anti-1-amino-3-fluorine 18-fluorocyclobutane-1-carboxylic acid PET/CT is more sensitive than 111In–capromab pendetide SPECT/CT in the detection of recurrent prostate carcinoma in the prostatic bed and extraprostatic sites.
Abstract
Purpose:
To compare the diagnostic performance of the synthetic amino acid analog radiotracer anti-1-amino-3-fluorine 18-fluorocyclobutane-1-carboxylic acid (anti-3-18F-FACBC) with that of indium 111 (111In)–capromab pendetide in the detection of recurrent prostate carcinoma.
Materials and Methods:
This prospective study was approved by the institutional review board and complied with HIPAA guidelines. Written informed consent was obtained. Fifty patients (mean age, 68.3 years ± 8.1 [standard deviation]; age range, 50–90 years) were included in the study on the basis of the following criteria: (a) Recurrence of prostate carcinoma was suspected after definitive therapy for localized disease, (b) bone scans were negative, and (c) anti-3-18F-FACBC positron emission tomography (PET)/computed tomography (CT) and 111In–capromab pendetide single photon emission computed tomography (SPECT)/CT were performed within 6 weeks of each other. Studies were evaluated by two experienced interpreters for abnormal uptake suspicious for recurrent disease in the prostate bed and extraprostatic locations. The reference standard was a combination of tissue correlation, imaging, laboratory, and clinical data. Diagnostic performance measures were calculated and tests of the statistical significance of differences determined by using the McNemar χ2 test as well as approximate tests based on the difference between two proportions.
Results:
For disease detection in the prostate bed, anti-3-18F-FACBC had a sensitivity of 89% (32 of 36 patients; 95% confidence interval [CI]: 74%, 97%), specificity of 67% (eight of 12 patients; 95% CI: 35%, 90%), and accuracy of 83% (40 of 48 patients; 95% CI: 70%, 93%). 111In–capromab pendetide had a sensitivity of 69% (25 of 36 patients; 95% CI: 52%, 84%), specificity of 58% (seven of 12 patients; 95% CI: 28%, 85%), and accuracy of 67% (32 of 48 patients; 95% CI: 52%, 80%). In the detection of extraprostatic recurrence, anti-3-18F-FACBC had a sensitivity of 100% (10 of 10 patients; 95% CI: 69%, 100%), specificity of 100% (seven of seven patients; 95% CI: 59%, 100%), and accuracy of 100% (17 of 17 patients; 95% CI: 80%, 100%). 111In–capromab pendetide had a sensitivity of 10% (one of 10 patients; 95% CI: 0%, 45%), specificity of 100% (seven of seven patients; 95% CI: 59%, 100%), and accuracy of 47% (eight of 17 patients; 95% CI: 23%, 72%).
Conclusion:
anti-3-18F-FACBC PET/CT was more sensitive than 111In–capromab pendetide SPECT/CT in the detection of recurrent prostate carcinoma and is highly accurate in the differentiation of prostatic from extraprostatic disease.
© RSNA, 2011
Supplemental material: http://radiology.rsna.org/lookup/suppl/doi:10.1148/radiol.11102023/-/DC1
Introduction
Prostate carcinoma is the second leading cause of death in men in the United States, with 32 050 estimated deaths in 2010 (1). The presence of an elevated prostate-specific antigen (PSA) level after definitive therapy for prostate cancer is suggestive of recurrence. Although approximately 30% of patients will experience recurrence of elevated PSA levels, those with biochemical failure may not manifest clinical disease (2–4).
Recurrence can occur within the prostate bed, in extraprostatic locations, or both. The restaging of patients with stage D0 disease (biochemical recurrence after definitive therapy) is crucial because the differentiation of prostatic bed from extraprostatic recurrence is vital for tailoring subsequent therapy, especially the use of salvage techniques. This differentiation cannot be made with PSA values alone, although a faster PSA doubling time may be indicative of a higher risk for distant disease (5,6).
Typically, restaging is performed with a combination of ultrasonography (US)–guided transrectal biopsy, bone scanning, computed tomography (CT), and magnetic resonance (MR) imaging (7–11). Molecular imaging with indium 111 (111In)–capromab pendetide (ProstaScint; EUSA Pharma, Langhorne, Pa) is also used to restage recurrent prostate cancer (9,11–14). However, accurate determination of the extent and location of recurrent disease with existing imaging techniques has proved challenging (14,15).
anti-1-amino-3-fluorine 18-fluorocyclobutane-1-carboxylic acid (anti-3-18F-FACBC) is a synthetic amino acid analog that has demonstrated promise in a pilot study for the staging and restaging of prostate carcinoma (16). The uptake of anti-3-18F-FACBC is likely mediated through one amino acid transport protein or a combination of amino acid transport proteins, and the radiotracer is not metabolized (17,18). Normal biodistribution of anti-3-18F-FACBC includes relatively intense uptake in the liver and pancreas and little renal excretion or brain uptake compared with 18F fluorodeoxyglucose (FDG) (19). In this study, we set out to compare the diagnostic performance of the synthetic amino acid analog radiotracer anti-3-18F-FACBC with that of 111In–capromab pendetide in the detection of recurrent prostate carcinoma.
Materials and Methods
One author (M.M.G.) and Emory University are eligible to receive royalties from the radiotracer being studied. The other authors had control of the data that might be a conflict for the author with a potential conflict of interest.
Preparation of anti-3-18F-FACBC
The preparation of anti-3-18F-FACBC has been previously reported (20). The decay-corrected radiochemical yield of the desired product was 24%, and its radiochemical purity was 99% 80 minutes after the end of bombardment. The mass of amino acids, predominantly anti-1-amino-3-hydroxycyclobutane-1-carboxylic acid, in the production batch was approximately 1.5 mg or 9.0 μmol, and the specific activity was 580–820 MBq/μmol based on anti-1-amino-3-hydroxycyclobutane-1-carboxylic acid at the end of synthesis.
Patient Selection
This prospective study was approved by the institutional review board and complied with Health Insurance Portability and Accountability Act guidelines. Written informed consent was obtained. Studies were performed between December 12, 2007, and June 17, 2010. All patients were evaluated at Emory Health Care. No adverse events were reported. Patients were included in this study if the following criteria were met: (a) Patients were originally diagnosed with localized (stage T1c, T2, or T3) prostate carcinoma and had undergone definitive therapy for localized disease; (b) recurrent prostate carcinoma was suspected on the basis of previous American Society for Radiology and Oncology (ASTRO) criteria of three consecutive increases in PSA level, the more recent ASTRO-Phoenix criteria of an increase in PSA level of at least 2.0 ng/mL above the nadir level after radiation therapy or cryotherapy, and/or an absolute PSA level of 0.3 mg/mL or greater after prostatectomy; (c) bone scans were negative for metastatic disease; and (d) 111In–capromab pendetide and anti-3-18F-FACBC studies were performed within 6 weeks of each other.
anti-3-18F-FACBC Imaging Protocol
Scanning was conducted by using a positron emission tomography (PET)/CT unit (Discovery DLS; GE Medical Systems, Milwaukee, Wis), and scans were interpreted on a workstation with use of software (MIMvista 4.2; MIMvista, Cleveland, Ohio). All patients fasted for 4–6 hours before undergoing scanning with anti-3-18F-FACBC.
After patients underwent CT of the abdomen and pelvis (80–120 mA, 120 kVp) with oral contrast material and without intravenous contrast material, anti-3-18F-FACBC (199.8–484.7 MBq) was injected intravenously over 2 minutes. After a 3-minute delay for blood pool clearance, PET was performed with three contiguous acquisitions (4 minutes per frame) starting from the pelvis below the prostate and extending superiorly to include the abdomen above the kidneys. This process was repeated twice. Thus, 5–16-minute (early), 17–28-minute (delayed 1), and 29–40-minute (delayed 2) acquisitions were performed. Images above the diaphragm were not acquired.
111In–Capromab Pendetide Imaging Protocol
Whole-body planar and abdominopelvic SPECT/CT examinations were performed 4 days after injection of 185-MBq 111In–capromab pendetide. Patients underwent imaging without fasting by using a protocol similar to that used by Soddee and co-workers (21). Imaging was performed with one of two single photon emission computed tomography (SPECT)/CT systems (VG/Hawkeye [GE Healthcare, Waukesha, Wis] or Symbia T6 [Siemens, Hoffman Estates, Ill]) equipped with medium-energy all-purpose parallel-hole collimation with two photopeak settings of 171 and 245 keV. Whole-body planar images were acquired with a 1024 × 256 matrix from head to midthigh; this was followed by CT without oral or intravenous contrast material at 2.5 mA and 140 kVp (VG/Hawkeye) or 120 mA and 130 kVp (Symbia T6). SPECT was performed with a 128 × 128 matrix with no zoom and with either 120 60-second projections (VG/Hawkeye) or 60 100-second projections (Symbia T6); images were reconstructed with and without attenuation correction by using iterative reconstruction with appropriate postreconstruction filtering. Images were then viewed on a workstation (Xeleris, GE Medical Systems). One patient underwent 111In–capromab pendetide SPECT at another facility, and his images were fused with separately acquired CT scans by using software.
Image Analysis
One nuclear radiologist with 14 years of experience (D.M.S.) and one nuclear medicine physician with 25 years of experience (R.K.H.) assessed both the anti-3-18F-FACBC and 111In–capromab pendetide studies. The investigators had access to the patient’s history but not to the results of recent imaging studies. The anti-3-18F-FACBC scans were interpreted individually by each reader, with disagreements to be resolved by consensus; however, there were no discrepancies. 111In–capromab pendetide scans were interpreted in a separate combined session by both readers; this reading session was performed at least 2 weeks later to minimize recall bias.
For anti-3-18F-FACBC scans, uptake in the focus was compared with that in background structures and classified as mild (higher than that of blood pool but less than that of marrow), moderate (higher than or equal to that of marrow but less than that of liver), or intense (equal to or higher than that of liver). Quantitative criteria (maximum standardized uptake value in the lesion/mean standardized uptake value in background) were used to aid the visual analysis. The maximum and mean standardized uptake values were recorded for each focus of abnormal uptake as well as for background structures including liver, marrow at L3, aorta, and bladder. An edge-seeking conformational volume of interest tool (PET Edge, MIMvista) was typically used. For prostate beds as well as extraprostatic sites (eg, lymph nodes and bone), abnormal moderate or intense focal uptake that was higher than that of background marrow and that persisted from early to delayed imaging was considered prospectively positive.
Well-established criteria were used to evaluate the 111In–capromab pendetide scans. Uptake was considered to be abnormal when there was activity or asymmetries of increased uptake compared to background expected biodistribution in normal adjacent organs or structures (22–24).
Reference Standard
The prostate bed in patients with either positive or negative imaging studies was typically investigated with transrectal US and biopsy as clinically appropriate. For patients who had previously undergone prostatectomy, biopsy was not performed if negative imaging studies were obtained at the surgical site and transrectal US did not show abnormal tissue in which to target.
Patients with abnormal foci in extraprostatic tissue at imaging were further investigated by using a combination of percutaneous imaging-guided needle biopsy, laparoscopic techniques, and open lymph node dissection. Because it is unusual for prostate carcinoma to metastasize to inguinal nodes, it was agreed that the inguinal regions would be evaluated with physical examination and percutaneous biopsy performed only if findings were suspicious for carcinoma.
A ground truth panel composed of a nuclear radiologist (D.M.S.), two urologists (P.T.N. and V.A.M., with 32 and 8 years of experience, respectively), and a radiation oncologist (P.J.R., with 5 years of experience) met at a regular conference and communicated via e-mail. Truth was ascertained by means of the criteria outlined below. If there were initial differences of opinion, further discussion ensued until a consensus was achieved.
The presence of disease in the prostate bed was confirmed by means of biopsy, with truth overridden only with use of clinical data. For example, a substantial reduction in PSA level after prostatic bed salvage therapy without a subsequent substantial increase in PSA level in 6 months would establish not only the presence of presumed prostatic bed disease but also the absence of extraprostatic disease. Extraprostatic nodal involvement per patient was confirmed by means of pathologic proof alone. Skeletal involvement was confirmed with either biopsy or a typical appearance on MR images (25). The absence of extraprostatic disease was confirmed with either a substantial reduction in PSA level after prostatic bed therapy without a substantial increase in 6 months as mentioned earlier and/or stable appearance on CT or MR images for more than 1 year without evidence of nodal or bone involvement. Because some patients had definitive follow-up results for prostate bed but not extraprostatic disease, and vice versa, the number of patients in each subanalysis differed.
Statistical Analyses
The statistical analyses provided measures of diagnostic performance (eg, sensitivity, specificity, negative predictive value, and positive predictive value) along with the associated confidence intervals (CIs). We determined the statistical significance of differences in sensitivity, specificity, and overall accuracy between anti-3-18F-FACBC and 111In–capromab pendetide by using the McNemar χ2 test to adjust for correlations in the accuracy measures. The significance of differences for other diagnostic performance measures (positive and negative predictive values) was assessed by using approximate tests based on the difference between two proportions. Statistical significance was determined by using a type I error rate of α = 0.05 for overall comparisons. Statistical analyses were performed by using MatLab software (version 7.10; MathWorks, Natick, Mass) and the R statistical package (R Foundation for Statistical Computing, Vienna, Austria, http://www.R-project.org).
Results
Demographics
Fifty patients met the inclusion criteria. The mean patient age (±standard deviation) was 68.3 years ± 8.1 (range, 50–90 years), and the mean PSA level was 6.62 ng/mL ± 7.63 (range, 0.11–44.74 ng/mL). PSA values were obtained within 16.8 days ± 37.4 of anti-3-18F-FACBC scanning. 111In–capromab pendetide studies were performed within 15.7 days ± 10.9 (range, 2–37 days) of anti-3-18F-FACBC scanning. Thirteen patients originally underwent radical prostatectomy, and 37 patients were treated with cryotherapy, high-frequency ultrasound, external beam radiation therapy, and/or brachytherapy. Forty-eight patients had definitive follow-up results for the prostate bed and 17 had definitive follow-up results for extraprostatic disease. Table E1 (online) lists individual patient data and results of imaging and follow-up.
Diagnostic Performance: Prostatic Bed
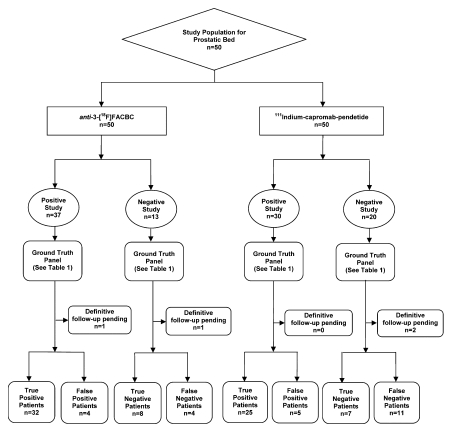
The diagnostic performance of anti-3-18F-FACBC in the prostate bed was better than that of 111In–capromab pendetide, with sensitivities of 89% (32 of 36 patients; 95% CI: 74%, 97%) and 69% (25 of 36 patients; 95% CI: 52%, 84%), respectively (P = .035). Figure 1 and Table 1 summarize data and reference standards applied for the prostatic bed. Table 2 includes a summary of diagnostic performances.
Figure 1:
Flow diagram of study patients and results obtained for disease in the prostatic bed with 111In–capromab pendetide and anti-3-18F-FACBC.
Table 1.
Reference Standards Applied to Prostate Bed Analysis
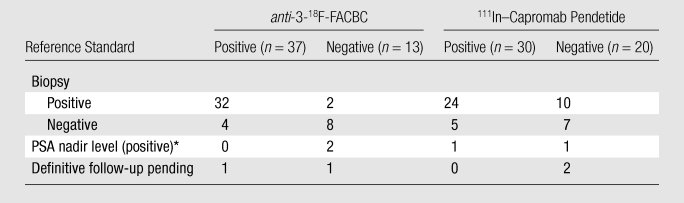
Note.—Data are numbers of patients.
Local disease was proved with PSA nadir level after local therapy only.
Table 2.
Diagnostic Performance of anti-3-18F-FACBC and 111In–Capromab Pendetide
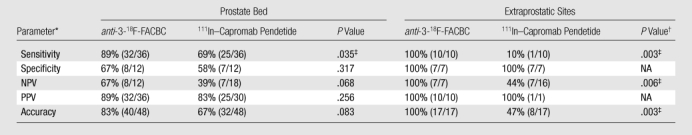
Note.—Data in parentheses are numbers of patients.
NPV = negative predictive value, PPV = positive predictive value.
NA = not applicable (sample size was too small).
Statistically significant (P ≤ .05).
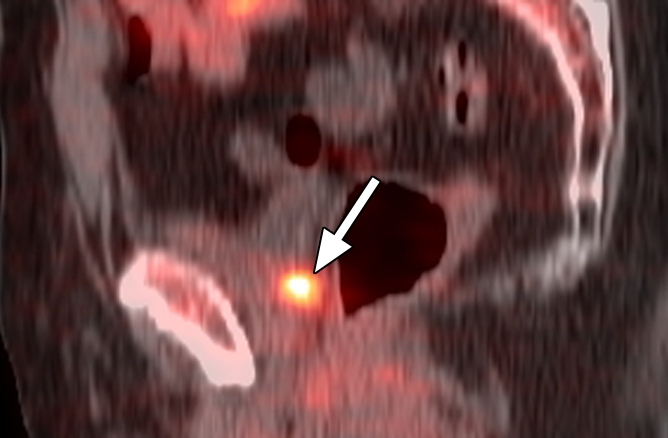
Four patients had false-positive findings in the prostate bed with anti-3-18F-FACBC; these patients also had false-positive findings with 111In–capromab pendetide. Three of these four patients originally underwent a nonradical prostatectomy treatment, and one patient underwent radical prostatectomy. Inflammation was not present in any biopsy sample. Benign glands and stroma were reported in two of the four patients, treatment-related changes were reported in one patient, and benign gland and stroma with fibromuscular tissue and a single lymphoid aggregate were reported in one patient. Four patients had false-negative findings with anti-3-18F-FACBC; three of these patients also had false-negative findings with 111In–capromab pendetide. Two of the four patients had undergone radical prostatectomy and two had undergone a nonradical prostatectomy treatment. Figure 2 shows an example of a patient with true-positive findings in the prostate bed with anti-3-18F-FACBC and false-negative findings with 111In-capromab-pendetide after radical prostatectomy. Figure E1 (online) is an example of true-positive uptake in the prostate bed with both modalities.
Figure 2a:

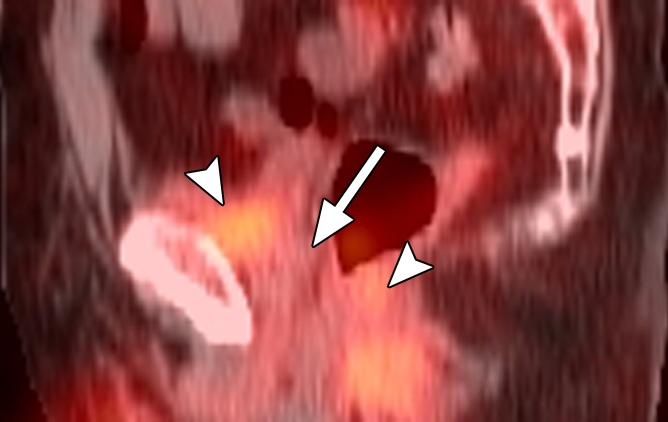
Images in a patient who had undergone radical prostatectomy (PSA level, 16.9 ng/mL). (a) Sagittal PET and (b) fused PET/CT scans obtained with anti-3-18F-FACBC. (c) SPECT and (d) fused SPECT/CT scans obtained with 111In–capromab pendetide. Images were fused to same CT scan. Intense uptake between bladder base and rectum on scans obtained with anti-3-18F-FACBC (arrow in a and b) corresponded to biopsy-proved recurrence. No uptake is seen in same region on images obtained with 111In–capromab pendetide (arrow in c and d). This is an example of a true-positive finding with anti-3-18F-FACBC and a false-negative finding with 111In–capromab pendetide. Note bladder and rectal activity on 111In–capromab pendetide images (arrowheads in c and d).
Figure 2b:
Images in a patient who had undergone radical prostatectomy (PSA level, 16.9 ng/mL). (a) Sagittal PET and (b) fused PET/CT scans obtained with anti-3-18F-FACBC. (c) SPECT and (d) fused SPECT/CT scans obtained with 111In–capromab pendetide. Images were fused to same CT scan. Intense uptake between bladder base and rectum on scans obtained with anti-3-18F-FACBC (arrow in a and b) corresponded to biopsy-proved recurrence. No uptake is seen in same region on images obtained with 111In–capromab pendetide (arrow in c and d). This is an example of a true-positive finding with anti-3-18F-FACBC and a false-negative finding with 111In–capromab pendetide. Note bladder and rectal activity on 111In–capromab pendetide images (arrowheads in c and d).
Figure 2c:
Images in a patient who had undergone radical prostatectomy (PSA level, 16.9 ng/mL). (a) Sagittal PET and (b) fused PET/CT scans obtained with anti-3-18F-FACBC. (c) SPECT and (d) fused SPECT/CT scans obtained with 111In–capromab pendetide. Images were fused to same CT scan. Intense uptake between bladder base and rectum on scans obtained with anti-3-18F-FACBC (arrow in a and b) corresponded to biopsy-proved recurrence. No uptake is seen in same region on images obtained with 111In–capromab pendetide (arrow in c and d). This is an example of a true-positive finding with anti-3-18F-FACBC and a false-negative finding with 111In–capromab pendetide. Note bladder and rectal activity on 111In–capromab pendetide images (arrowheads in c and d).
Figure 2d:
Images in a patient who had undergone radical prostatectomy (PSA level, 16.9 ng/mL). (a) Sagittal PET and (b) fused PET/CT scans obtained with anti-3-18F-FACBC. (c) SPECT and (d) fused SPECT/CT scans obtained with 111In–capromab pendetide. Images were fused to same CT scan. Intense uptake between bladder base and rectum on scans obtained with anti-3-18F-FACBC (arrow in a and b) corresponded to biopsy-proved recurrence. No uptake is seen in same region on images obtained with 111In–capromab pendetide (arrow in c and d). This is an example of a true-positive finding with anti-3-18F-FACBC and a false-negative finding with 111In–capromab pendetide. Note bladder and rectal activity on 111In–capromab pendetide images (arrowheads in c and d).
Diagnostic Performance: Extraprostatic Disease
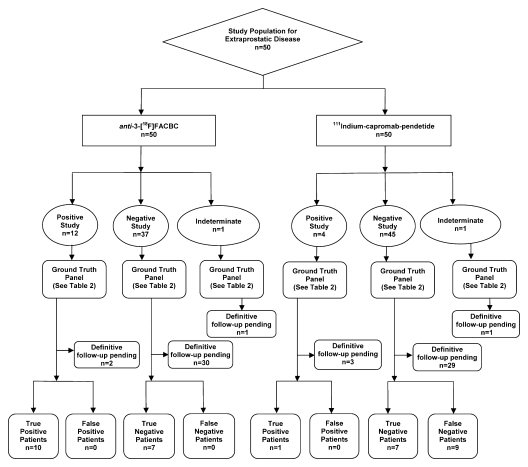
The diagnostic performance of anti-3-18F-FACBC in the detection of extraprostatic disease was better than that of 111In–capromab pendetide, with sensitivities of 100% (10 of 10 patients; 95% CI: 69%, 100%) and 10% (one of 10 patients; 95% CI: 0%, 45%), respectively (P = .003). Figure 3 and Table 3 summarize data and reference standards applied for extraprostatic disease. Table 2 includes a summary of diagnostic performances.
Figure 3:
Flow diagram of study patients and results obtained for extraprostatic disease with 111In–capromab pendetide and anti-3-18F-FACBC.
Table 3.
Reference Standards Applied to the Analysis of Extraprostatic Disease
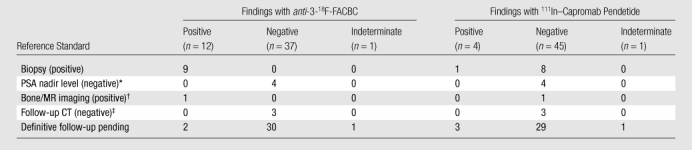
Note.—Data are numbers of patients.
Proved with PSA nadir level after local therapy only.
Characteristic imaging features of a skeletal lesion were seen at MR imaging.
The CT appearance of the abdomen and/or pelvis was stable for more than 1 year.
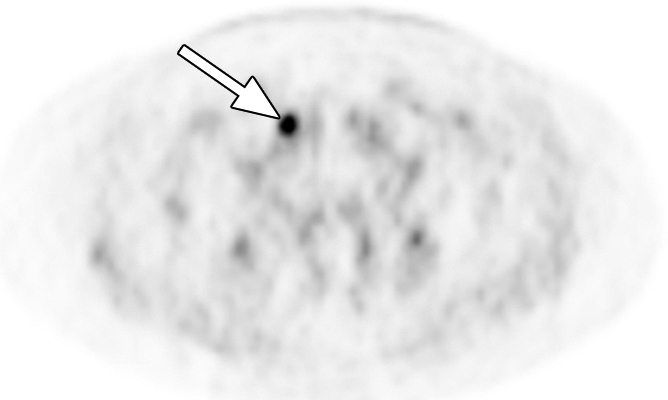
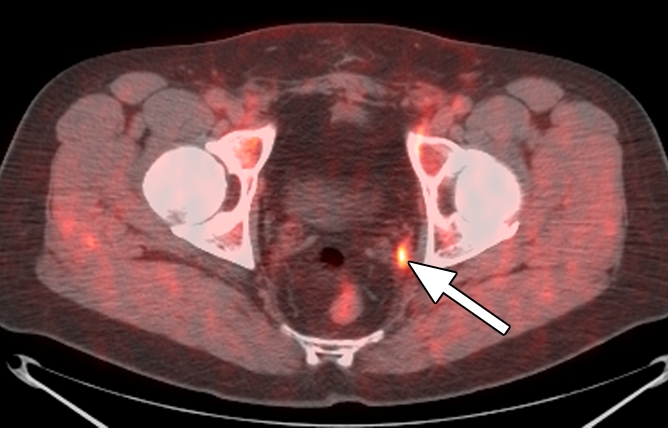
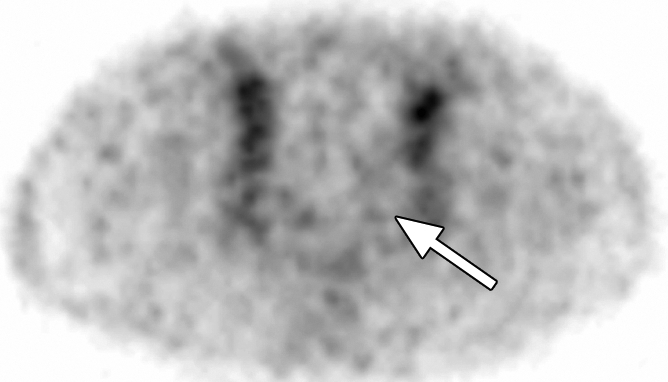
Extraprostatic recurrence was limited to lymph nodes in eight patients and to bone in one patient. One patient had both nodal and bone involvement. Figure 4 illustrates the detection of extraprostatic disease in a 5-mm node with anti-3-18F-FACBC PET/CT in a patient with a PSA level of 1.1 ng/mL. Figure 5 illustrates detection of a skeletal metastasis in a patient with a PSA level of 2.97 ng/mL. Both are examples of true-positive findings with anti-3-18F-FACBC and false-negative findings with 111In–capromab pendetide. Figures E2 and E3 (online) are more examples of the localization of extraprostatic disease with both modalities.
Figure 4a:
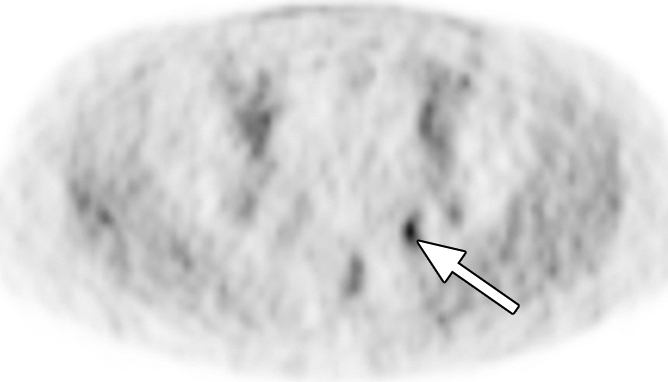
Images in a patient who had undergone radical prostatectomy (PSA level, 1.1 ng/mL). (a) Transverse PET and (b) fused PET/CT scans obtained with anti-3-18F-FACBC. (c) SPECT and (d) fused SPECT/CT scans obtained with 111In–capromab pendetide. Intense uptake on images obtained with anti-3-18F-FACBC (arrow in a and b) corresponded to a 5-mm recurrence in the left obturator lymph node, which was proved at biopsy. There is no uptake in same region on images obtained with 111In–capromab pendetide (arrow in c and d). This is an example of a true-positive finding with anti-3-18F-FACBC and a false-negative finding with 111In–capromab pendetide.
Figure 5a:
Images in a patient who had undergone radical prostatectomy (PSA level, 2.97 ng/mL). (a) Transverse PET and (b) fused PET/CT scans obtained with anti-3-18F-FACBC. (c) SPECT and (d) fused SPECT/CT scans obtained with 111In–capromab pendetide. Intense uptake on images obtained with anti-3-18F-FACBC (arrow in a and b) corresponded to a subtle lytic bone lesion in the right pubic ramus, which was proved as recurrence at biopsy. There is no uptake in same region on images obtained with 111In–capromab pendetide (arrow in c and d). This is an example of a true-positive finding with anti-3-18F-FACBC and a false-negative finding with 111In–capromab pendetide.
Figure 4b:
Images in a patient who had undergone radical prostatectomy (PSA level, 1.1 ng/mL). (a) Transverse PET and (b) fused PET/CT scans obtained with anti-3-18F-FACBC. (c) SPECT and (d) fused SPECT/CT scans obtained with 111In–capromab pendetide. Intense uptake on images obtained with anti-3-18F-FACBC (arrow in a and b) corresponded to a 5-mm recurrence in the left obturator lymph node, which was proved at biopsy. There is no uptake in same region on images obtained with 111In–capromab pendetide (arrow in c and d). This is an example of a true-positive finding with anti-3-18F-FACBC and a false-negative finding with 111In–capromab pendetide.
Figure 4c:
Images in a patient who had undergone radical prostatectomy (PSA level, 1.1 ng/mL). (a) Transverse PET and (b) fused PET/CT scans obtained with anti-3-18F-FACBC. (c) SPECT and (d) fused SPECT/CT scans obtained with 111In–capromab pendetide. Intense uptake on images obtained with anti-3-18F-FACBC (arrow in a and b) corresponded to a 5-mm recurrence in the left obturator lymph node, which was proved at biopsy. There is no uptake in same region on images obtained with 111In–capromab pendetide (arrow in c and d). This is an example of a true-positive finding with anti-3-18F-FACBC and a false-negative finding with 111In–capromab pendetide.
Figure 4d:
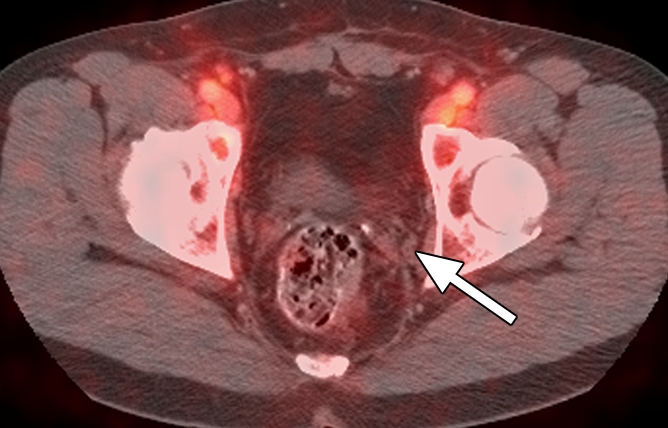
Images in a patient who had undergone radical prostatectomy (PSA level, 1.1 ng/mL). (a) Transverse PET and (b) fused PET/CT scans obtained with anti-3-18F-FACBC. (c) SPECT and (d) fused SPECT/CT scans obtained with 111In–capromab pendetide. Intense uptake on images obtained with anti-3-18F-FACBC (arrow in a and b) corresponded to a 5-mm recurrence in the left obturator lymph node, which was proved at biopsy. There is no uptake in same region on images obtained with 111In–capromab pendetide (arrow in c and d). This is an example of a true-positive finding with anti-3-18F-FACBC and a false-negative finding with 111In–capromab pendetide.
Figure 5b:
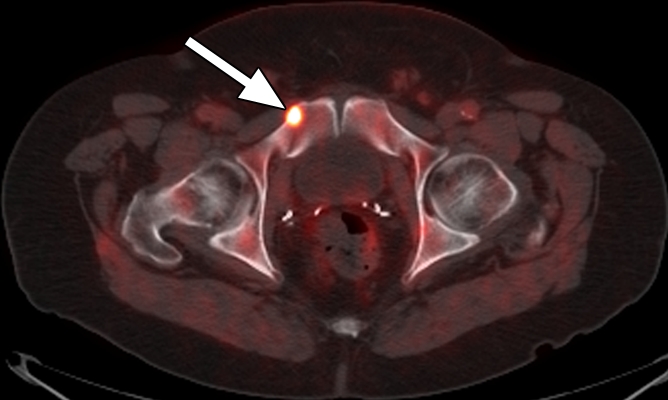
Images in a patient who had undergone radical prostatectomy (PSA level, 2.97 ng/mL). (a) Transverse PET and (b) fused PET/CT scans obtained with anti-3-18F-FACBC. (c) SPECT and (d) fused SPECT/CT scans obtained with 111In–capromab pendetide. Intense uptake on images obtained with anti-3-18F-FACBC (arrow in a and b) corresponded to a subtle lytic bone lesion in the right pubic ramus, which was proved as recurrence at biopsy. There is no uptake in same region on images obtained with 111In–capromab pendetide (arrow in c and d). This is an example of a true-positive finding with anti-3-18F-FACBC and a false-negative finding with 111In–capromab pendetide.
Figure 5c:

Images in a patient who had undergone radical prostatectomy (PSA level, 2.97 ng/mL). (a) Transverse PET and (b) fused PET/CT scans obtained with anti-3-18F-FACBC. (c) SPECT and (d) fused SPECT/CT scans obtained with 111In–capromab pendetide. Intense uptake on images obtained with anti-3-18F-FACBC (arrow in a and b) corresponded to a subtle lytic bone lesion in the right pubic ramus, which was proved as recurrence at biopsy. There is no uptake in same region on images obtained with 111In–capromab pendetide (arrow in c and d). This is an example of a true-positive finding with anti-3-18F-FACBC and a false-negative finding with 111In–capromab pendetide.
Figure 5d:
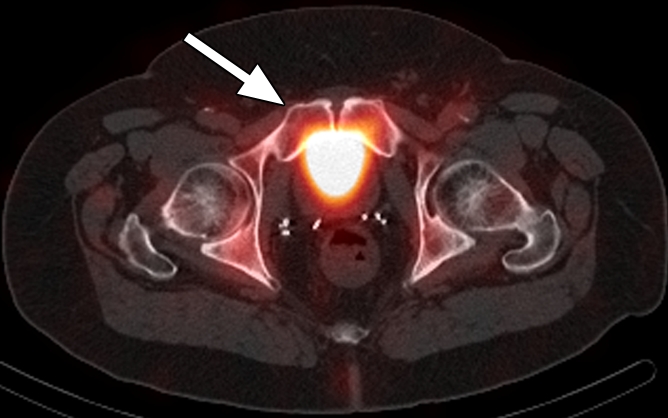
Images in a patient who had undergone radical prostatectomy (PSA level, 2.97 ng/mL). (a) Transverse PET and (b) fused PET/CT scans obtained with anti-3-18F-FACBC. (c) SPECT and (d) fused SPECT/CT scans obtained with 111In–capromab pendetide. Intense uptake on images obtained with anti-3-18F-FACBC (arrow in a and b) corresponded to a subtle lytic bone lesion in the right pubic ramus, which was proved as recurrence at biopsy. There is no uptake in same region on images obtained with 111In–capromab pendetide (arrow in c and d). This is an example of a true-positive finding with anti-3-18F-FACBC and a false-negative finding with 111In–capromab pendetide.
Discussion
We set out to determine the diagnostic performance of anti-3-18F-FACBC PET/CT in the detection of recurrent prostate carcinoma and to compare it with that of 111In–capromab pendetide SPECT/CT. We demonstrated that anti-3-18F-FACBC PET/CT enabled the detection of more recurrent disease than did 111In–capromab pendetide SPECT/CT.
Our findings are important because approximately one-third of patients will have biochemical evidence of recurrence and the therapeutic approach depends not only on confirming recurrence but, most important, determining whether recurrence is confined to the prostate bed or located at extraprostatic sites (2). The suspected location of distant disease may be evaluated with a combination of bone scanning, CT, and MR imaging. Each of these techniques has its limitations (9–11). A noninvasive method for guiding pathologic confirmation on a whole-body basis is important (14).
111In–capromab pendetide is a radiolabeled murine monoclonal antibody that binds to prostate-specific membrane antigen (26). Its sensitivity and specificity in the detection of recurrent prostate carcinoma was originally reported to be 62% and 72%, respectively (9). Yet, diagnostic performance in the subsequent literature varied widely, with sensitivities of 17%–92% and specificities of 47%–86%—likely reflecting differences in intraobserver variability and study population and design (9,11–14,27,28). Fusion with CT or MR images has been reported to improve accuracy (24,26,29). In our study, the sensitivity of 111In–capromab pendetide was 69% for detecting disease in the prostate bed and 10% for detecting disease in extraprostatic sites.
Seltzer and co-workers (13) also compared 111In–capromab pendetide images to biopsy-sampled nodes and reported that findings from 111In–capromab pendetide imaging were true positive in only one of six patients. The lower sensitivity of 111In–capromab pendetide is likely explained by several factors. The antibody in 111In–capromab pendetide helps detect the intracellular epitope of prostate-specific membrane antigen, which is problematic when the radiotracer is not internalized (23). In addition, the use of anti-3-18F-FACBC with PET results in images with higher spatial resolution than those obtained with SPECT, possibly contributing to the differences in detection between the two radiotracers in our study. Newly reported advanced SPECT reconstruction methods with 111In–capromab pendetide may improve accuracy (30,31).
CT is considered of low yield in the detection of recurrent local disease, with one study (32) reporting positive results in only 36% of cases, reflecting the difficulty in distinguishing recurrence from scar. Dynamic contrast material–enhanced MR imaging is used more frequently for the detection of local recurrence, with sensitivities of 70%–95% and specificities of 73%–100% depending on the type of previous therapy (33–36). Routine MR imaging and CT are limited in the detection of nodal involvement, with typical sensitivities in the lower range of 25%–78% and specificities of 66%–100% (26,37–41). MR imaging with superparamagnetic particles may be more promising for lymph node assessment, with a reported per-patient sensitivity of 80%–100% and specificity of 73%–95.7% (40–42).
FDG PET has little utility for normally slow-growing prostate carcinoma, with detection rates in the range of 31%–66% (43,44); better diagnostic performance was obtained for more aggressive disease (43). Interpretation of FDG PET scans of the pelvis is also hampered by renal excretion of FDG. PET/CT and iterative reconstruction techniques may result in improved accuracy.
Other PET and SPECT radiotracers are undergoing investigation (39,45–48). The most well studied of these radiotracers are carbon 11 (11C)–choline and 18F-choline, with published sensitivities of 36%–100% and specificities of 12.5%–100% (33,48–50). 11C-acetate has also been investigated, with sensitivities reported to be 75%–83% (48). 11C-methionine is an amino acid radiotracer that, in limited studies, demonstrated a sensitivity of 72% in the detection of metastatic prostate cancer (44) and an overall detection rate of 46.7% for primary carcinoma (51).
In this study, anti-3-18F-FACBC PET/CT demonstrated 89% sensitivity and 67% specificity in the detection of local recurrence in the prostate bed and 100% sensitivity and specificity in the detection of extraprostatic disease; these findings compare favorably to those with the above-mentioned techniques. Comparison on the basis of published studies alone, however, is problematic owing to differences in patient populations, study methodology, and reference standards used to establish truth. For example, diagnostic performance may be dependent on PSA level and scan method (eg, PET alone vs PET/CT).
We chose to make this comparison to conventional imaging with 111In–capromab pendetide because that is the current standard of care in our institution and many others. Direct comparisons of anti-3-18F-FACBC PET/CT to other promising imaging techniques such as choline PET/CT and/or advanced MR imaging, ideally with use of the patient as his or her own control in a multicenter environment, should be carried out before clinical recommendations can be made.
One limitation of a clinical study involving prostate carcinoma is the dilemma in establishing truth for absence of disease. PSA level in isolation is not an adequate end point and must be correlated in clinical context. Even an increase in PSA level after prostatectomy does not necessarily equate with clinical failure (2,4). Yet, prostate biopsies—especially those performed after therapy—are subject to sampling error and may underestimate true-positive disease (7,8,33,52,53). Most of our patients underwent nonradical prostatectomy procedures in which an increase in PSA level may be due to prostatitis or benign hypertrophy. A patient is more likely to receive salvage radiation therapy after radical prostatectomy with increasing PSA level and negative biopsy than after a nonradical prostatectomy technique in which biopsy proof is usually required at lower PSA levels before undergoing salvage surgery or cryotherapy.
There is no ideal method with which to establish truth in all circumstances, as reflected in the acceptable practice variations in the restaging of patients with prostate carcinoma (2). We chose to default to biopsy findings, which were overridden only with data obtained during the patient’s subsequent clinical and therapeutic course. If there was insufficient proof of the presence or absence of disease, the outcome was then considered indeterminate awaiting further follow-up. Thus, each patient was independently assessed by our multidisciplinary team in his unique circumstance, more closely mirroring clinical practice. In fact, it may take longer than the 1–2-year follow-up in this study for clinical disease to manifest (54,55). All patients are part of an ongoing clinical trial and will be followed up for a longer period of time.
Another potential limitation of this study is the fact that anti-3-18F-FACBC imaging was not carried out above the diaphragm. However, prostate skip metastases are rare, and there was no indication of such disease at either clinical examination or 111In–capromab pendetide imaging. Investigation of false-positive and false-negative findings in the prostate bed as well as diagnostic performance at different PSA levels will be the subject of future analysis.
In conclusion, anti-3-18F-FACBC PET/CT is more sensitive than 111In–capromab pendetide SPECT/CT in the detection of recurrent prostate carcinoma in the prostatic bed and in extraprostatic sites.
Advances in Knowledge.
Amino acid transport as characterized with anti-1-amino-3-fluorine 18-fluorocyclobutane-1-carboxylic acid (anti-3-18F-FACBC) PET/CT enabled detection of local recurrence with a sensitivity of 89% (95% confidence interval [CI]: 74%, 97%); conventional imaging with 111In–capromab pendetide SPECT/CT had a sensitivity of 69% (95% CI: 52%, 84%).
anti-3-18F-FACBC PET/CT helped detect extraprostatic recurrent disease with a sensitivity of 100% (95% CI: 69%, 100%); 111In–capromab pendetide SPECT/CT had a sensitivity of 10% (95% CI: 0%, 45%).
Implication for Patient Care.
anti-3-18F-FACBC PET/CT can be used to accurately restage prostate cancer.
Disclosures of Potential Conflicts of Interest: D.M.S. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: institution received a grant or grants are pending from Nihon Medi-Physics. Other relationships: none to disclose. B.S.B. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: none to disclose. Other relationships: none to disclose. P.T.N. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: institution received grants or grants are pending from Wilex. Other relationships: none to disclose. V.A.M. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: none to disclose. Other relationships: none to disclose. R.K.H. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: receives royalties from ELGEMS; institution receives royalties from ELGEMS. Other relationships: none to disclose. P.J.R. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: institution received grants or grants are pending from Nihon Medi-Physics. Other relationships: none to disclose. M.M.L. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: none to disclose. Other relationships: none to disclose. J.A.N. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: none to disclose. Other relationships: none to disclose. W.Y. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: none to disclose. Other relationships: none to disclose. F.D.B. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: none to disclose. Other relationships: none to disclose. M.M.G. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: received money for patent (planned, pending, or issued) from Nihon Medi-Physics; institution received money for patent (planned, pending, or issued) from Nihon Medi-Physics; received royalties from Nihon Medi-Physics; institution received royalties from Nihon Medi-Physics. Other relationships: none to disclose.
Acknowledgments
We acknowledge the hard work of Rianot Amzat, MD, Pooneh Taleghani, MD, Delicia Votaw, CNMT, Fenton G. Ingram, RT(R), CNMT, PET, Seraphinah Lawal, RT(R), CNMT, PET, Adam Brown, RT(N), CNMT, Ronald J. Crowe, RPh, BCNP, and the entire cyclotron and synthesis team, Leah-Madge Bellamy, RN, MSN, Beverly Hunter, RN, Michelle Faurot, BS, Adeboye Osunkoya, MD, James R. Galt, PhD, and John R. Votaw, PhD. We also gratefully acknowledge the contributions of William Torres, MD, and the Division of Body Imaging for their biopsy skills, James M. Provenzale, MD, for his editing skills, and Eric Jablonowski, AA, for media support.
Received October 8, 2010; revision requested November 17; revision received January 17, 2011; accepted January 26; final version accepted February 8.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Funding: This research was supported by the National Institutes of Health (grant 5R01CA129356).
Abbreviations:
- anti-3-18F-FACBC
- anti-1-amino-3-fluorine 18-fluorocyclobutane-1-carboxylic acid
- CI
- confidence interval
- FDG
- fluorine 18 fluorodeoxyglucose
- PSA
- prostate-specific antigen
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin 2010;60(5):277–300 [DOI] [PubMed] [Google Scholar]
- 2.Mohler J, Bahnson RR, Boston B, et al. NCCN clinical practice guidelines in oncology: prostate cancer. J Natl Compr Canc Netw 2010;8(2):162–200 [DOI] [PubMed] [Google Scholar]
- 3.Ward JF, Blute ML, Slezak J, Bergstralh EJ, Zincke H. The long-term clinical impact of biochemical recurrence of prostate cancer 5 or more years after radical prostatectomy. J Urol 2003;170(5):1872–1876 [DOI] [PubMed] [Google Scholar]
- 4.Jhaveri FM, Zippe CD, Klein EA, Kupelian PA. Biochemical failure does not predict overall survival after radical prostatectomy for localized prostate cancer: 10-year results. Urology 1999;54(5):884–890 [DOI] [PubMed] [Google Scholar]
- 5.Partin AW, Pearson JD, Landis PK, et al. Evaluation of serum prostate-specific antigen velocity after radical prostatectomy to distinguish local recurrence from distant metastases. Urology 1994;43(5):649–659 [DOI] [PubMed] [Google Scholar]
- 6.Okotie OT, Aronson WJ, Wieder JA, et al. Predictors of metastatic disease in men with biochemical failure following radical prostatectomy. J Urol 2004;171(6 Pt 1):2260–2264 [DOI] [PubMed] [Google Scholar]
- 7.Schöder H, Larson SM. Positron emission tomography for prostate, bladder, and renal cancer. Semin Nucl Med 2004;34(4):274–292 [DOI] [PubMed] [Google Scholar]
- 8.Fowler JE, Jr, Brooks J, Pandey P, Seaver LE. Variable histology of anastomotic biopsies with detectable prostate specific antigen after radical prostatectomy. J Urol 1995;153(3 Pt 2):1011–1014 [PubMed] [Google Scholar]
- 9.Brassell SA, Rosner IL, McLeod DG. Update on magnetic resonance imaging, ProstaScint, and novel imaging in prostate cancer. Curr Opin Urol 2005;15(3):163–166 [DOI] [PubMed] [Google Scholar]
- 10.Sartor O, McLeod D. Indium-111-capromab pendetide scans: an important test relevant to clinical decision making. Urology 2001;57(3):399–401 [DOI] [PubMed] [Google Scholar]
- 11.Kundra V, Silverman PM, Matin SF, Choi H. Imaging in oncology from the University of Texas M. D. Anderson Cancer Center: diagnosis, staging, and surveillance of prostate cancer. AJR Am J Roentgenol 2007;189(4):830–844 [DOI] [PubMed] [Google Scholar]
- 12.Lange PH. ProstaScint scan for staging prostate cancer. Urology 2001;57(3):402–406 [DOI] [PubMed] [Google Scholar]
- 13.Seltzer MA, Barbaric Z, Belldegrun A, et al. Comparison of helical computerized tomography, positron emission tomography and monoclonal antibody scans for evaluation of lymph node metastases in patients with prostate specific antigen relapse after treatment for localized prostate cancer. J Urol 1999;162(4):1322–1328 [PubMed] [Google Scholar]
- 14.Kelloff GJ, Choyke P, Coffey DS. Prostate Cancer Imaging Working Group Challenges in clinical prostate cancer: role of imaging. AJR Am J Roentgenol 2009;192(6):1455–1470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choo R. Salvage radiotherapy for patients with PSA relapse following radical prostatectomy: issues and challenges. Cancer Res Treat 2010;42(1):1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schuster DM, Votaw JR, Nieh PT, et al. Initial experience with the radiotracer anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid with PET/CT in prostate carcinoma. J Nucl Med 2007;48(1):56–63 [PubMed] [Google Scholar]
- 17.Martarello L, McConathy J, Camp VM, et al. Synthesis of syn- and anti-1-amino-3-[18F]fluoromethyl-cyclobutane-1-carboxylic acid (FMACBC), potential PET ligands for tumor detection. J Med Chem 2002;45(11):2250–2259 [DOI] [PubMed] [Google Scholar]
- 18.McConathy J, Martarello L, Simpson NE, et al. Uptake profiles of six 18F-labeled amino acids for tumor imaging: comparison of in vitro and in vivo uptake of branched chain and cyclobutyl amino acids by 9L gliosarcoma tumor cells [abstr]. J Nucl Med 2002;43(5):41P [Google Scholar]
- 19.Nye JA, Schuster DM, Yu W, Camp VM, Goodman MM, Votaw JR. Biodistribution and radiation dosimetry of the synthetic nonmetabolized amino acid analogue anti-18F-FACBC in humans. J Nucl Med 2007;48(6):1017–1020 [DOI] [PubMed] [Google Scholar]
- 20.McConathy J, Voll RJ, Yu W, Crowe RJ, Goodman MM. Improved synthesis of anti-[18F]FACBC: improved preparation of labeling precursor and automated radiosynthesis. Appl Radiat Isot 2003;58(6):657–666 [DOI] [PubMed] [Google Scholar]
- 21.Sodee DB, Nelson AD, Faulhaber PF, Maclennan GT, Resnick MI, Bakale G. Update on fused capromab pendetide imaging of prostate cancer. Clin Prostate Cancer 2005;3(4):230–238 [DOI] [PubMed] [Google Scholar]
- 22.Kahn D, Williams RD, Haseman MK, Reed NL, Miller SJ, Gerstbrein J. Radioimmunoscintigraphy with In-111-labeled capromab pendetide predicts prostate cancer response to salvage radiotherapy after failed radical prostatectomy. J Clin Oncol 1998;16(1):284–289 [DOI] [PubMed] [Google Scholar]
- 23.Thomas CT, Bradshaw PT, Pollock BH, et al. Indium-111-capromab pendetide radioimmunoscintigraphy and prognosis for durable biochemical response to salvage radiation therapy in men after failed prostatectomy. J Clin Oncol 2003;21(9):1715–1721 [DOI] [PubMed] [Google Scholar]
- 24.Schettino CJ, Kramer EL, Noz ME, Taneja S, Padmanabhan P, Lepor H. Impact of fusion of indium-111 capromab pendetide volume data sets with those from MRI or CT in patients with recurrent prostate cancer. AJR Am J Roentgenol 2004;183(2):519–524 [DOI] [PubMed] [Google Scholar]
- 25.Venkitaraman R, Cook GJ, Dearnaley DP, et al. Whole-body magnetic resonance imaging in the detection of skeletal metastases in patients with prostate cancer. J Med Imaging Radiat Oncol 2009;53(3):241–247 [DOI] [PubMed] [Google Scholar]
- 26.Manyak MJ. Indium-111 capromab pendetide in the management of recurrent prostate cancer. Expert Rev Anticancer Ther 2008;8(2):175–181 [DOI] [PubMed] [Google Scholar]
- 27.Hinkle GH, Burgers JK, Neal CE, et al. Multicenter radioimmunoscintigraphic evaluation of patients with prostate carcinoma using indium-111 capromab pendetide. Cancer 1998;83(4):739–747 [PubMed] [Google Scholar]
- 28.Manyak MJ, Hinkle GH, Olsen JO, et al. Immunoscintigraphy with indium-111-capromab pendetide: evaluation before definitive therapy in patients with prostate cancer. Urology 1999;54(6):1058–1063 [DOI] [PubMed] [Google Scholar]
- 29.Sodee DB, Sodee AE, Bakale G. Synergistic value of single-photon emission computed tomography/computed tomography fusion to radioimmunoscintigraphic imaging of prostate cancer. Semin Nucl Med 2007;37(1):17–28 [DOI] [PubMed] [Google Scholar]
- 30.Seo Y, Aparici CM, Cooperberg MR, Konety BR, Hawkins RA. In vivo tumor grading of prostate cancer using quantitative 111In-capromab pendetide SPECT/CT. J Nucl Med 2010;51(1):31–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seo Y, Franc BL, Hawkins RA, Wong KH, Hasegawa BH. Progress in SPECT/CT imaging of prostate cancer. Technol Cancer Res Treat 2006;5(4):329–336 [DOI] [PubMed] [Google Scholar]
- 32.Krämer S, Görich J, Gottfried HW, et al. Sensitivity of computed tomography in detecting local recurrence of prostatic carcinoma following radical prostatectomy. Br J Radiol 1997;70(838):995–999 [DOI] [PubMed] [Google Scholar]
- 33.Beresford MJ, Gillatt D, Benson RJ, Ajithkumar T. A systematic review of the role of imaging before salvage radiotherapy for post-prostatectomy biochemical recurrence. Clin Oncol (R Coll Radiol) 2010;22(1):46–55 [DOI] [PubMed] [Google Scholar]
- 34.Rouvière O, Vitry T, Lyonnet D. Imaging of prostate cancer local recurrences: why and how? Eur Radiol 2010;20(5):1254–1266 [DOI] [PubMed] [Google Scholar]
- 35.Haider MA, Chung P, Sweet J, et al. Dynamic contrast-enhanced magnetic resonance imaging for localization of recurrent prostate cancer after external beam radiotherapy. Int J Radiat Oncol Biol Phys 2008;70(2):425–430 [DOI] [PubMed] [Google Scholar]
- 36.De Visschere PJ, De Meerleer GO, Fütterer JJ, Villeirs GM. Role of MRI in follow-up after focal therapy for prostate carcinoma. AJR Am J Roentgenol 2010;194(6):1427–1433 [DOI] [PubMed] [Google Scholar]
- 37.Briganti A. How to improve the ability to detect pelvic lymph node metastases of urologic malignancies. Eur Urol 2009;55(4):770–772 [DOI] [PubMed] [Google Scholar]
- 38.Oyen RH, Van Poppel HP, Ameye FE, Van de Voorde WA, Baert AL, Baert LV. Lymph node staging of localized prostatic carcinoma with CT and CT-guided fine-needle aspiration biopsy: prospective study of 285 patients. Radiology 1994;190(2):315–322 [DOI] [PubMed] [Google Scholar]
- 39.Takahashi N, Inoue T, Lee J, Yamaguchi T, Shizukuishi K. The roles of PET and PET/CT in the diagnosis and management of prostate cancer. Oncology 2007;72(3-4):226–233 [DOI] [PubMed] [Google Scholar]
- 40.Heesakkers RA, Hövels AM, Jager GJ, et al. MRI with a lymph-node-specific contrast agent as an alternative to CT scan and lymph-node dissection in patients with prostate cancer: a prospective multicohort study. Lancet Oncol 2008;9(9):850–856 [DOI] [PubMed] [Google Scholar]
- 41.Harisinghani MG, Barentsz J, Hahn PF, et al. Noninvasive detection of clinically occult lymph-node metastases in prostate cancer. N Engl J Med 2003;348(25):2491–2499 [DOI] [PubMed] [Google Scholar]
- 42.Thoeny HC, Triantafyllou M, Birkhaeuser FD, et al. Combined ultrasmall superparamagnetic particles of iron oxide–enhanced and diffusion-weighted magnetic resonance imaging reliably detect pelvic lymph node metastases in normal-sized nodes of bladder and prostate cancer patients. Eur Urol 2009;55(4):761–769 [DOI] [PubMed] [Google Scholar]
- 43.Schöder H, Herrmann K, Gönen M, et al. 2-[18F]fluoro-2-deoxyglucose positron emission tomography for the detection of disease in patients with prostate-specific antigen relapse after radical prostatectomy. Clin Cancer Res 2005;11(13):4761–4769 [DOI] [PubMed] [Google Scholar]
- 44.Nuñez R, Macapinlac HA, Yeung HW, et al. Combined 18F-FDG and 11C-methionine PET scans in patients with newly progressive metastatic prostate cancer. J Nucl Med 2002;43(1):46–55 [PubMed] [Google Scholar]
- 45.Beer AJ, Eiber M, Souvatzoglou M, Schwaiger M, Krause BJ. Radionuclide and hybrid imaging of recurrent prostate cancer. Lancet Oncol 2011;12(2):181–191 [DOI] [PubMed] [Google Scholar]
- 46.Apolo AB, Pandit-Taskar N, Morris MJ. Novel tracers and their development for the imaging of metastatic prostate cancer. J Nucl Med 2008;49(12):2031–2041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hong H, Zhang Y, Sun J, Cai W. Positron emission tomography imaging of prostate cancer. Amino Acids 2010;39(1):11–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bouchelouche K, Tagawa ST, Goldsmith SJ, Turkbey B, Capala J, Choyke P. PET/CT imaging and radioimmunotherapy of prostate cancer. Semin Nucl Med 2011;41(1):29–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Picchio M, Briganti A, Fanti S, et al. The role of choline positron emission tomography/computed tomography in the management of patients with prostate-specific antigen progression after radical treatment of prostate cancer. Eur Urol. Published online September 15, 2010. Accessed March 11, 2011 [DOI] [PubMed] [Google Scholar]
- 50.Plathow C, Weber WA. Tumor cell metabolism imaging. J Nucl Med 2008;49(Suppl 2):43S–63S [DOI] [PubMed] [Google Scholar]
- 51.Tóth G, Lengyel Z, Balkay L, Salah MA, Trón L, Tóth C. Detection of prostate cancer with 11C-methionine positron emission tomography. J Urol 2005;173(1):66–69, discussion 69 [DOI] [PubMed] [Google Scholar]
- 52.Crook J, Malone S, Perry G, Bahadur Y, Robertson S, Abdolell M. Postradiotherapy prostate biopsies: what do they really mean? Results for 498 patients. Int J Radiat Oncol Biol Phys 2000;48(2):355–367 [DOI] [PubMed] [Google Scholar]
- 53.Cohen JH, Eastham J, Macchia RJ. Outcomes following negative prostate biopsy for patients with persistent disease after radiotherapy for prostate cancer. Int Braz J Urol 2010;36(1):44–48 [DOI] [PubMed] [Google Scholar]
- 54.Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. JAMA 1999;281(17):1591–1597 [DOI] [PubMed] [Google Scholar]
- 55.Trock BJ, Han M, Freedland SJ, et al. Prostate cancer–specific survival following salvage radiotherapy vs observation in men with biochemical recurrence after radical prostatectomy. JAMA 2008;299(23):2760–2769 [DOI] [PMC free article] [PubMed] [Google Scholar]