Abstract
Background
Although consensus guidelines for eosinophilic esophagitis (EoE) have been published, it is unclear whether gastroenterologists follow these recommendations.
Aim
To assess academic and community practice patterns for the evaluation and treatment of EoE and to compare these practices with current guidelines.
Methods
This was a prospective study of academic and community gastroenterologists using a self-administered online survey.
Results
A total of 60% (34 of 57) of academic and 29% (38 of 133) of community gastroenterologists completed the survey. Only 24% of academic and 3% of community gastroenterologists follow consensus guidelines to diagnose EoE (p=0.007). A proton pump inhibitor trial or negative pH study prior to diagnosis was required by just 25% of all gastroenterologists. The majority (60%) do not use the recommended threshold of 15 eosinophils per high powered field to diagnosis EoE. Half (51%) mistakenly require a positive endoscopic finding. For first line treatment, about half of the gastroenterologists surveyed treat with a swallowed topical steroid (53% academic, 56% community; p=NS), consistent with the guidelines.
Conclusions
There is variability in practice patterns for both diagnosis and treatment of EoE. Ongoing education and research concerning diagnosis and treatment is needed.
Keywords: Eosinophilic esophagitis, diagnosis, treatment, practice patterns
Introduction
Eosinophilic esophagitis (EoE) is a chronic allergic disease of the esophagus characterized by dysphagia and food impaction in adults.(1) This once rare diagnosis has become increasingly common, with various authors crediting a combination of growing awareness and increased incidence.(2–7) Given the initial heterogeneity of disease definitions (8) and rapidly evolving knowledge base for this disease, consensus guidelines for the diagnosis and treatment of EoE were published in 2007.(1)
According to these guidelines, a diagnosis of EoE should be made when a patient presents with symptoms of esophageal dysfunction and esophageal biopsy demonstrates 15 or more eosinophils in a high power field (eos/hpf) in the absence of competing causes such as gastroesophageal reflux disease (GERD).(1) To rule out GERD, a trial of a high dose proton-pump inhibitor (PPI) therapy or pH monitoring is recommended.(1) For treatment, guidelines recommend swallowed topical corticosteroids for mild to moderate cases or a finite course of systemic corticosteroids for more severe disease.(1) Recently, other treatment options for EoE have been investigated including mast cell inhibitors, leukotriene receptor antagonists, immune modulators and diet modification. There is, as yet, no universal approach to treatment.(9)
Little is known regarding real-world practices in the diagnosis and management of EoE. It is unclear whether most gastroenterologists follow the consensus guidelines when making a diagnosis of EoE. Further, in the setting of several treatment options and limited evidence, therapy for EoE may be heterogeneous. To date, no study has assessed the practice patterns of gastroenterologists in the United States who diagnosis and treat EoE and compared these practices with the guidelines. Understanding these practice patterns has the potential to identify areas in need of continuing education and to guide future research and guideline development. The purpose of this study was to assess academic and community practice patterns for the evaluation and treatment of eosinophilic esophagitis and to compare these practices with the current guidelines.
Methods
Study Design
This was a prospective study of academic and community gastroenterologists using a self-administered online survey. The survey was conducted between March 2010 and May 2010. This study was approved by the University of North Carolina Institutional Review Board. All participants consented to study participation.
Survey Design
The survey was composed of twenty-five questions which assessed four categories: (1) symptoms and endoscopic findings of EoE, (2) diagnosis of EoE, (3) treatment of EoE, and (4) respondent characteristics (see Online Appendix). The survey was piloted prior to the primary study to assess comprehensibility and comprehensiveness.
Study Population and Survey Administration
In order to assess findings across a spectrum of practice patterns, we surveyed two provider groups. The first was a sample of academic gastroenterologists primarily in adult patients from referral centers across the United States who concentrate in esophagology or international EoE experts. They were identified by their peer-reviewed publication record and/or national presentations related to research in esophageal disease, including EoE. The second group was a random sample of practicing North Carolina adult gastroenterologists identified through activity in state university-run continuing medical education programs.
Potential subjects were emailed an Institutional Review Board approved invitation to participate and a link to the survey. The survey could only be accessed via an email link and could only be completed once. Two follow-up e-mail reminders to participate were also sent. All responses were anonymous. No incentives to participate were provided.
Analysis
Means and standard deviations are reported for continuous variables. Proportions are reported for categorical data. To compare respondents’ characteristics, we used a 2 sample t-test, the Pearson’s chi-square test or the Fisher’s Exact Test, as appropriate. To assess respondent’s criteria for diagnosis, we asked “Which of the following do you require to make the diagnosis of EoE?” The respondent checked the answer (s) they felt most appropriate from a list of individual criteria including: consistent symptoms, positive endoscopy findings, positive biopsy findings, no clinical response to a PPI trial, no histologic response to a PPI trial, and negative pH and testing (see Appendix). Individual components were combined for the analysis. All tests of significance were two-tailed and alpha values <0.05 were considered significant. Analyses were performed with STATA software, version 11.0 (College Station, Texas).
Results
We distributed 190 surveys. Of the academic gastroenterologists, 34 of 57 completed the survey (60% response rate) and of the community gastroenterologists, 38 of 133 completed the survey (29% response rate). Respondent characteristics are detailed in Table 1. As expected, all of the academic gastroenterologists identified themselves as sub-specialized in esophageal disease or therapeutic endoscopy compared with 37% of the community gastroenterologists (p≤0.001). Academic gastroenterologists report caring for a greater volume of EoE patients per month compared with community gastroenterologists (mean 6 ± 8 vs. 2 ± 2; p=0.005). Academic gastroenterologists were also more likely to report that they were “very familiar” with the EoE consensus guidelines than community gastroenterologists (47% vs. 19%; p=0.01). Academic and community gastroenterologists did not differ significantly in years of practice (mean 14 ± 10 vs. 17 ± 10; p=NS).
Table 1.
Characteristics of respondents
| All Respondents (n= 72) | Academic GI (n= 34) | Community GI (n=38) | p-value* | |
|---|---|---|---|---|
| Years in practice (mean ± s.d.) | 16 ± 10 | 14 ± 10 | 17 ± 10 | NS |
| Sub-specialized in esophageal disease and/or therapeutic endoscopy (%) | 67% | 100% | 37% | ≤ 0.001 |
| General GI practice (%) | 29% | 3% | 53% | ≤ 0.001 |
| “Very familiar” with EoE consensus guidelines (%) | 33% | 47% | 19% | 0.01 |
| EoE patients per month (mean ± s.d.) | 4 ± 6 | 6 ± 8 | 2 ± 2 | 0.005 |
EoE, eosinophilic esophagitis; GI, gastroenterologist; NS, not significant
Comparison between academic and community GI performed with 2 sample t-test, Pearson’s chi-square test or Fisher’s Exact Test
Symptoms and Endoscopic Findings of EoE
A similar proportion of academic and community gastroenterologists identified heartburn, refractory reflux, chest pain, dysphagia, and food impaction as symptoms that would make them consider the diagnosis of EoE (Table 2). Significantly more academic gastroenterologists identified regurgitation as a symptom of EoE compared with community gastroenterologists (24% vs. 5%; p=0.03).
Table 2.
EoE Diagnosis -Symptoms and Endoscopic Findings
| All Respondents (n= 72) | Academic GI (n= 34) | Community GI (n=38) | p-value* | |
|---|---|---|---|---|
| What symptoms would make you consider the diagnosis of EoE: | ||||
| Heartburn (%) | 39% | 47% | 32% | NS |
| Regurgitation (%) | 14% | 24% | 5% | 0.03 |
| Refractory reflux (%) | 53% | 65% | 42% | NS |
| Chest pain (%) | 57% | 56% | 58% | NS |
| Abdominal pain (%) | 6% | 6% | 5% | NS |
| Dysphagia (%) | 97% | 97% | 97% | NS |
| Odynophagia (%) | 38% | 32% | 42% | NS |
| Food impaction (%) | 94% | 97% | 92% | NS |
| Nausea (%) | 8% | 6% | 11% | NS |
| Vomiting (%) | 10% | 12% | 8% | NS |
| Weight loss (%) | 14% | 12% | 16% | NS |
| What endoscopic findings do you consider consistent with the diagnosis of EoE: | ||||
| Esophageal rings (%) | 99% | 100% | 97% | NS |
| Esophageal stricture (%) | 56% | 65% | 47% | NS |
| Narrow caliber esophagus (%) | 76% | 85% | 68% | NS |
| Linear furrows (%) | 99% | 100% | 97% | NS |
| White plaques/exudates (%) | 86% | 97% | 76% | 0.01 |
| Erosive esophagitis (%) | 6% | 9% | 3% | NS |
| Decreased mucosal vascularity (%) | 15% | 27% | 5% | 0.01 |
| Congested esophageal mucosa (%) | 13% | 24% | 3% | 0.007 |
| Mucosal tears after passing the endoscope (%) | 86% | 85% | 87% | NS |
| Hiatal hernia (%) | 1% | 3% | 0% | NS |
| Normal appearing esophagus (%) | 50% | 50% | 50% | NS |
EoE, eosinophilic esophagitis; GI, gastroenterologist; NS, not significant
Comparison between academic and community GI performed Pearson’s chi-square test or Fisher’s Exact Test
A similar proportion of academic and community gastroenterologists identified esophageal rings, esophageal strictures, narrow caliber esophagus, linear furrows, mucosal tears after passing the endoscope and normal appearing esophagus as endoscopic findings that would make them consider the diagnosis of EoE (Table 2). However, more academic gastroenterologists identified decreased mucosal vascularity (27% vs. 5%; p=0.01), congested esophageal mucosa (24% vs. 3%; p=0.007) and white plaques (97% vs. 76%; p=0.01) as possible endoscopic findings of EoE compared with community gastroenterologists.
EoE Diagnosis
The consensus guidelines for EoE suggest that a diagnosis of EoE should be made when a patient presents with: 1) symptoms of esophageal dysfunction, 2) an esophageal biopsy demonstrates 15 or more eos/hpf, and 3) there is an absence of competing causes such as GERD. (1) Less than a quarter of academic gastroenterologists use all three components of the guideline recommendation to make a diagnosis of EoE, while only 3% of community gastroenterologists use all three (24% vs. 3%; p=0.007) (Table 3). Of all of the gastroenterologists surveyed, 44% make a diagnosis of EoE with limited criteria – specifically, 15% require only a positive biopsy, 19% require symptoms and a positive biopsy, and 10% require positive endoscopy findings and a positive biopsy. In addition, 51% require at least a positive endoscopic finding to make a diagnosis of EoE. A third of gastroenterologists surveyed (44% academic, 18% community; p=0.02) require that a patient is on a PPI prior to making the diagnosis of EoE with only 55% of those treating twice daily (Table 3). Just 25% required either a response to PPIs or a negative pH study to make a diagnosis of EoE.
Table 3.
EoE Diagnosis
| All Respondents (n= 72) | Academic GI (n= 34) | Community GI (n=38) | p-value * | |
|---|---|---|---|---|
| Do you put biopsies from different locations in different pathology jars: (% yes) | 65% | 79% | 53% | 0.02 |
| From where in the esophagus do you take biopsies: | ||||
| Proximal + Middle + Distal (%) | 44% | 47% | 42% | NS |
| Proximal + Distal (%) | 33% | 44% | 24% | NS |
| Middle + Distal (%) | 14% | 6% | 21% | NS |
| Proximal + Middle (%) | 4% | 3% | 5% | NS |
| Middle only (%) | 4% | 0% | 8% | NS |
| Do you require that a patient is on a PPI prior to making the diagnosis of EoE: (% yes) | 31% | 44% | 18% | 0.02 |
| If yes, do you use once daily or twice daily dosing? | ||||
| Once daily | 45% | 53% | 29% | NS |
| Twice daily | 55% | 47% | 71% | NS |
| If yes, what is the duration of treatment? | ||||
| Range (weeks) | 4 –12 | 4 –8 | 4 –12 | |
| Mean ± s.d. | 6 ± 2 | 6 ± 2 | 6 ± 3 | NS |
| Which of the following do you require to make the diagnosis of EoE: | ||||
| Positive biopsy only | 15% | 9% | 21% | NS |
| Consistent symptoms only | 0% | 0% | 0% | NS |
| Consistent symptoms + Positive biopsy | 19% | 24% | 16% | NS |
| Positive endoscopy findings + Positive biopsy | 10% | 3% | 16% | NS |
| Consistent symptoms + Positive Biopsy + One of the following: No clinical response to a PPI, No histologic response to a PPI, and/or Negative pH testing** | 13% | 24% | 3% | 0.007 |
| Consistent symptoms + Positive endoscopy findings + Positive biopsy + One of the following: No clinical response to a PPI, No histologic response to a PPI, and/or Negative pH testing | 15% | 15% | 16% | NS |
| Consistent symptoms + Positive endoscopy findings + Positive biopsy | 26% | 27% | 26% | NS |
EoE, eosinophilic esophagitis; GI, gastroenterologist; NS, not significant
Comparison between academic and community GI performed with 2 sample t-test, Pearson’s chi-square test or Fisher’s Exact Test
EoE Consensus Guidelines
The majority of all gastroenterologists surveyed (60%) do not use the currently recommended 15 eos/hpf as their cut-off point for a diagnosis of EoE. Academic gastroenterologists did use the threshold more frequently than community gastroenterologists (59% vs. 24%; p=0.002) (Figure 1b). In contrast, 61% of community gastroenterologists use a threshold equal to or greater than 20 eosinophils per high power field. A notable proportion of academic and community gastroenterologists report that they do not use a specific threshold for making a diagnosis of EoE (21% vs. 13%; p=NS).
Figure 1.
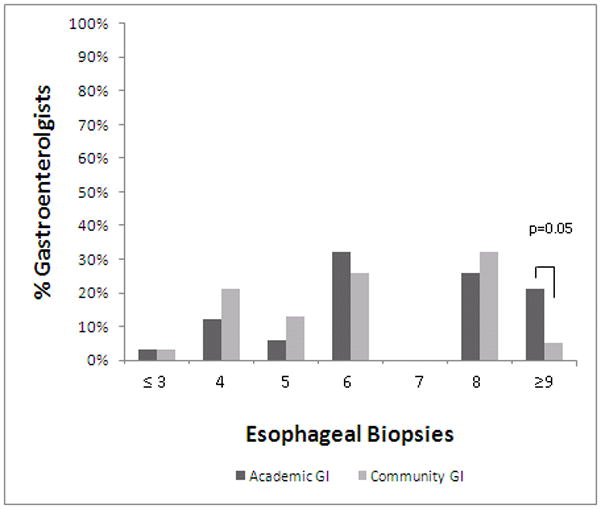
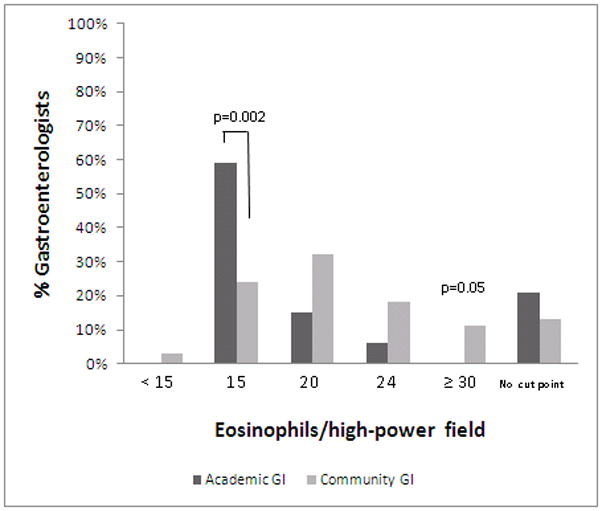
Figure 1a. Number of esophageal biopsies obtained when considering EoE diagnosis. The proportion of academic gastroenterologists obtaining biopsies are in dark bars, and the community gastroenterologists are in light bars. While there was heterogeneity in the number of biopsies taken overall, academic gastroenterologists are more likely to take 9 or more biopsies compared with community gastroenterologists.
Figure 1b. Threshold of eosinophils per high power when making a diagnosis of EoE. Academic gastroenterologists are in dark bars and community gastroenterologists are in light bars. Of the multiple cut-points reported, academic gastroenterologists are more likely to use a threshold of 15 eosinophils per high-power field.
For biopsy procurement, consensus guidelines recommend multiple biopsies throughout the esophagus.(1) There was heterogeneity in biopsy practices among both academic and community gastroenterologists. The majority of both academic and community gastroenterologists biopsy from multiple locations throughout the esophagus when considering a diagnosis of EoE (100% vs. 92%; p=NS) (Table 3). Specific biopsy locations, however, vary greatly (Table 3). The majority of academic and community gastroenterologists take between 4 and 8 biopsies when considering a diagnosis of EoE (76% vs. 92%; p=NS) (Figure 1a). Academic gastroenterologists are more likely to take 9 or more biopsies compared with community gastroenterologists (21% vs. 5%; p=0.05). They are also more likely to put their biopsies from different locations in different pathology jars than community gastroenterologists (79% vs. 53%; p=0.02).
EoE Therapy
For treatment, guidelines recommend swallowed topical corticosteroids for mild to moderate cases or a finite course of systemic corticosteroids for more severe disease.(1) Half of the gastroenterologists surveyed (53% academic, 56% community; p=NS) use a swallowed topical steroid from an inhaler for first line treatment of EoE (Table 4). Of those, the majority of both academic and community gastroenterologists use a total daily dose of 880mcg (53% v 48%; p=NS) or 1760mcg (29% v 14%; p=NS), consistent with consensus guidelines (Figure 2a). A notable proportion use a total daily dose of less than 880mcg per day (12% v 24%; p=NS) or greater than 1760mcg per day (6% v 14%; p=NS). There is also variability in the duration of swallowed topical steroid treatment −59% of gastroenterologists treat for 6–8 weeks, 27% less than 6 weeks and 15% more than 8 weeks (Figure 2b). Approximately one-third of gastroenterologists surveyed (33% academic, 30% community; p=NS) prescribe a PPI as first line treatment for EoE (Table 4).
Table 4.
EoE Medical Treatment
| All Respondents (n= 72) | Academic GI (n= 34) | Community GI (n=38) | p-value * | |
|---|---|---|---|---|
| What is your first line of therapy for treating EoE: | ||||
| Proton pump inhibitor once daily | 14% | 9% | 19% | NS |
| Proton pump inhibitor twice daily | 17% | 24% | 11% | NS |
| Swallowed topical steroid from an inhaler (ie fluticasone) | 54% | 53% | 56% | NS |
| Swallowed topical steroid in liquid form (ie budensonide) | 0% | 0% | 0% | NS |
| Swallowed topical steroid in a thickened liquid form (ie budesonide slurry) | 3% | 3% | 3% | NS |
| Systemic steroid (ie prednisone) | 0% | 0% | 0% | NS |
| Empiric dietary elimination therapy | 1% | 3% | 0% | NS |
| Targeted dietary elimination therapy based on allergy testing | 3% | 3% | 3% | NS |
| Elemental diet | 0% | 0% | 0% | NS |
| Leukotriene antagonist (ie montelukast) | 3% | 0% | 6% | NS |
| Immunomodulator (ie 6MP or azathioprine) | 0% | 0% | 0% | NS |
| Biologic (ie infliximab or mepolizumab) | 0% | 0% | 0% | NS |
| Other | 4% | 6% | 3% | NS |
| All Respondents (n= 41) | Academic GI (n= 19) | Community GI (n=22) | p-value * | |
| Of gastroenterologists who prescribe swallowed steroid first line, what is your second line therapy for EoE in patients who do not respond to initial therapy: | ||||
| Proton pump inhibitor once daily | 5% | 5% | 5% | NS |
| Proton pump inhibitor twice daily | 5% | 5% | 5% | NS |
| Swallowed topical steroid from an inhaler (ie fluticasone) | 5% | 0% | 9% | NS |
| Swallowed topical steroid in liquid form (ie budensonide) | 10% | 11% | 9% | NS |
| Swallowed topical steroid in a thickened liquid form (ie budesonide slurry) | 12% | 16% | 9% | NS |
| Systemic steroid (ie prednisone) | 29% | 32% | 27% | NS |
| Empiric dietary elimination therapy | 7% | 11% | 5% | NS |
| Targeted dietary elimination therapy based on allergy testing | 17% | 16% | 18% | NS |
| Elemental diet | 2% | 5% | 0% | NS |
| Leukotriene antagonist (ie montelukast) | 7% | 0% | 13% | NS |
| Immunomodulator (ie 6MP or azathioprine) | 0% | 0% | 0% | NS |
| Biologic (ie infliximab or mepolizumab) | 0% | 0% | 0% | NS |
| Other | 0% | 0% | 0% | NS |
EoE, eosinophilic esophagitis; GI, gastroenterologist; NS, not significant
Comparison between academic and community GI performed Pearson’s chi-square test or Fisher’s Exact Test
Figure 2.
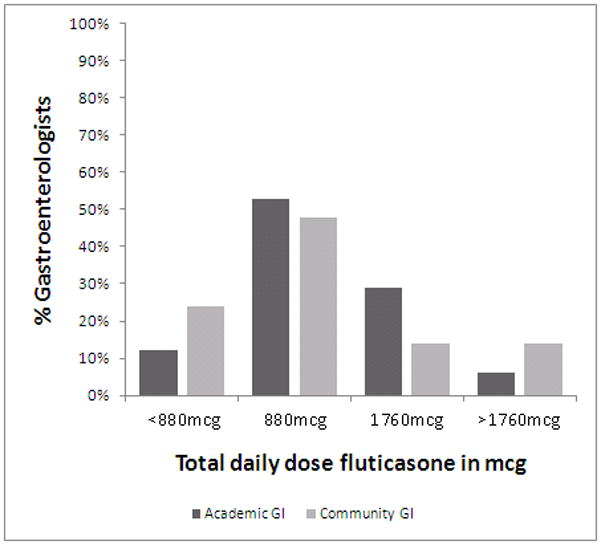
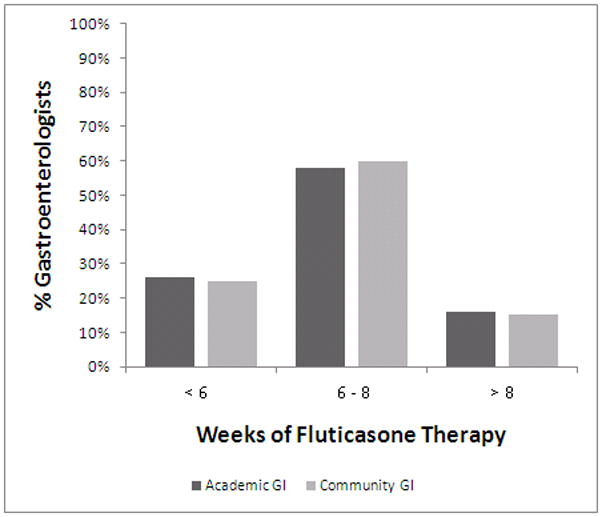
Figure 2a. Total daily dose fluticasone. Academic gastroenterologists are in dark bars and community gastroenterologists are in light bars. While there is a range of doses used, there are no differences between the two provider groups.
Figure 2b. Duration of fluticasone therapy. Academic gastroenterologists are in dark bars and community gastroenterologists are in light bars. While there is a range in the length of therapy prescribed, there are no differences between the two provider groups.
Of the gastroenterologists who prescribe a PPI as first line therapy, all (100%) prescribe a swallowed topical steroid for second line treatment of EoE. Of the gastroenterologists who prescribe a swallowed steroid as first line therapy, there is substantial variation in practice with regards to second line therapy. Specifically, 29% prescribe systemic steroids for second line therapy, 22% prescribe swallowed steroids in a liquid form, 26% prescribe diet modification, 10% prescribe a PPI and 7% prescribe montelukast (Table 4).
Regarding the use of dilation in the setting of EoE, consensus guidelines recommend esophageal dilation for symptomatic patients who present with fixed strictures causing impaction. Academic and community gastroenterologist do not differ significantly in their endoscopic treatment of EoE, although a sizable portion of each group demonstrates reluctance to dilate such patients. Approximately one half of gastroenterologists (academic 53% vs. community 63%; p=NS) would perform esophageal dilation for a patient with suspected EoE in the event of a critical stricture and symptoms of dysphagia (Online Table 5).
Table 5.
Endoscopic Therapy for EoE
| All Respondents (n= 72) | Academic GI (n= 34) | Community GI (n=38) | p-value * | |
|---|---|---|---|---|
| If you see an esophageal stricture when doing an endoscopy for a patient with suspected EoE, would you perform dilation at that initial endoscopy? | ||||
| Yes – always. | 10% | 12% | 8% | NS |
| Yes – but only if it is a critical stricture and the patient is having dysphagia. | 58% | 53% | 63% | NS |
| No – I would wait to confirm the diagnosis and perform dilation after the patient is on treatment but still having symptoms. | 32% | 35% | 29% | NS |
| No – never. | 0% | 0% | 0% | NS |
| If you perform a dilation, what method would you typically use? | ||||
| Wire-guided dilator (ie Savary) | 28% | 35% | 21% | NS |
| Through-the-scope balloon | 63% | 53% | 71% | NS |
| Maloney-dilator | 6% | 3% | 8% | NS |
| Depends on the length of the stricture | 4% | 9% | 0% | NS |
EoE, eosinophilic esophagitis; GI, gastroenterologist; NS, not significant
Comparison between academic and community GI performed with Pearson’s chi-square test or Fisher’s Exact Test
Consensus guidelines recommend that the treatment of EoE should initially target symptom improvement.(1) However, further endoscopic surveillance can be considered in patients willing to accept treatment based on these results.(1) To monitor response to EoE therapy, community gastroenterologists more frequently assess only a patient’s symptoms compared with academic gastroenterologists (86% vs. 50%; p=0.001) (Table 6). In contrast, 47% of academic gastroenterologists monitor and assess response to therapy with a combination of patient symptoms, endoscopic and/or biopsy findings (Online Table 6).
Table 6.
Response to Therapy
| All Respondents (n= 72) | Academic GI (n= 34) | Community GI (n=38) | p-value * | |
|---|---|---|---|---|
| How do you monitor response to therapy: | ||||
| Assess patient symptoms only | 69% | 50% | 86% | 0.001 |
| Repeat endoscopy and assess endoscopic findings only | 3% | 3% | 3% | NS |
| Repeat endoscopy and assess biopsy findings only | 6% | 9% | 3% | NS |
| Assess patient symptoms + Endoscopic findings + Biopsy findings | 11% | 21% | 3% | 0.02 |
| Assess patient symptoms + Biopsy findings | 7% | 9% | 6% | NS |
| Assess patient symptoms + Endoscopic findings | 3% | 6% | 0% | NS |
| Assess endoscopic + Biopsy findings | 1% | 3% | 0% | NS |
| Do you consider a response to therapy: | ||||
| Resolution of symptoms only | 66% | 53% | 78% | 0.03 |
| Resolution of endoscopy findings only | 0% | 0% | 0% | NS |
| Improvement in histology only (ie eosinophils present but less than initially) | 4% | 6% | 3% | NS |
| Normalization of histology only (ie no eosinophils on biopsy) | 3% | 3% | 3% | NS |
| Resolution of symptoms + Improvement or Normalization of histology | 15% | 18% | 13% | NS |
| Resolution of symptoms + Resolution of endoscopy findings + Improvement or Normalization of histology | 11% | 21% | 3% | 0.02 |
| In patients who respond to therapy, do you use maintenance therapy: | ||||
| Yes | 31% | 32% | 31% | NS |
| Sometimes | 56% | 53% | 58% | NS |
| No | 13% | 15% | 11% | NS |
EoE, eosinophilic esophagitis; GI, gastroenterologist; NS, not significant
Comparison between academic and community GI performed with Pearson’s chi-square test or Fisher’s Exact Test
Discussion
EoE is a poorly understood disease with what appears to be a rapidly evolving epidemiology. Practitioners initially struggled with heterogeneous disease definitions, and they are now limited by an underdeveloped literature and the need for additional education. Although consensus guidelines for the diagnosis and treatment of EoE have been published, it has long been unclear whether the diagnosis and management of these patients conformed to those guidelines. While our a priori hypothesis was that real-world practices would strongly diverge from consensus guidelines, we were surprised to find the magnitude of this divergence in both academic subspecialists and community practitioners.
We designed our study sample to enroll academic gastroenterologists who sub-specialized in esophageal disease and community gastroenterologists. Not surprisingly, these academic gastroenterologists care for a greater volume of EoE patients than community gastroenterologists and report greater familiarity with the consensus guidelines. Despite these differences, both the academic and community gastroenterologists deviated substantially from the recommendations of the consensus guidelines. Specifically, actual diagnostic practice diverges from the guidelines in three respects. First, few gastroenterologists formally exclude GERD. Second, the majority of gastroenterologists use a threshold of 20 or greater eos/hpf. Finally, many gastroenterologists require positive endoscopic finding to make a diagnosis of EoE, when endoscopic findings are not part of the current diagnostic criteria. Among those surveyed, there was no predominant diagnostic practice.
Why so few gastroenterologists follow the consensus guidelines for the diagnosis of EoE is unclear. It is possible that gastroenterologists are not familiar with the guidelines. Alternatively, some gastroenterologists may not agree with the diagnostic criteria recommended by the consensus guidelines. There is ongoing controversy about the relationship between EoE and GERD and whether GERD truly needs to be excluded to diagnose EoE. (10–12) Further, the recommendation of a threshold of 15 eosinophils per high power field also remains controversial.(1) These debates aside, it is well accepted that an endoscopically normal appearing esophagus does not preclude the diagnosis of EoE.(1, 8, 13) Nonetheless, our data show many gastroenterologists insist on endoscopic findings for the diagnosis of EoE. This finding suggests that it is lack of knowledge of the literature, rather than a careful weighing of evidence and rejection of the guidelines, that explains the poor adherence to the published guidelines.
The considerable variability in diagnostic criteria has important implications beyond the creation of a heterogeneous population of patients with a diagnosis of EoE. Patients with GERD may be falsely diagnosed with EoE and receive unnecessary and unhelpful steroids. Similarly, patients with EoE may be falsely diagnosed with GERD and exposed to the risks of GERD treatment, up to and including inappropriate anti-reflux surgery.(14) The risks and costs of a missed diagnosis of EoE are unknown.
In addition to variability in diagnostic practices, there were also differences in approaches to treatment. The majority of gastroenterologists use a swallowed topical steroid from an inhaler for first line treatment of EoE. While there is no universally accepted approach to the treatment of EoE, this practice is consistent with the consensus guidelines as well as data from small clinical trials. (1, 15–19) The differences in dose and duration of treatment are not surprising and are reflected in the variability in the literature. A notable proportion of respondents choose a PPI as first line treatment for EoE. This may be evidence that gastroenterologists misunderstand the importance of ruling out GERD or the timing of the PPI in relation to a diagnosis of EoE. Alternatively, there is controversy regarding the role of acid and PPIs in EoE. (10–12) Some authors have suggested that PPIs may have efficacy for EoE via an anti-inflammatory effect.(12, 20) For second line medical therapy, responses mirror the myriad options that have been reported for EoE. The lack of a consistent practice is understandable given that there are very few data to guide therapeutic decision-making in EoE patients that do not respond well to steroid therapy or PPI.
There has been one prior study of practice patterns in EoE but this was prior to the publication of the consensus guidelines.(21) In that survey of international adult and pediatric gastroenterologists, including trainees, King and colleagues found that the majority of their respondents biopsy both at proximal and distal locations within theesophagus when a diagnosis of EoE is being considered, use a threshold of 20 eosinophils per high power field to make a diagnosis of EoE and use fluticasone for treatment of EoE. Because the study population and specific questions differ between this study and ours, it is difficult to directly compare the results. However, a finding common to both studies was heterogeneity in diagnostic and therapeutic practices for this disease state.
Several limitations of our study deserve mention. First, gastroenterologists may have attempted to respond to questions with perceived correct answers or may have referred to the literature. Thus, the results of our study may not reflect actual decision making in clinical practice of those actually surveyed. If our results do reflect the use of the literature to respond, then one might expect such efforts to bias our data toward the more “correct” answers, with respect to compliance with the published guidelines – a concerning prospect, given the findings of our study. Second, in any study relying on surveys, a decrease in response rate threatens the validity of the study. Physician response rate to questionnaires is notoriously low, and our response is similar to other published questionnaire based studies in gastroenterology. (21–24) Finally, because we primarily surveyed adult gastroenterologists, the results cannot be directly applied to practice patterns of pediatric gastroenterologists.
In summary, there was a large degree of heterogeneity in the diagnosis and management of subjects with EoE both in community and sub-specialty GI practice. In many instances, both groups reported practices that did not conform to the current guidelines. A large proportion of gastroenterologists do not rule out GERD prior to making a diagnosis of EoE, use a threshold of 20 or greater eosinophils per high power field, and/or require positive endoscopic finding to make a diagnosis of EoE. These results suggest that ongoing education and research concerning diagnosis and treatment of EoE is needed regardless of the practice setting.
Acknowledgments
Grant support: This work is funded, in part, by NIH award number T32 DK 07634, NIH award number KL2RR025746 from the Nation Center for Research Resources and a Junior Faculty Development Award from the American College of Gastroenterology.
Declaration of funding interests: This study was funded in part, by NIH award number T32 DK 07634, NIH award number KL2RR025746 from the Nation Center for Research Resources and a Junior Faculty Development Award from the American College of Gastroenterology.
Abbreviations
- EoE
Eosinophilic esophagitis
- eos/hpf
eosinophils in a high power field
- GERD
gastroesophageal reflux disease
- PPI
proton-pump inhibitor
Footnotes
Author contributions: 1. Anne F. Peery MD - study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; and statistical analysis
2. Nicholas J. Shaheen MD MPH - study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; and statistical analysis
3. Evan S. Dellon MD MPH – study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; statistical analysis; study supervision
Statement of Interests: Authors’ declaration of personal interests: Evan Dellon has served as a consultant for Oncoscope, and has received research funding from AstraZeneca, ACG, and the NIH.
Nicholas Shaheen has served as a consultant or an advisory board member for AstraZeneca, Takeda, Oncoscope, NeoGenomics and CSA Medical and has received research funding from Astra Zeneca, Takeda, Oncoscope, BARRX Medical, CSA Medical, and Procter & Gamble.
Anne Peery has nothing to declare.
References
- 1.Furuta GT, Liacouras CA, Collins MH, Gupta SK, Justinich C, Putnam PE, et al. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology. 2007 Oct;133(4):1342–63. doi: 10.1053/j.gastro.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 2.Noel RJ, Putnam PE, Rothenberg ME. Eosinophilic esophagitis. N Engl J Med. 2004 Aug 26;351(9):940–1. doi: 10.1056/NEJM200408263510924. [DOI] [PubMed] [Google Scholar]
- 3.Prasad GA, Alexander JA, Schleck CD, Zinsmeister AR, Smyrk TC, Elias RM, et al. Epidemiology of eosinophilic esophagitis over three decades in Olmsted County, Minnesota. Clin Gastroenterol Hepatol. 2009 Oct;7(10):1055–61. doi: 10.1016/j.cgh.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Straumann A, Simon HU. Eosinophilic esophagitis: escalating epidemiology? J Allergy Clin Immunol. 2005 Feb;115(2):418–9. doi: 10.1016/j.jaci.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 5.Ronkainen J, Talley NJ, Aro P, Storskrubb T, Johansson SE, Lind T, et al. Prevalence of oesophageal eosinophils and eosinophilic oesophagitis in adults: the population-based Kalixanda study. Gut. 2007 May;56(5):615–20. doi: 10.1136/gut.2006.107714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dellon ES, Gibbs WB, Fritchie KJ, Rubinas TC, Wilson LA, Woosley JT, et al. Clinical, Endoscopic, and Histologic Findings Distinguish Eosinophilic Esophagitis From Gastroesophageal Reflux Disease. Clin Gastroenterol Hepatol. 2009 Sep 3; doi: 10.1016/j.cgh.2009.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gonsalves N, Kahrilas PJ. Eosinophilic oesophagitis in adults. Neurogastroenterol Motil. 2009 Oct;21(10):1017–26. doi: 10.1111/j.1365-2982.2009.01307.x. [DOI] [PubMed] [Google Scholar]
- 8.Dellon ES, Aderoju A, Woosley JT, Sandler RS, Shaheen NJ. Variability in diagnostic criteria for eosinophilic esophagitis: a systematic review. Am J Gastroenterol. 2007 Oct;102(10):2300–13. doi: 10.1111/j.1572-0241.2007.01396.x. [DOI] [PubMed] [Google Scholar]
- 9.Elliott EJ, Thomas D, Markowitz JE. Non-surgical interventions for eosinophilic esophagitis. Cochrane Database Syst Rev. 2010;3:CD004065. doi: 10.1002/14651858.CD004065.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spechler SJ, Genta RM, Souza RF. Thoughts on the complex relationship between gastroesophageal reflux disease and eosinophilic esophagitis. Am J Gastroenterol. 2007 Jun;102(6):1301–6. doi: 10.1111/j.1572-0241.2007.01179.x. [DOI] [PubMed] [Google Scholar]
- 11.Merwat SN, Spechler SJ. Might the use of acid-suppressive medications predispose to the development of eosinophilic esophagitis? Am J Gastroenterol. 2009 Aug;104(8):1897–902. doi: 10.1038/ajg.2009.87. [DOI] [PubMed] [Google Scholar]
- 12.Kedika RR, Souza RF, Spechler SJ. Potential anti-inflammatory effects of proton pump inhibitors: a review and discussion of the clinical implications. Dig Dis Sci. 2009 Nov;54(11):2312–7. doi: 10.1007/s10620-009-0951-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Attwood SE, Smyrk TC, Demeester TR, Jones JB. Esophageal eosinophilia with dysphagia. A distinct clinicopathologic syndrome. Dig Dis Sci. 1993 Jan;38(1):109–16. doi: 10.1007/BF01296781. [DOI] [PubMed] [Google Scholar]
- 14.Dellon ES, Farrell TM, Bozymski EM, Shaheen NJ. Diagnosis of eosinophilic esophagitis after fundoplication for ‘refractory reflux’: implications for preoperative evaluation. Dis Esophagus. 2010 Apr;23(3):191–5. doi: 10.1111/j.1442-2050.2009.01019.x. [DOI] [PubMed] [Google Scholar]
- 15.Konikoff MR, Noel RJ, Blanchard C, Kirby C, Jameson SC, Buckmeier BK, et al. A randomized, double-blind, placebo-controlled trial of fluticasone propionate for pediatric eosinophilic esophagitis. Gastroenterology. 2006 Nov;131(5):1381–91. doi: 10.1053/j.gastro.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 16.Schaefer ET, Fitzgerald JF, Molleston JP, Croffie JM, Pfefferkorn MD, Corkins MR, et al. Comparison of oral prednisone and topical fluticasone in the treatment of eosinophilic esophagitis: a randomized trial in children. Clin Gastroenterol Hepatol. 2008 Feb;6(2):165–73. doi: 10.1016/j.cgh.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 17.Dohil R, Newbury R, Fox L, Bastian J, Aceves S. Oral Viscous Budesonide Is Effective in Children With Eosinophilic Esophagitis in a Randomized, Placebo-controlled Trial. Gastroenterology. 2010 May 7; doi: 10.1053/j.gastro.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Straumann A, Degen L, Felder S, et al. Budesonide as induction treatment for active eosinophilic esophagitis in adolescents and adults: A randomized, double-blind, placebo-controlled study (Bee-I trial) Gastroenterology. 2008;134(Suppl 1 726):A104. [Google Scholar]
- 19.Straumann A, Conus S, Degen L, Felder S, Kummer M, Engel H, et al. Budesonide is Effective in Adolescent and Adult Patients with Active Eosinophilic Esophagitis. Gastroenterology. 2010 Jul 31; doi: 10.1053/j.gastro.2010.07.048. [DOI] [PubMed] [Google Scholar]
- 20.Zhang X, Cheng E, Huo X, Hormi-Carver K, Andersen J, Spechler S, et al. In esophageal squamous epithelial cell lines from patients with eosinophilic esophagitis (EoE), omeprazole blocks the stimulated secretion of eotaxin-3: A potential anti-inflammatory effect of omeprazole in EoE that is independent of acid inhibition. Gastroenterology. 2010;138(Suppl 1) abstract 877. [Google Scholar]
- 21.King J, Khan S. Eosinophilic esophagitis: perspectives of adult and pediatric gastroenterologists. Dig Dis Sci. 2010 Apr;55(4):973–82. doi: 10.1007/s10620-009-0801-9. [DOI] [PubMed] [Google Scholar]
- 22.Spiegel BM, Farid M, Esrailian E, Talley J, Chang L. Is irritable bowel syndrome a diagnosis of exclusion?: a survey of primary care providers, gastroenterologists, and IBS experts. Am J Gastroenterol. 2010 Apr;105(4):848–58. doi: 10.1038/ajg.2010.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spiegel BM, Farid M, van Oijen MG, Laine L, Howden CW, Esrailian E. Adherence to best practice guidelines in dyspepsia: a survey comparing dyspepsia experts, community gastroenterologists and primary-care providers. Aliment Pharmacol Ther. 2009 Apr 15;29(8):871–81. doi: 10.1111/j.1365-2036.2009.03935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adler DG, Hilden K, Wills JC, Quinney E, Fang JC. What drives US gastroenterology fellows to pursue academic vs. non-academic careers?: Results of a national survey. Am J Gastroenterol. 2010 Jun;105(6):1220–3. doi: 10.1038/ajg.2010.101. [DOI] [PubMed] [Google Scholar]


