Abstract
Methylphenidate (MPH) therapy for attention-deficit hyperactivity disorder is common in children and adults. Concerns regarding abuse of MPH prompted studies to better understand its pharmacology. We used an established drug discrimination task to determine if MPH could be discriminated by C57BL/6J(B6) mice. B6 mice learned to discriminate cues produced by racemic MPH (dl-MPH 5.0mg/kg), or half the dose of pure d-isomer (2.5mg/kg), and dose-response tests established appropriate reductions in discrimination with declining dose. Importantly, the two drug forms generalized to each other completely in substitution tests; consistent with reports that the l-isomer is pharmacodynamically inactive. An additional experiment indicated that lower doses (1 and 2mg/kg) of dl-MPH did not support acquisition of MPH discrimination despite extensive training. Mice acquired discrimination of dl-MPH only when the dose was increased to 4mg/kg. Thus, although these lower doses increased drug lever responding in mice trained on the higher dose, their stimuli were not sufficient to support acquisition of the discrimination task. These findings correspond to previous work conducted in our laboratory on threshold doses needed to produce stimulatory effects of motor activity in B6 mice. These pre-clinical findings provide insight into the relative potency, and by extension, efficacy of dl-MPH versus d-MPH doses.
Keywords: Methylphenidate, drug discrimination, mouse
Introduction
The stimulant drug methylphenidate (MPH) has been a first-line treatment for attention-deficit hyperactivity/disorder (ADHD) since the 1950s. The symptoms of ADHD were once viewed as being primarily limited to childhood and adolescence. However, the disorder is increasingly being recognized as persisting into adulthood (Biederman and Faraone, 2002, 2005; Okie, 2006). Consequently, MPH has become more widely available for abuse through diversion of this controlled substance (Kroutil et al., 2006; Darredeau et al., 2007), especially among high school (McCabe et al., 2004) and college students (McCabe et al., 2006; Godfrey, 2009). The neurochemical basis of MPH abuse liability appears to be based on the blockade of dopamine and norepinephrine reuptake to induce euphoric effects similar to that of cocaine (Volkow et al., 2002; Volkow and Swanson, 2003; Patrick et al., 2007). MPH is primarily prescribed as a racemic mixture, i.e., dl-MPH (Okie, 2006), though pure d-MPH formulations have more recently become available (Patrick et al., 2009). The l-isomer appears to exhibit little (Heal and Pierce, 2006) or no pharmacological activity (Patrick et al., 1987; Williard et al., 2007). Thus, the available evidence indicates that the therapeutic, reinforcing, as well as other pharmacological effects of MPH are produced only by the d-isomer.
The positive subjective effects of MPH reported by adult humans (Heil et al., 2002; Stoops et al., 2005; Patrick et al., 2007; Kollins et al., 2009) likely serve as discriminative stimuli. For psychoactive drugs, there is a high degree of concordance (Solinas et al., 2006) between human (Gatley et al., 1999; Kollins et al., 2001) and laboratory animal studies, including rats (Colpaert, 1999; Grant, 1999; Hodge et al., 2006) and mice (Middaugh et al., 1998; Gatley et al., 1999), regarding whether the drug will support discrimination. Surprisingly, while the general pharmacology of MPH has been extensively examined (Patrick et al., 1987; Markowitz et al., 2006; Askenasy et al., 2007; Markowitz and Patrick, 2008), the discriminative stimulus properties of MPH have been not been directly examined in many animal studies. Only two reports using rats indicate that rodents can learn to discriminate the stimulus cues provided by MPH (Overton and Shen, 1988; Perkins et al., 1991). Nevertheless, a substantial literature on rats indicates that the discriminative stimulus properties of MPH generalize to those produced by other psychoactive drugs (e.g. cocaine, amphetamine, methamphetamine) in substitution tests (Bondareva et al., 2002; Schweri et al., 2002; Wooters et al., 2008; Desai et al., 2010). Generally, these studies indicate that a wide range of MPH doses (1.25–10mg/kg) can substitute for cocaine (Kollins et al., 2001; Bondareva et al., 2002; Schweri et al., 2002; Li et al., 2006) and amphetamine (Bondareva et al., 2002; Czoty et al., 2004). In contrast, MPH alone did not substitute for the discriminative cue produced by nicotine, although MPH did amplify the cues provided by co-administration of a sub-threshold dose of nicotine (Wooters et al., 2008). The available information suggests that MPH produces a cue that can be discriminated by rodents and that the pharmacological effects of MPH are similar to other psychostimulants and not simply psychoactive drugs in general. However, information on discriminable MPH doses and the contribution of MPH isomers has not been well established.
Based on this gap in the literature, the present study characterized the discriminative stimulus properties of MPH in C57BL/6J (B6) mice, a strain that continues to serve as a reference strain in many behavioral/neurochemical investigations. Based on limited evidence that the l-isomer of MPH can influence the action of the d-isomer (Davids et al., 2002; Teo et al., 2003), we tested the possibility that its presence in the racemic mixture could influence the discriminative stimulus properties of the active d-isomer. To assess this, we compared the discriminative stimulus properties of racemic MPH against those of the pure d-isomer of MPH, with the hypothesis being: equimolar doses of the active d-isomer would substitute completely in the discrimination task. Further, recent evidence indicates that low doses of MPH (e.g. <2mg/kg) effectively modulate behaviors (Kuczenski and Segal, 2001; Berridge et al., 2006) and elevate extracellular levels of the neurotransmitters dopamine and norepinephrine in rats (Kuczenski and Segal, 1997, 2001; Berridge et al., 2006). In addition, we recently reported that a 2.5 mg/kg dose of dl-MPH, while producing no effect on motor activity of B6 mice by itself, did enhance ethanol-induced motor stimulation in this strain (Griffin et al., 2010). The relatively low MPH doses used in these studies provide for guarded extrapolation to clinical findings (Kuczenski and Segal, 2005). Therefore, we also investigated the discriminative effects of MPH using low doses (<5 mg/kg), to help establish a threshold for producing cues required for discrimination in an otherwise well-established drug discrimination task in B6 mice (Middaugh et al., 1998).
Methods
Subjects
C57/BL6 mice were obtained from Jackson Laboratories (Bar Harbor, ME) at 7 weeks of age. Animals were singly housed on a 12 hour reverse light cycle (lights on at 20.00h, lights off at 08.00h), with free access to water, and allowed to acclimate to home cages for ~2 weeks prior to behavioral testing. Animals were maintained at 85–90% of their free-feeding body weight throughout the studies. All experiments were approved by and conducted within the guidelines of the Institutional Animal Care and Use Committee (IACUC) at the Medical University of South Carolina and followed the guidelines of the NIH Guide for the Care and Use of Laboratory Animals (NIH Publication No. 80–23, Revised 1996).
Drug Discrimination Apparatus
Drug discrimination was assessed in 8 operant chambers enclosed in sound and light attenuating cabinets (MedAssociates Inc., St Albans, VT). The chambers (21.6 cm × 17.8 cm × 12.7 cm) were constructed of aluminum and Plexiglas with steel grid floors and were equipped with 2 levers located 2.2 cm above the floor. Lever presses of ~ 2g dead weight activated a microswitch and were recorded as responses. Animals were reinforced with a 5 second presentation of 0.01 cc of a 15% sucrose solution in a dipper located between the two levers. The operant chambers were controlled and responses were recorded by computer using MedPC software (MedAssociates Inc., St. Albans, Vermont).
Procedure
General
The experiments were conducted similarly to other previously published studies using other psychoactive drugs from our laboratory (Middaugh et al., 1988, 1998, 1999), although some differences are noted. Generally, both experiments had 3 phases; 1) lever acquisition, 2) drug discrimination training and 3) discrimination testing. During lever acquisition training, drug-free mice were trained to lever press on both levers for sucrose reinforcement. The number of responses required per reinforcement was gradually increased to a fixed ratio 15 schedule of reinforcement (FR15). During drug-discrimination training, mice were reinforced for responses on an assigned injection-appropriate lever (active lever), with 15 consecutive responses on the active lever producing reinforcement. After reaching criterion, 2-minute generalization tests were conducted under extinction conditions to establish dose-response functions and substitution of d- and dl- MPH.
Lever Acquisition Training
A shaping procedure was used to establish responding for the sucrose reinforcer (15% solution) with no drug administered. For the first 3 sessions, both levers were covered with metal panels like those used in the walls of the operant chamber, and the dipper arm was presented for 10 seconds every minute during the 15 minute sessions for the first experiment and the 20 minute sessions for the second experiment. Session time remained unchanged for the duration of each study. Animals were monitored throughout these sessions to ensure that they approached the dipper. During the fourth session, both levers were presented and responses on either lever were reinforced on a FR1 schedule of reinforcement. For the remainder of this phase, responses were restricted to either the left or right lever on an alternating pattern (L, R, L, R) and were reinforced on an FR1 schedule until 15 responses were made on each lever for 2 consecutive days. The reinforcement schedule was increased to FR3, FR5, FR10, and FR15 consecutively after receiving > 10 reinforcers on each of 4 consecutive days. Upon successful completion of the FR15 schedule acquisition requirements, mice advanced to the discrimination training phase.
Discrimination Training
To characterize the discriminative properties of MPH, we trained a group of mice to discriminate 5 mg/kg dl- MPH, a dose which significantly increases locomotor activity in B6 mice (Williard et al., 2007). Additionally, we trained a second group to discriminate the active d-isomer using 2.5 mg/kg d-MPH, which also significantly increases locomotor activity (Williard et al., 2007) and contains an equimolar dose of d-MPH compared to 5 mg/kg dl-MPH. The inclusion of the group of mice trained on the pure d-isomer afforded the opportunity to examine whether there were differences in discriminability of the racemic (i.e. dl-) mixture and the pure d-isomer.
Training was initiated with MPH or saline injected i.p. according to a semi-randomized schedule that ensured no more than 2 consecutive days of exposure to either MPH or saline and an equal number of exposures to each condition over a 2 week period. During discrimination training, mice were injected with MPH or saline 15 minutes prior to being placed in their assigned operant chamber. We selected this pretreatment time based on previous studies in our laboratory that showed peak stimulatory activity at 15 minutes post-injection and peak plasma and brain MPH levels at this time (Williard et al., 2007), and other studies that show peak elevation in DA levels at this time (Kuczenski and Segal, 1997). For the first 10 days of discrimination training, the mice had access only to the drug-paired lever after MPH injections or the saline-paired lever after saline injections. After 10 days, both levers were available for the remainder of the experiment. Half of the mice in each drug training group had the right lever paired with the drug and the left with saline; the other half of each group had the reverse pairing. After 15 consecutive responses on the injection appropriate lever, sucrose was presented. In the first experiment, responses on the inappropriate lever were counted but also reset the FR response counter on the active lever. However, in the second study, responses on the inappropriate lever were recorded, but had no consequence. Criteria for acquisition of drug discrimination were two-fold. The first criterion was that mice make ≥80% of responses on the injection appropriate lever prior to the first reinforcement (called FFR: First Fixed Ratio) for at least 3 consecutive days. Additionally, the mice were required to make ≥85% of total responses on the injection appropriate lever during the session for 3 consecutive days. Upon meeting these criteria, mice were eligible for MPH discrimination testing.
Discrimination Testing
All tests lasted 2 minutes and were conducted under extinction conditions. All other procedures were the same as during the training sessions. After every generalization test, mice resumed training and were required to meet discrimination criteria during at least 3 consecutive training sessions before another discrimination test session was conducted. For the dose substitution curves, mice were tested twice at each dose, on an escalating pattern of dosing, and the average used for data analysis, except there was only one test for the MPH doses greater than the training dose. Finally, mice were removed from the analysis if they did not make ≥80% of responses prior to the first reinforcement on the injection appropriate lever (i.e., FFR ≥80%) and greater than 85% of total responses on the appropriate lever in at least 5 of 7 test sessions with either the training dose or saline.
Drugs
Methylphenidate • HCl (dl- and d-; Sigma-Aldrich, Inc) was dissolved in 0.9% saline and administered i.p.at a volume of 0.01ml/g body weight.
Data Analysis
The primary dependent variables for these experiments were the FFR and the response ratios on the appropriate lever. Other dependent variables included the number of sessions to meet discrimination criteria and ED50 values. Comparison of group means was made by using Student’s T-Test and between group comparisons with multiple groups was made using Analysis of Variance (ANOVA), with repeated measures, as appropriate. Finally, GraphPad Prism®Version 4 (GraphPad Software, Inc) was used for non-linear curve fitting to calculate ED50 values for individual mice as well as for the groups. For all analyses, significance levels were set at p< 0.05.
Results
MPH Discrimination Training
We found that mice readily acquired discrimination of 5 mg/kg dl-MPH (12 of 14) and 2.5 mg/kg d-MPH (13 of 16), with mice meeting criteria to begin testing in an average of 16.2 ± 3.2 and 17.3 ± 2.8 sessions, respectively. The number of training sessions required to reach the discrimination criteria did not differ for d- vs. dl-MPH (t = 0.9, NS). To confirm that mice had learned the discrimination task, we conducted a total of 7 (4 drug, 3 saline) discrimination tests throughout the course of the experiment, with either the training compound or saline. Mice were included in all subsequent analysis if they met discrimination criteria on at least 5 out of 7 tests. This left n = 6 in the dl-MPH group and n = 6 in the d-MPH group for MPH dose substitution testing. These final groups of mice met criteria to begin the testing phase of the study within 15.8 ± 1.9 sessions for the dl-MPH group and 17.8 ± 0.6 sessions for the d-MPH group (t = 1.2, NS).
MPH Dose Response Testing
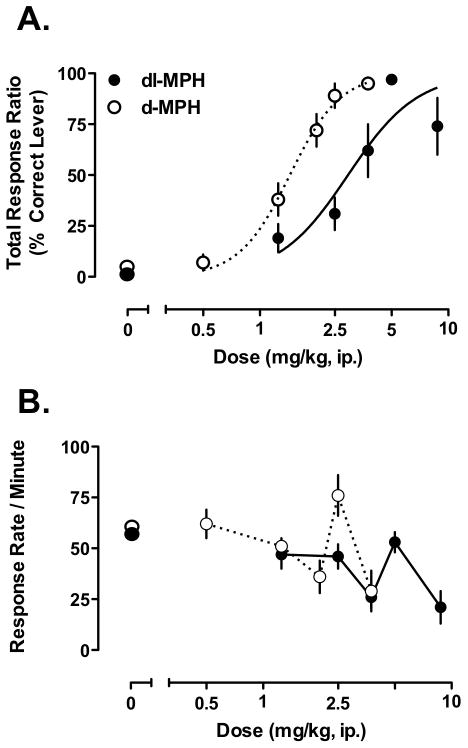
The results of the MPH dose-response study are summarized in Figure 1 and the response ratios on the injection appropriate lever are shown in Figure 1A. As expected, mice readily discriminated their training dose of MPH (dl- or d-) and saline on test sessions. Additionally, the percentage of responses on the MPH-paired lever declined with lower doses for each form. For doses greater than the training dose, there were slight differences between the d- and dl-forms. In the d-MPH trained group, a higher dose of d-MPH (3.75 mg/kg) generalized completely. For the dl-MPH group, the highest dl-MPH dose tested (8.75 mg/kg) produced a slight decrease in correct responding that fell short of criterion performance levels (74%) for the group. This was due primarily to 2 of the 6 mice having quite low (0% and 58%) total responses on the drug-paired lever versus >90% responding for the other 4 mice. Further, the dose-response functions were parallel as can be seen by the average non-linear curve fit estimates that are superimposed on the data in Figure 1A. As expected based on the use of lower doses, the dose-response curve for the d-MPH trained mice was shifted to the left relative to the dl-MPH trained mice. The mean ED50 values were 3.2 ± 0.3 and 1.5 ± 0.09 mg/kg for the dl- and d-MPH trained groups, respectively (t = 5.1, p < 0.001), supporting the idea of a leftward shift in the dose-response data for the d-MPH trained mice compared to the dl-MPH mice.
Figure 1.
Discrimination testing in mice trained to discriminate 5 mg/kg dl-MPH (n = 7) or 2.5 mg/kg d-MPH (n = 6). A) The dose substitution curves of dl-MPH and d-MPH are parallel and the d-MPH curve is shifted to the left, consistent with the use of lower, albeit equimolar, doses compared to the dl-MPH curve. The ED50 values are 3.2 ± 0.3 and 1.5 ± 0.09 mg/kg for the dose response functions of dl-MPH and d-MPH, respectively. B) Response rates for both compounds declined slightly with increasing dose. However, responses were higher when mice were tested on their training dose for both compounds, indicated by arrows (▲) Values are means ± S.E.M.
Finally, as shown in Figure 1B, response rates during testing generally declined with increasing dose, albeit there was an increase in both groups on tests with the training dose, producing response levels similar to those observed during testing with saline. The decline in response rates is not an uncommon finding in discrimination studies with psychoactive drugs (Middaugh et al., 1988). A repeated measures, 2-way ANOVA on the response rates from this experiment found only a significant main effect of Dose [F (5,50) = 21, p<0.001] consistent with the systematic decrease in responses, but neither the main effect of Group [F (1, 10) <3] nor the Group × Dose interaction [F (5,50)<1] were significant. Overall, the data shown in Figure 1 indicate that mice can readily discriminatedl-MPH and d-MPH from saline, in a dose-dependent manner, when the training doses are in a range associated with significant increases in locomotor activity (Williard et al., 2007).
Cross Substitution of dl- and d-MPH
Visual inspection of Figure 1A indicates that equivalent mg d-MPH doses of dl-MPH and d-MPH (e.g. 2.5 and 1.25 mg/kg, respectively, based on d-isomer content) produce similar response ratios, suggesting that the discriminative cue of the racemic mixture and the pure d-isomer are similar. The similarities are further supported by the parallel dose-response functions and the ED50 values of d-MPH being almost exactly one-half that of dl-MPH. We tested this hypothesis directly by administering an equivalent dose of d-MPH (i.e. 2.5 mg/kg) to mice trained to discriminate 5 mg/kg dl-MPH and likewise for mice trained to discriminate 2.5 mg/kg d-MPH (i.e., 5 mg/kg dl- MPH). We also administered one lower equivalent d-MPH dose of each compound in the respective groups. As can be seen in Figure 2, equivalent mg doses of the d-isomer produced nearly identical discrimination dose-effect curves, an observation supported by a repeated measures ANOVA that found only a significant effect of Dose [F (2,20) = 37, p<0.001] and no significant effects of Group or Group × Dose (both F<1). Interestingly, the lower equivalent doses of the d-isomer nearly reached criterion level responding (~75%) in both groups and this level of responding is higher than expected based on the doses given (note response levels in Figure 1A). Response rates in the cross-substitution experiment for the mice trained to discriminate d-MPH from saline were 43.6 ± 9.6 and 35.5 ± 14.2 when tested with 5 mg/kg dl-MPH and 2.5 mg/kg dl-MPH, respectively. For the mice trained to discriminate racemic MPH from saline, the response rates were 21.2 ± 5.1 and 35.1 ± 13.2 when tested with 2.5 mg/kg dl-MPH and 1.25 mg/kg dl-MPH, respectively. Therefore, the response rates were variable during cross-substitution testing, but generally were lower compared to tests with the training drug (compare to Figure 1B). The cross-substitution response rates and the relevant response rates from Figure 1B were analyzed using a 3-way, repeated-measures ANOVA, with Test Session (original test vs substituted test) as the within-subjects repeated measure and Dose (training dose vs. lower dose) and Group (d-isomer vs. racemic) as the between groups factors. The only significant finding was a main effect of Test Session [F (1,20) = 17, p<0.001], confirming that response rates during the cross-substitution test were lower than response rates during previous tests with the training drug. The 3-way factor interaction and 2-way factor interactions were not significant [all F<3.21, and all p>0.088]. Overall, these data are consistent with previous work indicating that the l-isomer is behaviorally inactive, because of the complete substitution using the appropriate concentrations of d-isomer in dl-MPH and d-MPH trained mice.
Figure 2.
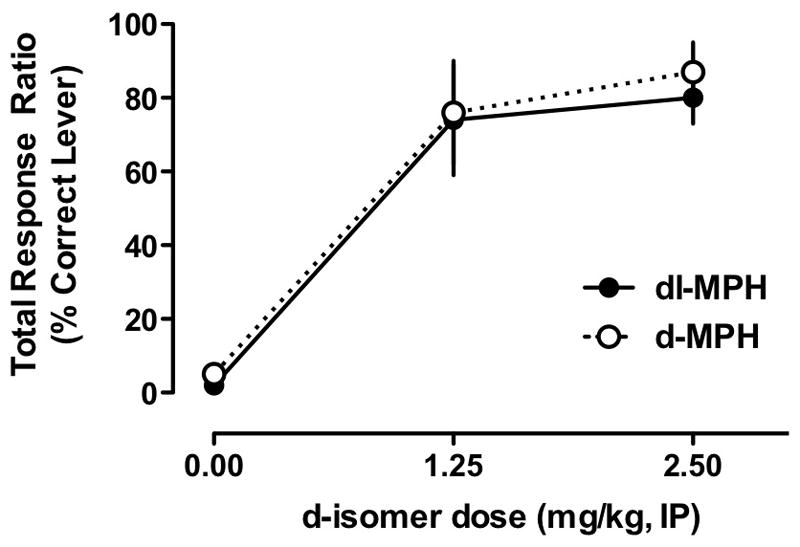
Cross substitution of dl-MPH and d-MPH in mice trained to discriminate 5 mg/kg dl-MPH (n = 7) or 2.5 mg/kg d-MPH (n = 6).. The same mice shown in Figure 2 were used to test whether dl-MPH and d-MPH would substitute in mice trained to discriminate the other form. Essentially, the data show that equivalent doses of either compound (based on the d-isomer) directly substitute for the other compound as anticipated. The data indicate that the presence of the l-isomer does not appear to contribute to the discriminative cue of dl-MPH. Values are means ± S.E.M.
Discrimination training with low MPH Doses
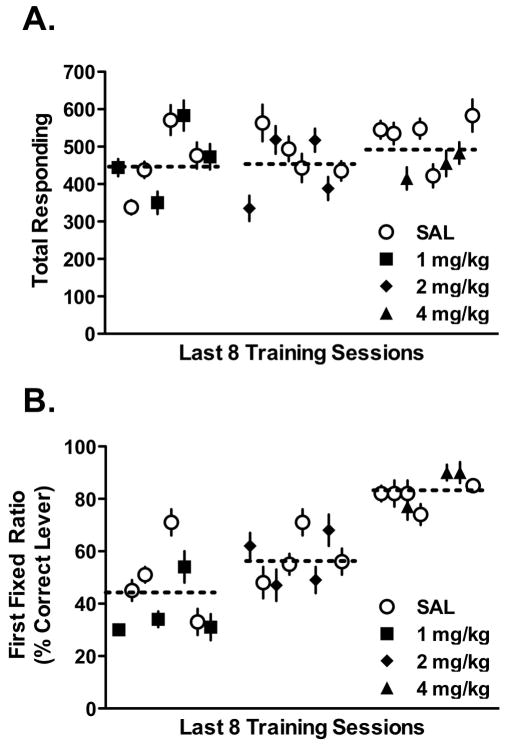
In our second experiment, we tested whether low doses of racemic MPH (1.0 and 2.0 mg/kg) would produce discriminative cues that could effectively support acquisition of drug discrimination. The results of this study are summarized in Figure 3. In this study, naive mice (n=24) began training in our paradigm to discriminate 1 mg/kg dl-MPH from vehicle (0.9% saline) and, after consistently failing to meet criteria for advancing to the Testing phase, were systematically advanced to two higher doses until there was evidence of discrimination. As can be seen in Figure 3A, the total responding for sucrose reinforcement was quite high, averaging between 450 and 500 responses across the entire experiment, suggesting the mice were motivated to press for the sucrose reinforcement. Furthermore, total responding was similar during the last eight days the mice were maintained on each of the three MPH doses, again supporting the absence of a drug effect on a behavioral response (mean responses across the eight day period for each dose (F (2, 69) = 0.6, NS). However, as shown in Figure 3B, the First Fixed Ratio (FFR), our primary measure of MPH discrimination during training, indicated that after 37 sessions of training with the 1.0 mg/kg against saline, the mice were responding slightly below chance, 43 ± 1.6%, on the correct lever. At this point, the MPH dose was doubled to 2.0 mg/kg and training continued as before. After 20 sessions of training with the 2.0 mg/kg dose against saline, FFR performance increased slightly to 54 ± 2.3% during the last 8 sessions, still far below the desired performance level. Further, the overall response ratio (% of responses on the drug-paired lever) on the correct lever during the last 8 sessions of either 1.0 or 2.0 mg/kg MPH doses indicated failure to meet the performance standards on this measure also (data not shown). The last 8 sessions of training with the 1.0 and 2.0 mg/kg MPH doses resulted in mean overall correct response ratios of 70 ± 0.8% and 78 ± 0.6%, respectively, when averaged across drug and saline levers. Collectively, the FFR and overall response ratio data suggest that the 1.0 and 2.0 mg/kg doses of MPH did not provide an adequate discriminative cue to support discrimination against saline.
Figure 3.
Training in the discrimination task with 1, 2 or 4 mg/kg dl-MPH (n = 24). Mice began training with 1 mg/kg, progressed to 2 mg/kg, and then finally were trained using 4 mg/kg. A) Total responses during the last 8 sessions on each dose on the appropriate lever (MPH or Saline) were similar across the entire experiment, regardless of dose. B) First Fixed Ratio (FFR) responses during the same sessions noted in panel A. Performance on the FFR for MPH or saline improved as the dose increased.. Thus, performance improved with increasing dose and mice reached criterion level responding (>80%) when the dose reached 4 mg/kg. In Panels A & B, the dotted lines indicate the mean responding for MPH and saline at each dose. Values are means ± S.E.M.
Training of the same mice was continued with a 4.0 mg/kg MPH dose. After 25 sessions of training with this dose, there was clear evidence of MPH discrimination. The average FFR across the last 8 sessions increased to 83 ± 1.6% (Figure 3B) indicating that B6 mice could indeed discriminate the 4.0 mg/kg MPH, a dose slightly less than that used in the above experiment. This observation was supported by a significant, repeated measures one-way ANOVA on the FFR data from the last 8 days of all three doses (F (2,69) = 117, p<0.001), indicating that performance increased with increasing dose. Similarly, the overall correct lever response ratio averaged 90 ± 0.4% across the last 8 sessions of training with 4mg/kg against saline, and a one-way ANOVA on these data over the 3 doses was also significant (F (2,69) = 74, p<0.001). Collectively, these data indicate that dl-MPH doses near 4.0 mg/kg are required for B6 mice to acquire drug discrimination.
Discussion
Consistent with earlier reports in rats (Overton and Shen, 1988; Perkins et al., 1991), mice reliably discriminated the cues of MPH from saline. Additionally, when low doses (1.0 or 2.0 mg/kg) were substituted for a 5.0 mg/kg training dose of MPH, total responses on the drug-paired lever, although greater than for saline, were less than 50%. Furthermore, the presence of the l-isomer in the racemic mixture of MPH did not contribute to the discriminative stimulus properties of dl-MPH as evidenced by complete cross substitution between the pure d-isomer and twice the mg/kg dose of the racemic mixture in mice trained to discriminate dl-MPH and d-MPH, respectively. This finding is consistent with the generally reported lack of pharmacological activity of the l-isomer (Patrick et al., 1987; Markowitz et al., 2006). The study also indicates that for B6 mice, doses of MPH (<2 mg/kg), although perhaps being detected, as noted by intermediate response levels on the drug-paired lever in our first experiment, did not support acquisition of the discrimination task, even with extensive training. Although evidence from other studies indicates pharmacological activity for these low MPH doses (Kuczenski and Segal, 1997, 2001; Berridge et al., 2006), they did not produce cues sufficient to allow B6 mice to acquire the ability to discriminate MPH from saline. Thus, the current experiments indicate that B6 mice can discriminate MPH at doses known to increase locomotor activity, 2.5 to 5mg/kg, but not at lower doses, and that the presence of the l-isomer does not influence the discriminability of MPH.
Both in vivo and in vitro studies indicate that the l-isomer component of racemic MPH is inactive (Patrick et al., 1987; Williard et al., 2007; Markowitz and Patrick, 2008) and the present results support this position for MPH discrimination. First, the dose-response function for the mice trained to discriminate d-MPH was simply shifted to the left in parallel, with an ED50 approximately half that of dl-MPH trained mice when given d-MPH doses. Further, the inactivity of the l-isomer was evidenced in the cross-substitution tests, in which MPH discrimination was similar when mice were given either the d-isomer or the racemate dosed at equivalent amounts of the d-isomer. Since the racemic mixture of MPH contains 50% each of d-MPH and l-MPH, titrating MPH doses based on the active d-isomer component was expected to produce substitution, given the anticipated pharmacological inactivity of the l-isomer. Consistent with this hypothesis, we found that doses of d-MPH substituted completely in mice trained to discriminate twice the dose of dl-MPH. Likewise, there was a complete substitution using equivalent dl-MPH (based on the d-isomer) in mice trained to discriminate d-MPH from saline. Thus, consistent with many previous studies indicating inactivity of the l-isomer, our experiments indicate that the l-isomer has no role in the discriminative stimulus cues that support MPH discrimination. This outcome contrasts, however, with some limited lines of evidence that l-MPH influences the pharmacology of d-MPH [for review, (Heal and Pierce, 2006; Patrick et al., 2009)]. Further, additional studies indicate that the l-isomer interferes with the metabolism of the d-isomer, but only when co-administered with ethanol (Patrick et al., 2007; Zhu et al., 2008). Although we did not test the l-isomer directly in mice trained to discriminate dl-MPH, it is highly unlikely the l-isomer could support discrimination because a large number of in vitro and in vivo studies indicate it is simply a passive component of the racemate [for review, (Markowitz and Patrick, 2008)].
As an additional observation, during the cross-substitution experiment we found that accuracy of MPH discrimination for the low, equimolar d-MPH doses approached that of the training doses (Figure 2). However, this was not observed when the mice were tested, to establish the dose-response curve, using the drug they were trained to discriminate (Figure 1). The reason for this unexpected increase in response accuracy during cross-substitution testing is not clear. It is possible that these two observations simply reflect of the amount of variance normally seen at borderline discriminative doses under our experimental conditions. This explanation, while plausible, is complicated by the fact that these two observations (Figure 1 and Figure 2) are not independent, but rather were made sequentially within the same group of mice. An alternative explanation could relate to the development of a sensitized locomotor response, specifically to the d-isomer of MPH, as has been previously described for MPH (Gaytan et al., 1997; McDougall et al., 1999; Valvassori et al., 2007). However, this appears unlikely because the overall response rate of these mice during the cross-substitution tests was actually reduced compared to response rates at equimolar doses in previous test sessions with the training drug. Finally, the increased response accuracy during cross-substitution testing could simply reflect improvement in testing performance over time, as the mice of both groups gained experience with the discriminative stimulus of the active isomer, d-MPH. Interestingly, the improved performance could be supported, at least in part, by the development of a sensitized response to the discriminative stimulus of d-MPH. This interpretation is consistent with a recent report demonstrating that pre-exposure to 2 mg/kg methamphetamine in comparison to saline, prior to discrimination training for 1 mg/kg methamphetamine produced a long-lasting, left-ward shift in the discriminative stimulus dose-response function (Suzuki et al., 2004). Note that a sensitized response, in the context of discriminative stimuli, can be detected only with drug doses lower than that used for training, since higher doses typically yield response ratios at or greater than established discrimination criteria. Thus, it is possible that sensitization to the discriminative stimulus effects of d-MPH could have occurred in the B6 mice and contributed to greater responding on the drug-paired lever when tested with low, equimolar d-MPH doses during cross-substitution testing. This intriguing possibility requires systematic experimentation to confirm, but suggests that the discriminative stimuli of d-MPH may be susceptible to sensitization processes.
The B6 mice in our second experiment did not acquire discrimination of MPH doses less than 4 mg/kg, even with extensive training. This was in spite of reports that lower doses impact behavior and elevate extracellular levels of neurotransmitters. For example, it was recently reported that MPH doses of 0.5 to 1.0 mg/kg, administered i.p. to rats, improved cognitive task performance, and elevated extracellular dopamine and norepinephrine by several-fold in the nucleus accumbens, prefrontal cortex and medial septal brain regions (Berridge et al., 2006). Although species difference might account for this difference, we recently reported that a 1.25 mg/kg dl-MPH dose enhanced EtOH-stimulated locomotor activity, although not when administered alone (Griffin et al., 2010). Therefore, doses of MPH less than 2.0 mg/kg, although behaviorally and neurochemically active in rats, and likely in B6 mice as evidenced by a reduction in EtOH consumption and an enhancement of ethanol stimulation (Griffin et al., 2010), did not produce cues sufficient for acquisition of the discrimination task by B6 mice. However, increasing the MPH dose into a range that produces greater stimulation of locomotor activity in B6 mice (e.g. 5 mg/kg) did result in a discriminable MPH cue, which is consistent with reports of MPH discrimination by rats (Overton and Shen, 1988; Perkins et al., 1991). Accordingly, the threshold dose of dl-MPH B6 mice required to support acquisition of discriminating its stimulus cues exceeds 2 mg/kg in this particular discrimination task.
The neurobiological mechanisms generating the discriminative stimulus cue of MPH in all likelihood is based on the activity of the MPH d-isomer on the inhibition of dopamine (DA) and norepinephrine (NE) transporters (Williard et al., 2007; Desai et al., 2010). The effects of MPH on these neurotransmitter systems in vivo appear to differ according to brain region as well as dose. For example, i.p. injections of low MPH doses produce larger increases in NE and DA in the prefrontal cortex than the nucleus accumbens (Berridge et al., 2006). Similarly, low MPH doses given orally were reported to increase NE in the hippocampus, while no increase in DA in the nucleus accumbens was observed (Kuczenski and Segal, 2001). Although the brain region supporting MPH discrimination is unknown, evidence from two microinjection studies indicate that the nucleus accumbens mediates cocaine discrimination (Wood and Emmett-Oglesby, 1989) and, further, that dopaminergic neurotransmission in the nucleus accumbens is critical for cocaine discrimination (Callahan et al., 1994). In B6 mice, the selective dopamine transporter inhibitor nomifensine (but not the selective norepinephrine transporter inhibitor nisoxetine) generalizes to the discriminative cues produced by cocaine (Middaugh et al., 1998), underscoring the importance of the dopaminergic system in the discriminative stimulus of cocaine. Given the pharmacological similarities between MPH and cocaine (Williard et al., 2007), it is likely that MPH discrimination is supported, at least in part, by dopaminergic neurotransmission in the nucleus accumbens. Thus, it is possible that the low MPH doses (<2 mg/kg) used in our studies simply did not engage the dopaminergic system to the extent required to generate an effective discriminative stimulus which resulted in poor discrimination of MPH versus saline. It is noted that the doses required for effectively acquiring MPH discrimination in these experiments are also those doses required to stimulate locomotor activity in B6 mice, which is also influenced by dopaminergic neurotransmission (Halberda et al., 1997). In previous studies, significant increases in locomotor activity were observed at 5 and 2.5 mg/kg for the dl- and d-forms, respectively, but not lower doses (Williard et al., 2007; Griffin et al., 2010). Thus, the MPH discrimination and MPH locomotor activation paradigms appear to be based on a common neurobiological substrate that may not be relevant at lower MPH doses, possibly explaining the requirement for higher MPH doses to evoke behavioral activity.
In summary, the current study revealed that the dose of MPH required for B6 mice to learn to discriminate cues was above 2.0 mg/kg and approximates the dose required to stimulate motor activity of this mouse strain. Additionally, our data indicate that the dl- and d-forms of MPH can be directly substituted, i.e., when used in equivalent doses of d-MPH, in mice trained to discriminate the other isomeric form. This finding supports numerous reports indicating that the l-isomer is behaviorally inactive. There are at least two implications of our findings for translational lines of research. First, if our animal model of MPH behavioral effects holds for humans, MPH abusers may ingest supra-therapeutic MPH doses in pursuit of euphoria; a scenario which predisposes the individual to adverse events. Second, emerging evidence indicates that low doses of MPH can potentiate the response to other psychoactive compounds. For example, in humans a typical therapeutic dose of MPH interacts with ethanol to potentiate pleasurable subjective effects (Patrick et al., 2007). Similarly, animal models have demonstrated that low doses of MPH interact with ethanol (Griffin et al., 2010) and nicotine (Wooters et al., 2008) to increase behavioral responses greater than that of either drug alone. Thus, the abuse liability of a low MPH dose may be accentuated by concomitant psychotropic substance(s).
Acknowledgments
This work was supported by NIH grant R01AA016707 (K.S.P.). The authors gratefully acknowledge the technical assistance of Andrew J. Novak.
References
- Askenasy EP, Taber KH, Yang PB, Dafny N. Methylphenidate (Ritalin): behavioral studies in the rat. Int J Neurosci. 2007;117:757–794. doi: 10.1080/00207450600910176. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Devilbiss DM, Andrzejewski ME, Arnsten AF, Kelley AE, Schmeichel B, Hamilton C, Spencer RC. Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biol Psychiatry. 2006;60:1111–1120. doi: 10.1016/j.biopsych.2006.04.022. [DOI] [PubMed] [Google Scholar]
- Biederman J, Faraone SV. Current concepts on the neurobiology of Attention-Deficit/Hyperactivity Disorder. J Atten Disord. 2002;6(Suppl 1):S7–16. doi: 10.1177/070674370200601s03. [DOI] [PubMed] [Google Scholar]
- Biederman J, Faraone SV. Attention-deficit hyperactivity disorder. Lancet. 2005;366:237–248. doi: 10.1016/S0140-6736(05)66915-2. [DOI] [PubMed] [Google Scholar]
- Bondareva TS, Young R, Glennon RA. Central stimulants as discriminative stimuli. Asymmetric generalization between (−)ephedrine and S(+)methamphetamine. Pharmacol Biochem Behav. 2002;74:157–162. doi: 10.1016/s0091-3057(02)00963-2. [DOI] [PubMed] [Google Scholar]
- Callahan PM, De la Garza R, 2nd, Cunningham KA. Discriminative stimulus properties of cocaine: modulation by dopamine D1 receptors in the nucleus accumbens. Psychopharmacology (Berl) 1994;115:110–114. doi: 10.1007/BF02244759. [DOI] [PubMed] [Google Scholar]
- Colpaert FC. Drug discrimination in neurobiology. Pharmacol Biochem Behav. 1999;64:337–345. doi: 10.1016/s0091-3057(99)00047-7. [DOI] [PubMed] [Google Scholar]
- Czoty PW, Makriyannis A, Bergman J. Methamphetamine discrimination and in vivo microdialysis in squirrel monkeys. Psychopharmacology (Berl) 2004;175:170–178. doi: 10.1007/s00213-004-1798-6. [DOI] [PubMed] [Google Scholar]
- Darredeau C, Barrett SP, Jardin B, Pihl RO. Patterns and predictors of medication compliance, diversion, and misuse in adult prescribed methylphenidate users. Hum Psychopharmacol. 2007;22:529–536. doi: 10.1002/hup.883. [DOI] [PubMed] [Google Scholar]
- Davids E, Zhang K, Tarazi FI, Baldessarini RJ. Stereoselective effects of methylphenidate on motor hyperactivity in juvenile rats induced by neonatal 6-hydroxydopamine lesioning. Psychopharmacology (Berl) 2002;160:92–98. doi: 10.1007/s00213-001-0962-5. [DOI] [PubMed] [Google Scholar]
- Desai RI, Paronis CA, Martin J, Desai R, Bergman J. Monoaminergic psychomotor stimulants: discriminative-stimulus effects and dopamine efflux. J Pharmacol Exp Ther. 2010 doi: 10.1124/jpet.110.165746. epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatley SJ, Volkow ND, Gifford AN, Fowler JS, Dewey SL, Ding YS, Logan J. Dopamine-transporter occupancy after intravenous doses of cocaine and methylphenidate in mice and humans. Psychopharmacology (Berl) 1999;146:93–100. doi: 10.1007/s002130051093. [DOI] [PubMed] [Google Scholar]
- Gaytan O, al-Rahim S, Swann A, Dafny N. Sensitization to locomotor effects of methylphenidate in the rat. Life Sci. 1997;61:101–107. doi: 10.1016/s0024-3205(97)00598-5. [DOI] [PubMed] [Google Scholar]
- Godfrey J. Safety of therapeutic methylphenidate in adults: a systematic review of the evidence. J Psychopharmacol. 2009;23:194–205. doi: 10.1177/0269881108089809. [DOI] [PubMed] [Google Scholar]
- Grant KA. Strategies for understanding the pharmacological effects of ethanol with drug discrimination procedures. Pharmacol Biochem Behav. 1999;64:261–267. doi: 10.1016/s0091-3057(99)00075-1. [DOI] [PubMed] [Google Scholar]
- Griffin WC, 3rd, Novak AJ, Middaugh LD, Patrick KS. The interactive effects of methylphenidate and ethanol on ethanol consumption and locomotor activity in mice. Pharmacol Biochem Behav. 2010;95(3):267–272. doi: 10.1016/j.pbb.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberda JP, Middaugh LD, Gard BE, Jackson BP. DAD1- and DAD2-like agonist effects on motor activity of C57 mice: differences compared to rats. Synapse. 1997;26:81–92. doi: 10.1002/(SICI)1098-2396(199705)26:1<81::AID-SYN9>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Heal DJ, Pierce DM. Methylphenidate and its isomers: their role in the treatment of attention-deficit hyperactivity disorder using a transdermal delivery system. CNS Drugs. 2006;20:713–738. doi: 10.2165/00023210-200620090-00002. [DOI] [PubMed] [Google Scholar]
- Heil SH, Holmes HW, Bickel WK, Higgins ST, Badger GJ, Laws HF, Faries DE. Comparison of the subjective, physiological, and psychomotor effects of atomoxetine and methylphenidate in light drug users. Drug Alcohol Depend. 2002;67:149–156. doi: 10.1016/s0376-8716(02)00053-4. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Grant KA, Becker HC, Besheer J, Crissman AM, Platt DM, Shannon EE, Shelton KL. Understanding how the brain perceives alcohol: neurobiological basis of ethanol discrimination. Alcohol Clin Exp Res. 2006;30:203–213. doi: 10.1111/j.1530-0277.2006.00024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollins SH, MacDonald EK, Rush CR. Assessing the abuse potential of methylphenidate in nonhuman and human subjects: a review. Pharmacol Biochem Behav. 2001;68:611–627. doi: 10.1016/s0091-3057(01)00464-6. [DOI] [PubMed] [Google Scholar]
- Kollins SH, English J, Robinson R, Hallyburton M, Chrisman AK. Reinforcing and subjective effects of methylphenidate in adults with and without attention deficit hyperactivity disorder (ADHD) Psychopharmacology (Berl) 2009;204:73–83. doi: 10.1007/s00213-008-1439-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroutil LA, Van Brunt DL, Herman-Stahl MA, Heller DC, Bray RM, Penne MA. Nonmedical use of prescription stimulants in the United States. Drug Alcohol Depend. 2006;84:135–143. doi: 10.1016/j.drugalcdep.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS. Effects of methylphenidate on extracellular dopamine, serotonin, and norepinephrine: comparison with amphetamine. J Neurochem. 1997;68:2032–2037. doi: 10.1046/j.1471-4159.1997.68052032.x. [DOI] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS. Locomotor effects of acute and repeated threshold doses of amphetamine and methylphenidate: relative roles of dopamine and norepinephrine. J Pharmacol Exp Ther. 2001;296:876–883. [PubMed] [Google Scholar]
- Kuczenski R, Segal DS. Stimulant actions in rodents: implications for attention-deficit/hyperactivity disorder treatment and potential substance abuse. Biol Psychiatry. 2005;57:1391–1396. doi: 10.1016/j.biopsych.2004.12.036. [DOI] [PubMed] [Google Scholar]
- Li SM, Campbell BL, Katz JL. Interactions of cocaine with dopamine uptake inhibitors or dopamine releasers in rats discriminating cocaine. J Pharmacol Exp Ther. 2006;317:1088–1096. doi: 10.1124/jpet.105.100594. [DOI] [PubMed] [Google Scholar]
- Markowitz JS, Patrick KS. Differential pharmacokinetics and pharmacodynamics of methylphenidate enantiomers: Does chirality matter? J Clin Psychopharmacol. 2008;28(3 Suppl 2):S54–61. doi: 10.1097/JCP.0b013e3181733560. [DOI] [PubMed] [Google Scholar]
- Markowitz JS, DeVane CL, Pestreich LK, Patrick KS, Muniz R. A comprehensive in vitro screening of d-, l-, and dl-threo-methylphenidate: an exploratory study. J Child Adolesc Psychopharmacol. 2006;16:687–698. doi: 10.1089/cap.2006.16.687. [DOI] [PubMed] [Google Scholar]
- McCabe SE, Teter CJ, Boyd CJ, Guthrie SK. Prevalence and correlates of illicit methylphenidate use among 8th, 10th, and 12th grade students in the United States, 2001. J Adolesc Health. 2004;35:501–504. doi: 10.1016/j.jadohealth.2004.02.004. [DOI] [PubMed] [Google Scholar]
- McCabe SE, Cranford JA, Morales M, Young A. Simultaneous and concurrent polydrug use of alcohol and prescription drugs: prevalence, correlates, and consequences. J Stud Alcohol. 2006;67:529–537. doi: 10.15288/jsa.2006.67.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall SA, Collins RL, Karper PE, Watson JB, Crawford CA. Effects of repeated methylphenidate treatment in the young rat: sensitization of both locomotor activity and stereotyped sniffing. Exp Clin Psychopharmacol. 1999;7:208–218. doi: 10.1037//1064-1297.7.3.208. [DOI] [PubMed] [Google Scholar]
- Middaugh LD, Favara JP, Boggan WO, Stringer AJ. Discriminative properties of phencyclidine in mice: generalization to ketamine and monohydroxy metabolites. Psychopharmacology (Berl) 1988;96:381–384. doi: 10.1007/BF00216066. [DOI] [PubMed] [Google Scholar]
- Middaugh LD, McGroarty KK, Groseclose CH, Adinoff B. Cocaine discrimination: relationship to local anesthetics and monoamine uptake inhibitors in C57BL/6 mice. Psychopharmacology (Berl) 1998;136:44–49. doi: 10.1007/s002130050537. [DOI] [PubMed] [Google Scholar]
- Middaugh LD, Kelley BM, Cuison ER, Jr, Groseclose CH. Naltrexone effects on ethanol reward and discrimination in C57BL/6 mice. Alcohol Clin Exp Res. 1999;23:456–464. [PubMed] [Google Scholar]
- Okie S. ADHD in adults. N Engl J Med. 2006;354:2637–2641. doi: 10.1056/NEJMp068113. [DOI] [PubMed] [Google Scholar]
- Overton DA, Shen CF. Comparison of four-drug discriminations in training compartments with four identical levers versus four different responses manipulanda. Pharmacol Biochem Behav. 1988;30:879–888. doi: 10.1016/0091-3057(88)90114-1. [DOI] [PubMed] [Google Scholar]
- Patrick KS, Caldwell RW, Ferris RM, Breese GR. Pharmacology of the enantiomers of threo-methylphenidate. J Pharmacol Exp Ther. 1987;241:152–158. [PubMed] [Google Scholar]
- Patrick KS, Straughn AB, Minhinnett RR, Yeatts SD, Herrin AE, DeVane CL, Malcolm R, Janis GC, Markowitz JS. Influence of ethanol and gender on methylphenidate pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther. 2007;81:346–353. doi: 10.1038/sj.clpt.6100082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick KS, Straughn AB, Perkins JS, Gonzalez MA. Evolution of stimulants to treat ADHD: transdermal methylphenidate. Hum Psychopharmacol. 2009;24:1–17. doi: 10.1002/hup.992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins AN, Eckerman DA, MacPhail RC. Discriminative stimulus properties of triadimefon: comparison with methylphenidate. Pharmacol Biochem Behav. 1991;40:757–761. doi: 10.1016/0091-3057(91)90081-c. [DOI] [PubMed] [Google Scholar]
- Schweri MM, Deutsch HM, Massey AT, Holtzman SG. Biochemical and behavioral characterization of novel methylphenidate analogs. J Pharmacol Exp Ther. 2002;301:527–535. doi: 10.1124/jpet.301.2.527. [DOI] [PubMed] [Google Scholar]
- Solinas M, Panlilio LV, Justinova Z, Yasar S, Goldberg SR. Using drug-discrimination techniques to study the abuse-related effects of psychoactive drugs in rats. Nat Protoc. 2006;1:1194–1206. doi: 10.1038/nprot.2006.167. [DOI] [PubMed] [Google Scholar]
- Stoops WW, Lile JA, Fillmore MT, Glaser PE, Rush CR. Reinforcing effects of methylphenidate: influence of dose and behavioral demands following drug administration. Psychopharmacology (Berl) 2005;177:349–355. doi: 10.1007/s00213-004-1946-z. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Fukuoka Y, Mori T, Miyatake M, Narita M. Behavioral sensitization to the discriminative stimulus effects of methamphetamine in rats. Eur J Pharmacol. 2004;498:157–161. doi: 10.1016/j.ejphar.2004.07.064. [DOI] [PubMed] [Google Scholar]
- Teo SK, Stirling DI, Thomas SD, Khetani VD. Neurobehavioral effects of racemic threo-methylphenidate and its D and L enantiomers in rats. Pharmacol Biochem Behav. 2003;74:747–754. doi: 10.1016/s0091-3057(02)01073-0. [DOI] [PubMed] [Google Scholar]
- Valvassori SS, Frey BN, Martins MR, Reus GZ, Schimidtz F, Inacio CG, Kapczinski F, Quevedo J. Sensitization and cross-sensitization after chronic treatment with methylphenidate in adolescent Wistar rats. Behav Pharmacol. 2007;18:205–212. doi: 10.1097/FBP.0b013e328153daf5. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Swanson JM. Variables that affect the clinical use and abuse of methylphenidate in the treatment of ADHD. Am J Psychiatry. 2003;160:1909–1918. doi: 10.1176/appi.ajp.160.11.1909. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Ding YS, Gatley SJ. Role of dopamine in the therapeutic and reinforcing effects of methylphenidate in humans: results from imaging studies. Eur Neuropsychopharmacol. 2002;12:557–566. doi: 10.1016/s0924-977x(02)00104-9. [DOI] [PubMed] [Google Scholar]
- Williard RL, Middaugh LD, Zhu HJ, Patrick KS. Methylphenidate and its ethanol transesterification metabolite ethylphenidate: brain disposition, monoamine transporters and motor activity. Behav Pharmacol. 2007;18:39–51. doi: 10.1097/FBP.0b013e3280143226. [DOI] [PubMed] [Google Scholar]
- Wood DM, Emmett-Oglesby MW. Mediation in the nucleus accumbens of the discriminative stimulus produced by cocaine. Pharmacol Biochem Behav. 1989;33:453–457. doi: 10.1016/0091-3057(89)90529-7. [DOI] [PubMed] [Google Scholar]
- Wooters TE, Neugebauer NM, Rush CR, Bardo MT. Methylphenidate enhances the abuse-related behavioral effects of nicotine in rats: intravenous self-administration, drug discrimination, and locomotor cross-sensitization. Neuropsychopharmacology. 2008;33:1137–1148. doi: 10.1038/sj.npp.1301477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu HJ, Patrick KS, Yuan HJ, Wang JS, Donovan JL, DeVane CL, Malcolm R, Johnson JA, Youngblood GL, Sweet DH, Langaee TY, Markowitz JS. Two CES1 gene mutations lead to dysfunctional carboxylesterase 1 activity in man: clinical significance and molecular basis. Am J Hum Genet. 2008;82:1241–1248. doi: 10.1016/j.ajhg.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]