Abstract
A convergent route featuring [3,3]-sigmatropic rearrangements of a linchpin azepinopyrrolidine served to install two of the four contiguous stereocenters present in the tricyclic Stemona alkaloids sessilifoliamide and stemoamide. In addition to the first total synthesis of (−)-sessilifoliamide C, a potential biosynthetic relationship between the sessilifoliamides and previously reported Stemona alkaloids is presented.
Natural products from Stemona and Croomia plants have served as an inspiration for chemical, biological, and synthetic studies since at least the 1930s, when the first derivatives were described in Western references.1,2,3 Extracts from these plants have been used for centuries in eastern cultures for the treatment of various respiratory problems, such as pertussis, bronchitis, and tuberculosis. However, with the exception of their well-documented insecticidal activities, validated evidence for other beneficial human health effects of the pure natural products is just beginning to emerge.4
In 2003, Takeya and co-workers isolated sessilifoliamides A–D (1–4) from the roots of the perennial herb Stemona sessilifolia.5 These alkaloids possess the characteristic pyrrolo[1,2-a]azepine core attached to a butenolide substituent (Scheme 1). The relative configuration of 3 was confirmed by a chemical degradation to a derivative in common with sessilifoliamide A, for which an x-ray crystal structure had been obtained.5 Interestingly, a possible biosynthetic relationship between the sessilifoliamides and the previously identified parvistemoline (5)6 is apparent upon C(16)-oxidation and Michael addition. Accordingly, we decided to investigate a unified and possibly biomimetic strategy toward the sessifoliamides and prepare sessilifoliamide C first, due to its similarity to the more complex ring system present in parvistemoline.
Scheme 1.
Biosynthetic hypothesis based on the structural similarities between sessilifoliamides and parvistemoline
Our retrosynthetic strategy for 3 was focused on the construction of the C(9)–C(10) adjacent stereocenters prior to butenolide formation (Scheme 2). We desired an approach that could accomplish this task in a single operation and envisioned that a [3,3]-sigmatropic Claisen rearrangement7 with precise stereocontrol in the transition state could meet these requirements. The precursor to this key transformation, alcohol 8, was accessible by ring-closing metathesis (RCM) of the diene formed from the alkylation of pyrrolidinone 10 with iodide 9.
Scheme 2.
Retrosynthetic approach for sessilifoliamide C
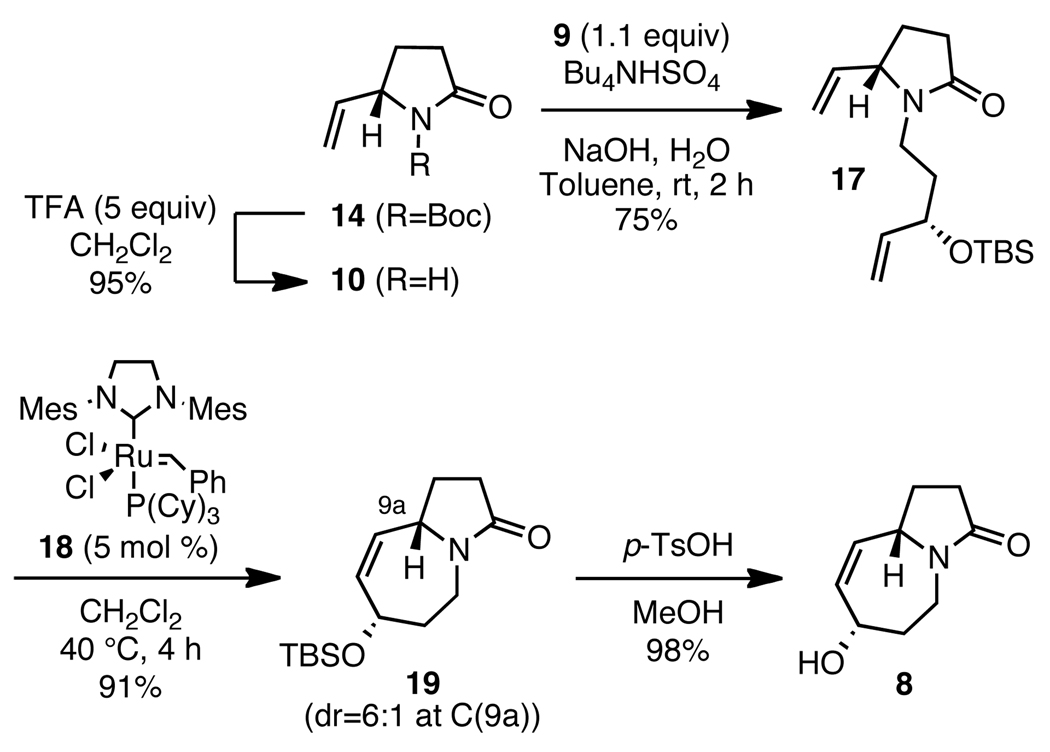
Several methods for the preparation of vinyl pyrrolidinone 10 are known in the literature.8 Preliminary RCM studies established that we needed a N-protective group on 10, and therefore we pursued a new, scaleable synthesis of the Boc-derivative 14 (Scheme 3). (S)-Pyroglutamic acid 11 was converted to the thioester with ethanethiol in the presence of carbodiimide, followed by N-Boc protection to afford carbamate 12. Direct conversion of 12 to the aldehyde 13 was successful by a Fukuyama reduction.9 Fukuyama conditions prior to N-Boc protection did not lead to the desired reduction product, and starting material was recovered cleanly despite modifications in the reaction time, catalyst loading and solvent. Pyrrolidinone protection facilitated the desired transformation. Immediate exposure of the aldehyde 13 to Wittig olefination delivered a 96% yield of alkene 14. Boc-protected 14 was found to be stable to bench-top storage, whereas amide 10 decomposed within hours. However, due to partial racemization prior to and/or during olefination, 14 was obtained in 72% ee, as determined by chiral HPLC analysis on a Chiralcel OD-H column.
Scheme 3.
Preparation of vinyl pyrrolidinone 14
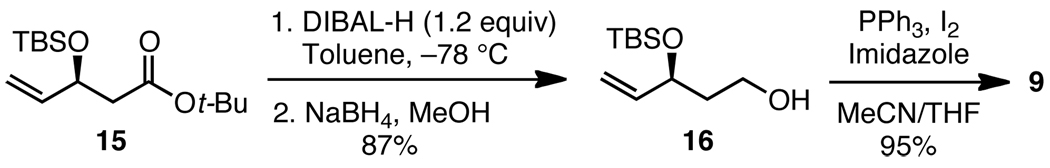
The second segment, iodide 9, was prepared by an enzymatic resolution strategy which provided the t-butyl ester 15 on a large scale (Scheme 4).10 A two-step reduction procedure was used to generate alcohol 16 in 87% yield; in the presence of 2 equiv of DIBAL-H, 16 was isolated in lower yield (ca. 70%). Finally, a straightforward iodination of 16 afforded 9.
Scheme 4.
Preparation of iodide 9
In preparation for the segment condensation, the N-Boc group in 14 was cleaved with TFA (Scheme 5). After some optimization, we discovered that alkylation of amide 10 under phase-transfer conditions was more reproducible and manageable on scale than NaH/DMF conditions, providing us with a reliable access to diene 17. Ring closure to the pyrrolo[1,2-a]azepine core characteristic for Stemona alkaloids by RCM of 17 afforded 19 in 91% yield as an inseparable 6:1 mixture of C(9a) diastereomers resulting from partially racemized 14. Removal of the TBS group under acidic conditions led to the allylic alcohol 8 as a hygroscopic solid.
Scheme 5.
Segment condensation, RCM and synthesis of linchpin intermediate 8
Prior difficulties with [3,3]-rearrangements on bicycles motivated us to probe whether this reaction could occur on the sterically congested concave face of pyrrolo[1,2-a]azepine 8. We selected an Eschenmoser-Claisen reaction7 to address this question, in analogy to our stenine3f and tuberostemonine3b syntheses (Scheme 6). The desired rearrangement occurred readily, and amide 21 was obtained in 92% yield under optimized conditions. Due to the structural similarity of 21 and stemoamide,11 we decided to complete the remaining 3 steps to the tricyclic core of this natural product. Iodolactonization of 21 and reduction of the resulting iodide proceeded in 98% and 97% yield, respectively, to give 23 as a 6:1 mixture of C(9a) diastereomers. Chromatographic removal of the remaining C(9a) isomer provided the major isomer of 23 in 71% yield for this step. Finally, lactone α-methylation led to 8-epi-stemoamide (24). While many syntheses of stemoamide and its stereoisomers have been reported,12 our effort represents the first synthesis of the C(8) epimer. Additionally, among the plethora of synthetic approaches to stemoamide, this is the first example of a [3,3]-rearrangement to install the C(9) stereocenter in this ring system.
Scheme 6.
Conversion of 8 to (−)-8-epi-stemoamide
Having demonstrated that the [3,3]-sigmatropic rearrangement and chain extension from C(7) to C(9) on 8 was indeed feasible, we studied the Ireland-Claisen rearrangement7,13 for the stereoselective installation of the tertiary methine C(11) in the sessilifoliamides. Acylation of alcohol 8 (still as a 6:1 mixture of C(9a) diastereomers derived from 19) with butyric acid under carbodiimide coupling conditions provided ester 25 in 92% yield (Scheme 7). Enolization with LiHMDS in a THF/HMPA mixture14 followed by trapping with TBSCl provided the (Z)-silyl ketene acetal as the sole stereoisomer according to 1H NMR analysis of the crude reaction product. The subsequent thermal rearrangement in toluene led to an approximately 2:1 ratio of silyl esters 26a and 26b, which were treated with TBAF followed by TMS-diazomethane to give the corresponding methyl esters. Unfortunately, both the yield of the four-step sequence and the ratio of diastereomeric products 27a and 27b remained low (21% yield, 2.2:1 dr) despite attempts to optimize the reaction time, temperature, and solvent polarity.
Scheme 7.
TBS-Mediated Ireland-Claisen rearrangement of 8
A significant improvement in this key transformation could be achieved after an analysis of the competing transition states of the Ireland-Claisen rearrangement (Scheme 8). Specifically, we noticed that the orientation of the trialkylsilyl group was remarkably different in 29‡ and 30‡, and therefore offered an opportunity to influence the course of the reaction. We hypothesized that a significant increase in the size of the alkyl groups on silicon would likely selectively destabilize chair transition state 30‡, where the silyl group is positioned underneath the 7-membered ring during the C-C bond formation, and be more readily tolerated in boat transition state 29‡, in which the silyl group is kept distant from the 5,7-ring system. This hypothesis could be readily put to test (Scheme 9). Ireland-Claisen rearrangement of the TIPS-silyl ketene acetal, formed by enolization of 25 in the presence of TIPSCl, required heating at reflux in degassed15 xylenes for 3 h to go to completion. The resulting 31a and 31b were again converted to the methyl esters to simplify analysis.
Scheme 8.
Transition state analysis of the Ireland-Claisen rearrangement
Scheme 9.
TIPS-Mediated Ireland-Claisen rearrangement of 8
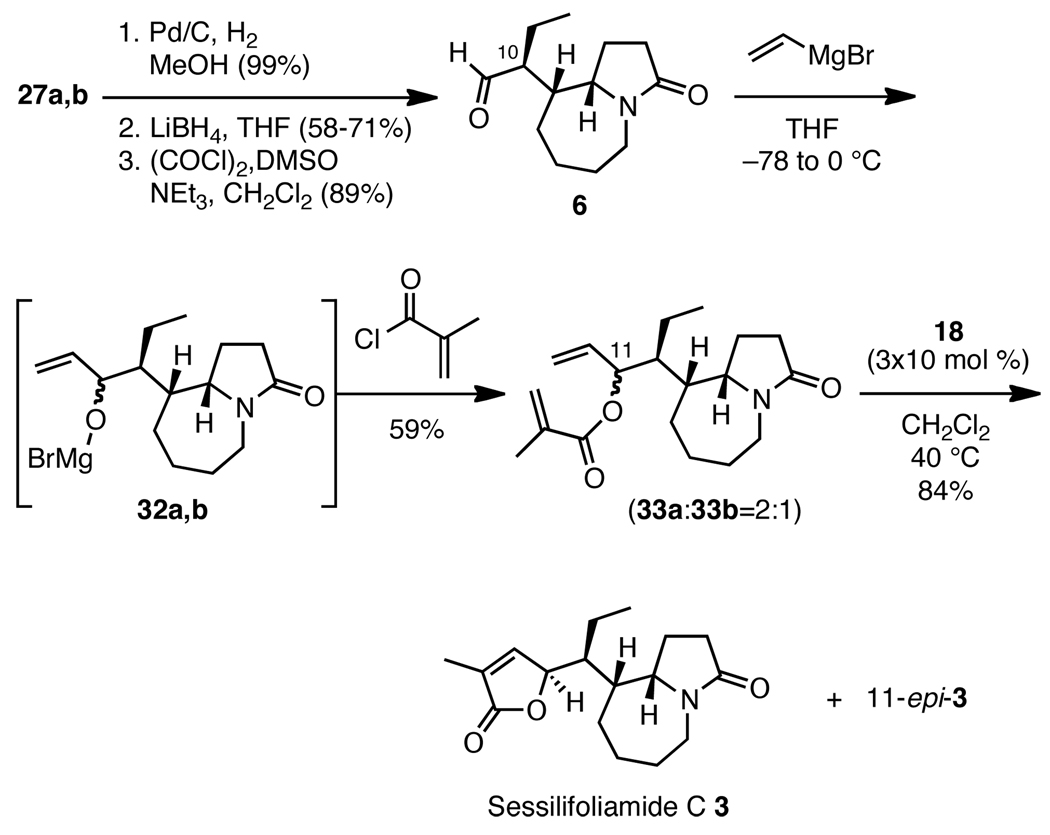
Gratifyingly, esters 27a and 27b were isolated in 51% yield from 25 in an improved 6:1 ratio of C(10) diastereomers, in support of our transition state analysis.16 Chromatography on SiO2 was used to remove the diastereomers at C(9a) from the 6:1 mixture of 27a and 27b. Confirmation of the assignment of the major isomer 27a was gained upon completion of the natural product and comparison of the spectroscopic data.
Surprisingly, methyl esters 27a and 27b proved to be resistant to nucleophilic addition of Grignard reagents and metalated dimethylhydroxylamine, even at elevated temperatures. We speculated that increased conformational flexibility and a lower oxidation state at carbonyl C(11) could promote reactivity. Therefore, the 6:1 mixture of 27a,b was hydrogenated and converted to the corresponding aldehyde by sequential treatment with Pd/C/H2, LiBH4 and Swern reagent (Scheme 10). The minor C(10)-stereoisomer could be removed chromatographically from either alcohol or aldehyde intermediate after the latter two steps to afford pure 6. Exposure of 6 to vinyl magnesium bromide afforded the allylic alkoxides 32a and 32b, which were acylated in situ with methacryloyl chloride to afford a 2:1 mixture of the labile 33a and 33b. A final RCM successfully assembled the butenolide ring in 84% yield, and MPLC separation revealed the major isomer to be (−)-sessilifoliamide C (3) by comparison of the spectroscopic data with the reported literature values of the natural product.17
Scheme 10.
Conversion of 27 to (−)-sessilifoliamide C
In summary, we have completed the first synthesis of the Stemona alkaloid (−)-sessilifoliamide C, which also represents the first member of the sessilifoliamide family to succumb to total synthesis. Noteworthy aspects of our approach include a convergent strategy featuring a [3,3]-rearrangement to install two contiguous stereocenters at C(9)–C(10), and the ability to access the stemoamide ring system in a unified strategy from linchpin intermediate 8. The synthetic efficiency of the key Ireland-Claisen rearrangement benefited from increasing the steric bulk of the ketene acetal silyl substituent to proceed through a preferred boatlike transition state while providing a level of diastereoselection that is notable in a bicyclic system.18
Supplementary Material
Acknowledgments
This work was supported by NIH/NIGMS CMLD program (GM067082). The authors thank Dr. John Williams (University of Pittsburgh) for High Resolution Mass Spectrometry and Mr. Mike Delk (University of Pittsburgh) for NMR spectrometer maintenance. ATH thanks the Goldblatt family for a University of Pittsburgh graduate fellowship.
Footnotes
Supporting Information Available: Experimental procedures and spectral data for all new compounds, including copies of 1H and 13C NMR spectra. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Lee HM, Chen KK. J. Amer. Pharm. Assoc. 1940;29:391. [Google Scholar]; (b) Edwards OE, Feniak G, Handa KL. Can. J. Chem. 1962;40:455. [Google Scholar]; (c) Uyeo S, Irie H, Harada H. Chem. Pharm. Bull. 1967;1:768. doi: 10.1248/cpb.15.768. [DOI] [PubMed] [Google Scholar]; (d) Alibes R, Figueredo M. Eur. J. Org. Chem. 2009:2421. [Google Scholar]; (e) Pilli RA, Rosso GB, Ferreira De Oliveira MDC. Nat. Prod. Rep. 2010;27:1908. doi: 10.1039/c005018k. [DOI] [PubMed] [Google Scholar]
- 2.For recent synthetic studies reported on Stemona alkaloids, see, for example: Chen Z-H, Tu Y-Q, Zhang S-Y, Zhang F-M. Org. Lett. 2011;13:724. doi: 10.1021/ol102955e. Chen Z-H, Zhang Y-Q, Chen Z-M, Tu Y-Q, Zhang F-M. Chem. Commun. 2011;47:1836. doi: 10.1039/c0cc02612c. Frankowski KJ, Setola V, Evans JM, Neuenswander B, Roth BL, Aube J. Proc. Nat. Acad. Sci. U.S.A. 2011 doi: 10.1073/pnas.1016558108. early edition.
- 3.For previous studies on Stemona alkaloids from our lab, see: Wipf P, Kim Y. Tetrahedron Lett. 1992;33:5477. Wipf P, Kim Y, Goldstein DM. J. Am. Chem. Soc. 1995;117:11106. Goldstein DM, Wipf P. Tetrahedron Lett. 1996;37:739. Wipf P, Li W. J. Org. Chem. 1999;64:4576. doi: 10.1021/jo9905064. Wipf P, Mareska DA. Tetrahedron Letters. 2000;41:4723. Wipf P, Spencer SR. J. Am. Chem. Soc. 2005;127:225. doi: 10.1021/ja044280k.
- 4.Greger H. Planta Med. 2006;72:99. doi: 10.1055/s-2005-916258. [DOI] [PubMed] [Google Scholar]
- 5.Kakua D, Hitotsuyanagi Y, Matsuura N, Fukaya H, Takeya K. Tetrahedron. 2003;59:7779. [Google Scholar]
- 6.Lin W, Xu R, Zhong Q. Huaxue Xuebao. 1991;49:927. [Google Scholar]
- 7.Wipf P. "Claisen rearrangements". In: Trost BM, Fleming I, Paquette LA, editors. Comprehensive Organic Synthesis. Vol 5. Oxford: Pergamon; 1991. pp. 827–874. [Google Scholar]
- 8.(a) Wei Z-Y, Knaus E. Synlett. 1993:295–296. [Google Scholar]; (b) Wei Z-Y, Knaus E. Tetrahedron. 1994;50:5569. [Google Scholar]; (c) Napoletano M, Della Bella D, Fraire C, Grancini G, Masotto C, Ricciardi S, Zambon C. Bioorg. Med. Chem. Lett. 1995;5:589. [Google Scholar]; (d) Gheorghe A, Schulte M, Reiser O. J. Org. Chem. 2006;71:2173. doi: 10.1021/jo0524472. [DOI] [PubMed] [Google Scholar]; (e) Mo F, Li F, Qiu D, Wang J. Tetrahedron. 2010;66:1274. [Google Scholar]
- 9.Tokuyama H, Yokoshima S, Lin S-C, Li L, Fukuyama T. Synthesis. 2002:1121. [Google Scholar]
- 10.(a) Ghosh AK, Kulkarni S. Org. Lett. 2008;10:3907. doi: 10.1021/ol8014623. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Ghosh AK, Yuan H. Tetrahedron Lett. 2009;50:1416. doi: 10.1016/j.tetlet.2009.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Vrielynck S, Vandewalle M. Tetrahedron Lett. 1995;36:9023. [Google Scholar]
- 11.Lin W, Ye Y, Xu R. J. Nat. Prod. 1992;55:571. [Google Scholar]
- 12.(a) Williams DR, Reddy JP, Amato GS. Tetrahedron Lett. 1994;35:6417. [Google Scholar]; (b) Khim S-K, Schultz AG. J. Org. Chem. 2004;69:7734. doi: 10.1021/jo049083i. [DOI] [PubMed] [Google Scholar]; (c) Bogliotti N, Dalko PI, Cossy J. J. Org. Chem. 2006;71:9528. doi: 10.1021/jo061628g. [DOI] [PubMed] [Google Scholar]; (d) Kohno Y, Narasaka K. Bull. Chem. Soc. Jpn. 1996;69:2063. [Google Scholar]; (e) Bates RW, Sridhar S. Synlett. 2009;12:1979. [Google Scholar]; (f) Jacobi PA, Lee K. J. Am. Chem. Soc. 1997;119:3409. [Google Scholar]; (g) Jacobi PA, Lee K. J. Am. Chem. Soc. 2000;122:4295. [Google Scholar]; (h) Kinoshita A, Mori M. J. Org. Chem. 1996;61:8356. [Google Scholar]; (i) Kinoshita A, Mori M. Heterocycles. 1997;46:287. [Google Scholar]; (j) Sibi MP, Subramanian T. Synlett. 2004:1211. [Google Scholar]; (k) Olivo HF, Tovar-Miranda R, Barragán E. J. Org. Chem. 2006;71:3287. doi: 10.1021/jo052364l. [DOI] [PubMed] [Google Scholar]; (l) Torssell S, Wanngren E, Somfai P. J. Org. Chem. 2007;72:4246. doi: 10.1021/jo070498o. [DOI] [PubMed] [Google Scholar]; (m) Gao P, Tong Z, Hu H, Xu P-F, Liu W, Sun C, Zhai H. Synlett. 2009:2188. [Google Scholar]; (n) Wang Y, Zhu L, Zhang Y, Hong R. Angew. Chem. Int. Ed. 2011;50:2787. doi: 10.1002/anie.201005833. [DOI] [PubMed] [Google Scholar]; (o) Honda T, Matsukawa T, Takahashi K. Org. Biomol. Chem. 2011;9:673. doi: 10.1039/c0ob00850h. [DOI] [PubMed] [Google Scholar]
- 13.Ireland RE, Mueller RH, Willard AK. J. Am. Chem. Soc. 1976;98:2868. [Google Scholar]
- 14.(a) Ireland RE, Wipf P, Armstrong JD. J. Org. Chem. 1991;56:650. [Google Scholar]; (b) Ireland RE, Wipf P, Xiang JN. J. Org. Chem. 1991;56:3572. [Google Scholar]
- 15.Degassing was necessary to suppress the formation of oxidative byproducts.
- 16.(a). Transition states were identified using a PM3 transition state search in Spartan 10 (Wavefunction, Inc., Irvine, CA). A substituent-simplified (R=Me, Me in place of Et) boat analog of 29‡ was found to be approximately equal in energy to the corresponding analog of chair 30‡ (Scheme 8). (b). For another observation of the effect of the steric bulk of the silyloxy group on the diastereoselectivity of the Ireland-Claisen rearrangement, see: Chen C-L, Namba K, Kishi Y. Org. Lett. 2009;11:409. doi: 10.1021/ol8027225.
- 17.The synthetic product was spectroscopically identical to the natural product,5 with the exception of the magnitude of the specific rotation (natural: [α]D26 –140 (c 0.17, CHCl3), synthetic: [α]D23 –84.6 (c 2.5, CHCl3).
- 18.(a) Castro AMM. Chem. Rev. 2004;104:2939. doi: 10.1021/cr020703u. [DOI] [PubMed] [Google Scholar]; (b) Gül S, Schoenebeck F, Aviyente V, Houk KN. J. Org. Chem. 2010;75:2115. doi: 10.1021/jo100033d. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.