Abstract
Aim:
This study has been designed to evaluate the effect of strong (MTAD) or soft (1- hydroxyethylidene – 1, 1-bisphosphonate (HEBP) final irrigating solution on the shear bond strength of AH plus sealer to coronal dentin. 17% EDTA was used as the reference.
Materials and Methods:
Forty freshly extracted human maxillary first premolars were prepared using different irrigation protocols (n=10). All the test groups had 1.3% NaOCl as initial rinse and followed by specific final rinse for each group: G1 – distilled water(control), G2 – 17% EDTA, G3- 18% HEBP and G4 – MTAD. Sections of polyethylene tubes that are 3mm long were filled with freshly mixed sealer and placed on the dentin surfaces. The bonding between the sealer and dentine surface was evaluated using shear bond testing. The values were statistically evaluated using one-way ANOVA followed by Tukey's test.
Result:
Significant difference was found among the irrigating regimes. EDTA showed highest bond strength followed by HEBP and MTAD
Keywords: AH plus, ethylenediaminetetraacetic acid, final irrigants, hydroxyethylidene-1, 1-bisphosphonate, mixture of tetracycline, acid and detergent
INTRODUCTION
The success of root canal treatment depends on the control of microorganisms in infected root canals. Root canal treatment is performed in several steps; one of the most important phases is cleaning and shaping, when instruments and irrigating solutions are used. During this phase, organic and inorganic matter remaining in the root canal must be removed so that root canal filling might adapt to the canal perfectly.[1–6] A sealer along with gutta- percha is used to achieve a fluid impervious apical seal. The sealer serves as a lubricant when inserting the gutta- percha point, as a filling material to fill the irregularities of preparation, and is necessary because gutta- percha does not bond spontaneously to the dentinal walls of the prepared canal.[7] During root canal preparation, a smear layer is formed on the walls of the canal[8] which consists of dentin debris, including pulp remnants, bacteria and endotoxins.[9] The smear layer prevents the penetration of the sealer into dentin tubule which increases the potential for microleakage.[10] Hence, it has to be removed.
In addition to NaOCl, the use of a chelating agent to remove the smear layer has been proved to be essential.[11] Hence, a final irrigant plays a key role in the success of a root canal treatment. Commonly used final irrigants in endodontics are ethylenediaminetetraacetic acid (EDTA),[12] mixture of tetracycline, acid and detergent (MTAD)[13] and 1-hydroxyethylidene-1, 1-bisphosphonate (HEBP).[14] Adhesion of the root canal filling on the dentinal walls is advantageous for two main reasons. In a static situation, it should eliminate any space that allows the percolation of fluids between the obturating material and the dentin wall.[15] In a dynamic situation, it is needed to resist dislodgement of the filling during subsequent manipulation.[16] A fluid tight seal cannot be obtained without the use of a sealer, because gutta-percha does not spontaneously bond to dentin walls.[17]
There have been no reports comparing the effect of HEBP with MTAD and EDTA when employed as final rinse on the shear bond strength, of commonly employed endodontic sealer. Hence the aim of our study has been to evaluate the effect of various final irrigants (EDTA, MTAD and HEBP) on shear bond strength of AH plus sealer.
MATERIALS AND METHODS
Forty freshly extracted human maxillary first premolars were scaled to remove all adhering soft tissue and debris, washed under running tap water, placed in distilled water, and refrigerated at 4°C. The coronal two-thirds were removed with a low speed diamond saw and the exposed dentin surfaces were employed. The teeth were fixed with cold cure acrylic resin to a plastic cylindrical ring (2cm in diameter and 2.5cm deep). The rings were filled with auto polymerizing polymethyl methacrylate resin (PMMA) mixed in accordance with the manufacturer's instructions to embed the tooth with its coronal surface exposed. After the PMMA had set, the coronal surface of tooth was ground with wet waterproof polishing paper through grades 240, 320, 400, and 600 on a Handimet grinder (Buehler, Lake Bluff, U.S.A) to get a flat, superficial layer of dentin. Three strokes in two directions perpendicular to each other for every grade served to standardize the surface preparation of the dentin. The smear layer was not disturbed. The prepared teeth were divided into four test groups comprising 10 teeth each: All the test groups had an initial rinse of 1.3% NaOCl for 20 min followed by the specific final rinses for each group: Group I samples were irrigated with distilled water (Control), Group II with 17% EDTA for 1 min, group III with 18% HEBP for 5 min and group IV with MTAD for 5 min.
Polyethylene tubes were cut to form 3mm high cylinders. These cylinders were used to apply the sealers on to the dentin with a constant surface area of 3.45cm2. To restrict the sealer to a particular area of dentin, adhesive Teflon tape with a hole with respect to the size of the cylinder's contact area was affixed to the dentin.[18] A window to the predetermined bond area was provided by this hole. The epoxy type sealer (AH plus, Dentsply De Trey, Gmbh, Konstanz, Germany) was left overnight in a conditioned temperature and humidity room (22°C and 25%). After their initial set, they were transferred to an incubator at 37°C. All the specimens were then stored for a period of 1 week. The specimens were removed from the incubator; air dried, and then they were engaged perpendicularly at their bases on a universal testing machine (Model 4411, Instron, warren, Michigan) at a crosshead speed of 0.5mm/min. The probe was positioned so that the chisel would travel parallel to the dentinal surface and contact the sealer cylinder at its interface with this surface. The shear force required to separate the cylinder from the dentin was recorded in Newtons (N) for each specimen, then divided by the contact surface area to determine the shear bond strength in mega pascals (MPa).
Statistical analysis
One - Way ANOVA followed by Tukey's test was used to analyze the data. Significance was established at P< 0.05 level.
RESULTS
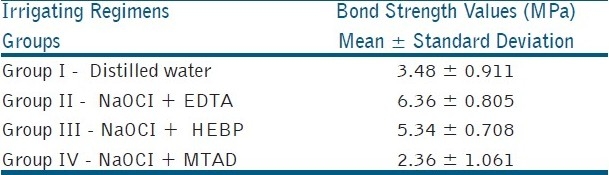
Comparing the shear bond strengths, EDTA (group II – 6.36 ± 0.805 Mpa) showed the highest bond strength which was followed by HEBP (group III - 5.34 ± 0.708 Mpa), control (group I – 3.48 Mpa), MTAD (group IV- 2.36 ± 1.061 Mpa) [Table 1].
Comparing EDTA and HEBP, no statistical difference was found between them.
Statistical difference was found between EDTA, HEBP to control and MTAD.
Comparing the control and MTAD, control was statistically better than MTAD.
Table 1.
Bond strength values of dentin treated with various irrigants
DISCUSSION
Leakage studies are much more common than adhesion studies in evaluating the fluid impervious apical seal of various endodontic sealers. However, it has been shown, on the teeth, that leakage study methods may provide varying results.[19] In addition, leakage studies do not reveal which of the two interfaces, dentine–sealer or gutta-percha–sealer, is leaking. They also do not furnish any insight on the mechanism of how the combination of two different materials can contribute to an apical seal.[20] Adhesion tests measure either tensile bond strength, where the bond is broken by a force perpendicular to the interface between material and surface, or shear strength where the force is parallel to the interface between the material and surface. The shear test was developed for measuring the bond of endodontic sealers to dentine and gutta-percha, and has been proven to be effective and reproducible.[21,22] This test model does not replicate clinical conditions. Attempts to closely duplicate these have resulted in complicated models that are difficult to reproduce and sometimes even to interpret.[23] Root dentine is not uniform and the surface of the canal walls that has been prepared during the endodontic treatment may differ widely. This is true not only between specimens, but also between sites in the same root, according to the level or even the direction of the wall-proximal or faciolingual. Therefore, coronal, rather than root dentine, was used for better reproducibility. Eldeniz et al, tested three resin-based sealers and found that AH plus exhibited the highest shear bond strength values to dentin.[24] Hence in our study we used AH plus sealer.
In our study EDTA showed higher bond strength, which is in concurrence with previous studies that have employed NaOCl followed by 17% EDTA as final irrigants.[24,25] EDTA showed higher bond strength followed by HEBP, the possible reason might be due to the removal of smear layer. Smear layer removal procedures allow the sealer penetration into the dentinal tubules and thus could increase the dentin bond strength of resin based sealer as well as an enhanced seal.[24,26] Other possible reason might be the depth of demineralized zone, which is a critical factor. Garcia-godoy et al,[27] showed that the EDTA created demineralized dentin zone about 2-4μm deep. Being a soft chelating agent, HEBP creates demineralized dentin zone less compared to EDTA.
Even though MTAD had better smear layer removal efficacy and demineralized dentin zone (8 to 12μm) compared to EDTA and HEBP, shear bond strength showed less. Reason for that might be the degradation product,[28] which might interfere in the sealing ability of the sealers. Tay et al, reported red-purple staining of light-exposed, root-treated dentine when root canals were rinsed with 1.3% NaOCl as initial rinse, followed by the use of MTAD as final rinse.[28] This reaction is of redox nature that highly resembled the previous mechanism of tetracycline staining. This process involves the oxidation of doxycycline by NaOCl wherein 1 mol of oxygen is absorbed per mole of adsorbed tetracycline and converted to a red-purple product. This red-purple degradation product that resulted from photo-oxidation of doxycycline was found to be 4-alpha, 12-alpha-anhydro- 4-oxo-4-dedi methylaminotetracycline (AODTC) with a high affinity for hydroxyl apatite. The conversion of dentine bound yellow precipitate to red-purple-stained dentine probably requires light exposure, as stained dentine was absent when the specimens were stored in the dark but appeared when the light-protected specimens were subsequently exposed to light.[28]
Conclusion
From the shear bond aspect, EDTA was the better irrigant to be used as a final rinse.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.McComb D, Smith DC. A preliminary scanning electron microscopic study of root canal after endodontic procedures. J Endod. 1975;1:238–42. doi: 10.1016/S0099-2399(75)80226-3. [DOI] [PubMed] [Google Scholar]
- 2.Dautel-Morazin A, Vulcain JM, Bonnaure-Mallet M. An ultrastructural study of the smear layer: Comparative aspects using secondary electron image and backscattered electron image. J Endod. 1994;20:531–4. doi: 10.1016/S0099-2399(06)80066-X. [DOI] [PubMed] [Google Scholar]
- 3.Torabinejad M, Handysides R, Khademi AA, Bakland LK. Clinical implications of the smear layer in endodontics: A review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94:658–66. doi: 10.1067/moe.2002.128962. [DOI] [PubMed] [Google Scholar]
- 4.Hülsmann M, Heckendorff M, Lennon A. Chelating agents in root canal treatment: Mode of action and indications for their use. Int Endod J. 2003;36:810–30. doi: 10.1111/j.1365-2591.2003.00754.x. [DOI] [PubMed] [Google Scholar]
- 5.Zehnder M. Root canal irrigants. J Endod. 2006;32:389–98. doi: 10.1016/j.joen.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 6.Sen BH, Wesselink PR, Türkün M. The smear layer: A phenomenon in root canal therapy. Int Endod J. 1995;28:141–8. doi: 10.1111/j.1365-2591.1995.tb00289.x. [DOI] [PubMed] [Google Scholar]
- 7.Hata G, Kawazoe S, Toda T, Weine FS. Sealing ability of thermafill with and without sealer. J Endod. 1992;18:322–6. doi: 10.1016/s0099-2399(06)80481-4. [DOI] [PubMed] [Google Scholar]
- 8.Goldman M, White RR, Moser CR, Tenca JI. A comparison of three methods of cleaning and shaping the root canal in vitro. J Endod. 1988;14:7–12. doi: 10.1016/s0099-2399(88)80235-8. [DOI] [PubMed] [Google Scholar]
- 9.Mader CL, Baumgartner JC, Peters DD. Scanning electron microscopic investigation of the smeared layer on root canal walls. J Endod. 1984;10:477–83. doi: 10.1016/S0099-2399(84)80204-6. [DOI] [PubMed] [Google Scholar]
- 10.Kennedy WA, Walker WA, Gough RW. Smear layer removal effects on apical leakage. J Endod. 1986;12:21–5. doi: 10.1016/S0099-2399(86)80277-1. [DOI] [PubMed] [Google Scholar]
- 11.Torabinejad M, Handysides R, Khademi A, Bakland L. Clinical implications of the smear layer in endodontics: A review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94:658–66. doi: 10.1067/moe.2002.128962. [DOI] [PubMed] [Google Scholar]
- 12.Aktener BO, Bikay U. Smear layer removal with different concentrations of EDTA mixtures. J Endod. 1993;19:228–31. doi: 10.1016/S0099-2399(06)81296-3. [DOI] [PubMed] [Google Scholar]
- 13.Beltz RE, Torabinejad M, Pouresmail M. Quantitative analysis of the solubilizing action of MTAD, sodium hypochlorite, and EDTA on bovine pulp and dentin. J Endod. 2003;29:334–7. doi: 10.1097/00004770-200305000-00004. [DOI] [PubMed] [Google Scholar]
- 14.De-Deus G, Zehnder M, Reis C, Fidel S, Fidel RA, Galan J, Jr, et al. Longitudinal co-site optical microscopy study on the chelating ability of etidronate and EDTA using a comparative single-tooth model. J Endod. 2008;34:71–5. doi: 10.1016/j.joen.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 15.Orstavik D, Eriksen HM, Beyer- Olsen EM. Adhesive Properties and leakage of root canal sealers in vitro. Int Endod J. 1983;16:59–63. doi: 10.1111/j.1365-2591.1983.tb01297.x. [DOI] [PubMed] [Google Scholar]
- 16.Stewart GG. A comparative study of three root canal sealing agents (Part 1) Oral Surg Oral Med Oral Pathol. 1958;11:1029–4. doi: 10.1016/0030-4220(58)90143-9. [DOI] [PubMed] [Google Scholar]
- 17.Skinner R, Van Himel T. The sealing ability of injection-molded thermoplasticized gutta-percha with without the use of sealers. J Endod. 1987;13:315–7. doi: 10.1016/S0099-2399(87)80112-7. [DOI] [PubMed] [Google Scholar]
- 18.Eldeniz AU, Erdemir A, Belli S. Shear bond strength of three resin based sealers to dentin with and without the smear layer. J Endod. 2005;31:293–6. doi: 10.1097/01.don.0000140577.99708.c8. [DOI] [PubMed] [Google Scholar]
- 19.Pommel L, About I, Pashely D, Camps J. Apical leakage of four endodontic sealers. J Endod. 2003;29:208–10. doi: 10.1097/00004770-200303000-00011. [DOI] [PubMed] [Google Scholar]
- 20.Lee KW, Williams MC, Camps JJ, Pashley DH. Adhesion of endodontic sealers to dentin and gutta-percha. J Endod. 2002;28:684–8. doi: 10.1097/00004770-200210000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Tagger M, Tagger E, Tjan AH, Bakland LK. Shearing bond strength of endodontic sealers to gutta percha. J Endod. 2003;29:191–3. doi: 10.1097/00004770-200303000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Tagger M, Tagger E, Tjan AH, Bakland LK. Measurement of adhesion of endodontic sealers to dentin. J Endod. 2002;28:351–4. doi: 10.1097/00004770-200205000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Orstavik WA. Adhesion of root canal sealers to bovine dentine and gutta percha. Int Endod J. 1990;23:13–9. doi: 10.1111/j.1365-2591.1990.tb00797.x. [DOI] [PubMed] [Google Scholar]
- 24.Eldeniz AU, Erdemir A, Belli S. Shear bond strength of three resin based sealers to dentin with and without the smear layer. J Endod. 2005;31:293–6. doi: 10.1097/01.don.0000140577.99708.c8. [DOI] [PubMed] [Google Scholar]
- 25.Gopikrishna V, Venkateshbabu N, Krithikadatta J, Kandaswamy D. Evaluation of the effect of MTAD in comparison with EDTA when employed as the final rinse on the shear bond strength of three endodontic sealers to dentine. Australian Endodontic Journal. doi: 10.1111/j.1747-4477.2010.00261.x. doi: 101111/j1747-4477201000261x. [DOI] [PubMed] [Google Scholar]
- 26.Behrend GD, Cutler CW, Gutmann JL. An in vitro study of smear layer removal and microbial leakage along the root canal fillings. Int Endod J. 1996;29:99–107. doi: 10.1111/j.1365-2591.1996.tb01169.x. [DOI] [PubMed] [Google Scholar]
- 27.García-Godoy F, Loushine RJ, Itthagarun A, Weller RN, Murray PE, Feilzer AJ, et al. Application of biologically oriented dentin bonding principles to the use of endodontic irrigants. Am J Dent. 2005;18:281–90. [PubMed] [Google Scholar]
- 28.Tay FR, Mazzoni A, Pashley DH, Day TE, Ngoh EC, Breschi L. Potential iatrogenic tetracycline staining of endodontically treated teeth via NaOCl/MTAD irrigation: A preliminary report. J Endod. 2006;32:354–9. doi: 10.1016/j.joen.2005.11.006. [DOI] [PubMed] [Google Scholar]


