Abstract
In pathologic radicular pain of lumbar spinal stenosis, cytokines such as tumor necrosis factor-alpha (TNF-α) and interleukins (ILs) play a crucial role in the pathogenesis of nerve degeneration and pain. We investigated TNF-α and IL-6 levels in the cerebrospinal fluid (CSF) of patients with radicular pain caused by lumbar spinal stenosis (LSS). A total of 30 LSS patients and 10 age-matched controls were examined. CSF samples were obtained adjacent to the level of stenosis in 30 LSS patients, and at the L4–L5 level in the 10 control patients. TNF-α and IL-6 levels in the samples were analyzed using enzyme-linked immunosorbent assays (ELISA). We compared the amounts of TNF-α and IL-6 with severity of pain (low back and leg pain), walking ability, and severity of stenosis (cross-sectional area of dural space). The concentration of IL-6 was significantly higher in LSS patients than in controls, but TNF-α levels were beneath the limit of detection. There was no correlation between IL-6 levels and severity of pain or walking ability (p > 0.05). However, there was a significant correlation between IL-6 levels and severity of stenosis (p < 0.05). The current study showed that the increased CSF IL-6 levels in LSS patients with radicular pain were not correlated with pain severity; although not proven in this study, the increase in CSF IL-6 concentration could indicate pathological nerve damage or degeneration of lumbar radiculopathy represented by the severity of stenosis.
Keywords: Proinflammatory cytokine, Cerebrospinal fluid, Pain, Radiculopathy, Spinal stenosis
Introduction
Radicular pain is a common symptom of lumbar disc herniation and spinal stenosis induced by mechanical compression and inflammation [8, 9]. Cytokines generated at the inflammatory site produce associated pain [6, 10, 11]. Compression of the spinal nerve roots by lumbar spinal stenosis (LSS) is a major clinical problem associated with intermittent claudication, numbness, and lack of normal sensitivity [5, 13]. It has been shown that compression of the spinal nerve roots may induce neurophysiologic dysfunction, degeneration, and reduced blood flow in nerve roots in both animal models and humans [5, 13].
Recently, cytokines such as interleukins 1, 6 (IL-1, IL-6), and tumor necrosis factor-alpha (TNF-α) have been strongly linked to radicular pain [4, 9, 13]. It has also been reported that IL-1, IL-6, and TNF-α are activated in the spinal cord, dorsal root ganglia, and Schwann cells in the spinal nerve roots following lumbar spinal stenosis, and that their expression is closely related to pain, and motor nerve dysfunction and degeneration [6, 13].
To our knowledge, there have been several clinical reports regarding the cytokines associated with compressed spinal nerves of patients with spinal degenerative disorders such as LSS [1, 7, 12]. However, the findings are controversial.
In the current study, we aimed to measure the levels of IL-6 and TNF-α in the CSF of patients with lumbar radiculopathy caused by LSS, and compared them with those in aged-matched controls. We further studied the correlations between the concentrations of these cytokines, and the severity of symptoms and the cross-sectional area of dural space at the compressed level.
Patients and methods
The ethics committee of our institution approved the protocol for the human procedures used in this study, and informed written consent was obtained from each subject.
Patients and aged-matched controls
Patients had low back and leg pain, continuing for at least 6 months (LSS group; mean age 63 ± 8 years, range 50–78 years; n = 30). Patients were diagnosed with lumbar spinal stenosis seen on magnetic resonance imaging (MRI). The patients showed one level of stenosis (L4–L5 level, 25 patients; L3–L4 level, 5 patients). If patients showed two or more levels of stenosis, they were excluded from the current study. Patients who had previously undergone spinal surgery were excluded. We also excluded spinal tumor, infection, and trauma. Ten patients who underwent lumbar spinal anesthesia for follow-up arthroscopic examination of their knee joint were included in the control group (mean age 61 ± 7 years; range 45–79 years). MRI at the lumbar level was performed in all patients in the control group, and no LSS was found in control group patients. The controls underwent arthroscopic surgery of the knee joint because of the 1-year old meniscus injuries. The controls did not have pain in the knee joint at the time of the follow-up examination, and none of the control group had any type of spinal disorder. There was no significant difference in age between the two groups (p > 0.05).
Pain score
To evaluate pain score (low back and leg pain), the visual analog scale (VAS) score (0, no pain; 10, worst pain) and the Japanese Orthopedic Association Score (JOAS: 0, worst pain; 3, no pain) were recorded. To evaluate waking distance without rest, JOAS evaluation was used (0: less than 100 m, 1: from 101 to 500 m, 2: can walk more than 500 m, but may feel leg pain during walking, 3: normal). In all patients, VAS scores were obtained 24 h before obtaining CSF samples.
Cross-sectional area of dural space at the stenosis level
We measured the cross-sectional area of the dural space at the level of stenosis and at a normal level (L1–L2) in 30 LSS patients using axial MRI sections. Dural space was defined as the area in the spinal canal of low intensity on T1-weighted images and of high intensity on T2-weighted images. The cross-sectional areas were measured with a computer-assisted imaging analysis system (NIH Image software, Springfield, VA). The percentage of dural space at the level of stenosis to normal level was calculated for each patient.
Enzyme-linked immunosorbent assay (ELISA) of cytokines
CSF samples were obtained from 30 LSS patients and 10 controls at the lumbar level. The CSF sample was obtained adjacent to the level of stenosis in 30 LSS patients and at the L2–L3 (L3–L4 stenosis) or L3–L4 (L4–L5 stenosis) level in the 10 control patients. The samples were stored immediately at 4°C. IL-6 and TNF-α levels were measured by a company providing comprehensive laboratory testing services (SRL, Tokyo, Japan), which was blinded to the groups of patients, using enzyme-linked immunosorbent assays (ELISAs) within 2 h. The detection limits were 0.16 pg/ml for IL-6 and 0.5 pg/ml for TNF-α.
We compared (1) the amounts of TNF-α and IL-6 between LSS patients and controls, and (2) the amounts of TNF-α and IL-6, and severity of pain (low back and leg pain), walking ability, and severity of stenosis (percentage of dural space at the level of stenosis to normal level and absolute value of the cross-sectional area at the level of stenosis) in the LSS patient group.
Statistical analysis
Data were compared using Mann–Whitney U, Kruskal–Wallis, and Pearson’s correlation coefficient tests. A p value of less than 0.05 was considered to be statistically significant. Data are expressed as mean ± standard deviation (SD).
Results
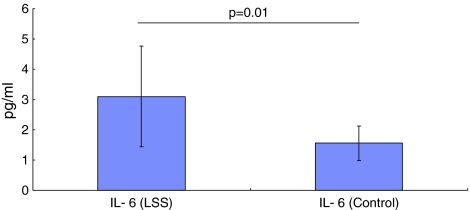
TNF-α was lower than the detection limit of 0.5 pg/ml in samples from the 30 LSS patients and 10 controls. The mean concentration of IL-6 in LSS patients was 3.1 ± 1.6 pg/ml (mean ± SD), which was significantly higher (p = 0.01) than that in controls (1.5 ± 0.5 pg/ml) (Fig. 1).
Fig. 1.
Mean IL-6 concentration in LSS and control patients. There were significantly higher levels in LSS patients compared with control patients (p = 0.01)
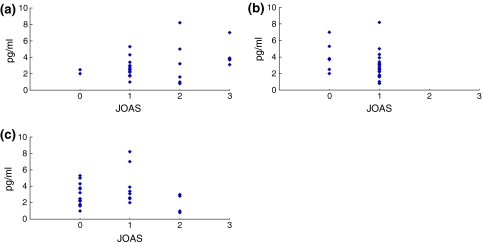
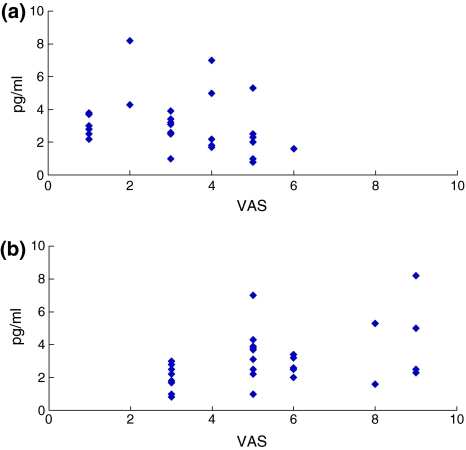
In the LSS patient group, the mean low back pain score was 1.5 ± 0.8 (JOAS) and 3.2 ± 1.5 (VAS), and the leg pain score was 0.8 ± 0.4 (JOAS) and 5.4 ± 2.0 (VAS). The walking score (JOAS) was 0.6 ± 0.7. In the LSS patient group, there was no significant correlation between the concentration of IL-6 and pain score (both for low back and leg pain: low back pain (JOAS) r2 = 0.51, p = 0.98; low back pain (VAS) r2 = 0.45, p = 0.87; leg pain (JOAS) r2 = 0.22, p = 0.18; leg pain (VAS) r2 = 0.41, p = 0.35). Furthermore, there was no significant correlation between the concentration of IL-6 and walking ability score (r2 = 0.28, p = 0.86) (Figs. 2, 3).
Fig. 2.
Correlation between IL-6 concentration and a low back pain (JOAS), b leg pain (JOAS), or c walking score (JOAS) in the LSS patient group. There was no significant correlation for any item
Fig. 3.
Correlation between IL-6 concentration and a low back pain (VAS) or b leg pain (VAS) in the LSS patient group. There was no significant correlation for any item
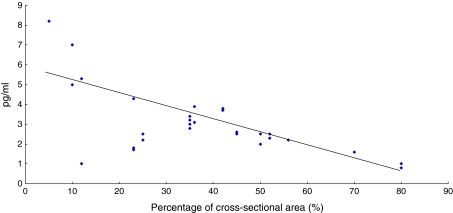
In the LSS patient group, the mean cross-sectional area of dural space at the compression site was 36 ± 19% of the normal dural space. There was a significant correlation between the concentration of IL-6 and severity of dural space restriction at the compression site (r2 = 0.64, p = 0.01) (Fig. 4). Furthermore, there was a significant correlation between the concentration of IL-6 and severity of absolute dural space stenosis at the compression site (r2 = 0.67, p = 0.01).
Fig. 4.
Correlation (r2 = 0.64, p = 0.01) between IL-6 concentration and reduction of cross-sectional area of dural space at the compression site
Discussion
In the current study, the concentration of IL-6 was significantly higher in LSS patients than in controls; however, TNF-α was lower than the detection limit in all samples obtained from both groups. There was no significant difference between the concentration of IL-6 and severity of pain or walking ability. On the other hand, there was significant difference between the concentration of IL-6 and the severity of stenosis of the dural space at the compression site. In the current study, we demonstrated a clear connection between these cytokines and nerve degeneration; however, there is a possibility that they are also linked to the pain experience.
Several authors have described a rat model of lumbar SCS in which cauda equina compression induces TNF-α expression in Schwann cells and macrophages, which is known to induce nerve degeneration as well as demyelination [5, 13]. IL-6 has been shown to be produced in Schwann cells following sciatic nerve injury, and IL-1 is also induced in resident Schwann cells in Wallerian degeneration of sciatic nerves [14]. In the current study, there was a significant difference between the concentration of IL-6 and the severity of the stenosis of the dural space. This finding indicates a relationship between the concentration of IL-6 and nerve damage or degeneration, which is consistent with the animal model.
There have been several clinical reports regarding the cytokines associated with the compressed nerves of patients with radicular pain caused by LSS. CSF and serum concentrations of IL-6, IL-8, and TNF-α were investigated using ELISAs in 39 patients with lumbar disc herniation and sciatica [1]. No significant increase in the concentrations of IL-6 or TNF-α was observed in almost all patients. Only the concentrations of IL-8 in CSF were increased in 12 of 39 patients; however, no relationship between CSF IL-8 concentration and pain intensity was found. On the other hand, the concentration of IL-6 and TNF-α in the CSF of patients with lumbar radiculopathy and its correlation with the severity of disease has been examined [7]. TNF-α levels were beneath the limit of detection, consistent with the data in the current study. The concentrations of IL-6 did not show any correlations with the symptom duration or pain score. Scuderi et al. studied many cytokines in the epidural space lavage obtained from patients with radicular pain. Cytokines including IL-6 and TNF-α were evaluated; however, none of the cytokines were isolated in a quantifiable concentration [12].
There appears to be a discrepancy between cytokine expression and pain between animal models and human LSS. In spinal nerve compression by disc herniation in humans, several studies were unable to show inflammatory cells in the vicinity of the nerve root, especially in chronic disc herniation [2, 3]. In this regard, cytokine expression in animal models may correlate with the acute phase in humans. Cadaveric studies of human disc herniation showed thrombosis of the local venous structure, basement membrane thickness, and intraneural fibrosis, but without histologic evidence of inflammation [3]. Furthermore, in patients with preoperative pain for 3 months or less, abundant macrophages and interleukins were observed in disc herniation tissue [2]. In contrast, in patients with pain for 6 months or longer, macrophages and interleukins were not observed in disc herniation tissue of most patients [2]. In the current study, patients had low back and leg pain, continuing for at least 6 months; therefore, the concentration of IL-6 may not correlate with symptoms such as low back and leg pain.
There were some limitations to the current study. First, we could not detect TNF-α, because the amount was below the limit of detection of ELISA. Further study using a more sensitive assay of TNF-α is required. Second, we only measured cytokines in the CSF; there is a possibility that this does not reflect the levels of cytokines directly in or around the compressed nerve tissue. Third, and most importantly, we did not directly show nerve degeneration and damage in LSS patients. We represented the severity of nerve damage by the severity of stenosis of the dural space seen by MRI, so further studies using other methods are needed to strengthen our hypothesis.
In conclusion, the concentration of IL-6 was significantly higher in the CSF of LSS patients than in the controls; however, TNF-α levels were below the limit of detection. There was no significant correlation between the concentration of IL-6 and the severity of pain or walking ability, but there was a significant correlation between the concentration of IL-6 and the severity of stenosis of the dural space. We propose that the concentration of IL-6 in LSS patients may reflect nerve damage or degeneration of the spinal nerve roots.
Conflict of interest
No funds were received in support of this study.
Footnotes
S. Ohtori, M. Suzuki, and T. Koshi contributed equally to this work.
References
- 1.Brisby H, Olmarker K, Larsson K, Nutu M, Rydevik B. Proinflammatory cytokines in cerebrospinal fluid and serum in patients with disc herniation and sciatica. Eur Spine J. 2002;11:62–66. doi: 10.1007/s005860100306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grönblad M, Virri J, Tolonen J, Seitsalo S, Kääpä E, Kankare J, Myllynen P, Karaharju EO. A controlled immunohistochemical study of inflammatory cells in disc herniation tissue. Spine. 1994;19:2744–2751. doi: 10.1097/00007632-199412150-00002. [DOI] [PubMed] [Google Scholar]
- 3.Hoyland JA, Freemont AJ, Jayson MI. Intervertebral foramen venous obstruction. A cause of periradicular fibrosis? Spine. 1989;14:558–568. doi: 10.1097/00007632-198906000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Ito T, Ohtori S, Inoue G, Koshi T, Doya H, Ozawa T, Saito T, Moriya H, Takahashi K. Glial phosphorylated p38 MAP kinase mediates pain in a rat model of lumbar disc herniation and induces motor dysfunction in a rat model of lumbar spinal canal stenosis. Spine. 2007;32:159–167. doi: 10.1097/01.brs.0000251437.10545.e9. [DOI] [PubMed] [Google Scholar]
- 5.Konno S, Yabuki S, Sato K, Olmarker K, Kikuchi S (1995) A model for acute, chronic, and delayed graded compression of the dog cauda equina: presentation of the gross, microscopic, and vascular anatomy of the dog cauda equina and accuracy in pressure transmission of the compression model. Spine 20:2758–2764 [DOI] [PubMed]
- 6.Myers RR, Wagner R, Sorkin LS (1999) Hyperalgesic actions of cytokines on peripheral nerves. In: Watkins LR, Maier SF (eds) Cytokines and Pain. Switzerland, pp 133–157
- 7.Nagashima H, Morio Y, Yamane K, Nanjo Y, Teshima R. Tumor necrosis factor-α, interleukin-1β, and interleukin-6 in the cerebrospinal fluid of patients with cervical myelopathy and lumbar radiculopathy. Eur Spine J. 2009;18:1946–1950. doi: 10.1007/s00586-009-1069-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olmarker K, Larsson K. Tumor necrosis factor α and nucleus-pulposus induced nerve root injury. Spine. 1998;23:2538–2544. doi: 10.1097/00007632-199812010-00008. [DOI] [PubMed] [Google Scholar]
- 9.Olmarker K, Rydevik B, Nordborg C. Autologous nucleus pulposus induces neurophysiologic and histologic changes in porcine cauda equina nerve roots. Spine. 1993;18:1425–1432. [PubMed] [Google Scholar]
- 10.Olmarker K, Nutu M, Størkson R. Changes in spontaneous behavior in rats exposed to experimental disc herniation are blocked by selective TNF-alpha inhibition. Spine. 2003;28:1635–1642. doi: 10.1097/01.BRS.0000083162.35476.FF. [DOI] [PubMed] [Google Scholar]
- 11.Onda A, Murata Y, Rydevik B, Larsson K, Kikuchi S, Olmarker K. Infliximab attenuates immunoreactivity of brain-derived neurotrophic factor in a rat model of herniated nucleus pulposus. Spine. 2004;29:1857–1861. doi: 10.1097/01.brs.0000137054.08788.b2. [DOI] [PubMed] [Google Scholar]
- 12.Scuderi GJ, Brusovamik GV, Anderson DG, Dunham CJ, Vaccaro AR, Demeo RF, Hallab N. Cytokine assay of the epidural space lavage in patients with lumbar intervertebral disk herniation and radiculopathy. J Spinal Disord Tech. 2006;19:266–269. doi: 10.1097/01.bsd.0000204501.22343.99. [DOI] [PubMed] [Google Scholar]
- 13.Sekiguchi M, Kikuchi S, Myers RR. Experimental spinal stenosis: relationship between degree of cauda equina compression, neuropathology, and pain. Spine. 2004;29:1105–1111. doi: 10.1097/00007632-200405150-00011. [DOI] [PubMed] [Google Scholar]
- 14.Shamash S, Reichert F, Rotshenker S. The cytokine network of Wallerian degeneration: tumor necrosis factor-α, interleukin-1α, and interleukin-1β. J Neurosci. 2001;22:3052–3060. doi: 10.1523/JNEUROSCI.22-08-03052.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]