Abstract
Cell therapy along with growth factor injection is currently widely investigated to restore the intervertebral disc. However, there is increasing evidence that transplanted unconditioned bone marrow-derived stromal cells (BMSCs) cannot thrive in the intervertebral disc “niche”. Moreover, uncertainty exists with respect to the cell phenotype that would be suitable to inject. The intervertebral disc cell phenotype only recently has been started to be characterised using transcriptomics profiling. Recent findings suggest that cytokeratin 19 (KRT-19) could be used as a potential candidate marker for the intervertebral disc, or more specifically the nucleus pulposus cell (NPC) phenotype. We present in vitro cell culture data using alginate bead culture of primary human BMSCs exposed to the standard chondrogenic stimulus, transforming growth factor beta-1 (TGF-β), the growth and differentiation factor 5 and/or bovine NPCs to induce a potential “discogenic” pathway. Chondrogenic induction via TGF-β pathway provoked down-regulation of KRT-19 gene expression in four out of five donors after 18 days of culture, whereas KRT-19 expression remained unchanged in the “discogenic” groups. In addition, the ratio of aggrecan/collagen II gene expression showed a remarkable difference (of at least 3 magnitudes) between the chondrogenic stimulus (low ratio) and the discogenic stimulus (high ratio). Therefore, KRT-19 and aggrecan/collagen II ratio may be potential markers to distinguish chondrogenic from “discogenic” differentiation.
Keywords: Coculture, Bone marrow stromal cells, Growth factor rhGDF-5, CDMP-1, Bovine nucleus pulposus cells, Gene expression, Differentiation
Introduction
Disc degeneration has been recognised as a major burden, and there is currently no biological treatment for regeneration or complete replacement available. Two regenerative approaches, which might prove success for the early degenerative lumbar intervertebral disc (IVD) are widely investigated, namely (1) injection of growth factors [33, 34] and (2) application of autologous stem cells [1, 23, 28]. On the side of growth factors, insulin-like growth factor-1, basic fibroblast growth factor (bFGF), and growth factors of the transforming growth factor beta (TGF-β) superfamily, mainly bone morphogenetic protein (BMP) 2 and 4 and TGF-β1, have been investigated in more detail. In this respect, a relatively recently identified growth factor of the TGF family is the growth and differentiation factor 5 (GDF-5) [16, 40], also known as “cartilage-derived morphogenetic protein-1” (CDMP-1), which became a synonym of GDF-5 [32]. GDF-5 plays a major role in skeletal development [5, 21], and functional mutation of GDF-5 has been shown to be involved in the development of osteoarthritis [37]. Application of GDF-5 has been shown to promote proliferation and matrix synthesis of chondrocytes [17] and of IVD cells [13, 30]. Gene expression of aggrecan and collagen type II of rat disc cells was significantly up-regulated upon over-expression of rhGDF-5 by viral transfection, while also adding the growth factor to the media led to dose-dependent responses [13, 56]. GDF-5 has also been identified as potential candidate to restore disc height in the rabbit degenerative model [10, 55]. In fact, these growth factors seem to be able to reverse disc height loss at least partially as demonstrated for the annular stab rabbit degeneration model and also most recently in a mouse lumbar annulus stab model [30]. The question remains to be addressed whether these growth factors might be equally successful for discs with larger dimensions and nutritional problems [2, 38].
Autologous mesenchymal stem cells (MSCs) have intensively been investigated as promising candidates for regenerative medicine [6, 7, 14, 44]. There are two major reasons for this; one is their multi-potentiality to differentiate into different cell types of the mesenchymal line, which promotes them ideal candidates for regeneration of bone, cartilage, muscle, tendon, and potentially IVD tissue [42]. The second reason is that these cells can be relatively easily isolated from various tissues, such as bone marrow or adipose tissue [14, 36, 64].
Different approaches of MSC implantation for IVD regeneration have been documented using a variety of animal models, types of cells and injection techniques [3, 24]. The fate of injected stem cells seems to be dependent on the cell dose, the ratio of injected to endogenous cells, and the possibility for cell–cell interaction [8, 12, 43, 48, 53, 57, 58]. Furthermore, there is recent evidence that unconditioned MSCs have a poor chance of survival in the “harsh” conditions, i.e. low pH and low oxygen and glucose concentration, of the IVD [8, 25, 59, 60, 63].
It therefore seems likely that MSCs that have been pre-conditioned towards the expression of the “IVD-like” phenotype may have improved regenerative potential. Methods reported to direct MSCs towards the “IVD-like” lineage include co-culture with nucleus pulposus cells (NPCs) and application of growth factors that are also known to induce chondrogenesis [9, 43, 45, 49, 51, 53, 62]. Besides the standard chondrogenic growth factor TGF-β, also GDF-5 has been reported to show chondrogenic effects in mesenchymal stem cells [4, 19, 20].
However, in spite of the phenotypical similarities between chondrocytes and disc cells, there are considerable differences in the mechanical function and hence the matrix composition of cartilage and disc [39]. Important issues are the cell phenotype that may be most suitable to regenerate the intervertebral disc and the question how to distinguish the chondrogenic from the IVD-like phenotype. In this context, cytokeratin 19 (KRT-19) was identified as a potential phenotype marker to differentiate NPCs from articular chondrocytes in a genome-wide microarray analysis of rat cells [27]. More recently, KRT-19 was found to be expressed more highly also in human NPCs compared to chondrocytes [35, 46]. However, the potential of KRT-19 as a marker for MSC differentiation towards the “IVD-like” phenotype has not been previously evaluated.
We addressed the hypothesis whether human bone-marrow derived stromal cells (hBMSCs) can be stimulated and committed towards a “discogenic” phenotype with recombinant human (rh) GDF-5 or by coculture with primary and healthy bovine NPCs. To monitor differentiation towards the “IVD-like” phenotype, KRT-19 was analysed in addition to standard chondrogenic markers, such as aggrecan (ACAN), collagen type II (COL2A1), and the transcription factor SOX-9, which is a key factor for the differentiation towards the chondrogenic lineage. Moreover, the ratio of gene expression between aggrecan and collagen type II was assessed to potentially differentiate chondrogenic from disc-like differentiation [39].
Methods
hBMSCs source
Fresh human bone-marrow aspirates were obtained from the Inselspital, University of Bern, after informed consent and approval from the local Ethical Committee (Ethical Approval KEK #164/07). Bone marrow stromal cells (hBMSCs) were isolated from aspirates of nine donors (7 males and 2 females, age ranged from 17 to 78 years) by standard density gradient procedure (Histopaque-1077, Sigma) and selection by plastic adherence [29]. Cells were expanded and passaged up to passage 4 in standard α-MEM (Gibco, Basel, Switzerland) containing 10% foetal calf serum (FCS) and 5 ng/mL recombinant human basic fibroblast growth factor-2 (rh bFGF-2) (Peprotech, London, UK) [50].
Bovine NPC source
The NPCs were derived from non-degenerate caudal IVDs (6–12 months old) of bovine tails obtained from local abattoir. Nucleus pulposus (NP) tissue was dissected and digested initially in 0.19% pronase (Roche) solution for 1 h with a subsequent overnight digestion in 0.4% collagenase II (Worthington) solution in serum-free Dulbeccos Modified Eagles Medium (DMEM, Gibco) containing antibiotics. The digested tissue/cell suspension was passed through a 100-μm cell strainer to remove tissue debris, and cells were pelleted by centrifugation at 400g for 5 min.
Coculture of MSCs and NPCs
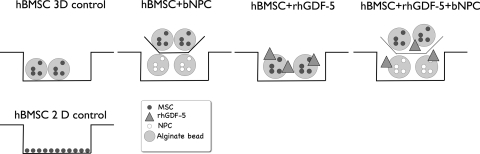
The NPCs and hBMSCs were seeded separately into 1.2% alginate (Sigma, Buchs, Switzerland) beads at a cell density of 4 × 106/mL by pressing the alginate through a 22G needle into 102 mM CaCl2 solution. Beads were then washed with 0.9% NaCl solution. All cocultures were conducted in duplicate in 12-well plates, using 0.4-μm pore size, high pore density, polyethylene terephthalate (PET) track-etched culture inserts (Becton, Dickinson and Company) to separate the beads of the two cell types. The hBMSC:NPC ratio was 1:1 for all experiments. Cells were cultured in serum-free high-glucose (4.5 g/L) DMEM containing 50 μg/mL ascorbic acid (Sigma), non-essential amino acids (Gibco), ITS+ (Sigma), 100 U/mL penicillin, 100 mg/mL streptomycin, 2.5 mg/mL amphotericin B, 100 mg/mL sodium pyruvate (all from Sigma), with or without 100 ng/mL rhGDF-5 (Advanced Technologies and Regenerative Medicine, LLC). Chondrogenic control beads were cultured with 10 ng/mL TGF-β1 (Peprotech, London, UK) and 100 nM dexamethasone (Sigma). Cells were cultured under six different conditions, i.e. (1) hBMSCs only (3D control), (2) co-culture hBMSCs + bNPCs, (3) hBMSCs + rhGDF-5, (4) hBMSCs + rhGDF-5 + dexamethasone, (5) co-culture hBMSCs + bNPCs + rhGDF-5, (6) hBMSCs + TGF-β1 + dexamethasone (“chondrogenic” medium) (Fig. 1). The cultures were kept at 37°C in a humid atmosphere containing 5% CO2, with medium change every 3 days. Data were collected after 6, 9, 12, 15, and 18 days.
Fig. 1.
Schematic illustration of the experimental setup and the different culture conditions
DNA and glycosaminoglycan (GAG) content
Alginate beads were digested in sodium citrate digestion buffer (150 mM NaCl, 55 mM Na3Citrate, 5 mM Cystein–HCl, pH 6.8) with 125 μg/mL papain from papaya latex (all Sigma) overnight at 60°C. The DNA content was determined by fluorimetry using HOECHST© dye and calf thymus DNA as a standard (all Sigma). Total GAG content was measured using the modified dimethylmethylene blue (DMMB) assay [18].
Histology and immunohistochemistry
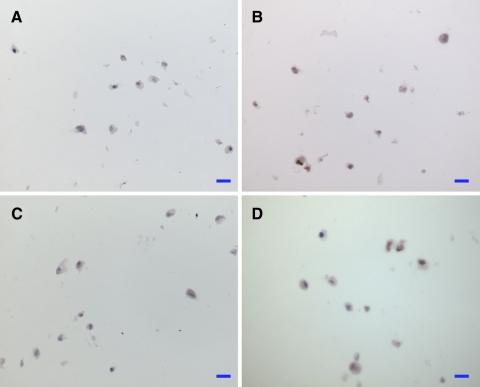
Alginate beads were fixed in Tissue-Tek O.C.T. compound (Sakura Inc.) for 20 min and flash-frozen in liquid nitrogen. Beads were then cryo-sectioned (12 μm) and stained by Alcian blue 8GX for 30 min at 37°C (Sigma). Slides were mounted in Eukit© and imaged using a Leica inverted microscope at a 10× and 40× magnification. High-resolution photographs were taken with a digital camera (Nikon 70D), with identical camera settings applied for all photographs without further image processing for an unbiased estimation of the proteoglycan content. For aggrecan immunostaining, a neo-epitope had to be generated by reduction and alkylation steps [52]. Cryosections were then incubated with primary antibody (12/21/1-C-6; Development Studies Hybridoma Bank DSHB, University of Iowa, Iowa City, IA; 1:10 dilution) for 1 h at room temperature, followed by biotinylated secondary antibody (30 min, RT) and the preformed avidin–biotin–peroxidase complex (30 min, RT). All detection reagents were taken from the Vectastain Elite ABC Kit (Vector Laboratories). As a chromogen 3,3′-diaminobenzidine monomer (DAB) was used. For the negative controls, the primary antibody was replaced by PBS.
Gene expression analyses
Alginate beads were dropped into 1 mL of TRI Reagent (Molecular Research Center, Cincinnati, OH, USA), incubated at room temperature for 20 min, flash-frozen in liquid nitrogen and stored at −80°C.
Beads were homogenised by Tissue Lyzer (Quiagen Inc.), and total RNA was extracted using a modified TRIspin method [46]. Reverse transcription (RT) was performed with TaqMan RT Reagents (Applied Biosystems, Foster City, CA). Oligonucleotide primers for human aggrecan (ACAN), collagen type I (COL1A1), collagen type II (COL2A1), and TaqMan© probes (all from Microsynth, Balgach, Switzerland) are listed in Table 1. Assay reagents for KRT-19, SOX-9, and 18S ribosomal RNA, which was used to normalise gene expression data, were from Applied Biosystems. Real-time PCR was performed using the ABI GeneAmp 7500 (Applied Biosystems). Gene expression was calculated by the 2−ΔΔCt method [31], normalising data to the expression level at day 0. Samples with no detectable RNA concentration of the target gene but with detectable concentration of the 18S ribosomal RNA (Ct < 18) were assigned a Ct of 45 (maximum number of cycles). Gene expression data were expressed relative to the values of the corresponding 3D control group (group 1) at the same day of culture.
Table 1.
RT-PCR primers used with TaqMan© design
| COL1A1 | Primer fw (5′–3′) | CCC TGG AAA GAA TGG AGA TGA T |
| Primer rev (5′–3′) | ACT GAA ACC TCT GTG TCC CTT CA | |
| Probe (5′FAM/3′TAMRA) | CGG GCA ATC CTC GAG CAC CCT | |
| COL2A1 | Primer fw (5′–3′) | GGC AAT AGC AGG TTC ACG TAC A |
| Primer rev (5′–3′) | GAT AAC AGT CTT GCC CCA CTT ACC | |
| Probe (5′FAM/3′TAMRA) | CCT GAA GGA TGG CTG CAC GAA ACA TAC | |
| ACAN | Primer fw (5′–3′) | AGT CCT CAA GCC TCC TGT ACT CA |
| Primer rev (5′–3′) | CGG GAA GTG GCG GTA ACA | |
| Probe (5′FAM/3′TAMRA) | CCG GAA TGG AAA CGT GAA TCA GAA TCA ACT | |
| SOX-9 | Gene expression assay | Hs_00165814_m1 (Applied Biosystems) |
| KRT-19 | Gene expression assay | Hs_00761767_s1 (Applied Biosystems) |
| 18S rRNA | Assay reagents | 4310893E (Applied Biosystems) |
Statistical analyses
Statistical analysis of DNA and GAG content was done using one-way ANOVA for time and using Tukey’s post-hoc multiple testing. Real-time PCR data were statistically tested using nonparametric Kruskal–Wallis and Dunn’s multiple comparison in GraphPad Prism version 5.0c, GraphPad Software, San Diego, CA, USA (http://www.graphpad.com).
Results
DNA content
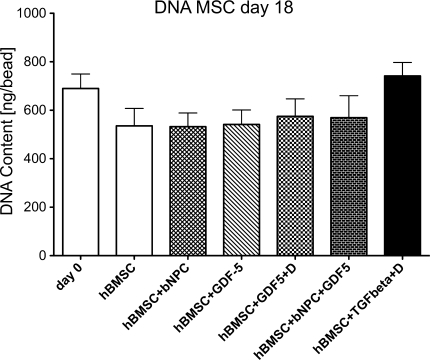
The DNA content of hBMSCs was 689.8 ± 59.63 ng/bead (mean ± SEM) at day 0 and dropped in the control group to 535.8 ± 71.73 ng/bead by day 18, which was not significant. Overall, there was no significant difference in DNA content with time (one-way Anova F = 1.800, P = 0.128) and between the groups. However, in the chondrogenic group, i.e. in presence of TGF-β1 and dexamethasone, the DNA content remained stable over the entire culture period and was 741.6 ± 55.79 ng/bead on day 18 (one-way ANOVA, P = 0.86) (Fig. 2), whereas the DNA content was slightly decreased in the other groups.
Fig. 2.
DNA content of hBMSCs in 3D alginate beads at 18 days of culture in serum-free medium either with or without coculture with NPC and growth factors. Values are mean ± SEM; N = 10
Extracellular matrix
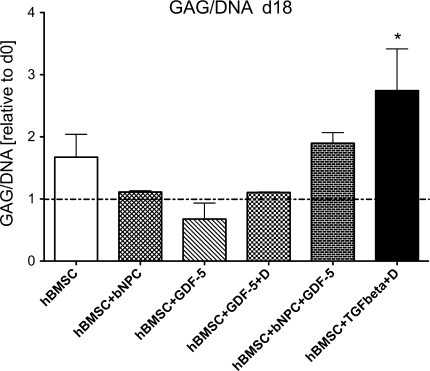
The GAG/DNA ratio increased 2.745 ± 0.671 fold in the hBMSC + TGF-β1 + dexamethasone group by day 18 (Fig. 3). A slight increase was also noted in the hBMSC + bNPC + rhGDF-5 group. However, except for the TGF-β1 + dexamethasone group (Wilcoxon signed-rank test from 1.0 ratio, P = 0.047), there was no significant difference in GAG/DNA measurements between the other groups and over time (one-way ANOVA: F = 1.283, P = 0.33 for day 18). Histology of alginate beads showed a relative increase in Alcian blue staining after 15 days of culture in all groups. Similar to the GAG/DNA content, an increased staining intensity relative to 3D control was only observed in the hBMSC + TGF-β1 + dexamethasone group (Fig. 4). Aggrecan immunostaining revealed some positive reaction in all treatment groups although supplementation with TGF-β1 + dexamethasone resulted in more aggrecan positive cells than rhGDF-5 and/or bNPC (Fig. 5).
Fig. 3.
GAG/DNA content of hBMSCs in 3D alginate beads at day 18 of culture normalised to day 0. Values are mean ± SEM; N = 10. *P < 0.05 t test from hypothetical mean 1.0
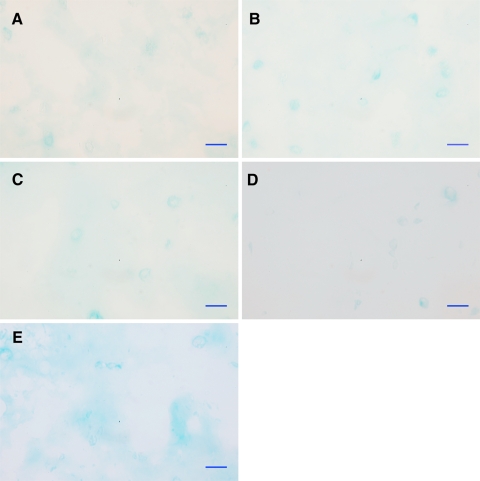
Fig. 4.
Cryosections (12 μm) of alginate beads at day 15 stained with alcian blue 8GX. a hBMSC control, b hBMSC + bNPC, c hBMSC + rhGDF-5, d hBMSC + bNPC + rhGDF-5, and e hBMSC + TGF-β + dexamethasone. Scale bar 20 μm
Fig. 5.
Aggrecan immunostaining of cryosections (12 μm) of alginate beads at day 15. a hBMSC control, b hBMSC + bNPC, c hBMSC + rhGDF-5, and d hBMSC + TGF-β + dexamethasone. Scale bar 20 μm
Gene expression
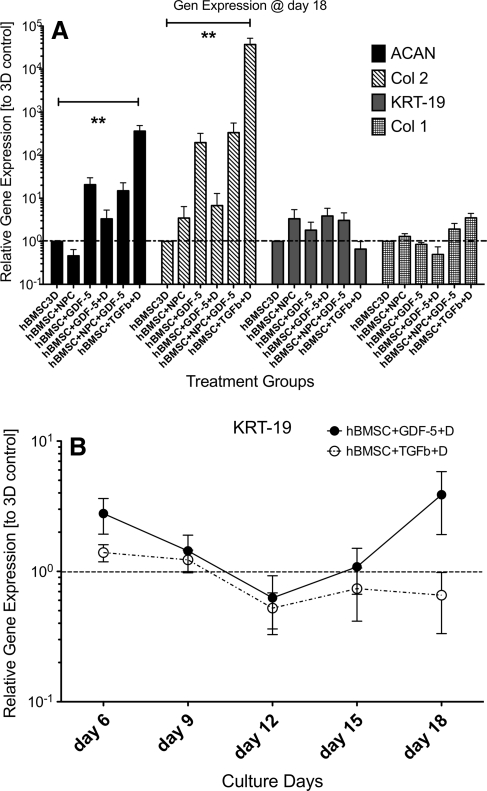
ACAN was significantly up-regulated relative to the 3D control at day 18 with mean up-regulations (±SEM) of 20.7 ± 9.21 times for the hBMSC + rhGDF-5, 14.89 ± 8.01 times for the hBMSC + rhGDF-5 + bNPC, and even 363.2 ± 125.1 times for the hBMSC + TGF-β1 group (Kruskal–Wallis P = 0.0139; Fig. 6a). Collagen type II was also significantly up-regulated relative to the 3D control after 18 days of culture: 196.4 ± 125.6 times in the hBMSC + rhGDF-5; 3.46 ± 2.93 times in the hBMSC + bNPC; 334.3 ± 225.8 times in the hBMSC + rhGDF-5 + bNPC; and 37,271 ± 14,800 times for hBMSC + TGF-β1 + dexamethasone group (Kruskal–Wallis P = 0.0002; Fig. 6a). Sox-9 was up-regulated 4.24 ± 1.96 times in the hBMSC + TGF-β1 + dexamethasone group, while there was a minor effect in groups containing rhGDF-5. For hBMSC + rhGDF-5, the Sox-9 expression was 1.57 ± 0.60 times and for hBMSC + rhGDF-5 + bNPC 1.73 ± 0.58 times up-regulated compared to 3D control, whereas the group hBMSC + bNPC remained around the “baseline” (Kruskal–Wallis P = 0.42) (data not shown). Collagen type I was up-regulated only in the presence of TGF-β1, but this difference was not significant (Kruskal–Wallis P = 0.42).
Fig. 6.
a RT-PCR results relative to 3D control for four gene loci. Data are normalised to the gene expression levels of the hBMSC control group at the respective time point. Stars mark significance of Dunn’s post-hoc testing (P < 0.01). Values are mean ± SEM, N = 10. b Gene expression of KRT-19 relative to 3D control. KRT-19 is down-regulated by the addition of TGF-β1 + dexamethasone after 18 days of culture
It is interesting to note that KRT-19 gene expression was down-regulated after day 12 of culture in the group of hBMSC + TGF-β1 + dexamethasone compared to the 3D control group although this was not significant (Kruskal–Wallis P = 0.0544). In contrast, KRT-19 was up-regulated in the other treatment groups compared to 3D control at day 18 (Fig. 6a). This pattern was observed as well at earlier culture time points (Fig. 6b).
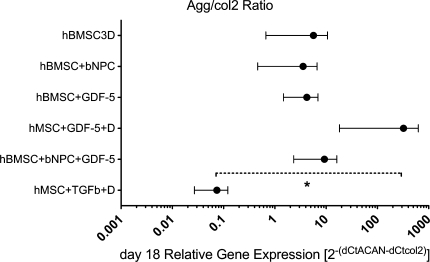
If the ratio of ACAN versus collagen II expression [calculated as 2−(∆CtACAN−∆Ctcol2)] is estimated, the hBMSC + rhGDF-5 + dexamethasone group was found to have the highest relative expression (higher ACAN compared to collagen II), while about equal ratios were found for most other groups, except for the hBMSC + TGF-β1 + dexamethasone group, where collagen type II expression was higher than ACAN expression (Fig. 7).
Fig. 7.
Relative gene expression ratio of ACAN to COL2A1 [expressed as 2−(∆CtACAN−∆Ctcol2)] in hBMSCs after 18 days of culture. Values are mean ± SEM. *P < 0.05 from Dunn’s post-hoc test
Discussion
Although differentiation of MSCs towards the disc-like phenotype has been demonstrated, there is still a certain ambiguity about how to monitor or to characterise the differentiation. Several recent studies have proposed different sets of markers for the distinction of the articular chondrocyte phenotype from the NP and annulus fibrosus-like phenotype [11, 27, 46, 47]. They found significant differences in expression profiles of collagen type 5, matrix gla protein, HtrA1 serine peptidase 1, alpha-2-macroglobulin, cytokeratin-18, neural cell adhesion molecule, glypican-3, and KRT-19. Furthermore, other molecules specific for NPCs were proposed, including CD24, HIF-1α, GLUT1, MMP2 [22, 45]. However, previous transcriptomics studies did not find any evidence for clear on–off signal among these genes, and inter-species comparison revealed considerable differences between rodent, larger animal, and human cell populations [47].
Recent gene expression comparison of human IVD and articular cartilage cells found strong evidence that KRT-19 might be a positive marker for NP-like cells among other markers [35, 46]. Although a negative correlation of KRT-19 gene expression in NPCs with donor age was found, KRT-19 was expressed more highly in NPCs than chondrocytes also in older individuals. At the protein level, KRT-19 was detected primarily in cells of the juvenile IVD, suggesting a role in the IVD development. This is the first report, which suggests a down-regulation of KRT-19 in hBMSCs in presence of TGF-β1, indicating that this potential NPC marker is negatively influenced in the chondrogenic TGF-β pathway. However, due to the large inter-donor variation in the responsiveness of BMSCs to the culture conditions, we can only report a trend of KRT-19 suppression upon chondrogenic stimulation (P = 0.07). In presence of rhGDF-5 and/or NPCs, KRT-19 expression was not changed or tended to be enhanced. The sustained expression of this cytokeratin in these groups could indicate the maintenance of a more primitive cell phenotype, whereas the cells exposed to the strong chondrogenic stimuli may lose KRT-19 expression during differentiation.
Our results confirm recent reports that ACAN and COL2A1 expression can be stimulated with a concentration of 100 ng/mL rhGDF-5 [13, 61]. Indeed, the concentration of 100 ng/mL was found to be ideal in a study where different doses of rhGDF-5, ranging from 1 to 100 ng/mL, were tested [13]. However, the chondrogenic induction using TGF-β1 was stronger than using rhGDF-5. Mwale et al. [39] found differences at the extracellular matrix level between NP and articular cartilage tissue. In particular, a GAG to hydroxyproline ratio of 27 was reported for the NP of young adults, while this ratio reached a value of about 2 for cartilage tissue. Looking at the related ACAN/COL2A1 ratio of the two respective gene expression levels, the group with hBMSC + rhGDF-5 + dexamethasone showed the highest value (Fig. 7), whereas the other treatments showed a moderate stimulation towards an increased ACAN/COL2A1 ratio. The chondrogenic pathway induced by TGF-β resulted in a further lower value (3 levels of magnitude) as expected from a chondrogenic induction [9, 11, 29]. These data suggest that the aggrecan/collagen II ratio may be considered to distinguish chondrogenic from disc-like differentiation also at the gene transcription level.
Co-culture with bNPCs only showed tendencies to up-regulate COL2A1 and KRT-19 expression. Based on previous reports, a ratio of 1:1 for hBMSCs/bNPCs was chosen for co-cultures [49]. However, other ratios such as the 1:3 ratio, which was found to be most successful for hBMSCs [43, 54] and muscle-derived MSCs [53], may have led to a different outcome. In a recent study by Kim et al. [26], rabbit MSCs were stimulated with autologous NPCs at three different MSC:NPC ratios, i.e. 1:1, 1:2, and 2:1. They reported a beneficial effect on the DNA content and on the GAG/DNA ratio when the MSC:NPC ratio was of 1:2. This supports the assumption that higher amounts of NPCs may be necessary to effectively stimulate proliferation and differentiation of MSCs in an indirect coculture system.
Sox-9 is known as a major transcription factor on the gateway for chondrogenesis [41]. In cooperation with Sox-5 and Sox-6, Sox-9 plays an important role in the proliferation and function of chondrocytes and can transactivate genes of cartilaginous matrix proteins such as collagen types II, IX, XI, and aggrecan core protein [15]. With a concentration of 10 ng/mL of TGF-β1 Sox-9 expression could slightly be increased, while no changes were observed in Sox-9 expression with rhGDF-5 or bNPCs. Thus, TGF-β1 apparently provoked up-regulation of chondrogenic transcription factors more strongly than rhGDF-5. It has been previously observed by Bai et al. [4] that GDF-5 is less stimulatory than TGF-β1 with respect to chondrogenic differentiation of MSCs.
A limitation of the present study is that it is mainly based on gene expression data, while little evidence is provided relating to protein and matrix production. It is conceivable that a culture time of more than 18 days may be required to detect changes in matrix production not only in the chondrogenic but also in the other treatment groups.
In conclusion, for MSC-based cell therapy and tissue engineering approaches for IVD regeneration, differentiation towards the disc cell phenotype may be important. This study shows the potential of rhGDF-5 for differentiation of hBMSCs, which might be considered for in vitro IVD tissue engineering and for in vivo therapeutic application in combination with MSCs. Furthermore, the present study indicates that KRT-19 regulation and ACAN/COL2A1 gene expression ratio may be useful molecular markers to distinguish between the chondrogenic and IVD-like differentiation. Further studies with longer culture times will be needed to confirm the potential of rhGDF-5 treatment with respect to protein synthesis and extracellular matrix accumulation.
Acknowledgments
We thank Dr. E. Brown (Advanced Technologies and Regenerative Medicine, LLC) for providing rhGDF-5 and Christoph Sprecher for technical support. This study was partially supported by the Swiss National Science Foundation (SNSF) (Grant #3320000-116818).
References
- 1.Acosta FL, Lotz J, Ames CP. The potential role of mesenchymal stem cell therapy for intervertebral disc degeneration: a critical overview. Neurosurg Focus. 2005;19(3):E4. doi: 10.3171/foc.2005.19.3.5. [DOI] [PubMed] [Google Scholar]
- 2.Alini M, Eisenstein SM, Ito K, Little C, Kettler AA, Masuda K, Melrose J, Ralphs J, Stokes I, Wilke HJ. Are animal models useful for studying human disc disorders/degeneration? Eur Spine J. 2008;17(1):2–19. doi: 10.1007/s00586-007-0414-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson DG, Albert TJ, Fraser JK, Risbud M, Wuisman P, Meisel HJ, Tannoury C, Shapiro I, Vaccaro AR. Cellular therapy for disc degeneration. Spine. 2005;30(17 Suppl):S14–S19. doi: 10.1097/01.brs.0000175174.50235.ba. [DOI] [PubMed] [Google Scholar]
- 4.Bai X, Xiao Z, Pan Y, Hu J, Pohl J, Wen J, Li L. Cartilage-derived morphogenetic protein-1 promotes the differentiation of mesenchymal stem cells into chondrocytes. Biochem Biophys Res Commun. 2004;325(2):453–460. doi: 10.1016/j.bbrc.2004.10.055. [DOI] [PubMed] [Google Scholar]
- 5.Buxton P, Edwards C, Archer CW, Francis-West P (2001) Growth/differentiation factor-5 (GDF-5) and skeletal development. J Bone Joint Surg 83-A Suppl 1(Pt 1):S23–S30 [PubMed]
- 6.Caplan AI. Review: mesenchymal stem cells: cell-based reconstructive therapy in orthopedics. Tissue Eng. 2005;11(7–8):1198–1211. doi: 10.1089/ten.2005.11.1198. [DOI] [PubMed] [Google Scholar]
- 7.Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25(11):2739–2749. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- 8.Chan SCW, Gantenbein-Ritter B, Leung VY, Chan D, Cheung KM, Ito K. Cryopreserved intervertebral disc with injected bone marrow-derived stromal cells: a feasibility study using organ culture. Spine J. 2010;10(6):486–496. doi: 10.1016/j.spinee.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 9.Chen S, Emery SE, Pei M. Coculture of synovium-derived stem cells and nucleus pulposus cells in serum-free defined medium with supplementation of transforming growth factor-beta1: a potential application of tissue-specific stem cells in disc regeneration. Spine. 2009;34(12):1272–1280. doi: 10.1097/BRS.0b013e3181a2b347. [DOI] [PubMed] [Google Scholar]
- 10.Chujo T, An HS, Akeda K, Miyamoto K, Muehleman C, Attawia M, Andersson G, Masuda K. Effects of growth differentiation factor-5 on the intervertebral disc—in vitro bovine study and in vivo rabbit disc degeneration model study. Spine. 2006;31(25):2909–2917. doi: 10.1097/01.brs.0000248428.22823.86. [DOI] [PubMed] [Google Scholar]
- 11.Clouet J, Grimandi G, Pot-Vaucel M, Masson M, Fellah HB, Guigand L, Cherel Y, Bord E, Rannou F, Weiss P, Guicheux J, Vinatier C. Identification of phenotypic discriminating markers for intervertebral disc cells and articular chondrocytes. Rheumatology. 2009;48(11):1447–1450. doi: 10.1093/rheumatology/kep262. [DOI] [PubMed] [Google Scholar]
- 12.Crevensten G, Walsh AJ, Ananthakrishnan D, Page P, Wahba GM, Lotz JC, Berven S. Intervertebral disc cell therapy for regeneration: mesenchymal stem cell implantation in rat intervertebral discs. Ann Biomed Eng. 2004;32(3):430–434. doi: 10.1023/B:ABME.0000017545.84833.7c. [DOI] [PubMed] [Google Scholar]
- 13.Cui M, Wan Y, Anderson DG, Shen FH, Leo BM, Laurencin CT, Balian G, Li X. Mouse growth and differentiation factor-5 protein and DNA therapy potentiates intervertebral disc cell aggregation and chondrogenic gene expression. Spine J. 2008;8(2):287–295. doi: 10.1016/j.spinee.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 14.da Silva Meirelles L, Caplan AI, Nardi NB. In search of the in vivo identity of mesenchymal stem cells. Stem Cells. 2008;26(9):2287–2299. doi: 10.1634/stemcells.2007-1122. [DOI] [PubMed] [Google Scholar]
- 15.Crombrugghe B, Lefebvre V, Behringer RR, Bi W, Murakami S, Huang W. Transcriptional mechanisms of chondrocyte differentiation. Matrix Biol. 2000;19(5):389–394. doi: 10.1016/S0945-053X(00)00094-9. [DOI] [PubMed] [Google Scholar]
- 16.Ducy P, Karsenty G. The family of bone morphogenetic proteins. Kidney Int. 2000;57(6):2207–2214. doi: 10.1046/j.1523-1755.2000.00081.x. [DOI] [PubMed] [Google Scholar]
- 17.Erlacher L, Ng CK, Ullrich R, Krieger S, Luyten FP. Presence of cartilage-derived morphogenetic proteins in articular cartilage and enhancement of matrix replacement in vitro. Arthritis Rheum. 1998;41(2):263–273. doi: 10.1002/1529-0131(199802)41:2<263::AID-ART10>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 18.Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue 1. Biochim Biophys Acta. 1986;883(2):173–177. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 19.Farng E, Urdaneta AR, Barba D, Esmende S, McAllister DR. The effects of GDF-5 and uniaxial strain on mesenchymal stem cells in 3-D culture. Clin Orthop Relat Res. 2008;466(8):1930–1937. doi: 10.1007/s11999-008-0300-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feng G, Wan Y, Balian G, Laurencin CT, Li X (2008) Adenovirus-mediated expression of growth and differentiation factor-5 promotes chondrogenesis of adipose stem cells. Growth Factors 26(3):132–142 [DOI] [PMC free article] [PubMed]
- 21.Francis-West PH, Abdelfattah A, Chen P, Allen C, Parish J, Ladher R, Allen S, MacPherson S, Luyten FP, Archer CW. Mechanisms of GDF-5 action during skeletal development. Development. 1999;126(6):1305–1315. doi: 10.1242/dev.126.6.1305. [DOI] [PubMed] [Google Scholar]
- 22.Fujita N, Miyamoto T, Imai J, Hosogane N, Suzuki T, Yagi M, Morita K, Ninomiya K, Miyamoto K, Takaishi H, Matsumoto M, Morioka H, Yabe H, Chiba K, Watanabe S, Toyama Y, Suda T. CD24 is expressed specifically in the nucleus pulposus of intervertebral discs. Biochem Biophys Res Commun. 2005;338(4):1890–1896. doi: 10.1016/j.bbrc.2005.10.166. [DOI] [PubMed] [Google Scholar]
- 23.Hiyama A, Mochida J, Iwashina T, Omi H, Watanabe T, Serigano K, Tamura F, Sakai D. Transplantation of mesenchymal stem cells in a canine disc degeneration model. J Orthop Res. 2008;26(5):589–600. doi: 10.1002/jor.20584. [DOI] [PubMed] [Google Scholar]
- 24.Hiyama A, Mochida J, Sakai D. Stem cell applications in intervertebral disc repair. Cell Mol Biol. 2008;54(1):24–32. [PubMed] [Google Scholar]
- 25.Holzwarth C, Vaegler M, Gieseke F, Pfister SM, Handgretinger R, Kerst G, Muller I. Low physiologic oxygen tensions reduce proliferation and differentiation of human multipotent mesenchymal stromal cells. BMC Cell Biol. 2010;11(1):11. doi: 10.1186/1471-2121-11-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim DH, Kim SH, Heo SJ, Shin JW, Lee SW, Park SA, Shin JW (2009) Enhanced differentiation of mesenchymal stem cells into NP-like cells via 3D co-culturing with mechanical stimulation. J Biosci Bioeng 108(1):63–67 [DOI] [PubMed]
- 27.Lee CR, Sakai D, Nakai T, Toyama K, Mochida J, Alini M, Grad S. A phenotypic comparison of intervertebral disc and articular cartilage cells in the rat. Eur Spine J. 2007;16(12):2174–2185. doi: 10.1007/s00586-007-0475-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leung VY, Chan D, Cheung KM. Regeneration of intervertebral disc by mesenchymal stem cells: potentials, limitations, and future direction. Eur Spine J. 2006;15(Suppl 3):S406–S413. doi: 10.1007/s00586-006-0183-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Z, Kupcsik L, Yao SJ, Alini M, Stoddart MJ. Chondrogenesis of human bone marrow mesenchymal stem cells in fibrin-polyurethane composites. Tissue Eng Part A. 2009;15(7):1729–1737. doi: 10.1089/ten.tea.2008.0247. [DOI] [PubMed] [Google Scholar]
- 30.Liang H, Ma SY, Feng G, Shen FH, Joshua Li X. Therapeutic effects of adenovirus-mediated growth and differentiation factor-5 in a mice disc degeneration model induced by annulus needle puncture. Spine J. 2010;10(1):32–41. doi: 10.1016/j.spinee.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 32.Luyten FP. Cartilage-derived morphogenetic protein-1. Int J Biochem Cell Biol. 1997;29(11):1241–1244. doi: 10.1016/S1357-2725(97)00025-3. [DOI] [PubMed] [Google Scholar]
- 33.Masuda K, An HS. Prevention of disc degeneration with growth factors. Eur Spine J. 2006;15(Suppl 3):S422–S432. doi: 10.1007/s00586-006-0149-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Masuda K, Lotz JC. New challenges for intervertebral disc treatment using regenerative medicine. Tissue Eng Part B Rev. 2010;16(1):147–158. doi: 10.1089/ten.teb.2009.0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Minogue BM, Richardson SM, Zeef LA, Freemont AJ, Hoyland JA. Transcriptional profiling of bovine intervertebral disc cells: implications for identification of normal and degenerate human intervertebral disc cell phenotypes. Arthritis Res Ther. 2010;12(1):R22. doi: 10.1186/ar2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mitchell JB, McIntosh K, Zvonic S, Garrett S, Floyd ZE, Kloster A, Di Halvorsen Y, Storms RW, Goh B, Kilroy G, Wu X, Gimble JM. Immunophenotype of human adipose-derived cells: temporal changes in stromal-associated and stem cell-associated markers. Stem Cells. 2006;24(2):376–385. doi: 10.1634/stemcells.2005-0234. [DOI] [PubMed] [Google Scholar]
- 37.Miyamoto Y, Mabuchi A, Shi D, Kubo T, Takatori Y, Saito S, Fujioka M, Sudo A, Uchida A, Yamamoto S, Ozaki K, Takigawa M, Tanaka T, Nakamura Y, Jiang Q, Ikegawa S. A functional polymorphism in the 5′ UTR of GDF5 is associated with susceptibility to osteoarthritis. Nat Genet. 2007;39(4):529–533. doi: 10.1038/2005. [DOI] [PubMed] [Google Scholar]
- 38.Miyazaki T, Kobayashi S, Takeno K, Meir A, Urban J, Baba H. A phenotypic comparison of proteoglycan production of intervertebral disc cells isolated from rats, rabbits, and bovine tails; which animal model is most suitable to study tissue engineering and biological repair of human disc disorders? Tissue Eng Part A. 2009;15(12):3835–3846. doi: 10.1089/ten.tea.2009.0250. [DOI] [PubMed] [Google Scholar]
- 39.Mwale F, Roughley P, Antoniou J. Distinction between the extracellular matrix of the nucleus pulposus and hyaline cartilage: a requisite for tissue engineering of intervertebral disc. Eur Cell Mater. 2004;8:58–63. doi: 10.22203/ecm.v008a06. [DOI] [PubMed] [Google Scholar]
- 40.Nakase T, Ariga K, Miyamoto S, Okuda S, Tomita T, Iwasaki M, Yonenobu K, Yoshikawa H. Distribution of genes for bone morphogenetic protein-4, -6, growth differentiation factor-5, and bone morphogenetic protein receptors in the process of experimental spondylosis in mice. J Neurosurg. 2001;94(1 Suppl):68–75. doi: 10.3171/spi.2001.94.1.0068. [DOI] [PubMed] [Google Scholar]
- 41.Paul R, Haydon RC, Cheng H, Ishikawa A, Nenadovich N, Jiang W, Zhou L, Breyer B, Feng T, Gupta P, He TC, Phillips FM. Potential use of Sox9 gene therapy for intervertebral degenerative disc disease. Spine. 2003;28(8):755–763. [PMC free article] [PubMed] [Google Scholar]
- 42.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 43.Richardson SM, Walker RV, Parker S, Rhodes NP, Hunt JA, Freemont AJ, Hoyland JA. Intervertebral disc cell-mediated mesenchymal stem cell differentiation. Stem Cells. 2006;24(3):707–716. doi: 10.1634/stemcells.2005-0205. [DOI] [PubMed] [Google Scholar]
- 44.Richardson SM, Hoyland JA, Mobasheri R, Csaki C, Shakibaei M, Mobasheri A. Mesenchymal stem cells in regenerative medicine: opportunities and challenges for articular cartilage and intervertebral disc tissue engineering. J Cell Physiol. 2010;222(1):23–32. doi: 10.1002/jcp.21915. [DOI] [PubMed] [Google Scholar]
- 45.Risbud MV, Albert TJ, Guttapalli A, Vresilovic EJ, Hillibrand AS, Vaccaro AR, Shapiro IM. Differentiation of mesenchymal stem cells towards a nucleus pulposus-like phenotype in vitro: implications for cell-based transplantation therapy. Spine. 2004;29(23):2627–2632. doi: 10.1097/01.brs.0000146462.92171.7f. [DOI] [PubMed] [Google Scholar]
- 46.Rutges J, Creemers LB, Dhert W, Milz S, Sakai D, Mochida J, Alini M, Grad S. Variations in gene and protein expression in human nucleus pulposus in comparison with annulus fibrosus and cartilage cells: potential associations with aging and degeneration. Osteoarthritis Cartilage. 2010;18(3):416–423. doi: 10.1016/j.joca.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 47.Sakai D, Nakai T, Mochida J, Alini M, Grad S. Differential phenotype of intervertebral disc cells: microarray and immunohistochemical analysis of canine nucleus pulposus and anulus fibrosus. Spine. 2009;34(14):1448–1456. doi: 10.1097/BRS.0b013e3181a55705. [DOI] [PubMed] [Google Scholar]
- 48.Serigano K, Sakai D, Hiyama A, Tamura F, Tanaka M, Mochida J. Effect of cell number on mesenchymal stem cell transplantation in a canine disc degeneration model. J Orthop Res. 2010;28(16):1267–1275. doi: 10.1002/jor.21147. [DOI] [PubMed] [Google Scholar]
- 49.Sobajima S, Vadala G, Shimer A, Kim JS, Gilbertson LG, Kang JD. Feasibility of a stem cell therapy for intervertebral disc degeneration. Spine J. 2008;8(6):888–896. doi: 10.1016/j.spinee.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 50.Solchaga LA, Penick K, Porter JD, Goldberg VM, Caplan AI, Welter JF. FGF-2 enhances the mitotic and chondrogenic potentials of human adult bone marrow-derived mesenchymal stem cells. J Cell Physiol. 2005;203(2):398–409. doi: 10.1002/jcp.20238. [DOI] [PubMed] [Google Scholar]
- 51.Steck E, Bertram H, Abel R, Chen B, Winter A, Richter W. Induction of intervertebral disc-like cells from adult mesenchymal stem cells. Stem Cells. 2005;23(3):403–411. doi: 10.1634/stemcells.2004-0107. [DOI] [PubMed] [Google Scholar]
- 52.Tischer T, Milz S, Maier M, Schieker M, Benjamin M. An immunohistochemical study of the rabbit suprapatella, a sesamoid fibrocartilage in the quadriceps tendon containing aggrecan. J Histochem Cytochem. 2002;50(7):955–960. doi: 10.1177/002215540205000709. [DOI] [PubMed] [Google Scholar]
- 53.Vadalà G, Sobajima S, Lee JY, Huard J, Denaro V, Kang JD, Gilbertson LG. In vitro interaction between muscle-derived stem cells and nucleus pulposus cells. Spine J. 2008;8(5):804–809. doi: 10.1016/j.spinee.2007.07.394. [DOI] [PubMed] [Google Scholar]
- 54.Vadalà G, Studer RK, Sowa G, Spiezia F, Iucu C, Denaro V, Gilbertson LG, Kang JD. Coculture of bone marrow mesenchymal stem cells and nucleus pulposus cells modulate gene expression profile without cell fusion. Spine. 2008;33(8):870–876. doi: 10.1097/BRS.0b013e31816b4619. [DOI] [PubMed] [Google Scholar]
- 55.Walsh AJ, Bradford DS, Lotz JC. In vivo growth factor treatment of degenerated intervertebral discs. Spine. 2004;29(2):156–163. doi: 10.1097/01.BRS.0000107231.67854.9F. [DOI] [PubMed] [Google Scholar]
- 56.Wang H, Kroeber M, Hanke M, Ries R, Schmid C, Poller W, Richter W. Release of active and depot GDF-5 after adenovirus-mediated overexpression stimulates rabbit and human intervertebral disc cells. J Mol Med. 2004;82(2):126–134. doi: 10.1007/s00109-003-0507-y. [DOI] [PubMed] [Google Scholar]
- 57.Watanabe T, Sakai D, Yamamoto Y, Iwashina T, Serigano K, Tamura F, Mochida J. Human nucleus pulposus cells significantly enhanced biological properties in a coculture system with direct cell-to-cell contact with autologous mesenchymal stem cells. J Orthop Res. 2010;28(5):623–630. doi: 10.1002/jor.21036. [DOI] [PubMed] [Google Scholar]
- 58.Wei A, Tao H, Chung SA, Brisby H, Ma DD, Diwan AD. The fate of transplanted xenogeneic bone marrow-derived stem cells in rat intervertebral discs. J Orthop Res. 2009;27(3):374–379. doi: 10.1002/jor.20567. [DOI] [PubMed] [Google Scholar]
- 59.Wuertz K, Godburn K, Neidlinger-Wilke C, Urban J, Iatridis JC. Behavior of mesenchymal stem cells in the chemical microenvironment of the intervertebral disc. Spine. 2008;33(17):1843–1849. doi: 10.1097/BRS.0b013e31817b8f53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wuertz K, Godburn K, Iatridis JC. MSC response to pH levels found in degenerating intervertebral discs. Biochem Biophys Res Commun. 2009;379(4):824–829. doi: 10.1016/j.bbrc.2008.12.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang X, Li X. Nucleus pulposus tissue engineering: a brief review. Eur Spine J. 2009;18(18):1–9. doi: 10.1007/s00586-009-1092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang SH, Wu CC, Shih TT, Sun YH, Lin FH. In vitro study on interaction between human nucleus pulposus cells and mesenchymal stem cells through paracrine stimulation. Spine. 2008;33(18):1951–1957. doi: 10.1097/BRS.0b013e31817e6974. [DOI] [PubMed] [Google Scholar]
- 63.Zeiter S, der Werf M, Ito K. The fate of bovine bone marrow stromal cells in hydrogels: a comparison to nucleus pulposus cells and articular chondrocytes. J Tissue Eng Regen Med. 2009;3(4):310–320. doi: 10.1002/term.167. [DOI] [PubMed] [Google Scholar]
- 64.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7(2):211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]