Abstract
There is a critical need for animal models to study aspects type 2 diabetes mellitus pathogenesis and prevention. While the rhesus macaque is such an established model, the common marmoset has added benefits including reduced zoonotic risks, shorter life span, and a predisposition to birth twins demonstrating chimerism. The marmoset as a model organism for the study of metabolic syndrome has not been fully evaluated. Marmosets fed high-fat or glucose-enriched diets were followed longitudinally to observe effects on morphometric and metabolic measures. Effects on pancreatic histomorphometry and vascular pathology were examined terminally. The glucose–enriched diet group developed an obese phenotype and a prolonged hyperglycemic state evidenced by a rapid and persistent increase in mean glycosylated hemoglobin (HgbA1c) observed as early as week 16. In contrast, marmosets fed a high-fat diet did not maintain an obese phenotype and demonstrated a delayed increase in HgbA1c that did not reach statistical significance until week 40. Consumption of either diet resulted in profound pancreatic islet hyperplasia suggesting a compensation for increased insulin requirements. Although the high fat diet group developed atherosclerosis of increased severity, the presence of lesions correlated with glucose intolerance only in the glucose-enriched diet group. The altered timing of glucose dysregulation, differential contribution to obesity, and variation in vascular pathology suggests mechanisms of effect specific to dietary nutrient content. Feeding nutritionally modified diets to common marmosets recapitulates aspects of metabolic disease and represents a model that may prove instrumental to elucidating the contribution of nutrient excess to disease development.
Introduction
Diabetes mellitus is a disease of great public health concern. In the United States alone, an estimated 23.6 million individuals have diagnosed or undiagnosed diabetes with the vast majority of undiagnosed cases likely to be type 2 diabetes (T2D) (1). As this population ages, the prevalence of associated cardiovascular, renal, and neurologic comorbidities is expected to rise, adding to the urgency of finding options for treatment and prevention. With over one billion in NIH dollars devoted to research initiatives in diabetes pathogenesis and prevention, there is a need to develop and refine animal models to facilitate this research (2). Of the mammalian systems available, non-human primate (NHP) models are highly beneficial due to their genetic and physiologic similarity to humans.
Currently there is a wide body of literature describing diabetes research in macaque species. A common model involves the study of spontaneous onset of T2D in aged rhesus macaques. Of macaques housed in captivity and fed standard ad libitum diets, a segment of the population remains lean with normal carbohydrate and lipid profiles while approximately one quarter develops overt diabetes mellitus (3). The remainder manifests features of metabolic syndrome characterized by varying degrees of insulin resistance, glucose intolerance, dyslipidemia, and obesity. Because macaques do not begin demonstrating clinical signs until age 10 or older and then with only a portion of animals developing disease, populations of animals with specific demographic and morphometric characteristics are required to generate a sufficient sample size to perform studies. Subsequently, these models are associated with significant financial constraints and specialized resource requirements.
New world primates, particularly the common marmoset, hold great potential as alternative primate models for the study of human disease. This species is easily handled, breeds well in captivity, and is associated with fewer zoonotic risks (4). There are additional benefits when performing longitudinal studies; the average marmoset life span is 10–12 years compared to 25–30 years in the rhesus macaque (5–6). As a result, marmosets develop age-related diseases within a shorter time span. Finally, female marmosets routinely ovulate multiple ova per cycle resulting in a high frequency of multiple births. This high prevalence of twinning allows for studies of gene-environment interaction (7).
Despite these advantages there are few publications reporting the utility of the common marmoset for the study of T2D or metabolic disease. Tardif and colleagues recently published a survey of 64 adult marmosets that identified variations in adiposity among study animals with obese animals demonstrating increased triglycerides, VLDL, fasting blood glucose, and glycosylated hemoglobin (HgbA1c) (8). Dietary studies have also been performed in marmosets to induce obesity in support of drug development for anti-obesity agents (9). Taken together these reports suggest that the marmoset is likely to respond to caloric manipulation and has the potential to develop metabolic alterations associated with a T2D phenotype.
NHP models become particularly relevant when examining the impact of diet on disease. Dietary studies are difficult to perform in human populations due to inaccuracies associated with self reported diet records (10). In contrast, animal models allow interventions in which diets consisting of specifically modified content can be fed in a controlled manner. Although diet and obesity are well known risk factors for the development of T2D, there is little consensus on the contribution of specific nutrients to disease pathogenesis. Performing dietary intake studies in a NHP model could prove instrumental in elucidating the role of various nutrient excesses.
In this study we examine the association between body composition and measures of lipid and glucose metabolism in common marmosets. To test the hypothesis that variations in dietary nutrient composition result in differentially altered glucose metabolism, a subset of randomly selected colony animals were fed either a high-fat or glucose-enriched diet and assessed for changes in glycosylated hemoglobin (HgbA1c), glucosuria, and defects in glucose tolerance. The effect of diet on lipid parameters, morphometric measures, and histopathologic outcomes was assessed as was the potential of these baseline parameters as predictors of dietary response.
Methods and Procedures
Animals
Marmosets were housed at the New England Primate Research Center (NEPRC) and maintained in accordance with the “Guide for the Care and Use of Laboratory Animals” of the Institute of Laboratory Resources. The facility is accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care International. Animals are housed in stainless steel caging in pairs or groups with room temperature maintained at 78°F +/− 4°F and humidity between 30–70%. Animals are fed once daily with standard commercial chow (Harlan Teklad New World Primate Chow 8791) supplemented with fruits, vegetables, seeds, and hard-boiled eggs. Fresh water is provided ad libitum.
Study design for dietary intervention
Work was approved by Harvard Medical School’s Standing Committee on Animals. Marmosets were selected at random from the NEPRC colony which houses, on average, 250 animals of which approximately 46% are male and 54% are female. Average age of animals within the colony is currently 4.16 years (SD 3.19) and ranges up to 14.4 years of age. Study animals were assigned to two dietary intervention groups (Table 1). Group one was fed a commercially available high-fat diet containing 20% added saturated fat (n=8; Laboratory Diets, Richmond IN). Group two was fed a commercially available diet enriched in glucose (n=16; Mazuri Callitrichid Diet 5MI5, PMI Nutrition, Henderson, CO). One animal from the glucose-enriched diet group diagnosed with small intestinal adenocarcinoma at necropsy was excluded from further analysis. Animals were fed once daily and the dietary supplements described above were continued. Baseline measures were obtained while animals were consuming standard chow allowing each animal to serve as its own control. Phlebotomy and measures of body composition were subsequently performed on all animals at weeks 16, 28, 40, and 52. Free catch urine samples were collected at week 40. Urine samples were collected in the early morning prior to feeding. Alterations in insulin-stimulated glucose uptake in response to diet were assessed using a modified intravenous glucose tolerance test (IVGTT) performed during the plateau phase for increases in HgbA1c between weeks 44–56. The study was terminated between weeks 70–78 when a subset of the dietary modified animals (n=8 from each diet group) was euthanized for complete necropsy. The remainder of the glucose-enriched diet group was maintained for further study.
Table 1.
Comparison of nutrient composition for standard diet, high-fat diet, and glucose-enriched diet.
| Composition | Standard chow | High-fat diet | Glucose-enriched diet |
|---|---|---|---|
| Metabolizable energy (kcal/g) | 3.38 | 4.02 | 3.42 |
| Fat, % | 10.4 | 22.2 | 7.8 |
| Cholesterol, ppm | 110b | 1042 | 500c |
| Protein, % | 23.7 | 19.6 | 21.3 |
| Fiber, % | 4.3 | 3.6 | 1.8 |
| Nitrogen free extract (by difference), % | 55.0 | 49.1 | 64.6 |
| Starch, % | 35.6b | 29. 6 | 14.2 |
| Glucose, % | 4.5b | 0.2 | 32.0c |
| Fructose, % | 0.3 | 0.1c | |
| Sucrose, % | 5.0b | 5.5 | 0.4c |
Unless otherwise indicated the composition is calculated on a dry matter basis according to data provided in published nutritional profiles.
Data provided by Harlan Teklad representative based on concentration predicted by diet ingredients. Estimated glucose concentration includes contribution from fructose.
Final calculated concentrations for carbohydrates and cholesterol were provided by Mazuri Exotic Animal Nutrition representative.
Body composition measures
Animals were habituated to entering an elongated holder. Total body weight was obtained by weighing the holder and animal on an electronic scale. As previously described, fat and lean mass were quantified by placing the holder in an EchoMRI quantitative magnetic resonance analyzer (QMR; Echo Medical Systems, Houston, TX) (8). Animals were measured two times during each session and the means were recorded.
Metabolic profiling
Blood samples were collected prior to the once daily feeding by routine phlebotomy techniques on hand caught marmosets sedated with Ketamine (15 mg/kg Ketaset IM; Fort Dodge, Overland Park, KS). HgbA1c was measured in whole blood using the DCA 2000+ Hemoglobin A1c Analyzer (Siemens Diagnostics, Tarrytown, NY). Serum triglycerides and total cholesterol were assayed by a veterinary diagnostic laboratory (Idexx Laboratories, Westbrook, ME). Urine glucose was quantified using Clinistix strips (Siemens Diagnostics, Tarrytown, NY) with measures ≥ 100 mg/dL indicative of glucosuria.
Intravenous glucose tolerance test (IVGTT)
Blood glucose measures were obtained from ketamine sedated animals at −5, −1, 5, 10, 20, 30, 40, and 60 minutes following an intravenous bolus of 50% dextrose (750 mg/kg). Dextrose was delivered via a catheter placed within the saphenous vein. Heel stick blood samples measured with a hand held glucometer (Lifescan, Inc., Milpitas, CA). A randomly selected group of animals fed standard chow served as the control group (n=8). Summary measures include peak, area under the curve (AUC) and 60-minute blood glucose response as well as glucose disappearance rate (KG; calculated as [logeGlucose5minute−logeglucose30minute]/25). KG was calculated from the linear portion of the curve and as previously referenced (11). Animals with AUC blood glucose response at or above the 80th percentile were classified as glucose intolerant.
Vascular pathology
Animals were euthanized via an intravenous pentobarbital overdose (≫50 mg/kg). Representative sections of all major organs were collected, fixed in 10% neutral buffered formalin, embedded, sectioned at 5μm, and stained using hematoxylin and eosin (HE). Depending on the vessel length, two to four cross-sections of carotid arteries, thoracic aorta, abdominal aorta, iliac arteries, and femoral arteries were evaluated for the presence of atherosclerotic lesions. Lesions were scored on a scale of I to VI using the American Heart Association lesion classification criteria (12–13). Type I lesions are characterized by presence of lipid-laden macrophages (foam cells) within the vascular intima. As the number of foam cells increases and they begin to accumulate in adjacent layers, the lesions progress to Type II. Type III lesions are defined by the presence of extracellular pools of lipid between smooth muscle layers. Extracellular lipid increases to the point of forming a well-defined core in Type IV lesions. Fibrous tissue deposition in the tunica intima represents a transition to Type V lesions and Type VI lesions demonstrate rupture and thrombus formation. The pathologist (JAK) was blinded to the animals’ diet while reviewing slides.
Pancreatic histomorphometry
Mean cross-sectional islet area was determined in HE stained pancreatic sections with the Nuance spectral imaging system (CRi, Woburn, MA). Mean islet size was calculated based on measures of all islets larger than four cells in 10 randomly selected, non-overlapping 20x fields with > 30 islets, on average, measured per animal. Archival tissues from eight healthy adult marmosets fed standard chow and previously submitted for necropsy were included as controls. Immunohistochemical staining for glucagon and insulin was performed. Preparation of tissue sections for immunohistochemistry has been described (14). Slides were incubated with rabbit anti-glucagon (1:275) or guinea pig anti-insulin polyclonal antibody (1:300; Dako Cytomation, Carpinteria, CA). Immunoglobulin fraction was used to stain irrelevant control slides. Sections were washed and incubated with biotinylated goat anti-rabbit immunoglobulins for glucagon (1:200) or biotinylated goat anti-guinea pig immunoglobulins for insulin (1:200). Slides were incubated with ABC solution (Vectastain ABC Kit, Vector Laboratories), color developed with diamino-benzidine-tetrahydrochloride-dihydrate (DAB), and counterstained (14). Each immunostain included negative control slides in which the primary antibody was excluded from the protocol. Sections were evaluated in a blinded fashion by JAK.
Data analysis
Analysis was performed using Stata software (Stata Press, College Station, TX). Quantitative variables are reported as means ± standard deviation (SD) or ± 95% confidence interval (CI) as indicated. Linear relationships were examined using scatter plots and correlations determined via calculation of Pearson correlation coefficient. AUC summary measures were calculated using the trapezoidal rule. Statistical comparisons within or between groups for continuous or categorical variables were performed with two-tailed Student’s t test (paired or unpaired) or Fischer exact test, respectively. The Wilcoxon signed-rank test or the Mann-Whitney U test was performed for comparisons of paired or unpaired non-normally distributed data. Kruskal-Wallis test was used to determine significant differences across the three diet groups. Bonferroni correction was performed to adjust for multiple comparisons. For all analysis, a p-value ≤ 0.05 was deemed significant.
Results
Associations between measures of body composition and metabolic parameters in colony animals
Total body weight, lean mass, and fat mass were determined in adult and sub-adult NEPRC colony housed marmosets (n= 187; 102 females, 85 males) ranging in age from 1.0–13.6 years. Breeding females were not measured to exclude potential confounding due to pregnancy. Variation in morphometric measures by gender was evaluated with female marmosets having significantly higher mean fat mass relative to males (Table 2). The contributions of fat and lean mass to total body mass were examined graphically (Figure 1). Calculation of Pearson’s correlation coefficients demonstrated that total mass and lean mass were highly correlated (r= 0.90, p< 0.00001) suggesting that 80% (coefficient of determination; r2) of the variability in total mass could be explained by the variability in lean mass. Total mass and fat mass were less highly correlated (r=0.77, p<0.00001) with only 60% of the variability in total mass explained by the variability in fat mass. These relationships were not modified by gender (data not shown). This data indicates that marmosets of comparable total mass were likely to have comparable lean mass but could present with widely variable body fat levels.
Table 2.
Morphometric and biochemical measures by sex and obesity status for colony marmosets.
| Female (n=102) | Male (n=85) | P value | |
|---|---|---|---|
| Age, mean (SD), years | 4.37 (3.18) | 4.61 (2.94) | N.S. |
| Morphometric measures, mean (SD) | |||
| Total mass, g | 385.70 (51.61) | 378.18 (49.12) | N.S. |
| Lean mass, g | 290.75 (29.92) | 293.53 (30.81) | N.S. |
| Fat mass, g | 44.04 (22.93) | 33.82 (22.12) | 0.002 |
| Biochemical measures, mean (SD), (n=51) | |||
| Blood glucose, mg/dL | 202.78 (56.81) | 190.61 (59.30) | N.S. |
| Serum triglycerides, mg/dL | 184.21 (224.32) | 222.91 (509.90) | N.S. |
| Serum cholesterol, mg/dL | 158.25 (63.95) | 196.56 (70.59) | <0.05 |
| Obeseb (n=39) | Non-obese (n=148) | P valuea | |
| Age, mean (SD), years | 2.74 (2.46) | 4.94 (3.06) | 0.0001 |
| Age category, No. (%) | |||
| 1–2 years | 18 (9.63) | 21 (11.23) | 0.0001 |
| 2–5 years | 18 (9.63) | 68 (36.36) | |
| 5–8 years | 1 (0.53) | 34 (18.18) | |
| > 8 years | 2 (1.07) | 25 (13.37) | |
| Sex, No. (%) | |||
| Female | 27 (14.44) | 75 (40.11) | 0.03 |
| Male | 12 (6.42) | 73 (39.04) | |
| Morphometric measures, mean (SD) | |||
| Total mass, g | 444.85 (48.97) | 365.79 (35.98) | 0.00001 |
| Lean mass, g | 313.72 (35.15) | 286.29 (26.13) | 0.00001 |
| Fat mass, g | 77.21 (13.40) | 29.43 (12.16) | 0.00001 |
| Biochemical measures, mean (SD), (n=51) | |||
| Blood glucose, mg/dL | 242.50 (56.30) | 176.63 (45.75) | 0.0001 |
| Serum triglyceride, mg/dL | 430.06 (620.14) | 97.25 (57.57) | <0.05 |
| Serum cholesterol, mg/dL | 175.75 (89.06) | 175.43 (59.31) | N.S. |
P value obtained by unpaired, two-tailed Student’s t test for continuous variables and Fisher exact test for categorical variables. N.S.-non-significant.
Obesity is defined by total grams body fat at or above the 80th percentile for colony housed animals.
Figure 1.
Linear correlations between lean mass or fat mass and total body mass in colony housed adult marmosets.
Serum chemistry panels collected within 12 months of body composition measurement were available from 51 (n=28 females, 23 males) of the animals surveyed. Examination of correlations between fat mass and lipid parameters indicated that fat mass was highly correlated to both triglyceride levels (r= 0.54, p<0.00001) and blood glucose levels (r=0.49, p=0.0003) but not to total serum cholesterol (r=0.11, p= 0.42). While there were significant sex differences in serum cholesterol (Table 2), the correlation trends for all parameters were similar in both male and female marmosets (data not shown).
A comparison of obese to non-obese marmosets was performed (Table 2). Marmosets at or above the 80th percentile of fat mass (≥ 60.24 g) were classified as obese with the remainder of surveyed animals considered non-obese. Obese marmosets demonstrated significantly increased total, fat, and lean mass. A significantly greater percentage of obese marmosets were female. Obese animals were significantly younger with the majority of obese animals representing sub-adults and adults younger than five years of age. Mean fat mass for sub- to young-adult animals ranging from 1–2 years of age (n=39; 53.90 g [SD, 24.70]) was significantly greater than that observed in prime adults ranging between 2–5 years of age (n=86; 38.01 g [SD, 24.72]), 5–8 years of age (n=35; 30.86 g [SD, 13.81]), and in aging animals greater than 8 years of age (n=27; 33.91 g [14.77], p=0.0004, Kruskal-Wallis test). Similar to previously published reports (8), obese marmosets had significant increases in mean blood glucose and mean serum triglycerides. There were no significant differences in serum cholesterol.
Effects of dietary manipulation
To examine how variation in dietary nutrient composition modulates morphometric and metabolic measures, a subset of randomly selected adult marmosets were fed either a high-fat (n=8) or glucose-enriched (n=15) diet and followed longitudinally for changes in body composition and metabolic parameters. The rationale for diet selection was based on interest in modeling response to extremes in nutrient composition. Results are presented in Table 3. Consumption of either diet resulted in a trend toward increased lean mass. Statistically significant differences from baseline were observed from week 28 onward in marmosets fed a glucose-enriched diet and at week 52 in marmosets fed a high-fat diet. Marmosets fed a glucose-enriched diet also demonstrated a marked increase in fat mass from week 16 onward with a 49% increase over mean baseline fat mass observed at week 52. Marmosets fed a high-fat diet also demonstrated increases in fat mass, although a statistically significant increase of 39% over mean baseline fat mass was observed only at week 16. While there were no significant differences in lean or fat mass when comparing the high-fat to glucose-enriched diet fed animals, marmosets fed a glucose-enriched diet demonstrated a trend toward a greater increase in the ratio of fat mass to total mass when compared to marmosets fed the high-fat diet with statistical significance achieved at week 40.
Table 3.
Longitudinal change in morphometric and biochemical measures in marmosets fed high-fat or glucose-enriched diets.
| Parameter | High-fat diet | Comparison to baselinea | Glucose-enriched diet | Comparison to baselinea | Comparison between dietsb | |
|---|---|---|---|---|---|---|
| Mean (SD) | P value | Mean (SD) | P value | P value | ||
| Lean mass, g | Wk 0 | 299.57 (27.68) | 286.81 (25.36) | NS | ||
| Wk 16 | 318.95 (40.13) | NS | 291.62 (25.00) | N.S. | NS | |
| Wk 28 | 305.33 (42.10) | NS | 299.54 (27.72) | 0.0004 | NS | |
| Wk 40 | 317.09 (34.91) | NS | 297.81 (31.60) | <0.05 | NS | |
| Wk 52 | 326.84 (39.14) | 0.005 | 297.22 (26.16) | 0.03 | NS | |
| AUC Lean mass by wk | 16291.93 (1873.42) | 15328.64 (1388.40) | NS | |||
| Fat mass, g | Wk 0 | 29.71 (16.77) | 35.76 (15.42) | NS | ||
| Wk 16 | 41.18 (23.61) | 0.01 | 44.68 (14.40) | 0.005 | NS | |
| Wk 28 | 39.06 (20.50) | NS | 54.04 (22.85) | 0.00004 | NS | |
| Wk 40 | 33.73 (20.30) | NS | 55.42 (25.30) | 0.002 | NS | |
| Wk 52 | 36.53 (10.60) | NS | 53.38 (27.54) | 0.02 | NS | |
| AUC Fat mass by wk | 1906.86 (951.30) | 2545.42 (1004.42) | NS | |||
| Fat:Total mass | Wk 0 | 0.08 (0.04) | 0.10 (0.04) | NS | ||
| Wk 16 | 0.10 (0.04) | 0.002 | 0.11 (0.03) | 0.01 | NS | |
| Wk 28 | 0.09 (0.04) | NS | 0.13 (0.05) | 0.00004 | NS | |
| Wk 40 | 0.08 (0.03) | NS | 0.13 (0.05) | 0.0004 | <0.05 | |
| Wk 52 | 0.09 (0.02) | NS | 0.13 (0.05) | 0.02 | NS | |
| AUC ratio by wk | 4.56 (1.79) | 6.30 (2.06) | NS | |||
| HgbA1c, % | Wk 0 | 4.08 (0.41) | 4.12 (0.30) | NS | ||
| Wk 16 | 4.12 (0.44) | NS | 4.58 (0.33) | 0.0004 | NS | |
| Wk 28 | 4.29 (0.30) | NS | 4.87 (0.51) | 0.0008 | 0.04 | |
| Wk 40 | 4.80 (0.52) | 0.002 | 5.11 (0.60) | 0.001 | NS | |
| Wk 52 | 4.74 (0.37) | 0.002 | 5.07 (0.42) | 0.00004 | NS | |
| AUC HgbA1c by wk | 227.82 (20.02) | 247.34 (17.70) | 0.03 | |||
| Trig., mg/dL | Wk 0c | 172.88 (180.92) | 76.33 (22.20) | NS | ||
| Wk 70–78 | 558.88 (1142.50) | NS | 157.00 (133.66) | NS | NS | |
| Chol., mg/dL | Wk 0c | 191.88 (58.02) | 155.33 (76.16) | NS | ||
| Wk 70–78 | 268.38 (163.69) | NS | 163.33 (69.13) | NS | NS | |
P value obtained by paired, two-tailed Student’s t test with Bonferroni adjustment or the Wilcoxon signed-rank test.
P value obtained by unpaired, two-tailed Student’s t test with Bonferroni adjustment or the Mann-Whitney U test.
Baseline lipid parameters available on a subset of animals (monosaccharide-enriched: n=6; high-fat: n=8).
Trig., Triglycerides; Chol., Cholesterol. NS-Non-significant.
HgbA1c, a common test utilized to assess glucose control in diabetic patients, was selected as a measure of the effect of diet on glucose metabolism (Table 3). This is a reliable index of the blood glucose average over a 1–4 month period and, unlike measures of circulating blood glucose, it is not subject to the effects of phlebotomy-associated capture stress. Marmosets fed a glucose-enriched diet demonstrated a rapid increase in mean HgbA1c. Statistically significant increases over baseline were observed from week 16 onward with a peak at week 40 and plateau through week 52. Marmosets fed a high-fat diet demonstrated a delayed increase over baseline mean HgbA1c that did not reach statistical significance until the peak at week 40 with plateau at week 52. Calculation of the mean AUC indicates that, overall, the observed increase in HgbA1c was greater in the glucose-enriched diet group compared to the high-fat diet group.
Serum triglycerides and cholesterol were measured at baseline on a subset of study animals (n=8 high-fat and n=6 glucose-enriched) and again at study termination on all animals (Table 3). While there was a trend toward diet-associated increases in lipid parameters over the duration of the study, particularly in the high-fat diet group, these differences were not statistically significant. Significant differences in lipid parameters were not detected between diet groups.
Effect of dietary manipulation on intravenous glucose tolerance test
Diet induced alteration in insulin stimulated glucose uptake was assessed using a modified IVGTT performed during the plateau phase of increased HgbA1c (weeks 44–56). Healthy, adult marmosets consuming standard chow (n=8) served as controls. Blood glucose was measured at specified time points pre- and post-administration of an intravenous 50% dextrose challenge. Sixty-minute, AUC, and peak blood glucose as well as the KG were examined as indicators of response. The glucose-enriched diet group demonstrated a significantly increased mean 60-minute blood glucose level (116.93 mg/dL [SD, 97.37]) compared to the high-fat diet (62.38 mg/dL [SD, 15.28]) and control groups (62.00 [SD, 16.10], p=0.02, Kruskal-Wallis test). There was a trend toward increased mean AUC blood glucose response in the glucose-enriched diet (10163.60 mg*minute/dL [SD, 3750.90]) compared to the high-fat diet (8094.81 mg*minute/dL [SD, 2080.86]) and control groups (8226.50 mg*minute/dL [SD, 1300.62], p=0.26, Kruskal-Wallis test) though this was not statistically significant. There was a similar non-significant trend towards reduced KG in the glucose-enriched diet (4.67 %/minute [SD 1.80]) compared to the high-fat diet (5.65 %/minute [SD 1.07]) and control groups (5.43 %/minute [SD 1.57], p=0.22, Kruskal-Wallis test). Significant differences in peak blood glucose response were not observed (data not shown). There was strong correlation between the 60-minute and AUC blood glucose response (r=0.69, p<0.00001), peak and AUC blood glucose response (r=0.76, p<0.00001), and KG and AUC blood glucose response (r=−0.76, p<0.00001). KG correlated with both the 60-minute (r=−0.49, p=0.005) and peak blood glucose responses (r=−0.42, p=0.02). The correlation between 60-minute and peak blood glucose response was less robust (r=0.30, p=0.10).
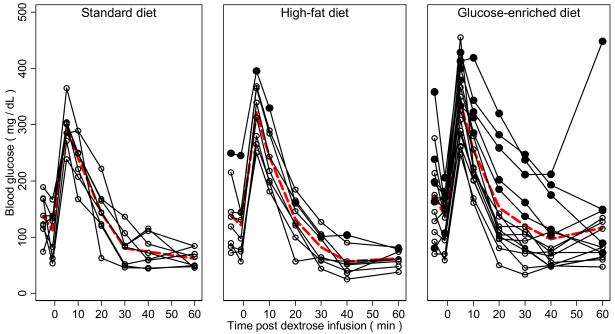
Interestingly, there was increased individual variability in the response to dextrose challenge among the dietary modified animals that was particularly evident in the glucose-enriched diet group (Figure 2). Animals at or above the 80th percentile for AUC blood glucose (≥ 10957.40 mg·minute/dL; indicated with solid circles in Figure 2) were classified as glucose intolerant. One (12.50%) glucose intolerant marmoset was present in the high-fat diet group and five (33.33%, p=0.37, Fisher exact test) were present in the glucose-enriched group. A greater percentage of intolerant animals (83.33%) had evidence of glucosuria compared to the remaining dietary modified animals (29.41%, p=0.05, Fisher exact test). Glucose intolerant marmosets demonstrated an increased mean HgbA1c and greater fat mass relative to the remaining dietary modified animals at week 52 (HgbA1c: 5.42% [SD 0.10] vs. 4.77% [SD 0.34] respectively, p=0.00001; Fat mass: 66.20 g [SD 28.94] vs. 40.93 [SD 19.14] respectively, p=0.02, Student’s t test). Glucose intolerant marmosets also had a reduced mean KG compared to the remaining dietary modified animals (3.23 %/minute [SD 1.29] vs. 5.64 %/minute [SD 1.23], p=0.0005). There were no significant differences in serum triglyceride or total cholesterol.
Figure 2.
Individual blood glucose responses measured at baseline and following intravenous challenge with 50% dextrose for marmosets fed standard, high-fat, and glucose-enriched diets. Dashed red line represents mean response for each diet group. Solid circles represent animals above the 80th percentile for area under the curve blood glucose response.
Vascular pathology
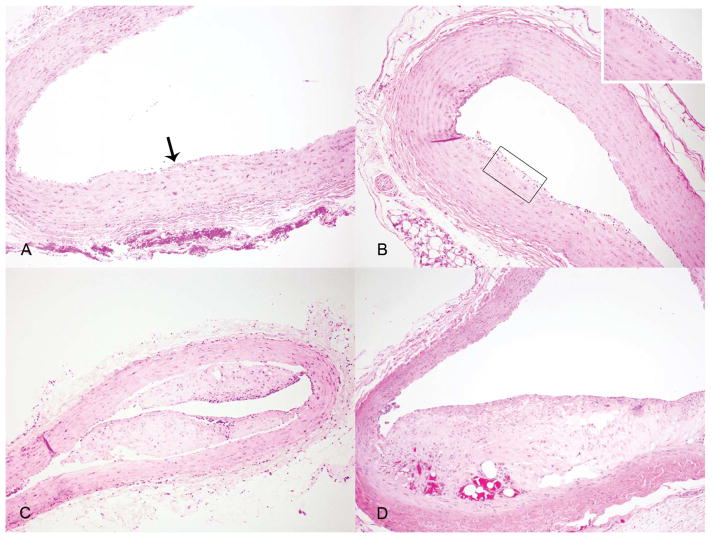
Marmosets consuming the high-fat diet (n=8) and a subset of those consuming the glucose-enriched diet (n=8) were euthanized for complete necropsy. To examine the association between diet and development of vascular lesions, the carotid arteries, thoracic aorta, abdominal aorta, iliac arteries, and femoral arteries were evaluated for the presence of atherosclerotic lesions. Lesion type by vessel for each animal is presented in Table 4 with representative histological sections presented in Figure 3. There were no significant differences in the number of animals demonstrating atherosclerotic lesions although marmosets in the high-fat diet group demonstrated a greater tendency for development of advanced lesions (≥ Type IV) while marmosets in the glucose-enriched group demonstrated only initial and intermediate lesions (≤ Type III). Interestingly, the presence of lesions correlated with development of glucose intolerance only in the glucose-enriched diet group. There was a trend toward increased mean total cholesterol in animals with lesions although this difference was not significant compared to animals lacking lesions (Total cholesterol: 239.18 [SD 151.64] vs. 157.00 mg/dL [SD 46.85] respectively, p=0.15, Mann-Whitney U test). Serum triglyceride was not significantly different when comparing animals with and without lesions (Triglyceride: 453.36 mg/dL [SD 973.33] vs. 155.2 mg/dL [SD 61.86] respectively, p=0.77, Mann-Whitney U test).
Table 4.
Vascular pathology for marmosets fed high-fat and glucose-enriched diets.
| Atherosclerotic lesion type by vessel a | ||||||
|---|---|---|---|---|---|---|
| Animal ID by diet | Glucose intolerance | Carotid arteries | Thoracic aorta | Abdominal aorta | Iliac arteries | Femoral arteries |
| High-fat | ||||||
| 1 | - | - | I | - | - | - |
| 2 | - | I | - | I | I | - |
| 3 | - | - | I | - | - | - |
| 4 | - | III | II | V | III | |
| 5 | - | - | - | - | - | - |
| 6 | - | - | I | - | - | - |
| 7 | - | III | III | I | VI | III |
| 8 | + | - | - | - | - | - |
| Glucose-enriched | ||||||
| 1 | - | - | - | - | - | - |
| 2 | - | - | - | - | - | - |
| 3 | - | - | - | - | - | - |
| 4 | + | II | II | I | I | |
| 5 | + | I | III | I | - | - |
| 6 | + | - | I | - | - | - |
| 7 | + | - | - | I | - | - |
| 8 | + | - | - | - | - | I |
Figure 3.
Histologic assessment of common marmoset arterial lesions. Fig 3A: Thoracic aorta from an animal fed a glucose-enriched diet with a type I arterial lesion characterized by localized accumulation of individual and small groups of foam cells. Fig 3B: Thoracic aorta from an animal fed a high-fat diet with a type II arterial lesion, characterized by more extensive infiltration of foam cells expanding the tunica intima and associated with intimal thickening. Fig 3C: Abdominal aorta from the same animal as in Fig 3B showing a type V lesion characterized by minimal extracellular lakes of lipid but severe fibrous thickening causing a narrowing of the arterial lumen. Fig 3D: Iliac artery from an animal fed a high-fat diet with a type VI arterial lesion characterized by fibrous thickening and extracellular lakes of lipid as in the type V lesion, but also accompanied by hemorrhage. This hemorrhage is most likely the result of a fissure in the surface of the arterial lesion which is out of the plane of section.
Pancreatic histomorphometry
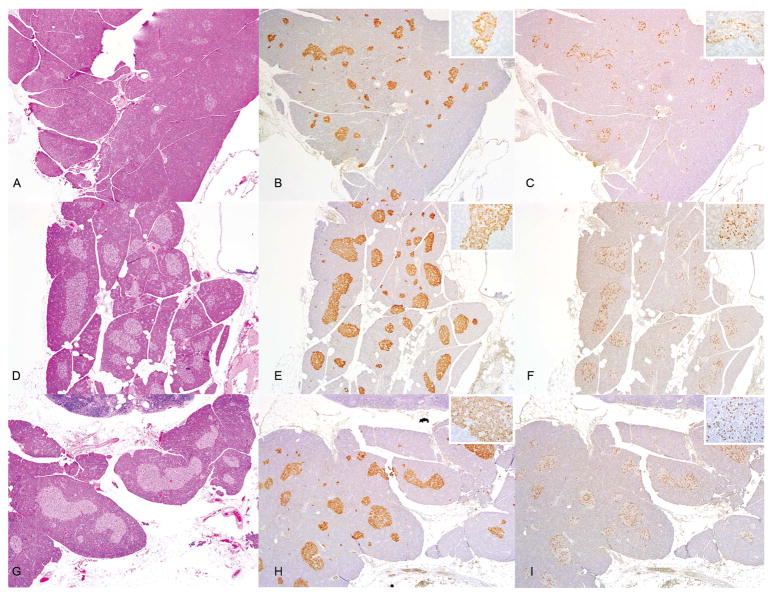
Pancreatic islet hyperplasia was evident in animals from both dietary modified groups (Figures 4A–C). Mean cross-sectional islet area was comparable between the high-fat and glucose-enriched diet fed animals (0.023 mm2 and 0.021 mm2 respectively) but demonstrated a nearly 2.5-fold increase when compared to controls fed a standard diet (0.0089 mm2, p=0.002, Kruskal-Wallis test). No abnormalities were detected in the islets on routine histopathologic examination. However, immunohistochemical analysis of the islets for insulin and glucagon expression did reveal differences between animals fed control and modified diets.
Figure 4.
Histologic and immunohistochemical assessment of common marmoset pancreases. Fig 4A: HE stained slide from a control marmoset fed standard monkey chow showing normal size and distribution of pancreatic islets. Fig 4B: Insulin immunostain of the same section of pancreas showing diffuse positive staining in all islets. Inset shows polarization of insulin staining with most intense staining towards the vascular pole of β cells. Fig 4C: Glucagon immunostain of same section. Inset shows glucagon staining localized mainly to the periphery of islets. In both animals fed a high-fat diet and glucose-enriched diet islets are hyperplastic on HE section (Figs 4D and 4G) and insulin immunostaining is maintained throughout all islets but with reduced intensity (Figs 4E and 4H). Insulin staining maintained polarity within cells (insets of Figs 4E and 4H). Unlike control animals, glucagon staining is consistent throughout the islet rather than focused on the periphery (Figs 4F and 4I).
In animals fed a control diet, insulin producing β cells were present throughout all islets and there were also positively staining individual cells or small cell clusters scattered throughout the exocrine pancreas. Staining intensity was polarized with most intense staining at the vascular end of each β cell as previously described (Figure 4D, inset) (15). Insulin staining was preserved and often intense in all islets of animals fed both glucose-enriched and high-fat diets (Figures 4E and 4F) and these animals continued to have numerous individual cells and small cell clusters that stained positive for insulin. However, animals with marked islet hyperplasia frequently showed a loss of staining intensity and occasionally a more granular staining appearance (Figures 4E and 4F, insets) compared with control animals. We did not observe a loss of insulin polarization within β cells as was previously reported in marmosets with islet hyperplasia (15).
Glucagon expression was highly variable in animals fed a control diet. Individual islets contained glucagon-positive α cells while adjacent islets showed no glucagon expression. Staining was often most intense in the outermost region of the islet and formed a rim around the islet (Figure 4G, inset) with only scattered cells in the islet’s interior staining positive, similar to findings from normal rodent islets but different from that in humans and rhesus macaques (16–17). In contrast, animals from both diet groups, especially those with hyperplastic islets, showed a more uniform expression of glucagon. Not only were a higher proportion of islets glucagon-positive overall, but within each islet glucagon-positive cells were scattered throughout the islet and not limited to the periphery (Figures 4H and 4I and insets).
Baseline predictors of response to dietary manipulation
The factors associated with obesity in the colony-wide survey were examined for their utility as predictors of altered glucose metabolism in response to dietary manipulation. These factors included age, sex, baseline serum triglycerides, and baseline blood glucose. Also examined as predictors of response to diet were lean mass, fat mass, cholesterol, and HgbA1c; all measured at baseline. Although obese colony animals tended to be younger, study marmosets identified as being glucose intolerant on IVGTT were older with an increased mean age of 8.5 years relative to the remaining dietary modified animals with a mean age of 5.29 years (p= 0.004, Student’s t test). A sex predisposition was not identified for response to IVGTT. The strongest predictor for altered glucose metabolism in response to diet was baseline serum triglycerides based on a robust correlation with week 52 HgbA1c (r=0.63, p=0.02) and the AUC blood glucose response to IVGTT (r=0.68, p=0.0008). Baseline fat mass was also predictive demonstrating correlations to week 52 HgbA1c (r=0.44, p= 0.04) and the AUC blood glucose response to IVGTT (r=0.41, p ≤ 0.05).
Discussion
Using a marmoset model we have demonstrated that variations in dietary fat and carbohydrate result in differentially altered glucose metabolism and body composition. Marmosets fed a diet enriched in simple sugars, specifically glucose, developed a prolonged hyperglycemic state as evidenced by the rapid and persistent elevation in mean HgbA1c as early as week 16. Animals in the glucose-enriched diet group also had a greater tendency towards development of an obese phenotype with an early and sustained increase in fat mass. In contrast, animals fed a high-fat diet demonstrated a delayed response and did not reach comparable elevations in HgbA1c until week 40. Animals in this group demonstrated only a transient increase in fat mass with return to basal levels for the remainder of the study.
There is little consensus regarding the contribution of specific nutrient excesses to the risk of T2D. Studies in human subjects and animal models present widely contrasting results on the benefits or risks of high carbohydrate versus high fat diets (18–22). Consumption of diets high in refined and simple sugars are thought to increase insulin demand, promote insulin resistance, and impair beta cell function. High fat diets are thought to increase postprandial mobilization of fatty acids which then augments peripheral and hepatic insulin resistance (23). It is also suggested that high fat diets alter the fatty acid composition of membrane lipids with subsequent effects on insulin mediated signal transduction (24). Using a marmoset model, we show that both diets ultimately contribute to altered glucose metabolism although prolonged intake of the high fat diet was required to achieve the effects seen relatively rapidly with the glucose-enriched diet. While more studies are required, the differential timing of dysregulated glucose metabolism suggests mechanisms of effect specific to nutrient content of the diet.
Consumption of modified diets by study marmosets resulted in profound pancreatic islet hyperplasia. Increased β cell mass has been documented in states of obesity and insulin resistance with the increased mass suggested, in part, to compensate for increased insulin requirements (25–29). β cell mass is dependent on both stem cell neogenesis and replication of differentiated β cells. Hyperglycemia and increased serum free fatty acids have been shown to stimulate β cell replication and cellular hypertrophy (26, 30). Progression to T2D is associated with β cell apoptosis and resultant reductions in β cell mass (27). Islet hyperplasia in study marmosets appeared to be due to β cell replication which suggests a compensation for increased insulin requirements. Because of the difficulties in systematically collecting human autopsy samples, a NHP model such as this may facilitate study of pancreatic islet morphology during the early, compensatory stages of disease.
Insulin immunostaining was performed as a surrogate measure for circulating serum insulin. Marmoset insulin has 85% identity of amino acid sequence with that of humans and old world NHP and a reduced affinity for anti-insulin antibodies (31–32). This may contribute to the current lack of validated immunoassays for measurement of marmoset insulin. While insulin staining was generally preserved in pancreata isolated from dietary modified animals, there was a tendency for a loss of staining intensity in animals with marked islet hyperplasia. While further examination is required, this alteration in staining character may represent the initial compromise of the compensatory ability of hypertrophied islets. Marmosets consuming modified diets also demonstrated alterations in glucagon staining characterized by a higher proportion of glucagon-positive islets and diffuse staining throughout the islets. Increased glucagon has been shown to worsen glucose tolerance in T2 diabetics likely via induction of glycogenolysis and increased postprandial glucose concentrations (33–34). Validation of assays to measure circulating insulin and other metabolic hormones in common marmosets is critically important to elucidating these relationships and advancing characterization of the model.
The common marmoset has great potential as a model organism for the study of diabetes due to a relatively short life span, high prevalence of twinning, development of bone-marrow chimerism, and close genetic relationship to humans (4–5). Surprisingly, there have been few studies that have examined the utility of this species as such a model. Common methods of inducing glucose dysregulation in other species include dietary modification, treatment with diabetogenic drugs, and pancreatectomy. Streptozotocin induction has limited usefulness in the marmoset due to reduced pancreatic β cell expression of the glucose transporter GLUT2 required for drug uptake (14). Surgical models are less desirable due to their possible complications and requirements for specialized expertise. As demonstrated by Tardif et. al. and confirmed in this study, marmosets do develop obesity that is accompanied by dyslipidemia and altered glucose metabolism (8). This study has extended the survey of morphometric parameters to sub-adults and aging animals and has demonstrated that obesity is highly prevalent in sub-adult marmosets ranging between 1–2 years of age. With 46% of animals in this age category demonstrating obesity, the common marmoset could potentially serve as a natural animal model of adolescent obesity. We additionally demonstrate that feeding a nutritionally modified diet, particularly one enriched in glucose, enhances a natural tendency to develop obesity resulting in a readily employable animal model.
One limitation of this model is the inter-individual variability in response to diet. Thirty-three percent and 12.5% of animals consuming a glucose-enriched or high-fat diet, respectively, had evidence of impaired glucose tolerance and, as a group, demonstrated glucosuria and HgbA1c measures greater than 5%. An examination of the predictors of dietary response indicate that factors such as older age, increased baseline fat mass, and elevated baseline triglycerides were associated with development of altered glucose metabolism. Selection of animals with these characteristics may allow for an increased incidence of the desired phenotype. In contrast to many rodent models, NHP represent an outbred population. An alternative research strategy would capitalize on this genetic and phenotypic heterogeneity to survey for additional factors contributing to varied response to diet.
Increased baseline triglyceride level was the strongest predictor of altered glucose metabolism in response to diet. The association of hypertriglyceridemia with abnormal glucose and insulin profiles has been previously described (18). In animal and human studies, intake of mono- and disaccharide enriched diets results in elevated triglycerides, the levels of which correlate to measures of insulin resistance (35–36). Normalization of triglyceride measures following administration of a hypolipidemic agent resulted in complete reversal of skeletal muscle insulin resistance in fructose fed rats and a partial reversal in high fat diet fed rats (37). Consumption of a high sucrose diet by hypertriglyceridemic human subjects was associated with greater increases in fasting insulin and insulin to glucose ratios compared to normolipidemic patients (35, 38). These studies suggest that hypertriglyceridemic patients represent a carbohydrate sensitive population.
Due to the link between metabolic syndrome and cardiovascular disease, we elected to survey for development of atherosclerosis. Marmosets fed a high fat diet developed advanced atherosclerotic lesions (Type V and VI) that, in humans, can be associated with clinical symptoms (13). These lesions developed despite the fact that this group did not demonstrate persistent gains in fat mass. This suggests that the increased amount or type of dietary fat consumed translates into a macronutrient-specific effect on atherosclerosis development. Animals fed the glucose-enriched diet developed less severe lesions. Interestingly, the presence of atherosclerotic lesions correlated with glucose intolerance only in the glucose-enriched diet group. It is well established that metabolic syndrome and its components are risk factors for atherosclerosis but the independent contribution of insulin resistance remains under debate (39–40). Most evidence points to a stronger association with the combined components of metabolic syndrome rather than individual contributing factors. Because of the association of atherosclerotic lesion development with glucose intolerance and increased fat mass, a marmoset model based on feeding a glucose-enriched rather than a high-fat diet may more accurately represent the human condition.
Surprisingly, we did not observe statistically significant elevations in serum cholesterol following transition of marmosets to modified diets containing 5–10 times the amount of cholesterol provided in the standard diet. This is likely due to phenotypic heterogeneity. Crook et. al. noted that marmosets fed a high fat/high cholesterol diet partitioned into groups designated hyper- and hypo-responders based on presence or absence of elevated serum cholesterol and risk of atherosclerosis (41). A similar phenomena was observed in cynomolgus macaques which, when provided with a 10-fold increase in dietary cholesterol, developed a wide range of perturbations in serum cholesterol with one-third designated as either hypo- or hyper-responsive (42). Our results confirm this phenotypic variability in marmosets fed the high fat diet whose total cholesterol ranged from a minimum of 82 mg/dL to a maximum of 658 mg/dL resulting in a 2.8 fold increase in the standard deviation relative to baseline measures.
A major limitation to this study is a lack of quantitative data on food intake. Marmosets find food items that are high in simple sugars extremely palatable raising the possibility that an overall increased caloric intake of the glucose-enriched diet, rather than the macronutrient content of the diet itself, contributed to gains in body fat and secondary metabolic effects. Although subjective, cage-side assessment of the amount of food consumed suggested that animals in each group had adequate and comparable dietary intake. The fact that marmosets placed on the high-fat diet demonstrated an upward trend in lean mass and a transient increase in fat mass supports this observation. This group also developed comparable, although delayed, increases in HgbA1c, comparable pancreatic islet hypertrophy, and atherosclerotic lesions of greater severity compared to animals placed on the glucose-enriched diet. Despite this, without robust measures of food consumption there is no way to say with certainty that a higher caloric intake of an extremely palatable glucose-enriched diet did not contribute in some fashion to the metabolic perturbations reported. Future studies should incorporate a mechanism for quantitatively assessing food consumption.
In summary, marmosets fed either a high-fat or glucose-enriched diet developed altered glucose metabolism, pancreatic islet hyperplasia, and atherosclerosis. Marmosets fed a glucose-enriched diet experienced a prolonged state of hyperglycemia due to a rapid response to diet. Feeding of nutritionally modified diets to common marmosets recapitulates aspects of metabolic disease and represents a useful animal model with which to study aspects of pathogenesis and prevention.
Acknowledgments
This work was supported by the National Center for Research Resources grant P51 RR000168-47.
References
- 1.CDC. National diabetes fact sheet: general information and national estimates on diabetes in the United States. U.S. Department of Health and Human Services, Center for Disease Control and Prevention; 2007. [Google Scholar]
- 2.NIH. Estimates of funding for various diseases, conditions, research areas. 2008. National Institutes of Health, Department of Health and Humans Services; 2008. [Google Scholar]
- 3.Hansen BC, Bodkin NL. Heterogeneity of insulin reponses: phases leading to Type 2 (non-insulin-dependent) diabetes mellitus in the rhesus monkey. Diabetologia. 1986;29:713–9. doi: 10.1007/BF00870281. [DOI] [PubMed] [Google Scholar]
- 4.Ludlage E, Mansfield K. Clinical care and diseases of the common marmoset (Callithrix jacchus) Comp Med. 2003;53:369–82. [PubMed] [Google Scholar]
- 5.Abbott DH, Barnett DK, Colman RJ, Yamamoto ME, Schultz-Darken NJ. Aspects of common marmoset basic biology and life history important for biomedical research. Comp Med. 2003;53:339–50. [PubMed] [Google Scholar]
- 6.Roth GS, Mattison JA, Ottinger MA, Chachich ME, Lane MA, Ingram DK. Aging in rhesus monkeys: relevance to human health interventions. Science. 2004;305:1423–6. doi: 10.1126/science.1102541. [DOI] [PubMed] [Google Scholar]
- 7.Tardif SD, Smucny DA, Abbott DH, Mansfield KG, Schultz-Darken N, Yamamoto ME. Reproduction in captive common marmosets (Callithrix jacchus) Comparative Medicine. 2003;53:364–8. [PubMed] [Google Scholar]
- 8.Tardif SD, Power ML, Ross CN, Rutherford JN, Layne-Colon DG, Paulik MA. Characterization of obese phenotypes in a small nonhuman primate, the common marmoset (Callithrix jacchus) Obesity (Silver Spring) 2009;17:1499–505. doi: 10.1038/oby.2009.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fong TM. Targeting metabolic syndrome. Expert Opin Investig Drugs. 2004;13:1203–6. doi: 10.1517/13543784.13.9.1203. [DOI] [PubMed] [Google Scholar]
- 10.Kelemen LE. GI Epidemiology: nutritional epidemiology. Aliment Pharmacol Ther. 2007;25:401–7. doi: 10.1111/j.1365-2036.2007.03244.x. [DOI] [PubMed] [Google Scholar]
- 11.Jen K-LC, Hansen BC. Glucose disappearance rate in rhesus monkeys: Some technical considerations. Am J Primatol. 1988;14:153–66. doi: 10.1002/ajp.1350140206. [DOI] [PubMed] [Google Scholar]
- 12.Stary HC, Chandler AB, Glagov S, et al. A definition of initial, fatty streak, and intermediate lesions of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation. 1994;89:2462–78. doi: 10.1161/01.cir.89.5.2462. [DOI] [PubMed] [Google Scholar]
- 13.Stary HC, Chandler AB, Dinsmore RE, et al. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation. 1995;92:1355–74. doi: 10.1161/01.cir.92.5.1355. [DOI] [PubMed] [Google Scholar]
- 14.Kramer J, Moeller EL, Hachey A, Mansfield KG, Wachtman LM. Differential expression of GLUT2 in pancreatic islets and kidneys of New and Old World nonhuman primates. Am J Physiol Regul Integr Comp Physiol. 2009;296:R786–93. doi: 10.1152/ajpregu.90694.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Juan-Salles C, Marco A, Ramos-Vara JA, et al. Islet hyperplasia in callitrichids. Primates. 2002;43:179–90. doi: 10.1007/BF02629646. [DOI] [PubMed] [Google Scholar]
- 16.Brissova M, Fowler MJ, Nicholson WE, et al. Assessment of human pancreatic islet architecture and composition by laser scanning confocal microscopy. J Histochem Cytochem. 2005;53:1087–97. doi: 10.1369/jhc.5C6684.2005. [DOI] [PubMed] [Google Scholar]
- 17.Wieczorek G, Pospischil A, Perentes E. A comparative immunohistochemical study of pancreatic islets in laboratory animals (rats, dogs, minipigs, nonhuman primates) Exp Toxicol Pathol. 1998;50:151–72. doi: 10.1016/S0940-2993(98)80078-X. [DOI] [PubMed] [Google Scholar]
- 18.Daly ME, Vale C, Walker M, Alberti KG, Mathers JC. Dietary carbohydrates and insulin sensitivity: a review of the evidence and clinical implications. Am J Clin Nutr. 1997;66:1072–85. doi: 10.1093/ajcn/66.5.1072. [DOI] [PubMed] [Google Scholar]
- 19.Marshall JA, Hamman RF, Baxter J. High-fat, low-carbohydrate diet and the etiology of non-insulin-dependent diabetes mellitus: the San Luis Valley Diabetes Study. Am J Epidemiol. 1991;134:590–603. doi: 10.1093/oxfordjournals.aje.a116132. [DOI] [PubMed] [Google Scholar]
- 20.Sahyoun NR, Anderson AL, Tylavsky FA, Lee JS, Sellmeyer DE, Harris TB. Dietary glycemic index and glycemic load and the risk of type 2 diabetes in older adults. Am J Clin Nutr. 2008;87:126–31. doi: 10.1093/ajcn/87.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Storlien LH, Baur LA, Kriketos AD, et al. Dietary fats and insulin action. Diabetologia. 1996;39:621–31. doi: 10.1007/BF00418533. [DOI] [PubMed] [Google Scholar]
- 22.Yang EJ, Kerver JM, Park YK, Kayitsinga J, Allison DB, Song WO. Carbohydrate intake and biomarkers of glycemic control among US adults: the third National Health and Nutrition Examination Survey (NHANES III) Am J Clin Nutr. 2003;77:1426–33. doi: 10.1093/ajcn/77.6.1426. [DOI] [PubMed] [Google Scholar]
- 23.Boden G. Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes. 1997;46:3–10. [PubMed] [Google Scholar]
- 24.Kamada T, Yamashita T, Baba Y, et al. Dietary sardine oil increases erythrocyte membrane fluidity in diabetic patients. Diabetes. 1986;35:604–11. doi: 10.2337/diab.35.5.604. [DOI] [PubMed] [Google Scholar]
- 25.Ahren B. Type 2 diabetes, insulin secretion and beta-cell mass. Curr Mol Med. 2005;5:275–86. doi: 10.2174/1566524053766004. [DOI] [PubMed] [Google Scholar]
- 26.Bonner-Weir S, Deery D, Leahy JL, Weir GC. Compensatory growth of pancreatic beta-cells in adult rats after short-term glucose infusion. Diabetes. 1989;38:49–53. doi: 10.2337/diab.38.1.49. [DOI] [PubMed] [Google Scholar]
- 27.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–10. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 28.Del Prato S, Wishner WJ, Gromada J, Schluchter BJ. Beta-cell mass plasticity in type 2 diabetes. Diabetes Obes Metab. 2004;6:319–31. doi: 10.1111/j.1462-8902.2004.00360.x. [DOI] [PubMed] [Google Scholar]
- 29.Flier SN, Kulkarni RN, Kahn CR. Evidence for a circulating islet cell growth factor in insulin-resistant states. Proc Natl Acad Sci U S A. 2001;98:7475–80. doi: 10.1073/pnas.131192998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Milburn JL, Jr, Hirose H, Lee YH, et al. Pancreatic beta-cells in obesity. Evidence for induction of functional, morphologic, and metabolic abnormalities by increased long chain fatty acids. J Biol Chem. 1995;270:1295–9. doi: 10.1074/jbc.270.3.1295. [DOI] [PubMed] [Google Scholar]
- 31.Seino S, Steiner DF, Bell GI. Sequence of a New World primate insulin having low biological potency and immunoreactivity. Proc Natl Acad Sci U S A. 1987;84:7423–7. doi: 10.1073/pnas.84.21.7423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wallis M. New insulin-like growth factor (IGF)-precursor sequences from mammalian genomes: the molecular evolution of IGFs and associated peptides in primates. Growth Horm IGF Res. 2009;19:12–23. doi: 10.1016/j.ghir.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 33.Shah P, Vella A, Basu A, Basu R, Schwenk WF, Rizza RA. Lack of suppression of glucagon contributes to postprandial hyperglycemia in subjects with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2000;85:4053–9. doi: 10.1210/jcem.85.11.6993. [DOI] [PubMed] [Google Scholar]
- 34.Shah P, Basu A, Basu R, Rizza R. Impact of lack of suppression of glucagon on glucose tolerance in humans. Am J Physiol. 1999;277:E283–90. doi: 10.1152/ajpendo.1999.277.2.E283. [DOI] [PubMed] [Google Scholar]
- 35.Reiser S, Handler HB, Gardner LB, Hallfrisch JG, Michaelis OEt, Prather ES. Isocaloric exchange of dietary starch and sucrose in humans. II. Effect on fasting blood insulin, glucose, and glucagon and on insulin and glucose response to a sucrose load. Am J Clin Nutr. 1979;32:2206–16. doi: 10.1093/ajcn/32.11.2206. [DOI] [PubMed] [Google Scholar]
- 36.Thorburn AW, Storlien LH, Jenkins AB, Khouri S, Kraegen EW. Fructose-induced in vivo insulin resistance and elevated plasma triglyceride levels in rats. Am J Clin Nutr. 1989;49:1155–63. doi: 10.1093/ajcn/49.6.1155. [DOI] [PubMed] [Google Scholar]
- 37.Storlien LH, Oakes ND, Pan DA, Kusunoki M, Jenkins AB. Syndromes of insulin resistance in the rat. Inducement by diet and amelioration with benfluorex. Diabetes. 1993;42:457–62. doi: 10.2337/diab.42.3.457. [DOI] [PubMed] [Google Scholar]
- 38.Reiser S, Bohn E, Hallfrisch J, Michaelis OEt, Keeney M, Prather ES. Serum insulin and glucose in hyperinsulinemic subjects fed three different levels of sucrose. Am J Clin Nutr. 1981;34:2348–58. doi: 10.1093/ajcn/34.11.2348. [DOI] [PubMed] [Google Scholar]
- 39.Sourij H, Schmoelzer I, Dittrich P, Paulweber B, Iglseder B, Wascher TC. Insulin resistance as a risk factor for carotid atherosclerosis: a comparison of the Homeostasis Model Assessment and the short insulin tolerance test. Stroke. 2008;39:1349–51. doi: 10.1161/STROKEAHA.107.502799. [DOI] [PubMed] [Google Scholar]
- 40.Razani B, Chakravarthy MV, Semenkovich CF. Insulin resistance and atherosclerosis. Endocrinol Metab Clin North Am. 2008;37:603–21. viii. doi: 10.1016/j.ecl.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Crook D, Weisgraber KH, Boyles JK, Mahley RW. Isolation and characterization of plasma lipoproteins of common marmoset monkey. Comparison of effects of control and atherogenic diets. Arteriosclerosis. 1990;10:633–47. doi: 10.1161/01.atv.10.4.633. [DOI] [PubMed] [Google Scholar]
- 42.Turley SD, Spady DK, Dietschy JM. Identification of a metabolic difference accounting for the hyper- and hyporesponder phenotypes of cynomolgus monkey. J Lipid Res. 1997;38:1598–611. [PubMed] [Google Scholar]