Abstract
The pedicle screw instrumentation represents the most rigid construct of the cervical and cervicothoracic spine and in spite of the risks to neurovascular structures clinical relevant complications do not occur frequently. The steep angles of the cervical pedicles result in a wide surgical exposure with extensive muscular trauma. The objective of this study was the evaluation of the accuracy of cervical pedicle screw insertion through a minimally invasive technique to reduce access-related muscular trauma. Therefore, percutaneous transpedicular instrumentation of the cervical and cervicothoracic spine was performed in 15 patients using fluoroscopy. All instrumentations from C2 to Th4 were inserted bilaterally through 2 to 3-cm skin and fascia incisions even in multilevel procedures and the rods were placed by blunt insertion through the incision. Thin-cut CT scan was used postoperatively to analyze pedicle violations. 76.4% of 72 screws were placed accurately. Most pedicle perforations were seen laterally towards the vertebral artery. Critical breaches >2 mm or narrowing of the transversal foramen occurred in 12.5% of screws; however, no revision surgery for screw displacement was needed in the absence of clinical symptoms. No conversion from percutaneous to open surgery was necessary. It was concluded that percutaneous transpedicular instrumentation of the cervical spine is a surgically demanding technique and should be reserved for experienced spine surgeons. The indications are limited to instrumentation-only procedures or in combination with anterior treatment, but with the potential to minimize access-related morbidity.
Electronic supplementary material
The online version of this article (doi:10.1007/s00586-011-1775-9) contains supplementary material, which is available to authorized users.
Keywords: CPS, Percutaneous, Cervical, Instrumentation, Fluoroscopy, Minimally invasive
Introduction
Cervical pedicle screw (CPS) constructs have been demonstrated to provide superior biomechanically stability compared to lateral mass plating or anterior plating especially in multi-level constructs [13, 20, 35]. However, this technique has a potential risk of nerve root, spinal cord and vertebral artery injury due to a small corridor of safe screw placement [31, 39, 41], recent clinical studies demonstrate that relevant complications do not occur frequently [2, 9, 11, 15, 18, 24, 26, 29, 47, 48]. The axis of the cervical pedicles to the sagittal midline plane vary between 25 and 60° and makes a wide exposure of the spine necessary [14]. Therefore, additional stab incisions are advised by several authors to maintain the necessary angle [12] and to reduce the morbidity of the surgical approach [15, 29]. In this context, multi-level posterior as well as combined anterior–posterior approaches to the cervical spine are proposed to be associated with increased incidence of perioperative complications [37]. Similar to stabilization procedures of the thoracolumbar spine with the objective to reduce access-related morbidity as muscular denervation and atrophy, length of hospital stay and blood loss [10, 16, 21, 22, 38, 44], minimally invasive procedures for cervical instrumentation represent the next step to reduce access-related muscular trauma. Therefore, several authors reported minimally invasive posterior procedures for stabilization and decompression of the cervical spine [8, 33, 40, 45]. However, this topic has not extensively investigated yet.
In the present study, we performed minimally invasive posterior instrumentation of the cervical and cervicothoracic spine, using percutaneously inserted CPS under fluoroscopic control in 15 patients. We analyzed the accuracy of screw placement and the limitations of the technique.
Materials and methods
From April 2008 to November 2009, 15 patients who needed posterior cervical or cervicothoracic stabilization were treated with minimally invasive transpedicular screw placement. A total of 72 screws were inserted. There were nine females and six male patients with mean age of 59 years (range 25–79 years). Mean ASA Score was 3.13 (range 2–4; Table 1). Table 1 provides information of demographic data and neurologic outcome. The cervical disorders included spine instabilities due to tumor (9 patients), infection (2 patients), trauma (3 patients) and cervical kyphotic deformity (1 patient). Patients were treated dorsal (4 patients), ventrodorsal (9 patients) and dorsoventral (2 patients). Detailed information of pathology, anterior and posterior treatment including used implants, number of fused levels and fluoroscopic and operating time is shown in Table 2. Percutaneous CPS instrumentation was performed in cases of high cervical instability as extensive multi-level corpectomies or one-level corpectomies with reduced bone quality due to osteoporosis, kyphotic deformity correction, fractures with disruption of the anterior and posterior column and in cases of palliative stabilization of metastatic lesions of the vertebral column. In the latter patients, the indication was limited to patients with an estimated life expectancy below 2 years to avoid implant failure after non-fusion instrumentation [24]. Patients with necessity for posterior decompression of neurologic structures or posterior fusion were excluded from percutaneous CPS placement.
Table 1.
Demographic data and neurologic status of patients
| Patient no. | Gender | Age | ASA | Frankel Grade pre-operative | Frankel Grade post-operative |
|---|---|---|---|---|---|
| 1 | m | 72 | 4 | A | D |
| 2 | f | 47 | 3 | E | E |
| 3 | f | 74 | 3 | E | E |
| 4 | m | 72 | 3 | E | E |
| 5 | f | 79 | 4 | E | E |
| 6 | f | 66 | 3 | E | E |
| 7 | m | 29 | 2 | E | E |
| 8 | f | 61 | 3 | E | E |
| 9 | m | 79 | 4 | C | D |
| 10 | f | 74 | 3 | E | E |
| 11 | m | 50 | 3 | E | E |
| 12 | f | 72 | 3 | C | D |
| 13 | f | 59 | 4 | E | E |
| 14 | f | 25 | 3 | E | E |
| 15 | m | 29 | 2 | * | * |
* Neurologic status not distinguishable from MVA-related severe brain trauma
Table 2.
Pathology, surgical procedures with used implants and duration of fluoroscopy and operation
| Patient no. | Pathology | Anterior treatment | Implants | Posterior treatment and screw diameter (mm)a | Fused levels | Fluoroscopic time (min) | Operation time (h) |
|---|---|---|---|---|---|---|---|
| 1 | Infection | Corp C5, VBR | Codman, ADD | C4 (3.5)–C6 (3.5) | C4-C6 | 3.3 | 2.1 |
| 2 | Tumor | Corp C7, BG | Reflex | C6 (3.5)–Th1 (4.0) | C6-Th1 | 4.8 | 2.13 |
| 3 | Tumor | Corp C3, VBR | Reflex, ADD | C2 (4.0)–C4 (4.0) | C2-C4 | 7.3 | 1.72 |
| 4 | Tumor | – | – | C2 (3.5), C4 (3.5), Th2 (4.0), Th3 (4.0) | – | 14 | 5.92 |
| 5 | Tumor | – | – | C2 (3.5), C4 (3.5), Th2 (4.0), Th3 (4.0) | – | 3.3 | 4.83 |
| 6 | Tumor | Corp C5 + C6 + C7, VBR | Codman, ADD | C4 (3.5)–Th1 (4.0) | C4-Th1 | 7.7 | 3.27 |
| 7 | Trauma | ACDF, BG | Reflex | C6 (3.5)–C7 (3.5) | C6-C7 | 5.5 | 2.0 |
| 8 | Tumor | – | – | C2 (3.5), C3 (3.5), Th1 (4.0), Th2 (4.0) | – | 6.7 | 4.65 |
| 9 | Infection | Corp C3-C4, VBR | Codman, ADD | C2 (3.5)–C5 (3.5) | C2-C5 | 10.9 | 3.92 |
| 10 | Spond | ACDF C2-C7 | Codman, PEEK-Cage | C2 (3.5)–C7 (3.5) | C2-C7 | 6.6 | 2.4 |
| 11 | Tumor | Corp C7, VBR | Reflex, ECD | C6 (3.5)–Th1 (3.5) | C6-Th1 | 6.1 | 3.17 |
| 12 | Trauma | Corp C4 + C5, VBR | Codman, ADD | C3 (3.5)–C6 (3.5) | C3-C6 | – | 2.57 |
| 13 | Tumor | Corp. C6 + C7, VBR | Codman, ADD | C5 (3.5)–Th4 (5.5)b | C5-Th1 | 7.5 | 2.13 |
| 14 | Tumor | – | – | C2 (3.5)–C4 (3.5) | – | 7.2 | 2.62 |
| 15 | Trauma | – | – | C3 (3.5)–C6 (3.5) | – | 10.5 | 3.15 |
Spond spondylosis with kyphosis, Corp corpectomy, VBR vertebral body replacement, Codman Codman plate (constrained plate), Reflex reflex plate (constrained plate), ADD expandable titanium cage (Ulrich, Germany), BG Bone graft, ACDF anterior cervical decompression and fusion, PEEK-Cage PEEK-Optima intervertebral Cage (Intromed, Germany), ECD expandable PEEK cage (Synthes, Switzerland)
aAll posterior instrumentations were performed with a constrained screw rod construct (Oasys, Stryker, Germany)
bTh4 Mantis (Stryker, Germany)
Presurgical management
All patients underwent a CT scan of the cervical or cervicothoracic spine for evaluation of the spinal stability and pedicle morphology as pedicle diameter and angle in multiplanar CT reconstruction. An additional MRI was performed for planning of the needed decompression of neurologic structures. MRI- or CT-angiography was not performed in each patient. The patients were evaluated clinically for neurologic status and pain.
Implants
In all cases, a constrained screw/rod system in combination with a trokar system (Oasys, Stryker, Germany) with polyaxial screws made of titanium with 3.5 and 4.0 mm diameter for dorsal cervical or cervicothoracic screw fixation was used as indicated in Table 2. In one case, the screw/rod system was combined with a percutaneous system for thoracolumbar instrumentation (Mantis, Stryker, Germany) using connecting rods (3.5/6 mm titanium, Synthes, Switzerland). For ventral stabilization, a constrained plate system (Reflex, Stryker, Germany) or a semiconstrained plate (Codman) was used. After corpectomy, ventral body replacement (ADD, Ulrich medical, Germany or ECD, Synthes, Switzerland) was performed.
Surgical technique of percutaneous pedicle screw insertion
All operations were performed by the senior spine surgeon (N.H.-A.). The patients were placed in prone position on a radiolucent table after fixation of the head in a carbon Mayfield clamp (Online Resource Fig. 1). The cervical spine was maintained in a neutral position and the shoulders were taped caudal to obtain a clear intraoperative radiographic imaging of the lower cervical spine. Prior to the operation, the fluoroscopic image quality in a.p., lateral, oblique and pedicle axis views was controlled. If the image quality was not sufficient, the percutaneous technique was abandoned.
The skin entry point of the surgical approach was determined by positioning of spinal needles with a 40° convergence with the tip lateral to the estimated pedicle entrance lateral to the midline of the lateral mass in the true anterior–posterior fluoroscopic image. The position in cranial-caudal direction was chosen just beyond the extension to the upper endplate of the vertebra in lateral fluoroscopic view (Online Resource Fig. 1). Following the lordosis of the cervical spine, the meeting trajectory required for instrumentation of adjacent levels allowed the same skin and fascia incision. A skin and fascia incision of 2–3 cm was sufficient for screw placement of 2 or 3 adjacent levels (Fig. 1 and Online Resource Fig. 2A). Following muscles were split in fiber course until the entry point on the articular mass was identified (Online Resource Fig. 2B).
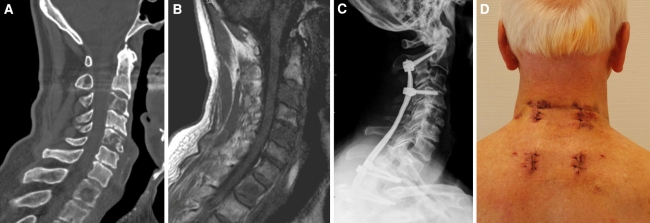
Fig. 1.
a A 73-year-old male with cervicothoracic instability due to osteolysis of C3, C5 and Th1. b No compression of the spinal cord was present as shown by presurgical MRI (T1). c A percutaneous stabilization C2 to Th3 was performed. d Postsurgical photograph of surgical approach. A 2–3 cm incision of skin and fascia was sufficient for instrumentation of 2–3 adjacent levels with rod insertion
The correct screw entry point was marked after placement of the trokar set using a bone awl (Online Resource Fig. 3A). Therefore, the awl was positioned just below the lower margin of the upper articular surface and lateral of the midline of the articular mass [1]. Using the lateral and oblique fluoroscopic view (perpendicular to the pedicle axis), the correct entry point and trajectory in the cranio-caudal direction was achieved (sagittal plane) (Online Resource Fig. 3B). The true a.p. fluoroscopic view and pedicle axis view [48] were used to determine the entry point on the lateral mass (Online Resource Fig. 3C, D). Therefore, the awl was positioned in the center of the pedicle after rotating the fluoroscope until the pedicle appears approximately circular. Using the trajectory of the pedicle axis view and the true a.p. view at the fluoroscope, the inclination of the pedicle from the mid-sagittal plane could be double checked with the preoperative CT scan.
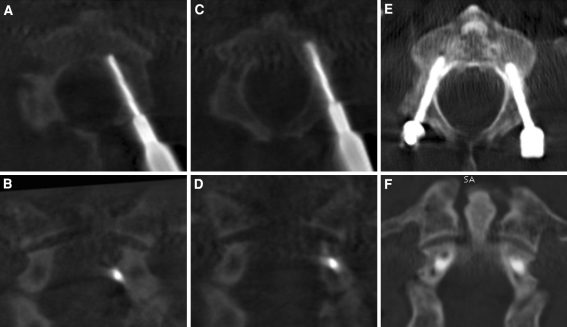
After opening of the cortical wall, the awl was removed with the trokar fixing the entry point and a drill bit for 3.5 and 4.0 mm screw, respectively with sleeve was inserted through the trokar and slow automatic drilling was performed (Online Resource Fig. 4). The inclination from the mid-sagittal plane was chosen according to the pedicle axis view and individual analysis of the vertebra on preoperative CT scan. The transverse angles of pedicles in the cervical spine average 40–50° in C3-C6 and 30–40° in C7 and C2 [14, 27, 28, 31, 39, 47]. Additionally, the additional rotation of the vertebra due to drilling was taken into account. The direction of the drilling trajectory was corrected according fluoroscopic oblique, lateral, true a.p. and pedicle axis view (Online Resource Fig. 4B–G). Thereafter, tapping with a tap sleeve was performed and the screw was inserted. The screw length as estimated on preoperative CT-scan was controlled by lateral fluoroscopy. In case of poor image quality, additionally an intraoperative three-dimensional fluoroscopic image was obtained using a C-arm to acquire further information about the position of the drill during the drilling process (ARCADIS Orbic 3D, Siemens medical, Erlangen, Germany). This C-arm generates a CT scan of a cubic volume of 12 cm × 12 cm × 12 cm acquiring 100 images in 60 s using the high-resolution imaging. The drill position could be evaluated in multiplanar reconstructions on the fluoroscope workstation and the drill trajectory was corrected accordingly (Fig. 2). After positioning of all screws, the rods were inserted and fixed with blocker screws (Online Resource Figs. 5, 6). Therefore, the rod bending was performed in cervical lordosis and in case of cervicothoracic instrumentation in lordosis and kyphosis. The correct curve could be controlled by lateral fluoroscopy. The rod was inserted by inserting it through the most caudal incision beyond the fascia and moved rostral by gently pushing it through the muscles. Thereby, the rod in multilevel constructs had to be rotated around its axis.
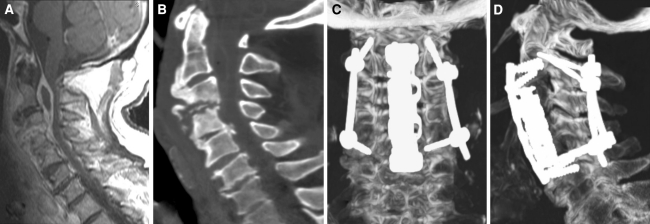
Fig. 2.
Intraoperative correction of screw position in C2. a Axial and b coronal view of multiplanar reconstruction obtained by isocentric C-arm after drill insertion. A clear displacement of the drill perforating the medial pedicle wall could be identified. c Axial and d coronal view of reconstructed images after correction of drill position. e Axial and f coronal view of postoperative CT scan showing correct screw placement
Postoperative management
Thin-cut computed tomography was performed postoperatively in all patients. The patient received a soft neck collar for 1–2 weeks after surgery. Patients were allowed to walk and sit the day after surgery if their condition permitted.
Evaluation of pedicle screw placement
Evaluation of pedicle screw position was performed using the CT scan postoperatively. Pedicle screw positions were analyzed using multiplanar reconstruction in sagittal, coronal and axial view independently by a spine surgeons and a radiologist (C.S. and P.B.). The pedicle screw positions were graded according to Richter et al. [29]. Group 1: correct screw placement without pedicle perforation or with pedicle perforation ≤1 mm. Group 2: pedicle perforation >1 mm without the need for screw revision. Group 3: pedicle perforation >1 mm with the need for screw revision because of (1) irritation or injury to nerve roots or spinal cord, or because of (2) injury of vertebral artery, (3) reduced biomechanical stability. Furthermore, directions and quantity of pedicle violations were measured and narrowing of transversal foramen was evaluated.
Results
Complications directly attributable to surgery
In all cases, no conversion to open procedure was necessary, even in multilevel procedures (see examples in Figs. 1 and 3). The mean operation and fluoroscopic time for percutaneous transpedicular instrumentation was 3.17 h (range 1.7–5.9 h) and 7.2 min (range 3.3–14 min) (Table 2). Neurologic symptoms according to Frankel improved in all patients with neurologic deterioration (Table 1).
Fig. 3.
a and b A 79-year-old male (patient 9) with incomplete tetraparesis (Frankel grade C) due to spondylodiscitis C3/C4 with epidural abscess from C2 to C4. He was treated with anterior corpectomy C3/C4, vertebral body replacement and anterior plating C2-C5. c and d Additional posterior stabilization was performed percutaneously C2-C5 to improve construct rigidity. An anterior revision surgery was necessary due to persistent deep tissue infection. At discharge from hospital neurologic symptoms were markedly improved (Frankel grade D)
No complications during dorsal treatment occured. Screw malpositions detected by three-dimensional fluoroscopic control with subsequent screw replacement were performed during two operations at the level of C2 and C4 and were corrected accordingly (Fig. 2). No postoperative neurologic impairment was observed. Anterior revision surgery was necessary in two patients. In one case (patient 1), the patient suffered previous to spinal pathology from a carcinoma of the larynx which was treated operatively in combination with a neck dissection and postoperative radiochemotherapy. Ventral decompression led to a dural tear with subsequent duraplastic, which was insufficient and needed revision. In the second patient (patient 9), revision was performed due to persistent deep tissue infection after corpectomy and vertebral body replacement for the reason of spondylodiscitis with paravertebral and epidural abscess.
Complications not directly attributable to surgery
In one patient, a pulmonary artery embolism occurred without further complication (patient 5) and a urinary tract infection occurred in a patient (patient 12).
Accuracy of pedicle screw position
Postoperative CT scans showed in 55 of 72 screws (76.4%) a correct position (Group 1). 17 of 72 screws (23.6%) showed perforations of the pedicle wall >1 mm (Group 2). The relative incidence of screw perforation was highest in C3 and C5 followed by C4 (Table 3). The portion of the pedicle perforated by the screw was the medial wall in 6.9%, lateral in 18.1%, superior in 1.4%, inferior in 0% and ventral in 0%. Medial malpositioned screws neither led to nerve root irritation or injury nor to compromise of the spinal cord. 2 out of 72 screws (2.8%) deviated medially more than 2 mm. Furthermore, 7 of 72 screws (9.7%) showed a narrowing of the transversal foramen of >25% (Fig. 4). In summary, 12.5% of the screws were considered critical. No alterations to perfusion of the vertebral artery as evaluated by duplex color-flow imaging, which has been proven as a reliable diagnostic tool for the assessment of vertebral artery lesions [3, 4], have been detected. None of the malpositioned screw led to clinical symptoms and had to be revised (Group 3).
Table 3.
Incidence of screw displacement on post-operative CT-scans
| Level | Number of screws | % of screws | ||
|---|---|---|---|---|
| Group 1 | Group 2 | Group 3 | ||
| C2 | 13 | 1 | 0 | 7.14 |
| C3 | 2 | 4 | 0 | 66.67 |
| C4 | 7 | 5 | 0 | 41.67 |
| C5 | 2 | 2 | 0 | 50.00 |
| C6 | 8 | 4 | 0 | 33.33 |
| C7 | 3 | 1 | 0 | 25.00 |
| Th1 | 8 | 0 | 0 | 0.00 |
| Th2 | 6 | 0 | 0 | 0.00 |
| Th3 | 4 | 0 | 0 | 0.00 |
| Th4 | 2 | 0 | 0 | 0.00 |
| Total | 55 | 17 | 0 | |
| Percentage | 76.4 | 23.6 | 0.0 | |
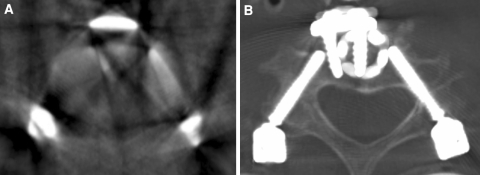
Fig. 4.
Limitations to intraoperative verification of correct screw placement after anterior vertebral body replacement of C3 with plating and percutaneous posterior transpedicular instrumentation of C2 to C4. a Axial view of multiplanar reconstruction obtained by isocentric C-arm (three-dimensional fluoroscopy). The poor image quality caused by metal artifacts allowed no evaluation of screw position. b A postoperative CT scan demonstrated a critical lateral pedicle wall perforation of the right screw in C4 with encroachment of the vertebral foramen (left side of the image)
Discussion
The decision for a particular internal fixation of the cervical spine should follow an analysis of the biomechanical stability, the type of neurologic compression, the underlying disease and the access morbidity. The anterior approach is often appropriate to achieve unrestricted spinal cord decompression and stabilization [42]. However, there are indications, such as multilevel stabilization in neoplastic instabilities, postsurgical deformities, injuries of the cervicothoracic junction and multilevel corpectomies, which make a solely or additional posterior stabilization necessary. These posterior instrumentation procedures have been shown to be associated with increased postoperative complications [37, 43]. Similar to stabilization procedures of the thoracolumbar spine, which have been proposed to reduce access-related morbidity as muscular denervation, atrophy, length of hospital stay, blood loss and postoperative pain [16, 21, 22], minimally invasive procedures for posterior cervical instrumentation represent the next logical step. Therefore, several authors reported minimally invasive posterior procedures for stabilization and decompression of the cervical spine [5, 8, 33, 40, 45]; however, the long-term benefit on clinical outcome remains to be proven. In this study, the feasibility and accuracy of percutaneous CPS placement using conventional fluoroscopy and three-dimensional fluoroscopy are presented and limitations as well as indications of this technique are discussed.
The cervical pedicle screw fixation is the most rigid fixation method of the cervical spine [13, 19, 20, 30, 35]. Despite its biomechanically advantages, the method is technically demanding and iatrogenic damage to neurovascular structures are feared. While lateral mass screw placement show low incidents (0–4%) of neurovascular complications [7, 25, 36, 46], recent reports concerning CPS are promising and clinical relevant complications do not occur frequently [2, 9, 11, 15, 18, 24, 26, 29, 47, 48].
Although the evaluation of correct screw placement is difficult to determine since postoperative CT scans overestimate the screw malposition due to metal artifacts [17], the main problem in comparing results of screw malposition results from the heterogeneous definition of the correct screw placement. Some authors did not provide a clear definition of the extent of pedicle wall perforation used for “correct” or “incorrect” screw placement in clinical studies [2, 18]. The correct screw placement in other studies was defined as no pedicle perforation [11, 23, 47, 48] or perforations up to 1 mm [15, 26, 29]. Screw malposition was classified as minor breach, exposure or partial perforation if the cortical breach did not extend 1.75–2 mm or encroachment of the transversal foramen up to 25% [11, 15, 23, 26, 47, 48]. Critical perforations of the pedicle was assumed if the violation measured more than 1.75 to 2 mm, with dural contact or narrowing of the transversal foramen >25%.
In our opinion, the screw placement should be evaluated according to the diagnostic or therapeutic consequences resulting from the grading of the pedicle screw position. Therefore, we used the definition of Richter et al. [29] combining radiological and clinical classification defining correct screw placement. In the present study, 76.4% of screws lay within 1 mm cortical breach and were classified as Group 1. Group 2, screw displacement >1 mm was seen in 23.6% of screws. Of these screws, 29.4% deviated medially, 76.4% laterally and 5.9% superiorly. 2 out of 72 screws (2.8%) deviated medially more than 2 mm. Furthermore, 7 of 72 screws (9.7%) showed a narrowing of the transversal foramen of >25%. In summary, 12.5% of the screws were considered critical. However, none of the cortical breaches resulted in clinical symptoms and had to be revised (Group 3). Screw perforations were reported in clinical studies using anatomic landmarks and fluoroscopy in 6.7% of 669 screws [18], 6.8% of 280 screws [47], 8.6% of 93 screws [29], 13.2% of 620 screws [48] and 29% of 86 screws [23]. With the use of navigation, the reported screw deviations ranged from 1.2% of 78 screws [18], 2% of 96 screws [9], 2.8% of 178 screws [11], 3% of 176 screws [29], 10% of 116 screws [26] and 30% of 94 screws [15]. The resulting clinical complications directly related to screw placement of 2,838 screws were 0.59% with eight radiculopathies and three bleedings from the vertebral artery [2, 11, 15, 18, 26, 29, 47, 48]. However, critical breaches detected by postoperative CT occur more frequently. Yukagawa et al reported critical violations of the pedicle in 3.9% (24 out of 620 screws), Kast et al. in 8.5% (8 out of 94 screws), Rath et al. in 4.3% (5 out of 116 screws), Yoshimoto et al. in 1.9% (5 out of 280 screws) and Neo et al. in 15.1% (13 out of 86 screws). In this context, the found rate of critical breaches in our study (12.5%) as well as the low complication rate due to screw placement fit into the clinical studies published previously. Lateral breaches are observed more frequently in the present study corroborating previous reports [26, 47, 48] due to the thinner cortical wall laterally of the cervical pedicles [14, 31]. Since the vertebral artery occupies between 8 and 75% of the transversal foramen [32], vertebral artery obstruction or injury rarely occurs [2, 23, 26]. However, taking into account that in our study lateral perforations with critical encroachment of the transverse foramen occurred in 9.7%, we changed our clinical protocol and started to perform an MRI- or CT-angiography regularly prior to CPS placement to exclude vertebral artery hypoplasia.
Subsequent to the published results, the opinion concerning the use of CPS varies. Some authors recommend the use of CPS only in cases where no alternative is possible [6], several authors advise the use of navigation [23, 26] and others stated that the use is relatively safe using a free-handed technique and the use of fluoroscopy if the surgeon is experienced and is familiar with the special anatomy of the cervical spine [29, 47].
One major problem of percutaneous placement of pedicle screws is the absence of anatomical landmarks, which reduces the information available to the surgeon. Without exposure of the spine, CT-navigation procedures could not be performed because of the necessity for surface matching [34]. In this context, three-dimensional fluoroscopy might represent a useful tool for transpedicular cervical stabilization to verify correct screw placement intraoperatively as suggested by Ito et al. [11]. In patients where fluoroscopic image quality is poor because of multiple dental implants, high-grade osteoporosis without definable pedicle walls, or regions like the cervicothoracic junction, the intraoperative three-dimensional fluoroscopy could prevent screw malpositions in our clinical setting (Fig. 2). However, in our experience, the image quality is sometimes not sufficient in patients with prior anterior surgery as plating or vertebral body replacement. Due to metal artifacts especially lateral pedicle violations could be not apparent (Fig. 4). From this experience, a sequential surgical strategy in combined therapeutic approaches containing the posterior stabilization prior to anterior procedures is advisable. This should improve image quality and prevent iatrogenic injury to neurovascular structures due to screw malposition. In patients where this strategy is not applicable, we recommend a conventional open approach which allows CT-navigation.
To further improve cervical pedicle screw accuracy in percutaneous stabilization of the cervical and cervicothoracic spine, the use of three-dimensional fluoroscopic navigation should be topic of further studies. Ito et al. [11] demonstrated the usefulness of this technique in CPS placement using a traditional open approach. Holly et al. [8] were able to demonstrate the benefit of the technique in minimally invasive placement of lateral mass screws in vitro. In this context, in vitro studies with the use of three-dimensional fluoroscopy might optimize the percutaneous technique and further reduce misplacement rates of CPS.
One major advantage of the described technique might be the reduction in muscular trauma subsequent to the surgical approach. As proposed already in lumbar spine surgery, this might reduce the denervation of muscles and hospital stay [10, 16, 21, 22, 38, 44] and reduces the increased complication rate following posterior or combined anterior–posterior approaches [37]. The disadvantage of the technique clearly resulted from stabilization-only character, which limits the indications. Since neither extensively decompression nor fusion is possible, the technique remains reserved to a narrow spectrum of patients with high-grade instabilities, which requires anterior procedures combined with posterior instrumentation or neoplastic instabilities without the necessity for fusion or decompression of neurovascular structures. Although no complication occurred from CPS placement in the present study, the high rate of misplaced screws including 12.5% critical screw placements should be kept in mind and the selection of the patients should be based on careful consideration of the potential risks.
The authors are aware of several limitations of this study. The number of cases treated with percutaneous CPS placement is small and the results are preliminary. Several clinical parameters as long-term outcome, pain, and blood loss, as well as operation time and complication rate, remained to be proven superior to the open technique. Therefore, a large prospective study is required to confirm the acquired data and compare the open versus percutaneous surgery.
In our experience, the percutaneous CPS placement is a surgically demanding technique even in the hands of experienced spine surgeons. Nevertheless, it could potentially minimize access-related morbidity in a carefully selected patient population.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Online Resource Fig 1 A,
The patients were placed in a prone position
,
the fluoroscope was checked for collision and whether the necessary fluoroscopic views are of sufficient quality.
B
, The skin entry point was determined by positioning a spinal needle and the correct trajectories were controlled by fluoroscopy pedicle axis (
C
) and lateral view (
D
).
Online Resource Fig 2
Surgical approach with skin incision of 2-3 cm (
A
) and subsequent muscle split in fiber course until the lateral mass was identified (
B
).
Online Resource Fig 3
After the surgical approach a trokar with blunt tip was positioned and a bone awl was inserted (
A
). The bone awl was positioned below the lower margin of the upper articular surface and lateral to the midline of the lateral mass. The correct position was controlled by lateral (
B
) and true a.p. fluoroscopic view (
C
) as well as oblique views perpendicular to the pedicle axis (not shown). The inclination of the bone awl from the mid-sagittal plane was chosen according to preoperative CT scan and controlled by fluoroscopy using the pedicle axis view (
D
). The pedicle axis view was achieved by rotation the fluoroscope until the pedicle appears approximately circular. The awl should be positioned in line between the medial and lateral boundaries of the pedicles and the cortical wall was opened.
Online Resource Fig 4
Process of automatic drilling with corresponding fluoroscopic views. Automatic drilling was performed after securing the entry point with the trokar (
A
). During drilling process, the correct trajectories of the drill were controlled by fluoroscopy using lateral, a.p. and pedicle axis views. If the drill reaches the vertebral body in oblique or lateral view (
B
), it should not cross the medial or lateral boundaries of the pedicle in a.p. view (
C
) and pedicle axis view (
D
). The correct inclination to the mid-sagittal plane was determined again using the preoperative CT scan and the pedicle axis view (
D
). Position of the drill at the end of the drilling process in fluoroscopic views in lateral (
E
), a.p. (
F
) and pedicle axis (
G
) direction.
Online Resource Fig 5
After drilling a 3.5 mm screw was inserted into the vertebra. During the insertion of the screw the position was checked by lateral (
A
+
D
), a.p. (
B
+
E
) and pedicle axis (
C
) fluoroscopy. Screw position can be seen on intraoperative image (
F
).
Online Resource Fig 6
After screw positioning the rod was inserted through the most caudal incision close to the bone and beyond the fascia and muscle (
A
) and fixed with blocker nuts (
B
). Intraoperative image (
C
) and intraoperative fluoroscopic views in a.p. (
D
) and oblique direction (
E
, perpendicular to the pedicle axis) demonstrating the fixed screw rod construct.
Acknowledgments
Conflict of interest N. Hansen Algenstaedt worked as a consultant of Stryker spine.
References
- 1.Abumi K, Kaneda K. Pedicle screw fixation for nontraumatic lesions of the cervical spine. Spine. 1997;22:1853–1863. doi: 10.1097/00007632-199708150-00010. [DOI] [PubMed] [Google Scholar]
- 2.Abumi K, Shono Y, Ito M, Taneichi H, Kotani Y, Kaneda K. Complications of pedicle screw fixation in reconstructive surgery of the cervical spine. Spine. 2000;25:962–969. doi: 10.1097/00007632-200004150-00011. [DOI] [PubMed] [Google Scholar]
- 3.Bartels E. Dissection of the extracranial vertebral artery: clinical findings and early noninvasive diagnosis in 24 patients. J Neuroimaging. 2006;16:24–33. doi: 10.1177/1051228405280646. [DOI] [PubMed] [Google Scholar]
- 4.Bartels E, Flugel KA. Evaluation of extracranial vertebral artery dissection with duplex color-flow imaging. Stroke. 1996;27:290–295. doi: 10.1161/01.str.27.2.290. [DOI] [PubMed] [Google Scholar]
- 5.Fong S, Duplessis S. Minimally invasive lateral mass plating in the treatment of posterior cervical trauma: surgical technique. J Spinal Disord Tech. 2005;18:224–228. doi: 10.1097/01.bsd.0000169062.77005.78. [DOI] [PubMed] [Google Scholar]
- 6.Hasegawa K, Hirano T, Shimoda H, Homma T, Morita O. Indications for cervical pedicle screw instrumentation in nontraumatic lesions. Spine (Phila Pa 1976) 2008;33:2284–2289. doi: 10.1097/BRS.0b013e31818043ce. [DOI] [PubMed] [Google Scholar]
- 7.Heller JG, Silcox DH, 3rd, Sutterlin CE., 3rd Complications of posterior cervical plating. Spine. 1995;20:2442–2448. doi: 10.1097/00007632-199511001-00013. [DOI] [PubMed] [Google Scholar]
- 8.Holly LT, Foley KT (2006) Percutaneous placement of posterior cervical screws using three-dimensional fluoroscopy. Spine 31:536–540; discussion 541 [DOI] [PubMed]
- 9.Hott JS, Papadopoulos SM, Theodore N, Dickman CA, Sonntag VK. Intraoperative Iso-C C-arm navigation in cervical spinal surgery: review of the first 52 cases. Spine. 2004;29:2856–2860. doi: 10.1097/01.brs.0000147742.20637.49. [DOI] [PubMed] [Google Scholar]
- 10.Isaacs RE, Podichetty VK, Santiago P, Sandhu FA, Spears J, Kelly K, Rice L, Fessler RG. Minimally invasive microendoscopy-assisted transforaminal lumbar interbody fusion with instrumentation. J Neurosurg. 2005;3:98–105. doi: 10.3171/spi.2005.3.2.0098. [DOI] [PubMed] [Google Scholar]
- 11.Ito Y, Sugimoto Y, Tomioka M, Hasegawa Y, Nakago K, Yagata Y. Clinical accuracy of 3D fluoroscopy-assisted cervical pedicle screw insertion. J Neurosurg. 2008;9:450–453. doi: 10.3171/SPI.2008.9.11.450. [DOI] [PubMed] [Google Scholar]
- 12.Jeanneret B, Gebhard JS, Magerl F. Transpedicular screw fixation of articular mass fracture-separation: results of an anatomical study and operative technique. J Spinal Disord. 1994;7:222–229. doi: 10.1097/00002517-199407030-00004. [DOI] [PubMed] [Google Scholar]
- 13.Jones EL, Heller JG, Silcox DH, Hutton WC. Cervical pedicle screws versus lateral mass screws. Anatomic feasibility and biomechanical comparison. Spine. 1997;22:977–982. doi: 10.1097/00007632-199705010-00009. [DOI] [PubMed] [Google Scholar]
- 14.Karaikovic EE, Daubs MD, Madsen RW, Gaines RW., Jr Morphologic characteristics of human cervical pedicles. Spine (Phila Pa 1976) 1997;22:493–500. doi: 10.1097/00007632-199703010-00005. [DOI] [PubMed] [Google Scholar]
- 15.Kast E, Mohr K, Richter HP, Borm W. Complications of transpedicular screw fixation in the cervical spine. Eur Spine J. 2006;15:327–334. doi: 10.1007/s00586-004-0861-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim DY, Lee SH, Chung SK, Lee HY. Comparison of multifidus muscle atrophy and trunk extension muscle strength: percutaneous versus open pedicle screw fixation. Spine. 2005;30:123–129. doi: 10.1097/01.brs.0000157172.00635.3a. [DOI] [PubMed] [Google Scholar]
- 17.Kim HS, Heller JG, Hudgins PA, Fountain JA. The accuracy of computed tomography in assessing cervical pedicle screw placement. Spine. 2003;28:2441–2446. doi: 10.1097/01.BRS.0000090830.94641.AE. [DOI] [PubMed] [Google Scholar]
- 18.Kotani Y, Abumi K, Ito M, Minami A. Improved accuracy of computer-assisted cervical pedicle screw insertion. J Neurosurg. 2003;99:257–263. doi: 10.3171/spi.2003.99.3.0257. [DOI] [PubMed] [Google Scholar]
- 19.Kotani Y, Cunningham BW, Abumi K, McAfee PC. Biomechanical analysis of cervical stabilization systems. An assessment of transpedicular screw fixation in the cervical spine. Spine. 1994;19:2529–2539. doi: 10.1097/00007632-199411001-00007. [DOI] [PubMed] [Google Scholar]
- 20.Kothe R, Ruther W, Schneider E, Linke B. Biomechanical analysis of transpedicular screw fixation in the subaxial cervical spine. Spine (Phila Pa 1976) 2004;29:1869–1875. doi: 10.1097/01.brs.0000137287.67388.0b. [DOI] [PubMed] [Google Scholar]
- 21.Lee SH, Choi WG, Lim SR, Kang HY, Shin SW. Minimally invasive anterior lumbar interbody fusion followed by percutaneous pedicle screw fixation for isthmic spondylolisthesis. Spine J. 2004;4:644–649. doi: 10.1016/j.spinee.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 22.Lowery GL, Kulkarni SS (2000) Posterior percutaneous spine instrumentation. Eur Spine J 9 Suppl 1:S126–130 [DOI] [PMC free article] [PubMed]
- 23.Neo M, Sakamoto T, Fujibayashi S, Nakamura T. The clinical risk of vertebral artery injury from cervical pedicle screws inserted in degenerative vertebrae. Spine (Phila Pa 1976) 2005;30:2800–2805. doi: 10.1097/01.brs.0000192297.07709.5d. [DOI] [PubMed] [Google Scholar]
- 24.Oda I, Abumi K, Ito M, Kotani Y, Oya T, Hasegawa K, Minami A. Palliative spinal reconstruction using cervical pedicle screws for metastatic lesions of the spine: a retrospective analysis of 32 cases. Spine (Phila Pa 1976) 2006;31:1439–1444. doi: 10.1097/01.brs.0000219952.40906.1f. [DOI] [PubMed] [Google Scholar]
- 25.Pateder DB, Carbone JJ. Lateral mass screw fixation for cervical spine trauma: associated complications and efficacy in maintaining alignment. Spine J. 2006;6:40–43. doi: 10.1016/j.spinee.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 26.Rath SA, Moszko S, Schaffner PM, Cantone G, Braun V, Richter HP, Antoniadis G. Accuracy of pedicle screw insertion in the cervical spine for internal fixation using frameless stereotactic guidance. J Neurosurg. 2008;8:237–245. doi: 10.3171/SPI/2008/8/3/237. [DOI] [PubMed] [Google Scholar]
- 27.Reinhold M, Bach C, Audige L, Bale R, Attal R, Blauth M, Magerl F. Comparison of two novel fluoroscopy-based stereotactic methods for cervical pedicle screw placement and review of the literature. Eur Spine J. 2008;17:564–575. doi: 10.1007/s00586-008-0584-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reinhold M, Magerl F, Rieger M, Blauth M. Cervical pedicle screw placement: feasibility and accuracy of two new insertion techniques based on morphometric data. Eur Spine J. 2007;16:47–56. doi: 10.1007/s00586-006-0104-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Richter M, Cakir B, Schmidt R. Cervical pedicle screws: conventional versus computer-assisted placement of cannulated screws. Spine. 2005;30:2280–2287. doi: 10.1097/01.brs.0000182275.31425.cd. [DOI] [PubMed] [Google Scholar]
- 30.Richter M, Wilke HJ, Kluger P, Neller S, Claes L, Puhl W. Biomechanical evaluation of a new modular rod-screw implant system for posterior instrumentation of the occipito-cervical spine: in vitro comparison with two established implant systems. Eur Spine J. 2000;9:417–425. doi: 10.1007/s005860000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sakamoto T, Neo M, Nakamura T (2004) Transpedicular screw placement evaluated by axial computed tomography of the cervical pedicle. Spine (Phila Pa 1976) 29:2510–2514; discussion 2515 [DOI] [PubMed]
- 32.Sanelli PC, Tong S, Gonzalez RG, Eskey CJ. Normal variation of vertebral artery on CT angiography and its implications for diagnosis of acquired pathology. J Comput Assist Tomogr. 2002;26:462–470. doi: 10.1097/00004728-200205000-00027. [DOI] [PubMed] [Google Scholar]
- 33.Scheufler KM, Kirsch E. Percutaneous multilevel decompressive laminectomy, foraminotomy, and instrumented fusion for cervical spondylotic radiculopathy and myelopathy: assessment of feasibility and surgical technique. J Neurosurg. 2007;7:514–520. doi: 10.3171/SPI-07/11/514. [DOI] [PubMed] [Google Scholar]
- 34.Schlenzka D, Laine T, Lund T (2000) Computer-assisted spine surgery. Eur Spine J 9(Suppl 1):S57–S64 [DOI] [PMC free article] [PubMed]
- 35.Schmidt R, Wilke HJ, Claes L, Puhl W, Richter M. Pedicle screws enhance primary stability in multilevel cervical corpectomies: biomechanical in vitro comparison of different implants including constrained and nonconstrained posterior instrumentations. Spine. 2003;28:1821–1828. doi: 10.1097/01.BRS.0000083287.23521.48. [DOI] [PubMed] [Google Scholar]
- 36.Sekhon LH. Posterior cervical lateral mass screw fixation: analysis of 1026 consecutive screws in 143 patients. J Spinal Disord Tech. 2005;18:297–303. doi: 10.1097/01.bsd.0000166640.23448.09. [DOI] [PubMed] [Google Scholar]
- 37.Shamji MF, Cook C, Pietrobon R, Tackett S, Brown C, Isaacs RE. Impact of surgical approach on complications and resource utilization of cervical spine fusion: a nationwide perspective to the surgical treatment of diffuse cervical spondylosis. Spine J. 2009;9:31–38. doi: 10.1016/j.spinee.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 38.Shunwu F, Xing Z, Fengdong Z, Xiangqian F. Minimally invasive transforaminal lumbar interbody fusion for the treatment of degenerative lumbar diseases. Spine (Phila Pa 1976) 2010;35:1615–1620. doi: 10.1097/BRS.0b013e3181c70fe3. [DOI] [PubMed] [Google Scholar]
- 39.Su P, Ma R, Li C, Liu S, Huang D. Pedicle screw fixation of the cervical spine: guidance by computed tomography. Clin Orthop Relat Res. 2007;462:99–104. doi: 10.1097/BLO.0b013e3180ebe4e5. [DOI] [PubMed] [Google Scholar]
- 40.Thongtrangan I, Le H, Park J, Kim DH. Minimally invasive spinal surgery: a historical perspective. Neurosurg Focus. 2004;16:E13. doi: 10.3171/foc.2004.16.1.14. [DOI] [PubMed] [Google Scholar]
- 41.Ugur HC, Attar A, Uz A, Tekdemir I, Egemen N, Caglar S, Genc Y (2000) Surgical anatomic evaluation of the cervical pedicle and adjacent neural structures. Neurosurgery 47:1162–1168; discussion 1168–1169 [DOI] [PubMed]
- 42.Ulrich C, Arand M, Nothwang J. Internal fixation on the lower cervical spine–biomechanics and clinical practice of procedures and implants. Eur Spine J. 2001;10:88–100. doi: 10.1007/s005860000233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang MC, Chan L, Maiman DJ, Kreuter W, Deyo RA. Complications and mortality associated with cervical spine surgery for degenerative disease in the United States. Spine (Phila Pa 1976) 2007;32:342–347. doi: 10.1097/01.brs.0000254120.25411.ae. [DOI] [PubMed] [Google Scholar]
- 44.Wang MY, Cummock MD, Yu Y, Trivedi RA. An analysis of the differences in the acute hospitalization charges following minimally invasive versus open posterior lumbar interbody fusion. J Neurosurg. 2010;12:694–699. doi: 10.3171/2009.12.SPINE09621. [DOI] [PubMed] [Google Scholar]
- 45.Wang MY, Levi AD (2006) Minimally invasive lateral mass screw fixation in the cervical spine: initial clinical experience with long-term follow-up. Neurosurgery 58:907–912; discussion 907–912 [DOI] [PubMed]
- 46.Wu JC, Huang WC, Chen YC, Shih YH, Cheng H (2008) Stabilization of subaxial cervical spines by lateral mass screw fixation with modified Magerl’s technique. Surg neurol 70 (Suppl 1):25–33; discussion S21:33 [DOI] [PubMed]
- 47.Yoshimoto H, Sato S, Hyakumachi T, Yanagibashi Y, Kanno T, Masuda T. Clinical accuracy of cervical pedicle screw insertion using lateral fluoroscopy: a radiographic analysis of the learning curve. Eur Spine J. 2009;18:1326–1334. doi: 10.1007/s00586-009-1109-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yukawa Y, Kato F, Ito K, Horie Y, Hida T, Nakashima H, Machino M. Placement and complications of cervical pedicle screws in 144 cervical trauma patients using pedicle axis view techniques by fluoroscope. Eur Spine J. 2009;18:1293–1299. doi: 10.1007/s00586-009-1032-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Below is the link to the electronic supplementary material.
Online Resource Fig 1 A,
The patients were placed in a prone position
,
the fluoroscope was checked for collision and whether the necessary fluoroscopic views are of sufficient quality.
B
, The skin entry point was determined by positioning a spinal needle and the correct trajectories were controlled by fluoroscopy pedicle axis (
C
) and lateral view (
D
).
Online Resource Fig 2
Surgical approach with skin incision of 2-3 cm (
A
) and subsequent muscle split in fiber course until the lateral mass was identified (
B
).
Online Resource Fig 3
After the surgical approach a trokar with blunt tip was positioned and a bone awl was inserted (
A
). The bone awl was positioned below the lower margin of the upper articular surface and lateral to the midline of the lateral mass. The correct position was controlled by lateral (
B
) and true a.p. fluoroscopic view (
C
) as well as oblique views perpendicular to the pedicle axis (not shown). The inclination of the bone awl from the mid-sagittal plane was chosen according to preoperative CT scan and controlled by fluoroscopy using the pedicle axis view (
D
). The pedicle axis view was achieved by rotation the fluoroscope until the pedicle appears approximately circular. The awl should be positioned in line between the medial and lateral boundaries of the pedicles and the cortical wall was opened.
Online Resource Fig 4
Process of automatic drilling with corresponding fluoroscopic views. Automatic drilling was performed after securing the entry point with the trokar (
A
). During drilling process, the correct trajectories of the drill were controlled by fluoroscopy using lateral, a.p. and pedicle axis views. If the drill reaches the vertebral body in oblique or lateral view (
B
), it should not cross the medial or lateral boundaries of the pedicle in a.p. view (
C
) and pedicle axis view (
D
). The correct inclination to the mid-sagittal plane was determined again using the preoperative CT scan and the pedicle axis view (
D
). Position of the drill at the end of the drilling process in fluoroscopic views in lateral (
E
), a.p. (
F
) and pedicle axis (
G
) direction.
Online Resource Fig 5
After drilling a 3.5 mm screw was inserted into the vertebra. During the insertion of the screw the position was checked by lateral (
A
+
D
), a.p. (
B
+
E
) and pedicle axis (
C
) fluoroscopy. Screw position can be seen on intraoperative image (
F
).
Online Resource Fig 6
After screw positioning the rod was inserted through the most caudal incision close to the bone and beyond the fascia and muscle (
A
) and fixed with blocker nuts (
B
). Intraoperative image (
C
) and intraoperative fluoroscopic views in a.p. (
D
) and oblique direction (
E
, perpendicular to the pedicle axis) demonstrating the fixed screw rod construct.