Abstract
Small interfering RNA (siRNA) has been widely proposed to treat various diseases by silencing genes, but its delivery remains a challenge. A well-controlled assembly approach was applied to prepare a protease assisted nanodelivery system. Onto a gold nanoparticles (AuNPs), protease degradable poly-L-lysine (PLL) and siRNA were fabricated by alternating the charged polyelectrolytes. In this study, up to 4 layers of PLL and 3 layers of siRNA (sR3P) were coated. Due to slow degradation of PLL, the incorporated siRNA was released gradually and showed extended gene silencing effect. Importantly, the inhibition effect in cells was found to correlate with the number of siRNA layers.
Keywords: siRNA, multilayer siRNA, delivery, gold nanoparticles, poly-L-lysine
1. Introduction
The discovery of RNA interference (RNAi) in Caenorhabditis elegans and the birth of the concept of short interfering RNA (siRNA) is only a bit longer than a decade. [1,2] Its gene silencing effect has been demonstrated in numerous biological models, thus siRNA has been widely proposed as a future therapy to treat many kinds of diseases including cancers. Although systemically delivered siRNA has been advanced into phase I clinical trials, more effective delivery systems are required to transport therapeutic siRNA to specific cells and tissues.[3,4] There are two major strategies for nucleic acid delivery, viral and non-viral vectors systems. Recombinant viral system has been commonly used in clinical trial, but the associated severe side reactions including immune response have led to death, mutagenesis and oncogenic potentials.[4–6] Because of such serious concerns on the recombinant viral vectors, various non-viral delivery systems, prepared by complexing with cationic lipids and polymers, have been validated.[7–13] Of many cationic polymers, polyethyleneimine (PEI) is one of the most widely used carriers to transport genes, oligonucleotides, and siRNAs.[14–17] Despite considerable transfection efficiencies, the properties of PEIs need to be further improved because of its associated toxicity and non-specific interactions with non-targeted cells.[15,18,19]
Layer by layer (LbL) fabrication technology is a gentle assembly procedure, which is based on charge-charge interaction between positively and negatively charged polymers, to add multiple layers of thin films onto a surface or a particle.[20, 21] The LbL method is simple and versatile, therefore a large variety of charged molecules can be used, including natural and synthetic polyelectrolytes.[22, 23] It has been applied to many different areas, including biological, material and electronic science.[24] We thought this simple LbL assembly approach would be suitable in preparing a new type of enzyme assisted siRNA delivery system. High loading of siRNA can be achieved by coating multiple layers of siRNA onto a nanoparticle, and the enzyme assisted release of siRNA can be controlled by the number of layers and the degradability of the positively charged polymers. In addition, the shielding layers could protect the siRNA from degradation.
In this paper in order to fabricate the proposed assembly, we selected AuNPs as the core for its unique properties including uniform size, shape-dependent optical and electronic features, biocompatibility and feasibility for surface modification.[25, 26] A polypeptide, poly-L-lysine (PLL) which has previously been used as a gene delivery vector and drug carrier was selected as the positively charged polyelectrolyte for its protease degradability.[27] Recently we have used PLL as a template to prepare self-quenched protease sensitive fluorescent probes for in vivo imaging of cancer and other diseases.[28–30] In vivo, the PLL was cleaved by lysosomal cathepsin B which is often up-regulated in cancer cells and inflamed cells, resulting in a bright fluorescent signal in the area with high enzymatic activity.[ 31–34] Similarly, the progressive degradation of PLL inside of cell is expected to trigger slow release of siRNA, resulting in a prolonged gene silencing effect.
2. Results and Discussion
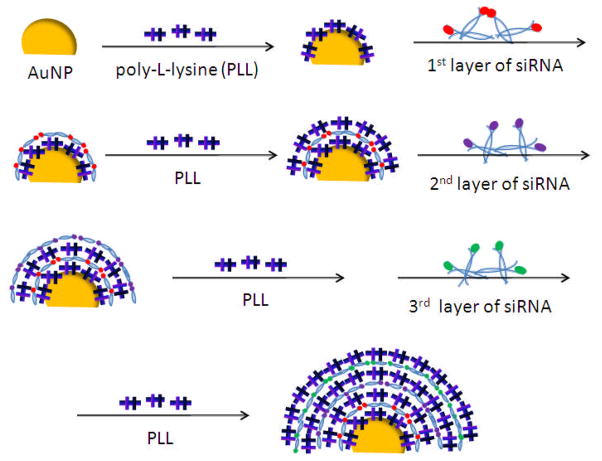
Figure 1 shows the preparition flow of multiple layered siRNA gold nanoparticles (sRAuNPs). It is known that the solubility of the coated AuNPs is largely affected by size and surface charges. The concentration and molecular weight of polycations and siRNA all have been optimized to prevent aggregation. [17,35] Using an optimized procedure, densely packed sRAuNPs were obtained. To assemble the multiple layered sRAuNPs, the negatively charged gold particles in water were dropped into the positive charged PLL solution (average Mw: 22.5 KDa) for the first layer of coating. The reaction solution was incubated for 30 min, and then the coated particles were spun down by centrifuge. After several washes with sterilized water, the PLL coated AuNPs were added to the negatively charged siRNA (21 bp against luciferase) solution. After incubation, free unbound siRNAs were removed by centrifugation and the particles were re-suspended in sterilized water. As shown in Figure 1, by repeating these procedures, multiple layers - total 4 layers of PLL and 3 layers of siRNA - were successfully deposited on Au surface by electrostatic interaction.
Figure 1.
Preparation of multilayer siRNA coated AuNPs (sRAuNPs) using siRNA and PLL as the charged polyelectrolytes.
Transmission electron micrographs (TEM) images of bare AuNPs (40 nm) and polyelectrolyte coated AuNPs were collected (Figure 2a). The visualization of the polyelectrolyte layers was achieved after negative staining with methylamine tungstate. Under TEM, all coated particles (sR1P, sR2P, and sR3P) were found about 50 nm in diameter. For comparison, the hydrodynamic diameter of the formulated particles was also measured by dynamic light scattering (DLS) after each layer of coating. The size of initial bare AuNPs was 40 nm, while the particle size increased steadily with the number of layers (sR1: 104 nm / sR1P: 151 nm / sR2P: 159 nm / sR3P: 183 nm). The differences between DLS and TEM might be caused by the hydodynamic structure of sRAuNPs. The initial zeta potential of bare AuNPs was -42 mV. The PLL loading brought up the surface charge to about +46 mV, while the subsequent siRNA layer dragged it down to about -30 mV again. This characteristic zigzag pattern of zeta potential indicated the successful layering of the alternatively charged molecules (Figure 2b).
Figure 2.
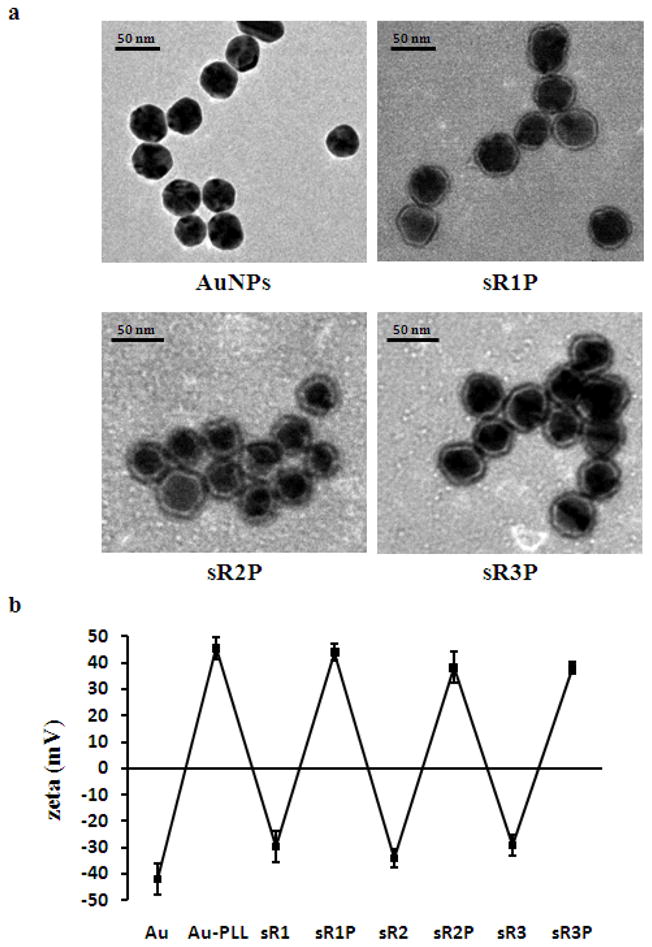
Characterization of sRAuNPs. (a) TEM images of bare AuNPs and polyelectrolyte coated AuNPs. Negative staining by methylamine tungstate used for all images. (b) zeta-potential after each coating of polyelectrolytes. The values represent the standard deviation of three independent experiments.
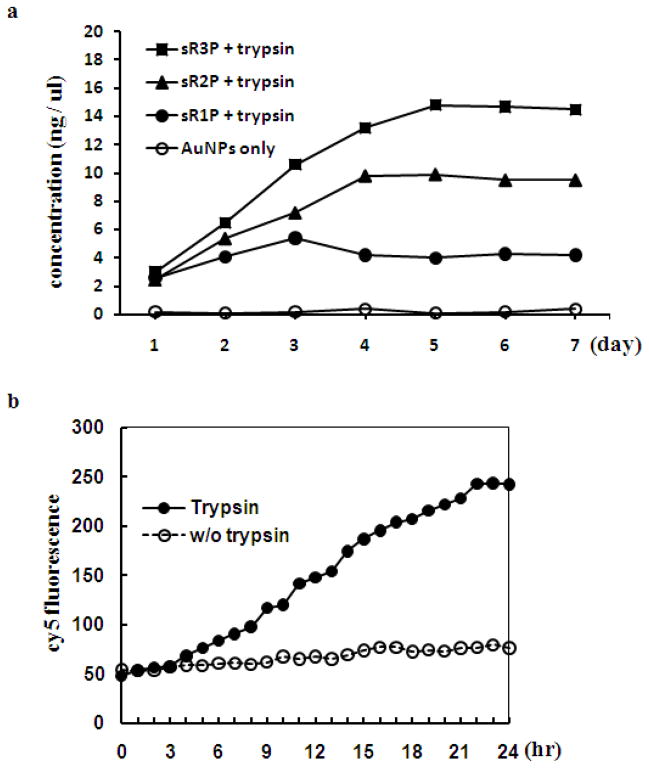
The next critical step is to confirm that the fabricated siRNA could be released from sRAuNPs by proteases. PLL is made of a nature amino acid, lysine, so that it could be degraded by many different types of proteases, including lysosomal cathepsin B, and trypsin. Enzyme assisted release of siRNA was determined by incubating various sRAuNPs with trypsin in buffer, and fractions of the solution were collected at different time points to determine the concentration of released siRNA. As expected, the release kinetic of siRNA from sRAuNPs depended on the number of layers (sR1P > sR2P > sR3P) (Figure 3a). It took about 3 days for siRNA to be fully released from the sR1P particles which had one layer of siRNA and two layers of PLL under the testing condition; whereas, it required 4 and 5 days for sR2P which had 2 siRNA/3 PLL and sR3P which had 3 siRNA/4 PLL, respectively. The data also validated the hypothesis that more siRNA could be carried on a single particle by multiple layering. The final siRNA concentration released from sRANPs for sR1P, sR2P and sR3P are 0.3, 0.7 and 1.1 μM, respectively. Similar results were observed in serum condition using sR1(cy5)P which coated with cyanine dye, cy5, tagged siRNA. As shown in Figure 3b, the fluorescence intensity of cy5 increased persistently because of the trypsin assisted release, and, importantly, the particle remained stable without trypsin, indicating no siRNA was released from the particle during the experimental period.
Figure 3.
Release of siRNA after proteolytic cleavage of PLL. (a) Multilayer sRAuNPs were incubated with or without trypsin in PBS and the concentration of released siRNA in supernatant were measured. (b) sR1(cy5)P AuNPs were incubated in RPMI 1640 medium containing serum with or without trypsin for 24 hr and the fluorescence was examined every hour.
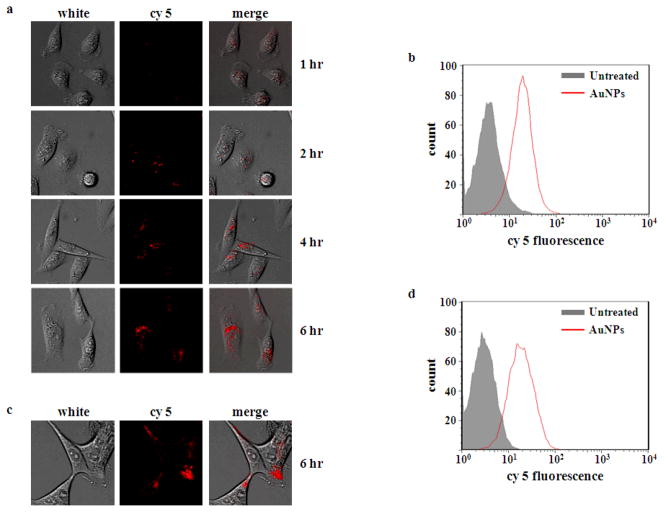
The ability of sRAuNPs to enter cells was investigated by real time fluorescence microscopy and flow cytometry. MDA-MB231-luc2 (Figure 4a) and LNCaP-luc2 (Figure 4c) cell lines were incubated with sR1(cy5)P AuNPs (1.58 × 109 particles) for 8 hours. Since cy5 label was anchored to the siRNA, the fluorescence images reveal the location of the siRNA. Punctate fluorescence signal was seen in the early time points, while the signal was diffused into cytoplasm as time went on (Figure 4a and 4c). Cellular uptakes of sRAuNPs by both cell lines were also confirmed by flow cytometry analysis. Strong cy5 fluorescent signal was obtained 24 hr after incubating with sR1(cy5)P AuNPs (Figure 4b and 4d). These cellular uptake data indicated that sRAuNPs require no transfection agent to enter cells, and, once it is internalized, the siRNA could be freed from particles slowly as proposed.
Figure 4.
Cellular uptake of sRAuNP. Real time image of the uptake of siRNA was visualized by fluorescence microscope after 8 hr incubation with sR(cy5)P AuNPs in MDA-MB231-luc2 (a) and LNCaP-luc2 (c). After 24 hr incubation with sR(cy5)P AuNPs, cellular uptake of siRNA was confirmed by flow cytometry analysis in both cell lines (b, d).
It has been reported that the toxicity of the formulated AuNPs depends on the chemical composition of the surface molecules, and high molecular weight polycationic carriers in non-viral vector delivery system could be toxic.[18, 36,37] Therefore the cytotoxicity of the prepared sRAuNPs was evaluated in MDA-MB231-luc2 and LNCaP-luc2 cell lines by comparing it with Lipofectamine 2000, which is widely used transfection agent. As shown in Figure 5, no significant toxicity was detected for all sRAuNPs, while some toxicity was observed with Lipofectamine 2000 in both cell lines (cell viability: less than 80 %).
Figure 5.
Cytotoxicity of multilayer sRAuNPs. Cell viability was evaluated by MTT assay after transfection with multilayer sRAuNPs or Lipofectamine 2000 in MDA-MB231-luc2 (a) and LNCaP-luc2 (b) cell lines. Results are representative of three independent experiments.
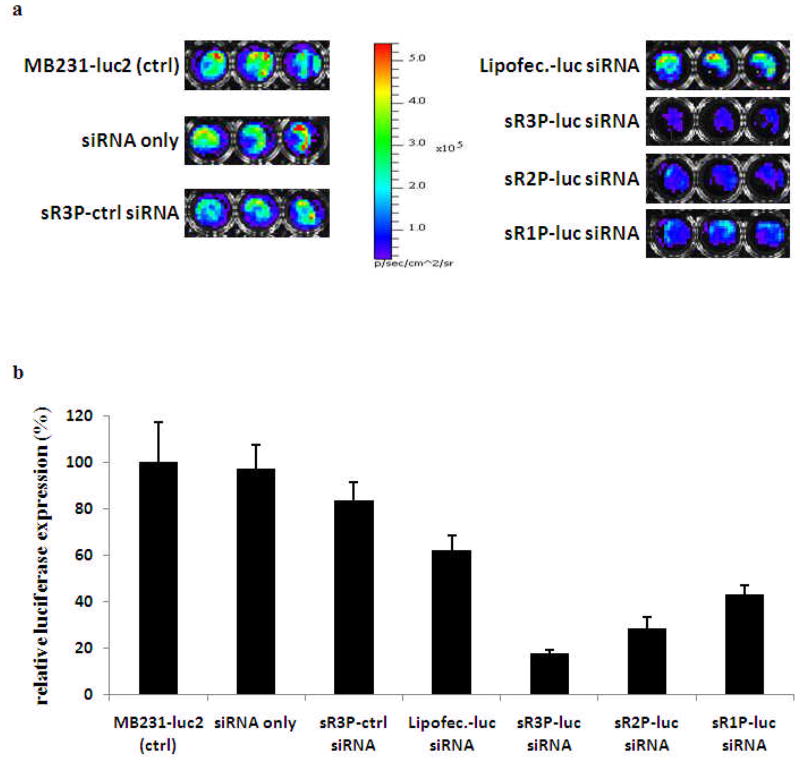
Finally the siRNA gene silencing effect was investigated by measuring the luciferase activity. MDA-MB231-luc2 cells stably expressing firefly luciferase were incubated with sR1P, sR2P, sR3P, siRNA/Lipofectamine, or free siRNA (Figure 6). In addition, a control sR3P was prepared with a nonsense siRNA. After incubation with different sRAuNPs for 5 days, the luminescence of MDA-MB231-luc2 cells was measured immediately after addition of luciferin. It was found that the luminescence was reduced to about 43% by sR1P (siRNA 0.3 μM : 1.26 × 108 particles). Furthermore, the luciferase silencing effects was depended on the number of siRNA layers. With the same amount of particles (1.26 × 108), the luminescence intensity was down to 28% by sR2P (siRNA 0.7 μM), and to 18% by sR3P (siRNA 1.1 μM). No significant silencing effect was observed, when the luciferase siRNA in sR3P was replaced by a nonsense control siRNA. In comparison, Lipofectamine formulation maintained 62% of luminescence, even though siRNA concentration has been doubled (2.2 μM). Free unformulated siRNA (2.2 μM) didn’t show any appreciable effect under the same condition, either. Similar results were observed with LNCaP-luc2 cell lines (data not shown). All results suggested that three layers siRNA coated (sR3P) AuNPs was the best formulation in gene silencing (>80%) among all different kinds of siRNA delivery formulations.
Figure 6.
Gene silencing effect of multilayer sRAuNP in MDA-MB231-luc2 cell line. Luminescence signal in MDA-MB231-luc2 cells after incubation with different sRAuNPs or Lipofectamine 2000 was evaluated by IVIS 200 (a). Value of luminescence intensity (photon/sec) in MDA-MB231-luc2 cells without treatment was set as 100 % (b). The results are representative of three independent experiments.
The positive charge has made the assembly straightforward, and also assisted the cellular delivery. It is known that positively charged NPs are most effective in crossing negatively charged cell membrane and localizing in the cytosol or nucleus.[38–40] Due to slow release of siRNA, excellent silencing effect was achieved even 5 or 6 days after single sRAuNPs treatment. Previously, the importance of biodegradable polymers for the improvement of siRNA release has been recognized.[35] PLL was degraded slowly inside of cell, resulting in a persistent siRNA effect. In addition, the multilayered sRAuNPs carried more siRNA into cell and silenced target gene more effectively than the monolayer sRAuNPs did.
LbL technology has been reported for sometime, however it was not popular in siRNA delivery. A monolayered siRNA particle formulated with PEI has been prepared for siRNA delivery,and a thin film-based multilayered siRNA was prepared with PEI for electroporation purpose.[35,41] Most recently PLL layered with siRNA has been applied to a thin film and albumin NPs.[42,43] In contrast, our design is a particle based multilayered sRAuNPs design which could have great impact in siRNA therapy. Our results suggested that multilayered sRAuNPs system is much more effective than the monolayered system. Furthermore, two or three different siRNAs which target different genes could be conveniently formulated on a single multilayered sRAuNPs, achieving synergistic gene silencing effect. Dual silencing of target siRNA has been reported to be more effectively than a single siRNA silencing.[44, 45]
In conclusion, we successfully fabricated densely packed multilayered sRAuNPs by layering oppositely charged PLL and siRNA on the surface of AuNPs. The prepared multilayered sRAuNPs, whose outer surface layer is PLL, could deliver siRNA into tumor cell and silence its target gene effectively, without side toxicity. Persistent siRNA inhibition effect was achieved as a result of incorporated protease assisted slow release design.
3. Experimental Section
Chemicals and materials
All siRNA and poly-L-lysine (Mw = 15,000–30,000 g mol−1) were obtained from Sigma-Aldrich (St. Louis, MO). Bare AuNPs (40 nm) were purchased from BB International (Cardiff, UK), Lipofectamine 2000 was from Invitrogen (Carlsbad, CA), D-Luciferin was from Regis Technologies (Morton Grove, IL) and MTT solution was from Promega (Madison, WI).
Preparation and characterization of multilayer sRAuNPs
Using layer by layer (LbL) method, multilayers of siRNA and PLL were successfully deposited on Au surface. The sequences of siRNA against luciferase are sense strand: 5’-CGUACGCGGAAUACUUCGAdTdT-3’, antisense strand: 5’ UCGAAGUAUUCCGCGUACGdTdT-3’, and of control nonsnese siRNA are sense strand: 5’-AGCUUCAUAAGGCGCAUGCdTdT-3’ and antisense strand: 5’-GCAUGCGCCUUAUGAAGCU-3dTdT-3’).[46] For cellular uptake experiments, a fluorochrome cyanine dye, cy5, was tagged on the 5' end of the sense siRNA. Au solution (3.15 × 109 particles in 0.7 mL) was added dropwisely onto a PLL solution (0.5 mL of 5 mg mL−1) in pure water. After incubating for 30 min in the dark with gentle shaking, the solution was centrifuged for 30 min at 16,100 g using a micro centrifuge (Eppendorf, Hauppauge, NY). The supernatant was removed, and the gel-like deep red pellet was re-suspended with pure water and centrifuged for 30 min at 16,100 g. After one more wash, PLL coated AuNPs were stored in pure water. Next polyelectrolyte layer was deposited by adding PLL coated AuNPs (in 0.5 mL pure water) to siRNA solution (4.0 μM, 0.5 mL). The reaction solution was incubated in the dark for 30 min with gentle shaking, followed by three washes. The deposition procedures were repeated to have total 7 layers of polyelectrolytes (4 layers of PLL and 3 layers of siRNA). Sizes and zeta potentials of AuNPs in water were measured by Zetasizer Nano-ZS (Malvern, Worcestershire, UK) according to the manufacturer's instruction.
Transmission electron micrographs (TEM) images
Size of AuNPs was measured by TEM using JEOL 2010 FasTEM (JEOL Ltd., Tokyo, Japan). In brief, all samples were prepared by placing a drop of the NPs solution onto a carbon coated copper TEM grid (Ted Pella Inc., Redding, CA) of mesh size 300. After 5 minutes, the excess amount of the solution was removed using a blotting paper. Negative staining of the sample was performed with one drop of Nano-WR (methylamine tungstate, Nanoprobes, Yaphank, NY) for 45 seconds. The excess of reagent was blotted away and the grids were allowed to dry overnight before the microscopy was performed. TEM measurements were operated at an accelerating voltage of 200 KV with a LaB6 filament.
Protease assisted siRNA release
To measure the release of siRNA from sRAuNPs, formulated particles (1.26 × 108 particles) were incubated in a 96-well culture plate at 37 °C with or without 50 μL trypsin-EDTA (0.25%, Sigma-Aldrich) in phosphate buffered saline (PBS). After incubation, the concentration of siRNA in supernatant (1.5 μL) was determined by ND-1000 spectrophotometer (NanoDrop, Wilmington, DE) at different time points. The protease-induced fluorescent change of siRNA was determined by incubating the sR1(cy5)P AuNPs (1.26 × 108 particles) in a 96-well culture plate with or without trypsin (50 μL) in RPMI 1640 medium (Thermo Scientific, Rockford, IL) containing serum (Sigma-Aldrich) at 37 °C and the increase of cy5 fluorescence signal was analyzed by spectramax M2 plate reader (Molecular Devices, Sunnyvale, CA) with a 649 nm excitation and a 670 nm emission for 24 hr.
Cell lines
The human breast cancer cell line stably expressing firefly luciferase (MDA-MB231-luc2) and the human prostate cancer cell line stably expressing firefly luciferase (LNCaP-luc2) were purchased from Caliper (Alameda, CA). MDA-MB231-luc2 cell line were cultured in minimum essential medium (Invitrogen, Carlsbad, CA), while LNCaP-luc2 cell line were cultured in RPMI 1640 medium (Thermo Scientific) and both cell lines were supplemented with 2 mM L-glutamine, 100 U mL−1 penicillin, 100 mg mL−1 streptomycin, and 10 % heat-inactivated fetal bovine serum (Sigma-Aldrich) in a humidified atmosphere of 5 % CO2 at 37 °C.
Cellular uptake of sRAuNPs
MDA-MB231-luc2 cells (5.0 × 104) and LNCaP-luc2 (2.5 × 105) cells were seeded on a 35 mm culture dish with a glass bottomed microwell (Met-Tek Inc, Clackamas, OR). After 24 hr or 48 hr, the culture medium was replaced with fresh sR1(cy5)P AuNPs (1.58 × 109 particles) containing medium, and further cultured for 8 hr. Cells were then washed twice with PBS and cultured in the phenol red free medium. Real time fluorescent images of cells were acquired by a LCV-110 incubator fluorescence microscopy (Olympus Corporation, Tokyo, Japan). For flow cytometry analysis, MDA-MB231-luc2 cells (5.0 × 104) and LNCaP-luc2 cells (2.5 × 105) were seeded on 6-well culture plate (BD Falcon, San Jose, CA) and cultured for 24 hr or 48 hr. sR1(cy5)P AuNPs (1.58 × 109 particles) were added and further cultured for 24 hr. After removal of the cultured medium, the cells were washed with PBS and then detatched from the wells by trypsin-EDTA. After three more washes with PBS in tube, the cy5 fluorescence signal inside of cells indicating the uptake of siRNA were measured by BD FACSAria III flow cytometry (BD Biosciences, San Jose, CA).
Cytotoxicity measurement of sRAuNPs
MTT assay was performed to determine the cytotoxicity of Lipofectamine 2000 (Invitrogen) and the preparations of sRAuNPs. Briefly, MDA-MB231-luc2 cells were collected by trypsinization, counted, and plated in a 96-well culture plate at a density of 5 × 103 (or 2.5 × 104 of LNCaP-luc2) cells per well. One day later, sRAuNPs (1.26 × 108 particles) and Lipofectamine 20000 (0.2 μL) were added and the cells were further cultured for 24 hr. MTT solutions (20 μL, Promega) were then added to each well. After incubation for additional 3 hr, absorbance was measured at 570 nm using a SpectraMax plate reader (Molecular Devices).
Gene silencing in MDA-MB231-luc2 and LNCaP-luc2 cell lines
For the examination of the gene silencing effect, bioluminescence measurement was performed after incubating with various multilayered AuNPs. Cells were seeded in a 96-well black clear bottom culture plate at a density of 2.5 × 103 (or 1.25 × 104 of LNCaP-luc2) cells per well. One day later, different sRAuNP (1.26 × 108 particles) were added to each well and cultured for additional 24 hr. The cells were further cultured in phenol red free medium for another 5 days. Manufacturer’s direction was followed for the transfection with Lipofectamine. Bioluminescence measurement was performed using IVIS 200 (Caliper) immediately after addition 125 μg mL−1 of D−Luciferin (Regis).
Acknowledgments
This research was supported in part by NIH CA135312, DOD W81XWH-10-1-0597 and Oshman Foundation.
References
- 1.Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Nature. 1998;391:806. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 2.Zamore PD. Cell. 2006;127:1083. doi: 10.1016/j.cell.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Crunkhorn S. Nat Rev Drug Discov. 2010;9:359. doi: 10.1038/nrd3161. [DOI] [PubMed] [Google Scholar]
- 4.Castanotto D, Rossi JJ. Nature. 2009;457:426. doi: 10.1038/nature07758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyce N. Nature. 2001;414:677. doi: 10.1038/414677a. [DOI] [PubMed] [Google Scholar]
- 6.Check E. Nature. 2002;420:116. doi: 10.1038/420116a. [DOI] [PubMed] [Google Scholar]
- 7.Gao K, Huang L. Mol Pharm. 2009;6:651. doi: 10.1021/mp800134q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim SS, Garg H, Joshi A, Manjunath N. Trends Mol Med. 2009;15:491. doi: 10.1016/j.molmed.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bumcrot D, Manoharan M, Koteliansky V, Sah DW. Nat Chem Biol. 2006;2:711. doi: 10.1038/nchembio839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim DH, Rossi JJ. Nat Rev Genet. 2007;8:173. doi: 10.1038/nrg2006. [DOI] [PubMed] [Google Scholar]
- 11.Elouahabi A, Ruysschaert JM. Mol Ther. 2005;11:336. doi: 10.1016/j.ymthe.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 12.Zhang S, Zhao B, Jiang H, Wang B, Ma B. J Control Release. 2007;123:1. doi: 10.1016/j.jconrel.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 13.Rosi NL, Giljohann DA, Thaxton CS, Lytton-Jean AK, Han MS, Mirkin CA. Science. 2006;312:1027. doi: 10.1126/science.1125559. [DOI] [PubMed] [Google Scholar]
- 14.Thomas M, Klibanov AM. Proc Natl Acad Sci USA. 2003;100:9138. doi: 10.1073/pnas.1233634100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kichler A. J Gene Med. 2004;6:S3. doi: 10.1002/jgm.507. [DOI] [PubMed] [Google Scholar]
- 16.Urban-Klein B, Werth S, Abuharbeid S, Czubayko F, Aigner A. Gene Ther. 2005;12:461. doi: 10.1038/sj.gt.3302425. [DOI] [PubMed] [Google Scholar]
- 17.Song WJ, Du JZ, Sun TM, Zhang PZ, Wang J. Small. 2010;6:239. doi: 10.1002/smll.200901513. [DOI] [PubMed] [Google Scholar]
- 18.Kunath K, von Harpe A, Fischer D, Petersen H, Bickel U, Voigt K, Kissel T. J Control Release. 2003;89:113. doi: 10.1016/s0168-3659(03)00076-2. [DOI] [PubMed] [Google Scholar]
- 19.Hu C, Peng Q, Chen F, Zhong Z, Zhuo R. Bioconjug Chem. 2010;21:836. doi: 10.1021/bc900374d. [DOI] [PubMed] [Google Scholar]
- 20.Jewell CM, Lynn DM. Adv Drug Delivery Rev. 2008;60:979. doi: 10.1016/j.addr.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peyratout CS, Dahne L. Angew Chem Int Ed. 2004;43:3762. doi: 10.1002/anie.200300568. [DOI] [PubMed] [Google Scholar]
- 22.Chanana M, Gliozzi A, Diaspro A, Chodnevskaja I, Huewel S, Moskalenko V, Ulrichs K, Galla HJ, Krol S. Nano Lett. 2005;5:2605. doi: 10.1021/nl0521219. [DOI] [PubMed] [Google Scholar]
- 23.Schneider GF, Subr V, Ulbrich K, Decher G. Nano Lett. 2009;9:636. doi: 10.1021/nl802990w. [DOI] [PubMed] [Google Scholar]
- 24.Ai H, Jones SA, Lvov YM. Cell Biochem Biophys. 2003;39:23. doi: 10.1385/CBB:39:1:23. [DOI] [PubMed] [Google Scholar]
- 25.Daniel MC, Astruc D. Chem Rev. 2004;104:293. doi: 10.1021/cr030698+. [DOI] [PubMed] [Google Scholar]
- 26.Lee JH, Lee K, Moon SH, Lee Y, Park TG, Cheon J. Angew Chem Int Ed. 2009;48:4174. doi: 10.1002/anie.200805998. [DOI] [PubMed] [Google Scholar]
- 27.Smith LC, Duguid J, Wadhwa MS, Logan MJ, Tung CH, Edwards V, Sparrow JT. Adv Drug Delivery Rev. 1998;30:115. doi: 10.1016/s0169-409x(97)00111-7. [DOI] [PubMed] [Google Scholar]
- 28.Weissleder R, Cheng HC, Marecos E, Kwong K, Bogdanov A., Jr Eur J Cancer. 1998;34:1448. doi: 10.1016/s0959-8049(98)00195-6. [DOI] [PubMed] [Google Scholar]
- 29.Tung CH, Bredow S, Mahmood U, Weissleder R. Bioconjug Chem. 1999;10:892. doi: 10.1021/bc990052h. [DOI] [PubMed] [Google Scholar]
- 30.Law B, Curino A, Bugge TH, Weissleder R, Tung CH. Chem Biol. 2004;11:99. doi: 10.1016/j.chembiol.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 31.Bervar A, Zajc I, Sever N, Katunuma N, Sloane BF, Lah TT. Biol Chem. 2003;384:447. doi: 10.1515/BC.2003.050. [DOI] [PubMed] [Google Scholar]
- 32.Podgorski I, Linebaugh BE, Sameni M, Jedeszko C, Bhagat S, Cher ML, Sloane BF. Neoplasia. 2005;7:207. doi: 10.1593/neo.04349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choi Y, Weissleder R, Tung CH. Cancer Res. 2006;66:7225. doi: 10.1158/0008-5472.CAN-06-0448. [DOI] [PubMed] [Google Scholar]
- 34.Funovics M, Weissleder R, Tung CH. Anal Bioanal Chem. 2003;377:956. doi: 10.1007/s00216-003-2199-0. [DOI] [PubMed] [Google Scholar]
- 35.Elbakry A, Zaky A, Liebl R, Rachel R, Goepferich A, Breunig M. Nano Lett. 2009;9:2059. doi: 10.1021/nl9003865. [DOI] [PubMed] [Google Scholar]
- 36.Giljohann DA, Seferos DS, Daniel WL, Massich MD, Patel PC, Mirkin CA. Angew Chem Int Ed. 2010;49:3280. doi: 10.1002/anie.200904359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Read ML, Singh S, Ahmed Z, Stevenson M, Briggs SS, Oupicky D, Barrett LB, Spice R, Kendall M, Berry M, Preece JA, Logan A, Seymour LW. Nucleic Acids Res. 2005;33:e86. doi: 10.1093/nar/gni085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Verma A, Stellacci F. Small. 2010;6:12. doi: 10.1002/smll.200901158. [DOI] [PubMed] [Google Scholar]
- 39.Arvizo RR, Miranda OR, Thompson MA, Pabelick CM, Bhattacharya R, Robertson JD, Rotello VM, Prakash YS, Mukherjee P. Nano Lett. 2010;10:2543. doi: 10.1021/nl101140t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cho EC, Xie J, Wurm PA, Xia Y. Nano Lett. 2009;9:1080. doi: 10.1021/nl803487r. [DOI] [PubMed] [Google Scholar]
- 41.Fujimoto H, Kato K, Iwata H. Anal Bioanal Chem. 2010;397:571. doi: 10.1007/s00216-010-3648-1. [DOI] [PubMed] [Google Scholar]
- 42.Zhang X, Kovtun A, Mendoza-Palomares C, Oulad-Abdelghani M, Fioretti F, Rinckenbach S, Mainard D, Epple M, Benkirane-Jessel N. Biomaterials. 2010;31:6013. doi: 10.1016/j.biomaterials.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 43.Singh HD, Wang G, Uludağ H, Unsworth LD. Acta Biomater. 2010;6:4277. doi: 10.1016/j.actbio.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 44.Tai W, Qin B, Cheng K. Mol Pharm. 2010;7:543. doi: 10.1021/mp9002514. [DOI] [PubMed] [Google Scholar]
- 45.Kumar P, Ban HS, Kim SS, Wu H, Pearson T, Greiner DL, Laouar A, Yao J, Haridas V, Habiro K, Yang YG, Jeong JH, Lee KY, Kim YH, Kim SW, Peipp M, Fey GH, Manjunath N, Shultz LD, Lee SK, Shankar P. Cell. 2008;134:577. doi: 10.1016/j.cell.2008.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Nature. 2001;411:494. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]