the bacteria.
Physicochemical changes.
The mean pH values of the control and mixed cultures are shown in Table 2. The initial pH values of both control and mixed cultures were in the range of 6.53 to 6.55. During growth of B. thermosphacta in gel cassettes either in control or mixed cultures the pH decreased to 5.56 to 5.45. It should be noted, however, that the decrease of pH in the mixed culture of B. thermosphacta and Pseudomonas sp. mainly occurred during the late-exponential-growth phase of the bacteria (Fig. 2 and Table 2). When these bacteria entered the stationary phase, the pH increased again to 6.30. The pH of the remaining cultures (CP, CS, and MPS) remained constant throughout incubation period.
TABLE 2.
Changes in pH of control cultures of Pseudomonas sp. (CP), B. thermosphacta (CB), and S. putrefaciens (CS) and their mixed cultures MPB, MPS, MBS, and MPBS in gel cassettes system at 5°C
| Time (h) | pH in:
|
||||||
|---|---|---|---|---|---|---|---|
| CP | CB | CS | MPB | MPS | MBS | MPBS | |
| 0 | 6.53 | 6.53 | 6.53 | 6.55 | 6.55 | 6.55 | 6.55 |
| 15 | 6.53 | 6.54 | 6.52 | 6.55 | 6.54 | 6.55 | 6.55 |
| 40 | 6.54 | 6.56 | 6.56 | 6.53 | 6.53 | 6.54 | 6.54 |
| 52 | NDa | ND | ND | 6.40 | 6.43 | 6.48 | 6.48 |
| 68 | 6.41 | 6.53 | 6.55 | 6.39 | 6.43 | 6.48 | 6.48 |
| 90 | 6.31 | 6.48 | 6.50 | 6.31 | 6.41 | 6.37 | 6.35 |
| 100 | ND | ND | ND | 6.17 | 6.45 | 6.21 | 6.21 |
| 116 | 6.38 | 6.41 | 6.42 | 6.10 | 6.39 | 5.68 | 5.98 |
| 138 | 6.52 | 6.54 | 6.44 | 5.77 | 6.52 | 5.81 | 5.81 |
| 168 | 6.47 | 5.84 | 6.45 | 5.86 | 6.44 | 5.76 | 5.77 |
| 212 | ND | ND | ND | 6.38 | 6.57 | 5.59 | 5.59 |
| 238 | 6.60 | 5.56 | 6.52 | 6.22 | 6.48 | 5.79 | 5.50 |
| 284 | ND | ND | ND | 6.30 | 6.49 | 5.45 | 5.45 |
ND, no data.
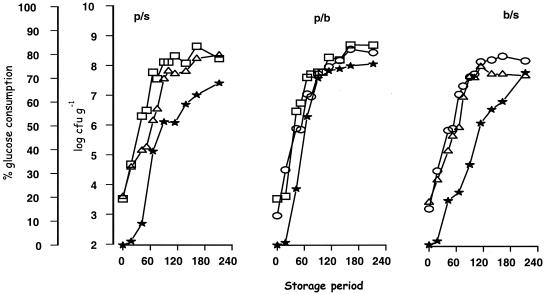
FIG. 2.
Growth curves of microorganisms in all possible combinations and changes in glucose concentration (★). (A) MPS. Symbols: □, Pseudomonas sp. strains; ▵, S. putrefaciens strains. (B) MPB. Symbols: □, Pseudomonas sp. strains; ○, B. thermosphacta strains. (C) MBS. Symbols: ○, B. thermosphacta strains; ▵, S. putrefaciens strains.
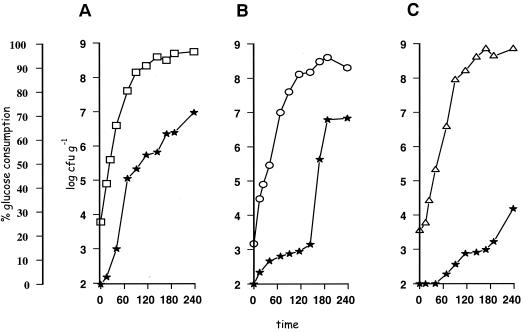
When we examined the physicochemical changes of the samples, we found that glucose was used by all of the tested bacteria (Fig. 1B). In particular, the decrease of glucose occurred mainly during the exponential growth of bacteria in control cultures, and the rate of glucose utilization was in the following order of magnitude: Pseudomonas sp. > B. thermosphacta > S. putrefaciens (Fig. 1). The latter result was not observed in the mixed cultures, in which the rates of glucose utilization were similar, and occurred in the early exponential phase (results not shown).
FIG. 1.
Growth curves of control cultures of Pseudomonas sp. strains (A), B. thermosphacta strains (B), and S. putrefaciens strains (C) and changes in glucose concentration (★).
The purity of the peaks of the eluted organic acids during storage was evaluated based on their RT and their UV spectra (results not shown). Of these, only three peaks were identified as lactic acid (RT = 10.30), formic acid (RT = 11.20), and acetic acid (RT = 12.40). It should be noted that two peaks (peak A, RT = 15.2; peak B, RT = 17.01), although found to contribute significantly in the organic acids profile, were not identifiable. In general, the profile of microbial metabolites as determined by HPLC analyses varied significantly among the different cases tested here. In particular, formic acid and peaks A and B were observed only in cultures in which Shewanella species were present, whereas the increase of the areas of these compounds was more pronounced in the single culture than in the mixed cultures (Table 3). Lactic acid was detected in all cultures of B. thermosphacta. The increase of this compound was evident by the end of the storage in the control culture. However, in all of the cocultures with this bacterium the increase of lactic acid was followed by a decrease (Table 3).
TABLE 3.
Changes in the areas under lactic, formic, and acetic acid peaks and unknown peaks with RT = 15.3 (peak A) and RT = 17.0 (peak B) in single cultures of Pseudomonas sp. (CP), B. thermosphacta (CB), and S. putrefaciens (CS) and their mixed cultures MPB, MPS, MBS, and MPBS in gel cassette systems at 5°C
| Cassette(s) | Area under peaka at time (h):
|
||||||
|---|---|---|---|---|---|---|---|
| 0 | 40 | 68 | 114 | 168 | 238 | 284 | |
| Lactic acid (RT = 10.4) | |||||||
| CP | ND | ND | ND | ND | ND | ND | NA |
| CB | ND | ND | ND | 0.024 | 0.060 | 0.087 | NA |
| CS | ND | ND | ND | ND | ND | ND | NA |
| MPB | ND | ND | ND | 0.078 | 0.088 | 0.049 | 0.035 |
| MPS | ND | ND | ND | ND | ND | ND | ND |
| MBS | ND | ND | TR | 0.076 | 0.135 | 0.182 | 0.122 |
| MPBS | ND | TR | TR | 0.074 | 0.116 | 0.191 | 0.083 |
| Formic acid (RT = 11.3) | |||||||
| CP | 0.100 | 0.082 | 0.065 | 0.030 | ND | ND | NA |
| CB | 0.100 | 0.080 | 0.072 | 0.058 | 0.056 | 0.055 | NA |
| CS | 0.100 | 0.090 | 0.190 | 1.017 | 0.888 | 0.703 | NA |
| MPB | 0.100 | 0.069 | 0.070 | 0.063 | 0.050 | 0.040 | TR |
| MPS | 0.100 | 0.065 | 0.073 | 0.040 | 0.062 | 0.058 | 0.051 |
| MBS | 0.100 | 0.020 | 0.020 | 0.059 | 0.066 | 0.122 | 0.027 |
| MPBS | 0.100 | 0.063 | 0.066 | 0.121 | 0.150 | 0.265 | 0.438 |
| Acetic acid (RT = 12.4) | |||||||
| CP | ND | ND | ND | ND | ND | ND | NA |
| CB | ND | TR | TR | 0.020 | 0.040 | 0.049 | NA |
| CS | ND | ND | TR | TR | 0.045 | 0.060 | NA |
| MPB | ND | TR | TR | TR | TR | TR | 0.020 |
| MPS | ND | TR | TR | 0.035 | 0.043 | 0.054 | 0.063 |
| MBS | ND | TR | TR | 0.032 | 0.045 | 0.060 | 0.060 |
| MPBS | ND | TR | TR | 0.030 | 0.043 | 0.049 | 0.056 |
| Peak A (RT = 15.2) | |||||||
| CP | 0.101 | 0.105 | 0.050 | 0.040 | TR | TR | NA |
| CB | 0.101 | 0.120 | 0.115 | 0.080 | 0.060 | 0.030 | NA |
| CS | 0.101 | 0.100 | 0.113 | 0.480 | 1.567 | 1.850 | NA |
| MPB | 0.101 | 0.121 | 0.090 | 0.030 | TR | TR | TR |
| MPS | 0.101 | 0.106 | 0.090 | 0.143 | 1.051 | 1.400 | 1.123 |
| MBS | 0.101 | 0.133 | 0.129 | 0.100 | 0.170 | 0.210 | 0.192 |
| MPBS | 0.101 | 0.134 | 0.094 | 0.091 | 0.125 | 0.171 | 0.994 |
| Peak B (RT = 17) | |||||||
| CP | ND | ND | ND | ND | ND | ND | NA |
| CB | ND | ND | ND | ND | ND | ND | NA |
| CS | ND | ND | TR | 0.302 | 0.580 | 0.674 | NA |
| MPB | ND | ND | ND | ND | ND | ND | ND |
| MPS | ND | ND | ND | 0.246 | 0.211 | 0.272 | 0.249 |
| MBS | ND | ND | TR | 0.180 | 0.228 | 0.271 | 0.182 |
| MPBS | ND | ND | TR | 0.244 | 0.247 | 0.227 | 0.315 |
Each sample value is the mean of two samples taken from different experiments (coefficient of variation of the mean of samples taken from different experiments of <5%). Each sample was analyzed in duplicate (coefficient of variation of samples from the same experiment of <0.65%). ND, not detected; NA, not analyzed; TR, trace amounts.
DISCUSSION
Numerous attempts have been made over the last two decades to associate given metabolites with the degree of food spoilage (15, 21). To identify an effective metabolite (indicator) for reliable monitoring quality and safety for control purposes, factors such as dynamic storage (fluctuation of temperature, packaging [vacuum packaging or modified atmosphere packaging], film permeability, etc.) and implicit factors (e.g., microbial competition) should be taken into account. Indeed, by understanding where specific metabolites originate from (i.e., responsible organism or substrate) and the effects of food characteristics on the rate and type of metabolite formation, we will be able to determine when and how to exploit them for the benefit of industry, government, and the consumer.The identification of the ideal metabolite that can be used for spoilage assessment has proved a difficult task for the following reasons (22): the compound must (i) be absent or at least present at only low levels in food, (ii) increase during storage, and (iii) be produced by the dominant flora, and (iv) show good correlation with sensory evaluation.
However, the rate of microbial metabolite production and the metabolic pathways of particular microbial association that will inevitably spoil food are affected by the prevailing intrinsic, extrinsic, and implicit parameters. An implicit parameter can be considered the result of a microbial association or of a single microorganism that has a positive, negative, or neutral response with other microorganism(s) present in the same food (17, 21).
There are a number of examples related to positive responses (synergistic or syntrophic) of bacteria in nature that have been reported (9). In food, this has been evident mainly with the transformation of a substratum into edible food (e.g., yogurt, sausages, olives, etc.). Similar observations have been reported with the ecophysiological characteristics of certain pathogenic bacteria. For example, the growth of Listeria monocytogenes was enhanced from the presence of pseudomonads, possibly due to increase of the available nutrients arising from their hydrolytic activity (16, 26, 27, 34, 35, 37). On the other hand, competition for nutrients (e.g., under excess, limitation, or starvation) or oxygen or hydrogen sources (in aerobic or anaerobic ecosystems, respectively) and the production of substances (i.e., bacteriocins, acids, or volatile compounds) that can restrict growth can be considered a negative response (antagonistic or competitive interaction) of synergisms (11, 29).
The contribution of nutrients to either “positive” or “negative” responses was also evident in the present study. This would be the case with glucose, which is the principal and simplest nutrient carbon source in muscle food (23, 28). In singly cultured bacteria, this compound is metabolized more rapidly with the obligate aerobe strains of pseudomonads compared to the facultative anaerobic strains of B. thermosphacta and the oxidative (mostly negative) strains of S. putrefaciens (Fig. 1). Although the coculture of pseudomonads with the above-mentioned bacteria did not affect the growth rate (Table 2), an acceleration of glucose consumption was evident (Fig. 2). A higher specific activity for glucose in pseudomonads versus other spoilage microorganisms of muscle foods is well documented in the literature (23, 28). Indeed, the ability of Pseudomonas species to transform glucose rapidly to gluconate has been considered a competitive advantage for the success of these bacteria (28). In the present study, the data is not sufficient to support the concept of competition for utilization of this compound by the cocultured pseudomonads and B. thermosphacta. However, since the growth rate of B. thermosphacta was higher in coculture samples with pseudomonads than in singly cultured samples, it can be said that the latter bacterium can play a syntrophic role for the former. This observation is important since B. thermosphacta has a much greater spoilage potential than lactobacilli and can be important in both aerobic and anaerobic spoilage of muscle foods. This organism utilizes glucose and glutamate but not any other amino acid during aerobic incubation (19). It produces a mixture of end products, including acetoin, acetic acid, iso-butyric acid, and iso-valeric acid, 2,3-butanediol, diacetyl, 3-methylbutanal, 2-methylpropanol, and 3-methylbutanol, during its aerobic metabolism in medium containing glucose, ribose, or glycerol as the main carbon and energy source (2, 7). The precise proportions of these end products is affected by the glucose concentration, pH, and temperature (28, 29).
A negative response (antagonistic) can be regarded also as a factor governing the selection of spoilage flora, particularly of fish. This is the case with pseudomonads and S. putrefaciens. It is well established in the literature that the inhibitory effect of the former bacterium on the latter is attributed to the ability of the Pseudomonas sp. to produce siderophores (20). This inhibition was also evident in the present study (Fig. 1 and 2 and Table 1). However, the type and rate of metabolic products in cocultured samples of this organism with B. thermosphacta suggested that other responses (negative or positive) might also account for this inhibition. For example, despite the extremely high levels of formic acid formation and the unknown peaks a and b, evident in singly cultured samples of S. putrefaciens (Table 3), the corresponding levels were significantly lower in samples in which this orgamism was cocultured with pseudomonads or B. thermosphacta.The lower production of these metabolic products was found in the latter case. In our opinion, competition for glucose may also be a key factor for this negative response.
Although some bibliographic data support this conclusion (13, 29), further studies that replace the nutrient broth with muscle juice and use inoculation with specific spoilage organisms in various combinations are needed to elucidate the effect of parameters such as structure and nutrients on the spoilage of muscle food.
REFERENCES
- 1.Baranyi, J., T. A. Roberts, and P. McClure. 1993. A non-autonomous differential equation to model bacterial growth. Food Microbiol. 10:43-59. [Google Scholar]
- 2.Blickstad, E., and G. Molin. 1984. Growth and end-product formation in fermenter cultures of Brochothrix thermosphacta ATCC 11509T and two psychrotrophic Lactobacillus spp. in different gaseous atmospheres. J. Appl. Bacteriol. 57:213-220. [DOI] [PubMed] [Google Scholar]
- 3.Brocklehurst, T. F., G. A. Mitchell, W. A. Pleass, and A. C. Smith. 1995. The effect of step changes in sucrose concentration on the growth of Salmonella typhimurium LT2. J. Appl. Bacteriol. 78:495-500. [DOI] [PubMed] [Google Scholar]
- 4.Brocklehurst, T. F., G. A. Mitchell, Y. P. Ridge, R. Seale, and A. C. Smith. 1995. The effect of transient temperatures on the growth of Salmonella typhimurium LT2 in gelatin gel. Int. J. Food Microbiol. 27:45-60. [DOI] [PubMed] [Google Scholar]
- 5.Brocklehurst, T. F., G. A. Mitchell, and A. C. Smith. 1997. A model experimental gel surface for the growth of bacteria on foods. Food Microbiol. 14:303-311. [Google Scholar]
- 6.Dainty, R. H. 1996. Chemical/biochemical detection of spoilage. Int. J. Food Microbiol. 33:19-33. [DOI] [PubMed] [Google Scholar]
- 7.Dainty, R. H., and C. M. Hibbard. 1983. Precursors of the major end products of aerobic metabolism of Brochothrix thermosphacta. J. Appl. Bacteriol. 54:127-133. [DOI] [PubMed] [Google Scholar]
- 8.Dalgaard, P. 2002. Modelling and predicting the shelf-life of seafood p. 191-219. In H. A. Bremner (ed.), Safety and quality issues in fish processing. Woodhead Publishing, Cambridge, United Kingdom.
- 9.Dolfing, J., and J. C. Gottschal. 1996. Microbe-microbe interactions, p. 372-433. In R. I. Mackie, B. A. White, and E. R. E. Isaacson (ed.), Gastrointestinal microbiology, vol. 2. Gastrointestinal microbes and host interaction. Chapman & Hall, New York, N.Y.
- 10.Drosinos, E. H., and R. G. Board. 1994. Metabolic activities of pseudomonads in batch cultures in extract of minced lamb. J. Appl. Bacteriol. 77:613-620. [DOI] [PubMed] [Google Scholar]
- 11.Drosinos, E. H., K. Lambropoulou, E. Mitre, and G.-J. E. Nychas. 1997. Attributes of fresh gilt Seabream (Sparus aurata) fillets treated with potassium sorbate, sodium gluconate and stored under a modified atmosphere at 0 ± 1°C. J. Appl. Microbiol. 83:569-575. [Google Scholar]
- 12.Drosinos, E. H., and G.-J. E Nychas. 1996. Brochothrix thermosphacta, the climax micro-organism on Greek fish tsipoura (Sparus aurata) and gopa (Boops boops) stored under a modified atmosphere at 0-4°C Ital. J. Food Sci. Technol. 4:323-330. [Google Scholar]
- 13.Drosinos, E. H., and G.-J. E. Nychas. 1997. Production of acetate and lactate in relation to glucose content during modified atmosphere storage of gilt-head seabream (Sparus aurata) at 0 ± 1oC. Food Res. Int. 30:711-717. [Google Scholar]
- 14.Drosinos, E. H., C. Tassou, K. Kakiomenou, and G.-J. E. Nychas. 2000. Microbiological, physico-chemical and organoleptic attributes of a country tomato salad and fate of Salmonella enteritidis during storage under aerobic or modified atmosphere packaging conditions at 4°C and 10°C. Food Control 11:131-135. [Google Scholar]
- 15.Ellis, D. I., and R. Goodacre. 2001. Rapid and quantitative detection of the microbial spoilage of muscle foods: current status and future trends. Trends Food Sci. Technol. 12:414-424. [Google Scholar]
- 16.Farrag, S. A., and E. H. Marth. 1989. Growth of Listeria monocytogenes in the presence of Pseudomonas fluorescens at 7 or 13°C in skim milk. J. Food Prot. 52:852-855. [DOI] [PubMed] [Google Scholar]
- 17.Fredrickson, A. G. 1977. Behavior of mixed cultures of microorganisms. Annu. Rev. Microbiol. 31:63-87. [DOI] [PubMed] [Google Scholar]
- 18.Gardner, G. A. 1966. A selective medium for the enumeration of Microbacterioum thermosphactum in meat and meat products. J. Appl. Bacteriol. 29:455-460. [DOI] [PubMed] [Google Scholar]
- 19.Gill, C. O., and K. G. Newton. 1977. The development of aerobic spoilage on meat stored at chill temperatures. J. Appl. Bacteriol. 43:189-195. [DOI] [PubMed] [Google Scholar]
- 20.Gram, L., and J. Melchiorsen. 1996. Interaction between fish spoilage bacteria Psudomonas sp. and S. putrefaciens in fish extracts and on fish tissue. J. Appl. Bacteriol. 80:589-595. [DOI] [PubMed] [Google Scholar]
- 21.Huis in't Veld, J. H. J. 1996. Microbial and biochemical spoilage of foods: an overview. Int. J. Food Microbiol. 33:1-18. [DOI] [PubMed] [Google Scholar]
- 22.Jay, J. M. 1986. Microbial spoilage indicators and metabolites, p. 219-240. In M. D. Pierson and N. J. Sterm (ed.), Food-borne microorganisms and their toxins: developing methodology. Marcel Dekker, Inc., New York, N.Y.
- 23.Koutsoumanis, K., and G.-J. E. Nychas. 1999. Chemical and sensory changes associated with microbial flora of Mediterranean boque (Boops boops) stored aerobically at 0, 3, 7 and 10°C. Appl. Environ. Microbiol. 65:698-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koutsoumanis, K., P. Taoukis, E. H. Drosinos, and G.-J. E. Nychas. 2000. Applicability of an Arrhenius model for the combined effect of temperature and CO2 packaging on the spoilage microflora of fish. Appl. Environ. Microbiol. 66:3528-3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lambropoulou, K. A., E. H. Drosinos, and G.-J. E. Nychas. 1996. The effect of glucose supplementation on the spoilage microflora and chemical composition of minced beef stored aerobically or under a modified atmosphere at 4°C. Int. J. Food Microbiol. 30:281-291. [DOI] [PubMed] [Google Scholar]
- 26.Marshall, D. L., L. S. Andrews, J. H. Wells, and A. J. Farr. 1992. Influence of modified atmosphere packaging on the competitive growth of Listeria monocytogenes and Pseudomonas fluorescens on precooked chicken. Food Microbiol. 9:303-309. [Google Scholar]
- 27.Marshall, D. L., and R. H. Schmidt. 1991. Physiological evaluation of stimulated growth of Listeria monocytogenes by Pseudomonas species in milk. Can. J. Microbiol. 37:594-599. [DOI] [PubMed] [Google Scholar]
- 28.Nychas, G.-J. E., E. Drosinos, and R. G. Board. 1998. Chemical changes in stored meat, p. 288-326. In R. G. Board and A. R. Davies (ed.), The microbiology of meat and poultry. Blackie Academic and Professional, London, United Kingdom.
- 29.Pin, C., D. García de Fernando, J. Gonzalo, and A. Ordóñez. 2002. Effect of modified atmosphere composition on the metabolism of glucose by Brochothrix thermosphacta. Appl. Environ. Microbiol. 68:4441-4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Skandamis, P. N., and G.-J. E. Nychas. 2002. Preservation of fresh meat with active and modified atmosphere packaging conditions. Int. J. Food Microbiol. 79:35-45. [DOI] [PubMed] [Google Scholar]
- 31.Tassou, C. C., and I. S. Boziaris. 2002. Survival of Salmonella enteritidis and physicochemical changes in grated carrots inoculated or not with Lactobacillus sp. and stored under atmospheres at 4°C. J. Sci. Food Agric. 82:1122-1127. [Google Scholar]
- 32.Tryfinopoulou, P., E. H. Drosinos, and G.-J. E. Nychas. 2001. Performance of Pseudomonas CFC-selective medium in the fish storage ecosystems. J. Microbiol. Methods 47:243-247. [DOI] [PubMed] [Google Scholar]
- 33.Tryfinopoulou, P., E. Tsakalidou, and G.-J. E. Nychas. 2002. Characterization of Pseudomonas spp. associated with spoilage of gilt-head sea-bream fish stored under various storage conditions. Appl. Environ. Microbiol. 68:5-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsigarida, E., and G.-J. E. Nychas. 2001. Ecophysiological attributes of a Lactobacillus sp. and a Pseudomonas sp. on sterile beef fillets in relation to storage temperature and film permeability. J. Appl. Microbiol. 90:696-705. [DOI] [PubMed] [Google Scholar]
- 35.Tsigarida, E., P. Skandamis, and G.-J. E. Nychas. 2000. Behaviour of Listeria monocytogenes and autochthonous flora on meat stored under aerobic, vacuum and modified atmosphere packaging conditions with or without the presence of oregano essential oil at 5°C. J. Appl. Microbiol. 89:901-909. [DOI] [PubMed] [Google Scholar]
- 36.Wilson, P. D. G., T. F. Brocklehurst, S. Arino, D. Thuault, M. Jakobsen, M. Lange, J. Farkas, J. W. T. Wimpenny, and J. F. Van Impe. 2002. Modelling microbial growth in structured foods: toward a unique approach. Int. J. Food Microbiol. 73:275-289. [DOI] [PubMed] [Google Scholar]
- 37.Worm, J., L. E. Jensen, S. T. Hansen, M. Søndergaard, and O. Nybroe. 2000. Interactions between proteolytic and non-proteolytic Pseudomonas fluorescens affect protein degradation in a model community. FEMS Microbiol. Ecol. 32:103-109. [DOI] [PubMed] [Google Scholar]