Abstract
Background and study aims
The impact of the diagnosis and treatment of dysplastic Barrett’s esophagus (BE) on quality of life (QoL) is poorly understood. This study assessed the influence of dysplastic BE on QoL and evaluated if endoscopic treatment of dysplastic BE with radiofrequency ablation (RFA) improves QoL.
Patients and methods
We analyzed changes in QoL in the AIM Dysplasia Trial—a multicenter study of patients with dysplastic BE randomized to RFA therapy or a sham intervention. We developed a 10-item questionnaire to assess the influence of dysplastic BE on QoL. The questionnaire was completed by subjects at baseline and 12 months.
Results
One hundred and twenty-seven patients were randomized to RFA (n=84) or sham (n=43). At baseline, the majority of subjects reported esophageal cancer worry (71% RFA, 85% sham) and esophagectomy worry (61% RFA, 68% sham). Subjects reported depression, impaired QoL, worry, stress and dissatisfaction with the condition of their esophagus. Of those randomized, 117 subjects completed the 12 month endpoint. Compared to sham, subjects treated with RFA had significantly reduced esophageal cancer worry (p=0.003) and esophagectomy worry (p=0.009). They also had significantly reduced depression (p=0.02), general worry with the condition of their esophagus (p≤0.001), impact on daily QoL (p=0.009), stress (p=0.03), dissatisfaction with the condition of their esophagus (p≤0.001) and impact on work and family life (p=0.02).
Conclusions
Inclusion in the treatment group of this randomized, sham-controlled trial of RFA was associated with improvement in disease-specific health-related quality of life. This improvement appears secondary to a perceived decrease in the risk of cancer.
Barrett’s esophagus (BE) is a metaplastic change in the lining of the esophagus from stratified squamous epithelium to specialized columnar epithelium.[1] This intestinal metaplasia may progress through low-grade (LGD) and high-grade dysplasia (HGD) to esophageal adenocarcinoma. Intestinal metaplasia is associated with an approximately 0.5% per patient-year risk of developing esophageal adenocarcinoma. For patients with high-grade dysplasia, this risk increases to 5–10% or more per patient-year.[2–5] Esophageal adenocarcinoma has a 5-year survival rate of less than 15%.[6]
QoL has been defined as “those attributes valued by patients, including their resultant comfort or sense of well-being; the extent to which they were able to maintain reasonable physical, emotional, and intellectual function; and the degree to which they retain their ability to participate in valued activities within the family, in the workplace, and in the community.”[7] Several investigators have assessed quality of life (QoL) in patients with BE. A diagnosis of BE with intestinal metaplasia is associated with a decrease in QoL on generic and organ system-specific measures, along with increased health care cost and increased health care utilization.[8] To date, no studies have evaluated QoL in patients with dysplastic BE. In contrast to patients with intestinal metaplasia, patients with dysplastic BE may feel additional anxiety about their risk of developing cancer. Further, management of dysplastic BE requires close follow-up, repeat endoscopy, and invasive procedures. Financial stress may also be greater—a BE diagnosis has been shown to cause increases in life insurance premiums and restricts the availability of health insurance.[9]
Historically, BE with HGD was treated with esophagectomy, a procedure associated with significant morbidity and some mortality.[10] The impact of esophagectomy on QoL in HGD has been rarely studied.[11–13] Endoscopic ablation has evolved as a less invasive alternative to esophagectomy. Successful endoscopic eradication of BE is associated with a decreased risk of esophageal adenocarcinoma; however, the effect of successful endoscopic treatment of dysplastic BE on QoL is not known. [3,14] To evaluate QoL before and after endoscopic treatment of dysplastic BE with radiofrequency ablation (RFA), we have performed an analysis of QoL in the AIM Dysplasia Trial.[14]
Methods
Parent Study Design
The AIM Dysplasia Trial is a multicenter, randomized, sham-controlled study of patients with BE and low- or high-grade dysplasia. Patients were randomized to receive RFA therapy plus endoscopic surveillance or a sham intervention plus surveillance. A detailed description of the study methods have been reported, but are described here in brief.[14] Patients were considered for study inclusion if they were between the ages of 18 and 80 years and had 8 cm in length or less of non-nodular dysplastic BE. All patients with high grade dysplasia were required to have an endoscopic ultrasonography examination negative for lymphadenopathy and esophageal-wall abnormalities within 12 months of enrollment. Endoscopic mucosal resection 8 weeks or more prior to study enrollment was permitted if subsequent endoscopy demonstrated non-nodular dysplasia. Exclusion criteria were pregnancy, active esophagitis or stricture precluding passage of the endoscope, a history of esophageal cancer, esophageal varices, uncontrolled coagulaopathy, or a life expectancy of less than 2 years as judged by the site investigator. All management options including conservative management and esophagectomy were reviewed with all patients who provided written informed consent.
Subjects were randomly assigned in a 2:1 ratio with the use of computer-generated block-randomization to receive either RFA or a sham endoscopic procedure, stratified by degree of dysplasia (high-grade dysplasia (HGD) & low-grade dysplasia (LGD)). All subjects had an upper endoscopy, esophageal intubation with a study catheter and measurement of the esophageal inner diameter. Among subjects in the ablation group, the entire segment of BE was ablated. Among those in the control group, the study catheter was removed and the procedure was terminated.
Subjects in the ablation arm received up to four endoscopic RFA treatments (HALO360 and HALO90, BÂRRX Medical, Sunnyvale, CA). All subjects received esomeprazole 40 mg twice daily (AstraZeneca, LLP, Wilmington, DE) throughout the study. All subjects underwent endoscopic surveillance at 3 month (HGD cohort) or 6 month (LGD cohort) intervals. The primary outcomes at 12 months were complete eradication of dysplasia and complete eradication of intestinal metaplasia.[14]
The study protocol was approved by each site’s Institutional Review Board. All subjects gave written informed consent. The study was monitored by an independent data and safety monitoring committee. The parent study was supported by BÂRRX Medical, maker of the ablation devices used in the protocol. Study medication was provided by AstraZeneca. Support for the preliminary work for the quality of life tool (patient interviews, interview coding and analysis) was provided by NIH R03 DK075842 & NIH P30 DK56350. Statistical analysis and data management were supported by NIH P30 DK034987 & NIH T32 DK 07634.
Assessment of QoL
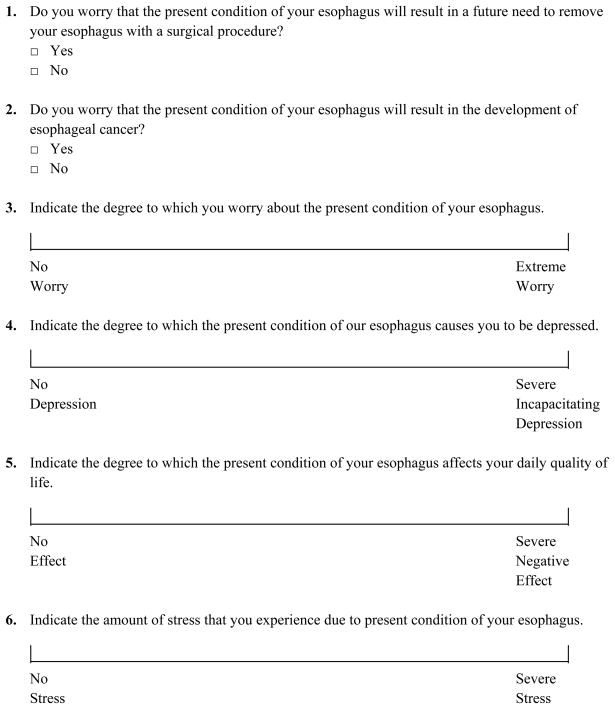
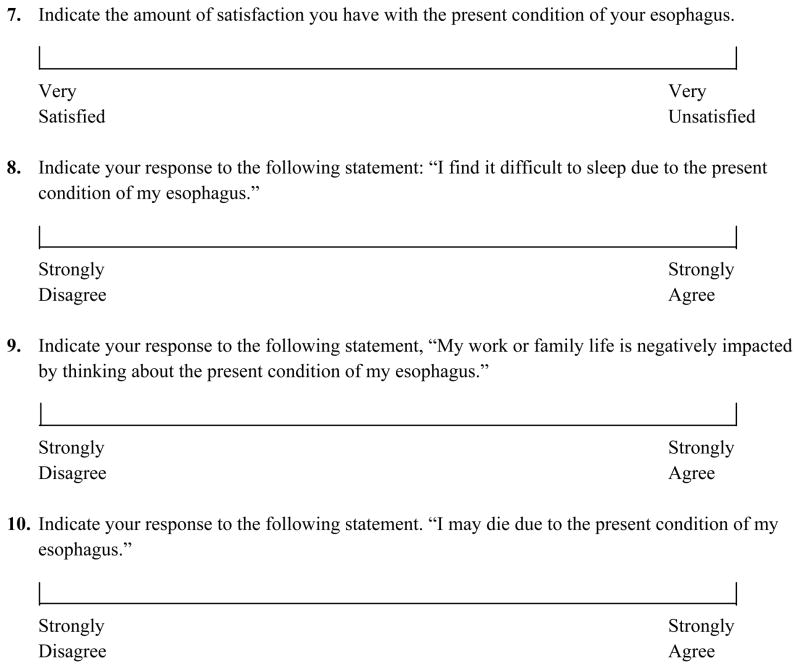
At baseline, each subject completed a self-administered BE QoL questionnaire (Figure 1). Because subjects may misunderstand the current condition of their esophagus, and because quality of life may depend on the subjects’ perception of their condition more so than the actual disease state, subjects also answered a question asking their understanding of the current disease state of their esophagus, with choices including normal esophagus, non-dysplastic BE, BE with LGD, BE with HGD or BE with cancer. Subjects were then randomized to RFA or sham and received care as detailed above. After the 12 month endoscopy, the subject was contacted by study site personnel and their 12 month esophageal biopsy results were disclosed and discussed. Thereafter, the subject again completed the BE QoL questionnaire and again reported their perceived disease state as above. The randomization group to which the patient was assigned (RFA vs. sham) was revealed only after the completed BE QoL questionnaire was received.
Figure 1.
Quality of Life Questionnaire
QoL Questionnaire Development
No validated quality of life measure for BE exists, and measures designed to assess QoL in GERD may miss important domains of QoL in BE. For that reason, we assessed factors impacting QoL in BE. Twenty-five subjects with BE underwent one-on-one interviews with three individuals skilled in qualitative research. These interviews solicited areas of most concern for subjects, and queried the impact of the diagnosis of BE on the subject’s QoL. After thematic saturation had been reached (no new themes were identified), interviews were coded using a qualitative data analysis program, and summary frequency tables of patient concerns were created. A questionnaire was developed based on relevant QoL domains and recurrent thematic responses to the interviews encompassing areas commonly identified by patients as sources of reduction in QoL (Figure 1). This questionnaire consists of 2 yes/no questions, and 8 visual analog scale questions. Pilot testing suggested it was easily understood by patients, and responsive to changes in the subjects’ condition.
Statistical Analysis
Means and standard deviations are reported for continuous variables. Proportions are reported for categorical data. To compare RFA and sham subject’s baseline characteristics, we used a 2 sample t-test, the Pearson’s chi-square test or the Fisher’s Exact Test, as appropriate. To compare RFA and sham baseline QoL scores, we used the Pearson’s chi-square for categorical QoL outcomes and Wilcoxon Rank-sums for continuous QoL outcomes. To further assess the impact of dysplastic BE on QoL, baseline QoL scores were stratified by sex, age, race, dysplasia grade, BE length, and BMI. The stratified analysis was performed with the Fisher’s Exact test and Wilcoxon Rank-sums for categorical variables and Spearman Correlation for continuous variables. To perform bivariate analyses comparing QoL at 12 months based on the outcome of treatment, we used the Fisher’s Exact test for categorical QoL outcomes and Wilcoxon Rank-sums for continuous QoL outcomes.
We performed bivariate analyses to assess the impact of RFA and subject’s histology findings at 12 months on QoL. Seven analyses were performed. The first analysis compared the RFA group to the sham group. The second analysis compared the RFA group achieving complete eradication of intestinal metaplasia to the sham group. The next analysis compared the RFA group without complete eradication of intestinal metaplasia to the sham group. The fourth analysis compared the RFA group with complete eradication of intestinal metaplasia to the RFA group with incomplete eradication of intestinal metaplasia. Analyses 5–7were repeats of analyses 2–4, however, in these three analyses patients were classified using their self-reported perceived disease state as opposed to their actual pathology results.
All tests of significance were two-tailed and alpha values <0.05 were considered significant. No adjustments for multiple comparisons were made. Quantitative analyses were performed with SAS software, version 9.0 (SAS Institute).
Results
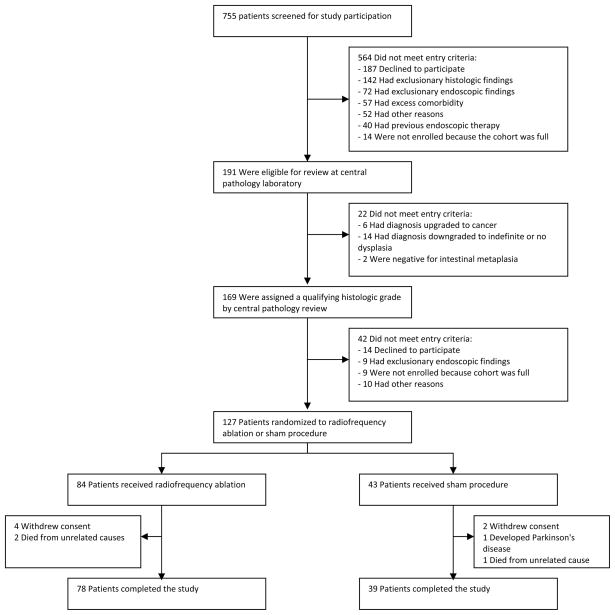
Seven hundred and fifty-five patients were screened for participation and 127 patients met study criteria and were randomized to RFA or sham procedure (Figure 2). Of those randomized, 117 subjects completed the 12 month endoscopy (primary endpoint). In brief, in an intention to treat analysis, we found that among subjects with LGD, 90.5% of those in the RFA group had complete eradication of dysplasia compared with 22.7% of those in the sham group. Among subjects with HGD, 81.0% of those in the RFA group had complete eradication of dysplasia compared with 19.0% of those in the sham group. Among all subjects, 77.4% of those in the RFA group had complete eradication of intestinal metaplasia compared to 2.3% of those in the sham group (p<0.01 for all). By the 12 month endoscopy, 1 subject in the RFA group and 4 subjects in the sham group progressed to esophageal adenocarcinoma (1.2% vs. 9.3%, p<0.05).
Figure 2.
Study enrollment and 12-month outcomes
QoL and Dysplastic BE
At baseline, QoL data was collected on the 127 subjects who met study criteria and were enrolled (intent-to-treat population). Subject demographics and characteristics are detailed in Table 1. At baseline, the majority of subjects reported worry that they would develop esophageal cancer (71% RFA, 85% sham) and worry that they would need an esophagectomy (61% RFA, 68% sham) (Table 2). Given the present state of their esophagus, subjects also reported worry, depression, impaired QoL, stress and dissatisfaction with their esophagus (Table 2). Randomization was successful, in that QoL scores did not differ significantly between the RFA and the sham group (Table 2).
Table 1.
Patient Demographics and Characteristics
| RFA | Sham | |
|---|---|---|
| Patients (n) | 84 | 43 |
| Age (yrs) -mean | 66.1 ± 9.1 | 65.9 ± 8.5 |
| Sex -no. (%) | ||
| Female | 14 (17%) | 3 (7%) |
| Male | 70 (83%) | 40 (93%) |
| Race -no. (%) | ||
| White | 78 (93%) | 43 (100%) |
| Black | 3 (4%) | 0 (0%) |
| Latino | 3 (4%) | 0 (0%) |
| Body mass index -mean* | 28.5 ± 4.9 | 31.3 ± 5.8 |
| Length of Barrett's esophagus (cm) -mean | 4.9 ± 2.2 | 5.0 ± 2.4 |
| High grade dysplasia -no. (%) | 42 (50%) | 21 (49%) |
| Time since diagnosis of Barrett's esophagus (yrs) -mean | 5.2 ± 4.5 | 4.8 ± 5.3 |
| Time since diagnosis of dysplasia (yrs) -mean | 2.1 ± 2.8 | 1.9 ± 2.7 |
RFA = Radiofrequency ablation
Means arereported as means plus-minus the standard deviation
p < 0.05 for the comparison between the RFA and sham group based on 2-sample t-test
Table 2.
Baseline Dysplastic BE QoL
| RFA | Sham | |
|---|---|---|
| Patients (n) § | 84 | 43 |
| Esophagectomy, worry no. (% yes) | 51 (61%) | 28 (68%) |
| Adenocarcinoma, worry no. (% yes) | 60 (71%) | 35 (85%) |
| Worry – mean (0 = none to 100 = extreme) | 44 ± 26 | 41 ± 23 |
| Depression – mean (0 = none to 100 = extreme) | 20 ± 23 | 21 ± 23 |
| Daily QoL – mean (0 = no effect to 100 severe negative effect) | 18 ± 20 | 18 ± 22 |
| Amount of Stress – mean (0 = noneto 100 = severe stress) | 22 ± 25 | 19 ± 23 |
| Amount of Satisfaction – mean (0 = very satisfiedto 100 = very unsatisfied) | 54 ± 31 | 61 ± 31 |
| Difficulty to Sleep – mean (0 = disagreeto 100 = agree) | 21 ± 25 | 24 ± 26 |
| Work or Family Life Negatively Impacted – mean (0 = disagree to 100 = agree) | 22 ± 25 | 23 ± 27 |
| May Die Due to Esophagus – mean (0 = disagreeto 100 = agree) | 24 ± 27 | 27 ± 27 |
RFA = Radiofrequency ablation
Means are reported as means plus-minus the standard deviation
p = not significant for all comparisons between RFA and Sham based on Pearson’s chi-square for categorical QoL outcomes and Wilcoxon Rank-sums for continuous QoL outcomes.
2 Missing Values in Sham Arm
At baseline, women reported a trend toward greater worry that they would develop esophageal cancer than men (94% versus 73%, respectively; p=0.07). Given the present state of their esophagus, women also reported greater worry with the condition of their esophagus (p=0.02), impact on daily QoL (p=0.004) and difficulty with sleep (p=0.03). Increasing age was associated with less esophageal cancer worry (p=0.03) and esophagectomy worry (p=0.04). Increasing age was also associated with less worry with the condition of their esophagus (p≤0.001), depression (p=0.005), amount of stress (p=0.009), difficulty with sleep (p=0.005) and concern regarding dying (p=0.03). Increasing BMI was positively correlated with depression (p=0.02), dissatisfaction with the condition of the esophagus (p=0.02) and difficulty with sleep (p=0.007). Grade of dysplasia, race and BE length was not associated with a statistically significant difference in QoL scores at baseline.
QoL and Dysplastic BE status post RFA
Of the subjects randomized, 117 completed the 12 month endoscopy (Figure 2). One subject in the RFA group and 4 subjects in the sham group progressed to esophageal adenocarcinoma, leaving 112 subjects available for the current QoL analysis. After the 12 month endoscopy, patients were informed of the disease state of their esophagus (normal esophagus, non-dysplastic BE, BE with LGD, BE with HGD or BE with cancer). However, after being given this information, only 59% of subjects correctly reported their current disease state while 32% reported an upgraded diagnosis and 9% a downgraded diagnosis (Table 3). Disease misclassification did not differ by gender or treatment type.
Table 3.
Subject Reported Disease Misclassification
| Final (12 month) Biopsy Results | n | (%) Reported Correct Diagnosis | (%) Reported Upgraded Diagnosis | (%) Reported Downgraded Diagnosis |
|---|---|---|---|---|
| Adenocarcinoma | 4 | 100% | NA | 0% |
| High grade dysplastic BE | 17 | 47% | 0% | 53% |
| Low grade dysplastic BE | 15 | 80% | 20% | 0% |
| Nondysplastic IM | 15 | 33% | 53% | 13% |
| Complete Eradication of IM | 66 | 60% | 40% | NA |
| All Subjects | 117 | 59% | 32% | 9% |
BE = Barrett’s esophagus
IM = Intestinal metaplasia
NA = Not applicable
Compared to the sham group, subjects treated with RFA had improved QoL (Table 4). Specifically, they demonstrated significantly reduced worry that they would develop esophageal cancer (p=0.003) and worry that they would need an esophagectomy (p=0.009) (Table 4). They also had significantly reduced worry about the present condition of their esophagus (p≤0.001), depression (p=0.02), impact on daily QoL (p=0.009), stress (p=0.03), dissatisfaction with the condition of their esophagus ( p≤0.001) and impact on work and family life (p=0.02) compared to sham. Similar results were obtained when we performed the same analysis comparing subjected treated with RFA achieving complete eradication of intestinal metaplasia to the sham group (Table 5) and when comparing those who believed they had achieved complete eradication of intestinal metaplasia to the sham group (Table 5). Compared to the sham group, subjects in the RFA group who did not achieve complete eradication of intestinal metaplasia still had improvement in multiple domains. Specifically, they demonstrated significantly reduced worry that they would develop esophageal cancer (p=0.03), a significant reduction in stress (p=0.05) and dissatisfaction with the condition of their esophagus (p=0.04) (Table 6). The same analysis performed with subject perceived pathology demonstrated that subjects treated with RFA who reported incomplete eradication of intestinal metaplasia compared to the sham group had significantly reduced worry that they would develop esophageal cancer (p≤0.001) and worry that they would need an esophagectomy (p=0.01) (Table6). They also had a significant reduction in worry about the present condition of their esophagus (p=0.005), as well as trends toward improvement in dissatisfaction with their esophagus (p=0.06) and impact on work or family life (p=0.05) (Table6).
Table 4.
QoL after RFA compared to sham
| RFA | Sham | p-value‡ | |
|---|---|---|---|
| Patients (n) | 77 | 35 | |
| Esophagectomy, worry – % no at 12 months | 83% | 43% | 0.009 |
| Adenocarcinoma, worry – % no at 12 months | 78% | 34% | 0.003 |
| Worry – mean change frombaseline | −24 ± 24 | 2 ± 30 | ≤0.001 |
| (0 = none to 100 = extreme) | |||
| Depression – mean change from baseline | −10 ± 23 | 0 ± 25 | 0.02 |
| (0 = none to 100 = extreme) | |||
| Daily QoL – mean change from baseline | −10 ± 21 | 0 ± 28 | 0.009 |
| (0 = no effect to 100 severe negative effect) | |||
| Amount of Stress – mean change from baseline | −12 ± 24 | −1 ± 24 | 0.03 |
| (0 = none to 100 = severe stress) | |||
| Amount of Satisfaction – mean change from baseline | −42 ± 37 | −17 ± 30 | ≤0.001 |
| (0 = very satisfied to 100 = very unsatisfied) | |||
| Difficulty to Sleep – mean change from baseline | −16 ± 23 | −10 ± 24 | NS |
| (0 = disagree to 100 = agree) | |||
| Work or Family Life Negatively Impacted – mean change from baseline | −16 ± 25 | −3 ± 24 | 0.02 |
| (0 = disagree to 100 = agree) | |||
| May Die Due to Esophagus – mean change from baseline | −14 ± 27 | −6 ± 31 | NS |
| (0 = disagree to 100 = agree) |
NS = Not Significant
RFA = Radiofrequency ablation
QoL = Quality of life
CE-IM = Complete eradication of intestinal metaplasia
Means are reported as the mean plus-minus the standard deviation
comparison between RFA and Sham based on Fisher’s Exact test for categorical QoL outcomes and Wilcoxon Rank-sums for continuous QoL outcomes
Table 5.
QoL after RFA with CE-IM (actual and perceived) compared to sham
| RFA with CE-IM actual | p-value§ | RFA with CE-IM perceived | p-value‡ | Sham | |
|---|---|---|---|---|---|
| Patients (n) | 65 | 41 | 35 | ||
| Esophagectomy, worry – % no at 12 months | 85% | ≤0.001 | 90% | ≤0.001 | 43% |
| Adenocarcinoma, worry – % no at 12 months | 80% | ≤0.001 | 85% | ≤0.001 | 34% |
| Worry – mean change from baseline (0 = none to 100 = extreme) | −27 ± 24 | ≤0.001 | −30 ± 21 | ≤0.001 | 2 ± 30 |
| Depression – mean change from baseline (0 = none to 100 = extreme) | −10 ± 24 | 0.02 | −11 ± 21 | 0.01 | 0 ± 25 |
| Daily QoL – mean change from baseline (0 = no effect to 100 severe negative effect) | −11 ± 20 | 0.006 | −13 ± 16 | 0.003 | 0 ± 28 |
| Amount of Stress – mean change from baseline (0 = none to 100 = severe stress) | −11 ± 25 | 0.05 | −14 ± 20 | 0.03 | −1 ± 24 |
| Amount of Satisfaction – mean change from baseline (0 = very satisfied to 100 = very unsatisfied) | −43 ± 39 | ≤0.001 | −52 ± 33 | ≤0.001 | −17 ± 30 |
| Difficulty to Sleep – mean change from baseline (0 = disagree to 100 = agree) | −16 ± 23 | NS | −17 ± 24 | NS | −10 ± 24 |
| Work or Family Life Negatively Impacted – mean change from baseline (0 = disagree to 100 = agree) | −16 ± 24 | 0.03 | −15 ± 23 | 0.03 | −3 ± 24 |
| May Die Due to Esophagus – mean change from baseline (0 = disagree to 100 = agree) | −13 ± 25 | NS | −15 ± 22 | NS | −6 ± 31 |
NS = Not Significant
RFA = Radiofrequency ablation
QoL = Quality of life
CE-IM = Complete eradication of intestinal metaplasia
Means are reported as the mean plus-minus the standard deviation
comparison between RFA with CE-IM and Sham based on Fisher’s Exact test for categorical QoL outcomes and Wilcoxon Rank-sums for continuous QoL outcomes
comparison between RFA with perceived CE-IM and Sham based on Fisher’s Exact test for categorical QoL outcomes and Wilcoxon Rank-sums for continuous QoL outcomes
Table 6.
QoL after RFA without CE-IM (actual and perceived) compared to sham
| RFA without CE-IM actual | p-value§ | RFA without CE-IM perceived | p-value‡ | Sham | |
|---|---|---|---|---|---|
| Patients (n) | 13 | 37 | 35 | ||
| Esophagectomy, worry – % no at 12 months | 77% | NS | 76% | 0.01 | 43% |
| Adenocarcinoma, worry – % no at 12 months | 69% | 0.03 | 70% | ≤0.001 | 34% |
| Worry – mean change from baseline (0 = none to 100 = extreme) | −11 ± 20 | NS | −18 ± 25 | 0.005 | 2 ± 30 |
| Depression – mean change from baseline (0 = none to 100 = extreme) | −9 ± 22 | NS | −8 ± 26 | NS | 0 ± 25 |
| Daily QoL – mean change from baseline (0 = no effect to 100 severe negative effect) | −6 ± 26 | NS | −7 ± 25 | NS | 0 ± 28 |
| Amount of Stress – mean change from baseline (0 = none to 100 = severe stress) | −16 ± 22 | 0.05 | −11 ± 28 | NS | −1 ± 24 |
| Amount of Satisfaction – mean change from baseline (0 = very satisfied to 100 = very unsatisfied) | −34 ± 27 | 0.04 | −31 ± 40 | NS | −17 ± 30 |
| Difficulty to Sleep – mean change from baseline (0 = disagree to 100 = agree) | −15 ± 24 | NS | −14 ± 23 | NS | −10 ± 24 |
| Work or Family Life Negatively Impacted – mean change from baseline (0 = disagree to 100 = agree) | −18 ± 27 | NS | −17 ± 27 | 0.05 | −3 ± 24 |
| May Die Due to Esophagus – mean change from baseline (0 = disagree to 100 = agree) | −20 ± 33 | NS | −13 ± 31 | NS | −6 ± 31 |
NS = Not Significant
RFA = Radiofrequency ablation
QoL = Quality of life
CE-IM = Complete eradication of intestinal metaplasia
Means are reported as the mean plus-minus the standard deviation
comparison between RFA without CE-IM and Sham based on Fisher’s Exact test for categorical QoL outcomes and Wilcoxon Rank-sums for continuous QoL outcomes
comparison between RFA without CE-IM perceived and Sham based on Fisher’s Exact test for categorical QoL outcomes and Wilcoxon Rank-sums for continuous QoL outcomes
Subjects in the RFA group who had complete eradication of intestinal metaplasia compared to those in the RFA group with incomplete eradication of intestinal metaplasia had significantly reduced worry about the present condition of their esophagus (p=0.03), but no other differences on the other nine areas queried (Table7). The same analysis, performed with subject perceived pathology demonstrated that subjects treated with RFA who reported complete eradication of intestinal metaplasia compared to those treated with RFA who reported incomplete eradication of intestinal metaplasia had significant reductions in worry about the present condition of their esophagus (p=0.05) and dissatisfaction with their esophagus (p=0.03) (Table7).
Table 7.
QoL after RFA with CE-IM (actual and perceived) compared to RFA without CE-IM (actual and perceived)
| RFA with CE-IM actual | RFA without CE-IM actual | p-value§ | RFA withCE-IM perceived | RFA without CE-IM perceived | p-value‡ | |
|---|---|---|---|---|---|---|
| Patients (n) | 65 | 13 | 41 | 37 | ||
| Esophagectomy, worry – % no at 12 months | 85% | 77% | NS | 90% | 76% | NS |
| Adenocarcinoma, worry – % no at 12 months | 80% | 70% | NS | 85% | 70% | NS |
| Worry – mean change from baseline (0 = none to 100 = extreme) | −27 ± 11 | −11 ± 20 | 0.03 | −30 ± 21 | −18 ± 25 | 0.05 |
| Depression – mean change from baseline (0 = none to 100 = extreme) | −10 ± 23 | −9 ± 22 | NS | −11 ± 21 | −8 ± 26 | NS |
| Daily QoL – mean change from baseline (0 = no effect to 100 severe negative effect) | −11 ± 20 | −6 ± 26 | NS | −13 ± 7 | −7 ± 25 | NS |
| Amount of Stress – mean change from baseline (0 = none to 100 = severe stress) | −11 ± 24 | −16 ± 22 | NS | −14 ± 20 | −11 ± 28 | NS |
| Amount of Satisfaction mean change from baseline (0 = very satisfied to 100 = very unsatisfied) | −43 ± 39 | −34 ± 27 | NS | −52 ± 33 | −31 ± 40 | 0.03 |
| Difficulty to Sleep – mean change from baseline (0 = disagree to 100 = agree) | −16 ± 23 | −15 ± 24 | NS | −17 ± 24 | −14 ± 23 | NS |
| Work or Family Life Negatively Impacted – mean change from baseline (0 = disagree to 100 = agree) | −16 ± 24 | −18 ± 27 | NS | −15 ± 23 | −17 ± 27 | NS |
| May Die Due to Esophagus – mean change from baseline (0 = disagree to 100 = agree) | −13 ± 25 | −20 ± 33 | NS | −15 ± 22 | −13 ± 31 | NS |
NS = Not Significant
RFA = Radiofrequency ablation
QoL = Quality of life
CE-IM = Complete eradication of intestinal metaplasia
Means are reported as the mean plus-minus the standard deviation
comparison between RFA with CE-IM and RFA without CR-IM on Fisher’s Exact test for categorical QoL outcomes and Wilcoxon Rank-sums for continuous QoL outcomes
comparison between RFA with CE-IM perceived and RFA without CE-IM perceived based on Fisher’s Exact test for categorical QoL outcomes and Wilcoxon Rank-sums for continuous QoL outcomes
Discussion
While quality of life is among the most difficult research endpoints to measure, it is perhaps the most important of all clinical outcomes. Much research to date in the treatment of BE has focused on the methodologically simpler outcomes of morbidity and mortality. While most patients want to live longer, many of our patients are less interested in these outcomes as we define them. Our patients want to feel better physically and mentally. They want to live longer, but also to live well.
Our study found that the majority of patients with dysplastic BE report substantial worry about cancer and the need for esophagectomy, as well as a negative impact on their quality of life secondary to their diagnosis. Women appear to be disproportionately affected by the condition, a finding common to multiple quality of life analyses of different disease states.[15] Regardless of final histological diagnosis, randomization to the RFA group was associated with improvement in QoL (Table4). Not surprisingly, subjects who underwent successful endoscopic RFA resulting in complete eradication of intestinal metaplasia had marked improvement in QoL as compared to subjects undergoing a sham procedure. Specifically, these subjects reported significant decreases in worry that the present condition of their esophagus would result in the development of cancer and future need for an esophagectomy. They also reported decreased symptoms of general worry relative to the present condition of their esophagus, depression, and stress along with improved satisfaction with their esophagus. Work and family life were improved as was overall daily QoL.
Most subjects (77%) randomized to the RFA group achieved complete eradication of all intestinal metaplasia, accounting for the improvement in QoL seen in the treatment arm. The majority of RFA-treated subjects who did not achieve complete eradication of intestinal metaplasia had downgrading of their disease, with eradication of dysplasia. Because non-dysplastic BE presumably has a lower risk of progression to cancer, it is not surprising that these subjects with incomplete eradication of IM also reported a significant decrease in worry that the present condition of their esophagus would result in the development of cancer and had reduced general worry, dissatisfaction with the condition of their esophagus, and impact on work or family life as compared to subjects who underwent sham procedure. There were few significant differences in QoL when comparing those treated with complete eradication of IM to those treated without complete eradication of IM. Because all subjects treated with RFA had either complete eradication of IM or downgrading of disease, there were significant improvements in QoL for the majority of subjects in the RFA group compared to the sham group.
Perceived health is an important predictor of health outcomes, independent of clinical health status.[16] In our population, a remarkable 41% of subjects misclassified the disease state of their esophagus even after being informed of the results of their 12 month biopsies, with most over-estimating their disease severity. The etiology of this misunderstanding is unclear and deserves further study, but points to suboptimal communication between the physician and the patient regarding this complex clinical situation. To assess the impact of this misunderstanding of disease on our data, we performed an analysis using both actual pathology and self-reported pathology. The magnitude and directionality of the impact of treatment with RFA was largely unchanged in analyses using self-reported pathology. Our data suggest that the positive impact of RFA therapy was largely secondary to manipulation of the patients’ perceived risk of cancer. This is attested to by the observation that the quality of life measures in subjects who wrongfully believed they were clear of BE were similar to those of subjects who truly achieved a complete eradication of BE. While the impact of RFA treatment on cancer mortality remains unclear, it is obvious that informing subjects that their BE had been eradicated led to the logical belief that their chance of developing or dying of cancer had been diminished.
The alternative to RFA for dysplastic BE is esophagectomy. Three studies have described the impact of QoL after esophagectomy for dysplastic BE.[11–13] Each study retrospectively assessed QoL approximately 5 years status post esophagectomy with the SF-36, a generic assessment of health related quality of life. All three studies found that the cohort post-esophagectomy had SF-36 scores similar to or better then age- and sex-matched US general population normal values.[11–13] In addition to the SF-36, Chang et al assessed common post esophagectomy symptoms and found that 59% had reflux or regurgitation, 55% diarrhea, 45% bloating, 28% nausea, 28% dysphagia, 17% postprandial diaphoresis, 17% abdominal pain and 7% hoarseness.[12] There is a single retrospective cohort study of QoL in patients with a history of dysplastic BE or intramucosal carcinoma status post endoscopic ablation (photodynamic therapy, argon photo coagulation and or endoscopic mucosal resection) compared to esophagectomy.[17] QoL was assessed with the SF-36 and did not differ by treatment group in a model adjusted for age and gender.[17] It is unclear why the SF-36 scores suggest no change in QoL post-esophagectomy while patients do report the common post-esophagectomy symptoms noted above. This may result from the use of generic instruments instead of disease specific instruments, which are designed to identify issues that are important to patients with specific conditions as well as measure clinically important changes from specific treatments.[18] There may also be selection bias in these studies, in that only subjects well enough to undergo esophagectomy were included in the intervention groups, and only those who survived the surgery were available later for survey. Comparing such a cohort to the general population may not be appropriate.
To date, no validated quality of life measure for Barrett’s esophagus has been described in the literature. Generic instruments may be insensitive to clinically significant changes specific to BE. Given these concerns, we created a disease-targeted measure to assess aspects of QoL in BE. This measure was constructed after conducting interviews to identify areas of greatest concern for patients with BE. Disease specific measures, by design, usually have adequate validity and reliability.[19] While the methodology used to develop our instrument provides high content validity, the test-retest reliability of the instrument and convergent validity have not been established. In addition, a reference timeframe was not established in the questionnaire and may need adjustment based on the intervention considered. Further work will be necessary to define the operating characteristics of this tool. Finally, we chose not to adjust for multiple comparisons because each question addressed a unique quality of life dimension.
In summary, our data suggest that the majority of patients with dysplastic BE report a substantial negative impact on their QoL. Eradication of dysplasia by ablation leads to a marked improvement in disease-related QoL in the first 12 months of treatment. The durability of these changes will be assessed with serial follow-up of this cohort.
Supplementary Material
References
- 1.Spechler SJ. Clinical practice. Barrett’s Esophagus. N Engl J Med. 2002;346:836–842. doi: 10.1056/NEJMcp012118. [DOI] [PubMed] [Google Scholar]
- 2.Sharma P, Falk GW, Weston AP, et al. Dysplasia and cancer in a large multicenter cohort of patients with Barrett’s esophagus. Clin Gastroenterol Hepatol. 2006;4:566–572. doi: 10.1016/j.cgh.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Overholt BF, Lightdale CJ, Wang KK, et al. Photodynamic therapy with porfimer sodium for ablationof high-grade dysplasia in Barrett’s esophagus: international, partially blinded, randomized phase III trial. Gastrointest Endosc. 2005;62:488–498. doi: 10.1016/j.gie.2005.06.047. [DOI] [PubMed] [Google Scholar]
- 4.Reid BJ, Levine DS, Longton G, Blount PL, Rabinovitch PS. Predictors of progression to cancer in Barrett’sesophagus: baseline histology and flow cytometry identify low-and high-risk patient subsets. Am J Gastroenterol. 2000;95:1669–1676. doi: 10.1111/j.1572-0241.2000.02196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rastogi A, Puli S, El-Serag HB, et al. Incidence of esophageal adenocarcinoma in patients with Barrett’s esophagus and high-grade dysplasia: a meta-analysis. Gastrointest Endosc. 2008;67:394–398. doi: 10.1016/j.gie.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 6.Eloubeidi MA, Mason AC, Desmond RA, El-Serag HB. Temporal trends (1973–1997) in survival of patients with esophageal adenocarcinoma in the United States: a glimmer of hope? Am J Gastroenterol. 2003;98:1627–1633. doi: 10.1111/j.1572-0241.2003.07454.x. [DOI] [PubMed] [Google Scholar]
- 7.Wenger N, Furberg CD. Quality of life assessments in clinical trials. New York: Raven Press; 1990. [Google Scholar]
- 8.Crockett SD, Lippmann QK, Dellon ES, Shaheen NJ. Health-related quality of life in patients with Barrett’s esophagus: a systematic review. Clin Gastroenterol Hepatol. 2009;7:613–623. doi: 10.1016/j.cgh.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shaheen NJ, Dulai GS, Ascher B, Mitchell KL, Schmitz SM. Effect of a new diagnosis of Barrett’s esophagus on insurance status. Am J Gastroenterol. 2005;100:577–580. doi: 10.1111/j.1572-0241.2005.41422.x. [DOI] [PubMed] [Google Scholar]
- 10.Birkmeyer JD, Stukel TA, Siewers AE, et al. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349:2117–2127. doi: 10.1056/NEJMsa035205. [DOI] [PubMed] [Google Scholar]
- 11.Moraca RJ, Low DE. Outcomes and health-related quality of life after esophagectomy for high-grade dysplasia and intramucosal cancer. Arch Surg. 2006;141:545–549. doi: 10.1001/archsurg.141.6.545. discussion 549–551. [DOI] [PubMed] [Google Scholar]
- 12.Chang LC, Oelschlager BK, Quiroga E, et al. Long-term outcome of esophagectomy for high-grade dysplasia or cancer found during surveillance for Barrett’s esophagus. J Gastrointest Surg. 2006;10:341–346. doi: 10.1016/j.gassur.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 13.Headrick JR, Nichols FC, 3rd, Miller DL, et al. High-grade esophageal dysplasia: long-term survival and quality of life after esophagectomy. Ann Thorac Surg. 2002;73:1697–1702. doi: 10.1016/s0003-4975(02)03496-3. discussion 1702–1693. [DOI] [PubMed] [Google Scholar]
- 14.Shaheen NJ, Sharma P, Overholt BF, et al. Radiofrequency ablation in Barrett’s esophagus with dysplasia. N Engl J Med. 2009;360:2277–2288. doi: 10.1056/NEJMoa0808145. [DOI] [PubMed] [Google Scholar]
- 15.Cherepanov D, Palta M, Fryback DG, Robert SA. Gender differences in health-related quality-of-life are partly explained by sociodemographic and socioeconomic variation between adult men and women in the US: evidence from four US nationally representative data sets. Qual Life Res. 2010 doi: 10.1007/s11136-010-9673-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaplan GA, Camacho T. Perceived health and mortality: a nine-year follow-up of the human population laboratory cohort. Am J Epidemiol. 1983;117:292–304. doi: 10.1093/oxfordjournals.aje.a113541. [DOI] [PubMed] [Google Scholar]
- 17.Schembre D, Arai A, Levy S, Farrell-Ross M, Low D. Quality of life after esophagectomy and endoscopic therapy for Barrett’s esophagus with dysplasia. Dis Esophagus. 2010 doi: 10.1111/j.1442-2050.2009.01042.x. [DOI] [PubMed]
- 18.Patrick DL, Deyo RA. Generic and disease-specific measures inassessing health status and quality of life. Med Care. 1989;27:S217–232. doi: 10.1097/00005650-198903001-00018. [DOI] [PubMed] [Google Scholar]
- 19.McSweeny AJ, Creer TL. Health-related quality-of-life assessment in medical care. Dis Mon. 1995;41:1–71. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.