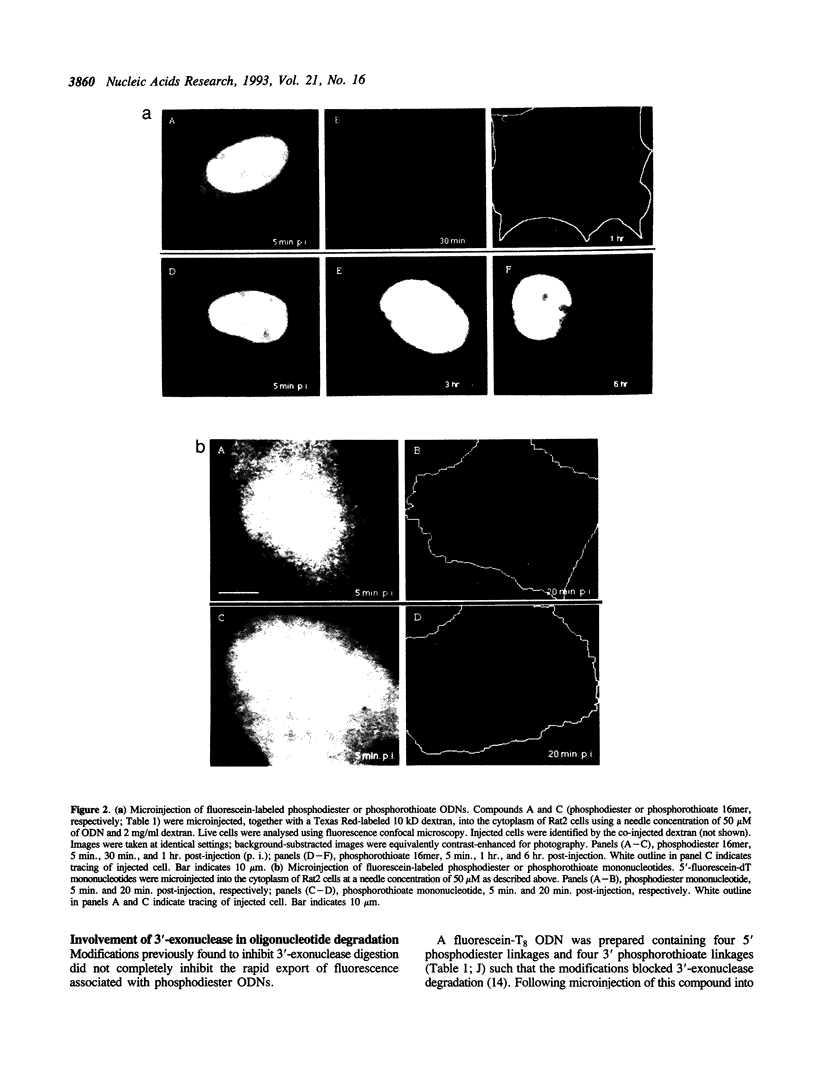
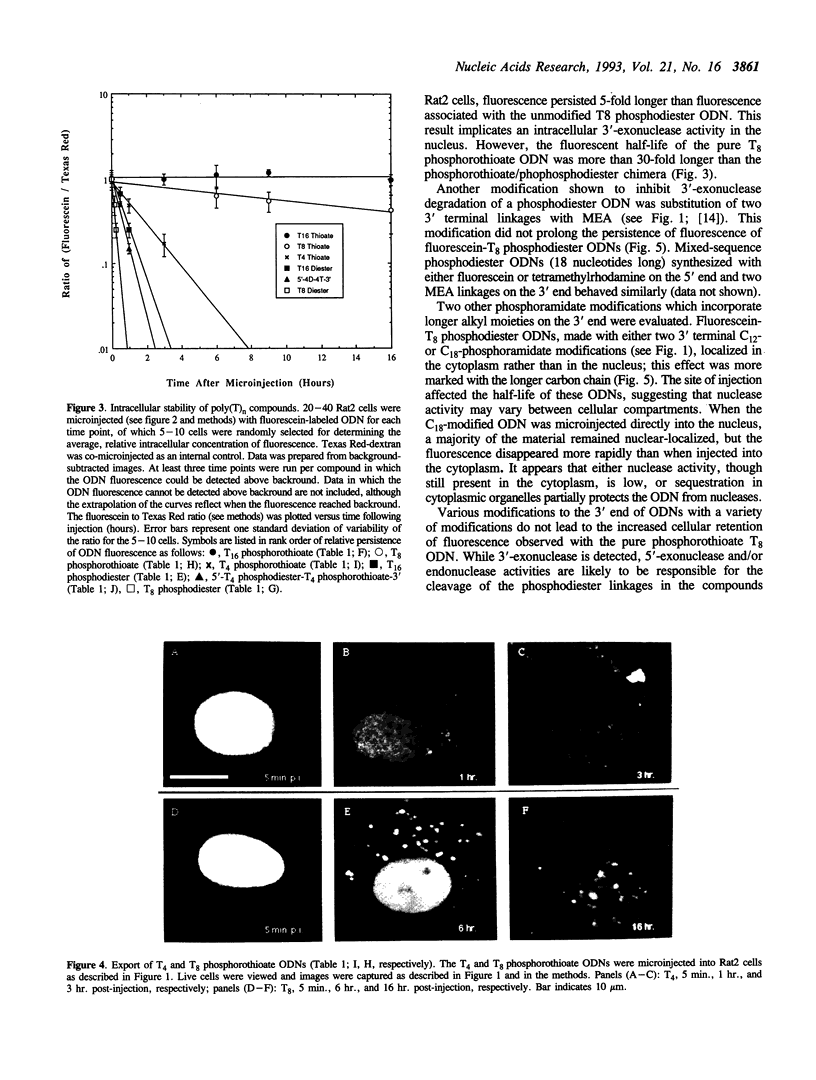
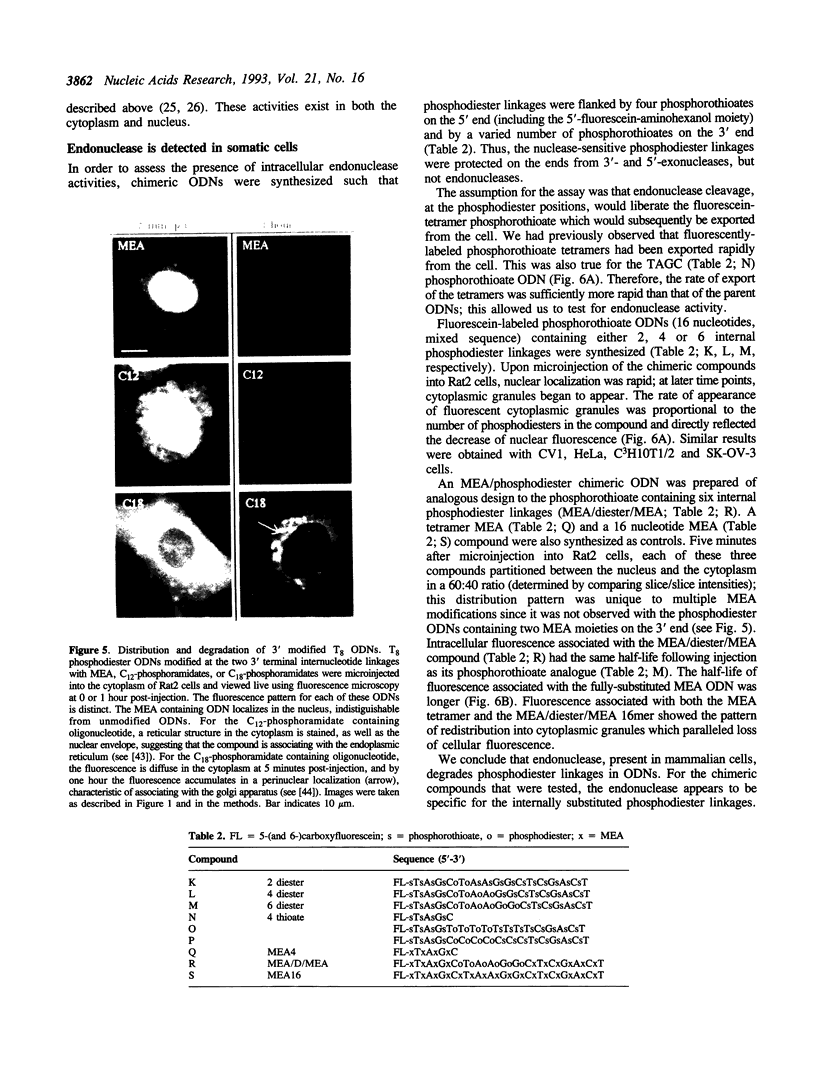
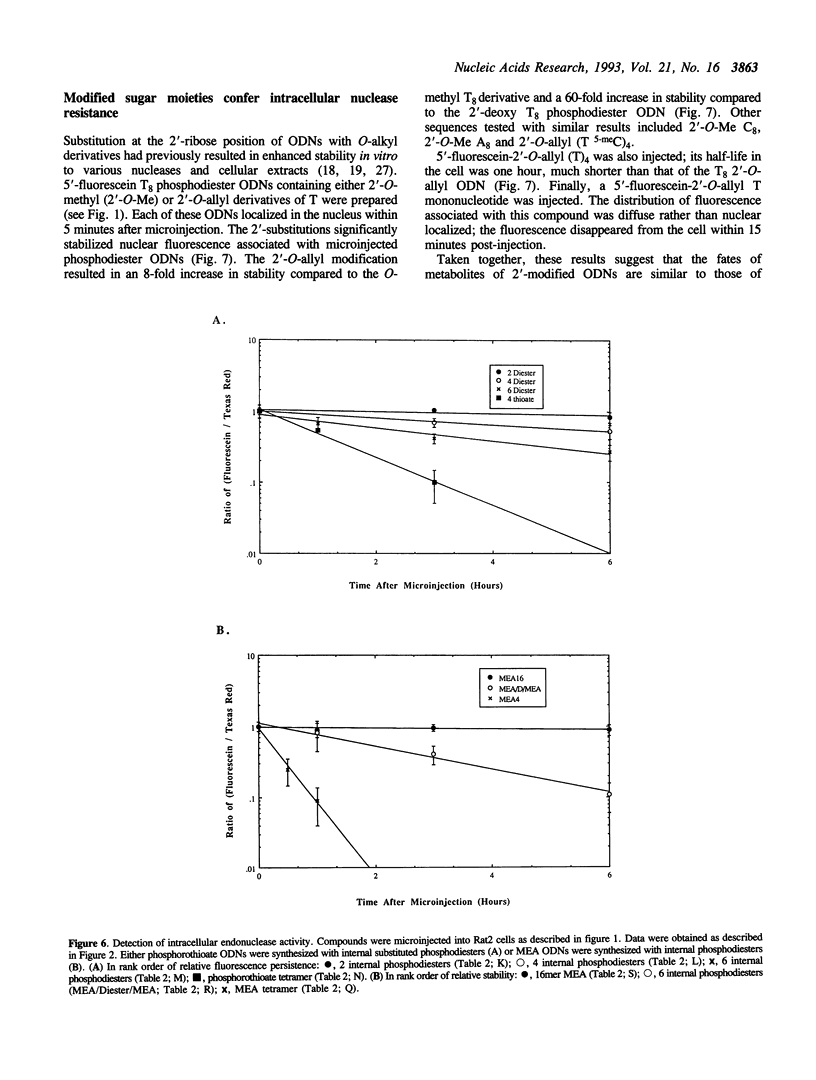
Abstract
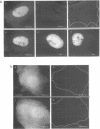
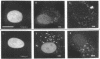
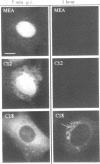
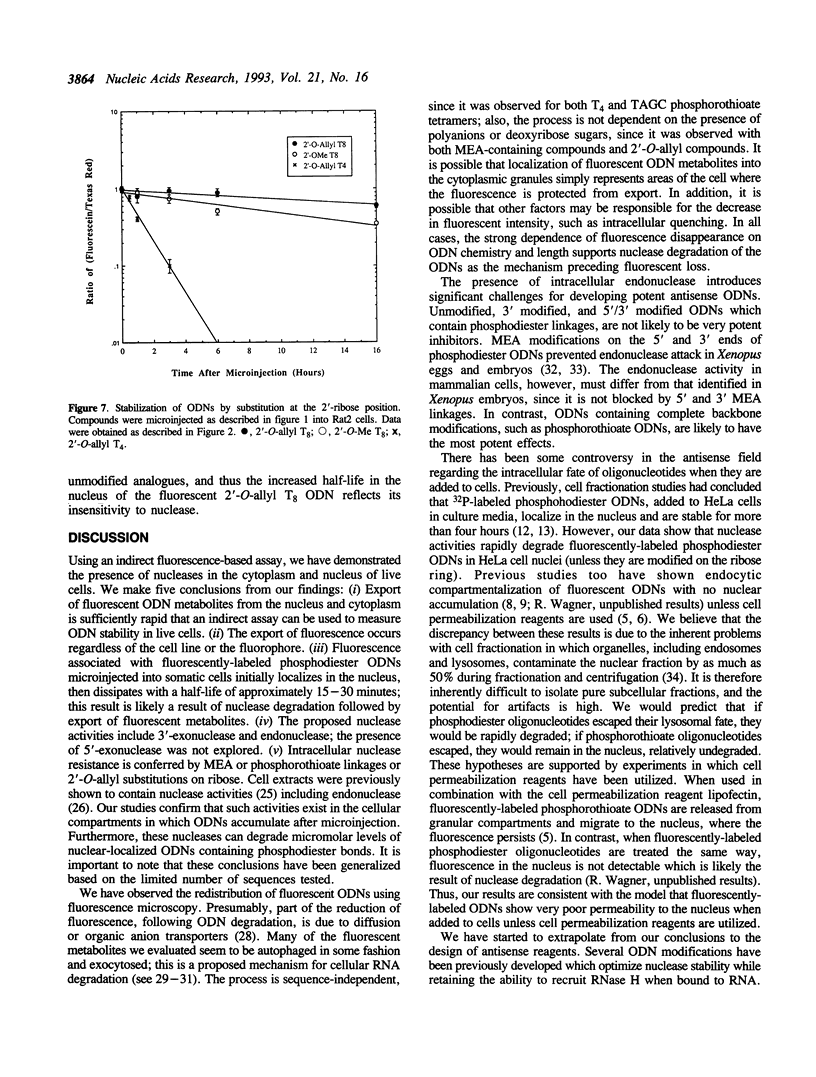
The intracellular distribution and metabolism of microinjected fluorescently-labeled oligonucleotides (ODNs) have been evaluated using confocal fluorescence microscopy. Fluorescent phosphodiester ODNs, microinjected into the cytoplasm of mammalian cells, rapidly accumulate within the nucleus; the fluorescence disappears with a half-life of 15-20 minutes. Microinjected fluorescent phosphorothioate ODNs remain in the nucleus for more than 24 hours. The persistence of fluorescence depends on the length of the ODN. Modification of the 3' end of phosphodiester ODNs does not significantly slow the rapid disappearance of fluorescence, although certain 3' modifications localize ODNs into the cytoplasm. Using specially designed ODNs, endonuclease activity is demonstrated to exist in the cytoplasm and nucleus. Modification of the 2' position of the ribose rings of a fluorescent phosphodiester oligodeoxynucleotide with O-methyl or O-allyl does not alter its intracellular distribution; however, the 2'-O-allyl modification stabilizes the persistence of fluorescence more than 60-fold compared to the 2'-deoxy control. Thus, the experiments indicate that somatic cells contain nucleolytic activities which degrade microinjected ODNs; however, chemical modification can dramatically circumvent this process.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agrawal S., Mayrand S. H., Zamecnik P. C., Pederson T. Site-specific excision from RNA by RNase H and mixed-phosphate-backbone oligodeoxynucleotides. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1401–1405. doi: 10.1073/pnas.87.4.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker C., Holland D., Edge M., Colman A. Effects of oligo sequence and chemistry on the efficiency of oligodeoxyribonucleotide-mediated mRNA cleavage. Nucleic Acids Res. 1990 Jun 25;18(12):3537–3543. doi: 10.1093/nar/18.12.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett C. F., Chiang M. Y., Chan H., Shoemaker J. E., Mirabelli C. K. Cationic lipids enhance cellular uptake and activity of phosphorothioate antisense oligonucleotides. Mol Pharmacol. 1992 Jun;41(6):1023–1033. [PubMed] [Google Scholar]
- Carmo-Fonseca M., Pepperkok R., Carvalho M. T., Lamond A. I. Transcription-dependent colocalization of the U1, U2, U4/U6, and U5 snRNPs in coiled bodies. J Cell Biol. 1992 Apr;117(1):1–14. doi: 10.1083/jcb.117.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmo-Fonseca M., Pepperkok R., Sproat B. S., Ansorge W., Swanson M. S., Lamond A. I. In vivo detection of snRNP-rich organelles in the nuclei of mammalian cells. EMBO J. 1991 Jul;10(7):1863–1873. doi: 10.1002/j.1460-2075.1991.tb07712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin D. J., Green G. A., Zon G., Szoka F. C., Jr, Straubinger R. M. Rapid nuclear accumulation of injected oligodeoxyribonucleotides. New Biol. 1990 Dec;2(12):1091–1100. [PubMed] [Google Scholar]
- Cotten M., Oberhauser B., Brunar H., Holzner A., Issakides G., Noe C. R., Schaffner G., Wagner E., Birnstiel M. L. 2'-O-methyl, 2'-O-ethyl oligoribonucleotides and phosphorothioate oligodeoxyribonucleotides as inhibitors of the in vitro U7 snRNP-dependent mRNA processing event. Nucleic Acids Res. 1991 May 25;19(10):2629–2635. doi: 10.1093/nar/19.10.2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagle J. M., Walder J. A., Weeks D. L. Targeted degradation of mRNA in Xenopus oocytes and embryos directed by modified oligonucleotides: studies of An2 and cyclin in embryogenesis. Nucleic Acids Res. 1990 Aug 25;18(16):4751–4757. doi: 10.1093/nar/18.16.4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagle J. M., Weeks D. L., Walder J. A. Pathways of degradation and mechanism of action of antisense oligonucleotides in Xenopus laevis embryos. Antisense Res Dev. 1991 Spring;1(1):11–20. doi: 10.1089/ard.1991.1.11. [DOI] [PubMed] [Google Scholar]
- Eadie J. S., McBride L. J., Efcavitch J. W., Hoff L. B., Cathcart R. High-performance liquid chromatographic analysis of oligodeoxyribonucleotide base composition. Anal Biochem. 1987 Sep;165(2):442–447. doi: 10.1016/0003-2697(87)90294-6. [DOI] [PubMed] [Google Scholar]
- Froehler B. C., Ng P. G., Matteucci M. D. Synthesis of DNA via deoxynucleoside H-phosphonate intermediates. Nucleic Acids Res. 1986 Jul 11;14(13):5399–5407. doi: 10.1093/nar/14.13.5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehler B., Ng P., Matteucci M. Phosphoramidate analogues of DNA: synthesis and thermal stability of heteroduplexes. Nucleic Acids Res. 1988 Jun 10;16(11):4831–4839. doi: 10.1093/nar/16.11.4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furdon P. J., Dominski Z., Kole R. RNase H cleavage of RNA hybridized to oligonucleotides containing methylphosphonate, phosphorothioate and phosphodiester bonds. Nucleic Acids Res. 1989 Nov 25;17(22):9193–9204. doi: 10.1093/nar/17.22.9193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamper H. B., Reed M. W., Cox T., Virosco J. S., Adams A. D., Gall A. A., Scholler J. K., Meyer R. B., Jr Facile preparation of nuclease resistant 3' modified oligodeoxynucleotides. Nucleic Acids Res. 1993 Jan 11;21(1):145–150. doi: 10.1093/nar/21.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W. Y., Storm C., Egan W., Cheng Y. C. Cellular pharmacology of phosphorothioate homooligodeoxynucleotides in human cells. Mol Pharmacol. 1993 Jan;43(1):45–50. [PubMed] [Google Scholar]
- Goodchild J. Conjugates of oligonucleotides and modified oligonucleotides: a review of their synthesis and properties. Bioconjug Chem. 1990 May-Jun;1(3):165–187. doi: 10.1021/bc00003a001. [DOI] [PubMed] [Google Scholar]
- Graessmann M., Graessmann A. Microinjection of tissue culture cells. Methods Enzymol. 1983;101:482–492. doi: 10.1016/0076-6879(83)01033-2. [DOI] [PubMed] [Google Scholar]
- Heydrick S. J., Lardeux B. R., Mortimore G. E. Uptake and degradation of cytoplasmic RNA by hepatic lysosomes. Quantitative relationship to RNA turnover. J Biol Chem. 1991 May 15;266(14):8790–8796. [PubMed] [Google Scholar]
- Hoke G. D., Draper K., Freier S. M., Gonzalez C., Driver V. B., Zounes M. C., Ecker D. J. Effects of phosphorothioate capping on antisense oligonucleotide stability, hybridization and antiviral efficacy versus herpes simplex virus infection. Nucleic Acids Res. 1991 Oct 25;19(20):5743–5748. doi: 10.1093/nar/19.20.5743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt J. T., Redner R. L., Nienhuis A. W. An oligomer complementary to c-myc mRNA inhibits proliferation of HL-60 promyelocytic cells and induces differentiation. Mol Cell Biol. 1988 Feb;8(2):963–973. doi: 10.1128/mcb.8.2.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell K. E., Devaney E., Gruenberg J. Subcellular fractionation of tissue culture cells. Trends Biochem Sci. 1989 Feb;14(2):44–47. doi: 10.1016/0968-0004(89)90040-6. [DOI] [PubMed] [Google Scholar]
- Inoue H., Hayase Y., Imura A., Iwai S., Miura K., Ohtsuka E. Synthesis and hybridization studies on two complementary nona(2'-O-methyl)ribonucleotides. Nucleic Acids Res. 1987 Aug 11;15(15):6131–6148. doi: 10.1093/nar/15.15.6131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iribarren A. M., Sproat B. S., Neuner P., Sulston I., Ryder U., Lamond A. I. 2'-O-alkyl oligoribonucleotides as antisense probes. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7747–7751. doi: 10.1073/pnas.87.19.7747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lardeux B. R., Mortimore G. E. Amino acid and hormonal control of macromolecular turnover in perfused rat liver. Evidence for selective autophagy. J Biol Chem. 1987 Oct 25;262(30):14514–14519. [PubMed] [Google Scholar]
- Leonetti J. P., Mechti N., Degols G., Gagnor C., Lebleu B. Intracellular distribution of microinjected antisense oligonucleotides. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2702–2706. doi: 10.1073/pnas.88.7.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loke S. L., Stein C. A., Zhang X. H., Mori K., Nakanishi M., Subasinghe C., Cohen J. S., Neckers L. M. Characterization of oligonucleotide transport into living cells. Proc Natl Acad Sci U S A. 1989 May;86(10):3474–3478. doi: 10.1073/pnas.86.10.3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McShan W. M., Rossen R. D., Laughter A. H., Trial J., Kessler D. J., Zendegui J. G., Hogan M. E., Orson F. M. Inhibition of transcription of HIV-1 in infected human cells by oligodeoxynucleotides designed to form DNA triple helices. J Biol Chem. 1992 Mar 15;267(8):5712–5721. [PubMed] [Google Scholar]
- Pagano R. E., Longmuir K. J. Phosphorylation, transbilayer movement, and facilitated intracellular transport of diacylglycerol are involved in the uptake of a fluorescent analog of phosphatidic acid by cultured fibroblasts. J Biol Chem. 1985 Feb 10;260(3):1909–1916. [PubMed] [Google Scholar]
- Pagano R. E., Martin O. C. A series of fluorescent N-acylsphingosines: synthesis, physical properties, and studies in cultured cells. Biochemistry. 1988 Jun 14;27(12):4439–4445. doi: 10.1021/bi00412a034. [DOI] [PubMed] [Google Scholar]
- Postel E. H., Flint S. J., Kessler D. J., Hogan M. E. Evidence that a triplex-forming oligodeoxyribonucleotide binds to the c-myc promoter in HeLa cells, thereby reducing c-myc mRNA levels. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):8227–8231. doi: 10.1073/pnas.88.18.8227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quartin R. S., Brakel C. L., Wetmur J. G. Number and distribution of methylphosphonate linkages in oligodeoxynucleotides affect exo- and endonuclease sensitivity and ability to form RNase H substrates. Nucleic Acids Res. 1989 Sep 25;17(18):7253–7262. doi: 10.1093/nar/17.18.7253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seglen P. O., Gordon P. B., Holen I. Non-selective autophagy. Semin Cell Biol. 1990 Dec;1(6):441–448. [PubMed] [Google Scholar]
- Shaw J. P., Kent K., Bird J., Fishback J., Froehler B. Modified deoxyoligonucleotides stable to exonuclease degradation in serum. Nucleic Acids Res. 1991 Feb 25;19(4):747–750. doi: 10.1093/nar/19.4.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha N. D., Cook R. M. The preparation and application of functionalised synthetic oligonucleotides: III. Use of H-phosphonate derivatives of protected amino-hexanol and mercapto-propanol or -hexanol. Nucleic Acids Res. 1988 Mar 25;16(6):2659–2669. doi: 10.1093/nar/16.6.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sproat B. S., Lamond A. I., Beijer B., Neuner P., Ryder U. Highly efficient chemical synthesis of 2'-O-methyloligoribonucleotides and tetrabiotinylated derivatives; novel probes that are resistant to degradation by RNA or DNA specific nucleases. Nucleic Acids Res. 1989 May 11;17(9):3373–3386. doi: 10.1093/nar/17.9.3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein C. A., Subasinghe C., Shinozuka K., Cohen J. S. Physicochemical properties of phosphorothioate oligodeoxynucleotides. Nucleic Acids Res. 1988 Apr 25;16(8):3209–3221. doi: 10.1093/nar/16.8.3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg T. H., Newman A. S., Swanson J. A., Silverstein S. C. Macrophages possess probenecid-inhibitable organic anion transporters that remove fluorescent dyes from the cytoplasmic matrix. J Cell Biol. 1987 Dec;105(6 Pt 1):2695–2702. doi: 10.1083/jcb.105.6.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Fluorescent labeling of endocytic compartments. Methods Cell Biol. 1989;29:137–151. doi: 10.1016/s0091-679x(08)60192-2. [DOI] [PubMed] [Google Scholar]
- Thierry A. R., Dritschilo A. Intracellular availability of unmodified, phosphorothioated and liposomally encapsulated oligodeoxynucleotides for antisense activity. Nucleic Acids Res. 1992 Nov 11;20(21):5691–5698. doi: 10.1093/nar/20.21.5691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickstrom E. L., Bacon T. A., Gonzalez A., Freeman D. L., Lyman G. H., Wickstrom E. Human promyelocytic leukemia HL-60 cell proliferation and c-myc protein expression are inhibited by an antisense pentadecadeoxynucleotide targeted against c-myc mRNA. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1028–1032. doi: 10.1073/pnas.85.4.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]