Abstract
Epidermal growth factor receptors (EGFR), upregulated in many tumor types, have been a target for therapeutic development and molecular imaging. The objective of this study was to evaluate the distribution and metabolic characteristics of fluorine-18 labeled anilinoquinazolines as potential imaging agents for EGFR tyrosine kinase expression. Fluorine-18 labeled fluoronitrobenzenes were prepared by reaction of potassium cryptand [18F]fluoride with 1,2- and 1,4-dinitrobenzenes, and 3-nitro-N,N,N-trimethylanilinium triflate in 5 min. Decay-corrected radiochemical yields of [18F]fluoride incorporation into the nitro-aromatic compounds were 81 ± 2%, 44 ± 4% and 77 ± 5% (n = 3–5) for the 2-, 3- and 4-fluoro isomers, respectively. Sodium borohydride reduction to the corresponding [18F]fluoroanilines was achieved with greater than 80% conversion in 5 min. Coupling of [18F]fluoroaniline-hydrochlorides to 6,7-dimethoxy-4-chloro-quinazoline gave the corresponding 6,7-dimethoxy-4-(2-, 3- and 4-[18F]fluoroanilino)quinazolines in 31 ± 5%, 17 ± 2% and 55 ± 2% radiochemical yield, respectively, while coupling to the 6,7-diethoxy-4-chloro-quinazoline produced 6,7-diethoxy-4-(2-, 3- and 4-[18F]fluoroanilino)quinazolines in 19 ± 6%, 9 ± 3% and 36 ± 6% radiochemical yield, respectively, in 90 minutes to end of synthesis from [18F]fluoride. Biodistribution of 2- and 4-[18F]fluoroanilinoquinazolines was conducted in tumor-bearing mice (MDA-MB-435 and MDA-MB-468 xenografts). Low tumor uptake (<1% injected dose per gram (ID/g) of tissue up to 3 h post injection of the radiotracers) was observed. High bone uptake (5 – 15% ID/g) was noted with the 4-[18F]fluoroanilino-quinazolines, The metabolic stabilities of radiolabeled quinazolines were further evaluated by incubation with human female cryopreserved isolated hepatocytes. Rapid degeneration of the 4-fluoro-substituted compounds to baseline polar metabolites was observed by radio-TLC whereas the 2- and 3-[18F]fluoroaniline derivatives were significantly more stable, up to 2 h, corroborating the in vivo biodistribution studies. Para-substituted [18F]fluoroanilines, a common structural motif in radiopharmaceuticals, are highly susceptible to metabolic degradation.
Keywords: PET, Fluorine-18, Fluoroaniline, Metabolism, Quinazoline, EGFR, Hepatocytes
1. Introduction
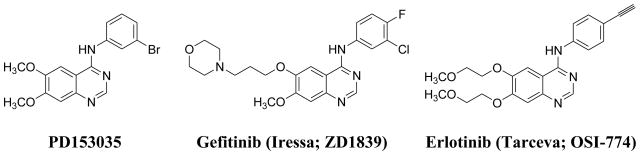
The design of potent and selective protein kinase inhibitors is a major goal of drug discovery programs and within the last decade several small molecule kinase inhibitors have been approved for clinical use.1 The epidermal growth factor receptor (EGFR) is a member of the ErbB family of receptor tyrosine kinases, cell surface receptors that activate a variety of cellular events including cell proliferation and differentiation. Overexpression of these receptors in a variety of tumors is often an indicator of poor prognosis.2 EGFR, as well as other ErbB family members, are established biomarkers and targets for cancer therapy.3–5 Of the therapeutic approaches taken to develop EGFR-based drugs, one of the most actively pursued involves small molecule tyrosine kinase inhibitors that compete with the adenosine triphosphate (ATP)-binding pocket of the cytoplasmic domain of the receptor, inhibiting receptor phosphorylation and blocking the transduction of downstream signaling cascades.6 The first drug candidates, 6,7-dimethoxy-4-(3-bromoanilino)quinazoline7,8 (PD153035; Figure 1) and its diethoxy- derivative, were very potent and selective inhibitors of EGFR tyrosine kinase activity (IC50 = 25 and 6 pM, respectively). Subsequently, several anilino-quinazoline-based EGFR inhibitors have been evaluated, with Gefitnib (ZD1839, Iressa, AstraZeneca) and Erlotinib (Tarceva, OSI-774, Genentech and OSI Pharmaceuticals; Figure 1) achieving clinical use approval by the US FDA.9
Figure 1.
Quinazoline-Based Inhibitors of the EGFR tyrosine kinase.
An effective single photon emission computed tomography (SPECT) or positron emission tomography (PET) imaging agent for the EGFR could facilitate the development of therapeutics and provide improved criteria for patient selection.10,11 Consequently, targeting of the extracellular EGFR domain with radiolabeled monoclonal antibodies, affibodies or peptides, or employing radiolabeled small molecules to target the intracellular kinase domain has emerged as a major molecular imaging initiative. This field has been extensively reviewed by several laboratories,12–21 including ours.22 The majority of radiolabeled EGFR tyrosine kinase inhibitors are 4-anilino-quinazoline derivatives, based on the prototypical reversibly binding inhibitor, PD153035. Recently, [11C]-PD153035 has advanced to preliminary human studies.23 The short half-life of carbon-11 (20.4 min) may limit the widespread use of this radiopharmaceutical to sites that have an on-site cyclotron, and may not allow adequate time for the radiotracer to accumulate in tumor cells that overexpress EGFR. In an attempt to overcome the limitations of the short half-life of carbon-11, we24 and several other laboratories22 have directed our research efforts towards preparing fluorine-18 (half-life = 109.7 min) labeled EGFR-tyrosine kinase inhibitors that may achieve adequate tumor to background contrast.
The metabolism of pharmaceutical compounds is important for the delivery and availability of the molecules to reach the intended target site in vivo. Likewise radiopharmaceutical metabolism is also a critical parameter for the development of new tracers. Species-specific metabolism precludes the prediction of compound metabolism in humans. Isolated hepatocytes have been applied to evaluate the metabolism of radiopharmaceuticals in several species and can be used to evaluate a series of compounds in the same species.25 Hepatocyte assays may be used to portend the metabolic fate of short-lived radiotracers in humans, providing an important parameter for tracer development.
We previously reported a systematic study that screened several EGFR tyrosine kinase inhibitors that could be readily radiolabeled for PET imaging in an array of in vitro assays.26 This report identified two compounds that warrant radioisotope labeling with fluorine-18, namely, 4-(2-fluoroanilino)- and 4-(3-fluoroanilino)-6,7-diethoxyquinazoline, for further evaluation in vivo. The goals of the present work are: 1) to efficiently synthesize 6,7-dimethoxy- and 6,7-diethoxy-4-(2-, 3- and 4-[18F]fluoroanilino)quinazolines; 2) to evaluate the imaging potential of select compounds in tumor-bearing mice; and 3) to investigate the metabolic stability of these radiotracers in human hepatocytes compared to the in vivo mice distribution data.
2. Results and discussion
2.1 Radiochemistry
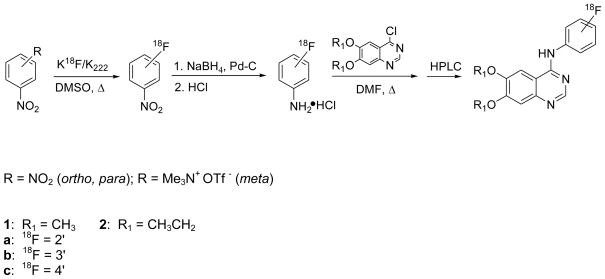
In order to investigate the positional effect of the fluorine-18 bound to the [18F]fluoroanilino-group, our first goal was to optimize the preparation of 2-, 3- and 4-[18F]fluoroanilines for rapid coupling to the respective 4-chloro-quinazoline derivatives, to prepare the title radiotracers. The preparation of fluorine-18 labeled dialkoxy-fluoroanilinoquinazolines is shown in Scheme 1. Intermediates, 2- and 4-[18F]fluoronitrobenzene, were produced by reaction of potassium [18F]fluoride/cryptand complex (K18F/Kryptofix® (K222)) with the corresponding 1,2- and 1,4-dinitrobenzenes, and 3-[18F]fluoronitrobenzene was prepared from 3-nitro-N,N,N-trimethylanilinium triflate. The radiochemical yields, as well as the respective time course for these syntheses, are shown in Table 1.
Scheme 1.
Syntheses of [18F]1 and [18F]2 via [18F]fluoroanilines.
Table 1.
Time Course and Radiochemical Yields During [18F]Fluoroanilinoquinazoline Syntheses.
| Compound | Time Coursea (min) | % Radiochemical Yieldb | ||
|---|---|---|---|---|
| a (ortho-F) | b (meta-F) | c (para-F) | ||
| [18F]Fluoronitrobenzenec | 20 | 81± 2 | 44± 4 | 77± 5 |
| [18F]Fluoroaniline | 30 | 68± 2 | 35± 3 | 72± 6 |
| [18F]1 (dimethoxy-) | 90d | 31± 5 | 17± 2 | 55± 2 |
| [18F]2 (diethoxy-) | 90d | 19± 6 | 9± 3 | 36± 6 |
100% of activity at time of 0 min is attributed to [18F]Fluoride and represents the start of synthesis,
Decay-corrected relative to [18F]fluoride; n = 3–5,
Precursor; 1,2- and 1,4-dinitrobenzene (a and c); 3-nitro-N,N,N-trimethylanilinium triflate (b),
Following preparative HPLC and sample concentration/formulation.
The syntheses of 2- and 4-[18F]fluoronitrobenzene was efficient, consistent with previous work.27–30 The incorporation of [18F]fluoride into the 1,3-substituted dinitrobenzene has historically been a low yielding (< 17%) reaction.27 The use of 3-nitro-N,N,N-trimethylanilinium triflate gave 3-[18F]fluoronitrobenzene in reasonable yield (44 ± 4 %) after 5 min using a modified method.31 A competing side reaction of [18F]fluoride ion with one of the methyl groups on the trimethylammonium moiety produces [18F]fluoromethane.32
The [18F]fluoronitrobenzenes were purified by C18 solid phase extraction (SPE) and reduced to the corresponding [18F]fluoroanilines in 5 min at room temperature using a modification of a previously reported method (Scheme 1).33 The reduction reaction was quenched with excess HCl to give the [18F]fluoroaniline-hydrochloride salt. Formation of the salt was necessary to prevent the complete loss of the [18F]fluoroaniline during the solvent evaporation process.
Coupling the [18F]fluoroanilines to 6,7-dimethoxy- or 6,7-diethoxy-4-chloro-quinazoline yielded [18F]1a–c and [18F]2a–c, respectively, as shown in Scheme 1. Moderate yields were obtained for the coupled quinazolines ([18F]1a–c and [18F]2a–c; Table 1). The yields for the dimethoxy-derivatives ([18F]1a–c) were consistently greater than the respective diethoxy-compounds ([18F]2a–c). The relatively low measured specific activities of 44 to 308 Ci/mmol (1.5–11.4 MBq/mmol) are attributed to the small initial amounts of fluoride used (2.5–6.5 mCi; 93–241 MBq) for the synthesis. Greater than 100 mCi (3.7 GBq) of starting [18F]-fluoride was used for all compounds prepared for biological evaluations and specific activities correspond to >1 Ci/μmol at the time of injection. The total synthesis time for the combined reactions was optimized at 90 min. Our yields improved upon the previously reported34 non-decay corrected radiochemical yield for compounds [18F]1b and [18F]1c (ca. 1% and 4 – 12%, respectively). The more facile and less time consuming syntheses reported herein enabled biological evaluations of [18F]1b to be carried out for the first time.
2.2. Biological Studies
2.2.1. Metabolism of [18F]-fluoroanilinoquinazolines by human hepatocytes
Species differences in metabolism are well-known. This phenomenon has been seen with radiopharmaceuticals as well.35 The distribution differences amongst the positional isomers seen in the current mouse study (see section 2.2.2) and the previously reported degradation of the 4-[18F]fluoroanilinoquinazline34 prompted a further evaluation of the metabolic fate of these compounds. Human hepatocytes were chosen in order to determine whether the same metabolic pathways active in the mouse were present in humans.
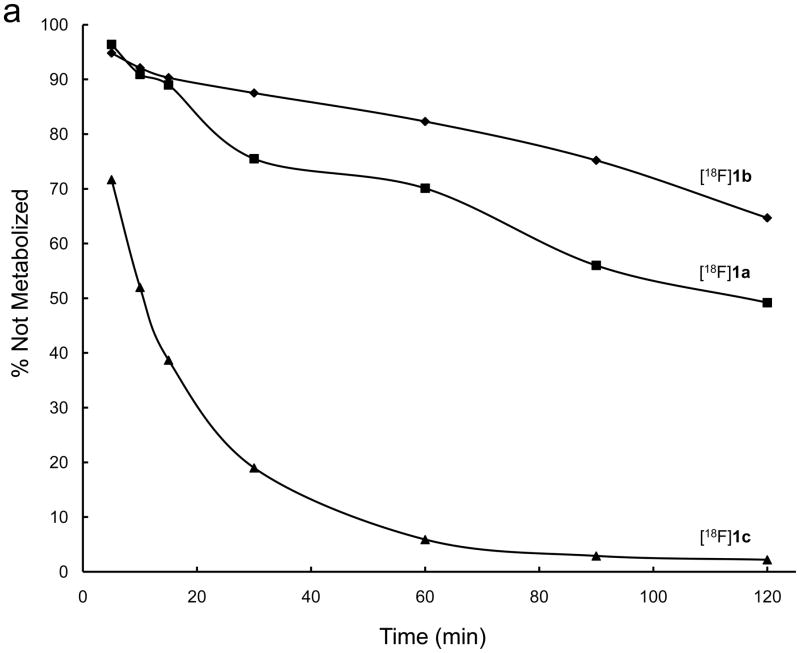
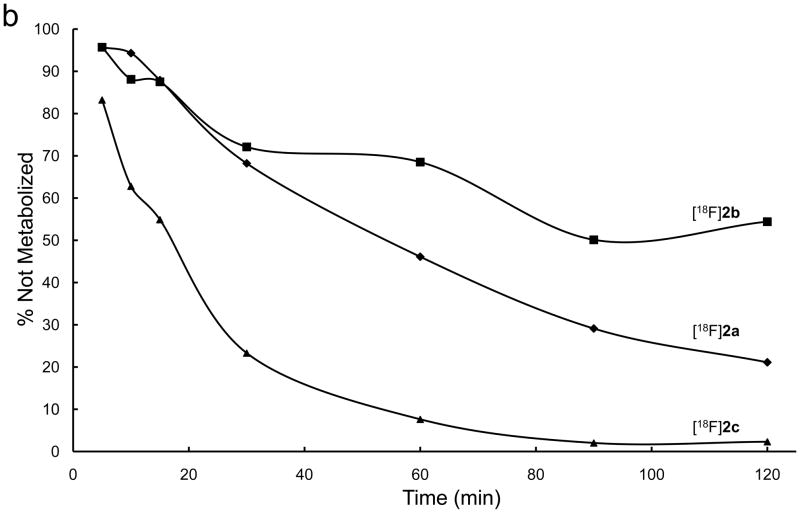
The six [18F]-fluoroanilinoquinazolines were added to human hepatocyte preparations and the time course of tracer metabolism over 120 min was monitored by radioTLC on aliquots of the media. The metabolic degradation of [18F]1a–c and [18F]2a–c is shown in Figures 2a and 2b, respectively. The pattern of parent radiotracer loss over time, 4-[18F]fluoro > 2-[18F]fluoro > 3-[18F]fluoro, was similar for both series. The 4-[18F]fluoroanalino compounds in both series showed comparable rapid exponential metabolism with complete consumption of the tracer within ~90 min. The dimethoxy-2-[18F]fluoro and 3-[18F]fluoro analogs were consumed more slowly than the corresponding diethoxy derivatives. All of the degradation products were more polar than the parent radiotracer with the majority of activity at the baseline. Thorough characterization of the polar metabolites was not conducted.
Figure 2.
Metabolic stability of a) [18F]1 and b) [18F]2 by female human hepatocytes over 2 hours.
The rapid metabolism of 4-[18F]fluoroanilinoquinazolines in the present and previous work34,36 is likely due to defluorination. These results are consistent with reported defluorination of 19F 4-fluoroaniline-containing compounds.37–39 The 2-[18F] and 3-[18F]fluoranilinoquinazolines are more stable in the hepatocyte assay suggesting that they are more suitable for in vivo imaging.
2.2.2. Ex vivo biodistribution studies in tumor-bearing mice
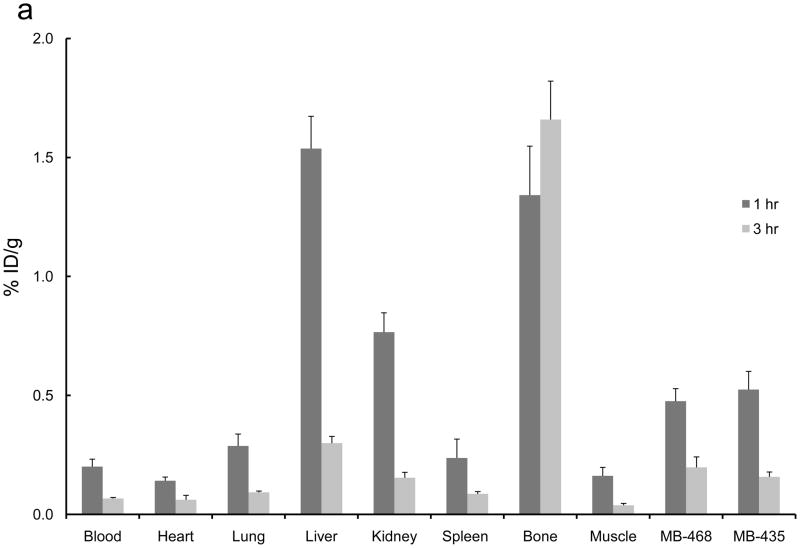
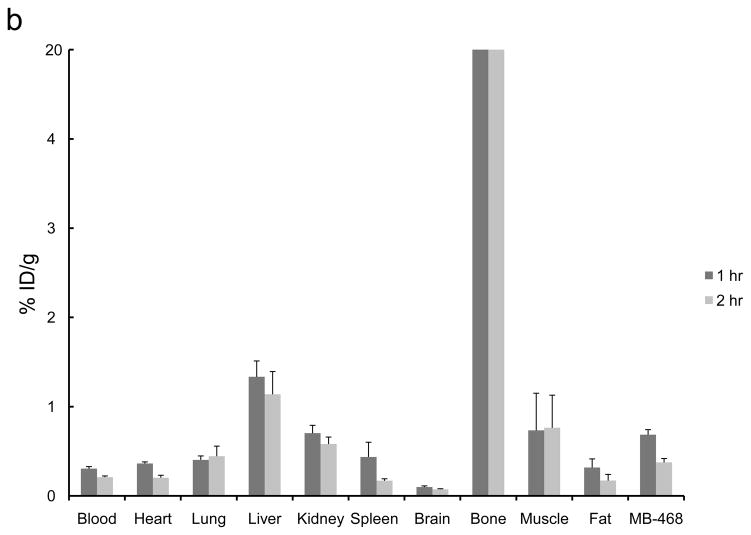
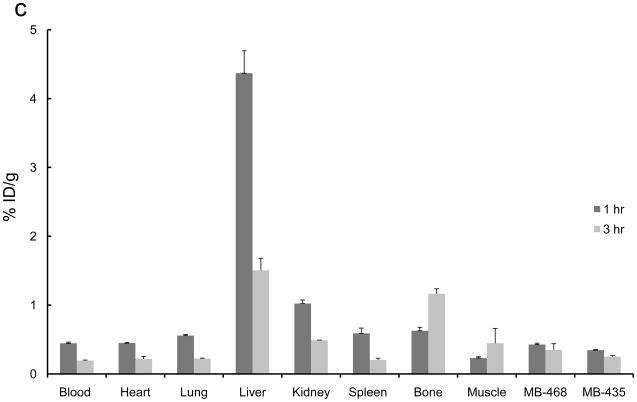
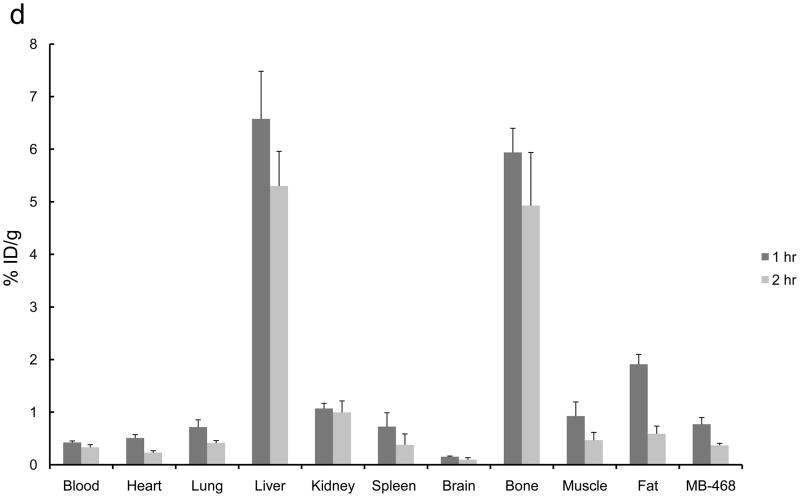
The 2-[18F]- and 4-[18F] fluoroanilinoquinazolines were selected for evaluation in the mouse xenograft models as they were prepared in higher radiochemical yields compared to the 3-[18F] analogs. Ex vivo biodistribution studies were carried out in mice with both EGFR positive (MDA-MB-468) and EGFR negative (MDA-MB-435) or only EGFR positive (MDA-MB-468) xenografts. The tumors, normal tissues, blood and bone were excised and counted for radioactivity from one to three hours post injection of the radiotracers. The organ distributions for all four compounds is shown in Figure 3. These compounds showed limited tumor uptake (<0.6% injected dose (ID) per gram of tumor) at all time points. There was no difference in uptake between the EGFR positive and EGFR negative xenografts. A blocking study was carried out with [18F]1a by pre-injection of PD153035 (1 mg/kg administered 1 h prior to radiotracer injection). The pre-blocking failed to reduce radiotracer uptake compared to the control group.
Figure 3.
Ex vivo biodistributions of a) [18F]1a, b) [18F]1c, c) [18F]2a, and d) [18F]2c in tumor-bearing mice.
Bone uptake for the 2-[18F] analogs (Figure 3a,c) was less than 2% ID/g at all timepoints. The 4-[18F] analogs (Figure 3b,d) demonstrated significantly greater bone uptake, 5 – 15% ID/g. The high bone accumulation of these compounds is indicative of in vivo defluorination and corroborates the hepatocyte studies that also show a rapid degradation of these compounds to polar species, likely [18F]fluoride. This study corroborates the high bone uptake that was attributed to in vivo defluorination following injection of 4-(3-chloro-4-[18F]fluoroanilino)-6,7-dimethoxy-quinazoline in mice.34,36 Rapid metabolism of [18F]1c was previously noted yet the bone uptake was not analyzed in that study.34,36 This further demonstrates the general metabolic instability of 4-fluoroanilino compounds and serves as a cautionary note for its use in pharmaceutical and radiopharmaceutical design. It is also interesting to note that this metabolic defluorination process is active in assays from different species.
“The fluorine effect” describes the phenomenon that occurs when differences in pharmacological properties between fluorinated and non-fluorinated derivatives of a compound are displayed, and is attributed to the specific site of fluorine substitution.40,41 The specific site of aryl ring fluorination with 18F is well known to alter pharmacokinetic properties of radiopharmaceuticals in vivo. For example, an evaluation of aromatic ring-substituted 2-, 5- or 6-[18F]fluoro-L-dihydroxyphenylalanine ([18F]fluoro-L-DOPA) revealed dramatically different biological properties in human imaging studies.42 Therefore it is not surprising that 2-, 3- and 4-substituted [18F]fluoroanilino-quinazolines also demonstrated different biological properties in vivo. This study further illustrates the importance of isomeric considerations in radiopharmaceutical design.
3. Conclusions
Fluorine-18 labelled 2-, 3- and 4-fluoroanilines were prepared as key intermediates in the synthesis of a series of 6,7-dimethoxy- and 6,7-diethoxy-4-(2-, 3- and 4-[18F]fluoroanilino)quinazolines in yields suitable for further study as EGFR imaging agents. Rapid degradation of 6,7-dialkoxy-4-(4-[18F]fluoroanilino)quinazolines was seen in the in vitro human hepatocyte assay as well as by high bone uptake in mouse studies. This is consistent with metabolic defluorination in this class of compounds. As 4-[18F]fluoroanilines are extensively used in PET radiopharmaceutical development, this work shows the importance of the position of the radiolabel on the in vivo behaviour of [18F]-fluoroaniline-based radiopharmaceuticals in multiple species.
4. Experimental Section
4.1 Radiochemistry
4.1.1 Materials
Anhydrous acetonitrile, dimethyl sulfoxide and N,N-dimethylformamide were purchased from Aldrich (99.8%) and used without further purification. Kryptofix® 222 (Aldrich, 98%), K2CO3 (Aldrich, > 99%) NaBH4 (Spectrum), palladium, 10 wt % on activated carbon (Aldrich), HCl (Aldrich), absolute EtOH USP (Aaper), HPLC grade MeOH (Burdick & Jackson) and [18O]H2O (Rotem Industries, Ltd, Israel, > 94.1 atom %) were used without further purification. The 1,4-dinitrobenzene (Aldrich, 98%) and 1,2-dinitrobenzene (> 99%) compounds were purchased from Aldrich and recrystallized from ethyl acetate prior to use, while and 1-nitro-3-aryltrimethylammonium trifluoromethanesulfonate was synthesized as previously described.31
4.1.2 Standard Techniques
No-carrier-added 18F-fluoride was produced by 10 MeV proton irradiation of 30% enriched [18O]H2O in a silver target by the 18O(p,n)18F nuclear reaction on the LBNL Biomedical Isotope Facility CTI RDS 111 cyclotron. The [18O]H2O/18F− (ca. 100–450 μL) was transferred into a conical glass vial containing K222 (5 mg, 13 μmol), K2CO3 (0.5 mg, 3.6 μmol) in 500 μL of CH3CN. The water was removed by azeotropic distillation with anhydrous CH3CN (1 mL) at 100 °C in vacuo under a stream of nitrogen. The drying process was repeated.
4.2 Synthesis of [18F]Fluoroanilines
The conversion of [18F]fluoronitrobenzenes to [18F]fluoroanilines described here is a modification of previously reported methods.24,33 The 1,2- or 1,4-dinitrobenzene (ca. 0.5 mg, 3 μmol), or 1-nitro-3-aryltrimethylammonium trifluoromethanesulfonate (ca. 0.5 mg, 2 μmol) was dissolved in 250 μL of DMSO and transferred into a conical vial containing the dried K18F/K222. The vessel was sealed with a Teflon-faced silicone septum and heated at 130 °C for 5 min. The sample was diluted to 5 mL with water and loaded onto an activated (MeOH) and equilibrated (water) C18 Sep-Pak® cartridge (Waters). The cartridge was flushed with 4 mL of water and the [18F]fluoronitrobenzene was eluted with 2 mL of MeOH directly into a flat-bottomed glass vial (4 mL) containing NaBH4 (10 mg, 260 μmol) and 10 wt % Pd-C (4 mg). The reduction was allowed to proceed for 5 min at room temperature prior to quenching with 100 μL of concentrated HCl. The solution was filtered through Celite, then evaporated to dryness at 100 °C in vacuo with the aid of CH3CN.
4.3 Synthesis of [18F]Fluoroanilino-quinazolines
The dried [18F]fluoroaniline-hydrochlorides were coupled to 6,7-diethoxy- (2.2 mg, 9 μmol) or 6,7 dimethoxy-4-chloro-quinazoline (2.2 mg, 10 μmol) in 250 μL of DMF and reacted at 100 °C for 15 min. The reaction mixture was made basic with 1 N NaOH (1 mL) and loaded onto an activated C18 Sep-Pak® cartridge. The cartridge was flushed with 4 mL of H2O, eluted with 2 mL of MeOH and filtered through Celite and a 0.45 mm nylon membrane filter prior to preparative HPLC purification.
Preparative HPLC purification (column; Beckman Ultrasphere ODS-5 μm, 10 mm × 25 cm, mobile phase; 65 : 35 (v/v) CH3OH : (1% Et3N in H2O adjusted to pH = 7.4 with H3PO4), flow rate; 4 mL min−1 ([18F]1a – c), 6 mL min−1 ([18F]2a – c)). The column eluent was monitored for UV absorbance at 254 nm (UV detector: Linear, UV 106) and radioactivity (detector: Carroll and Ramsey Associates, Model 105S). All [18F]fluoroanilinoquinazolines were identified by HPLC via co-injection of an aliquot of the final product with authentic sample, prepared as previously described by our group.26 Compounds [18F]1a–c were isolated at a flow rate of 4.0 mL min−1 and had retention times of 7, 13 and 10.5 min, respectively. Compounds [18F]2a–c were isolated at a flow rate of 6 mL min−1 and had retention times of 9.5, 12 and 14.5 min, respectively. Compounds [18F]1 and [18F]2 had high radiochemical purities (>95%). Specific activities were determined by comparing the integration of the HPLC peak area to a standard curve generated by an authentic sample of the compound at various concentrations.
4.4 Metabolic Studies with Hepatocytes
2-, 3- or 4-[18F]fluoroanilino-6,7-dialkoxyquinazoline were independently synthesized and dissolved in modified Krebs-Henseleit buffer. Cryopreserved female human hepatocytes (In Vitro Technologies; currently Celsis International) were thawed and resuspended in the buffer and divided into 4 wells of a 12 well plate. The buffer solution containing the 2-, 3- or 4-[18F]fluoroanilino-6,7-dialkoxyquinazolines (0.5 mL) was transferred into 3 of the wells. The prepared cells were maintained at 37 °C in a CO2 (5%) incubator for the entire experiment. Aliquots (100 μL) were taken at 5, 10, 15, 30, 60, 90 and 120 min and transferred into a microcentrifuge tube containing 200 μL of MeOH. Tubes were vortexed for 30 sec and centrifuged for 5 min at 10,000 G. Aliquots (5 μL) of the supernatant were spotted on SiO2 TLC plates and eluted with 10% EtOH/EtOAc. TLC plates were scanned using a Bioscan radio-TLC system. Cell viability was greater than 95% throughout the duration of the experiment, as assessed by staining cells with Trypan Blue.
4.5 Biodistribution in Tumor-Bearing Mice
All animal experiments were carried out under humane conditions, with approval from the Institutional Animal Care and Use Committee at the E.O. Lawrence Berkeley National Laboratory. Tumor-bearing nude SCID mice were purchased from Sierra Biosource. The mice were subcutaneously implanted with MDA-MB-468 (ca. 1 million cells in the flank) and in some cohorts, the MDA-MB-435 (ca. 1 million cells) were also implanted in the shoulder.
The mice were anesthetized with isoflurane and given a tail vein injection of 1 – 2 MBq (20 – 50 μCi) of radiotracer formulated in saline. The mice (n = 4 per time point) were euthanized between one and three hours post injection of the radiotracer. Blood, tumor, and tissues were removed, weighed and counted. The decay-corrected percent injected dose per gram of tissues and blood were calculated. For the blocking study, 1 mg/kg of PD153035 was administered by intravenous injection at one hour prior to radiotracer injection.
Acknowledgments
We thank Dr. Andrew Gibbs and Dr. Erathodiyil Nandanan for their synthetic expertise and helpful discussions. This work was supported by the Director, Office of Science, Office of Biological and Environmental Research, Medical Sciences Division of the U.S. Department of Energy under Contract No. DE-AC03-76SF00098 and NIH grant #CA94253 (HFV) and #CA79823 (HFV). This work was also sponsored by the Department of the Army under Grant No. DAMD17-98-1-8064 (HFV) and the State of California under Grant No. UCBCRP 4IB-0059 (HFV). We thank the Natural Sciences and Engineering Research Council of Canada for a Postdoctoral Fellowship (NV). We also thank Chris Ramsey for technical expertise and production of [18F]fluoride.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Liao JJ. J Med Chem. 2007;50:409. doi: 10.1021/jm0608107. [DOI] [PubMed] [Google Scholar]
- 2.Jorissen RN, Walker F, Pouliot N, Garrett TP, Ward CW, Burgess AW. Exp Cell Res. 2003;284:31. doi: 10.1016/s0014-4827(02)00098-8. [DOI] [PubMed] [Google Scholar]
- 3.Ritter CA, Arteaga CL. Semin Oncol. 2003;30:3. doi: 10.1053/sonc.2003.50027. [DOI] [PubMed] [Google Scholar]
- 4.Stern DF. Exp Cell Res. 2003;284:89. doi: 10.1016/s0014-4827(02)00103-9. [DOI] [PubMed] [Google Scholar]
- 5.Fry DW. Exp Cell Res. 2003;284:131. doi: 10.1016/s0014-4827(02)00095-2. [DOI] [PubMed] [Google Scholar]
- 6.Arteaga CL. Exp Cell Res. 2003;284:122. doi: 10.1016/s0014-4827(02)00104-0. [DOI] [PubMed] [Google Scholar]
- 7.Bridges AJ, Zhou H, Cody DR, Rewcastle GW, McMichael A, Showalter HD, Fry DW, Kraker AJ, Denny WA. J Med Chem. 1996;39:267. doi: 10.1021/jm9503613. [DOI] [PubMed] [Google Scholar]
- 8.Fry DW, Kraker AJ, McMichael A, Ambroso LA, Nelson JM, Leopold WR, Connors RW, Bridges AJ. Science. 1994;265:1093. doi: 10.1126/science.8066447. [DOI] [PubMed] [Google Scholar]
- 9.Hartmann JT, Haap M, Kopp HG, Lipp HP. Curr Drug Metab. 2009;10:470. doi: 10.2174/138920009788897975. [DOI] [PubMed] [Google Scholar]
- 10.Aaronson SA. Science. 1991;254:1146. doi: 10.1126/science.1659742. [DOI] [PubMed] [Google Scholar]
- 11.Dancey J, Sausville EA. Nat Rev Drug Discov. 2003;2:296. doi: 10.1038/nrd1066. [DOI] [PubMed] [Google Scholar]
- 12.Cai W, Niu G, Chen X. Eur J Nucl Med Mol Imaging. 2008;35:186. doi: 10.1007/s00259-007-0560-9. [DOI] [PubMed] [Google Scholar]
- 13.Gelovani JG. Cancer Metast Rev. 2008;27:645. doi: 10.1007/s10555-008-9156-5. [DOI] [PubMed] [Google Scholar]
- 14.Mishani E, Abourbeh G. Curr Top Med Chem. 2007;7:1755. doi: 10.2174/156802607782507457. [DOI] [PubMed] [Google Scholar]
- 15.Mishani E, Abourbeh G, Eiblmaier M, Anderson CJ. Curr Pharm Des. 2008;14:2983. doi: 10.2174/138161208786404326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mishani E, Hagooly A. J Nucl Med. 2009;50:1199. doi: 10.2967/jnumed.109.062117. [DOI] [PubMed] [Google Scholar]
- 17.Pantaleo MA, Nannini M, Maleddu A, Fanti S, Nanni C, Boschi S, Lodi F, Nicoletti G, Landuzzi L, Lollini PL, Biasco G. Ann Oncol. 2009;20:213. doi: 10.1093/annonc/mdn625. [DOI] [PubMed] [Google Scholar]
- 18.Reilly RM. Monoclonal antibodies and peptide-targeted radiotherapy of cancer. Hoboken, New Jersey: John Wiley & Sons, Inc; 2010. [Google Scholar]
- 19.Tolmachev V, Stone-Elander S, Orlova A. Lancet Oncol. 2010;11:992. doi: 10.1016/S1470-2045(10)70088-7. [DOI] [PubMed] [Google Scholar]
- 20.McLarty K, Reilly RM. Clin Pharmacol Ther. 2007;81:420. doi: 10.1038/sj.clpt.6100096. [DOI] [PubMed] [Google Scholar]
- 21.Wadas TJ, Wong EH, Weisman GR, Anderson CJ. Chem Rev. 110:2858. doi: 10.1021/cr900325h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hicks J, Wilson AA, VanBrocklin HF, Houle S, Vasdev N. Molecules. 2010;15:8260. doi: 10.3390/molecules15118260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu N, Li M, Li X, Meng X, Yang G, Zhao S, Yang Y, Ma L, Fu Z, Yu J. J Nucl Med. 2009;50:303. doi: 10.2967/jnumed.108.056556. [DOI] [PubMed] [Google Scholar]
- 24.Vasdev N, Dorff PN, Gibbs AR, Nandanan E, Reid L, O’Neil JP, VanBrocklin HF. J Labelled Compd Radiopharm. 2005;48:109. [Google Scholar]
- 25.Moerlein SM, Weisman RA, Beck D, Li AP, Welch MJ. Nucl Med Biol. 1993;20:49. doi: 10.1016/0969-8051(93)90135-h. [DOI] [PubMed] [Google Scholar]
- 26.VanBrocklin HF, Lim JK, Coffing SL, Hom DL, Negash K, Ono MY, Gilmore JL, Bryant I, Riese DJ., 2nd J Med Chem. 2005;48:7445. doi: 10.1021/jm050607w. [DOI] [PubMed] [Google Scholar]
- 27.Attina M, Cacace F, Wolf AP. J Labelled Compd Radiopharm. 1983;20:501. [Google Scholar]
- 28.Attina M, Cacace F, Wolf AP. J Chem Soc Chem Commun. 1983:108. [Google Scholar]
- 29.Karramkam M, Hinnen F, Bramoulle Y, Jubeau S, Dolle F. J Labelled Compd Radiopharm. 2002;45:1103. [Google Scholar]
- 30.Olma S, Ermert J, Coenen HH. J Labelled Compd Radiopharm. 2006;49:1037. [Google Scholar]
- 31.Haka MS, Kilbourn MR, Watkins GL, Toorongian SA. J Labelled Compd Radiopharm. 1989;27:823. [Google Scholar]
- 32.Pun M, Joseph S, Blecha J, Vanbrocklin H. J Labelled Compd Radiopharm. 2009;52:S173. [Google Scholar]
- 33.Feliu AL. J Labelled Compd Radiopharm. 1988;25:1245. [Google Scholar]
- 34.Bonasera TA, Ortu G, Rozen Y, Krais R, Freedman NM, Chisin R, Gazit A, Levitzki A, Mishani E. Nucl Med Biol. 2001;28:359. doi: 10.1016/s0969-8051(01)00200-1. [DOI] [PubMed] [Google Scholar]
- 35.Pike VW. Trends Pharmacol Sci. 2009;30:431. doi: 10.1016/j.tips.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Snyder SE, Sherman PS, Blair JB. J Nucl Med. 2000;41:1035. [Google Scholar]
- 37.Park BK, Kitteringham NR. Drug Metab Rev. 1994;26:605. doi: 10.3109/03602539408998319. [DOI] [PubMed] [Google Scholar]
- 38.Renson J, Bourdon V. Arch Int Pharmacodyn Ther. 1968;171:240. [PubMed] [Google Scholar]
- 39.Rietjens IM, Vervoort J. Chem Biol Interact. 1991;77:263. doi: 10.1016/0009-2797(91)90036-7. [DOI] [PubMed] [Google Scholar]
- 40.Kirk KL, Creveling CR. Med Res Rev. 1984;4:189. doi: 10.1002/med.2610040204. [DOI] [PubMed] [Google Scholar]
- 41.Kirk KL, Olubajo O, Buchhold K, Lewandowski GA, Gusovsky F, McCulloh D, Daly JW, Creveling CR. J Med Chem. 1986;29:1982. doi: 10.1021/jm00160a030. [DOI] [PubMed] [Google Scholar]
- 42.Chirakal R, Vasdev N, Asselin MC, Schrobilgen GJ, Nahmias C. J Fluorine Chem. 2002;115:33. [Google Scholar]