Abstract
The Aspergillus aculeatus rhaA gene encoding an α-l-rhamnosidase has been expressed in both laboratory and industrial wine yeast strains. Wines produced in microvinifications, conducted using a combination of the genetically modified industrial strain expressing rhaA and another strain expressing a β-glucosidase, show increased content mainly of the aromatic compound linalool.
Monoterpenes such as geraniol, linalool, and α-terpineol present in grapes determine the varietal flavor properties of young quality wines made from Muscat varieties (for reviews, see references 19 and 21). Geraniol and linalool are considered to be the most important of the monoterpene alcohols, as they are present in greater concentrations and have lower flavor thresholds than other major wine monoterpenes. In particular, linalool is thought to be responsible for the grapelike aroma of wines produced from the Muscat variety. A large proportion of these compounds are found as odorless diglycoside conjugates, such as 6-O-α-l-arabinofuranosyl-β-d-glucopyranosides, 6-O-α-l-rhamnopyranosyl-β-d-glucopyranosides, and 6-O-β-d-apiofuranosyl-β-d-glucopyranosides. This fraction constitutes a potential pool of aroma precursors, and its enzymatic hydrolysis has been studied as a method to release free monoterpenes and enhance grape juice and wine flavor (10, 34, 35). It is now well established that this enzymatic hydrolysis occurs in two steps (11). During the first step, and depending on the conjugate, the glycosidic linkage is cleaved by either a β-d-apiosidase, an α-l-arabinofuranosidase, or an α-l-rhamnosidase, and the corresponding monoterpenyl-β-d-glucosides are released. In the second step, monoterpenes are liberated by the action of a β-d-glucosidase. Consequently, research interest has been focused on those glycosidases able to release flavor compounds from glycosides. Several glycosidases, such as α-l-arabinofuranosidases (14, 30, 31, 36), β-d-apiosidases (13), α-l-rhamnosidases (2, 3, 15, 17, 18, 20, 22, 32, 33), and β-d-glucosidases (1, 12, 20, 26, 30, 31), from different microbial sources have been characterized for their potential application in increasing the aroma of wine.
An alternative way to achieve increases in the content of volatile compounds is the inoculation of must with genetically modified wine yeasts expressing genes coding for these enzymes (25). Previous work in our laboratory has reported the use of several transgenic wine yeasts expressing a Candida molischiana β-d-glucosidase and an Aspergillus niger α-l-arabinofuranosidase as tools to increase the free monoterpene content in wine (27, 28). We have also expressed genes encoding a Trichoderma longibrachiatum endoglucanase (23) and different Aspergillus nidulans xylanases (4, 5) in industrial wine yeast strains, which resulted in increased levels of some volatile compounds.
Recently, we cloned two genes from Aspergillus aculeatus encoding the α-l-rhamnosidases RhaA and RhaB (18). In the present work, the expression of the gene encoding the A. aculeatus RhaA in a commercial wine yeast strain was investigated. For this purpose, a 2.2-kb BamHI fragment carrying the rhaA cDNA (18) (which includes a 19-amino-acid signal sequence) was ligated between the Saccharomyces cerevisiae glyceraldehyde-3-phosphate dehydrogenase gene promoter (i.e., GPDp) and the phosphoglycerokinase (i.e., PGKt) terminator and polyadenylation signals in plasmid pG-1 (29), thereby generating plasmid pR31. Plasmids pR31 and pG-1 (a control) were used independently to transform the laboratory yeast strain W303-1A (MATa ade2-1 his3-11,15 leu2-3,112 can1-100 trp1-1 ura3-1) using the LiAc-ss-DNA-polyethylene glycol protocol described by Gietz and Woods (7). Tryptophan prototrophic transformants were selected on synthetic dextrose minimal supplemented medium (0.67% yeast nitrogen base without amino acids, 20 g of glucose per liter, 20 mg of adenine per liter, 20 mg of uracil per liter, 20 mg of histidine per liter, and 30 mg of leucine per liter) and analyzed for α-l-rhamnosidase production by plating on synthetic dropout minimal-supplemented media containing 4-methylumbelliferyl α-l-rhamnopyranoside (MUR) as the substrate (16). The hydrolysis of MUR by the action of α-l-rhamnosidase resulted in the release of 4-methylumbelliferone, which can be visualized under UV illumination as fluorescent halos surrounding yeast colonies. Only yeast cells transformed with pR31 showed the corresponding activity. Eight randomly selected pR31 transformants and a control strain transformed with pG-1 were grown in liquid selective medium for 18 h, and α-l-rhamnosidase activity was determined in culture filtrates by using p-nitrophenyl-α-l-rhamnopyranoside(pNPR) as the substrate (18). As expected, activity was detected only in pR31 transformants. These data confirm that the A. aculeatus rhaA gene encodes an α-l-rhamnosidase and demonstrate that it can be efficiently expressed in S. cerevisiae. From those transformants exhibiting the highest levels of α-l-rhamnosidase activity, one of them, designated YR6, was isolated for further studies. This transformant produces 130 mU of α-l-rhamnosidase activity per ml (specific activity of 2.6 U/mg) after 72 h of growth in yeast extract-peptone-dextrose (YPD) medium (1% yeast extract, 2% bacteriological peptone, 2% glucose).
Following the protocol previously developed except for the use of 10 mM sodium citrate buffer (pH 3.8) (18), the A. aculeatus RhaA enzyme was purified to apparent homogeneity from transformant YR6 by using cation-exchange chromatography. The level of recovery was 12% of that of the original α-l-rhamnosidase activity. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of RhaA revealed a single protein band after N deglycosylation of an apparent molecular mass of 70 kDa, a finding which was in agreement with the molecular mass of RhaA purified from A. aculeatus (18). Optimum pH and stability as well as kinetic constants using pNPR as the substrate were similar to those described for wild-type RhaA (18). Certain factors that may affect enzyme activity under enological conditions were also tested (28). Ethanol at a concentration of 15% (vol/vol) reduced RhaA activity by 55%, whereas at 20% (vol/vol) ethanol, the enzyme retained 35% of its original activity. The presence of SO2 at concentrations of up to 150 mg/liter, a level that can be found at different stages during wine fermentation, did not significantly affect enzyme activity. Glucose concentration did however affect RhaA activity, and inhibition levels of 59, 84, and 93% were observed for glucose concentrations of 0.15, 0.50, and 1 M, respectively. Finally, to test the ability of RhaA to hydrolyze Muscat must glycosides, the release of l-rhamnose was analyzed by high-performance liquid chromatography using a Waters Sugar-Pak I column (dimensions, 6.5 by 300 mm) at 80°C and a refraction index detector. From our data, it is possible to conclude that the heterologously expressed RhaA at a final concentration of 48 μg/ml in the incubation mixture is able to release 46 nmol of l-rhamnose after 24 h of incubation at 40°C. These experiments indicate that the A. aculeatus enzyme has enologically relevant properties.
In order to express the A. aculeatus rhaA gene in a commercial wine yeast strain, we isolated the GPDp::rhaA::PGKt expression cassette from plasmid pR31 as a 4.2-kb HindIII fragment and ligated it into the HindIII site of plasmid YEpCR21 (23), yielding plasmids pR37 and pR38 (corresponding to each of the two possible orientations with respect to the vector). The industrial wine yeast strain T73 (24) was transformed with plasmids YEpCR21 (control), pR37, and pR38, and positive colonies were tested for α-l-rhamnosidase activity on YPD plates supplemented with 1 μg of cycloheximide per ml and containing MUR as the substrate. No activity was detected in T73 or in a T73 strain (YR13) transformed with the vector YEpCR21. However, transformants generated with pR37 and pR38 gave similar levels of α-l-rhamnosidase activity, demonstrating that all the signals necessary for expression (i.e., promoter and terminator) are present on the 4.2-kb HindIII fragment and are independent of orientation within the vector. From the latter, transformant YR8 was selected and used for microvinification experiments. This transformant secretes 600 mU of α-l-rhamnosidase per ml after 72 h of growth in YPD medium, a sixfold increase in production with respect to strain YR6. Following previously described protocols (9), we detected no differences in RhaA cellular location between the strains YR6 and YR8 (30% intracellular, 10% cell wall bound, and 60% extracellular).
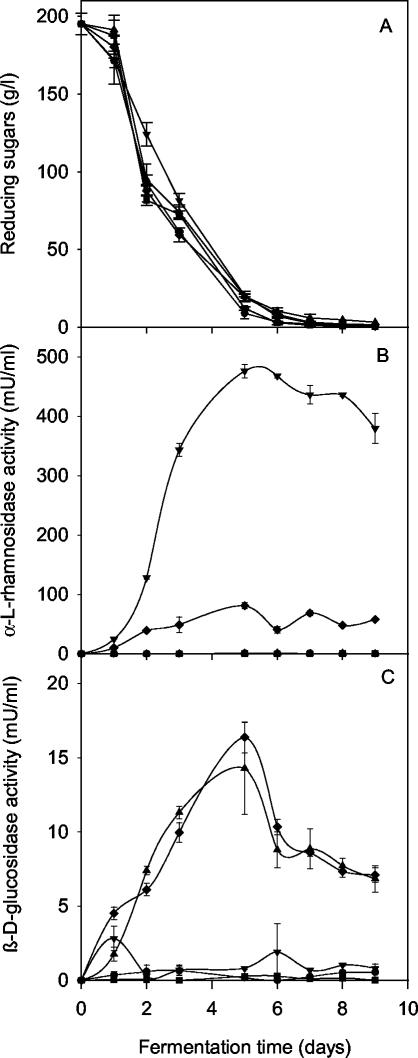
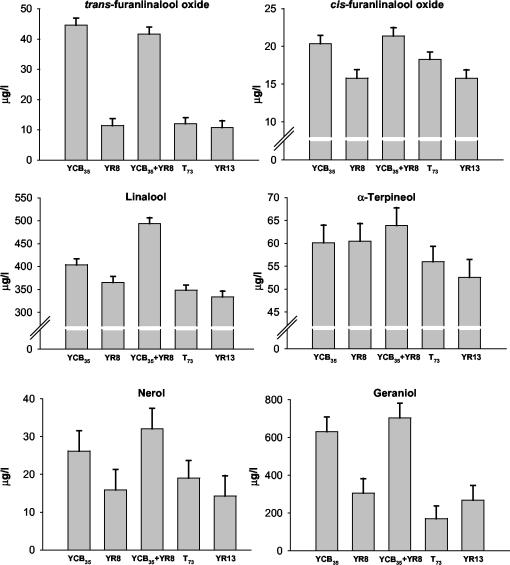
Due to the fact that the enzymatic hydrolysis of grape rhamnoglucosides occurs in two steps and that the actions of an α-l-rhamnosidase and a β-d-glucosidase are needed, microvinification experiments were carried out both singly and with a combination of transformant YR8 expressing the A. aculeatus rhaA gene and a T73 strain (YCB35) expressing the C. molischiana gene bgln, which codes for a β-d-glucosidase (28). Controls were done with the untransformed T73 strain and the transformant YR13. Microvinifications were performed in triplicate and carried out at 18°C in 375-ml glass bottles containing 300 ml of Muscat grape must inoculated with 105 cells/ml. The progress of the fermentation was similar in all the microvinifications (Fig. 1A). After 9 days of fermentation, the final wine products contained approximately the same ethanol concentration: 11.8% (vol/vol) in wines produced by T73 and 12.0% (vol/vol) in those produced by the YR13 transformant and the combination of the transformants YR8 and YCB35. Figure 1B and C show that α-l-rhamnosidase activity is only found in the presence of transformant YR8 and that although β-d-glucosidase activity was mainly detected in the presence of YCB35 strain, as previously reported (28), very slight levels of activity could be detected in all fermentations. The highest levels of both enzyme activities occurred during the first 5 days of fermentation. High levels of α-l-rhamnosidase activity (470 mU/ml) were reached in the fermentation carried out by strain YR8, whereas in the presence of the YCB35 transformant, around 90 mU of α-l-rhamnosidase activity per ml was found. β-d-Glucosidase activity reached levels of approximately 15 mU/ml in the YCB35 fermentation and also in the presence of the YR8 transformant. Further studies will be done to find out if the differences in α-l-rhamnosidase activity between single and double fermentations are a consequence of differences in growth or enzyme production rates. Regarding possible effects on aroma compounds, volatiles were extracted by using C18 cartridges and analyzed by gas chromatography-mass spectrometry (8) (Fig. 2). Fermentations carried out by YCB35 alone showed marked increases in trans-furanlinalool oxide and geraniol contents. These data suggest the possible existence of monoglucosides of these terpenes or the capability of the C. molischiana β-d-glucosidase to hydrolyze diglycosides with different specificities, depending on the aglycon. Similar results have been reported for purified C. molischiana β-d-glucosidase added directly to Muscat wine (6). Although a slight increase in linalool content was also detected, greater levels of linalool were observed for wines cofermented by YCB35 and the novel genetically modified yeast strain YR8 producing the A. aculeatus α-l-rhamnosidase A. Moreover, significant increases in α-terpineol and nerol were detected only in wines produced by both yeast strains in combination compared to individual T73 and YR13 fermentations. These data indicate the biotechnological feasibility of the combination of wine yeast strains producing α-l-rhamnosidase and β-d-glucosidase to increase free monoterpenol content. To our knowledge, this is the first report on the use of a genetically modified wine yeast strain producing an α-l-rhamnosidase of enological relevance. Problems related to the social acceptance of genetically modified foods, mainly in the European Union, make the use of such products an alternative for the future but not for the present.
FIG. 1.
Analysis of microvinifications carried out with the commercial wine yeast strain T73 (•), the YR13 control strain transformed with the vector YEpCR21 (▪), the YCB35 transformant strain expressing the C. molischiana β-d-glucosidase (▴), the YR8 transformant strain expressing the A. aculeatus α-l-rhamnosidase (▾), and a combination of the YCB35 and the YR8 strains (♦). Shown are the kinetics of reducing sugar consumption (A) and α-l-rhamnosidase (B) and β-d-glucosidase (C) activities, respectively, during the course of fermentation.
FIG. 2.
Concentrations expressed as mean values with Tukey's honestly significant difference intervals at 95% confidence level of various terpenols in the final wines produced in the microvinifications (aroma threshold values in micrograms per liter: trans-furanlinalool oxide and cis-furanlinalool oxide, >6,000; linalool, 100; α-terpineol and nerol, 400 to 500; geraniol, 130) (19).
Acknowledgments
P.M. and M.O. contributed equally to this report.
We thank E. Ibáñez and J. A. Tamayo for their invaluable assistance and Andrew P. MacCabe for critical reading of the manuscript.
This work was supported by a Ramón Areces grant and also by the CICYT AGL2002-01906 project (Spanish Ministry of Science and Technology-FEDER).
REFERENCES
- 1.Belancic, A., Z. Günata, M. J. Vallier, and E. Agosin. 2003. β-Glucosidase from the grape native yeast Debaryomyces vanrijiae: purification, characterization, and its effect on monoterpene content of a Muscat grape juice. J. Agric. Food Chem. 51:1453-1459. [DOI] [PubMed] [Google Scholar]
- 2.Gallego, M. V., F. Piñaga, D. Ramón, and S. Vallés. 1996. Production and characterization of an Aspergillus terreus α-l-rhamnosidase of oenological interest. Z. Lebensm.-Unters.-Forsch. 203:522-527. [Google Scholar]
- 3.Gallego, M. V., F. Piñaga, D. Ramón, and S. Vallés. 2001. Purification and characterization of an α-l-rhamnosidase from Aspergillus terreus of interest in winemaking. J. Food Sci. 66:1-6. [Google Scholar]
- 4.Ganga, M. A., A. Querol, S. Vallés, D. Ramón, A. P. MacCabe, and F. Piñaga. 1998. Heterologous production in Saccharomyces cerevisiae of different Aspergillus nidulans xylanases of potential interest in oenology. J. Sci. Food Agric. 78:315-320. [Google Scholar]
- 5.Ganga, M. A., F. Piñaga, S. Vallés, D. Ramón, and A. Querol. 1999. Aroma improving in microvinification processes by the use of a recombinant wine yeast expressing the Aspergillus nidulans xlnA gene. Int. J. Food Microbiol. 47:171-178. [DOI] [PubMed] [Google Scholar]
- 6.Genovés, S., J. V. Gil, P. Manzanares, J. L. Aleixandre, and S. Vallés. 2003. Candida molischiana β-glucosidase production by Saccharomyces cerevisiae and its application in winemaking. J. Food Sci. 68:2096-2100. [Google Scholar]
- 7.Gietz, R. D., and R. A. Woods. 1994. High efficiency transformation with lithium acetate, p. 121-134. In J. R. Johnson (ed.), Molecular genetics of yeasts. IRL Press, Oxford, United Kingdom.
- 8.Gil, J. V., and S. Vallés. 2001. Effect of maceration enzymes on red wine aroma at laboratory scale: exogenous addition or expression by transgenic wine yeast. J. Agric. Food Chem. 49:5515-5523. [DOI] [PubMed] [Google Scholar]
- 9.González-Candelas, L., A. Cortell, and D. Ramón. 1995. Construction of a recombinant wine yeast strain expressing a fungal pectate lyase gene. FEMS Microbiol. Lett. 126:263-270. [DOI] [PubMed] [Google Scholar]
- 10.Günata, Z. 1994. Etude de exploitation par voie enzymatique des précurseurs d'arômes du raisin de nature glycosidique. Rev. Oenol. Tech. Vitivinic. Oenol. 74:22-27. [Google Scholar]
- 11.Günata, Z., S. Bitteur, J.-M. Brillouet, C. Bayonove, and R. E. Cordonnier. 1988. Sequential enzymic hydrolysis of potentially aromatic glycosides from grape. Carbohydr. Res. 184:139-149. [Google Scholar]
- 12.Günata, Z., C. L. Bayonove, C. Tapiero, and R. E. Cordonnier. 1990. Hydrolysis of grape monoterpenyl β-d-glucosides by various β-glucosidases. J. Agric. Food Chem. 38:1232-1236. [Google Scholar]
- 13.Günata, Z., I. Dugelay, M. J. Vallier, J. C. Sapis, and C. Bayonove. 1997. Multiple forms of glycosidases in an enzyme preparation from Aspergillus niger: partial characterization of a β-apiosidase. Enzyme Microb. Technol. 21:39-44. [Google Scholar]
- 14.Le Clinche, F., F. Piñaga, D. Ramón, and S. Vallés. 1997. α-l-Arabinofuranosidases from Aspergillus terreus with potential application in enology: induction, purification and characterization. J. Agric. Food Chem. 45:2379-2383. [Google Scholar]
- 15.Manzanares, P., L. H. de Graaff, and J. Visser. 1997. Purification and characterization of an α-l-rhamnosidase from Aspergillus niger. FEMS Microbiol. Lett. 157:279-283. [DOI] [PubMed] [Google Scholar]
- 16.Manzanares, P., D. Ramón, and A. Querol. 1999. Screening of non-Saccharomyces wine yeasts for the production of β-d-xylosidase activity. Int. J. Food Microbiol. 46:105-112. [DOI] [PubMed] [Google Scholar]
- 17.Manzanares, P., M. Orejas, E. Ibañez, S. Vallés, and D. Ramón. 2000. Purification and characterization of an α-l-rhamnosidase from Aspergillus nidulans. Lett. Appl. Microbiol. 31:198-202. [DOI] [PubMed] [Google Scholar]
- 18.Manzanares, P., H. C. van den Broeck, L. H. de Graaff, and J. Visser. 2001. Purification and characterization of two different α-l-rhamnosidases, RhaA and RhaB, from Aspergillus aculeatus. Appl. Environ. Microbiol. 67:2230-2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marais, J. 1983. Terpenes in the aroma of grapes and wines: a review. S. Afr. J. Enol. Vitic. 4:49-58. [Google Scholar]
- 20.Martino, A., C. Schiraldi, A. Lazzaro, I. Fiume, G. Spagna, P. G. Pifferi, and M. Rosa. 2000. Improvement of the flavor of Falanghina white wine using a purified glycosidase preparation from Aspergillus niger. Process Biochem. 36:93-102. [Google Scholar]
- 21.Mateo, J. J., and M. Jiménez. 2000. Monoterpenes in grape juice and wines. J. Chromatogr. A 881:557-567. [DOI] [PubMed] [Google Scholar]
- 22.Orejas, M., E. Ibáñez, and D. Ramón. 1999. The filamentous fungus Aspergillus nidulans produces an α-l-rhamnosidase of potential oenological interest. Lett. Appl. Microbiol. 28:383-388. [Google Scholar]
- 23.Pérez-González, J. A., R. González, A. Querol, J. M. Sendra, and D. Ramón. 1993. Construction of a recombinant yeast strain expressing β-(1,4)-endoglucanase and its use in microvinification processes. Appl. Environ. Microbiol. 59:2801-2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Querol, A., E. Barrio, T. Huerta, and D. Ramón. 1992. Strain for use as dry yeast in fermentation of Alicante wines: selection and DNA patterns. J. Food Sci. 57:183-185. [Google Scholar]
- 25.Querol, A., and D. Ramón. 1996. The application of molecular techniques in wine microbiology. Trends Food Sci. Technol. 7:73-78. [Google Scholar]
- 26.Rosi, I., M. Vinella, and P. Domizio. 1994. Characterization of β-glucosidase activity in yeasts of oenological origin. J. Appl. Bacteriol. 77:519-527. [DOI] [PubMed] [Google Scholar]
- 27.Sánchez-Torres, P., L. González-Candelas, and D. Ramón. 1996. Expression in a wine yeast strain of the Aspergillus niger abfB gene. FEMS Microbiol. Lett. 145:189-194. [DOI] [PubMed] [Google Scholar]
- 28.Sánchez-Torres, P., L. González-Candelas, and D. Ramón. 1998. Heterologous expression of a Candida molischiana anthocyanin-β-glucosidase in a wine yeast strain. J. Agric. Food Chem. 46:354-360. [DOI] [PubMed] [Google Scholar]
- 29.Schena, M., D. Picard, and K. R. Yamamoto. 1991. Vectors for constitutive and inducible gene expression in yeast. Methods Enzymol. 194:389-398. [DOI] [PubMed] [Google Scholar]
- 30.Spagna, G., F. Andreani, E. Salatelli, D. Romagnoli, D. Casarini, and P. G. Pifferi. 1998. Immobilization of the glycosidases: α-l-arabinofuranosidase and β-d-glucopyranosidase from Aspergillus niger on a chitosan derivative to increase the aroma of wine. Part II. Enzyme Microb. Technol. 23:413-421. [Google Scholar]
- 31.Spagna, G., D. Romagnoli, M. Angela, G. Bianchi, and P. G. Pifferi. 1998. A simple method for purifying glycosidases: α-l-arabinofuranosidase and β-d-glucopyranosidase from Aspergillus niger to increase the aroma of wine. Part I. Enzyme Microb. Technol. 22:298-304. [Google Scholar]
- 32.Spagna, G., R. N. Barbagallo, A. Martino, and P. G. Pifferi. 2000. A simple method for purifying glycosidases: α-l-rhamnopyranosidase from Aspergillus niger to increase the aroma of Moscato wine. Enzyme Microb. Technol. 27:522-530. [DOI] [PubMed] [Google Scholar]
- 33.Spagna, G., R. N. Barbagallo, D. Casarini, and P. G. Pifferi. 2001. A novel chitosan derivative to immobilize α-l-rhamnopyranoside from Aspergillus niger for application in beverage technologies. Enzyme Microb. Technol. 28:427-438. [DOI] [PubMed] [Google Scholar]
- 34.Williams, P. J., C. R. Strauss, and B. Wilson. 1981. Classification of the monoterpenoid composition of Muscat grapes. Am. J. Enol. Vitic. 32:230-235. [Google Scholar]
- 35.Williams, P. J., C. R. Strauss, B. Wilson, and R. A. Massy-Westropp. 1982. Novel monoterpene disaccharide glycosides of Vitis vinifera grapes and wines. Phytochemistry 21:2013-2020. [Google Scholar]
- 36.Yanai, T., and S. Sato. 2000. Purification and characterization of a novel α-l-arabinofuranosidase from Pichia capsulata X91. Biosci. Biotechnol. Biochem. 64:1181-1188. [DOI] [PubMed] [Google Scholar]